Aguirre D.A. et al. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at multi-detector row CT. Radiographics 2005;25(6):1501–1520.
Alam A. et al. The accuracy of ultrasound in the diagnosis of clinically occult groin hernias in adults. Eur Radiol 2005;15(12):2457–2461.
Atr M. et al. Surgically important bowel and/or mesenteric injury in blunt trauma: accuracy of multidetector CT for evaluation. Radiology 2008;249(2):524–533.
Burkhardt J.H. et al. Diagnosis of inguinal region hernias with axial CT: the lateral crescent sign and other key findings. Radiographics 2011;31(2):E1–E12.
Carucci L.R. et al. Internal hernia following Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of radiographic findings at small-bowel examination. Radiology 2009;251(3):762–770.
Catalano O.A. et al. Internal hernia with volvulus and intussusception: case report. Abdom Imaging 2004;29(2):164–165.
Chavhan G.B. et al. Multimodality imaging of the pediatric diaphragm: anatomy and pathologic conditions. Radiographics 2010;30(7):1797–1817.
Cherian P.T. et al. Radiologic anatomy of the inguinofermal region: insights from MDCT. AJR 2007;189(4):W177–W183.
Cherian P.T. et al. The diagnosis and classification of inguinal and femoral hernia on multisection spiral CT. Clin Radiol 2008;63(2):184–192.
Cioppa T. et al. Cytoreduction and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis from pseudomyxoma peritonei. World J Gastroenterol 2008;14(44):6817–6823.
Cronin C.G. et al. Retroperitoneal fibrosis: a review of clinical features and imaging findings. AJR 2008;191(2):423–431.
Cronin C.G. et al. Pictorial essay: multitechnique imaging findings of prolene plug hernia repair. AJR 2010;195:701–706.
Demir M.K. et al. Case 108: sclerosing encapsulating peritonitis. Radiology 2007;242(3):937–939.
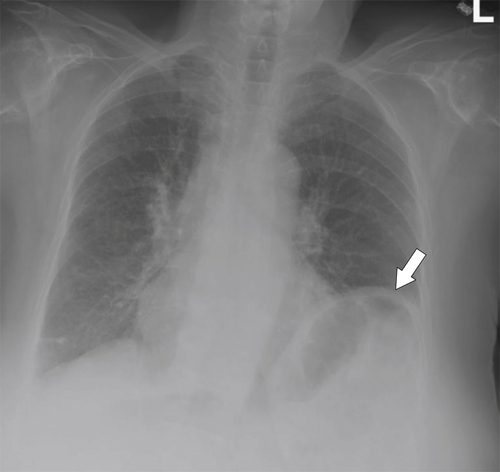
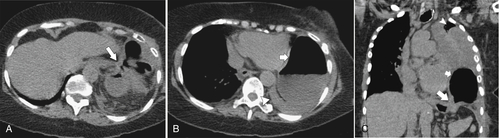
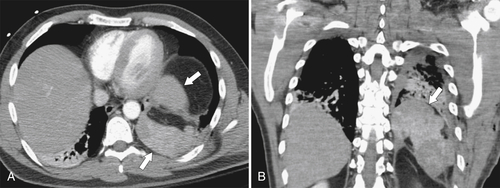
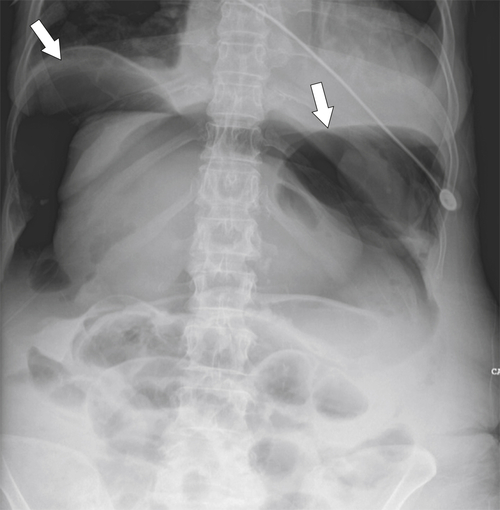
Desir A., Ghaye B. CT of blunt diaphragmatic rupture. Radiographics 2012;32(2):477–498.
Dinauer P.A. et al. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics 2007;27(1):173–187.
Fuks D. et al. CT can help the surgeon consider conversion from laparoscopic to open cholecystectomy. Radiology 2012;263(1):128–138.
Fukukura Y. et al. Autoimmune pancreatitis associated with idiopathic retroperitoneal fibrosis. AJR 2003;181(4):993–995.
Garg P.K. et al. Subcutaneous and breast metastasis from asymptomatic gallbladder carcinoma. Hepatobiliary Pancreat Dis Int 2009;8(2):209–211.
Gayer G. et al. Foreign objects encountered in the abdominal cavity at CT. Radiographics 2011;31(2):409–428.
George C. et al. Computed tomography appearances of sclerosing encapsulating peritonitis. Clin Radiol 2007;62(8):732–737.
Hanbidge A.E. et al. US of the peritoneum. Radiographics 2003;23(3):663–684: discussion 684-685.
Harshen R. et al. Pseudomyxoma peritonei. Clin Oncol (R Coll Radiol) 2004;15(2):73–77.
Horton K.M. et al. CT findings in sclerosing mesenteritis (panniculitis): spectrum of disease. Radiographics 2003;23(6):1561–1567.
Iannuccilli J.D. et al. Sensitivity and specificity of eight CT signs in the preoperative diagnosis of internal mesenteric hernia following Roux-en-Y gastric bypass surgery. Clin Radiol 2009;64(4):373–380.
Jacquemin G. et al. Pseudomyxoma peritonei: review on a cluster of peritoneal mucinous diseases. Acta Chir Belg 2005;105(2):127–133.
Jaffe T.A. et al. Practice patterns in percutaneous image-guided intraabdominal abscess drainage: survey of academic and private practice centers. Radiology 2004;233(3):750–756.
Jayne D.G. The molecular biology of peritoneal carcinomatosis from gastrointestinal cancer. Ann Acad Med Singapore 2003;32(2):219–225.
Johnson P.T. et al. The elephant trunk procedure for aortic aneurysm repair: an illustrated guide to surgical technique with CT correlation. AJR 2011;197:W1052–W1059.
Kamaya A. et al. Imaging manifestations of abdominal fat necrosis and its mimics. Radiographics 2011;31(7):2021–2034.
Kandpal H. et al. Combined transmesocolic and left paraduodenal hernia: barium, CT and MRI features. Abdom Imaging 2007;32(2):224–227.
Kim S.H. et al. Esophageal varices in patients with cirrhosis: multidetector CT esophagography—comparison with endoscopy. Radiology 2007;242(3):759–768.
Kreuzberg B. et al. Diagnostic problems of abdominal desmoids tumors in various locations. Eur J Radiol 2007;62(2):180–185.
Larici A.R. et al. Helical CT with sagittal and coronal reconstructions: accuracy for detection of diaphragmatic injury. AJR 2002;179(2):451–457.
Lee J.C. et al. Aggressive fibromatosis: MRI features with pathologic correlation. AJR 2006;186(1):247–254.
Lee W.K. et al. Infected (mycotic) aneurysms: spectrum of imaging appearances and management. Radiographics 2008;28(7):1853–1868.
Levy A.D. et al. From the archives of the AFIP: benign fibrous tumors and tumorlike lesions of the mesentery: radiologic-pathologic correlation. Radiographics 2006;26(1):245–264.
Lockhart M.E. et al. Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR 2007;188:745–750.
Low R.N. Diffusion-weighted MR imaging for the whole body metastatic disease and lymphadenopathy. Magn Reson Imaging Clin N Am 2009;17(2):245–261.
Ly J.Q. The Rigler sign. Radiology 2003;228(3):706–707.
Martin L.C. et al. Review of internal hernias: radiographic and clinical findings. AJR 2006;186(3):703–717.
McCarville M.B. et al. MRI and biologic behavior of desmoids tumors in children. AJR 2007;189(3):633–640.
McDonald E.S. et al. Best cases from the AFIP: extraabdominal desmoids-type fibromatosis. Radiographics 2008;28(3):901–906.
Morikawa T. et al. Recurrent prostatic stromal sarcoma with massive high-grade prostatic intraepithelial neoplasia. J Clin Pathol 2007;60(3):330–332.
Nason L.K. et al. Imaging of the diaphragm: anatomy and function. Radiographics 2012;32(2):E51–E70.
Nishie A. et al. Fitz-Hugh-Curtis syndrome: radiologic manifestation. J Comput Assist Tomogr 2003;27(5):786–791.
Nishino M. et al. Primary retroperitoneal neoplasms: CT and MR findings with anatomic and pathologic diagnostic clues. Radiographics 2003;23(1):45–57.
Osadchy A. et al. Small bowel obstruction related to left side paraduodenal hernia: CT findings. Abdom Imaging 2005;30(1):53–55.
Park C.M. et al. Recurrent ovarian malignancy: patterns and spectrum of imaging findings. Abdom Imaging 2003;28(3):404–415.
Power N. et al. CT assessment of anastomotic bowel leak. Clin Radiol 2007;62(1):37–42.
Purysko A.S. et al. Beyond appendicitis: common and uncommon gastrointestinal causes of right lower quadrant abdominal pain at multidetector CT. Radiographics 2011;31(4):927–947.
Raptopoulos V. et al. Peritoneal carcinomatosis. Eur Radiol 2001;11(11):2195–2206.
Reddy S.A. et al. Clinical observations: diagnosis of transmesocolic internal hernia as a complication of retrocolic gastric bypass: CT imaging criteria. AJR 2007;189:52–55.
Rees O. et al. Multidetector-row CT of right hemidiaphragmatic rupture caused by blunt trauma: a review of 12 cases. Clin Radiol 2005;60(12):1280–1289.
Robinson P. et al. Inguinofemoral hernia: accuracy of sonography in patients with indeterminate clinical features. AJR 2006;187(5):1168–1178.
Sai V.F. et al. Colonoscopy after CT diagnosis of diverticulitis to exclude colon. Radiology 2012;263:383–390.
Schwartz S.A. et al. CT findings of rupture, impending rupture, and contained rupture of abdominal aortic aneurysms. AJR 2007;188(1):W57–W62.
Shadbolt C.L. et al. Imaging of groin masses: inguinal anatomy and pathologic conditions revisited. Radiographics 2001;21(Spec. No):S261–S271.
Shinagare A.B. et al. Pictorial essay: A to Z of desmoid tumors. Am J Roentgenol 2011;197(6):W1008–W1014.
Singh A.K. et al. Neoplastic iliopsoas masses in oncology patients: CT findings. Abdom Imaging 2008;33(4):493–497.
Singh A.K. et al. Omental infarct: CT imaging features. Abdom Imaging 2006;31:549–554.
Sunnapwar A. et al. Taxonomy and imaging spectrum of small bowel obstruction after Roux-en-Y gastric bypass surgery. AJR 2010;194:120–128.
Tamsma J.T. et al. Pathogenesis of malignant ascites: Starling’s law of capillary hemodynamics revisited. Ann Oncol 2001;12(10):1353–1357.
Tateishi U. et al. Nodal status of malignant lymphoma in pelvic and retroperitoneal lymphatic pathways: PET/CT. Abdom Imaging 2010;35(2):232–240.
Thornton E. et al. Pattern of the month: patterns of fat stranding. AJR 2011;197:W1–W14.
Ti J.P. et al. Pictorial essay: imaging features of encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. AJR 2010;195:W50–W54.
Tirkes T. et al. Peritoneal and retroperitoneal anatomy and its relevance for cross-sectional imaging. Radiographics 2012;32(2):437–451.
Tombak M.C. et al. Clinical perspective: an unusual cause of intestinal obstruction: abdominal cocoon. AJR 2010;194:W176–W178.
Wong W.L. et al. Best cases from the AFIP: multicystic mesothelioma. Radiographics 2004;24(1):247–250.
Zarvan N.P. et al. Abdominal hernias: CT findings. AJR 1995;164:1391–1395.