Chapter 32 Peritoneal Cavity and Gastrointestinal Tract
Introduction
The network of connections formed by the peritoneal reflections serves to provide continuity between the abdominal walls and the organs therein. In addition, it also bridges the retroperitoneum with the peritoneum. These connections not only serve a physiologic and life-sustaining role but also act as a pathway for spread of disease.1 These processes include inflammation, infection, trauma, and importantly, tumor.
Primary tumors of the peritoneum are rare.2 Most commonly, primary tumor elsewhere metastasize to the peritoneum. Patients present with vague and nonspecific symptoms. Therefore, imaging plays a key role in the diagnosis of peritoneal disease. Because of the spaces and compartments formed by the peritoneal reflections, there are predictable patterns of disease spread that can be anticipated with certain tumors. Common tumors that spread and metastasize to the peritoneum include stomach, colon, ovarian, and pancreatic cancer and lymphoma.
Peritoneal disease could not be assessed in a direct manner radiographically before the advent of cross-sectional imaging.3 With improving technologies such as multidetector row computed tomography (CT), positron-emission tomography/computed tomography (PET/CT), magnetic resonance imaging (MRI), and ultrasound, peritoneal metastasis can now be readily evaluated and followed on scans for surveillance.
Anatomy
Embryology
To fully understand the peritoneum, a basic understanding of its embryologic development is necessary.4–7 However, to explain the complex and detailed embryologic development of the peritoneum is not the purpose of this chapter. Briefly, the primitive gut is suspended within the peritoneal cavity by a dorsal and ventral primitive mesentery, which divides the peritoneum into a right and a left cavity. Unlike the ventral mesentery, the dorsal mesentery does not stop its attachment at the stomach, but continues to connect the primitive gut to the posterior abdominal wall inferiorly. The absence of the ventral mesentery in the lower abdomen allows for communication between the left and the right abdominal cavity.
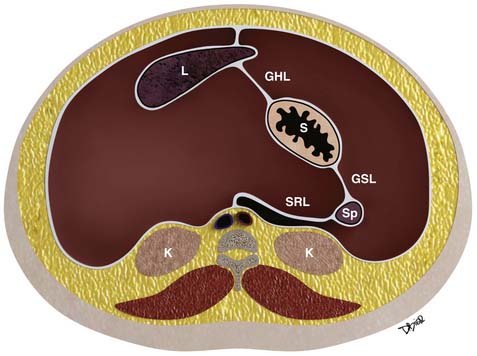
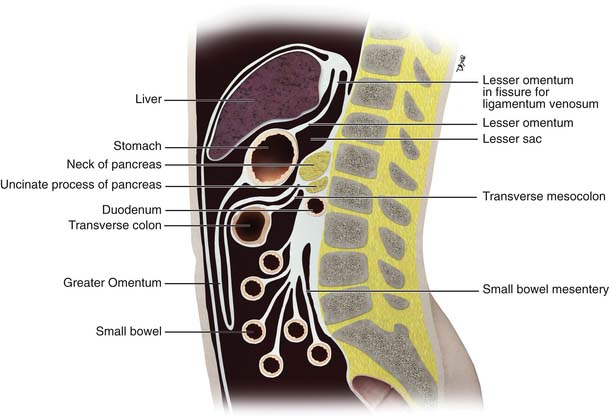
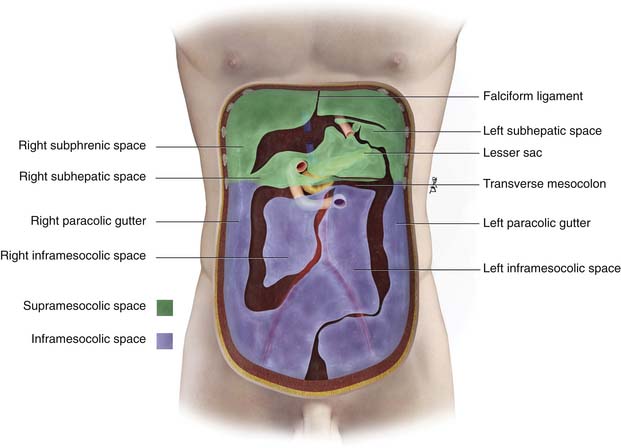
Further growth, organ development, elongation, cavitation, and rotation form the adult peritoneum (Figures 32-1 and 32-2). This includes the formation of the lesser sac, which is isolated from the remainder of the peritoneum or greater sac, except at a small opening called the foramen of Winslow.
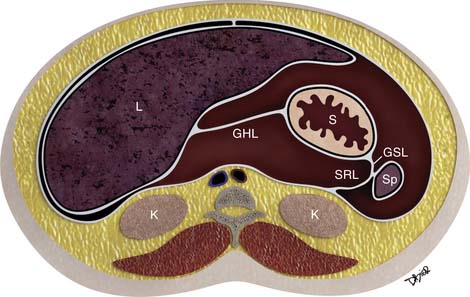
Figure 32-2 Peritoneal structures in the adult, position and relationships.
(Illustration courtesy of David Bier.)
A single layer of mesothelial cells forms the peritoneum. It is separated from the submesothelial layer of connective tissue by a basal lamina. The submesothelial layer of connective tissue consists of collagen, elastic fibers, fibroblast-like cells, arteries, veins, and lymphatics.6 The submesothelial layer or subperitoneal space is a virtual space that allows continuity between the mesenteries, the ligaments, the abdominal wall, and the retroperitoneum, as are described further.
Visceral and Parietal Peritoneum
The peritoneum is classified as either visceral or parietal.8 The abdominal and pelvic walls are lined by the parietal peritoneum. This includes the anterior surface of the retroperitoneum, the abdominal wall, and the undersurface of the hemidiaphragm. Conversely, the visceral peritoneum covers the intraperitoneal organs or viscera and forms the omenta and mesenteries.
In males, the greater peritoneal cavity is a closed continuous cavity. Conversely, in females, it is discontinuous at the ostia of the oviducts, providing a communication between the peritoneal cavity and the lower pelvis, which is extraperitoneal.6
Double folds of the peritoneum result in formation of ligaments and mesenteries. They suspend and form the supporting structure for the peritoneal organs. For example, the mesentery suspends the small bowel within the peritoneal cavity. The mesentery serves also to carry the arteries, lymphatics, and nerves. The omenta are formed from a double fold of the visceral peritoneum that extends from the stomach (Figures 32-3 and 32-4). The peritoneal cavity is divided into various interconnecting compartments by the ligaments and mesenteries.
Supramesocolic and Inframesocolic Space
The peritoneal cavity is divided into a supramesocolic and an inframesocolic space by the transverse mesocolon9 (Figure 32-5). The supramesocolic space is further divided into the right and left supramesocolic space by the falciform ligament. The left supramesocolic space is then further subdivided into the anterior and posterior perihepatic spaces. The right supramesocolic space also is subdivided into the anterior perihepatic space and a posterior compartment known as the lesser sac. The right and left supramesocolic space communicates via the foramen of Winslow, allowing communication between the lesser sac and the remainder of the peritoneal cavity or greater sac. There is continuity between the right paracolic gutter and the right supramesocolic space. However, the phrenicocolic ligament acts as a barrier between the left paracolic gutter and the left supramesocolic space.
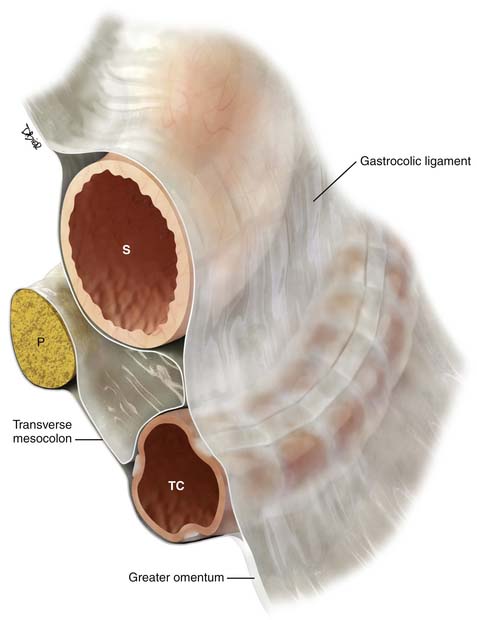
Important supporting ligaments in the supramesocolic space include the gastrohepatic ligament, hepatoduodenal ligament, gastrocolic ligament, gastrosplenic ligament, and splenorenal ligament (Figures 32-6 to 32-8). The ligaments are anatomically connected and continuous and their location and relationship can be identified by certain landmarks, mostly vasculature (Table 32-1).
Table 32-1 Supramesocolic Ligaments: Their Organ Relationship and Landmarks
| LIGAMENT | ORGAN RELATIONSHIP | LANDMARKS |
|---|---|---|
| Gastrohepatic ligament | Lesser curvature of the stomach to left hepatic lobe | Left gastric vessels and left gastric nodal station |
| Hepatoduodenal ligament | Lesser curvature of the stomach to the hepatic hilum | Portal vein, hepatic artery, extrahepatic bile duct, and nodal stations |
| Gastrocolic ligament/supracolic omentum | Greater curvature of the stomach to the body of the transverse colon | Perigastric branches of the left gastroepiploic vessels with anastomosis to the right gastroepiploic vessels |
| Greater omentum | Transverse colon extending as an apron anterior to the small bowel | Epliploic vessels and branches of the gastroepiploic vessels |
| Gastrosplenic ligament | Continuous and to the left of the gastrocolic ligament, from the greater curvature of the fundus and upper body of the stomach to the splenic hilum | Short gastric vessels and left gastroepiploic vessels |
| Splenorenal ligament | Continuity between the spleen and the tail of the pancreas | Distal splenic artery or proximal splenic vein |
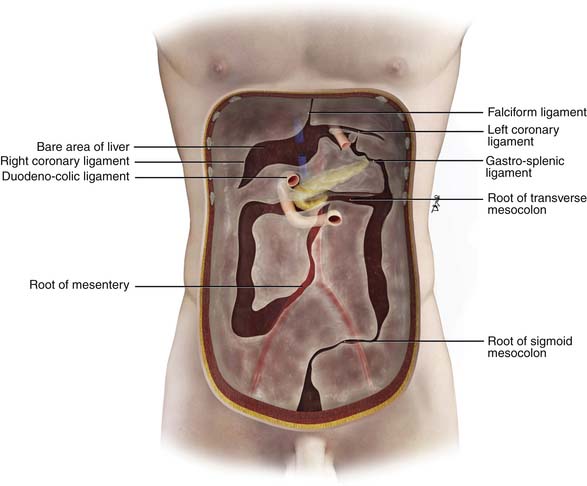
The inframesocolic compartment is divided into a right and a left inframesocolic space by the obliquely oriented small bowel mesentery. The ascending colon provides the lateral border of the right inframesocolic space. The inframesocolic compartment consists of the root of the mesentery, jejunal mesentery, ileal mesentery, ascending mesocolon, descending mesocolon, sigmoid mesentery, and the pelvic floor and peritoneal folds (Table 32-2).
Table 32-2 Inframesocolic Compartment, Organ Relationship, and Landmarks
| LIGAMENT | ORGAN RELATIONSHIP | VASCULAR LANDMARKS |
|---|---|---|
| Root of mesentery | From the horizontal portion of the duodenum to the right iliac fossa | SMA, SMV, and ileocolic artery and vein |
| Ileal mesentery | From the root of the mesentery to the ileum | Ileal artery and veins |
| Ascending mesocolon | Root of the mesentery to the ascending colon | Right colic artery and vein, cecal artery and vein |
| Jejunal mesentery | From the base of the mesentery to the jejunum | Jejunal artery and vein |
| Descending mesocolon | Base of the transverse mesocolon along the tail of the pancreas to the descending colon | Left colic artery and vein |
| Sigmoid mesocolon | Root of the sigmoid mesocolon | Sigmoid arteries, superior hemorrhoidal artery and vein |
SMA, superior mesenteric artery; SMV, superior mesenteric vein.
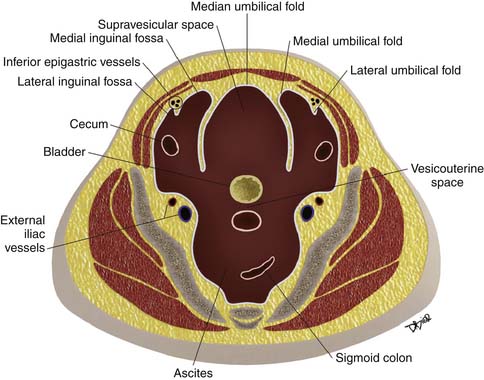
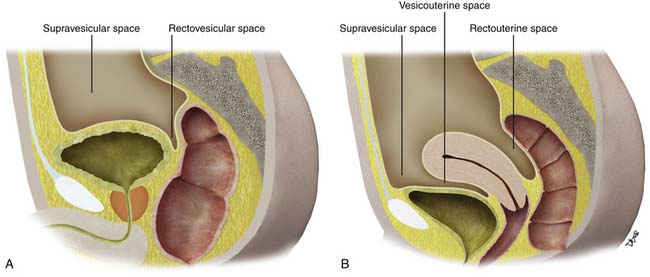
Paravesicular Spaces
Peritoneal folds or reflections in the pelvis also result in potential spaces and compartments10–12 (Figures 32-9 and 32-10). The urinary bladder, obliterated umbilical arteries, and inferior epigastric vessels indent upon the parietal peritoneum to form the anterior and posterior paravesicular spaces.
The lateral inguinal fossa is the site for the internal inguinal ring and can contain portions of the cecum or sigmoid colon. The medial inguinal fossa can also contain portions of the cecum or sigmoid colon and is the site for the femoral canal.
These large potential spaces can be easily delineated when there is accumulation of ascites, abscesses, metastasis, and other disease processes (Figures 32-11 and 32-12).
Key Points Anatomy
• Embryologic development of the peritoneum is complex; basic knowledge of its development aids in an understanding of disease spread.
• Peritoneum lines the abdominal and pelvic walls and organs, aiding in lubrication, organ support, movement, and host defense.
• Foldings of the peritoneum form the ligaments and mesenteries, suspending and supporting internal organs.
• Ligaments and mesenteries create the compartments and potential spaces within the abdominal and pelvic cavity.
Spread of Disease
There are four established pathways of tumor spread and metastasis in the peritoneal cavity.1,13 First, tumor can spread directly from the tumor through the serosa, through the peritoneal lining, and to an adjacent organ. It can also spread directly within the peritoneal ligaments, mesenteries, and omenta along the “subperitoneal space” to noncontiguous organs in a perilymphatic, perineural, perivascular, or periductal manner. Second, tumor can spread by intraperitoneal seeding along the flow of ascitic fluid. Third, they can follow the lymphatics. Finally, tumor can also embolize hematogenously.
Direct Spread
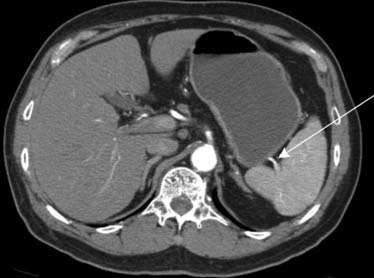
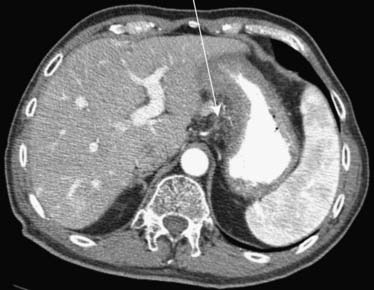
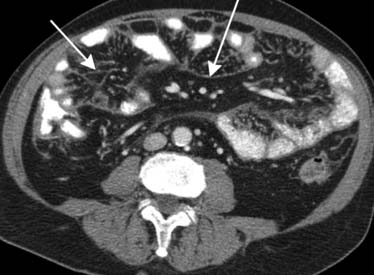
Carcinoma of the stomach, colon, and pancreas commonly involves the subperitoneal space of the ligaments, mesenteries, and omenta.14 For instance, gastric cancer will commonly use the lesser omentum/gastrohepatic ligament to spread metastatic disease to the left hepatic lobe. This can be identified when there is loss of a fat plane in the lesser omentum as seen on CT imaging (Figure 32-13).
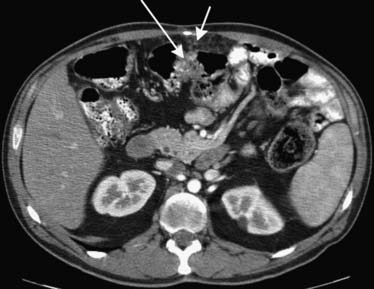
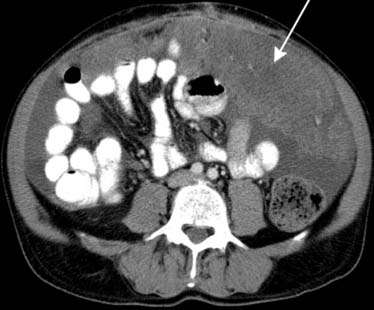
Similarly, the greater omentum and transverse mesocolon can serve as pathways for subperitoneal spread of cancer of the stomach, pancreas, and colon. When cancer has spread to the greater omentum, “omental caking” can be identified15 (Figures 32-14 and 32-15).
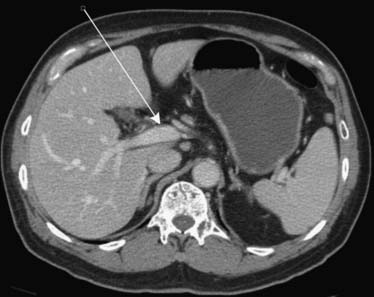
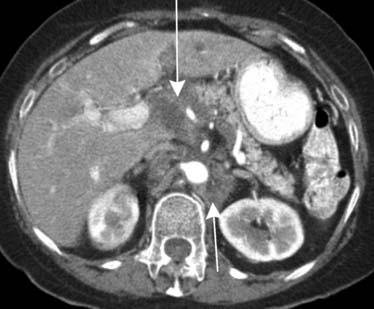
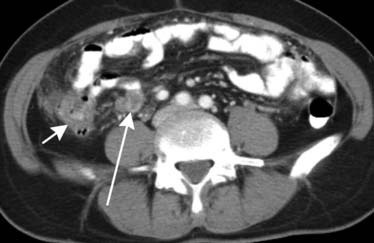
Pancreatic cancer can spread directly to the liver via the hepatoduodenal ligament. The hepatoduodenal ligament again contains the portal vein, common bile duct, and hepatic artery (Figure 32-16). Less frequently, the lesser omentum can serve as a bidirectional bridge. That is, disease can spread in a reverse fashion from the liver back to the stomach.
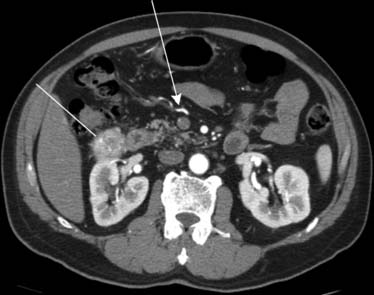
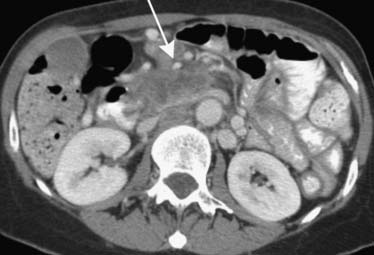
The root of the mesentery extends from the horizontal portion of the duodenum to the iliac fossa. Lymphoma, carcinoid, pancreatic, and colonic cancer will commonly involve the root of the mesentery and utilize it as a pathway for spread of disease. Imaging will reveal mesenteric soft tissue thickening, perivascular encasement, and tethering of bowel1 (Figure 32-17).
Intraperitoneal Seeding
Spread of disease such as metastasis within the peritoneal cavity is influenced by physical forces such as gravity and negative subdiaphragmatic pressure.1 Dynamic circulation, flow, and pooling of ascites within dependent peritoneal recesses also serve to spread and deposit disease.
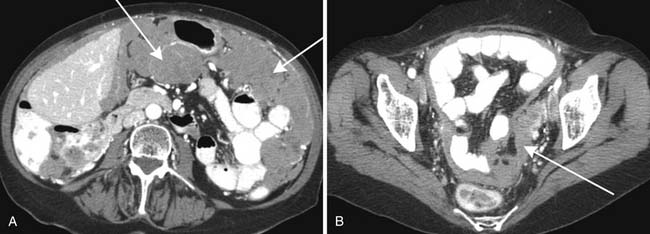
Common sites for intraperitoneal spread of metastatic disease are the pouch of Douglass/rectovesicular space in the pelvis, right lower quadrant at the inferior junction of the small bowel mesentery, superior aspect of the sigmoid mesocolon, and right paracolic gutter. When there are intraperitonal metastases, they can be identified as soft tissue nodules or plaquelike soft tissue masses on imaging such as CT. There may be associated ascites. Peritoneal metastatic nodules as small as 5 mm can be identified with CT imaging. Likewise, parietal peritoneal involvement will produce smooth or nodular thickening with enhancement when visualized by CT imaging.
Serous cystadenocarcinoma may produce diffuse peritoneal calcifications, especially after treatment.16 Carcinoid and, less commonly, gastric and colon cancer will also elicit peritoneal calcifications.8
Pseudomyxoma peritonei from mucinous tumor of the appendix or mucinous carcinomatosis from a gastrointestinal tract or ovarian primary can also be identified in the peritoneal cavity. Mucin secretion will be cystic in appearance by imaging. They exert pressure upon the viscera, causing scalloping along the surface of organs such as the liver, spleen, or bowel17 (Figure 32-18). The pressure from the mucin will occasionally prevent bowel from floating upward, unlike ascites. Mucin will also occasionally calcify.
Lymphatic Spread
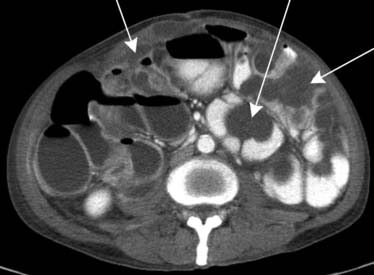
Spread of disease can also occur via the lymphatics.18 However, the lymphatics play a minor role, except in lymphoma, where 50% will be non-Hodgkin’s disease. Round or oval mesenteric masses will be manifested with displacement of bowel. A sandwich sign has also been described.19 This is seen when mesenteric and retroperitoneal lymphadenopathy are separated by a fat plane (Figure 32-19).
Hematogenous Spread
Finally, metastatic disease to the peritoneum can spread hematogenously. For instance, the mesenteric arteries can carry metastasis to the antimesenteric side of the bowel, producing nodules, thickening, or ulceration. Common tumors that spread hematogenously include melanoma, breast, and lung (Figure 32-20).
Imaging Techniques
Computed Tomography
CT is widely used for imaging of the peritoneum and its associated disease processes. For metastatic disease, spiral CT demonstrated sensitivities ranging from 85% to 93%, specificities of 78% to 91%, positive predictive values of 88% to 98%, and negative predictive values of 78% to 88%. The sensitivity, however, is greatly reduced with nodules less than 1 cm (25-50%).3 Some studies found that sensitivities were highest for metastatic disease to the right paracolic gutter and infracolic omentum and when thin slices and multiplanar reconstruction were obtained.20
Magnetic Resonance Imaging
Although MRI is not as commonly used to assess for peritoneal metastasis as CT, it can be effective.2,21 As stated, CT sensitivity for peritoneal nodules less than 1 cm is low (25-50%). However, with superior contrast resolution, MRI sensitivities for peritoneal nodules less than 1 cm have been reportedly better (85-90%).22
Positron-Emission Tomography/Computed Tomography
PET/CT has also been found to be effective in the evaluation of peritoneal metastatic disease.23–27 Reported sensitivities for PET/CT in detection of peritoneal metastasis range from 78% to 100%.22 However, cystic or mucinous peritoneal carcinomatosis can be non–fluoro-2-deoxy-D-glucose (FDG)–avid and produce false-negative results on PET/CT owing to their low cellularity. In addition, like CT and MRI, PET/CT will be limited by spatial resolution. Lesions less than 1 cm may not have sufficient FDG uptake to be detectable.
Sonography
Key Points Imaging
• CT is widely available and is a robust imaging modality for the evaluation of peritoneal metastasis and tumor.
• MRI, PET/CT, and sonography are also available imaging modalities for the evaluation of peritoneal metastasis, within the appropriate clinical indication and expertise of the radiologist.
Approach to and Classifying of Peritoneal Disease
When encountering peritoneal disease, patient history, risk factors, laboratory studies, and radiographic evaluation will aid in the diagnosis. Classification of peritoneal disease can be divided into primary tumors of the peritoneum and secondary tumors and tumor-like lesions.22 A few examples of disease classifications are outlined in Tables 32-3 and 32-4.
| Mesothelial Tumors |
| Malignant mesothelioma |
| Epithelial Tumors |
| Primary peritoneal serous carcinoma |
| Smooth Muscle Tumor |
| Leiomyomatosis peritonealis disseminate |
| Tumors of Uncertain Origin |
| Solitary fibrous tumor |
Modified from Levy AD, Shaw JC, Sobin LH, from the Archives of the AFIP. Continuing medical education: secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
Table 32-4 Secondary Tumors and Other Diseases of the Peritoneal Cavity
| Metastic Neoplasms |
| Carcinomatosis |
| Pseudomyxoma peritonei |
| Lymphomatosis |
| Infection and Inflammation |
| Granulomatous peritonitis such as tuberculosis |
| Inflammatory pseudotumor |
| Miscellaneous |
| Endometriosis |
| Melanosis |
| Splenosis |
Modified from Levy AD, Shaw JC, Sobin LH, from the Archives of the AFIP. Continuing medical education: secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
When there are peritoneal masses with or without associated ascites, a differential diagnosis can be generated. With a history of prior asbestos exposure, malignant mesothelioma should be suspected. Imaging findings of sheetlike thickening of the peritoneum, lack of a primary malignancy, lack of lymphadenopathy, or lack of metastasis elsewhere should aid in the differential diagnosis6,28,29 (Figure 32-21).
Malignant peritoneal mesothelioma usually develops in individuals exposed to high levels of asbestos. Lower levels of exposure are usually associated with pleural malignant mesothelioma. Women exposed to absbestos are noted to have a lower rate of developing malignant mesothelioma.6 Only 23% of women with previous asbestos exposure will develop the disease. Other risk factors suspected of contributing to the development of malignant mesothelioma can include erionite (a mineral fiber found in Turkey), therapeutic irradiation, exposure to simian virus 40, and chronic pleural or peritoneal irritation.6 Younger patients have been found to occasionally develop the disease with no history of exposure.
Fatty and soft tissue peritoneal masses may reveal benign lipomas and neurofibromas, respectively. A history of neurofibromatosis type 1 can aid in the diagnosis. A more cystic multilocuated mass can be due to a rare benign condition found in women known as multicystic mesothelioma.30,31
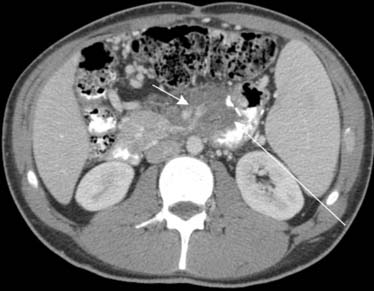
Secondary disease of the peritoneum can result from inflammation and infection. Tuberculosis (TB) and histoplasmosis are examples. Patients with human immunodeficiency virus (HIV) or other immunosupression, young adults between the ages of 20 and 40, patients with a history of illicit drug use, and patients from or with recent travel to endemic areas should be suspected of TB peritonitis in the appropriate setting. Clues to their diagnosis can be found when there is associated lymphadenopathy in the mesentery and retroperitoneum. Furthermore, the lymphadenopathy will usually contain low attenuation centrally with an enhancing rim.22,32 This is due to casseating necrosis (Figure 32-22). Calcification occasionally involves the lymph nodes.
Metastatic disease is the most common manifestation of a peritoneal neoplastic process. Indeed, when peritoneal masses are discovered, it will likely be due to metastasis. Culprits in the order of most common will be colorectal and ovarian carcinoma, followed by gastric, pancreatic, breast, lung, and melanoma.33
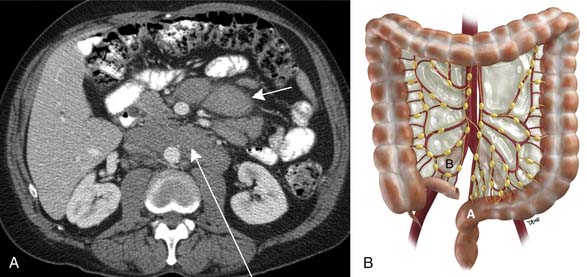
Understanding the peritoneal anatomy and the route of disease spread aids in suggesting the most likely diagnosis. For instance, if there is isolated lymphadenopathy along the superior hemorrhoidal distribution, a rectosigmoid primary tumor can be suspected to have spread along this route (see Figure 32-19B).
Findings of an adnexal pelvic mass with ascites, peritoneal nodules in dependent locations, and omental caking will be suspicious for a primary ovarian carcinoma. Intraperitoneal seeding is the most common form of disease spread for this cancer. Overall, peritoneal carcinomatosis usually will consist of peritoneal nodules and masses (Figure 32-23). Common locations for metastatic peritoneal nodules are found in the pouch of Douglass, ileocecal region, right paracolic gutter, sigmoid mesocolon, greater omentum, and right subdiaphragmatic parietal peritoneum. Ascites may or may not be present.
The diagnosis of peritoneal disease will be apparent for most patients in certain instances, because they will present with history of a known primary malignancy confirmed by clinical, pathologic, and radiologic evidence. Conversely, without a known malignancy, occasionally, it will be difficult to differentiate between a primary malignant peritoneal disease (such as malignant peritoneal mesothelioma), peritoneal metastatic disease, or infection such as TB. Clinical presentation will be nonspecific. Patients will often present with localized or generalized abdominal pain or discomfort, abdominal distention, digestive disturbances, pelvic or abdominal mass, and bowel obstruction.
1. Meyers M.A., Oliphant M., Berne A.S., et al. The peritoneal ligaments and mesenteries: pathways of intraabdominal spread of disease. Radiology. 1987;163:593-604.
2. Elsayes K.M., Staveteig P.T., Narra V.R., et al. MRI of the peritoneum: spectrum of abnormalities. AJR Am J Roentgenol. 2006;186:1368-1379.
3. Healy J.C., Reznek R.H. Peritoneal metastasis. In: Husband J.E.S., Reznek R. Imaging in Oncology. London: Martin Dunitz Ltd; 1998:841-852.
4. Sompayrac S.W., Mindelzun R.E., Silverman P.M., Sze R. The greater omentum. AJR Am J Roentgenol. 1997;168:683-687.
5. Silverman P. The subperitoneal space: mechanism of tumor spread within the peritoneal cavity, mesentery and omentum. Cancer Imaging. 2004;4:25-29.
6. Levy A.D., Arnáiz J., Shaw J.C., et al. Continuing medical education: from the archives of the AFIP: primary peritoneal tumors: imaging features with pathologic correlation. Radiographics. 2008;28:583-607.
7. Jeong Y.J., Kim S., Kwak S.W., et al. Neoplastic and nonneoplastic conditions of serosal membrane origin: CT findings. Radiographics. 2008;28:801-818.
8. Hamrick-Turner J.E., Chiechi M.V., Abbitt P.L., Ros P.R. Neoplastic and inflammatory processes of the peritoneum, omentum, and mesentery: diagnosis with CT. Radiographics. 1992;12:1051-1068.
9. Healy J.C., Reznek R.H. The peritoneum, mesenteries and omenta: normal anatomy and pathological processes. Eur Radiol. 1998;8:886-900.
10. Auh Y.H., Rubenstein W.A., Markisz J.A., et al. Intraperitoneal paravesical spaces: CT delineation with US correlation. Radiology. 1986;159:311-317.
11. Gambino J., Cohen A.J., Friedenberg R.M. The direction of bladder displacement by adnexal masses. Clin Imaging. 1983;17:8-11.
12. Auh Y.H., Rubenstein W.A., Schneider M., et al. Extraperitoneal paravesical spaces: CT delineation with US correlation. Radiology. 1986;159:319-328.
13. Sheth S., Horton K.M., Garland M.R., Fishman E.K. Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics. 2003;23:457-473.
14. Oliphant M., Berne A.S. Computed tomography of the subperitoneal space: demonstration of direct spread of intraabdominal disease. J Comput Assist Tomogr. 1982;6:1127-1137.
15. Woodward P.J., Hosseinzadeh K., Saenger J.S. from the archives of the AFIP: radiologic staging of ovarian carcinoma with pathologic correlation. Radiographics. 2004;24:225-246.
16. Agarwal A., Yeh B.M., Breiman R.S., et al. Peritoneal calcification: causes and distinguishing features on CT. AJR Am J Roentgenol. 2004;182:441-445.
17. Sulkin T.V.C., O’Neill H., Amin A.I., Moran B. CT in pseudomyxoma peritonei: A review of 17 cases. Clin Radiol. 2002;57:608-613.
18. Karaosmanoglu D., Karcaaltincaba M., Oguz B., et al. CT findings of lymphoma with peritoneal, omental and mesenteric involvement: Peritoneal lymphomatosis. Eur J Radiol. 2009;71:313-317.
19. Hokama A., Nakamoto M., Kinjo F., Fujita J. ‘The sandwich sign’ of mesenteric lymphoma. Eur J Haematol. 2006;77:363-364.
20. Pannu H.K., Horton K.M., Fishman E.K. Thin section dual-phase multidetector-row computed tomography detection of peritoneal metastases in gynecologic cancers. J Comput Assist Tomogr. 2003;27:333-340.
21. Low R.N., Barone R.M., Lacey C., et al. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology. 1997;204:513-520.
22. Levy A.D., Shaw J.C., Sobin L.H., from the archives of the AFIP. Continuing medical education: secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
23. Turlakow A., Yeung H.W., Salmon A.S., et al. Peritoneal carcinomatosis: role of 18F-FDG PET. J Nucl Med. 2003;44:1407-1412.
24. Anthony M.-P., Khong P.-L., Zhang J. Spectrum of 18F-FDG PET/CT appearances in peritoneal disease. AJR Am J Roentgenol. 2009;193:W523-W529.
25. Schwarz J.K., Grigsby P.W., Dehdashti F., et al. The role of 18F-FDG PET in assessing therapy response in cancer of the cervix and ovaries. J Nucl Med. 2009;50(Suppl 1):64S-73S.
26. Weber W.A. Assessing tumor response to therapy. J Nucl Med. 2009;50(Suppl 1):1S-10S.
27. Funicelli L., Travaini L.L., Landoni F., et al. Peritoneal carcinomatosis from ovarian cancer: the role of CT and [(18)F]FDG-PET/CT. Abdom Imaging. 2010;35:701-707.
28. Guest P.J., Reznek R.H., Selleslag D., et al. Peritoneal mesothelioma: the role of computed tomography in diagnosis and follow up. Clin Radiol. 1992;45:79-84.
29 Puvaneswary M., Chen S., Proietto T. Peritoneal mesothelioma: CT and MRI findings. Australas Radiol. 2002;46:91-96.
30. Safioleas M.C., Constantinos K., Michael S., et al. Benign multicystic peritoneal mesothelioma: a case report and review of the literature. World J Gastroenterol. 2006;12:5739-5742.
31. Jain K.A. Imaging of peritoneal inclusion cysts. AJR Am J Roentgenol. 2000;174:1559-1563.
32. Ha H.K., Jung J.I., Lee M.S., et al. CT differentiation of tuberculous peritonitis and peritoneal carcinomatosis. AJR Am J Roentgenol. 1996;167:743-748.
33. Gupta P. The straight line sign. Radiology. 2006;240:611-612.