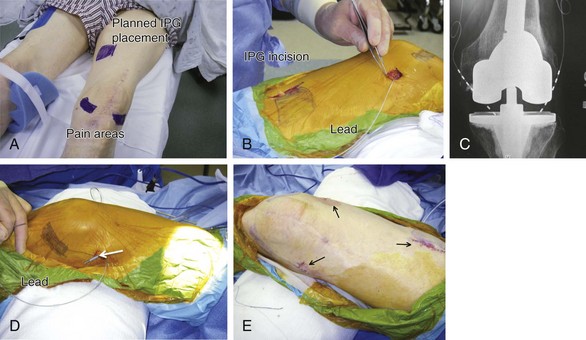
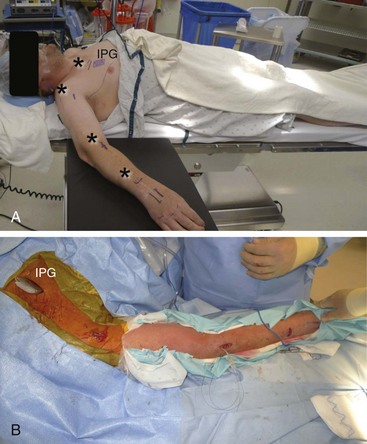
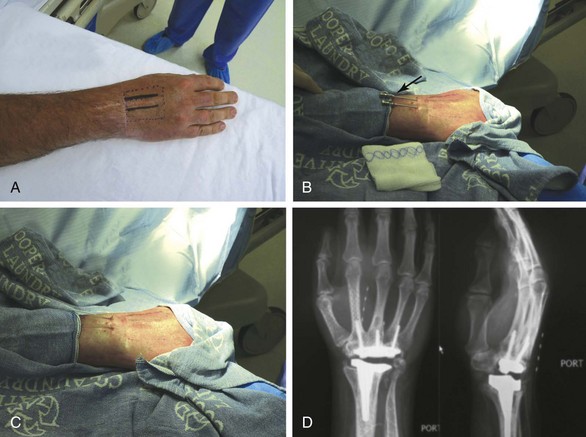
Chapter 20 Peripheral Subcutaneous Stimulation for Intractable Pain
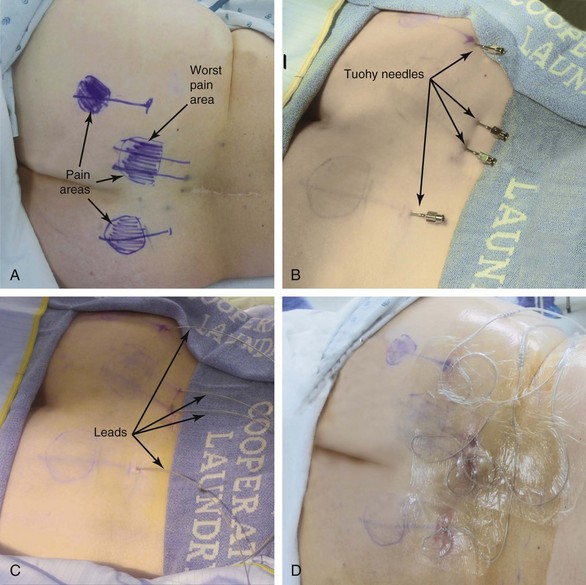
 If the areas of worse pain are very extensive (several square inches), the patient might not be a candidate for the procedure.
If the areas of worse pain are very extensive (several square inches), the patient might not be a candidate for the procedure. Always cover with the lead placement either the areas of “worse pain” or the areas where the pain starts (or both, if possible).
Always cover with the lead placement either the areas of “worse pain” or the areas where the pain starts (or both, if possible).Introduction
The technique is known with several different names, such as subcutaneous stimulation, peripheral nerve field stimulation, regional stimulation, and peripheral nerve stimulation.1–6 All of these definitions point to the fact that the target is the small peripheral nervous system fibers in the subcutaneous tissue. This is a paradigm switch from previous neurostimulation modalities, in which the stimulation is applied to a well-defined large neural structure (i.e., a large peripheral nerve, the nerve roots, or the spinal cord).
Although the mechanisms of action are unknown, they are most likely similar to the ones described for peripheral nerve stimulation.7
Technique1
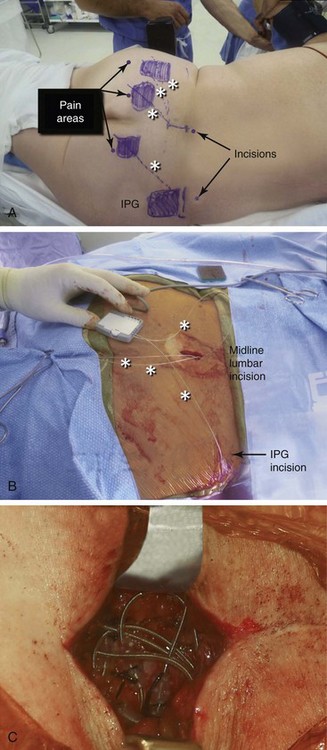
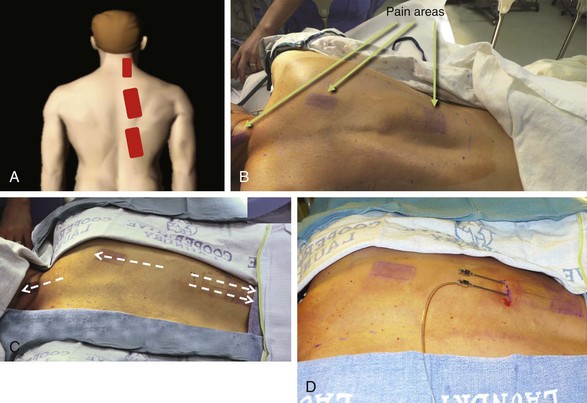
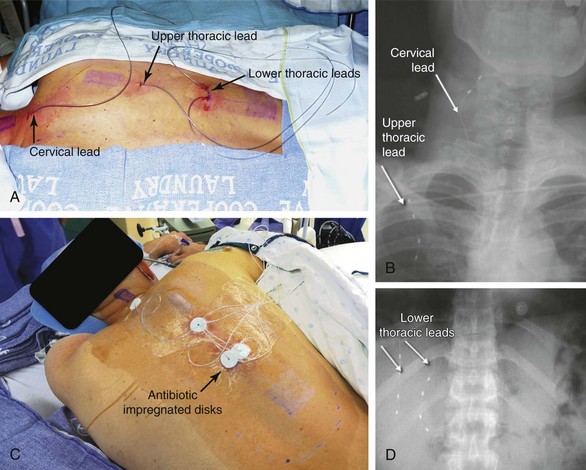
Almost any area of the body can be reached with this technique. The most commonly addressed areas include lumbar, posterior thoracic, scapular, inguinal, and various regions of the head and face1–6, 8–17 (Figs. 20-1 to 20-4).
The depth of the needle should satisfy three criteria:
 There should be no indentations in the skin. Indentations mean that the needle is actually in the dermis. If the needle is in the dermis, advancing it is very difficult, and one meets a lot of resistance. If the patient is semiawake, he or she demonstrates a lot of resistance since advancing a needle in the dermis is extremely painful.
There should be no indentations in the skin. Indentations mean that the needle is actually in the dermis. If the needle is in the dermis, advancing it is very difficult, and one meets a lot of resistance. If the patient is semiawake, he or she demonstrates a lot of resistance since advancing a needle in the dermis is extremely painful. One should be able to visualize a slight “tenting up” caused by the needle in the subcutaneous tissue, and certainly one should be able to readily feel the needle with finger palpation. If the needle cannot be felt, its location is too deep.
One should be able to visualize a slight “tenting up” caused by the needle in the subcutaneous tissue, and certainly one should be able to readily feel the needle with finger palpation. If the needle cannot be felt, its location is too deep.Indications
Both nociceptive and neuropathic pain can respond positively to this modality.
Criteria for the trial procedure include: (1) severe pain of at least a 6-month duration, (2) failure of conservative treatment, and (3) psychological stability. Both axial and limb painful areas can be targeted (Figs. 20-5 to 20-7). In the author’s practice the most common indications include intractable axial lumbar pain (whether the patient has received surgical intervention or not), intractable posterior thoracic pain, intractable scapular pain, inguinal pain, thoracic wall pain, shoulder pain, headaches, and atypical facial pain. I have not placed subcutaneous leads in the perineum. I have implanted many patients with severe intractable low back pain whose other alternative was a multilevel spine fusion. When dealing with exclusively axial pain and in the presence of multilevel lumbar spine degenerative disease, a trial with PSS seems to be a reasonable option.
PSS can also be used in conjunction with intraspinal stimulation and/or large peripheral nerve stimulation.8 I have several patients in whom both modalities were successfully implemented jointly, either as separate procedures or as part of a single implant procedure. It is not unusual to be able to adequately cover pain in the lower extremities with intraspinal leads, but to rely on leads placed subcutaneously to address pain in the axial low lumbar area. Another common situation for a “mixed” lead placement is in patients who have upper-extremity and scapular pain. In this instance one lead can be placed intraspinally in the cervical area, and another in the scapular area subcutaneously. Both the intraspinal and the subcutaneous leads can be plugged in the same IPG (provided that the total number of contacts does not exceed the capacity of the system). The current can be programmed to flow between the intraspinal and the subcutaneous leads; this might lead to an even broader field of stimulation.
Peripheral Subcutaneous Stimulation and Transcutaneous External Nerve Stimulation
There are four reasons why TENS might not work:
 The patient might have skin hypersensitivity or allodynia in the pain areas and might not tolerate the electrodes on the skin.
The patient might have skin hypersensitivity or allodynia in the pain areas and might not tolerate the electrodes on the skin.Results
Only case reports are available in the literature at the time of writing of this chapter. There are no large series reports or prospective studies. Positive results have been reported in patients with intractable lumbar axial pain, inguinal neuralgia, neuropathic pain, abdominal pain, postherpetic neuralgia, and cervicogenic headaches, to name a few. 3–6, 8–19
The most comprehensive retrospective study is the one by Verrills and associates.3 The authors collected data on 13 consecutive patients who had successful trials and were subsequently implanted with Octrode percutaneous leads placed subcutaneously within the major area of pain. Eleven patients met diagnostic criteria for failed back surgery syndrome. A questionnaire assessed outcomes, including pain, analgesic use, and patient satisfaction. The response rate was 93% (13/14; average follow-up time was 7 months). There was a significant decrease in pain levels: an average reduction of 3.77 visual analog scale (VAS) points. Eleven patients (85%) reported successful outcomes and an average pain reduction of 4.18 points, but two reported a poor response. Pain relief was highly correlated with reduced analgesia and patient satisfaction. No complications were reported. Before peripheral nerve stimulation (PNS), the mean VAS was 7.42 ± 1.16 (range 5 to 10). Following PNS, the mean VAS was 3.92 ± 1.72 (range 1 to 6), representing a mean improvement in VAS of 3.77 ± 1.65 (range 1 to 6.5). As a response to PNS, patients were asked to rank their satisfaction with the procedure. One patient considered the outcome to be completely satisfactory. Two patients were very satisfied, and seven were satisfied with the procedure. Two patients were not completely satisfied, and one was not satisfied with PNS.
1 Barolat G. Techniques for subcutaneous peripheral nerve field stimulation for intractable pain. In: Krames E, Peckham H, Rezai A, editors. Neuromodulation. St Louis: Elsevier; 2009:1017-1020.
2 Abejón D, Elliot S. Krames peripheral nerve stimulation or is it peripheral subcutaneous field stimulation; What is in a moniker? Neuromodulation. 2009;12:1-4.
3 Verrills P, et al. Peripheral nerve stimulation: a treatment for chronic low back pain and failed back surgery syndrome? Neuromodulation. 2009;12:68-75.
4 Al Tamimi M, et al. Successful treatment of chronic neuropathic pain with subcutaneous peripheral nerve stimulation: four case reports. Neuromodulation. 2009;3:210-214.
5 Bernstein CA, et al. Spinal cord stimulation in conjunction with peripheral nerve field stimulation for the treatment of low back and leg pain: a case series. Neuromodulation. 2008;11:116-123.
6 Krutsch JP, et al. A case report of subcutaneous peripheral nerve stimulation for the treatment of axial back pain associated with postlaminectomy syndrome. Neuromodulation. 2008;11:112-115.
7 Ellrich J, Lamp S. Peripheral nerve stimulation inhibits nociceptive processing: an electrophysiological study in healthy volunteers. Neuromodulation. 2005;8:225-232.
8 Lipov EG, Joshi JR, Slavin KV. Hybrid neuromodulation technique: use of combined spinal cord stimulation and peripheral nerve stimulation in treatment of chronic pain in back and legs. Acta Neurochir (Wien). 2008;150:971.
9 Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007;14:216-221.
10 Paicius RM, Bernstein CA, Lempert-Cohen C. Peripheral nerve field stimulation for the treatment of chronic low back pain: preliminary results of long-term follow-up: a case series. Neuromodulation. 2007;10:279-290.
11 Paicius RM, Bernstein CA, Lempert-Cohen C. Peripheral nerve field stimulation in chronic abdominal pain. Pain Physician. 2006;9:261-266.
12 Rodrigo-Royo MD, et al. Peripheral neurostimulation in the management of cervicogenic headache: four case reports. Neuromodulation. 2005;8:241-248.
13 Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58:112-119.
14 Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008;5:100-106.
15 Stinson LW, et al. Peripheral subcutaneous electrostimulation for control of intractable postoperative inguinal pain: a case report series. Neuromodulation. 2001;4:99-104.
16 Yakovlev AE, Peterson A. Peripheral nerve stimulation in treatment of intractable postherpetic neuralgia. Neuromodulation. 2007;10:373-375.
17 Lipov E, et al. Use of peripheral subcutaneous field stimulation for the treatment of axial neck pain: a case report. Neuromodulation. 2009;12:292-295.
18 Ordia J, Vaisman J. Subcutaneous peripheral nerve stimulation with paddle lead for treatment of low back pain: case report. Neuromodulation. 2009;12:205-209.
19 Reverberi C, Bonezzi C, Demartini L. Peripheral subcutaneous neurostimulation in the management of neuropathic pain: five case reports. Neuromodulation. 2009;12:146-155.