9 Peripheral nerves
General Features
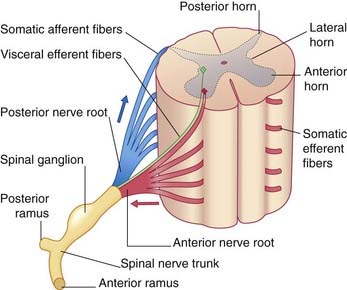
The peripheral nerves comprise the cranial and spinal nerves linking the brain and spinal cord to the peripheral tissues. The spinal nerves are formed by the union of anterior and posterior nerve roots at their points of exit from the vertebral canal (Figure 9.1). The swelling on each posterior root is a spinal or posterior root ganglion. The spinal nerve is only 1 cm long and occupies an intervertebral foramen. On emerging from the foramen, it divides into anterior and posterior rami.
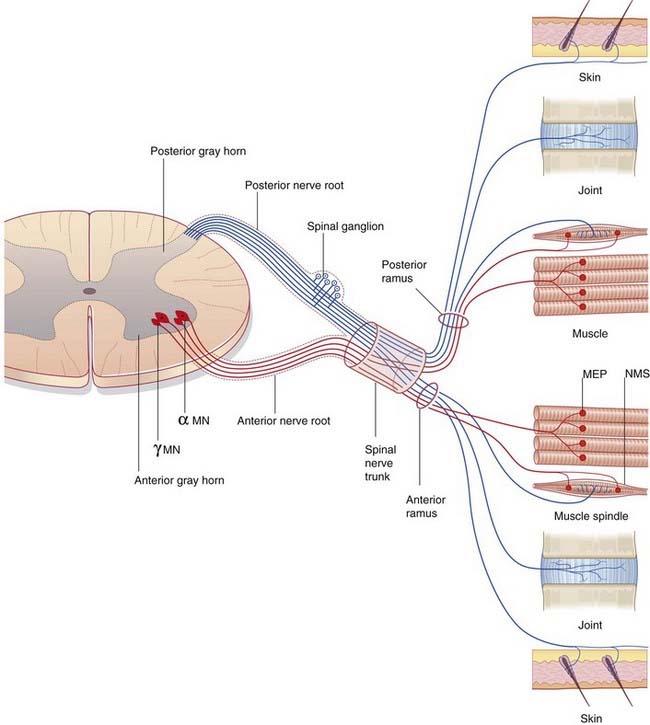
The neurons contributing to peripheral nerves are partly contained within the central nervous system (CNS) (Figure 9.2). The cells giving rise to the motor (efferent) nerves to skeletal muscles are multipolar alpha and gamma neurons of similar configuration to the one depicted in Figure 6.4; in the spinal cord, they occupy the anterior horn of gray matter. Further details are in Chapter 10. Those giving rise to posterior nerve roots are unipolar neurons whose cell bodies lie in posterior root ganglia and whose sensory (afferent) central processes enter the posterior horn of gray matter.
Microscopic Structure of Peripheral Nerves
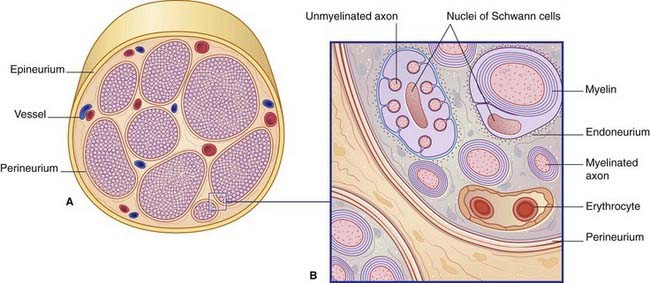
Figure 9.3 illustrates the structure of a typical peripheral nerve. It is not possible to designate individual nerve fibers as motor or sensory on the basis of structural features alone.
Myelin formation
The Schwann cell is the representative neuroglial cell of the peripheral nervous system (PNS). It forms chains of neurolemmal cells along the nerves. Modified Schwann cells form satellite cells in posterior root ganglia and in autonomic ganglia, and teloglia at encapsulated sensory nerve endings (Ch. 11).
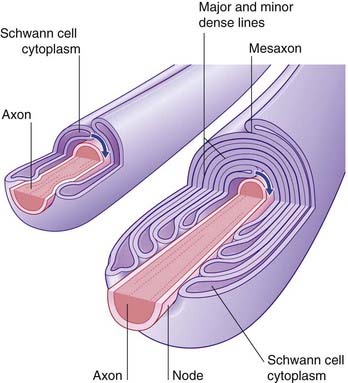
If an axon is to be myelinated, it receives the simultaneous attention of a sequence of Schwann cells along its length. Each one encloses the axon completely, creating a ‘mesentery’ of plasma membrane, the mesaxon (Figure 9.4). The mesaxon is displaced progressively, being rotated around the axon. Successive layers of plasma membrane come into apposition to form the major and minor dense lines (Figure 9.4).
Myelin expedites conduction
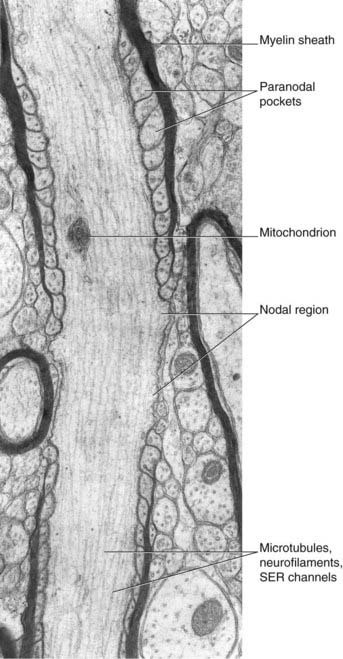
Details of diameters and sources are given in Tables 9.1 and 9.2.
| Fiber type | Origin |
|---|---|
| Sensory | |
| Ia | Muscle spindle annulospiral endings |
| Ib | Golgi tendon organs |
| II (Aβ) | Muscle spindle flower spray endings; touch or pressure receptors in skin and elsewhere |
| III (Aδ) | Follicular endings; fast pain and thermal receptors |
| IV (C) | Slow pain, itch, touch receptors |
| Motor | |
| Aα | Alpha motor neurons supplying extrafusal muscle fibers |
| Aγ | Gamma motor neurons supplying intrafusal muscle fibers |
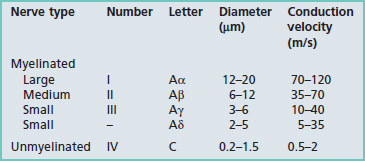
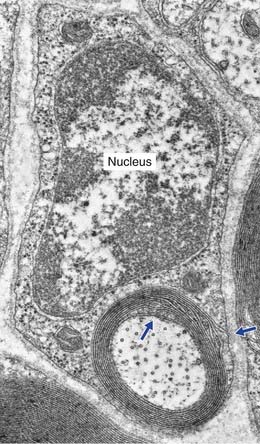
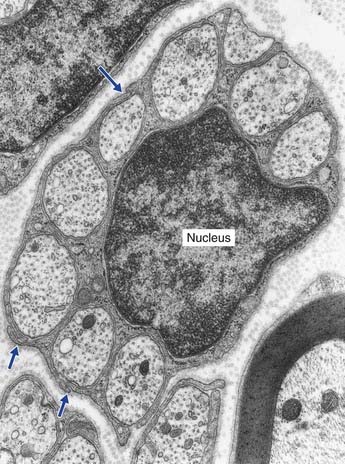
The electron micrograph in Figure 9.5 illustrates a myelinated peripheral nerve fiber with attendant Schwann cell, that in Figure 9.6 a group of unmyelinated fibers bedded in the cytoplasm of a Schwann cell, and Figure 9.7 a nodal region within the CNS.
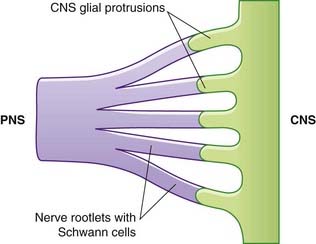
Central nervous system–peripheral nervous system transitional region
Close to the brainstem and spinal cord, peripheral nerves enter the CNS–PNS transitional zone (Figure 9.8). Astrocyte processes reach out of the CNS into the endoneurial compartments of peripheral nerve rootlets and interdigitate with the Schwann cells. In unmyelinated fibers, the astrocytes burrow into the space between axons and Schwann cells. In myelinated fibers, nodes are bounded by Schwann cell myelin (showing some transitional features) on the peripheral side, and by oligodendrocytic myelin centrally.
Degeneration and Regeneration
Wallerian degeneration of peripheral nerves
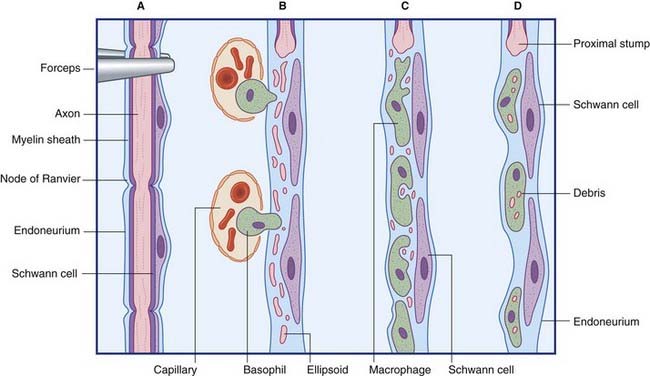
The principal events in peripheral nerve degeneration are represented in Figure 9.9 and described in the caption. Following a crush or cut injury to a nerve, the axons and myelin sheaths distal to the cut break up into ‘elipsoids within the first 48 h – mainly because of Ca2+-activated release of proteases by Schwann cells. The debris is cleared by monocytes that enter the damaged endoneurial sheaths from the blood and become macrophages. In addition to their phagocytic function, the macrophages are mitogenic to Schwann cells and participate with Schwann cells in provision of trophic (feeding) and tropic (guidance) factors for regenerating axons.
Regeneration of peripheral nerves
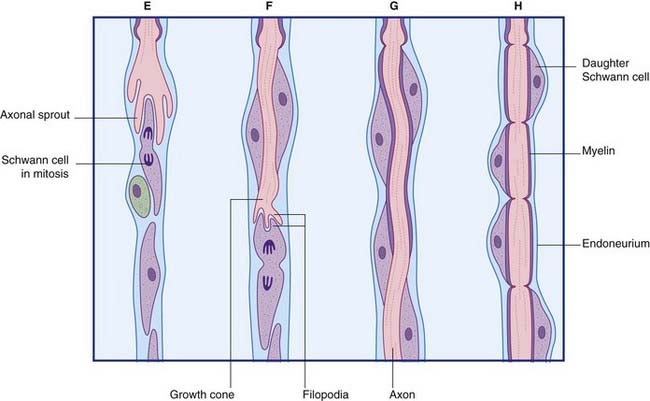
The principal events in regeneration of a peripheral nerve are summarized in Figure 9.9B. Following a clean cut, axons begin to sprout from the face of the proximal stump within a few hours, but in the more common crush or tear injuries seen clinically, the axons die back for 1 cm or more and sprouting may be delayed for a week. Successful regeneration requires that the axons make contact with Schwann cells of the distal stump. Failure to make contact leads to production of a pseudoneuroma consisting of whorls of regenerating axons trapped in scar tissue at the site of the initial injury. Following amputation of a limb, an amputation pseudoneuroma can be a source of severe pain.
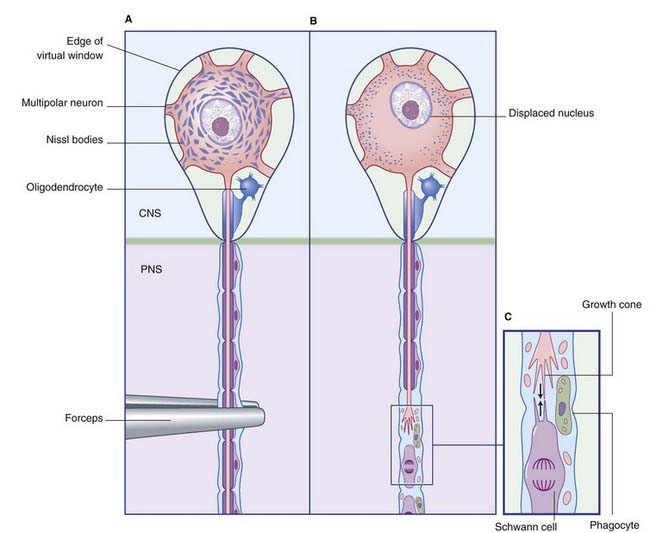
Upstream effects of nerve section are as follows: