Chapter 16 Peripheral Nerve Stimulation
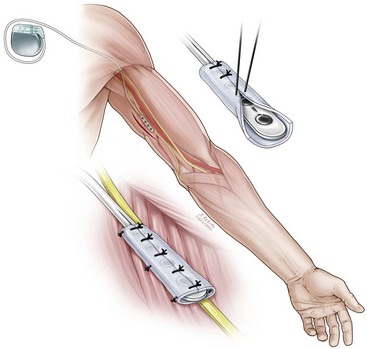
 Fascial cuff can be placed between peripheral nerve and plate electrode during surgical implantation (serves an analogous to role to the dura in spinal cord stimulation).
Fascial cuff can be placed between peripheral nerve and plate electrode during surgical implantation (serves an analogous to role to the dura in spinal cord stimulation).Introduction
Peripheral nerve stimulation (PNS) has been recognized as a treatment modality for peripheral neuropathic pain beginning with Wall and Sweet’s original description in 1967.1 By inserting percutaneous needle electrodes into their own infraorbital regions, these authors were able to test the effects of stimulation on peripheral nerves. Through stimulation with square-wave pulses at 100 Hz and with a pulse width of 100 msec, they were able to induce diminished sensation to pin prick in the area of the stimulation. This led the way to investigations of PNS as a modality for treating neuropathic pain.
The last two decades have seen an increased interest in the use of PNS with application to occipital neuralgia, facial pain, and complex regional pain syndrome.2 In addition to targeting defined peripheral nerves, clinicians are also applying the techniques of PNS to subcutaneous and regional stimulation.3,4 This has led to a number of trials assessing the efficacy of PNS for a wide array of conditions, including lower back pain, postherniorrhaphy inguinal pain, sacral pain, and migraine headaches.5
Basic Science
Our understanding of the mechanism underlying PNS is still being developed. One hypothesis for achieving pain control with PNS involves the gate control theory of pain management proposed by Melzack and Wall in 1965.6 In their initial description of the gate theory, the authors postulated that large- and small-diameter fibers both send input to inhibitory neurons within the substantia gelatinosa. They theorized that small-diameter fibers provided inhibitory input to the substantia gelatinosa and the large-diameter fibers provided excitatory input. The summation of these inputs modulated the overall inhibitory connections from the substantia gelatinosa neurons to the dorsal horn transmission (T) cells, the projections of which formed the anterolateral system. In accordance with this theory, an increase in large-diameter afferent input would lead to increased inhibitory output from the substantia gelatinosa and consequently decreased transmission of nociceptive input to suprasegmental centers. Thus neurostimulation of peripheral nerves through its effect on the lowest threshold large-diameter fibers would act to “close the gate,” effectively inhibiting the transmission of small-diameter pain fibers. Although the gate control theory provides a framework for understanding the mechanism of PNS, the specifics have been challenged.7
Other investigators have shown additional mechanisms that may contribute to the potential efficacy of PNS. Campbell and Taub8 explored the mechanism of PNS through transcutaneous nerve stimulation of the median nerve. The authors stimulated the median nerve proximally with a 100-Hz, 1-msec stimulus. They found that the response elicited depended on the stimulus amplitude. At 10 V to 12 V touch threshold was elevated. With increased voltage pain thresholds were also elevated, and at intensities of 50 V analgesia was elicited. In addition, the development of analgesia was correlated with the loss of the Aδ portion of the compound action potential, suggesting that peripheral nerve transmission block may underlie the suppression of pain by PNS.
Ignelzi and Nyquist9 further explored the mechanism of PNS by placing cuff PNS electrodes around the sural and superficial radial nerves of cats. They found that, with stimulation, all of the components of the compound action potential were affected, although the Aδ fiber peak was more affected following neurostimulation than were the Aα or Aβ peaks. The changes were represented by either a reduction in amplitude or an increase in the latency of these waves. These findings also support the involvement of a more peripheral mechanism underlying the analgesic effect of PNS.
Indications/Contraindications
Indications
PNS is usually indicated for patients with peripheral neuropathic pain that can be attributed to a single peripheral nerve.10 Candidates for PNS should have undergone multimodal therapy, including medical management, anesthetic blockade, and physical therapy. As with patients who are being evaluated for spinal cord stimulation, neuropsychological testing can be valuable. In addition, before permanent implantation of the internal pulse generator, patients should have undergone a successful trial of stimulation with a predetermined therapeutic benefit.
More recent studies of subcutaneous target stimulation (STS) and regional stimulation have broadened the traditional inclusion criteria of neuropathic pain attributable to a single peripheral nerve.11 Retrospective case series of subcutaneous stimulation applied to painful areas has demonstrated therapeutic benefit for lower back pain, neck pain, inguinal pain, and others. However, the longer-term efficacy for this application is unknown.
Equipment
The system for PNS includes the electrode through which stimulation is applied to the target nerve and the implantable pulse generator (IPG). Initial electrode designs were cuff electrodes, which were wrapped around the target nerves. This electrode design was found to lead to an increase in perineural fibrosis with some association of peripheral nerve injury. Newer electrode designs are either plate electrodes, which are surgically implanted, or wire electrodes, which may be implanted percutaneously.12
There has been significant recent research on BION (bionic neuron) technology, which is being applied to the field of PNS.13 BION is an implantable stimulator that can be used to target nerves and muscles. The design is quite unique from currently used technologies since it is a leadless system, in which the generator and electrode are incorporated into a single miniature apparatus. Technology is being developed to percutaneously implant these devices to be used as peripheral nerve stimulators.14 Reports have been published with this technique targeting the occipital and pudendal nerves, among others.
Technique
Peripheral nerve stimulators can be implanted through either an open surgical or a percutaneous approach. Box 16-1 lists nerves that are commonly targeted for PNS. If using a surgical approach, the first step is the exposure of the target nerve. An approximate 4-cm distance of the nerve is dissected free from the surrounding tissues. Care is taken not to excessively disrupt the vascularity of the nerve. Once the nerve has been dissected free, the next step is placing the electrode under the nerve so the electrode contacts lie in proximity to the nerve. The electrode can then be sutured to the surrounding tissues to prevent migration. Some authors have advocated placing a piece of fascia between the stimulating electrodes and the nerve.12 This is thought to be analogous to the dura in spinal cord stimulation, which separates the spinal cord–stimulating electrode from the underlying spinal cord. Fig. 16-1 illustrates the surgical technique for placement of an ulnar nerve stimulator.
The techniques for percutaneous implantation of peripheral nerve stimulators to specific target nerves are discussed in the following paragraphs. A recent report by Huntoon and Burgher15 describes an ultrasound-guided technique for percutaneously implanting PNS in close proximity to the target peripheral nerve. They have used this technique to implant PNS around the median, radial, ulnar, peroneal, and posterior tibial nerves with efficacy similar to surgically implanted leads.
Occipital Nerve Stimulation
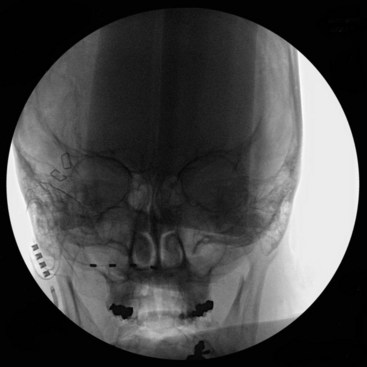
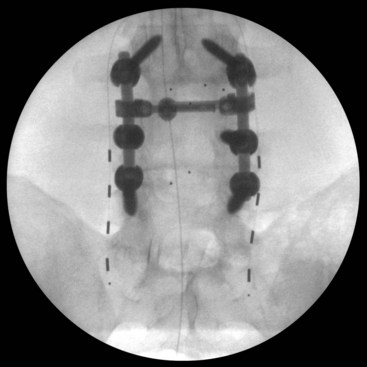
A trial of occipital nerve stimulation may be performed under local or general anesthesia. The senior author (KJB) has found radiographic targeting effective and thus performs the trial under general anesthesia to aid in patient comfort. For a unilateral trial the patient may be positioned supine on the operating table with head turned contralateral to the side of implantation. If a bilateral trial is to be performed, the patient may be positioned either in the lateral position or prone on the operating room table. Anteroposterior (AP) fluoroscopy is used to guide the positioning of the electrode. A Tuohy needle can be gently curved to approximate the curvature of the suboccipital region, and an entry point is chosen slightly medial to the mastoid tip. The needle can then be advanced subcutaneously with the beveled edge down radiographically angled toward the tip of the odontoid as a guide to the superior/inferior plane (Fig. 16-2). The electrode is then passed to the midline through the Tuohy needle. Alternatively some authors12,16 prefer to aim the electrode along the posterior arch of C1. Care is taken so the Tuohy needle does not penetrate the scalp as it passes medially.
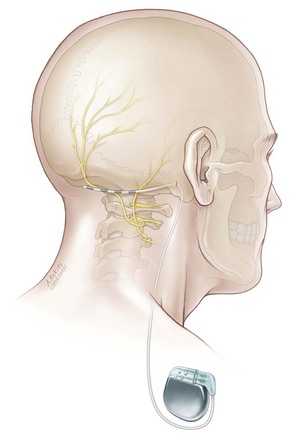
Following a successful trial, permanent implantation can be performed under general anesthesia with the IPG placed in the infraclavicular region. During the permanent implantation a small 2-cm vertical incision is made in the retromastoid incision to allow anchoring of the lead to the underlying fascia (Fig. 16-3).
Supraorbital and Infraorbital Stimulation
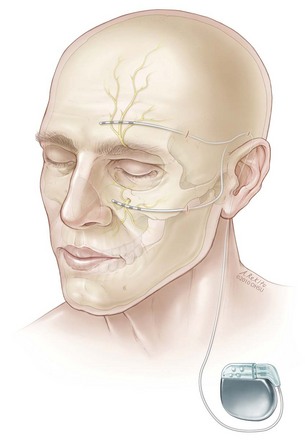
Implantation of supraorbital and infraorbital stimulator electrodes may be performed with the patient awake or under general anesthesia. Similarly to occipital nerve stimulation, we prefer to implant the trial electrode using radiographic guidance under general anesthesia for patient comfort. For supraorbital stimulation, a small stab incision is made in the anterior portion of the temple above the orbital ridge. A gently curved Tuohy needle is advanced subcutaneously toward the midline, and the electrode is passed through the needle with the goal of spanning the supraorbital foramen. For permanent implantation the trial electrode is removed, and a new percutaneous electrode is inserted as described. This electrode is then tunneled to the ipsilateral retromastoid region, where it can be anchored to the underlying fascia through a small 2-cm incision. The electrode and extension cable can then be tunneled and connected to the IPG through an infraclavicular incision (Fig. 16-4).
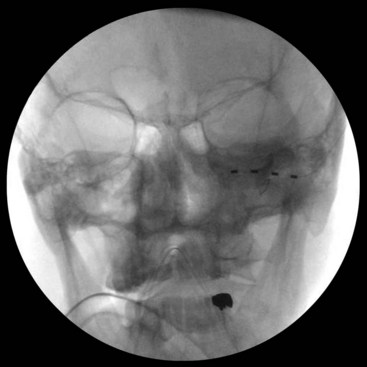
For infraorbital stimulation, an entry point is chosen lateral and inferior to the orbital floor, and the electrode is advanced so it spans the infraorbital foramen (Fig. 16-5). During permanent lead implantation, a 2-cm incision is made approximately 1 cm in front of the tragus, and the electrode is tunneled to this incision where it is anchored to the fascia. The electrode and extension cable can then be tunneled to an infraclavicular incision where the IPG will be implanted.
Subcutaneous Stimulation
Trial and implantation of electrodes for subcutaneous stimulation is a newer technique. Technically, however, the implantation of the electrode and pulse generator is identical as for other percutaneously placed peripheral nerve stimulators. For subcutaneous stimulation, leads are implanted with the goal of placing the active contacts in the center of the painful area.11
Some authors have advocated peripheral nerve field stimulation techniques by subcutaneously placing two parallel electrodes on either side of the painful region (Fig. 16-6). In applying regional stimulation, one lead can serve as the anode, and the other parallel lead can serve as the cathode.3
Outcomes Evidence
Peripheral Nerve Stimulation
In 1976 Campbell and Long17 reported their series of 23 patients who had been treated with PNS for a variety of conditions, including peripheral nerve injuries, sciatica, and lower back pain. The authors used a bipolar electrode that was wrapped around the affected peripheral nerve. At an average follow-up of 12 months, 14 of the 23 patients were found to have failed treatment. Four patients in the series had excellent outcomes, and five patients had intermediate results. In reviewing the patient characteristics and clinical outcomes, the authors found that the patient’s diagnosis was the factor most correlated with their outcome. None of the patients who were implanted with a sciatic nerve stimulator for lower back pain had good outcomes. Conversely, patients implanted with PNS for chronic peripheral nerve injury had the best outcomes and the highest chance for success.
Reports of PNS applied to patients with peripheral nerve injury have been very favorable. Law, Swett, and Kirsch18 reported their results with 22 patients who had been treated with PNS for chronic posttraumatic neuropathy. At a mean follow-up of 25 months, 13 (59%) patients reported benefit from their stimulators and continued to use them.18 Similarly Waisbrod and associates19 reported their experience using a cuff electrode from Avery in the treatment of 19 patients with traumatic peripheral nerve injuries. Their series incorporated a range of targets, including the sciatic, femoral, median, posterior tibial, and other nerves. At a mean follow-up of 11.5 months, the authors report complete pain relief in 11 patients (58%) and significant pain relief in four other patients (21%). Poor pain relief was seen in the remaining four (21%) patients.
Van Calenbergh and associates20 reported long-term outcomes of a small group of patients who had been implanted with PNS in the treatment of neuropathic pain after nerve injury. The authors used a circumferential Avery electrode that was surgically implanted around the injured nerve proximally. Of the 10 patients available for follow-up, four were not using the device because of hardware complications or infection. The remaining five patients were found to have excellent pain relief from the PNS at a mean follow-up of 22 months.
Occipital Nerve Stimulation
Slavin, Nersesyan, and Wess16 and Slavin and associates21 reported their experience with unilateral and bilateral occipital nerve stimulation for occipital neuralgia. In their series five patients underwent a trial of unilateral stimulation, and nine patients had a bilateral electrode trial. A successful trial was determined by a 50% reduction in pain by the visual analog pain scale. On the basis of this criterion, 10 patients (71%) underwent permanent implantation. In a mean follow-up of 22 months, 70% of patients who were implanted were found to have beneficial effects of stimulation.
In a series of patients who underwent implantation of an occipital nerve stimulator following a successful trial, Weiner12 reported excellent pain relief in 55%, good relief in 27%, and fair relief in 15%.
Trigeminal Branch Stimulation
Amin and associates22 reported their experience with 16 patients who underwent a trial of PNS in the treatment of supraorbital neuralgia. Ten patients experienced a greater than 50% reduction in the visual analog score and consequently were implanted with the IPG. In the 30-week follow-up after implantation, patients were found to have a significantly decreased pain score and a significantly reduced requirement for morphine equivalents.
In a longer-term follow-up study, Johnson and Burchiel23 reported a series of 10 patients who had undergone implantation of supraorbital or infraorbital nerve stimulators for trigeminal neuropathic pain following facial trauma or herpes zoster infection. At a mean follow-up of 26 months, 70% of patients were found to have continued benefit from the stimulation and experienced greater than 50% reduction in their pain compared with preimplantation. All of the patients with posttraumatic neuropathic pain had greater than 50% pain reduction and reduced their intake of medications.
Slavin and associates21 shared their follow-up of 12 patients who had undergone a trial of PNS to the trigeminal branches for facial pain, reporting that 9 patients had a successful trial warranting implantation. Over a mean follow-up of 44 months, five of these nine patients maintained greater than 50% pain relief. One patient underwent explantation because of infection, another was explanted as his pain improved, and the remaining two patients did not maintain benefit.
Subcutaneous Target Stimulation
Subcutaneous stimulation of painful areas of the body without targeting a specific peripheral nerve is a newer technique that has been applied since the late 1990s. In 2006 Goroszeniuk, Kothari, and Mamann4 reported their technique and outcomes with a series of three patients who suffered from chronic pain that had been unresponsive to multimodal therapy. During a subcutaneous trial of stimulation using slow frequency stimulation at 2 Hz for 2 minutes, the authors were able to achieve several hours of pain control in each of the patients. The patients went on to permanent implantation with positive results. Of note, the authors found that each of these patients did not receive significant benefit with a transcutaneous electrical nerve stimulation (TENS) unit.
In 2010 Sator-Katzenschlager and associates11 reported a multicenter retrospective series of 119 patients who underwent a trial of STS between 1999 and 2007. The indications for STS included low back pain, failed back surgery syndrome, neck pain, and postherpetic neuralgia. Of the 119 patients, 111 reported a more than 50% reduction in the numerical rating scale (NRS) for pain with the trial of stimulation and underwent permanent implantation. In short-term follow-up of 3 months, the average reduction in the NRS was from 8.2 before surgery to 4.0 after surgery. As the authors conclude, prospective long-term studies will be needed to fully assess the efficacy of this treatment modality.
Risk and Complication Avoidance
Risks associated with PNS are generally related to those of any implantable device. The most notable risks include infection, lead breakage, migration of the stimulating electrode, and nerve injury. The rates of complications range from 5% to 43% in published series, with infection remaining the major risk.10 As a means for limiting the risk of device explantation, the present authors have found local injection of antibiotics before skin closure to be effective in reducing the risk of surgical site infections. Miller, Acar, and Burchiel24 found a 5.7% risk of infection associated with implantation of neurostimulation and intrathecal devices with prophylaxis by intravenous antibiotics before surgery. When a local injection of neomycin/polymyxin before skin closure was used in addition to intravenous antibiotics, the infection rate dropped to 1.2%.
1 Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108-109.
2 Hassenbusch S. Peripheral nerve stimulation. In: Follett K, editor. Neurosurgical pain management. Philadelphia: Elsevier; 2004:144-149.
3 Falco FJE, et al. Cross talk: a new method for peripheral nerve stimulation: an observational report with cadaveric verification. Pain physician. 2009;12:965-983.
4 Goroszeniuk T, Kothari S, Hamann W. Subcutaneous neuromodulating implant targeted at the site of pain. Reg Anesth Pain Med. 2006;31:168-171.
5 Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapy. 2008;5:100-106.
6 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
7 Nathan PW. The gate-control theory of pain: a critical review. Brain. 1976;99:123-158.
8 Campbell JN, Taub A. Local analgesia from percutaneous electrical stimulation: a peripheral mechanism. Arch Neurol. 1973;28:347-350.
9 Ignelzi RJ, Nyquist JK. Direct effect of electrical stimulation on peripheral nerve evoked activity: implications in pain relief. J Neurosurg. 1976;45:159-165.
10 Gybels J, Nuttin B. Peripheral nerve stimulation. In: Loeser J, editor. Bonica’s management of pain. ed 3. Philadelphia: Lippincott; 2000:1851-1855.
11 Sator-Katzenschlager S, et al. Subcutaneous target stimulation (STS) in chronic noncancer pain: a nationwide retrospective study. Pain Pract. 2010;10(4):279-286.
12 Weiner RL. Peripheral nerve stimulation. In: Burchiel K, editor. Surgical management of pain. New York: Thieme Medical Publishers; 2002:498-504.
13 Loeb GE, Richmond FJR, Baker LL. The BION devices: injectable interfaces with peripheral nerves and muscles. Neurosurgery. 2006;20:E2.
14 Kaplan HM, Loeb GE. Design and fabrication of an injection tool for neuromuscular microstimulators. Ann Biomed Eng. 2009;37:1858-1870.
15 Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009;10:1369-1377.
16 Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58:112-119. discussion 112-119
17 Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 1976;45:692-699.
18 Law JD, Swett J, Kirsch WM. Retrospective analysis of 22 patients with chronic pain treated by peripheral nerve stimulation. J Neurosurg. 1980;52:482-485.
19 Waisbrod H, et al. Direct nerve stimulation for painful peripheral neuropathies. J Bone Joint Surg Br. 1985;67:470-472.
20 Van Calenbergh F, et al. Long term clinical outcome of peripheral nerve stimulation in patients with chronic peripheral neuropathic pain. Surg Neurol. 2009;72:330-335. discussion 335
21 Slavin KV, et al. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurgery. 2006;21:E5.
22 Amin S, et al. Peripheral nerve stimulator for the treatment of supraorbital neuralgia: a retrospective case series. Cephalalgia. 2008;28:355-359.
23 Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery. 2004;55:135-141. discussion 141-132
24 Miller JP, Acar F, Burchiel KJ. Significant reduction in stereotactic and functional neurosurgical hardware infection after local neomycin/polymyxin application. J Neurosurgery. 2009;110:247-250.