Chapter 18 Peripheral Nerve Stimulation
Percutaneous Technique
 Minimally invasive PNS is a novel technique that may allow a trial of peripheral nerve stimulation before permanent placement and potentially allow long-term implantation.
Minimally invasive PNS is a novel technique that may allow a trial of peripheral nerve stimulation before permanent placement and potentially allow long-term implantation. Percutaneous leads were engineered for spinal cord stimulation indications; thus long-term safety studies of these leads near target nerves have not been performed.
Percutaneous leads were engineered for spinal cord stimulation indications; thus long-term safety studies of these leads near target nerves have not been performed. The operator should be familiar with ultrasound-guided nerve blocks and have a strong working knowledge of cross-sectional anatomy to maximize the safety of these techniques.
The operator should be familiar with ultrasound-guided nerve blocks and have a strong working knowledge of cross-sectional anatomy to maximize the safety of these techniques. One must understand that discrete fascicles within the peripheral nerve may be targeted differentially based on the location of the electrode relative to the internal configuration of the fascicles.
One must understand that discrete fascicles within the peripheral nerve may be targeted differentially based on the location of the electrode relative to the internal configuration of the fascicles. A narrow therapeutic window for stimulation (unwanted motor stimulation) is the result of the internal fascicular arrangement relative to the location of the external lead.
A narrow therapeutic window for stimulation (unwanted motor stimulation) is the result of the internal fascicular arrangement relative to the location of the external lead. Over time epineurial fibrosis occurs, which may change the impedance and stimulation energy requirements significantly.
Over time epineurial fibrosis occurs, which may change the impedance and stimulation energy requirements significantly. Further research will help define the appropriate role of minimally invasive techniques for clinical applications.
Further research will help define the appropriate role of minimally invasive techniques for clinical applications. Preimplantation nerve block is extremely useful in patient selection, particularly if the patient receives complete relief of pain in a single nerve distribution.
Preimplantation nerve block is extremely useful in patient selection, particularly if the patient receives complete relief of pain in a single nerve distribution. A transverse (axial) view of the nerve with an in-line approach to lead placement is technically easiest.
A transverse (axial) view of the nerve with an in-line approach to lead placement is technically easiest. Similar to spinal cord stimulation, PNS patients should limit activity in the relevant extremity for 4 to 6 weeks.
Similar to spinal cord stimulation, PNS patients should limit activity in the relevant extremity for 4 to 6 weeks. Transmuscular placement is undesirable and with muscular movement may cause a ratcheting effect and promote migration.
Transmuscular placement is undesirable and with muscular movement may cause a ratcheting effect and promote migration. When placing leads and generators permanently using this technique, avoiding crossing joint lines and keeping the parts of the system closer together are important considerations.
When placing leads and generators permanently using this technique, avoiding crossing joint lines and keeping the parts of the system closer together are important considerations.Introduction
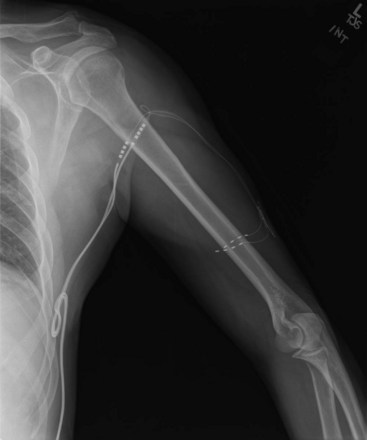
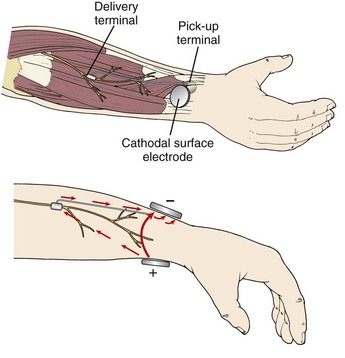
Peripheral nerve stimulation (PNS) is a technique of direct electrical stimulation of peripheral nerve(s) to relieve pain or to assist with the functional restoration of patients with severe neurological injuries, including those to the spinal cord. The term PNS has also been used to describe the superficial placement of electrical leads in areas of the body such as the back, thorax, or abdomen where no discrete named nerves exist but small nerve fibers near the dermis can be targeted to produce local analgesia. This type of stimulation is mechanistically poorly understood and will be referred to as peripheral field stimulation (not the subject of this chapter). PNS became conceptually possible after the development of the gate control theory.1 Wall and Sweet’s experiments with PNS tested the concept of electrical stimulation–produced analgesia, initially of the authors’ infraorbital nerves and subsequently in eight patients.2 Soon many neurological and orthopedic surgeons became interested in the technique, and several case series of these peripheral nerve surgeries were described 3–6 The early history of the development of these techniques is chronicled by Michael Stanton-Hicks and colleagues,6 who describe the indications, outcomes, and complications of a series of 91 patients at the Cleveland Clinic. Despite early investigator interest and the development of novel lead and battery technology, innovations in programmability, and other technical improvements for stimulation of the peripheral nerves, the application and study of PNS has been less vigorous in scope than that for spinal cord stimulation. The absence of a comparable minimally invasive trial system that became available with the introduction of percutaneous spinal cord stimulation electrodes in the 1980s may be one reason that peripheral nerve surgeries have not advanced as rapidly. Other potential reasons for decreased interest in PNS may be frequent complications such as lead migration (requiring revision surgery), neurological damage to the axons from mechanical or stimulation-induced nerve injury, and poor patient selection leading to poor outcomes. PNS has always been an open technique since its inception more than 40 years ago, but recently minimally invasive techniques were described.7–9 Possible advantages to peripheral nerve stimulator placement via a minimally invasive technique might include: (1) minimal nerve trunk dissection and manipulation (potential trauma); (2) the omission of the need for a fascial graft or fat pad interposed between the electrode and the nerve (the fat around the epineurium in situ is minimally perturbed by the percutaneous electrode); (3) a true percutaneous trial avoiding dissection and open surgery; and (4) the ability to perform intraoperative electrical stimulation testing in an awake patient with minimal sedation compared to a patient under general anesthesia. Cadaver feasibility studies were performed in 2008,7,8 suggesting that ultrasound (US) image guidance might allow safe placement of peripheral nerve stimulating electrodes near target peripheral nerves, similar to nerve catheter placement. Following these feasibility studies, a case series of nine patients who received PNS placements using US was published, based on the principles described in the cadaver studies.9 In two of the nine cases, patients with good paresthesia coverage did not achieve analgesia and were not implanted, thus avoiding an unnecessary surgical dissection. Most cases produced durable analgesia beyond 1 year. The programming of multiple different stimulation parameters intraoperatively allowed the additional placement of second electrodes parallel to the first or on either side of a nerve trunk to more optimally stimulate the desired fascicles in some cases (Fig. 18-1).9 Others have described a stimulus router concept that eliminates the need for an implanted generator. A surgeon places a passive implant delivery terminal near the target nerve. Current is passed between a surface cathodal electrode and a pick-up terminal, a small fraction of which (10% to15%) is passed to the delivery terminal. This results in PNS at threshold. It is unclear at this time if this type of implant would be less prone to migration without additional study (Fig. 18-2).10
Current Evidence
At this juncture no prospective randomized and blinded studies have been performed. A recent paper by Bittar and Teddy11 reviewed the current evidence for PNS. Unfortunately the greatest need for evidence at this time is from prospective, randomized controlled, and blinded studies. Until now these studies have been difficult if not impossible to perform because of patient perception of a physical sensation (paresthesia), which hampers blinding, and the lack of minimally invasive, ethical controls. In an editorial on the subject of peripheral neuromodulation, Davis12 discussed some of the factors that may make interpretation of currently available studies difficult. Questions regarding the role of external neurolysis (the removal of external scarring around the nerve) on the analgesia seen after PNS, unclear placebo effects, the unequal application of physical therapy in some patient groups, lack of standardization of potentially analgesic drugs (including neuromodulators and opioids), or the increased attention to the patient needs during study protocols are all possible confounding factors. Long-term studies are difficult, but some studies seem to suggest an extremely long-standing effect of PNS, with the possibility of nerve healing over time.13 Van Calenbergh and colleagues13 looked back at a group of 11 patients who had been chronically implanted with radiofrequency-coupled peripheral nerve stimulators over an average period of 22 years! Of these 11, four had been explanted, one had died of unrelated causes to his or her stimulator, and one could not participate because of distance from the study center. The remaining five patients had long-term circumferential electrode arrays. These patients demonstrated a durable improvement in their pain, level of function, and use of analgesics when comparing stimulation off to stimulation on. They had sustained minimal complications. Remarkably, benefits persisted despite the passage of over two decades on average. One patient had even demonstrated gradual resolution of his neuropathic pain in the face of neural stimulation. The largest clinical series in print to date are those from Eisenberg, Waisbrod, and Gerbershagen14 and the Cleveland Clinic.6 In Eisenberg, Waisbrod, and Gerbershagen’s series, 46 patients with isolated peripheral nerve syndromes were treated. Positive results were noted in 78% of patients, whereas 22% had poor results. Decreases in visual analog pain scores from 69 ± 12 before surgery to 24 ± 28 after surgery were seen.12 The major pathologies treated in the PNS protocols were for the following: (1) nerve lesions incurred from surgeries around the hip or knee, (2) nerve entrapments, (3) persistent pain after nerve graft surgeries, or (4) postaccidental nerve injection injuries.14 In the previously unpublished Cleveland Clinic series,6 results on average were positive, with a frequency of revision surgery of 1.6 per patient, not including battery replacements.
Patient Selection
In general patients with neuropathic pain syndromes involving the periphery may qualify for these procedures when they have failed to improve with more conventional and conservative therapies. Patients should be evaluated thoroughly, with the medical history concentrating on previous diagnostic studies, previous failed medication trials, any previous surgical procedures, electrodiagnostic results, and results of any previous nerve block procedures. In the physical examination the physician should concentrate on the neurological, musculoskeletal, and skin examinations. Presence of any motor deficits, sensory deficits, specific dermatomal or peripheral nerve distributions, presence or absence of allodynia, hyperesthesia, hypesthesia, or hyperalgesia should be noted. Vasomotor, sudomotor, or trophic changes should be noted since they may point to a complex regional pain syndrome. Electrodiagnostic studies are extremely important but may be normal in primary pain syndromes. Electromyography (EMG) and nerve conduction studies (NCSs) are also important to the classification, grading, and prognostication of many injuries. In some cases serial evaluations of patients with EMG/NCS can show important trends in reinnervation or ongoing denervation of specific areas. Nerve blocks are an important modality in preoperative patient selection as well. Blocking the suspected nerve proximal to the site of injury helps confirm the involvement of that nerve in the pathophysiology of the pain. One protocol has evolved with the use of local anesthetic coupled with clonidine (Catapres) for neural blockade. In addition to its ability to prolong the effects of the local anesthetic in many cases, clonidine may be important in the inflammatory pain response by blocking production of cytokines involved in hyperalgesia. Indeed, Lavand’homme and Eisenbach15 have demonstrated prolonged relief of some neuropathic pain states with clonidine. Whether the nerve block is performed with US or electrical stimulation and surface landmarks is perhaps not of paramount importance. However, one advantage of US guidance is the ability to fully scan the patient’s area of interest. In some cases one might diagnose a specific underlying cause such as a neuroma (Fig. 18-3), a constriction caused by mechanical factors, a foreign body, or an anatomical variant that might pose problems with the technique. Other imaging modalities such as magnetic resonance studies are increasingly sophisticated and capable of influencing treatment decisions.
Safety
It seems intuitively obvious that objects placed in close proximity to a peripheral nerve or abutting against the nerve might potentially cause neural injury. Indeed, previous studies have demonstrated the possibility of neural damage from the surgical procedure itself but also from friction on the epineurium by normal nerve movement (translocation) in an extremity. There have been only minimal studies with respect to a purely mechanical effect. Agnew and colleagues16 have studied the effects of perineurial electrodes relative to both mechanical and stimulation effects on nerve function long term. These studies were done with helical electrode arrays with three to seven circumferential twists. In one experiment a control side was used to evaluate whether the mechanical or stimulation effects were responsible for adverse changes in histology. Prolonged stimulation with higher frequencies and continuous application of bipolar current have both been shown to cause neurological injury. Interestingly the use of local anesthetics seems to be protective against the development of peripheral nerve injuries from the stimulation.17 The local anesthetic protective effect suggests that the conduction of long-term high-frequency electrical charge is causative for global axonal changes seen in histological specimens. Other studies of long-term application of stimulation to the other nerves (e.g., phrenic nerves) have shown inconsistent effects. Although significant changes were noted in some, many patients had no histological changes.18 However, since the phrenic is a motor nerve, any major axonal injury might be more clinically evident. In the initial case series of PNS patients with US-guided placements, stimulation frequencies have been low. Subthreshold stimulation (amplitudes below the perception of a physical paresthesia) at frequencies below 100 Hz and with more narrow pulse widths was frequently used.9 There were no episodes of neural injury caused by US-guided PNS, despite three episodes of lead migration. In addition, there is a long history of PNS implant surgery over the last four decades, with rare reports of permanent nerve damage from the stimulation implant.
Anatomical Considerations: Nerve Fascicular Arrangement
One issue that complicates any peripheral nerve electrode placement in the four extremities is that nerves must freely glide within fascial/muscle planes along with their vascular supply as the extremity moves. Nerves can be entrapped by scar tissue, and the rough edges of an external electrode could cause constriction and scarring over time. Mixed peripheral nerves are also characterized by a complex internal fascicular arrangement. Briefly, nerve trunks may have sensory, motor, and mixed axons at various locations within the peripheral nerve. This complex cross-sectional anatomical configuration means that optimal stimulation of the desired sensory fascicle might, for example, be at the medial aspect of the ulnar nerve in a supracondylar placement but change location within a matter of a few millimeters to a posterior location. If the amplitude of stimulation is too high above sensory threshold, motor fascicles deeper within the trunk may easily be activated, causing muscle cramping and/or pain. Sunderlund19 studied the complex fascicular arrangements in the upper-extremity nerves more than 60 years ago. Very little additional work has been done on this topic. Major findings from his work are that even intraindividual (side-to-side) differences, branch points, and number of fascicles at a given anatomical location may vary. Thus stimulation intraoperatively could be useful to determine the potential for good-to-excellent results of PNS. A recent study looked at issues of cross-sectional anatomy more closely; specifically the effects of the fascicle perineurial thickness, diameter, and position within the nerve trunk on axonal excitation thresholds and neural recruitment. A model of human femoral nerve within a nerve circumferential cuff electrode was studied. The study showed that stimulation of target fascicles depends strongly on the cross-sectional anatomy of the nerve being stimulated. The mean thickness of perineurium was 3% ± 1% of the fascicle diameter. Increased thickness of the human perineurium or larger fascicle diameter increases the threshold for electrical activation. If a large neighbor fascicle were present, it could also affect stimulation activation of the target fascicle by as much as 80% ± 11%.20
Common Approaches to Ultrasound-Guided Peripheral Nerve Stimulation
Radial Nerve Stimulation
The radial nerve lies close to the lateral surface of the lower third of the humerus at a point 10 to 14 cm proximal to the lateral epicondyle. The nerve is somewhat elliptical in shape, and US scanning is reasonably clear in most cases with a high-frequency probe. An in-plane approach, in which the nerve is viewed in cross section (transverse scan) while the needle is slowly advanced in plane with the transducer is easier, but longitudinal scans are also possible. It is important to prescan the area around the nerve and humerus to define the anatomy. The lateral head of the triceps muscle is overlying the nerve here; and, although one would desire to avoid transgression of large amounts of muscular tissue, there is no more optimal approach to the nerve in a superficial location above the humerus. The profunda brachii artery and recurrent radial artery branch are often seen when scanning by using color Doppler features on the US machine.7 Electrode migration is prevented by anchoring firmly to muscle fascia and creating a tension loop of the lead. Placing the pulse generator close to the lead is also desirable to prevent migration.9 Intraoperative stimulation testing can define potential early motor axon recruitment and allow alternate locations of the lead to be attempted.
Ulnar Nerve Stimulation
US scanning with the probe in a transverse orientation to the ulnar nerve is facile in the medial epicondylar groove (cubital tunnel). From this location the nerve can be scanned more proximally in the arm approximately 9 to 13 cm above the condyle. The nerve lies superior to the medial triceps muscle here. In cases of previous transposition surgery, one can target the area just proximal to the patient’s scar to begin scanning the nerve, moving from presumed normal areas of nerve to areas of potential pathology such as scarring or neuroma formation. The needle may be advanced, using an in-plane approach with a high-frequency transducer from posterior to anterior on the medial aspect of the arm (Fig. 18-4). Anatomically the nerve passes into the cubital tunnel in the ulnar groove behind the medial epicondyle. The cubital tunnel is a vulnerable area for the nerve, particularly in males, and also a potential area of intraoperative compression.
Posterior Tibial Nerve Stimulation
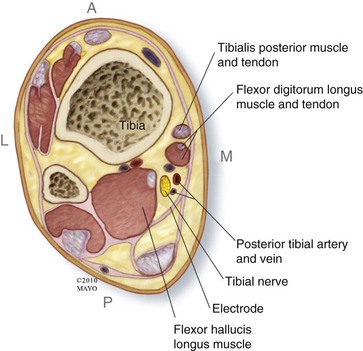
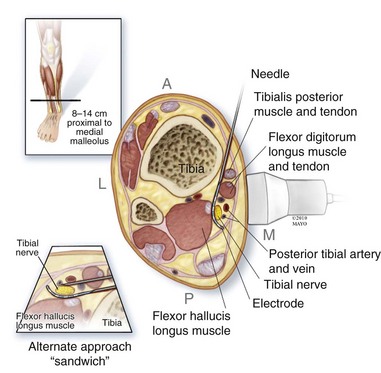
The posterior tibial nerve can stimulated more distally in the leg superior to the medial malleolus. The tarsal tunnel is a common area of potential compression; however, the tissue immediately superior to the tarsal tunnel is very compact and not easily approached with a US-guided approach. Moving a bit more superior to the malleolus (e.g., 10 to 14 cm superior) was found to be a reasonable approach in feasibility testing.8 The tibialis posterior muscle, the digitorum profundus, one or two large veins, the flexor hallucis longus, and the tibial bone surface are surrounding structures in this location (Fig. 18-5). US scanning of the ankle near the medial malleolus, with transverse probe orientation, is continued proximally until an adequate exposure of the nerve is realized. Location of the posterior tibial artery is often not an obstacle but should be noted during electrode placement. When approaching the posterior tibial nerve in the lower leg, the needles may be passed on either side to “sandwich” the nerve, providing stimulation of both sides; or, conversely, the electrodes may be placed parallel to each other if two leads are required (Fig. 18-6). The pulse generator may be placed in the upper leg, superficial to the fascia of the quadriceps, or on the fascia of medial gastrocnemius muscle in the lower leg if adequate soft tissue is noted.
1 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
2 Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108-109.
3 Nashold BS, et al. Long-term pain control by direct peripheral nerve stimulation. J Bone Joint Surg Am. 1982;64:1-10.
4 Strege DW, et al. Chronic peripheral nerve pain treated with direct electrical nerve stimulation. J Hand Surg (Am). 1994;19:931.
5 Hassenbusch SJ, et al. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84:415-423.
6 Stanton-Hicks M, et al. Miscellaneous and experimental therapies. In: Wilson PR, Stanton-Hicks M, Harden R, editors. CRPS: Current diagnosis and therapy, progress in pain research and management, vol. 32. Seattle: IASP Press; 2005.
7 Huntoon MA, et al. Feasibility of ultrasound guided percutaneous placement of peripheral nerve stimulation electrodes and anchoring during simulated movement. Part Two: Upper extremity. Reg Anesth Pain Med. 2008;33:558-565.
8 Huntoon MA, et al. Feasibility of ultrasound guided percutaneous placement of peripheral nerve stimulation electrodes in a cadaver model. Part One: Lower extremity. Reg Anesth Pain Med. 2008;33:551-557.
9 Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009;10:1369-1377.
10 Gan LS, et al. A new means of transcutaneous coupling for neural prostheses. IEEE Transact Biomed Engin. 2007;54:509-517.
11 Bittar RG, Teddy PJ. Peripheral neuromodulation for pain. J Clin Neurosci. 2009:1259-1261.
12 Davis GA. Commentary: peripheral neuromodulation for pain. J Clin Neurosci. 2009;16:1262.
13 Van Calenbergh F, et al. Long-term clinical outcome of peripheral nerve stimulation in patients with chronic peripheral neuropathic pain. Surg Neurol. 2009;72:330-335.
14 Eisenberg E, Waisbrod H, Gerbershagen HU. Long- term peripheral nerve stimulation for painful nerve injuries. Clin J Pain. 2004;20:143-146.
15 Lavand’homme P, Eisenach JC. Perioperative administration of the alpha-2-adrenoceptor agonist clonidine at the site of nerve injury reduces the development of mechanical hypersensitivity and modulates local cytokine expression. Pain. 2003;105:247-254.
16 Agnew WF, et al. Histologic and physiologic evaluation of electrically stimulated peripheral nerve: considerations for the selection parameters. Ann Biomed Engin. 1989;17:39-60.
17 Agnew WF, et al. Local anesthetic protects against electrically induced damage in peripheral nerve. J Biomed Engin. 1990;12:301-308.
18 Kim JH, et al. Diaphragm-pacing histopathological changes in the phrenic nerve following long-term electrical stimulation. J Thoracic Cardiovasc Surg. 1976;72:602-608.
19 Sunderland S. The intraneural topography of the radial, median, and ulnar nerves. Brain. 1945;68:243-298.
20 Grinberg Y, et al. Fascicular perineurium thickness, size, and position affect model predictions of neural excitation. IEEE Transact Neural Syst Rehabil Engin. 2008;16:572-581.