CHAPTER 116 Peripheral Magnetic Resonance Angiography
MR angiography (MRA) is a highly reliable technique that is widely used for imaging large and medium-sized arteries of the pelvis and lower extremities. In many hospitals worldwide, this technique has become an important adjunct to duplex ultrasonography (DUS) and x-ray catheter angiography, specifically intra-arterial digital subtraction angiography (IA DSA), in the workup of suspected peripheral artery disease (PAD). MRA, in many cases, is replacing diagnostic x-ray catheter angiography because it can provide similar diagnostic vascular road maps (Fig. 116-1) without the associated clinical concerns and risks related to invasive catheterization, ionizing radiation exposure, and use of iodinated contrast agents.
PAD (also called peripheral arterial occlusive disease or peripheral vascular occlusive disease) is almost invariably the result of advanced atherosclerosis of the pelvic and lower extremity arteries. With increasing age, atherosclerotic plaque develops in the walls of the lower extremity arteries, leading to luminal narrowing and often arterial occlusion. This progression of events results in a recognizable clinical constellation of signs and symptoms that typically begins as intermittent claudication and progresses to lower extremity pain at rest and even nonhealing skin ulceration. The diagnosis of PAD is typically initially made on the basis of a single measurement of the ankle-brachial index (ABI) below 0.9.1
PREVALENCE AND EPIDEMIOLOGY
Atherosclerotic PAD is an important health care problem in Western society, with an estimated prevalence of about 3% of the general population in those older than 50 years. The prevalence rises to about 15% to 20% of the general population in those older than 70 years.1
ETIOLOGY AND PATHOPHYSIOLOGY
In a minority of patients with intermittent claudication, the PAD will subsequently progress to critical ischemia, whereby even at rest the lower extremity arterial flow is insufficient to meet basal resting metabolic needs (i.e., oxygen and nutrient demand) of the lower extremity. Clinically, this is manifested by pain at rest and, in more severe cases, as nonhealing ulcers, cellulitis, and even gangrene. See Chapter 113 for an extensive discussion of the clinical aspects of PAD.
MANIFESTATIONS OF DISEASE
Clinical Presentation
The diagnosis of PAD is made on the basis of the typical history, physical examination (palpation of arterial pulsations), and measurement of the ABI. When a patient presents to the general practitioner or vascular surgeon with complaints of PAD, first-line treatment consists of modification of and/or treatment for atherosclerotic risk factors, such as smoking, hypertension, hypercholesterolemia, and the institution of (supervised) exercise training.2,3 Only when the patient’s complaints become too limiting to pursue regular activities will invasive interventional treatments be considered. For patients with intermittent claudication, the decision to intervene is largely dependent on relative criteria (patient and surgeon preference), but for patients with chronic critical ischemia, the need to intervene is more urgent because tissue perfusion does not meet basic metabolic demands, even at rest. Of 100 patients presenting with PAD, 5 eventually undergo percutaneous or surgical treatment.1 Although this is only a small minority of patients with PAD, the estimated annual number of percutaneous and surgical procedures performed for PAD in the United States alone was well over 200,000 in 2000, with sharp increases expected.4
Imaging Indications and Algorithm
Because the diagnosis of PAD is usually made from the typical history, physical examination, and ABI measurements, the need for imaging of the peripheral arteries only arises when a percutaneous or surgical intervention is considered. Imaging is needed to explore the extent of the disease process (e.g., number, location, and severity of atherosclerotic lesions) and to plan the correct approach for therapy.5
Traditionally, the standard of reference for imaging PAD has been x-ray catheter angiography, which initially had been through a translumbar aortic approach. In 1953, the transfemoral approach was developed by Seldinger, in which arterial access is gained through the superficial or common femoral artery.6 Having been refined and technically optimized, this is the procedure most widely used in state of the art angiography today, and is still considered the standard of reference. When combined with digital subtraction techniques, high-resolution projection arteriograms of the peripheral arterial circulation can be obtained in a routine fashion. However, substantial rates of local and systemic procedure-related complications have sparked the search for noninvasive alternatives to IA DSA.
Imaging Techniques and Findings
Ultrasound
DUS is widely used to determine the location, length, and severity of aortoiliac and femoropopliteal stenoses and obstructions. Duplex ultrasound was developed in the 1980s as an alternative to invasive angiography to avoid the inherent complications associated with the latter procedure. With DUS, the severity of a stenosis can be determined by using peak systolic velocity (PSV) measurements in arteries with reduced luminal diameter, PSV ratio at the site of stenosis and adjacent normal artery, end-diastolic velocity, and other less firmly established criteria. The sensitivity and specificity of DUS are generally moderate to high, ranging from 70% to 90%.7 However, a relatively recent meta-analysis,8 as well as a large prospective comparison study between CE MRA and DUS in 295 patients,9 has found that CE MRA is more sensitive and specific compared with DUS for the detection of PAD.
Computed Tomography
Recent advances in CT technology have enabled fast and robust CT angiography (CTA) of the peripheral vascular tree. Although there are fewer reports comparing CTA with conventional angiography for the detection of PAD as compared with CE MRA, it is widely believed that CTA is a valid and reliable method.10 The drawback of CTA is the enormous number of data sets that it generates—up to several thousand images per patient—and that heavily calcified arteries demand extensive user interaction to assess the underlying degree of stenosis adequately. In addition, the newest generation of multidetector row CT scanners is so fast that the contrast bolus may progress more slowly down the leg than the CT acquisition, leading to suboptimal opacification of the distal lower extremity arteries. For an in-depth discussion of CTA of the peripheral arteries, including these issues, see Chapter 115.
Magnetic Resonance Angiography
Although there are a variety of different MR angiography techniques, CE MRA is the most widely used method. Phase contrast (PC) and time of flight (TOF) MRA11 were the subjects of intense investigation about a decade ago, but the intrinsic drawbacks associated with these methods, such as long imaging times and their propensity to overestimate the degree and length of arterial stenoses, have led to the abandonment of these techniques in favor of CE MRA. The superiority of CE MRA over other MRA methods for peripheral artery imaging has been confirmed in several meta-analyses.12,13
Contrast-Enhanced Magnetic Resonance Angiography of the Peripheral Arteries
Synchronization of Three-Dimensional Contrast-Enhanced Magnetic Resonance Angiography Acquisition with Contrast Arrival
For successful CE MRA, care must be taken to synchronize peak arterial enhancement with image data acquisition, specifically acquisition of the central k-space data. The time of peak arterial enhancement is a function of many variables, the most important of which are injection rate and volume, amount and rate of saline flush,14 and cardiac output.15 Because the time of peak arterial enhancement can vary substantially among patients, the CE MRA examination needs to be tailored to the individual contrast arrival time. This is important for two main reasons: (1) to prevent “ringing” image artifacts and poor arterial opacification, which may occur if imaging is performed too early; and (2) to prevent suboptimal arterial enhancement and excessive venous and/or background enhancement, which occurs if imaging is performed too late.
Strategies to Optimize Vessel to Background Contrast
For multistation peripheral CE MRA (i.e., bolus chase CE MRA), the arterial T1 shortening associated with the sustained injection of a 0.1- to 0.3-mmol/kg dose of a standard (0.5 M) gadolinium-chelate contrast agent is generally insufficient to view the arteries preferentially over the extended FOVs over that of background tissue, especially in distal infrapopliteal arteries. The elimination of signal from background tissues, especially fat, because it has the shortest T1, is typically necessary for successful multistation peripheral CE MRA. The most commonly used technique to suppress background signal is image subtraction of nonenhanced mask three-dimensional MRA images from those of similarly acquired contrast-enhanced three-dimensional CE MRA. Although image subtraction decreases the signal-to-noise ratio by a factor of about 1.4 (v2 when the number of signals acquired is 1), vessel to background contrast improves to the extent that whole-volume MIPs become clinically useful, especially when using injection rates below 1.0 mL/sec.16 A disadvantage of using mask scans is that patients may move in between acquisition of the mask and contrast-enhanced parts of the scan, which can lead to subtraction misregistration artifacts. Subtraction misregistration artifacts may also occur if table positioning between the precontrast mask and postcontrast CE MRA is not accurate, on the order of 1 mm or less.
Because the T1 of fat is close to that of contrast-enhanced arterial blood, another way to suppress background tissue is by spectral saturation of signal from protons in fat. Although a fat saturation prepulse can be integrated into the three-dimensional CE MRA sequence, this takes a significant amount of time, which in turn must be offset by decreasing spatial resolution to achieve the same desired overall acquisition duration. Results of using fat saturation pulses are mixed and their use can, therefore, not be universally recommended.17,18
A dedicated peripheral vascular surface coil is mandatory for high-quality imaging of the pelvic and lower leg arteries. Image quality and anatomic coverage are vastly improved when compared with imaging without these dedicated lower extremity coils.19
Strategies to Decrease Venous Enhancement
Venous contamination is an important problem for CE MRA. This problem is particularly prevalent in patients with cellulitis or arteriovenous fistulas or malformations.20 Venous and background soft tissue contamination of arterial illustration are particularly prevalent in patients with diabetes mellitus.21 Diabetic patients, furthermore, are more likely to have limb-threatening ischemia and to require peripheral distal bypass surgery, making them prime candidates for preoperative peripheral artery imaging using CE MRA.
To avoid the limitations of imaging three consecutive stations, an alternative approach is to use two separate injections for peripheral CE MRA. In this approach, the distal lower legs (calves-feet or infrapopliteal region) are imaged during the first contrast medium bolus, and then a second contrast medium injection is administered to image the remaining two more proximal stations (the aortoiliac region and upper legs) using a two-station bolus chase CE MRA method. A benefit of this hybrid approach22 is that it can provide more reliable high spatial resolution three-dimensional MRA of the distal lower leg station, because timing of imaging is specific for contrast arrival to the distal lower extremities versus arbitrary timing based on progression of the stepping table during a standard three-station multistation peripheral CE MRA. The initial acquisition of the lower legs is typically done using up to 15 to 20 mL of a standard 0.5 M Gd-chelate contrast agent; it can be performed as a single arterial phase or multiphase (arterial and delayed phase) acquisition. Because this first acquisition is a dedicated single-station MRA of the infrapopliteal region, synchronization of central k-space lines is optimized for peak contrast enhancement in the lower leg arteries, and venous enhancement is almost eliminated. After imaging the infrapopliteal region, a moving table acquisition is performed to image the proximal two stations (aortoiliac region and upper legs) using the remaining dose of 20 to 40 mL Gd-chelate contrast medium. Note that it is not recommended that any cumulative Gd-chelate contrast medium dose exceed an agent’s approved dose levels, which in some cases is up to 0.3 mmol/kg dose for an adult patient. The greatest benefit of using the hybrid approach can be expected in patients with fast or highly variable arterial flow velocities, such as patients with chronic critical ischemia, arteriovenous fistulas, diabetes mellitus, and/or cellulitis.
Over the past few years, all major MR vendors have implemented dedicated centric k-space filling algorithms. Centric k-space filling is useful for CE MRA because the time between arterial and venous opacification is usually shorter than the duration of a high spatial resolution three-dimensional CE MRA acquisition. The underlying principle is to collect central k-space data, which primarily determine image contrast, during peak contrast enhancement of the target arterial territory and before significant venous enhancement has taken place.23,24 Peripheral k-space data primarily provide information related to edge detail of the image and can be acquired later during the bolus progression, with nominal impact on overall image quality. Therefore, use of centric k-space filling schemes for CE MRA timed for arterial phase of a bolus will result in preferential arterial images, with minimal venous contamination in most cases, even if the period between arterial and venous enhancement is shorter than the total duration of image acquisition.
Preferential arterial imaging can also be provided using time-resolved CE MRA, but it is crucial that temporal sampling be sufficient to image the arterial phase of the contrast medium bolus prior to significant venous enhancement. One popular time-resolved CE MRA technique uses repetitive centric k-space filling to obtain high spatial resolution MR angiograms with high temporal frame rate (i.e., time-resolved imaging at several seconds per frame). Korosec and colleagues25 were first to describe this concept, which they termed time-resolved imaging of contrast kinetics (TRICKS). With TRICKS, the contrast-sensitive central part of k-space is oversampled (i.e., more often) than the peripheral resolution-sensitive views. After the acquisition is finished, central k-space lines are combined with peripheral lines through a process of temporal interpolation so that a series of time-resolved three-dimensional images of the vasculature are obtained. More recently, keyhole contrast-enhanced timing robust angiography (CENTRA) was described. With keyhole CENTRA, temporal resolution is increased by repetitive acquisition of the central part of k-space only. This information is later combined with a data set containing the peripheral part of k-space, which is acquired as part of the last frame of the time-resolved series.26 Subsequently, these hybrid k-spaces can be reconstructed as a series of time-resolved three-dimensional CE MR angiograms. Combining keyhole imaging with parallel imaging can further increase temporal resolution. Time-resolved three-dimensional CE MRA is well suited for the initial dedicated distal lower leg (infrapopliteal) three-dimensional CE MRA of a hybrid peripheral MRA method.
Another final method to reduce venous contamination is by using midfemoral or infragenual venous compression with infrasystolic pressures of 50 to 60 mm Hg.27,28 It remains to be determined whether patients with critical ischemia and/or ulcers, in which high-quality lower leg images are most important, can tolerate this type of compression.
Resolution Requirements
To describe the degree of stenosis accurately, it is paramount that the resolution of the three-dimensional data set meet minimal standards. For example, it is known from studies by Hoogeveen and associates29 and Westenberg and coworkers30 that at least three pixels are needed across the lumen of an artery to quantify the degree of stenosis with an error of less than 10%. When this constraint is kept in mind, it is rather obvious that a higher spatial resolution is needed to characterize stenoses in the hepatic or renal arteries accurately, which are smaller in diameter than the abdominal aorta or iliac arteries. In general, voxel dimensions should be kept as close to isotropic (equal length in all dimensions) as possible; otherwise, vessels become blurred when they are viewed on postprocessed oblique projections. Recommended voxel sizes resolution are about 4 to 5 mm3 in the aortoiliac arteries, 3 to 4 mm3 in the upper legs, and 1 mm3 or more for hepatic, renal, or lower leg arteries.
Contrast Media and Injection Protocols
CE MRA relies on synchronizing maximum T1 shortening with acquisition of central k-space information. However, injection of gadolinium-chelate contrast medium only leads to transient T1 shortening of the blood pool. After having briefly enhanced the intravascular space, these contrast agents rapidly diffuse into the extracellular space. The intravascular half-life of commercially approved agents is about 90 seconds.31 Enough contrast must be injected to decrease the T1 of blood to values smaller than those of stationary background tissues. To depict the vasculature selectively, this means that the T1 of blood must be reduced to a value well below that of fat (T1 at 1.5 T = 270 ms). The rate of gadolinium injection is dictated by the following equation:
where T1 denotes the T1 of arterial blood at a given gadolinium concentration, 1200 is the T1 of arterial blood at 1.5 T, R1 is the T1 relaxivity of the contrast medium, and [Gd] is the arterial concentration of the gadolinium chelate. The rate of contrast injection should be such that an arterial T1 of about 50 ms or less is achieved. For example, when injecting a double dose (0.2 mmol/kg) of a 0.5 mmol/mL Gd-chelate contrast medium at 1.0 mL/sec in a 75-kg patient (i.e., 30 mL of contrast medium), assuming a relaxivity of 3.9 mmol−1 · s−1 and a cardiac output of 5 L/min, the lowest achievable T1 in arterial blood under first-pass conditions will be about 41 ms.
At present, there is no single preferred injection protocol, although an empiric strategy that works well in clinical practice is that the contrast injection duration should be about 40% to 60% of the acquisition duration. The rationale for this strategy is twofold. First, because of contrast dilution at the leading and trailing edges of the contrast medium bolus, as well as variable transit times through different portions of the pulmonary circulation, contrast bolus duration will increase in the body (usually to about 5 to 7 seconds).32 The second reason is that contrast injected after about half of the typical scan duration (on the order of 10 to 20 seconds) will not arrive in the arterial bed of interest before k-space lines contributing to contrast enhancement in the image are acquired.
The amount of Gd-chelate contrast medium to be injected, as well as injection speed and amount of saline flush, are dependent on other variables, such as the scan duration and technique used (i.e., single vs. multiple injection). Boos and colleagues15 have found that increasing the amount of contrast injected, as well as saline flush volume, increases bolus length and improves small vessel conspicuity, but does not necessarily result in higher vessel to background contrast.
Novel Contrast Media
Vessel to background signal can also be improved by using other contrast agents than the standard 0.5 M gadolinium chelate contrast agents. Recently, the first 1.0-M agent (Gadobutrol, Schering AG, Berlin) was approved for clinical use in Europe for CE MRA. Gadobutrol is formulated at a higher Gd concentration of 1.0 mol/L and has about 20% higher relaxivity (T1 relaxivity in blood, 5.2 mmol−1 · s−1 at 37° C and 1.5 T) than traditional 0.5-M Gd-chelate contrast agents, generating lower blood T1 values compared with traditional contrast agents and thus offering an attractive method to increase intravascular signal.33 In direct head to head comparisons between 1.0- and 0.5-M Gd-chelate contrast agents for pelvic MRA, Goyen and associates34 found that the use of the 1.0-M agent led to significantly higher signal- and contrast-to-noise ratios and better delineation of especially small pelvic arteries.
Other promising agents for peripheral arterial imaging are gadobenate dimeglumine (Gd-BOPTA; MultiHance, Bracco Diagnostics, Milan, Italy) and gadofosveset trisodium (Ablavar; Lantheus Medical Imaging, Billerica, Mass). Both these agents exhibit reversible albumin binding (5% of injected dose for gadobenate dimeglumine and 85% of injected dose for gadofosveset trisodium), which leads to high-contrast images at lower doses compared with the conventional extracellular agents. Because of the high fraction that is protein-bound, gadofosveset trisodium is excreted much slower than extracellular agents, as evidenced by a mean intravascular half-life of about 30 minutes.35 This leads to the possibility of not only acquiring images during the first pass, but also during the so-called steady-state or equilibrium phase of the contrast bolus, a period during which the contrast agent has opacified both the arterial and venous systems. Because of the contrast agents’ prolonged intravascular circulation time, images can be acquired with much higher spatial resolution compared with first-pass imaging, thus promising to increase the sensitivity and specificity of the resultant images for arterial disease detection and grading.36
Nonenhanced Magnetic Resonance Angiography of the Peripheral Arteries
Improvements in MR hardware and software, coupled with concerns about the safety of gadolinium-based contrast agents, have contributed to a renaissance of interest in nonenhanced MRA. An excellent and very comprehensive overview of the different nonenhanced techniques has recently been published by Miyazaki and Lee,37 and the reader is referred to this article for further information about the technical details of these techniques.
However, despite the promising initial results, there is a paucity of studies in the literature in which noncontrast medium–enhanced techniques are compared with CE MRA or IA DSA. As of mid-2009, only a single study in 36 patients has been published38 in which three-dimensional noncontrast medium–enhanced electrocardiograam (ECG)-gated MRA of the distal lower extremities was compared with CE MRA. Although the noncontrast medium–enhanced technique demonstrates a high negative predictive value 92.3%, the overall accuracy of 79.4% can be considered poor compared with CE MRA techniques.
Classic Signs
The typical appearance of an atherosclerotic lesion is a focal excentric arterial narrowing, that may be relatively smooth or more serrated in appearance (Fig. 116-2). Lesions can be very short (several millimeters) or may extend over the entire vessel length. Long-standing high-grade stenoses and occlusions may be bridged by collateral vessels with a typical corkscrew appearance. Acute or very recent occlusions tend not to be very well collateralized.
DIFFERENTIAL DIAGNOSIS
From Clinical Presentation
Although PAD in the lower extremities is almost invariably caused by atherosclerosis, there are well-known nonatherosclerotic diseases that may present with intermittent claudication or critical ischemia. An atypical history or clinical characteristics incongruent with those typically seen in atherosclerotic PAD should prompt consideration of alternative causes. There is an extensive list of differential diagnostic considerations. Some of the more frequently seen diseases are vasculitis, Buerger’s disease, popliteal entrapment, cystic adventitial disease, and radiation-induced arteritis.11
From Imaging Findings
If a lesion in the peripheral vascular tree does not have the typical atherosclerotic appearance as described earlier, one should consider other diseases. Concentric smooth luminal narrowing is atypical in atherosclerosis and is generally suggestive of more uncommon causes of PAD, such as vasculitis (Fig. 116-3) or cystic adventitial disease (Fig. 116-4). Evaluation of the soft tissues surrounding the artery of interest may reveal additional clues about other causes, such as aberrant insertion of the medial head of the gastrocnemius muscle in popliteal entrapment syndrome or the presence of high-signal intensity proteinaceous material in the popliteal artery wall in cystic adventitial disease.
SYNOPSIS OF TREATMENT OPTIONS
See Chapters 113 and 114 for an overview of treatment options for PAD.
REPORTING: INFORMATION FOR THE REFERRING PHYSICIAN
Aorta and Iliac Arteries
For lesions involving the aortic bifurcation and proximal iliac artery (Fig. 116-5), involvement of the contralateral iliac artery should also be noted, because this will change the preferred interventional treatment strategy. In case of aortic occlusion (Leriche syndrome; Fig. 116-6) or unilateral iliac artery occlusion, the site of distal reconstitution should be noted, because this will determine the surgical approach. The easiest and most widely used way of measuring diameter reduction is simply to measure the maximum degree of luminal reduction on MIP images, analogous to how stenoses are measured on IA DSA images. A stenosis is generally considered to be hemodynamically significant when reduction of the luminal diameter exceeds 50%.
Femoropopliteal Arteries
CE MRA is an excellent modality to image infrainguinal disease, because inflow and outflow arteries can be imaged in a single examination. The key differentiation that must be made when evaluating the upper leg vasculature is whether there is a relatively short focal stenosis (see Fig. 116-2) or complete occlusion over a long segment (Fig. 116-7). This differentiation is particularly important in the setting of intermittent claudication, because patients and their vascular surgeons may only be interested in invasive treatment in case endovascular options can be considered. The most common site of stenoses or occlusions is where the superficial femoral artery courses through the adductor (Hunter) canal. Current best clinical practice is to attempt endovascular treatment in patients in whom lesion length does not exceed 3 cm. Available evidence indicates that surgery is still the best treatment option when lesion length exceeds 5 cm.1
Lower Leg and Pedal Arteries
Although depiction of the infragenicular arterial system in patients with intermittent claudication is important, it is usually not the location of the lesions that causes symptoms, nor the target for invasive intervention, except in patients with diabetes mellitus.39 This is opposed to the group of patients with chronic critical ischemia, those with rest pain and/or tissue loss. The angiographic hallmark of chronic critical ischemia is bilateral multiple stenoses and occlusions at different levels in the peripheral arterial tree. Patients with diabetes are a well-recognized subgroup, with primarily distal atherosclerotic occlusive disease and preservation of normal inflow (Fig. 116-8). Obtaining a full anatomic study from the infrarenal aorta down to the lower leg and pedal arteries is essential for the preinterventional workup of distal peripheral artery disease.
The guiding principle behind vascular surgical reconstruction in the lower legs of patients with chronic critical ischemia has evolved from conservative treatment, with eventual amputation, to restoration of pulsatile flow to the distal lower leg to end rest pain and achieve wound healing.40,41 Distal bypass grafting has a much better limb salvage rate than conservative treatment.42 Consequently, the vascular surgeon will bypass into the best available outflow vessel, regardless of anatomic level, provided that inflow into the artery and the origin of the graft are uncompromised. To determine the best possible treatment plan, it is essential for the vascular surgeon to obtain adequate anatomic information about lower leg arteries and the pedal arteries in addition to functional information from other tests, such as transcutaneous oxygen measurements and targeted high-resolution duplex ultrasonography.1
A thorough knowledge of below-knee and pedal vasculature and their anatomic variations is mandatory when reporting lower leg MRA studies. The most frequently encountered variations are high origins of the anterior and posterior tibial arteries. From a vascular surgical perspective, it is important to recognize variations in the dorsal pedal artery and medial and lateral plantar branches of the posterior tibial artery because nonfilling of one of the named segments does not necessarily mean that the vessel is occluded.43 The crucial distinction to make in a report is whether the anterior circulation of the foot (most often the dorsalis pedis artery) anastomoses to the posterior circulation (posterior tibial, lateral and medial plantar arteries) via the deep plantar artery to constitute the pedal arch. Occlusion of the entire pedal arch is prognostic for a poor outcome of below-knee bypass grafting.44
The most important pitfall for CE MRA is venous enhancement and contamination of arterial images, which occurs primarily in patients with cellulitis and diabetes (Fig. 116-9). A priori identification of these conditions helps determine the optimal imaging approach.45 Exclusion of pedal arterial anatomy because of too small an imaging volume is a common mistake that can be avoided by meticulous review of localizer original partitions. Stenosis of the dorsalis pedis artery can be induced artifactually by tight straps and when the foot is imaged in plantar flexion. The cause of this latter so-called ballerina sign artifact is compression of the dorsal pedal artery by the distal part of the retinaculum extensorum.46
Postinterventional Imaging and Evaluation of Peripheral Arterial Bypass Grafts
Considering the chronic nature of the atherosclerotic disease process, many patients will ultimately present with renewed complaints after having been treated successfully for intermittent claudication or chronic critical ischemia (Figs. 116-10 and 116-11). Consequently, a substantial number of patients undergoing peripheral vascular imaging will have metallic implants such as vascular stent grafts, endoprostheses, and vascular surgical ligating clips. These implants are sometimes known to cause serious artifacts with MRI and MRA as a result of differences in susceptibility between the metal of which they are manufactured and human tissue. Susceptibility artifacts are highly variable but can be recognized by local or regional distortions or complete signal voids. In addition, artifacts may also be present after hip and knee joint replacement surgery. It is important to realize that artifact severity not only depends on the stent graft material, but also on the diameter of the stent graft or size of the ligating clip, field strength, echo time, angle to the main magnetic field, and orientation to the readout gradient.47,48 Stents made of Nitinol suffer least from artifactual signal loss and stainless steel stents have the most signal loss. The proximal and distal anastomoses of the graft should be assessed carefully for the presence of stenoses. Clips may also be found in the vicinity of renal transplants, on side branches of venous grafts, and at sites of vein harvesting.
KEY POINTS
 Peripheral artery disease is prevalent, particulary in older patients. Up to 20% of the general population older than 70 years have signs of PAD.
Peripheral artery disease is prevalent, particulary in older patients. Up to 20% of the general population older than 70 years have signs of PAD. Technical advances, such as parallel imaging, centric k-space sampling, k-space view sharing, and dedicated multielement peripheral vascular coils enable the acquisition of high spatial resolution arterial images of the peripheral vascular tree in routine clinical practice.
Technical advances, such as parallel imaging, centric k-space sampling, k-space view sharing, and dedicated multielement peripheral vascular coils enable the acquisition of high spatial resolution arterial images of the peripheral vascular tree in routine clinical practice. Contrast-enhanced MRA remains the standard of reference for the workup of PAD because this method has been shown to be highly reliable in comparison to IA DSA.
Contrast-enhanced MRA remains the standard of reference for the workup of PAD because this method has been shown to be highly reliable in comparison to IA DSA.Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334:1257.
Kaufman JA. Lower extremity arteries. In: Thrall JA, editor. Vascular and Interventional Radiology: The Requisites. Philadelphia: Mosby; 2004:407-444.
Leiner T. Magnetic resonance angiography of abdominal and lower extremity vasculature. Top Magn Reson Imaging. 2005;16:21-66.
Miyazaki M, Lee VS. Nonenhanced MR angiography. Radiology. 2008;248:20-43.
Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl):S5-S67.
Rofsky NM, Adelman MA. MR angiography in the evaluation of atherosclerotic peripheral vascular disease. Radiology. 2000;214:325-338.
1 Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl):S5-S67.
2 Beebe HG. Intermittent claudication: effective medical management of a common circulatory problem. Am J Cardiol. 2001;87:14D-18D.
3 Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975-980.
4 Krajcer Z, Howell MH. Update on endovascular treatment of peripheral vascular disease: new tools, techniques, and indications. Tex Heart Inst J. 2000;27:369-385.
5 Rofsky NM, Adelman MA. MR angiography in the evaluation of atherosclerotic peripheral vascular disease. Radiology. 2000;214:325-338.
6 Seldinger SI. Catheter replacement of the needle in percutaneous arteriography. Acta Radiol. 1953;39:368-375.
7 Koelemay MJ, den Hartog D, Prins MH, et al. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. Br J Surg. 1996;83:404-409.
8 Visser K, Hunink MG. Peripheral arterial disease: gadolinium-enhanced MR angiography versus color-guided duplex US—a meta-analysis. Radiology. 2000;216:67-77.
9 Leiner T, Kessels AG, Nelemans PJ, et al. Peripheral arterial disease: comparison of color duplex US and contrast-enhanced MR angiography for diagnosis. Radiology. 2005;235:699-708.
10 Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334:1257.
11 Leiner T. Magnetic resonance angiography of abdominal and lower extremity vasculature. Top Magn Reson Imaging. 2005;16:21-66.
12 Koelemay MJ, Lijmer JG, Stoker J, et al. Magnetic resonance angiography for the evaluation of lower extremity arterial disease: a meta-analysis. JAMA. 2001;285:1338-1345.
13 Nelemans PJ, Leiner T, de Vet HC, van Engelshoven JM. Peripheral arterial disease: meta-analysis of the diagnostic performance of MR angiography. Radiology. 2000;217:105-114.
14 Schoenberg SO, Londy FJ, Licato P, et al. Multiphase-multistep gadolinium-enhanced MR angiography of the abdominal aorta and runoff vessels. Invest Radiol. 2001;36:283-291.
15 Boos M, Scheffler K, Haselhorst R, et al. Arterial first pass gadolinium-CM dynamics as a function of several intravenous saline flush and Gd volumes. J Magn Reson Imaging. 2001;13:568-576.
16 Ho KY, de Haan MW, Kessels AG, et al. Peripheral vascular tree stenoses: detection with subtracted and nonsubtracted MR angiography. Radiology. 1998;206:673-681.
17 Ruehm SG, Nanz D, Baumann A, et al. Three-dimensional contrast-enhanced MR angiography of the run-off vessels: value of image subtraction. J Magn Reson Imaging. 2001;13:402-411.
18 Leiner T, de Weert TT, Nijenhuis RJ, et al. Need for background suppression in contrast-enhanced peripheral magnetic resonance angiography. J Magn Reson Imaging. 2001;14:724-733.
19 Leiner T, Nijenhuis RJ, Maki JH, et al. Use of a three-station phased array coil to improve peripheral contrast-enhanced magnetic resonance angiography. J Magn Reson Imaging. 2004;20:417-425.
20 Wang Y, Chen CZ, Chabra SG, et al. Bolus arterial-venous transit in the lower extremity and venous contamination in bolus chase three-dimensional magnetic resonance angiography. Invest Radiol. 2002;37:458-463.
21 Zhang HL, Kent KC, Bush HL, et al. Soft tissue enhancement on time-resolved peripheral magnetic resonance angiography. J Magn Reson Imaging. 2004;19:590-597.
22 Morasch MD, Collins J, Pereles FS, et al. Lower extremity stepping-table magnetic resonance angiography with multilevel contrast timing and segmented contrast infusion. J Vasc Surg. 2003;37:62-71.
23 Wilman AH, Riederer SJ. Improved centric phase encoding orders for three-dimensional magnetization-prepared MR angiography. Magn Reson Med. 1996;36:384-392.
24 Willinek WA, Gieseke J, Conrad R, et al. Randomly segmented central k-space ordering in high-spatial-resolution contrast-enhanced MR angiography of the supraaortic arteries: initial experience. Radiology. 2002;225:583-588.
25 Korosec FR, Frayne R, Grist TM, Mistretta CA. Time-resolved contrast-enhanced three-dimensional MR angiography. Magn Reson Med. 1996;36:345-351.
26 Hoogeveen RM, von Falkenhausen M, Gieseke J. Fast, dynamic high resolution contrast-enhanced MR angiography with CENTRA keyhole and SENSE. Twelfth Scientific Meeting of the International Society for Magnetic Resonance in Medicine. Kyoto, Japan, May 2004.
27 Herborn CU, Ajaj W, Goyen M, et al. Peripheral vasculature: whole-body MR angiography with midfemoral venous compression—initial experience. Radiology. 2004;230:872-878.
28 Bilecen D, Schulte AC, Aschwanden M, et al. MR angiography with venous compression. Radiology. 2004;233:617-618.
29 Hoogeveen RM, Bakker CJ, Viergever MA. Limits to the accuracy of vessel diameter measurement in MR angiography. J Magn Reson Imaging. 1998;8:1228-1235.
30 Westenberg JJ, van der Geest RJ, Wasser MN, et al. Vessel diameter measurements in gadolinium contrast-enhanced three-dimensional MRA of peripheral arteries. Magn Reson Imaging. 2000;18:13-22.
31 Schmiedl U, Moseley ME, Ogan MD, et al. Comparison of initial biodistribution patterns of Gd-DTPA and albumin-(Gd-DTPA) using rapid spin echo MR imaging. J Comput Assist Tomogr. 1987;11:306-313.
32 Prince MR, Grist TM, Debatin JF. Three-dimensional Contrast MR Angiography. Berlin: Springer; 2003.
33 Hentsch A, Aschauer MA, Balzer JO, et al. Gadobutrol-enhanced moving-table magnetic resonance angiography in patients with peripheral vascular disease: a prospective, multi-centre blinded comparison with digital subtraction angiography. Eur Radiol. 2003;13:2103-2114.
34 Goyen M, Lauenstein TC, Herborn CU, et al. 0.5 M Gd chelate (Magnevist) versus 1.0 M Gd chelate (Gadovist): dose-independent effect on image quality of pelvic three-dimensional MR-angiography. J Magn Reson Imaging. 2001;14:602-607.
35 Perreault P, Edelman MA, Baum RA, et al. MR angiography with gadofosveset trisodium for peripheral vascular disease: phase II trial. Radiology. 2003;229:811-820.
36 Hadizadeh DR, Gieseke J, Lohmaier SH, et al. Peripheral MR angiography with blood pool contrast agent: prospective intraindividual comparative study of high-spatial-resolution steady-state MR angiography versus standard-resolution first-pass MR angiography and DSA. Radiology. 2008;249:701-711.
37 Miyazaki M, Lee VS. Nonenhanced MR angiography. Radiology. 2008;248:20-43.
38 Lim RP, Hecht EM, Xu J, et al. Three-dimensional nongadolinium-enhanced ECG-gated MRA of the distal lower extremities: preliminary clinical experience. J Magn Reson Imaging. 2008;28:181-189.
39 Menzoian JO, LaMorte WW, Paniszyn CC, et al. Symptomatology and anatomic patterns of peripheral vascular disease: differing impact of smoking and diabetes. Ann Vasc Surg. 1989;3:224-228.
40 Hughes K, Domenig CM, Hamdan AD, et al. Bypass to plantar and tarsal arteries: an acceptable approach to limb salvage. J Vasc Surg. 2004;40:1149-1157.
41 Pomposelli FB, Kansal N, Hamdan AD, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37:307-315.
42 Holstein PE, Sorensen S. Limb salvage experience in a multidisciplinary diabetic foot unit. Diabetes Care. 1999;22(Suppl 2):B97-B103.
43 Alson MD, Lang EV, Kaufman JA. Pedal arterial imaging. J Vasc Interv Radiol. 1997;8:9-18.
44 Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517-538.
45 Maki JH, Wilson GJ, Eubank WB, Hoogeveen RM. Predicting venous enhancement in peripheral MRA using a two-station timing bolus. Eleventh Scientific Meeting of the International Society for Magnetic Resonance in Medicine. Toronto, Ontario, May 2003.
46 Kaufman JA. Lower extremity arteries. In: Thrall JA, editor. Vascular and Interventional Radiology: The Requisites. Philadelphia: Mosby; 2004:407-444.
47 Weishaupt D, Quick HH, Nanz D, et al. Ligating clips for three-dimensional MR angiography at 1.5 T: in vitro evaluation. Radiology. 2000;214:902-907.
48 Meissner OA, Verrel F, Tato F, et al. Magnetic resonance angiography in the follow-up of distal lower-extremity bypass surgery: comparison with duplex ultrasound and digital subtraction angiography. J Vasc Interv Radiol. 2004;15:1269-1277.

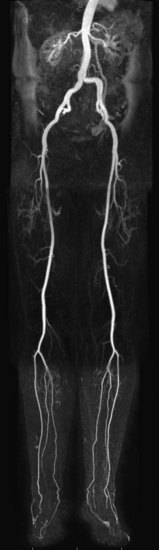
 FIGURE 116-1
FIGURE 116-1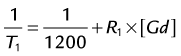
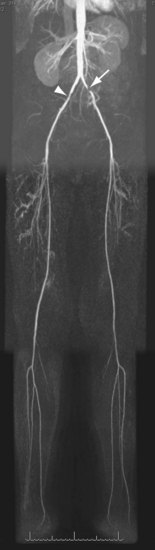
 FIGURE 116-2
FIGURE 116-2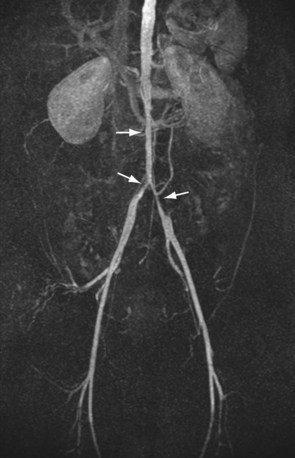
 FIGURE 116-3
FIGURE 116-3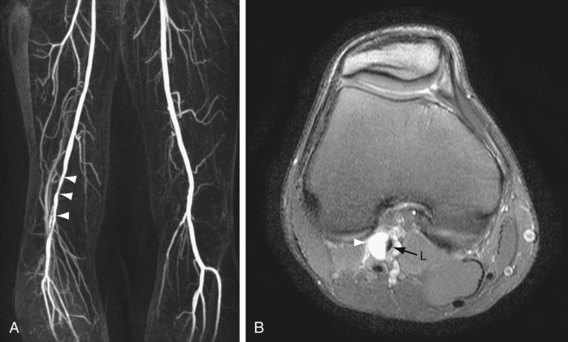
 FIGURE 116-4
FIGURE 116-4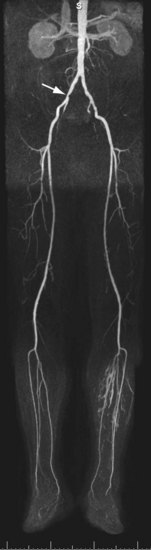
 FIGURE 116-5
FIGURE 116-5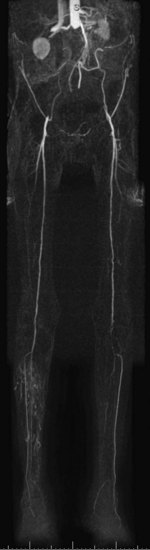
 FIGURE 116-6
FIGURE 116-6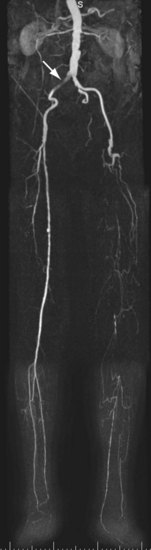
 FIGURE 116-7
FIGURE 116-7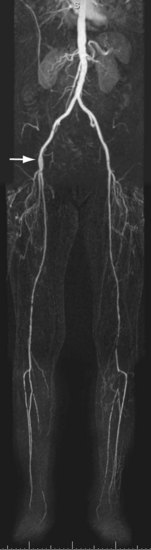
 FIGURE 116-8
FIGURE 116-8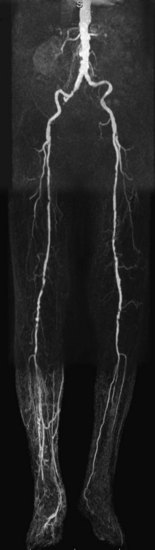
 FIGURE 116-9
FIGURE 116-9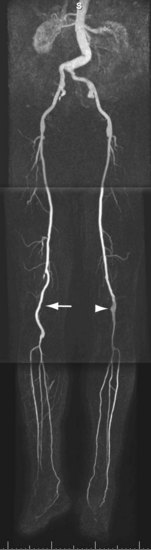
 FIGURE 116-10
FIGURE 116-10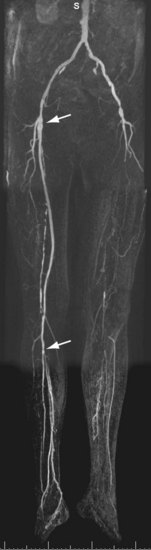
 FIGURE 116-11
FIGURE 116-11

