Chapter 24 Peripheral Joint and Soft Tissue Injection Techniques
Overview and History
The first published description of intraarticular corticosteroid injection was by Hollander et al.79 in 1951, who demonstrated clinical improvement with the injection of hydrocortisone in a case series of patients with inflammatory joint conditions. Hollander78 subsequently reported on 250,000 injections in 8000 patients and was the first to describe complications associated with intraarticular corticosteroid injections. These included postinjection flare, aseptic necrosis in weight-bearing joints, and infection (infections occurred at a frequency of 1 in 15,000 injections). Practitioners have since expanded the use of corticosteroids to include injections near tendon insertions for enthesitis and other periarticular structures such as bursae.183 These injections have other known complications including localized fat atrophy and tendon rupture.25 Despite these known complications, physicians continue to use corticosteroids as evidenced by a survey showing that 89% of physiatrists171 and 93% of rheumatologists use intraarticular and soft tissue corticosteroid injections for the treatment of pain.75 Fortunately, other medications such as local anesthetics and hyaluronan derivatives are available that can also be used to help with musculoskeletal pain conditions when corticosteroids are not indicated or are not beneficial.
Purpose: Diagnostic or Therapeutic
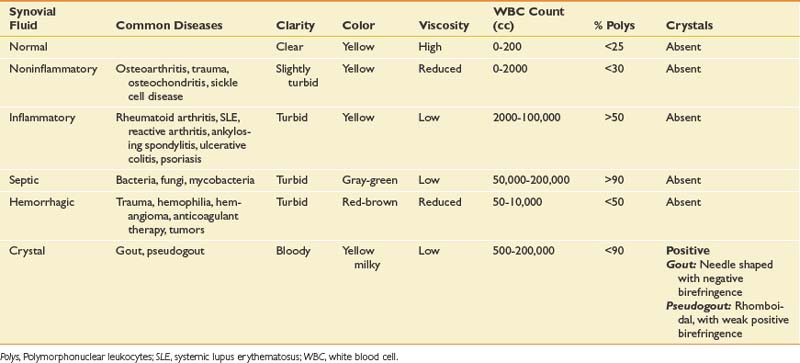
Intraarticular space or soft tissue injections can be used either for diagnostic or therapeutic purposes. Obtaining fluid from a joint, bursa, or cystic structure can help determine the cause of a fluid collection. Although a complete discussion is beyond the scope of this chapter, certain laboratory studies and bedside analyses can help categorize the nature of the fluid (Table 24-1). Cell count classifies the type of fluid as being noninflammatory, inflammatory, infectious, or septic. Culture and sensitivity help in the identification of a specific organism and its susceptibility to antibiotics. Birefringence classifies the type of crystals present. Although laboratory analysis is the most specific and sensitive way to analyze aspirated fluid, the string test allows for a nonspecific bedside screen for inflammation. Because of its highly viscous nature, healthy synovial fluid should be able to stretch at least 2.5 cm before breaking when dripped from a syringe. Inflammation decreases the overall viscosity and makes the fluid drip instead of stretch. If the aspirated fluid is noninflammatory, it should be clear enough to read 0.25-inch font through a test tube.134 The fluid should be sent for formal analysis when an infection or a crystal arthropathy is suspected, or when the cause of the fluid collection is uncertain.92
Although aspiration can help classify the etiology of the fluid, injecting an anesthetic into a structure might aid in identifying or confirming whether the structure is a pain generator.94 For example, a diagnostic injection into the hip joint using fluoroscopic guidance can help distinguish whether pain is emanating from a pathologic disorder of the hip joint or is referred from the lumbar spine. Because single anesthetic diagnostic injections have a known false-positive rate, a second confirmatory injection might be necessary.45
Not only can injections be used for diagnostic purposes, they can also be effective in the treatment of painful conditions. Although isolated aspiration of accumulated fluid can be helpful in some cases, any benefits obtained from solely removing fluid are often short-lived.201 Consequently various injected medications and preparations have been used alone or in combination to provide a longer therapeutic benefit for various musculoskeletal conditions. Performing these injections should help lessen the need for oral medication, improve function, and reduce disability. Optimally injections should not be performed in isolation, but in combination with physical therapy and exercise.26 Injected medications such as anesthetics, corticosteroids, and various preparations of viscosupplementation have different adverse effects, durations of action, and costs. These factors should be thoroughly understood before use of injections in the clinical setting.
Medications
Corticosteroids
Mechanisms, Types, and Adverse Effects
Corticosteroids are commonly used for intraarticular or soft tissue musculoskeletal conditions and have multiple mechanisms of action. Although corticosteroids can cause various systemic effects, the antiinflammatory and immunosuppression properties are due to the glucocorticoid effects. Although glucocorticoids act directly on nuclear steroid receptors to control the rate of synthesis of messenger ribonucleic acid and proteins in T and B cells, they also produce changes in white blood cell traffic and alterations in levels of cytokines and enzymes, and inhibit the function of phospholipase A2. The overall effect of these reactions is a reduction in proinflammatory derivatives such as bradykinin, histamine, prostaglandins, and leukotrienes. Bradykinin and histamine are capable of directly stimulating primary afferent nociceptive fibers, whereas prostaglandins and leukotrienes sensitize nociceptors.37 One objective measure of these antiinflammatory effects is the reduction of erythrocyte sedimentation rates and C-reactive protein levels.188 In addition to the effect on inflammation, corticosteroids have also been reported to have a direct stabilizing effect on neural membranes and inhibit C-fiber transmission, thereby reducing ectopic discharges from neural fibers, including those from within the spinal cord.128,150 This could help explain how corticosteroids might have antinociceptive effects even in noninflammatory conditions.86 These antiinflammatory and antinociceptive effects are often delayed, explaining why the therapeutic onset of corticosteroids can take 24 to 48 hours to produce any benefit.206
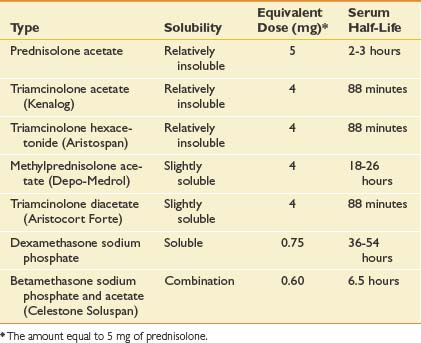
Multiple corticosteroids are available, each varying in potency, solubility, and duration of action. In one survey of rheumatologists, methylprednisolone acetate was the most frequently used corticosteroid at 35%, followed closely by triamcinolone hexacetonide (31%) and triamcinolone acetonide (22%). Each corticosteroid preparation has known properties pertaining to potency, relative doses, half-life, and solubility (Table 24-2).
Corticosteroid solubility is a key feature that varies among the preparations available. It is postulated that corticosteroids with lower solubility (such as depot preparations) might remain at the injection site longer and maintain higher effective synovial levels.34 In fact, corticosteroid depot preparations have such low solubility that their crystals have been found at the site of an injection up to 1 month postinjection.63 Because of their lower solubility and greater duration of action, there can be a greater concern that injecting depot corticosteroids around soft tissue structures might lead to local adverse effects.34 This is in contrast to more water soluble corticosteroids like dexamethasone, which can diffuse away more easily and potentially create more systemic effects.34
Intraarticular injections have generally demonstrated an excellent safety record and are described by the American College of Rheumatology as “safe and effective when administered by an experienced physician.”10 Corticosteroid injections do, however, have known adverse effects. A postinjection flare can occur in 2% to 6% of patients63 and is thought to be the result of a chemical synovitis from the injected particulate corticosteroid crystals.81,116 Although this postinjection flare typically occurs within 24 hours, it is usually self-limited and can be effectively managed with ice packs, analgesic therapy, or in rare cases, aspiration of joint fluid.164 Corticosteroids can also cause fat atrophy and skin changes, but at a rate less than 1%.97 There are case reports of less common adverse effects, including tendon rupture with direct intratendon injection∗ and avascular necrosis.3,56,67,72,127 Recent evidence has also shown that patients who receive intraarticular corticosteroids in the 6 months preceding total joint arthroplasty have an increased risk of infection postoperatively.43,104,138,200
Corticosteroids also have known adverse systemic effects. Facial flushing occurs in up to 15% of patients, and has an onset within hours and can linger for several days.140 Intraarticular corticosteroid injections have also been shown to suppress the hypothalamic-pituitary-adrenal (HPA) axis.99,135,157 Although usually mild and transient, prolonged HPA suppression can last up to 11 weeks and in one case has caused Cushing’s syndrome.135 Other systemic effects include increased hepatic glucose synthesis and antagonism of insulin for up to 2 weeks after the injection, potentially worsening any preexisting hyperglycemia.132 Any potential effect on bone metabolism appears to be transient and mild, without known clinical relevance.49 There have been no case reports of corticosteroid-induced myopathy after intraarticular injections.
There is a concern that intraarticular injections can cause joint degradation because of the direct catabolic effect of the corticosteroid, or because of the increased use of the less painful but still diseased joint. In rabbits, corticosteroids have been shown to damage chondrocytes,137 but this effect has not been confirmed in primates.60 In a prospective trial comparing intraarticular injections of either saline or triamcinolone every 2 months for up to 2 years in patients with knee osteoarthritis, there were no differences in joint space narrowing seen in either group at the 2-year follow-up.154 In another study in patients with rheumatoid arthritis, there was no difference in joint arthroplasty rates between those who received four or more intraarticular injections annually and those receiving less frequent injections.159 In children with juvenile rheumatoid arthritis, intraarticular injections of corticosteroids did not appear to affect cartilage integrity.82,182 Because of the known and unknown adverse effects, it is generally accepted that physicians should limit the total number of corticosteroid injections to three or four annually per patient.10,143
Efficacy
Most reviews andTM large prospective studies have demonstrated a positive efficacy of corticosteroid injections in inflammatory conditions at the shoulder,12,22 hip,58,151,213 and knee joints.11,14,62,213 There are additional uncontrolled trials and reports for small joints23,123,205 and soft tissues70,91,142,162,172 that mostly consist of heterogeneous studies with small patient populations, differing disease pathology, and varying methodological quality.14 When taken as a whole, the literature demonstrates that corticosteroids may provide short-term pain relief for various musculoskeletal conditions.8,108,113,158 Currently the American College of Rheumatology guidelines recommend intraarticular corticosteroid injections for selected patients with signs of inflammation.10 Too few studies are available to make a recommendation for a specific corticosteroid preparation (i.e., triamcinolone vs. betamethasone).213
Carette et al.26 studied the effect of fluoroscopically guided corticosteroid shoulder joint injections on symptoms from adhesive capsulitis. Patients were randomly assigned to one of four groups: corticosteroid injection and physical therapy, corticosteroid injection alone, saline injection and physical therapy, and saline injection alone. At 6 weeks the group that received the corticosteroid injection and physical therapy had significant improvements in pain and range of motion, but by 1 year all four groups had similar results. The data suggested that a corticosteroid injection helped pain in the short-term but did not affect long-term outcomes.
Studies using US-guided subacromial bursa corticosteroid injections have demonstrated variable efficacy.29,31,48,129,161 No studies to date have analyzed the efficacy of US-guided corticosteroid injections into the biceps tendon sheath, glenohumeral joint, or acromioclavicular (AC) joint.
Corticosteroid injections have been studied extensively for carpal tunnel syndrome.8,108,113 A prospective study of surgical decompression versus local corticosteroid injection in patients with carpal tunnel syndrome resulted in symptomatic relief in both groups followed up for 1 year.108 A Cochrane Review looked at 12 studies of carpal tunnel syndrome and determined that “local corticosteroid injection provides significantly greater clinical improvement than oral corticosteroid for up to three months,” including improvements in pain and paresthesias.113 There also could be long-term improvement in hand function and electrophysiologic studies.8
Corticosteroid injections can be considered a definitive treatment for certain conditions, such as de Quervain’s tenosynovitis.158 Kullenberg et al.96 conducted a prospective, randomized, blinded trial comparing the use of either 80 mg triamcinolone acetonide or mepivacaine 1% in patients with osteoarthritis who were awaiting hip replacement surgery. Injection with corticosteroids under fluoroscopic guidance resulted in the greatest improvement in pain and function at 3 weeks and 12 weeks. The group that received mepivacaine did not show significant improvement in either parameter at 3 weeks and withdrew from the study before 12 weeks because of lack of effect. Robinson et al.160 compared 56 patients with hip pain injected under fluoroscopic guidance with 40 mg of methylprednisolone, and 36 patients with hip pain injected with 80 mg of methylprednisolone, with follow-up at weeks 6 and 12. When the doses were compared, the group that received the 80-mg dose demonstrated a significant improvement compared with the 40-mg group at 6 and 12 weeks. Imaging findings did not relate to severity of symptoms or response to injections. Plant et al.146 studied the effect of 80 mg of methylprednisolone and lignocaine on pain at the hip from osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. Fluoroscopic guidance was used to confirm needle placement in the hip joint. At 12 weeks those with some evidence of a hypertrophic (visible osteophytes) pattern on plain radiographs had a significant response to the injection.96 In a controlled pilot study, Qvistgaard et al.151 showed that patients treated with repeated corticosteroid injections into the hip using US guidance had significant improvement during the study period, which indicated a moderate clinical effect.
Several studies have shown that intraarticular corticosteroid knee injections provide 1 to 4 weeks of relief of pain for osteoarthritis of the knee.11,37 Sustained relief has not been demonstrated in the literature. There is a paucity of studies examining the efficacy of various conditions with US-guided knee injections. Bliddal20 compared the efficacy of US-guided injections of methylprednisolone with that of etanercept in multiple joints of patients with rheumatoid arthritis. Although a subgroup analysis was not completed specifically for the knee, no significant differences were found between the two groups for the relief of pain. Another uncontrolled trial showed good short-term outcomes when US guidance was used to aspirate and inject corticosteroids into perimeniscal cysts.109
Viscosupplementation
Mechanisms, Types, and Adverse Effects
In osteoarthritis the hyaluronic acid found in synovial fluid decreases in both molecular weight and concentration. Because of this reduction, injectable hyaluronan derivatives (collectively known as viscosupplementation) were developed for therapeutic purposes. Although their exact mechanism of action is unclear, some theories do exist including possible antiinflammatory or antinociceptive effects, or stimulation of in vivo hyaluronic acid synthesis.207 Restoration of the viscoelastic properties of the synovial fluid appears the most logical explanation, but other mechanisms must exist because studies show that the injected hyaluronic acid only remains in the joint space for hours to days.32 In fact, an optimal effect might only develop after repeated weekly injections, but can persist for several months.194
Adverse reactions associated with these medications are uncommon, and no long-term systemic effects have been reported.155 The most common adverse reaction reported is a pseudoseptic reaction that occurs 24 to 72 hours after the injection, but at a rate of less than 3%.185 This reaction should be differentiated from the much less frequent complication of an infected joint.
Efficacy
The literature on the efficacy of intraarticular viscosupplementation is primarily focused on the knee, with limited studies of other joints. Although the literature covering viscosupplementation for knee osteoarthritis has been conflicting and has a known publication bias,15,105 metaanalyses suggest an improvement in pain compared with placebo.105 In patients who have failed conservative treatment, there is evidence that viscosupplementation is beneficial for the treatment of knee pain caused by osteoarthritis.15,105 In one large placebo-controlled, randomized trial of 660 patients with moderate to severe shoulder pain caused by osteoarthritis, rotator cuff tear, or adhesive capsulitis, a significant improvement in pain relief was found in all groups up to 13 weeks after viscosupplementation.18 Thirty patients with symptomatic osteoarthritis of the shoulder who failed conservative treatment and were treated with intraarticular viscosupplementation demonstrated improvement in function and pain levels.170
Although viscosupplementation has been studied extensively and with high-quality studies for knee osteoarthritis,105 few high-quality studies have evaluated its use in the treatment of hip osteoarthritis. Van den Bekerom et al.194 performed a systematic review of 16 studies, with only two high-quality studies of viscosupplementation injections for hip osteoarthritis using fluoroscopic or US guidance. In the level 1 study that was done, the efficacy of low-molecular-weight viscosupplementation was compared with that of high-molecular-weight viscosupplementation but not with placebo.190 Patients had significant improvements with both types of viscosupplementation for up to 6 months. Because the placebo effect can be significant,105 these results should be viewed cautiously. In uncontrolled trials using different US approaches, repeated viscosupplementation injections demonstrate significant improvement in visual analogue scale (VAS) and functional outcomes.24,124,125,149,191 Despite the lack of high-quality research, viscosupplementation has been shown to improve symptoms in uncontrolled studies and can be considered on an individual basis.
Anesthetics
Mechanisms, Types, and Adverse Effects
Local anesthetics are frequently used in intraarticular and soft tissue injections to not only decrease the pain from the injection but also for diagnostic purposes. There are two classes of anesthetics, the esters and the amides. They differ in solubility, pH, and vasodilatory effects, resulting in unique onset of action, potency, and duration (Table 24-3). These properties can be altered by the addition of other agents such as epinephrine and sodium bicarbonate. Epinephrine and sodium bicarbonate can increase the pH, thereby prolonging the duration of action of local anesthetics. Epinephrine acting via vasoconstriction prolongs the duration of action and decreases systemic absorption.98
| Agent | Duration of Action | Maximum Dose (per Procedure) |
|---|---|---|
| Esters | ||
| Procaine (Novocain) | Short (15-60 minutes) | 7 mg/kg; not to exceed 350-600 mg |
| Chloroprocaine (Nesacaine) | Short (15-60 minutes) | |
Anesthetic dosage is determined by the target tissue and the desired level of local anesthesia (Table 24-4). Increasing the dose shortens the onset of action and increases the potency and duration, but also increases the possibility of an adverse reaction.98
Anesthetics can have adverse effects on the central nervous system, cardiovascular system, and immune system. Cardiovascular effects include direct myocardial depression and bradycardia, which can lead to cardiovascular collapse. Patients who have renal or hepatic compromise, are older or pregnant, or who have preexisting cardiac disease can be at an increased risk of toxicity. The biggest cause of toxicity is direct intravascular injection, making it necessary to routinely aspirate before injection. Lower concentrations of local anesthetics are typically used for joint and infiltrative soft tissue injections (see Table 24-4). Given the small doses for joint injections and these lower concentrations, systemic toxicity should be exceedingly rare.
The esters are derivatives of para-aminobenzoic acid and have been associated with significantly higher rates of allergic reactions than the amide anesthetics. For this reason, most physicians prefer using amide anesthetics.98 Recent animal and in vitro studies have demonstrated a dose- and time-dependent toxicity on cartilage that has not been demonstrated in vivo.44,90,167 There is also a concern that local anesthetics might be directly neurotoxic.95
General Considerations
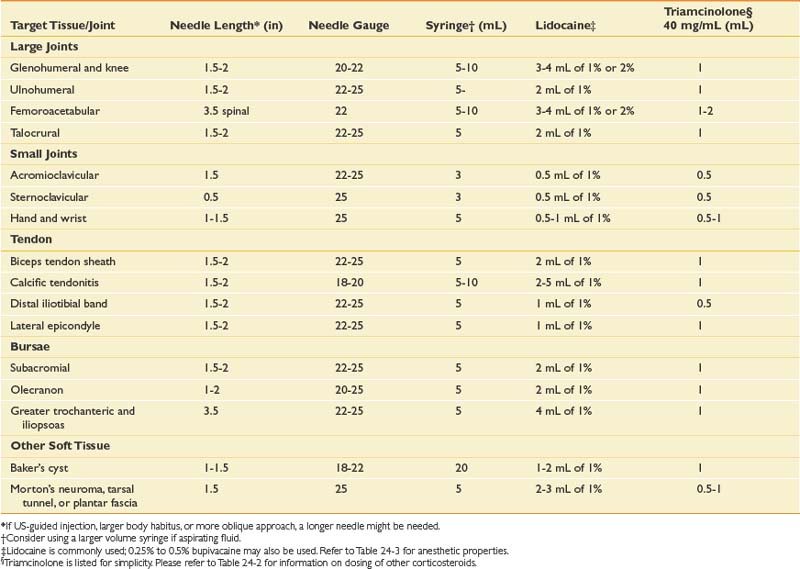
Before any injection, several factors must be appropriately addressed, including the equipment needed and obtaining informed consent. Specific needle type, length, and gauge will vary depending on the target tissue, type, and volume of fluid to be injected or aspirated (see Table 24-4). Informed consent should include all potential risks and benefits of the procedure, including possible alternative treatments. The risk profile will vary depending on the medication given, and should be adjusted accordingly. Aside from the previously mentioned adverse effects of the medications, there are also risks associated with insertion of a needle through the skin, including infection, bleeding, hematoma formation, and neurovascular injury.
Joint infections are rare, with an incidence from 1 in 3000 to 1 in 50,000.28 Symptoms typically occur 3 to 4 days postinjection, whereas postinjection flares usually are reported within the first 24 hours.163 Infection can be minimized through the use of sterile technique.
Bleeding can be minimized through proper technique, the use of epinephrine in the local anesthetic, and assessing for any bleeding diathesis. Patients receiving warfarin only have a mild increased bleeding risk if the international normalized ratio is at an appropriate therapeutic level. For this reason some practitioners state that it is not typically necessary to discontinue warfarin before a peripheral joint injection.189
Non–Image-Guided Injections
Most practitioners do not use any image guidance for the majority of peripheral joint injections. The overall accuracy of a non–image-guided “blind injection” varies between joints, approach, and experience. Overall the literature demonstrates a 10% to 40% “miss rate” of blind injections when compared with US-guided or fluoroscopic-guided injections. Although the use of either fluoroscopic or US image guidance has been shown to enhance the accuracy of injections, it has not been conclusively proven that enhanced accuracy is related to an enhanced efficacy.68
Ultrasound-Guided Injections
With improved technology and subsequently better resolution, musculoskeletal US has been increasingly used to help guide peripheral joint and soft tissue injections.4,7,87,177,202
Unlike other imaging modalities, US has the unique advantage of being able to visualize soft tissues, bony landmarks, and the needle with real-time scanning, thereby allowing dynamic visualization.4,7,87,177
Unlike fluoroscopy, which uses radiation and primarily visualizes bone and joints, diagnostic US is unique because it uses acoustic waves, which are safe in all patient populations. US offers superb soft tissue resolution, surpassing that of magnetic resonance imaging for certain conditions.130 With this superb resolution, nearby neurovascular structures can be localized and avoided during any injection. Doppler imaging not only helps identify normal vasculature, but also aids in identifying the neovascularization that has been associated with pathologic conditions such as tendinopathy.46 Studies have suggested that US-guided destruction of these neovessels results in clinical and morphologic improvements.76,103,212
The biggest disadvantage of using musculoskeletal US is that it is largely operator dependent, requiring proper technique to adequately visualize and inject underlying structures.84,118,195 Most injections using US are performed with the “free hand technique,” where one hand holds the probe while the other hand positions the needle.7,87,180 Because of this, performing US-guided injections requires manual dexterity and has a documented learning curve.141 It is highly recommended that before attempting US-guided injections, individuals gain experience with diagnostic US imaging and have an understanding of its capabilities and limitations. Injection experience can be obtained by practicing on phantom models or participating in instructional courses; however, these are not equivalent to hands-on supervised training.
A strong knowledge of musculoskeletal anatomy and the ability to discern pertinent structures with diagnostic US are essential. An important step that helps in identifying these structures is selecting the most appropriate transducer or probe.84,177 For example, high-frequency (5 to 12 MHz) linear array transducers are helpful for imaging more superficial structures, whereas low-frequency (3 to 5 MHz) curvilinear transducers might be needed for injections of deeper structures such as the hip joint.102,175 The “hockey” stick probe with its smaller footprint, however, might better enable visualization of superficial structures near the wrist and ankle (Figure 24-1).177
It is recommended that a preliminary scan be carried out before performing any US-guided injection. This preliminary scan serves several purposes. The first purpose is to identify any neurovascular structures that need to be avoided during the procedure. The preliminary scan also allows adjustment of the depth, focus, gain, and time-gain compensation so that optimal visualization of the intended target can be obtained.84,177,196,197 The decision can then be made regarding the best approach, with marking of the planned entry site.84
For each US-guided injection, various approaches can be used, each having advantages and disadvantages.7,118,180 The chosen approach is often dependent on the practitioner’s comfort level, training, and experience. Because there are numerous approaches to injecting musculoskeletal structures, the techniques described below are merely suggestions, and crucial concepts will be highlighted.
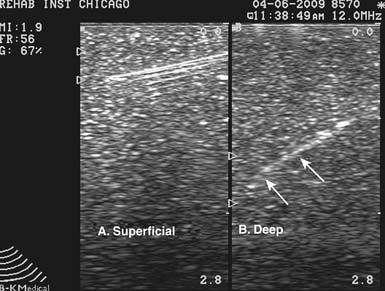
US can image an injection needle in longitudinal or transverse planes.7,177 When the longitudinal view is used, the transducer lies parallel to the needle. This is often referred to as the needle being “in plane” with the transducer.177 With the short axis or transverse view of the needle, the transducer is oriented perpendicular to the needle, and the needle is considered to be “out of plane” (Figure 24-2).177 Because a longitudinal view of the needle should yield consistent visualization of the needle shaft and tip, this is the preferred approach and is especially useful when first learning US-guided procedures.177 The out-of-plane technique makes identifying the needle tip difficult because the shaft will appear the same anywhere it is viewed along its length. To identify the needle tip, the acoustic outline of the bevel must be visualized, which can be challenging.4,87,177,181 It cannot be stressed enough that when performing an US-guided injection, it is crucial that the needle tip be constantly visualized.87 Without constant visualization of the needle tip, injury to nearby structures and inaccurate placement of medication can occur.
Proper visualization of the needle tip can sometimes be improved by slightly spinning or by jiggling the needle gently to see perturbations of the nearby tissue, with or without the use of Doppler imaging. Hydrodissection can also lead to better identification of the needle tip. With this technique a small amount of sterile saline or local anesthetic can be injected during the US-guided procedure.7,177 The relatively hypoechoic fluid provides a darker background compared with the relatively hyperechoic needle, making the needle more conspicuous.
Certain factors affect the brightness or echogenicity of the needle, including needle depth and obliquity, with needle gauge being less important. When the US beam does not strike the needle at a 90-degree angle, some of the beam will be reflected away from the transducer, and the needle will appear less bright or hypoechoic.7,84,118,177,196 Consequently, it is ideal to keep the transducer and US beam perpendicular to the needle to make it as bright or hyperechoic as possible.7,84,177 Deeper tissues often require the needle to be directed at an oblique angle, resulting in the US beam striking the needle at an angle other than 90 degrees. Regardless of its gauge, the needle will appear less echogenic with a more oblique approach, and visualizing the needle while injecting deeper tissues can be more difficult (Figure 24-3). These injections might be better suited for the more experienced ultrasonographer.7
Injecting very superficial targets such as tendon sheaths, cystic structures, and some joints can also be technically demanding. When an intended target lies close to the skin surface, there is often not enough subcutaneous tissue to place an injection needle under the transducer. This can be circumvented by using a standoff approach. With this technique, sterile US gel is heaped up on the skin under the probe overlying the superficial target. Then the injection needle is placed directly into the gel, providing initial visualization under the probe and eventually guided down into the intended target (Figure 24-4).4 Essentially the standoff gel serves as a vehicle that helps to place the needle under the probe near the superficial structure without having to traverse the needle through tissue. Angling the transducer as needed serves to improve the echogenicity of the needle even before its entering into the skin.
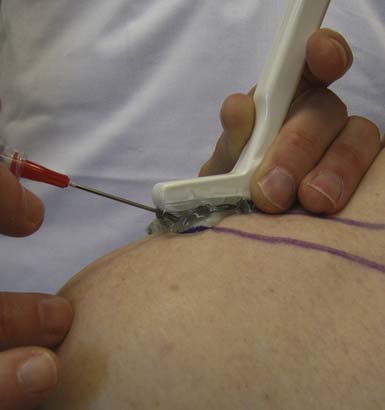
FIGURE 24-4 Standoff demonstration: acromioclavicular joint injection with ultrasound guidance using hockey stick probe.
Because needle echogenicity is highly dependent on technique, commercially prepared echogenic needles might not be worth the added expense. It is much more important that the needle be visualized perpendicular to the US beam. With this in mind, a 27-gauge needle can be as easily recognized on US as some larger gauge needles.87,177
Although there are no absolute recommendations as to whether to perform these injections with sterile technique, every attempt at minimizing the risk of infection should be undertaken.4 Commercially prepared packets that contain both sterile US gel and sterile transducer covers are available. Although this might limit the risk of infection, patients can perceive such an elaborate setup as somewhat overwhelming. Other patients will be reassured knowing that this injection will be placed accurately, because the needle and pertinent tissue will be visualized during the entire procedure. Although some US-guided injections can be performed with the patient in a seated position, having the patient lie down can help ease any anxiety and also mitigate a potential vasovagal reaction. Proper technique includes gripping the transducer while maintaining good contact with the patient’s skin. This reduces hand fatigue and, more importantly, provides tactile feedback to the clinician.177 Although no absolute standard exists, there are guidelines that recommend appropriate documentation of the study.131 This can include archiving preinjection and postinjection images, as well as images that verify needle position at the time of the actual injection.
Technical Guide
Upper Limb Techniques
Glenohumeral Joint
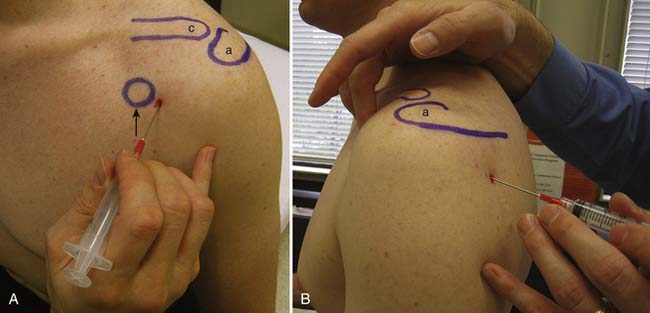
There are multiple ways to perform blind glenohumeral joint injections.51,69,148,168,169 Among these techniques, the accuracy of an anterior approach ranges from 27% to 99%, and a posterior approach ranges from 50% to 91%. When a blind posterior approach is used, the patient is seated with the ipsilateral hand and forearm resting on the lap. A 1½- or 2-inch needle then is placed into the skin 1 to 1½ inches below the posterolateral aspect of the acromion (Figure 24-5). The needle is then advanced anteriorly and slightly medially toward the coracoid process until it is felt to rest within the joint. For the anterior approach, the needle is inserted just lateral to the coracoid process and advanced posteriorly and slightly medially (see Figure 24-5). The typical volumes used for glenohumeral joint injections are found in Table 24-4. In cases of adhesive capsulitis, the overall volume of the glenohumeral joint is reduced, but injecting as much volume as tolerated will help in breaking up the adhesions limiting the range of motion and contributing to this condition.193
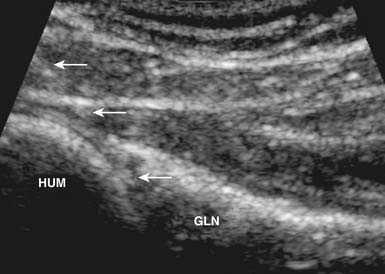
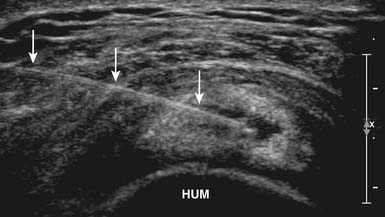
With the use of US guidance, the glenohumeral joint can be injected with the patient prone and the affected arm hanging off the table, or alternatively with the patient side-lying with the affected side up. A posterior short-axis approach provides the best access into the joint.4,102 The needle enters the skin in plane with the transducer from a lateral approach and enters the joint posterior to the humeral head (Figure 24-6). Because of the relative depth of this joint, a spinal needle often is needed to perform this injection.4,55
Subacromial Bursa
Several studies have evaluated the accuracy of blind subacromial injections using various techniques, including posterior, lateral, and anterior approaches.∗ Accuracy rates for these blind approaches are 70% to 91%, 29% to 90%, and 69% to 83%, respectively.51 Although any of these approaches can place the medication into the subacromial bursa, some studies demonstrated that frequently the medication is actually injected into the rotator cuff.139 The ramifications of placing corticosteroid into the rotator cuff are unclear at this time.
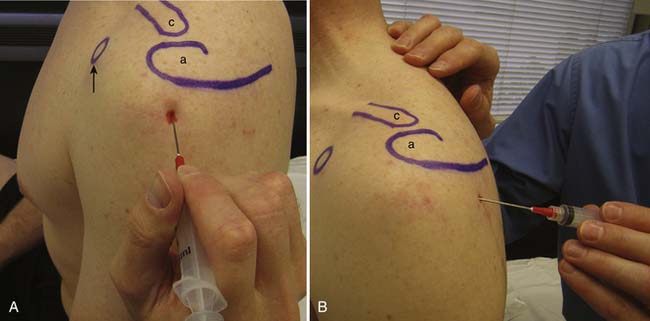
To perform the lateral subacromial injection, the needle is inserted at the midpoint and just inferior to the lateral aspect of the acromion (Figure 24-7). Once through the skin, the needle is advanced medially under the acromion. If significant resistance is felt while injecting the medication, then the needle might be near bone or in tendon and should be repositioned. For the posterior approach, a 1½- to 2-inch needle is inserted approximately 2 cm inferior to the posterolateral aspect of the acromion and advanced anteriorly and slightly medially under the acromion where the medication is then injected (see Figure 24-7).
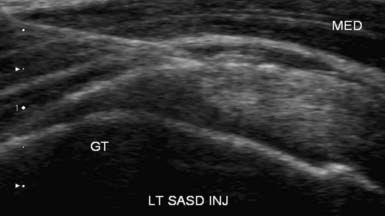
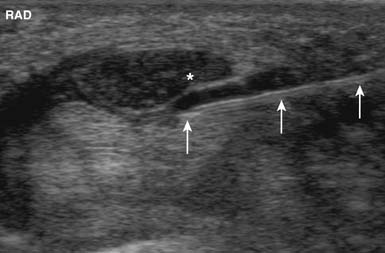
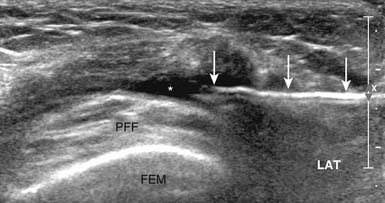
The US-guided subacromial bursa injection can be performed with the patient upright or side-lying with the affected side up. If tolerated, the arm should be placed into extension and internal rotation to optimize visualization of the supraspinatus and overlying subacromial bursa. The transducer is placed on the shoulder so that a longitudinal view of the subacromial bursa and supraspinatus tendon is obtained. The bursa is identified as a hypoechoic structure deep to the more hyperechoic bony acromion and peribursal fat but superficial to the supraspinatus tendon.7,118 A 22- to 25-gauge needle is then placed under the skin parallel to the transducer via the anterolateral approach and directed into the subacromial bursa. A helpful tip while performing this injection is to infiltrate anesthetic locally while visualizing needle entry into the bursa. While fluid is injected, the bursa will fill, causing delamination of the bursa away from the overlying hyperechoic peribursal fat, making identification of the needle easier and confirming proper placement (Figure 24-8).4
Acromioclavicular Joint
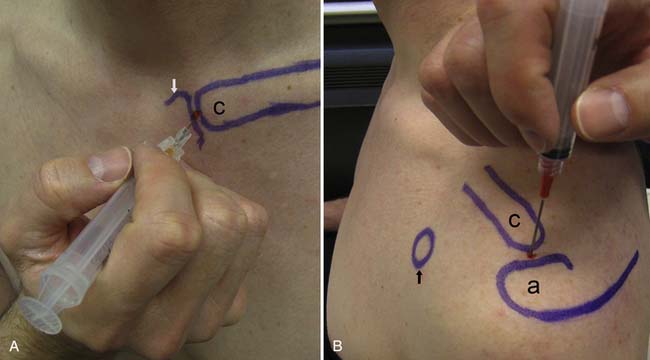
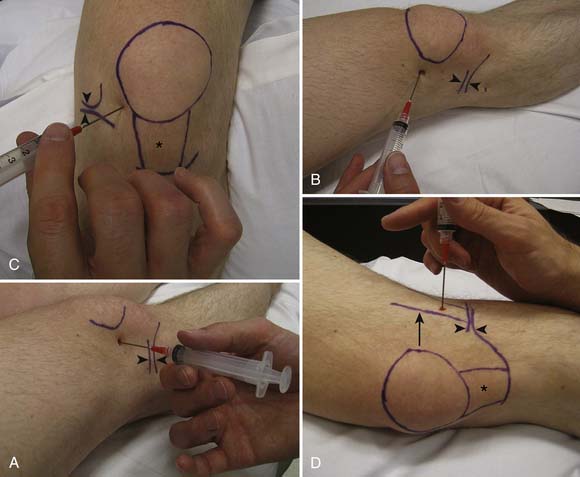
The accuracy of blind injections into the AC joint is 60%.to 67%.17,139 Being a superficial structure, the AC joint space is accessible from the superior aspect of the joint. To determine the point of entry, the distal aspect of the clavicle is palpated. Here there is usually a slight depression between the distal clavicle and the adjacent acromion, defining the borders of the AC joint. The needle is advanced into this depression inferiorly until the needle is in the joint (Figure 24-9). Fluoroscopic guidance is not routinely used for this joint, but use of image guidance should be considered if the injection is being done for diagnostic purposes.
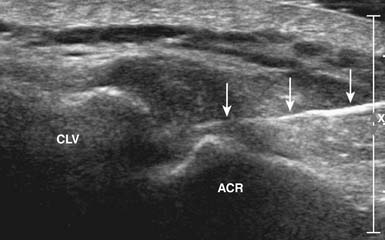
The AC joint can be injected with US guidance, with the patient again in the seated or side-lying position. The joint is most easily imaged with the transducer lying across the joint where the hyperechoic bony acoustic landmarks of the acromion and clavicle meet with the interposition of the hypoechoic joint and overlying hyperechoic capsule. The joint is injected using a standoff approach as described above. A 22- to 25-gauge needle enters into the gel in parallel with the transducer, then punctures the skin and AC joint capsule. Once into the joint, usually only a small amount of fluid is injected (see Table 24-4; Figure 24-10). This technique can also be used for any small articulation.
An alternative US approach to the AC joint can be performed by first obtaining a long axis view of the joint.4 The skin over the bony articulation on either side of the joint is labeled with a marking pen. The transducer is rotated 90 degrees, and a needle is then placed between the marks into the joint in plane with the probe. The US is then rotated back 90 degrees to the long-axis view of the joint, confirming placement of the needle within the joint.4
Sternoclavicular Joint
The sternoclavicular joint can be approached anteriorly with the patient supine or sitting. To avoid injuring the lung, a ½- to ⅝-inch needle is advanced posteriorly into the joint (see Figure 24-9). Fluoroscopic guidance is not used routinely for the injection. A similar approach described above for the AC joint can be used for US-guided sternoclavicular joint injection.
Biceps Tendon
With the patient seated or supine, the superior aspect of the intertubercular groove is palpated between the greater and lesser tuberosities of the humerus. A 25-gauge needle is then inserted and advanced posteriorly until contact is made with the periosteum. The needle is then withdrawn slightly and the medication injected (see Table 24-4; Figure 24-11). Fluoroscopic guidance is not used routinely for this injection.
The biceps tendon sheath can be injected with US guidance using a transverse view, with the patient supine and the forearm supinated.4 A 22- to 25-gauge needle is then placed in plane with the transducer from a lateral approach into the subcutaneous tissues and guided down into the tendon sheath where the medication is injected. After medication has been injected, a halo of hypoechoic fluid may be noticeable around the tendon, thereby confirming placement of the medication in the tendon sheath.4
Calcific Tendonitis
Calcific tendonitis of the shoulder is caused by hydroxyapatite crystal deposition within the rotator cuff tendon and is quite readily identified with US imaging.4,9,87,118 This deposition is identified as a hyperechoic mass within the tendon accompanied by acoustic shadowing. Occasionally these deposits can result in a significant mass effect, resulting in painful subacromial impingement. Multiple studies have demonstrated excellent short and long-term outcomes when treating this painful disabling condition with aspiration or fragmentation.9,38,54,101 This procedure can be performed with the patient in a position similar to that used for the subacromial injection. Adequate local anesthesia should be achieved before and while performing the treatment. Although smaller gauge needles have been used9 and might be better tolerated, typically an 18- to 20-gauge needle is placed into the center of the calcification.53,54,87 A 5- to 10-mL syringe filled with 1% lidocaine is then used to repeatedly lavage the area under direct visualization (Figure 24-12).53 The aspirated crystals appear as white and chalky when they return back into the syringe, and have a tendency to deposit into the bottom of the syringe. It is therefore advisable to keep the syringe parallel to the floor so that the crystals are not reinjected into the tendon.9 An alternate technique that avoids the accumulation of the crystals in the syringe is to use a two-needle technique as described by Joines et al.87 Lavage continues until no more chalky material is obtained. If the calcifications are well formed and hard, they can be repeatedly probed with the needle tip to fragment them.87 After either the lavage or fragmentation, it is highly recommended that a subacromial corticosteroid be placed to help reduce a postprocedure pain flare.87
Ulnohumeral Joint
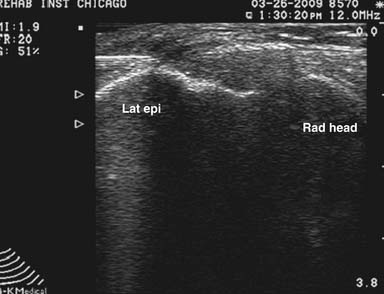
Multiple approaches have been used to inject this joint blindly.52 The posterolateral approach is relatively simple to perform and because of the absence of pertinent neurovascular structures, it is also the safest.55 The elbow is flexed into a comfortable position. Next the bony landmarks of the olecranon, radial head, and lateral epicondyle are palpated, marked, and connected. The soft spot in the center of this triangle demarcates the entry point for a relatively small gauge needle. The needle is advanced until it enters the joint space (Figure 24-13). Fluoroscopic guidance is not used routinely for this injection, but could be used to confirm intraarticular placement.
With US guidance, the posterior elbow joint can be injected easily with the arm supported and the elbow placed into 90 degrees of flexion.118 A longitudinal view of the humerus and posterior elbow joint is then obtained. A needle is then placed in plane with the transducer, entering proximally and proceeding distally into the posterior ulnohumeral articulation.118 Effusions, if present, can be aspirated from this location.
Wrist Extensor or Flexor Origin Pain
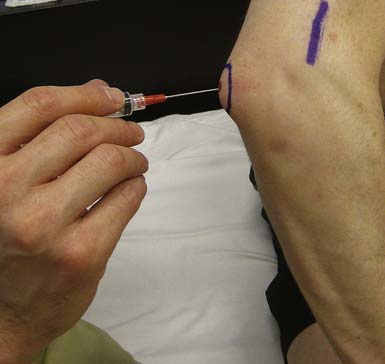
Although long-term relief has been inconsistent, injections of corticosteroid for medial or lateral epicondylar pain can help with short-term pain relief, especially when symptoms are refractory to other conservative measures.172 The lateral epicondyle is injected at the area of maximal tenderness near the origin of the common wrist extensors. Typically the needle contacts the periosteum of the lateral epicondyle, then is withdrawn slightly before injecting medication (Figure 24-14). A similar technique is used for medial epicondylar pain.52 Direct injection of the tendon is generally not recommended.
If US guidance is deemed necessary, the common wrist extensor mass can be approached with an in-plane technique. The first step is to obtain a longitudinal view of the radiocapitellar joint.118 Just proximal to this is the bony acoustic landmark of the lateral epicondyle. The common extensor tendon origin lies just superficial to this and can be injected from either a proximal or distal approach (Figure 24-15). The origin of the common flexor mass can be injected in a similar manner, except that the medial epicondyle is used as the bony landmark.
Olecranon Bursa
The normal olecranon bursa is usually not palpable or visible with US. Pathologic conditions caused by trauma, rheumatologic conditions, and infection, however, can result in distension of this bursa. Because patients who receive this injection will likely have swelling of the bursa, the swelling over the olecranon dictates placement of the needle. After sterilizing the skin, a 22- to 25-gauge needle is typically sufficient for an injection. In cases in which complex fluid is to be aspirated, however, a larger gauge needle might be needed (Figure 24-16).52 Depending on the condition, corticosteroid with or without local anesthetic can then be injected.
Carpal Tunnel Injections
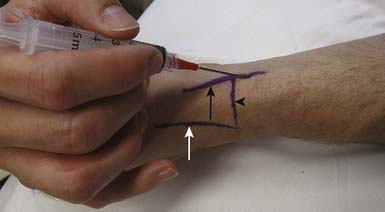
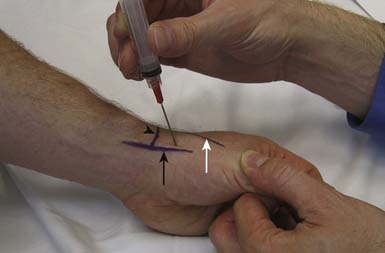
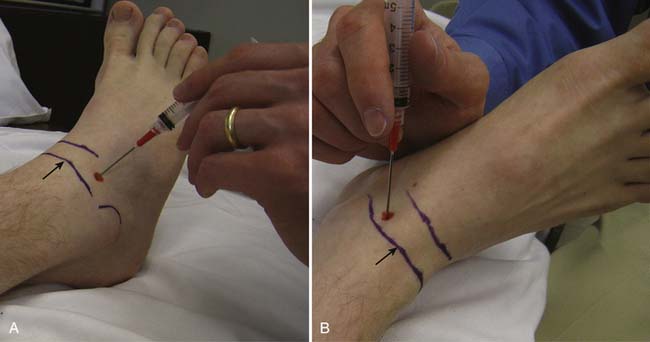
The most significant complication of a carpal tunnel injection is needle injury to the median nerve. Using a blind approach, Dubert and Racasan47 attempted to determine the best way to enter the carpal tunnel without contacting the median nerve. This was done by evaluating the anatomy of 124 consecutive patients undergoing carpal tunnel release surgery. Given the proximity of the median nerve to the palmaris longus, a 25-gauge needle placed through the flexor carpi radialis (FCR) via a lateral or medial approach was determined to be the best approach to the carpal tunnel. In a cadaveric study, Ozturk et al.136 examined three blind approaches to determine the safest and most accurate approach into the carpal tunnel. They found that injecting through the FCR tendon resulted in no needle injuries to the median nerve and produced a 96% accuracy rate. With this approach, the needle is inserted 1 cm proximal to the wrist crease at a 45-degree angle from the skin and directed distally in an ulnar direction through the FCR tendon (Figure 24-17). When there is no more resistance, the needle is thought to be in the carpal tunnel. Alternatively, the needle can be inserted 1 cm proximal to the wrist crease medial to the palmaris longus and angled 30 degrees from the skin toward the carpal tunnel52 (see Figure 24-17). Unfortunately, any of these techniques can potentially injure the median nerve. Therefore if paresthesias are experienced by the patient, the needle should be withdrawn slightly.
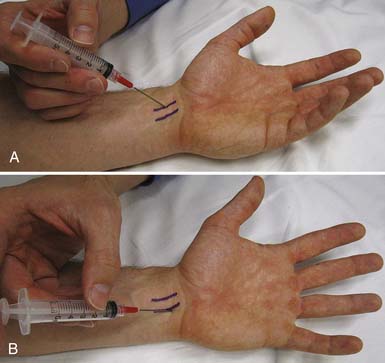
FIGURE 24-17 Carpal tunnel injection. A, Through the flexor carpi radialis tendon. B, Medial to the palmaris longus tendon.
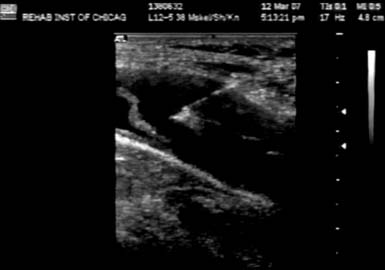
Smith et al.176 have described an US-guided technique whereby the carpal tunnel is injected with an ulnar-sided approach while using a short-axis view of the distal wrist crease. With this approach, a 27-gauge needle passes superficial to the ulnar nerve and artery in plane with the transducer and places the medication both superficially and deep to the median nerve (Figure 24-18). In addition to the therapeutic effects of the corticosteroid, using this approach can mechanically separate the nerve from the restrictive connective tissue.176 Grassi et al.64 have shown improvement with US-guided corticosteroid injections for carpal tunnel syndrome in a subset of rheumatoid patients with flexor tenosynovitis.
Abductor Pollicis Longus and Extensor Pollicis Brevis Tenosynovitis (de Quervain’s Tenosynovitis)
To inject the sheath around the abductor pollicis longus and extensor pollicis brevis tendons, the needle is inserted 1 cm proximal to the radial styloid process at a 30- to 45-degree angle from the skin and parallel to the direction of the tendons (Figure 24-19). In order not to penetrate the tendon, the needle is advanced only slightly in either a proximal or distal direction. If paresthesias are experienced, the needle should be repositioned to avoid injuring the superficial radial nerve.52
Because there is a known miss rate with blind injections,214 the first dorsal wrist compartment can be injected with US guidance. Although the thumb extensor sheath can be injected with either a longitudinal or short-axis view of the tendon, the transverse view is preferred (having the needle entering the sheath while in plane with the transducer).Using a standoff approach enables the needle to enter directly into the sheath without having to traverse a large amount of subcutaneous tissue. Ideally when fluid is injected, it will be seen distending the sheath, confirming proper placement of the medication.118 US has been shown to be helpful in identifying intertendinous septa, which if present, can theoretically limit the spread of medication.40 Although no studies exist examining the efficacy of US-guided injections for de Quervain’s syndrome, Zingas et al.214 have shown that accurate US-guided placement of medication in this condition results in greater clinical improvement than does blind injection.
Carpometacarpal Joint
Osteoarthritis of the first carpometacarpal joint is a common source of wrist pain. If symptoms are refractory to usual treatments, a corticosteroid injection might help with pain relief. The needle enters the skin just proximal to the first metacarpal on the extensor surface, between the first metacarpal and trapezium bones. Care must be taken to avoid the radial artery and the extensor pollicis tendons. To avoid the radial artery, the needle should enter toward the ulnar side of the extensor pollicis brevis tendon. If the needle doesn’t fall into the joint, traction can be applied, which can further open the joint space (Figure 24-20) This approach was described by Mandl et al.110 and Tallia and Cardone.186
Because approximately 10% to 40% of blind carpometacarpal joint injections are misplaced,73,110,144,147 US guidance can ensure more accurate placement of medication. Injecting small joints about the wrist and hand with US can be performed using a similar approach as described above for the AC joint.7 The only difference is that the bony landmarks of the targeted joint as well as any important neurovascular structures must be properly identified before the actual injection.
Stenosing Flexor Tenosynovitis (Trigger Finger)
Symptomatic trigger fingers are typically caused by abnormal thickening of the A1 pulley near the metacarpophalangeal joint and are associated with stenosing tenosynovitis of the finger flexors.61,66,166 Although some patients might benefit from just a subcutaneous placement of corticosteroid for this condition, some authors have suggested that accurate placement of the corticosteroid could improve outcomes.61,187 The A1 pulley on US appears thickened with decreased echogenicity and hypervascularity.66 One US-guided technique begins with having the patient lie supine with the palm up. A longitudinal view of the finger flexor tendon and a subsequent transverse view of the A1 pulley are then obtained.61 With the use of an in-plane technique with sterile standoff gel, the A1 pulley is injected under direct visualization. Although surgical release of the pulley88 has been described using a similar approach, no controlled studies exist examining the efficacy of US-guided corticosteroid injections of the trigger finger compared with the efficacy of blind injections.
Lower Limb Techniques
Femoroacetabular Joint
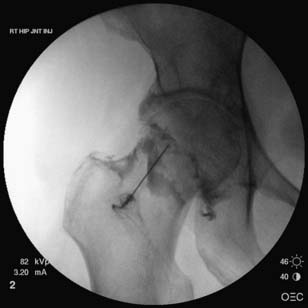
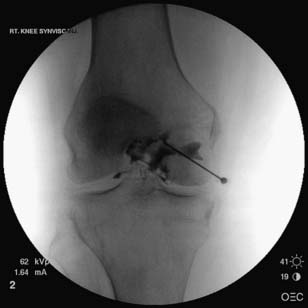
When the femoroacetabular joint is injected without image guidance, the miss rate is relatively high,41,42 and the femoral nerve can be injured about 30% of the time.100 Consequently it is highly suggested that image guidance be used. With a fluoroscopic approach, the patient first lies in a supine position with the hip in a neutral position. An anteroposterior view of the hip is used to visualize the head of the femur and adjacent acetabulum. Before placing a marker over the skin to determine the entrance point for the needle, it is prudent to verify the location of the femoral pulse so that it is avoided. Once the skin is prepared and draped sterilely, a marker is placed over the superior and lateral aspect of the femoral head, away from the femoroacetabular articulation. A 22-gauge, 3½-inch needle can be advanced posteriorly until it is felt that the needle has hit bone. Another approach is to advance the needle toward the head-neck junction of the femur, which minimizes the chance of injuring the articular cartilage. A common pattern of contrast flow is seen in (Figure 24-21). Typical volumes of injectate are similar to that used for the knee joint (see Table 24-4).
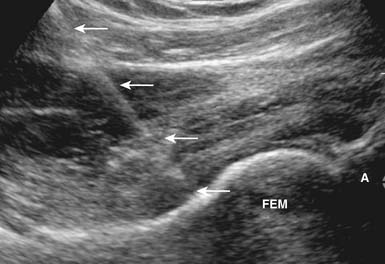
Aspiration and injection of the hip joint can also be performed with US guidance.149,177 Sonography of the hip joint can help identify joint effusions, bursitis, and tendinopathy, as well as the nearby femoral vessels and nerve.111,199 To perform a sonographically guided hip injection, the patient is placed supine with the hip in neutral alignment. Because the joint is deep, a low-frequency curvilinear transducer might be needed. It is placed in parallel with the femoral neck, yielding a longitudinal view of the femoral head–neck junction.174 After local anesthesia is placed, a 22-gauge spinal needle is then directed under sonographic guidance in parallel with the transducer into the anterior synovial recess of the hip joint (Figure 24-22). Because of the oblique direction of this injection, the needle is not as echogenic as with a more superficial injection. Injection of a small amount of anesthetic into the joint confirms placement when free intraarticular flow is identified.174 It takes about 10 injections to typically become proficient at performing US-guided hip injections.13
Greater Trochanteric Bursa
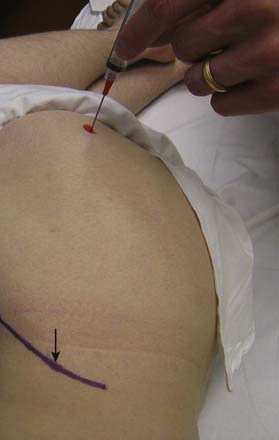
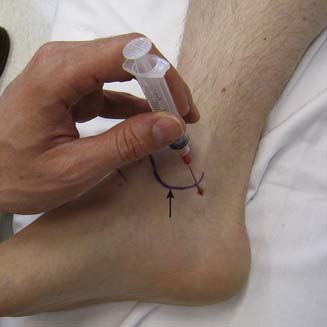
There are more than 20 different bursae about the hip, many of which can cause clinical symptoms.119 The greater trochanteric bursa is composed of three separate bursae, with the subgluteus maximus being the largest and located posterior to the greater trochanter.199 Although no randomized trial exists examining efficacy, some studies suggest some short-term improvement of symptoms in patients who receive a local corticosteroid injection.21 Cohen et al.33 studied the accuracy of blind trochanteric bursa injections. A bursagram seen by fluoroscopy confirmed accurate placement 53% of the time when the injection was performed by experienced physicians. The greater trochanteric bursa can be injected blindly with the patient side-lying with the affected side up. Next the area of maximal tenderness over the greater trochanter is palpated and the needle directed toward this area until periosteum is contacted. The needle is then pulled back slightly and the medication injected (Figure 24-23). Depending on the body habitus, a 3½-inch needle might be needed. Fluoroscopic guidance is not routinely used for this type of injection.
Because these are potential spaces, they are only clearly identified on US when abnormal.198 To inject the subgluteus maximus bursa, the patient lies in a lateral decubitus position with the affected hip up. Depending on the patient’s size, either a linear or curvilinear transducer is placed longitudinally over the greater trochanter. The transducer is then moved posterior to the greater trochanter and rotated 90 degrees, localizing the subgluteus maximus bursa. Then a 22-gauge spinal needle is directed in plane from a posterior to anterior approach until the bursa is entered. If the gluteus medius or minimus bursa is to be injected, a similar approach is performed except that these bursae are located more anteriorly.118 The needle enters from an anterior approach and is directed posteriorly towards the greater trochanter. No studies examining the effectiveness of US-guided bursa injections are available.
Iliopsoas Bursa
The iliopsoas bursa is the largest bursa in the body, located between the hip capsule and the iliacus and psoas muscles.57,199 This bursa is not normally visualized unless it is pathologically enlarged. Iliopsoas bursitis can produce pain, neuropathy, and a palpable mass, and has been implicated in snapping hip syndrome.19,198 Adler and Finzel6 have described an US-guided bursa technique that can provide relief for patients with or without previous hip replacement who have anterior hip and groin pain caused by iliopsoas bursitis. The patient is placed supine, and a linear or curvilinear transducer is used, depending on the patient’s size. The tendon is visualized in short axis at the iliopectineal eminence of the acetabulum. If fluid is present, a 20- or 22-gauge needle is then positioned in plane with the transducer by using a lateral approach into the bursa. If the bursa is not visible, then the needle is positioned between the tendon and hip capsule. If the needle is placed accurately and fluid is injected, bursal distension should occur.6 Patients who get relief after iliopsoas bursa injection can benefit from surgical release of the tendon, especially if they have had previous hip arthroplasty.6,19,203
Knee Joint
At least seven different blind injection approaches into the tibiofemoral joint have been described in the literature. These include the modified Waddell, lateral midpatellar, anterolateral, anteromedial, medial midpatellar, superolateral, and superomedial approaches.∗ In these studies the success rates varied from 43% to 100%, depending on the approach and means of confirming accurate placement within the joint. Although no study compared different needle lengths, it appears that using a 2-inch needle provides more accurate placement than when using a 1½-inch needle. Because they are more accurate than the other approaches mentioned above, only the superolateral, lateral midpatellar, and anteromedial approaches will be described. The anteromedial approach is the least accurate of these three, with studies showing accuracy rates from 55% to 75%.50,83,191
For the lateral midpatellar approach, the patient lies supine with the knee extended (Figure 24-24). Before sterilizing the skin, a mark is made over the lateral midportion of the patella. A needle is then placed just posterior to the patella, entering into the joint just anterior to the lateral femoral condyle. For the superolateral approach, the knee remains in the extended position; however, the needle enters the skin 1 cm lateral to the patella at the junction of the superior one third and inferior two thirds of the patella and posterolateral to the attachment of the quadriceps tendon (see Figure 24-24). The needle is then advanced at an angle of 45 degrees and directed toward the center of the joint. For the anteromedial approach, the patient is lying supine with the knee bent anywhere from 60 to 90 degrees (see Figure 24-24). Although the entrance point of the needle is inferior and medial to the patella, it is directed posteriorly or posterolaterally toward the intercondylar notch. Once the needle is deep enough or hits periosteum, the medication is injected. Because the injectate should flow easily, if any resistance is encountered during injection of the medication, the needle should be withdrawn slightly and then redirected. Care should be taken with this approach because superficial injections can place the medication into Hoffa’s fat pad, potentially resulting in atrophy if corticosteroid is used. Fluoroscopy, if needed, helps guide needle placement and confirms proper intraarticular needle placement, and can be used with any of these three approaches (Figure 24-25). Typical medications and volumes used for intraarticular knee injections are shown in Table 24-4.
Because the suprapatellar recess communicates with the knee joint proper in 85% of adult knees, it is an ideal location to detect joint effusions with US.85,118 Cadaveric studies have demonstrated that effusions of 7 mL or more can be detected on US.39 Although this recess can be accessed with either a longitudinal or short-axis view, the needle can be obscured by the overlying patella when a longitudinal view is used.55,118 Therefore a short-axis view of the suprapatellar bursa with an in-plane view of the needle is preferred when injecting or aspirating fluid from this space. With this approach, the knee is supported in slight flexion, and the needle enters the suprapatellar recess laterally in plane with the transducer (Figure 24-26).55 The length and gauge of the needle used will depend on the body habitus of the patient and the nature of fluid being aspirated or injected.
Baker’s Cyst
When distended, the semimembranosus–medial gastrocnemius bursa is referred to as a Baker’s cyst. It communicates with the knee joint in 50% of adults older than 50 years.85 Symptoms related to this cyst include posterior knee pain, swelling, and stiffness or tightness often aggravated by activity.70 Because of a valve-like mechanism, fluid can become trapped in the bursa with repetitive knee flexion and extension.70 Potential injury to nearby popliteal neurovascular structures makes a blind injection into this area risky. The use of US allows confirmation of a Baker’s cyst, which appears as a well-defined hypoechoic structure located between the medial head of the gastrocnemius and semimembranosus tendon, and which communicates with the posterior knee joint adjacent to the medial femoral condyle.7,177,198,204 The use of sonographic guidance to aspirate or inject these cysts helps not only to locate the nearby popliteal neurovascular structures, but also to identify septae within the cyst that can limit the effectiveness of the injection.36 The procedure is performed with the patient lying prone with the knee in slight flexion. Then using a longitudinal view of the cyst, an 18-gauge needle is placed into the cyst proximally, entering in plane with the transducer (Figure 24-27). Local anesthetic infiltration during the injection can help not only with local pain control, but also with identification of septae or borders of the cyst. The length of the needle required depends on the size of the patient. Although intraarticular corticosteroid injections are known to effectively reduce the size of Baker’s cysts,1 no study has been done showing the effectiveness of direct US-guided injections for this disorder.
Distal Iliotibial Band or Lateral Femoral Epicondyle
For distal iliotibial band (ITB) syndrome, in which pain occurs from friction between the ITB and lateral femoral epicondyle, a blind or US-guided injection can be done with the patient supine and the knee extended. An alternative approach is with the patient side-lying with the affected side up. After the lateral femoral epicondyle is located superior to the lateral tibiofemoral joint line, the needle is directed towards this bony prominence. Once the needle contacts the periosteum, the needle is withdrawn slightly to allow for injection of medication (see Figure 24-24).
Talocrural Joint
Either an anteromedial or anterolateral approach can be used to inject the talocrural joint.52 The patient is typically supine with the ankle either in neutral or slight plantar flexion. With the anteromedial approach, the needle enters the skin just lateral to the medial malleolus, but medial to the tendon of the tibialis anterior, along an imaginary line connecting the medial and lateral malleoli. The needle is advanced laterally, superiorly, and posteriorly (Figure 24-28). The needle should be advanced until it is felt to be in the joint and a decrease in resistance is appreciated. With the anterolateral approach, the needle is inserted into the skin anterior and medial to the lateral malleolus, but lateral to the extensor digitorum longus (along the line between the medial and lateral malleoli) (see Figure 24-28). The needle is directed medially, superiorly, and posteriorly. Fluoroscopic guidance can be used with either of these approaches. Obtaining relief from a fluoroscopically placed ankle injection can help determine the source of ankle pain, which in turn can help guide surgical interventions.93
Sonographic guidance can also assist with the accurate placement of injections about the foot and ankle.156,180 The anterior ankle joint can be injected using US guidance with the patient again in the supine position with the foot in slight plantar flexion.55,117 A longitudinal view of the tibiotalar joint is then obtained. The needle enters distally in plane with the transducer into the anterior synovial recess while avoiding the overlying ankle dorsiflexors.55,156 Because some ankle effusions can be complex, the gauge of the needle used will depend on what is being injected or aspirated.
Tarsal Tunnel
Although using US is recommended, the tarsal tunnel can be injected with a blind approach.52 The patient is either lying on the side of the limb to be injected or is lying supine with the limb externally rotated to expose the medial ankle. Because the posterior tibial nerve lies posterior to the tendon of the posterior tibialis, the needle should be angled 30 degrees from the skin and directed posterior to the medial malleolus and the tendon of the posterior tibialis (Figure 24-29). If paresthesias are provoked, the needle should be withdrawn and redirected.
The posteromedial malleolar structures can be injected with US guidance using a transverse view of the posterior medial malleolar structures. The needle is placed posteriorly in plane with the transducer and directed toward the visualized tibial nerve if tarsal tunnel syndrome is suspected, or into the sheaths of any of the posterior malleolar tendons if treating tenosynovitis.178 The use of a standoff approach with sterile gel as described above can help with needle placement.
Intermetatarsal Head (for Morton’s Neuroma)
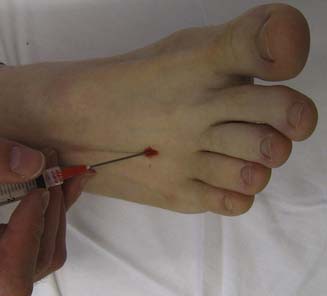
Morton’s neuroma is due to fibrosis of the interdigital nerve causing symptoms of pain in the forefoot pain and paresthesias in the toes. Typically the neuroma is located between the third and fourth metatarsals and can be treated with various nonsurgical methods, including corticosteroid injections.65,71,112,153 A symptomatic Morton’s neuroma can be injected with the patient lying supine. With a dorsal approach, the needle is placed 1 cm proximal to the metatarsal web space and advanced between the heads of the adjacent metatarsals, where the medication is injected around the common digital nerve (Figure 24-30). Care should be taken not to advance the needle too far, because it might then enter the plantar fat pad, potentially resulting in fat pad atrophy if corticosteroid is injected.52
Some studies have demonstrated that US-guided injections for Morton’s neuroma can result in better clinical outcomes and delay the need for surgery.71,80,112,179 With the patient supine, a longitudinal dorsal view is obtained of the intermetatarsal space. A 25-gauge needle then is directed volarly, entering the neuroma in plane with the transducer from either a distal or proximal approach.80
Plantar Fascia
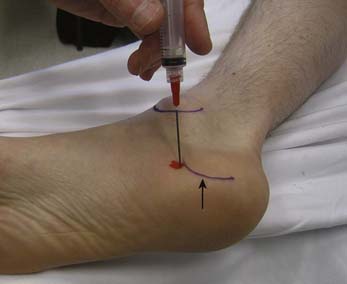
The plantar fascia is a common source of heel pain, which typically is worse with the first few steps taken in the morning. If treatment with conservative measures fails, a corticosteroid injection might help provide clinical improvement. There is, however, a documented association of increased fascia rupture after this procedure.2,165,210 To perform an injection for plantar fasciitis, the patient can either be in a side-lying position or supine with the limb externally rotated to expose the medial heel. After obtaining adequate anesthesia either by local infiltration or by performing a tibial nerve block, a 25-gauge needle is inserted medially and directed laterally on the plantar surface just superior and anterior to the calcaneous. The needle is advanced to the periosteum of the anterior aspect of the calcaneous and withdrawn slightly (Figure 24-31). Care should be taken to avoid injecting corticosteroid into the calcaneal or plantar fat pad.52
US findings of plantar fasciitis include thickening of the plantar fascia with concomitant fat pad edema.59 The plantar fascia tissue can be injected with either a transverse or longitudinal view.180 Care should be taken not to inject directly into the plantar fascia.180 With a transverse view of the plantar fascia, the needle enters medially on the plantar surface in plane with the transducer, just distal to the calcaneus. There is evidence that US-guided plantar fascia injection can help with a reduction in plantar fascia thickness and a reduction in pain.210
Novel Treatments for Tendinopathy
Chronic tendinopathy on US appears as a thickened structure with decreased echogenicity often associated with neovascularization.5,46,77,114,192 Patients who are refractory to traditional forms of treatment such as medication, physical therapy, and corticosteroid injections might benefit from nontraditional forms of interventional musculoskeletal US procedures.152 McShane et al.121,122 have shown improvement in patients with chronic elbow extensor tendinosis with US-guided percutaneous tenotomy. Others have used either autologous blood injections or platelet-rich plasma injections placed under US guidance for the same diagnosis, noting an improvement not only in pain scores but also morphology.35,126 Destruction of neovascularization with sclerosing medications placed under US guidance resulted in a significant improvement in knee function in patients with refractory patellar tendinopathy76 and also reduced pain in patients with wrist extensor, patellar, and Achilles tendinopathy.76,103,211
1. Acebes J.C., Sánhez-Pernaute O., Diaz-Oca, et al. Ultrasonographic assessment of Baker’s cysts after intra-articular corticosteroid injection in knee osteoarthritis. J Clin Ultrasound. 2006;34(3):113-117.
2. Acevedo J.I., Beskin J.L. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19(2):91-97.
3. Adleberg J.S., Smith G.H. Corticosteroid-induced avascular necrosis of the talus. J Foot Surg. 1991;30(1):66-69.
4. Adler R.S., Allen A. Percutaneous ultrasound guided injections in the shoulder. tech shoulder elbow surg. 2004;5(2):122-133.
5. Adler R.S., Buly R., Ambrose R., et al. Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am J Roentgenol. 2005;185(4):940-943.
6. Adler R.S., Finzel K.C. The complementary roles of MR imaging and ultrasound of tendons. Radiol Clin North Am. ix, 2005;43(4):771-804.
7. Adler R.S., Sofka C.M. Percutaneous ultrasound-guided injections in the musculoskeletal system. Ultrasound Q. 2003;19(1):3-12.
8. Agarwal V., et al. A prospective study of the long-term efficacy of local methyl prednisolone acetate injection in the management of mild carpal tunnel syndrome. Rheumatology (Oxford). 2005;44(5):647-650.
9. Aina R., Cardinal E., Bureau N.J., et al. Calcific shoulder tendinitis: treatment with modified US-guided fine-needle technique. Radiology. 2001;221(2):455-461.
10. Anonymous. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46(2):328-346.
11. Arroll B., Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328(7444):869-873.
12. Arroll B., Goodyear-Smith F. Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract. 2005;55(512):224-228.
13. Atchia I., Birrell F., Kane D. A modular, flexible training strategy to achieve competence in diagnostic and interventional musculoskeletal ultrasound in patients with hip osteoarthritis. Rheumatology (Oxford). 2007;46(10):1583-1586.
14. Bellamy N., Campbell J., Welch V., et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. (2):2006. CD005328
15. Bellamy N., Campbell J., Welch V., et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. (2):2006. CD005321
16. Bickel K.D. Flexor pollicis longus tendon rupture after corticosteroid injection. J Hand Surg [Am]. 1996;21(1):152-153.
17. Bisbinas I., Belthur M., Said M., et al. Accuracy of needle placement in ACJ injections. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):762-765.
18. Blaine T., Moskowitz R., Udell J., et al. Treatment of persistent shoulder pain with sodium hyaluronate: a randomized, controlled trial: a multicenter study. J Bone Joint Surg Am. 2008;90(5):970-979.
19. Blankenbaker D.G., De Smet A.A., Keene J.S. Sonography of the iliopsoas tendon and injection of the iliopsoas bursa for diagnosis and management of the painful snapping hip. Skeletal Radiol. 2006;35(8):565-571.
20. Bliddal H. Placement of intra-articular injections verified by mini air-arthrography. Ann Rheum Dis. 1999;58(10):641-643.
21. Brinks A., van Rijn R.M., Bohnen A.M., et al. Effect of corticosteroid injection for trochanter pain syndrome: design of a randomised clinical trial in general practice. BMC Musculoskelet Disord. 2007;8:95.
22. Buchbinder R., Green S., Youd J.M. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. (1):2003. CD004016
23. Buttaci C.J., Stitik T.P., Yonclas P.P., et al. Osteoarthritis of the acromioclavicular joint: a review of anatomy, biomechanics, diagnosis, and treatment. Am J Phys Med Rehabil. 2004;83(10):791-797.
24. Caglar-Yagci H., Unsal S., Yagci I., et al. Safety and efficacy of ultrasound-guided intra-articular hylan G-F 20 injection in osteoarthritis of the hip: a pilot study. Rheumatol Int. 2005;25(5):341-344.
25. Cardone D.A., Tallia A.F. Joint and soft tissue injection. Am Fam Physician. 2002;66(2):283-288.
26. Carette S., Moffet H., Tardif J., et al. Intraarticular corticosteroids, supervised physical therapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: a placebo-controlled trial. Arthritis Rheum. 2003;48(3):829-838.
27. Chakravarty K., Pharoah P.D., Scott D.G. A randomized controlled study of post-injection rest following intra-articular steroid therapy for knee synovitis. Br J Rheumatol. 1994;33(5):464-468.
28. Charalambous C.P., Tryfonidis M., Sadiq S., et al. Septic arthritis following intra-articular steroid injection of the knee: a survey of current practice regarding antiseptic technique used during intra-articular steroid injection of the knee. Clin Rheumatol. 2003;22(6):386-390.
29. Chávez-Lopez M.A., Navarro-Soltero L.A., Rosas-Cabralet A., et al. Methylprednisolone versus triamcinolone in painful shoulder using ultrasound-guided injection. Mod Rheumatol. 2008;19:147-150.
30. Chechick A., Amit Y., Israeli A., et al. Recurrent rupture of the Achilles tendon induced by corticosteroid injection. Br J Sports Med. 1982;16(2):89-90.
31. Chen M.J., Lew H.L., Hsu T.C., et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006;85(1):31-35.
32. Cohen M.D. Hyaluronic acid treatment (viscosupplementation) for OA of the knee. Bull Rheum Dis. 1998;47(7):4-7.
33. Cohen S.P., Narvaez J.C., Lebovits A.H., et al. Corticosteroid injections for trochanteric bursitis: is fluoroscopy necessary? A pilot study. Br J Anaesth. 2005;94(1):100-106.
34. Cole B.J., Schumacher H.R.Jr. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13(1):37-46.
35. Connell D.A., Ali K.E., Ahmad M., et al. Ultrasound-guided autologous blood injection for tennis elbow. Skeletal Radiol. 2006;35(6):371-377.
36. Court-Payen M. Sonography of the knee: intra-articular pathology. J Clin Ultrasound. 2004;32(9):481-490.
37. Creamer P. Intra-articular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol. 1999;11(5):417-421.
38. del Cura J.L., Torre I., Zabala R., et al. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am J Roentgenol. 2007;189(3):W128-W134.
39. Delaunoy I., Feipel V., Appelboom T., et al. Sonography detection threshold for knee effusion. Clin Rheumatol. 2003;22(6):391-392.
40. Diop A.N., Ba-Diop S., Sane J.C., et al. [Role of US in the management of de Quervain’s tenosynovitis: review of 22 cases] [French]. J Radiol. 2008;89(9 pt 1):1081-1084.
41. Dobson M.M. A check on the anatomical accuracy of intra-articular hip injections in relation to the therapy of coxarthritis. Ann Rheum Dis. 1948;7(3):172-174.
42. Dobson M.M. A further anatomical check on the accuracy of intra-articular hip injections in relation to the therapy of coxarthritis. Ann Rheum Dis. 1950;9(3):237-240.
43. Dodd L.E. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 2007;89(3):422. author reply 422
44. Dragoo J.L., Korotkova T., Kanwar R., et al. The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med. 2008;36(8):1484-1488.
45. Dreyfuss P., Dryer S., Griffin J., et al. Positive sacroiliac screening tests in asymptomatic adults. Spine. 1994;19(10):1138-1143.
46. du Toit C., Stieler M., Saunders R., et al. Diagnostic accuracy of power Doppler ultrasound in patients with chronic tennis elbow. Br J Sports Med. 2008;42(11):572-576.
47. Dubert T., Racasan O. A reliable technique for avoiding the median nerve during carpal tunnel injections. Joint Bone Spine. 2006;73(1):77-79.
48. Ekeberg O.M., Bautz-Holter E., Tveitå E.K., et al. Subacromial ultrasound guided or systemic steroid injection for rotator cuff disease: randomised double blind study. BMJ. 2009;338:a3112.
49. Emkey R.D., Lindsay R., Lyssy J., et al. The systemic effect of intraarticular administration of corticosteroid on markers of bone formation and bone resorption in patients with rheumatoid arthritis. Arthritis Rheum. 1996;39(2):277-282.
50. Esenyel C., Demirhan M., Esenyel M., et al. Comparison of four different intra-articular injection sites in the knee: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):573-577.
51. Eustace J.A., Brophy D., Gibney R., et al. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
52. Falco F.J.E., Irwin F.L., Kim D.W., et al. Peripheral joint, soft tissue, and spinal injection techniques. In Braddom R., editor: Physical medicine & rehabilitation, ed 3, Philadelphia: Saunders, 2006.
53. Farin P.U., Jaroma H., Soimakallio S. Rotator cuff calcifications: treatment with US-guided technique. Radiology. 1995;195(3):841-843.
54. Farin P.U., Räsänen H., Jaroma H., et al. Rotator cuff calcifications: treatment with ultrasound-guided percutaneous needle aspiration and lavage. Skeletal Radiol. 1996;25(6):551-554.
55. Fessell D.P., Jacobson J.A., Craig J., et al. Using sonography to reveal and aspirate joint effusions. AJR Am J Roentgenol. 2000;174(5):1353-1362.
56. Fisher D.E., Bickel W.H. Corticosteroid-induced avascular necrosis: a clinical study of seventy-seven patients. J Bone Joint Surg Am. 1971;53(5):859-873.
57. Flanagan F.L., Sant S., Coughlan R.J., et al. Symptomatic enlarged iliopsoas bursae in the presence of a normal plain hip radiograph. Br J Rheumatol. 1995;34(4):365-369.
58. Flanagan J., Casale F.F., Thomas T.L., et al. Intra-articular injection for pain relief in patients awaiting hip replacement. Ann R Coll Surg Engl. 1988;70(3):156-157.
59. Gibbon W.W., Long G. Ultrasound of the plantar aponeurosis (fascia). Skeletal Radiol. 1999;28(1):21-26.
60. Gibson T., Burry H.C., Poswillo D., et al. Effect of intra-articular corticosteroid injections on primate cartilage. Ann Rheum Dis. 1977;36(1):74-79.
61. Godey S.K., Bhatti W.A., Watson J.S., et al. A technique for accurate and safe injection of steroid in trigger digits using ultrasound guidance. Acta Orthop Belg. 2006;72(5):633-634.
62. Godwin M., Dawes M. Intra-articular steroid injections for painful knees: systematic review with meta-analysis. Can Fam Physician. 2004;50:241-248.
63. Gordon G.V., Schumacher H.R. Electron microscopic study of depot corticosteroid crystals with clinical studies after intra-articular injection. J Rheumatol. 1979;6(1):7-14.
64. Grassi W., Farina A., Filippucci E., et al. Intralesional therapy in carpal tunnel syndrome: a sonographic-guided approach. Clin Exp Rheumatol. 2002;20(1):73-76.
65. Greenfield J., Rea J.Jr., Ilfeld F.W. Morton’s interdigital neuroma: indications for treatment by local injections versus surgery. Clin Orthop Relat Res. 1984;(185):142-144.
66. Guerini H., Pessis E., Theumann N., et al. Sonographic appearance of trigger fingers. J Ultrasound Med. 2008;27(10):1407-1413.
67. Gunal I., Karatosun V. Avascular necrosis of the femoral heads after single corticosteroid injection. CMAJ. 2006;175(1):31.
68. Hall S., Buchbinder R. Do imaging methods that guide needle placement improve outcome? Ann Rheum Dis. 2004;63(9):1007-1008.
69. Hanchard N., Shanahan D., Howe T., et al. Accuracy and dispersal of subacromial and glenohumeral injections in cadavers. J Rheumatol. 2006;33(6):1143-1146.
70. Handy J.R. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31(2):108-118.
71. Hassouna H., Singh D., Taylor H., et al. Ultrasound guided steroid injection in the treatment of interdigital neuralgia. Acta Orthop Belg. 2007;73(2):224-229.
72. Heimann W.G., Freiberger R.H. Avascular necrosis of the femoral and humeral heads after high-dosage corticosteroid therapy. N Engl J Med. 1960;263:672-675.
73. Helm A.T., Higgins G., Rajkumar P., et al. Accuracy of intra-articular injections for osteoarthritis of the trapeziometacarpal joint. Int J Clin Pract. 2003;57(4):265-266.
74. Henkus H.E., Cobben L.P., Coerkamp E.G., et al. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
75. Hochberg M.C., Perlmutter D.L., Hudson J.I., et al. Preferences in the management of osteoarthritis of the hip and knee: results of a survey of community-based rheumatologists in the United States. Arthritis Care Res. 1996;9(3):170-176.
76. Hoksrud A., Ohberg L., Alfredson H., et al. Ultrasound-guided sclerosis of neovessels in painful chronic patellar tendinopathy: a randomized controlled trial. Am J Sports Med. 2006;34(11):1738-1746.
77. Hoksrud A., Ohberg L., Alfredson H., et al. Color Doppler ultrasound findings in patellar tendinopathy (jumper’s knee). Am J Sports Med. 2008;36(9):1813-1820.
78. Hollander J.L. Intrasynovial corticosteroid therapy in arthritis. Md State Med J. 1970;19(3):62-66.
79. Hollander J.L., Brown E.M., Jessar R.A., et al. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc. 1951;147(17):1629-1635.
80. Hughes R.J., Ali K., Jones H., et al. Treatment of Morton’s neuroma with alcohol injection under sonographic guidance: follow-up of 101 cases. AJR Am J Roentgenol. 2007;188(6):1535-1539.
81. Hunter J.A., Blyth T.H. A risk-benefit assessment of intra-articular corticosteroids in rheumatic disorders. Drug Saf. 1999;21(5):353-365.
82. Huppertz H.I., Tschammler A., Horwitz A.E., et al. Intraarticular corticosteroids for chronic arthritis in children: efficacy and effects on cartilage and growth. J Pediatr. 1995;127(2):317-321.
83. Jackson D.W., Evans N.A., Thomas B.M. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84(9):1522-1527.
84. Jacobson J.A. Introduction. In: Jacobson J.A., editor. Fundamentals of musculoskeletal ultrasound. Philadelphia: Saunders, 2007.
85. Jacobson J.A. Knee ultrasound. In: Jacobson J.A., editor. Fundamentals of musculoskeletal ultrasound. Philadelphia: Saunders, 2007.
86. Johansson A., Hao J., Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34(5):335-338.
87. Joines M.M., Motamedi K., Seeger L.L., et al. Musculoskeletal interventional ultrasound. Semin Musculoskelet Radiol. 2007;11(2):192-198.
88. Jou I.M., Chern T.C. Sonographically assisted percutaneous release of the A1 pulley: a new surgical technique for treating trigger digit. J Hand Surg [Br]. 2006;31(2):191-199.
89. Kang M.N., Rizio L., Prybicien M., et al. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(suppl 1):61S-66S.
90. Karpie J.C., Chu C.R. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35(10):1621-1627.
91. Karpinski M.R., Piggott H. Greater trochanteric pain syndrome: a report of 15 cases. J Bone Joint Surg Br. 1985;67(5):762-763.
92. Kerolus G., Clayburne G., Schumacher H.R.Jr. Is it mandatory to examine synovial fluids promptly after arthrocentesis? Arthritis Rheum. 1989;32(3):271-278.
93. Khoury N.J., el-Khoury G.Y., Saltzman C.L., et al. Intraarticular foot and ankle injections to identify source of pain before arthrodesis. AJR Am J Roentgenol. 1996;167(3):669-673.
94. Kirkley A., Litchfield R.B., Jackowski D.M., et al. The use of the impingement test as a predictor of outcome following subacromial decompression for rotator cuff tendinosis. Arthroscopy. 2002;18(1):8-15.
95. Kitagawa N., Oda M., Totoki T. Possible mechanism of irreversible nerve injury caused by local anesthetics: detergent properties of local anesthetics and membrane disruption. Anesthesiology. 2004;100(4):962-967.
96. Kullenberg B., Runesson R., Tuvhag R., et al. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
97. Kumar N., Newman R.J. Complications of intra- and peri-articular steroid injections. Br J Gen Pract. 1999;49(443):465-466.
98. Latifzai K., Sites B.D., Koval K.J. Orthopaedic anesthesia: part 1. Commonly used anesthetic agents in orthopaedics. Bull NYU Hosp Jt Dis. 2008;66(4):297-305.
99. Lazarevic M.B., Skosey J.L., Djordjevic-Denic G., et al. Reduction of cortisol levels after single intra-articular and intramuscular steroid injection. Am J Med. 1995;99(4):370-373.
100. Leopold S.S., Battista V., Oliverio J.A. Safety and efficacy of intraarticular hip injection using anatomic landmarks. Clin Orthop Relat Res. 2001;391:192-197.
101. Lin J.T., Adler R.S., Bracilovic A., et al. Clinical outcomes of ultrasound-guided aspiration and lavage in calcific tendinosis of the shoulder. HSS J. 2007;3(1):99-105.
102. Lin J., Jacobson D.P., Fessell W.J., et al. An illustrated tutorial of musculoskeletal sonography. part 2. upper extremity. AJR Am J Roentgenol. 2000;175(4):1071-1079.
103. Lind B., Ohberg L., Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1327-1332.
104. Little N.J., Chipperfield A., Ricketts D.M. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 2007;89(3):423. author reply 423
105. Lo G.H., LaValley M., McAlindon T., et al. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290(23):3115-3121.
106. Lopes R.V., Furtado R.N.V., Parmigiani L., et al. Accuracy of intra-articular injections in peripheral joints performed blindly in patients with rheumatoid arthritis. Rheumatology (Oxford). 2008;47(12):1792-1794.
107. Luc M., Pham T., Chagnaud C., et al. Placement of intra-articular injection verified by the backflow technique. Osteoarthritis Cartilage. 2006;14(7):714-716.
108. Ly-Pen D., Andréu J.L., de Blas G., et al. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled clinical trial. Arthritis Rheum. 2005;52(2):612-619.
109. Macmahon P.J., Brennan D.D., Duke D., et al. Ultrasound-guided percutaneous drainage of meniscal cysts: preliminary clinical experience. Clin Radiol. 2007;62(7):683-687.
110. Mandl L.A., Hotchkiss R.N., Adler R.S., et al. Can the carpometacarpal joint be injected accurately in the office setting? Implications for therapy. J Rheumatol. 2006;33(6):1137-1139.
111. Marchal G.J., Van Holsbeeck M.T., Raes M., et al. Transient synovitis of the hip in children: role of US. Radiology. 1987;162(3):825-828.
112. Markovic M., Crichton K., Read J.W., et al. Effectiveness of ultrasound-guided corticosteroid injection in the treatment of Morton’s neuroma. Foot Ankle Int. 2008;29(5):483-487.
113. Marshall S., Tardif G., Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. (2):2007. CD001554
114. Martinoli C., Bianchi S., Derchi L.E. Tendon and nerve sonography. Radiol Clin North Am. 1999;37(4):691-711.
115. Mathews P.V., Glousman R.E. Accuracy of subacromial injection: anterolateral versus posterior approach. J Shoulder Elbow Surg. 2005;14(2):145-148.
116. McCarty D.J.Jr., Faires J.S. A comparison of the duration of local anti-inflammatory effect of several adrenocorticosteroid esters: a bioassay technique. Curr Ther Res Clin Exp. 1963;5:284-290.
117. McNally E.G. Lower limb: anatomy and technique. In: McNally E.G., editor. Practical musculoskeletal ultrasound. Philadelphia: Saunders, 2005.
118. McNally E.G. Musculoskeletal interventional ultrasound. In: McNally E.G., editor. Practical musculoskeletal ultrasound. Philadelphia: Saunders, 2005.
119. McNally E.G. Ultrasound of the hip. In: McNally E.G., editor. Practical musculoskeletal ultrasound. Philadelphia: Saunders, 2005.
120. McQuillan R., Gregan P. Tendon rupture as a complication of corticosteroid therapy. Palliat Med. 2005;19(4):352-353.
121. McShane J.M., Nazarian L.N., Harwood M.I. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25(10):1281-1289.
122. McShane J.M., Shah V.N., Nazarian L.N. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow: is a corticosteroid necessary? J Ultrasound Med. 2008;27(8):1137-1144.
123. Meenagh G.K., Patton J., Kynes C., et al. A randomised controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63(10):1260-1263.
124. Migliore A., Tormenta S., Martin Martin L.S., et al. The symptomatic effects of intra-articular administration of hylan G-F 20 on osteoarthritis of the hip: clinical data of 6 months follow-up. Clin Rheumatol. 2006;25(3):389-393.
125. Migliore A., Tormenta S., Valente C., et al. [Intra-articular treatment with Hylan G-F 20 under ultrasound guidance in hip osteoarthritis: clinical results after 12 months follow-up] [Italian]. Reumatismo. 2005;57(1):36-43.
126. Mishra A., Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
127. Mont M.A., Myers T.H., Krackow K.A., et al. Total knee arthroplasty for corticosteroid associated avascular necrosis of the knee. Clin Orthop Relat Res. 1997;338:124-130.
128. Nacimiento A.C., Bartels M., Loew F. Acute effects of dexamethasone on normal and on posttraumatic spinal cord polysynaptic reflex activity and axonal conduction. Surg Neurol. 1986;26(1):13-16.
129. Naredo E., Cabero F., Beneyto P., et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
130. Nazarian L.N. The top 10 reasons musculoskeletal sonography is an important complementary or alternative technique to MRI. AJR Am J Roentgenol. 2008;190(6):1621-1626.
131. Nazarian LN. AIUM practice guideline for the performance of the musculoskeletal ultrasound examination: Available at: http://www.aium.org/publications/guidelines/musculoskeletal.pdfSeries. Accessed May 19, 2009.
132. Nesbitt L.T.Jr. Minimizing complications from systemic glucocorticosteroid use. Dermatol Clin. 1995;13(4):925-939.
133. Newnham D.M., Douglas J.G., Legge J.S., et al. Achilles tendon rupture: an underrated complication of corticosteroid treatment. Thorax. 1991;46(11):853-854.
134. Nicholas J.L. Joint and soft tissue injection techniques. In Braddom R., editor: Physical medicine & rehabilitation, ed 2, Philadelphia: Saunders, 2000.
135. O’Sullivan M.M., Rumfeld W.R., Jones M.K., et al. Cushing’s syndrome with suppression of the hypothalamic-pituitary-adrenal axis after intra-articular steroid injections. Ann Rheum Dis. 1985;44(8):561-563.
136. Ozturk K., Esenyel C.Z., Sonmez M., et al. Comparison of carpal tunnel injection techniques: a cadaver study. Scand J Plast Reconstr Surg Hand Surg. 2008;42(6):300-304.
137. Papacrhistou G., Anagnostou S., Katsorhis T. The effect of intraarticular hydrocortisone injection on the articular cartilage of rabbits. Acta Orthop Scand. 1997;275(suppl):132-134.
138. Papavasiliou A.V., Isaac D.L., Marimuthu R., et al. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 2006;88(3):321-323.
139. Partington P.F., Broome G.H. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
140. Pattrick M., Doherty M. Facial flushing after intra-articular injection of steroid. Br Med J (Clin Res Ed). 1987;295(6610):1380.
141. Pekkafahli M.Z., Kiralp M.Z., Basekim C.C., et al. Sacroiliac joint injections performed with sonographic guidance. J Ultrasound Med. 2003;22(6):553-559.
142. Peters-Veluthamaningal C., van der Windt D.A.W.M., Winters J.C., et al. Corticosteroid injection for trigger finger in adults. Cochrane Database Syst Rev. (1):2009. CD005617
143. Pfenninger J.L. Injections of joints and soft tissue. I. General guidelines. Am Fam Physician. 1991;44(4):1196-1202.
144. Pichler W., Grechenig W., Grechenig S., et al. Frequency of successful intra-articular puncture of finger joints: influence of puncture position and physician experience. Rheumatology (Oxford). 2008;47(10):1503-1505.
145. Pilgaard S. [Achilles tendon rupture during corticosteroid therapy.] [Danish]. Ugeskr Laeger. 1962;124:1408-1409.
146. Plant M.J., Borg A.A., Dziedzic K., et al. Radiographic patterns and response to corticosteroid hip injection. Ann Rheum Dis. 1997;56(8):476-480.
147. Pollard M.A., Cermak M.B., Buck W.R., et al. Accuracy of injection into the basal joint of the thumb. Am J Orthop. 2007;36(4):204-206.
148. Porat S., Leupold J.A., Burnett K.R., et al. Reliability of non-imaging-guided glenohumeral joint injection through rotator interval approach in patients undergoing diagnostic MR arthrography. AJR Am J Roentgenol. 2008;191(3):W96-99.
149. Pourbagher M.A., Ozalay M., Pourbagher A. Accuracy and outcome of sonographically guided intra-articular sodium hyaluronate injections in patients with osteoarthritis of the hip. J Ultrasound Med. 2005;24(10):1391-1395.
150. Qin C., Greenwood-Van Meerveld B., Myers D.A., et al. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol. 2003;89(3):1343-1352.
151. Qvistgaard E., Christensen R., Torp-Pedersen S., et al. Intra-articular treatment of hip osteoarthritis: a randomized trial of hyaluronic acid, corticosteroid, and isotonic saline. Osteoarthritis Cartilage. 2006;14(2):163-170.
152. Rabago D., Best T.M., Zgierska A.E., et al. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet rich plasma. Br J Sports Med. 2009;43(7):471-481.
153. Rasmussen M.R., Kitaoka H.B., Patzer G.L. Nonoperative treatment of plantar interdigital neuroma with a single corticosteroid injection. Clin Orthop Relat Res. 1996;326:188-193.
154. Raynauld J.P., Buckland-Wright C., Ward R., et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377.
155. Raynauld J.P., Goldsmith C.H., Bellamy N., et al. Effectiveness and safety of repeat courses of hylan G-F 20 in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2005;13(2):111-119.
156. Reach J.S., Easley M.E., Chuckpaiwong B., et al. Accuracy of ultrasound guided injections in the foot and ankle. Foot Ankle Int. 2009;30(3):239-242.
157. Reid D.M., Patel S., Reid I.W., et al. Hypothalamic-pituitary-adrenal (HPA) axis function in patients receiving long-term intra-articular corticosteroids. Clin Rheumatol. 1983;2(2):159-161.
158. Richie I, C.A., Briner W.W. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16(2):102-106.
159. Roberts W.N., Babcock E.A., Breitbach S.A., et al. Corticosteroid injection in rheumatoid arthritis does not increase rate of total joint arthroplasty. J Rheumatol. 1996;23(6):1001-1004.
160. Robinson P., Keenan A.M., Conaghan P.G. Clinical effectiveness and dose response of image-guided intra-articular corticosteroid injection for hip osteoarthritis. Rheumatology (Oxford). 2007;46(2):285-291.
161. Rutten M.J., Maresch B.J., Jager G.J., et al. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
162. Saygi B., Yildirim Y., Saygi E.K., et al. Morton neuroma: comparative results of two conservative methods. Foot Ankle Int. 2005;26(7):556-559.
163. Schumacher H.R., Chen L.X. Injectable corticosteroids in treatment of arthritis of the knee. Am J Med. 2005;118(11):1208-1214.
164. Schumacher H.R.Jr. Aspiration and injection therapies for joints. Arthritis Rheum. 2003;49(3):413-420.
165. Sellman J.R. Plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1994;15(7):376-381.
166. Serafini G., Derchi L.E., Quadri P., et al. High resolution sonography of the flexor tendons in trigger fingers. J Ultrasound Med. 1996;15(3):213-219.
167. Seshadri V., Coyle C.H., Chu C.R. Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy. 2009;25(4):337-347.
168. Sethi P.M., El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
169. Sethi P.M., Kingston S., Elattrache N. Accuracy of anterior intra-articular injection of the glenohumeral joint. Arthroscopy. 2005;21(1):77-80.
170. Silverstein E., Leger R., Shea K.P. The use of intra-articular hylan G-F 20 in the treatment of symptomatic osteoarthritis of the shoulder: a preliminary study. Am J Sports Med. 2007;35(6):979-985.
171. Skedros J.G., Hunt K.J., Pitts T.C. Variations in corticosteroid/anesthetic injections for painful shoulder conditions: comparisons among orthopaedic surgeons, rheumatologists, and physical medicine and primary-care physicians. BMC Musculoskelet Disord. 2007;8:63.
172. Smidt N., Assendelft W.J., van der Windt D.A., et al. Corticosteroid injections for lateral epicondylitis: a systematic review. Pain. 2002;96(1-2):23-40.
173. Smith A.G., Kosygan K., Williams H., et al. Common extensor tendon rupture following corticosteroid injection for lateral tendinosis of the elbow. Br J Sports Med. 1999;33(6):423-424. discussion 424–425
174. Smith J., Hurdle M.F. Office-based ultrasound-guided intra-articular hip injection: technique for physiatric practice. Arch Phys Med Rehabil. 2006;87(2):296-298.
175. Smith J., Hurdle M.F., Weingarten T.N. Accuracy of sonographically guided intra-articular injections in the native adult hip. J Ultrasound Med. 2009;28(3):329-335.
176. Smith J., Steve J., Wisniewski M.D., Finnoff J.T., et al. Sonographically guided carpal tunnel injections: the ulnar approach. J Ultrasound Med. 2008;27(10):1485-1490.
177. Smith J.F., Finnoff J.T. Diagnostic and interventional musculoskeletal ultrasound. 1. Fundamentals. Phys Med Rehabil. 2009;1(1):64-75.
178. Sofka C.M., Adler R.S. Ultrasound-guided interventions in the foot and ankle. Semin Musculoskelet Radiol. 2002;6(2):163-168.
179. Sofka C.M., Adler R.S., Ciavarra G.A., et al. Ultrasound-guided interdigital neuroma injections: short-term clinical outcomes after a single percutaneous injection: preliminary results. HSS J. 2007;3(1):44-49.
180. Sofka C.M., Collins A.J., Adler R.S. Use of ultrasonographic guidance in interventional musculoskeletal procedures: a review from a single institution. J Ultrasound Med. 2001;20(1):21-26.
181. Sofka C.M., Saboeiro G., Adler R.S. Ultrasound-guided adult hip injections. J Vasc Interv Radiol. 2005;16(8):1121-1123.
182. Sparling M., Malleson P., Wood B., et al. Radiographic followup of joints injected with triamcinolone hexacetonide for the management of childhood arthritis. Arthritis Rheum. 1990;33(6):821-826.
183. Speed C.A. Injection therapies for soft-tissue lesions. Best Pract Res Clin Rheumatol. 2007;21(2):333-347.
184. Sweetnam R. Corticosteroid arthropathy and tendon rupture. J Bone Joint Surg Br. 1969;51(3):397-398.
185. Tahiri L., Benbouazza K., Amine B., et al. Acute pseudoseptic arthritis after viscosupplementation of the knee: a case report. Clin Rheumatol. 2007;26(11):1977-1979.
186. Tallia A.F., Cardone D.A. Diagnostic and therapeutic injection of the wrist and hand region. Am Fam Physician. 2003;67(4):745-750.
187. Taras J.S., Raphael J.S., Pan W.T., et al. Corticosteroid injections for trigger digits: is intrasheath injection necessary? J Hand Surg [Am]. 1998;23(4):717-722.
188. Taylor H.G., Fowler P.D., David M.J., et al. Intra-articular steroids: confounder of clinical trials. Clin Rheumatol. 1991;10(1):38-42.
189. Thumboo J., O’Duffy J.D. A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium. Arthritis Rheum. 1998;41(4):736-739.
190. Tikiz C., Ünlü Z., Şener A., et al. Comparison of the efficacy of lower and higher molecular weight viscosupplementation in the treatment of hip osteoarthritis. Clin Rheumatol. 2005;24(3):244-250.
191. Toda Y., Tsukimura N. A comparison of intra-articular hyaluronan injection accuracy rates between three approaches based on radiographic severity of knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(9):980-985.
192. Torp-Pedersen T.E., Torp-Pedersen S.T., Qvistgaard E., et al. Effect of glucocorticosteroid injections in tennis elbow verified on colour Doppler ultrasonography: evidence of inflammation. Br J Sports Med. 2008;42(12):978-982.
193. Vad V.B., Sakalkale D., Warren R.F. The role of capsular distention in adhesive capsulitis. Arch Phys Med Rehabil. 2003;84(9):1290-1292.
194. van den Bekerom M.P., Lamme B., Sermon A., et al. What is the evidence for viscosupplementation in the treatment of patients with hip osteoarthritis? Systematic review of the literature. Arch Orthop Trauma Surg. 2008;128(8):815-823.
195. Van Holsbeeck M. Artifacts in musculoskeletal ultrasound. In: van Holsbeeck M., editor. Musculoskeletal Ultrasound. St Louis: Mosby, 2001.
196. Van Holsbeeck M. Interventional musculoskeletal ultrasound. In: van Holsbeeck M., editor. Musculoskeletal ultrasound. St Louis: Mosby, 2001.
197. Van Holsbeeck M. Physical principles of ultrasound imaging. In: van Holsbeeck M., editor. Musculoskeletal ultrasound. St Louis: Mosby, 2001.
198. Van Holsbeeck M. Sonography of bursae. In: van Holsbeeck M., editor. Musculoskeletal ultrasound. St Louis: Mosby, 2001.
199. Van Holsbeeck M. Sonography of the hip. In: van Holsbeeck M., editor. Musculoskeletal ultrasound. St Louis: Mosby, 2001.
200. Vashista G.N. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 2007;89(3):422-423. author reply 423
201. Waddell D.D., Marino A.A. Chronic knee effusions in patients with advanced osteoarthritis: implications for functional outcome of viscosupplementation. J Knee Surg. 2007;20(3):181-184.
202. Wang S.C., Chhem R., Cardinal E., et al. Joint sonography. Radiol Clin North Am. 1999;37(4):653-668.
203. Wank R., Miller T.T., Shapiro J.F. Sonographically guided injection of anesthetic for iliopsoas tendinopathy after total hip arthroplasty. J Clin Ultrasound. 2004;32(7):354-357.
204. Ward E.E., Jacobson J.A., Fessellet D.P., et al. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol. 2001;176(2):373-380.
205. Ward S.T., Williams P.L., Purkayastha S. Intra-articular corticosteroid injections in the foot and ankle: a prospective 1-year follow-up investigation. J Foot Ankle Surg. 2008;47(2):138-144.
206. Weiss M.M. Corticosteroids in rheumatoid arthritis. Semin Arthritis Rheum. 1989;19(1):9-21.
207. Wen D.Y. Intra-articular hyaluronic acid injections for knee osteoarthritis. Am Fam Physician. 2000;62(3):565-572.
208. Wind W.M.Jr., Smolinski R.J. Reliability of common knee injection sites with low-volume injections. J Arthroplasty. 2004;19(7):858-861.
209. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
210. Yucel I., Yazici B., Degirmenci E., et al. Comparison of ultrasound-, palpation-, and scintigraphy-guided steroid injections in the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2009;129(5):695-701.
211. Zeisig E., Fahlström M., Öhberget L., et al. Pain relief after intratendinous injections in patients with tennis elbow: results of a randomised study. Br J Sports Med. 2008;42(4):267-271.
212. Zeisig E., Ohberg L., Alfredson H. Sclerosing polidocanol injections in chronic painful tennis elbow: promising results in a pilot study. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1218-1224.
213. Zhang W., Moskowitz R.W., Nukiet G., et al. OARSI recommendations for the management of hip and knee osteoarthritis. II. OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162.
214. Zingas C., Failla J.M., Van Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surg [Am]. 1998;23(1):89-96.