Chapter 18 Peripheral Artery Disease
Clinical Evaluation
The least often recognized of the commonly occurring manifestations of atherosclerosis is peripheral artery disease (PAD). Epidemiological studies suggest that approximately 7.1 million people in the United States have PAD.1 Among 7458 participants aged 40 years and older from the 1999 to 2004 National Health and Nutrition Examination Survey (NHANES), prevalence of PAD was 5.9%.1,2 Despite its relative frequency, predictable patient population, and prognostic implications for life and limb, many cardiovascular physicians do not undertake clinical evaluation of PAD. This chapter will focus on the history, physical examination, and diagnostic tests important to management of limb atherosclerosis.
Patient History
Diagnosis of PAD begins with clinical suspicion in the typical patient population. This includes avid questioning and seeking to elicit historical evidence of limb and systemic atherosclerosis. Clinical suspicion should be heightened in older persons, in those with coronary or cerebral atherosclerosis, and in patients with atherosclerotic risk factors such as diabetes or tobacco use, as well as renal failure (see Chapter 16). Peripheral artery disease is uncommon before the age of 40 years. In the German Epidemiological Trial on Ankle Brachial Index (getABI) of 6990 unselected patients aged 65 years or older, prevalence of PAD in men and women was 20% and 17%, respectively.3 In the PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) program, a study of 6979 patients in 350 primary care practices across the United States, ankle-brachial index (ABI) screening was performed in subjects older than age 70, or older than age 50 if they were smokers or had diabetes.4 In this primary care population, 29% of those screened with an ABI met the criteria for PAD.
Despite the relative frequency of disease, diagnosis of PAD is not often considered because the majority of patients with PAD are asymptomatic. In the PARTNERS program, only 11% of PAD patients had classic symptoms.5 Similar data have been reported in other large cross-sectional studies (see Chapter 16). Even in high-risk subgroups with a higher population frequency of PAD, the diagnosis may be missed because PAD is often asymptomatic. The decision to look for PAD in the outpatient should be predicated on the pretest probability of finding it. Application of the PARTNERS criteria, for example, demonstrated the importance of risk factors in enriching the population with PAD to make ABI screening worthwhile. Thus, the presence of risk factors for atherosclerosis should lower the threshold for routine screening.
Symptoms of Peripheral Artery Disease
The most commonly ascribed symptom that develops as a result of PAD is intermittent claudication. The word claudication derives from the Latin word claudicatio, which was used to describe the limp gait of a lame horse. As defined in the Rose questionnaire,6 claudication is development of an ischemic muscular pain on exertion. The pain can be characterized as aching, burning, heaviness, feeling leaden, tightness, or cramping. Pain should originate in a muscular bed, such as the calf, thigh, hip, or buttock, and not localize to a joint. The area of the worst blood flow limitation usually subtends the site of muscular discomfort. For example, patients who develop hip or buttock discomfort with walking most likely have distal aorta or iliac artery occlusive disease, whereas patients with calf claudication likely have superficial femoral or popliteal arterial stenoses or occlusions. Reduction of muscular work on activity cessation rebalances available blood supply with muscle demand and quickly resolves the pain.
Both time of activity to pain onset and time to pain resolution should be consistent and predictable. The distance walked to the onset of leg discomfort is called the initial claudication distance, and the maximal distance the patient can walk without stopping because of leg discomfort is called the absolute claudication distance. Several classification schemes are used to categorize the severity of claudication, including the Fontaine (Table 18-1) and Rutherford classifications (Table 18-2).7 When the interview is complete, the physician should have insight into the nature of discomfort, how long it has been present, the typical duration of exercise required to cause the discomfort, and the amount of rest necessary to relieve the symptoms.
| Stage | Description |
|---|---|
| I | Asymptomatic, ABI < 0.9 |
| II | Intermittent claudication |
| III | Daily rest pain |
| IV | Focal tissue necrosis |
ABI, ankle-brachial index.
Table 18-2 Rutherford Classification
| Grade | Category | Description |
|---|---|---|
| 0 | 0 | Asymptomatic |
| I | 1 | Mild claudication |
| I | 2 | Moderate claudication |
| I | 3 | Severe claudication |
| II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss |
| IV | 6 | Major tissue loss |
Classic symptoms of claudication do not occur in all patients with PAD, including those with functional limitations. The application of questionnaires for claudication, such as the World Health Organization (WHO)/Rose questionnaire or the Walking Impairment Questionnaire,6,8 may underestimate PAD prevalence by 50%. Data from McDermott et al. indicate that complaints other than claudication are common.9 They evaluated functional tolerance across a range of symptoms in cross-sectional analyses of patients with and without PAD.5,10 Peripheral artery disease patients demonstrated several types of leg discomfort, including leg pain at rest and with walking, pain with walking alone requiring cessation of activity, and pain patients could “walk through.” This variety of presentations would be missed with questioning only for classic symptoms. Moreover, the type of discomfort predicted function. Patients with pain at rest and with walking had worse functional capacity than those whose pain occurred with walking and stopped with walking cessation, and those who were able to “walk through” the pain. Quality of leg pain, whether it is atypical or classic, does not predict severity of reduction in limb perfusion pressure as measured by the ABI.11
Presence of intermittent claudication has important prognostic implications regarding functional capacity and mortality. Three quarters of patients with intermittent claudication will have stable symptoms over the next 10 years; approximately 25% will progress to more disabling claudication or critical limb ischemia requiring revascularization or culminating in amputation.6 Moreover, they will suffer a mortality more than twice that of the general population, approximating 30% at 5 years.12
Differential Diagnosis of Claudication
Once exercise-related discomfort has been established, several alternate vascular and nonvascular diagnoses should be considered (Box 18-1). Vascular disorders include popliteal artery entrapment (see Chapter 62), compartment syndrome, fibromuscular dysplasia, venous insufficiency (see Chapter 55), and vasculitis (see Chapters 41 through 45). Popliteal artery entrapment typically occurs in very active persons or athletes. Because of an abnormal origin of the medial (or less commonly, lateral) head of the gastrocnemius muscle, the popliteal artery may be compressed with walking and yield symptoms of claudication. Endofibrosis of the external iliac artery (EIA), a relatively rare occurrence in highly trained cyclists and other endurance athletes, may cause claudication.
Fibromuscular dysplasia is a noninflammatory arterial occlusive disease that most commonly affects the renal and carotid arteries but may involve other arterial beds (see Chapter 63).13 Any of the arteries in the lower extremities may be affected, but the iliac arteries are the most common. Fibroplasias may involve the intima, media, or adventitial layer of the artery. The most common variety is medial fibroplasia. It can be diagnosed from the “string of beads” appearance on angiography and by its predilection for the nonbranching points of vessels. The etiology of fibromuscular dysplasia remains unknown.
Critical Limb Ischemia
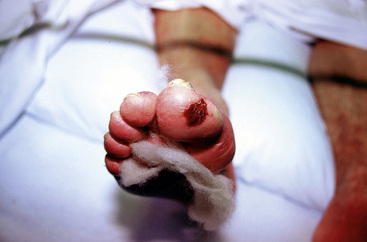
Critical limb ischemia (CLI) is the most debilitating manifestation of PAD (Fig. 18-1). The TransAtlantic Inter-Society Consensus (TASC) Working Group estimates that the incidence of CLI is between 300 and 1000 persons per million per year.6 Across the globe, frequency of amputation varies from 28 per million people per year in Madrid to 439 amputations per million people per year among Navajo Americans.14 Prevalence of CLI was 1.2% and affected more women than men in a population study of 8000 persons between 60 and 90 years of age.15
Diabetes and smoking increase the risk of developing CLI. Diabetes is the cause of most nontraumatic lower-extremity amputations in the United States.16 Diabetes increases the risk of amputation nearly fourfold, even with similar levels of blood flow limitation as in nondiabetic patients.17 Cigarette smoking also increases the risk that PAD will progress to CLI. In a study of 343 consecutive patients with intermittent claudication, 16% of those who continued to smoke developed CLI, compared to none in those who were able to stop smoking.18 In 190 patients undergoing lower-extremity revascularization followed for 3 years, those who smoked more than 15 cigarettes a day had a 10-fold higher risk (21%) of amputation compared to those who smoked fewer than 15 cigarettes a day (2%).19 Critical limb ischemia occurs as a consequence of tissue ischemia at rest, and it manifests as foot pain, nonhealing ulcers, or tissue gangrene. The pain is often severe and unremitting and localized to the acral portion of the foot or toes, notably at the site of ulceration or gangrene. Blood flow limitation is so severe that the gravitational effects of leg position may affect symptoms. Patients commonly report that leg elevation worsens pain. This is typically worse at night when the patient is in bed and the leg, now at heart level, no longer benefits from the dependent position. Placing the foot on the floor beside the bed is a common action used by patients to reduce pain. Inability to use the leg and chronically placing the leg in a dependent position may cause peripheral edema, a finding occasionally mistaken for venous disease in these patients. With severe ischemia, any skin perturbation, including bedclothes or blankets, may cause pain; in ischemic neuropathy, this causes a lancinating pain in the foot. Other symptoms of CLI include hypesthesia, cold intolerance, muscular weakness, and joint stiffness of the affected limb. Severity of CLI is categorized in both the Fontaine and Rutherford classification schemes (see Tables 18-1 and 18-2).
Differential Diagnosis of Critical Limb Ischemia
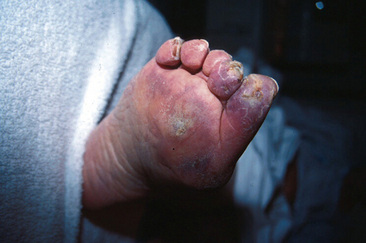
Differential diagnosis of CLI includes vascular and nonvascular diseases (Box 18-2). Atheroembolism, or blue toe syndrome, occurs when components of large-vessel atherosclerotic plaque embolize to distal vessels (Fig. 18-2) (see Chapter 47). The embolized material is composed of fibroplatelet debris and cholesterol crystals. A common cause of atheroembolism is iatrogenic disturbance of the vessel, whether from catheterization or surgery. Several features may help in differentiating atheroembolism from traditional CLI. Patients typically have pulses palpable down into the feet, because the emboli require a patent pathway to distal portions of the extremities. Other clinical clues include new renal insufficiency and blood eosinophilia. On examination, the patient will have areas of cyanosis or violaceous discoloration of the toes or portions of the feet and areas of livedo reticularis.
Acute limb ischemia may occur from thrombosis in situ or from thromboemboli of large fibroplatelet accumulations that originate in the heart or large arteries and occlude conduit arteries (see Chapter 46). These patients have an accelerated course and may present with the “five Ps” of acute ischemia: pain, pallor, poikilothermia, paresthesia, and paralysis. Other causes of limb ischemia include vasospasm, TAO, other vasculitides, and connective tissue disorders (see Chapters 41 44, and 48). Other causes of ulcers include neuropathy, venous disease, and trauma (see Chapter 61).
Nonvascular causes of foot pain include neuropathy, arthritides such as gout, fasciitis, and trauma (see Box 18-1).
Physical Examination
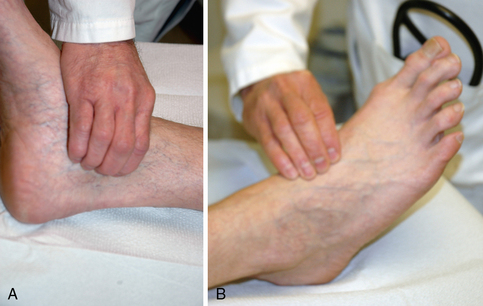
A comprehensive physical examination that includes general appearance of the patient, integument, heart, lungs, abdomen, and limbs should be performed during the initial patient encounter to elucidate evidence of systemic disease and provide insight into cause and manifestation of the patient’s vascular disease. The entire vascular system should be examined. Blood pressure is measured in each arm. A blood pressure difference of 10 mmHg or more may be indicative of innominate, subclavian, axillary, or brachial artery stenosis. The carotid, brachial, radial, ulnar, femoral, popliteal, dorsalis pedis, and posterior tibial pulses should be palpated in every patient (Fig. 18-3). Several pulse-descriptive schemes have been promulgated. One is to grade the pulses as 0 (absent), 1 (diminished), and 2 (normal). A very prominent or forceful pulse may occur in patients with aortic regurgitation or high cardiac output states. Absence of any pulse in the lower extremity, except in the dorsalis pedis, increases the likelihood of PAD.20 The dorsalis pedis pulse is not palpable in approximately 8% of healthy patients.20 Absence of a peripheral pulse may indicate a significant stenosis between the present and absent pulse. Occasionally, pulses may be palpable below the level of a significant stenosis. This most commonly occurs in the setting of iliac artery disease when there may be sufficient collateral vessels to maintain perfusion to distal arteries.
Changes in skin appearance with elevation and dependency may provide a gauge for PAD severity. The leg should be elevated to 45 to 60 degrees for 1 minute. If pallor develops quickly (within 10-15 seconds), severe PAD is likely. After 1 minute, the patient sits up and the leg is placed in a dependent position. The time to pedal vein refill should be recorded. Ischemic-induced arteriolar and venular dilation may lead to development of a violaceous appearance of the foot with dependency, called dependent rubor (Fig. 18-4). Normal refill occurs rapidly, typically within 10 to 15 seconds. Prolongation of venous filling or the development of numbness beyond 1 minute suggests severe PAD.
Two classification schemes are used to categorize the clinical assessment of patients with PAD: the Fontaine Stage Classification of PAD and the Rutherford Categorical Classification of PAD. In the system described by Fontaine, the severity of PAD is classified into 1 of 4 stages ranging from asymptomatic in stage 1, intermittent claudication in stage 2, daily rest pain in stage 3, and focal tissue necrosis in stage 4 (see Table 18-1). The Rutherford system employs seven categories, dividing severity of claudication into three categories (mild, moderate, and severe) and CLI into three categories (rest pain, minor tissue loss, and major tissue loss) (see Table 18-2).
Diagnostic Testing
Office-Based Ankle-Brachial Index
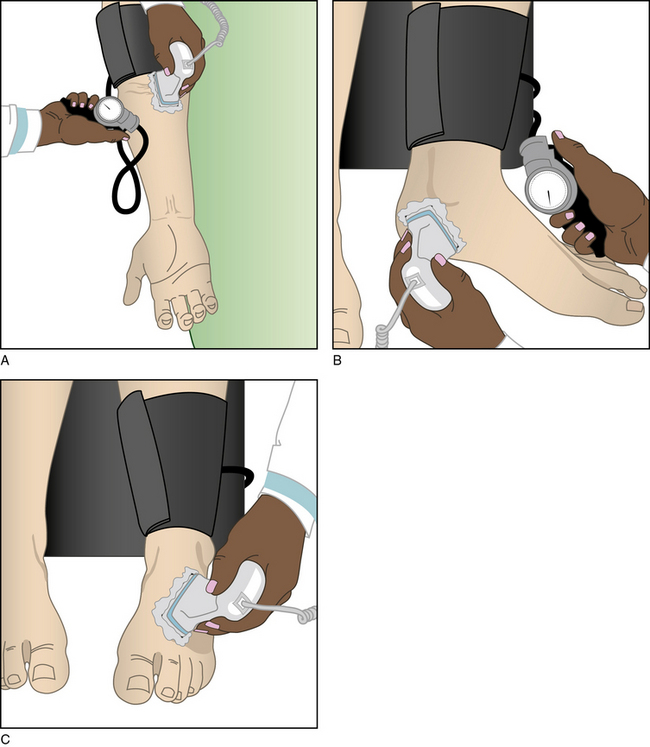
Measurement of the ABI is a simple method employed to corroborate the historical and physical findings of PAD. The ABI is the ratio of the systolic blood pressure at the ankle and brachial artery. The latter is an estimate of central aortic pressure. Brachial artery systolic blood pressure is measured in both arms and ankles using a handheld 5- or 10-MHz Doppler ultrasound device and sphygmomanometric cuff. The cuffs are placed on each arm above the antecubital fossa and above each ankle. The cuffs are sequentially inflated above systolic pressure and then are slowly depressurized. The Doppler probe, placed over the brachial artery and the dorsalis pedis and posterior tibial arteries, monitors the pressure (Fig. 18-5). As the cuff is slowly deflated, reappearance of a Doppler signal indicates the systolic pressure at the level of the cuff.
Brachial artery pressures must be measured in both arms because atherosclerosis may occur in subclavian and axillary arteries. The higher of the two brachial systolic blood pressures is used for reference in the ABI calculation. Hence, the ABI is the systolic pressure at each ankle divided by the higher pressure of the two brachial artery pressures; an ABI may be generated for each leg. When assessing foot perfusion, the ABI uses the highest of the pedal pressures in each leg.21 Both pedal pressures (dorsalis pedis and posterior tibial artery) are considered when seeking evidence of atherosclerosis. Some have suggested that ankle pressure used to calculate ABI should be an average of the two pedal pressures.22
Normal systolic pressure at the ankle should be at least the same as in the arm, yielding an ABI of 1.0 or greater. As a result of reflected arterial pressure waves, healthy persons tend to have an ABI ranging from 1.0 to 1.43. Recognizing an intrinsic (up to 10%) variability of blood pressure when measured sequentially, since ankle pressures are not measured simultaneously, an abnormal ABI consistent with the diagnosis of PAD is categorized as 0.9 or less, whereas an ABI above 0.9 to 1.0 is borderline abnormal.23 The ABI is a reliable determinant of PAD, with a sensitivity for ABI 0.9 or less ranging from 79% to 95% and specificity of 96% to 100%.21,24 An ABI of 0.4 or less is extremely abnormal and typically present in patients with CLI. Arterial calcification may introduce a false elevation in ABI, typically greater than 1.4, as a result of noncompressible vessels at the ankle. Arterial calcification occurs more commonly in patients with diabetes or end-stage renal disease and in the elderly.25,26
The ABI provides prognostic information because it is a barometer of the burden of systemic atherosclerosis. In the ABI Collaboration, the incidence of adverse events rose as the ABI dropped below 1.0, even in the absence of symptoms.27 The lower the ABI, the greater the cardiovascular morbidity and mortality.28,29 An ABI of 0.8 or less is associated with twice the age-adjusted 10-year mortality, and an ABI of 0.4 or less, is associated with a fourfold increase in mortality.21,27 Measuring the ABI provides an assessment of both PAD and cardiovascular risk.
There are limitations to ABI measurement. The correlation between ABI, functional capacity, and symptoms is weak. Resting ABI is occasionally normal in patients with PAD; this may occur in patients with aortoiliac stenoses and a well-collateralized arterial system that maintains perfusion pressure. In patients with a normal ABI but a strong suspicion of significant PAD, office-based exercise testing may be performed. Exercise accentuates arterial gradients by increasing the turbulence across the flow-limiting lesion and decreasing muscular arteriolar resistance to significantly attenuate lower-extremity perfusion pressure. In fact, arterial pressure at the ankle may reach zero in patients who develop claudication and recover more than 10 minutes after exercise cessation. In the office, stair climbing or active pedal plantar flexion30 may be used to elicit symptoms and document a decrease in ankle pressure (and ABI) to confirm the diagnosis of intermittent claudication.
Noninvasive Laboratory Testing for Peripheral Artery Disease
Physiological testing
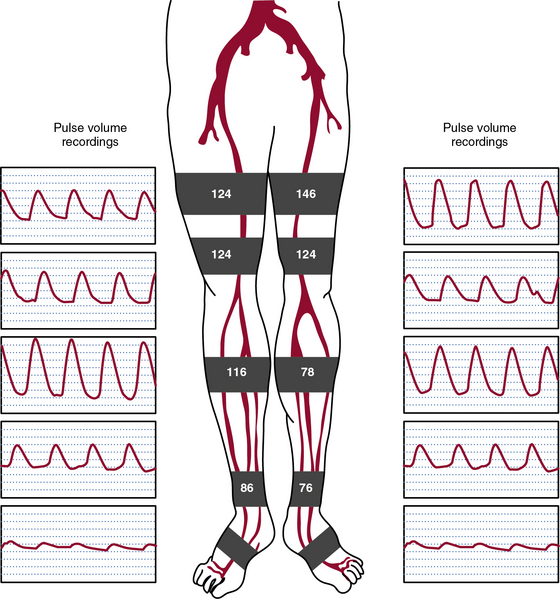
Physiological or functional testing most commonly occurs in a noninvasive vascular laboratory (see Chapter 12). Measurement of limb segmental systolic pressures employs methods similar to ABI measurement (Fig. 18-6). Sphygmomanometric cuffs are placed on the proximal thigh, distal thigh, calf, and ankle. The cuffs are inflated sequentially to suprasystolic pressure and then deflated to determine systolic pressure at each site. A Doppler probe is placed on the posterior tibial or dorsalis pedis artery. Arterial stenosis or occlusion will decrease the perfusion pressure. Arterial pressure gradients of more than 20 mmHg between thigh cuffs and 10 mmHg between cuffs below the knee indicate presence of a stenosis. As with the ABI, the most common source of error for the test is vascular calcification. In the setting of vascular calcification, a toe brachial index may be obtained. Pressures in the toe may be measured with strain gauge photoplethysmography. Pressure is measured in the toes and a ratio of toe pressure to brachial artery pressure is generated; a value of 0.7 or less is consistent with PAD.
Pulse volume recordings (PVRs) or segmental pneumatic plethysmography determines the relative change in limb volume with each pulse and can be obtained along with segmental pressure measurements. The pulse-volume waveform represents the product of pulse pressure and vascular wall compliance. In a healthy person, the pulse-volume waveform is similar to a normal arterial pressure waveform and includes rapid upstroke, dicrotic notch, and downstroke (see Fig. 18-6). The waveform changes when it is recorded distal to a significant stenosis as perfusion pressure falls. Initially, there is a loss of the dicrotic notch. As the stenosis worsens, waveform upstroke (anacrotic slope) is delayed, amplitude is less, and the downstroke (catacrotic slope) is slower. Combined use of segmental pressure measurements and PVRs improves the accuracy of identifying significant stenosis.
Treadmill Testing
As described earlier, eliciting symptoms through exercise may permit the diagnosis of PAD, despite a normal or near-normal ABI. When a vessel has a significant stenosis, increasing flow through the lesion decreases energy delivered beyond the area of stenosis. Treadmill exercise increases blood flow through a stenosed vessel and can increase sensitivity of the ABI. In the vascular laboratory, a diagnosis of intermittent claudication and a quantification of exercise tolerance may be obtained through treadmill exercise testing. Many protocols exist to test walking ability, but each falls into one of two types: constant or graded exercise. In constant exercise protocols (e.g., Carter protocol), a specific speed (1.5-2.0 mph) and treadmill grade (0%-12%) is chosen, whereas in the graded exercise protocols (e.g., Hiatt or Gardner protocol), speed and/or treadmill grade may increase.31 In both protocols, the brachial and ankle pressures are determined pretest at rest, patients exercise until they are unable to continue, and brachial and ankle pressures are redetermined within 1 minute of exercise cessation. Patients with PAD as a cause of exercise limitation will have an attenuated rise in ankle pressure compared to brachial artery pressure or, more commonly, a fall in ankle pressure, thus lowering the ABI. The fall in ankle pressure is directly related to severity of arterial occlusive disease. Analogously, length of recovery is also directly related to disease severity. Two parameters are recorded in addition to the ABI: the time claudication begins (initial claudication time), and the time until exhaustion or cessation (absolute claudication time). Variability of walking distance is greater in the constant exercise protocols than the graded protocols, making the latter more commonly used.
Anatomical Imaging of the Peripheral Circulation
Duplex Ultrasonography
Duplex ultrasonography of the lower extremities is performed in most vascular laboratories (see Chapter 12). The combination of bright (B)-mode ultrasound, color Doppler imaging, and pulsed-Doppler velocity analysis can accurately identify the location and severity of atherosclerotic lesions in the legs. Normally, flow through each arterial segment should be laminar, with a uniform homogeneous color appearance. Blood flow becomes turbulent and velocity increases at sites of sclerosis, creating areas of color discordance. Pulsed Doppler measurements in the area of stenosis demonstrate increased flow velocity and spectral broadening.
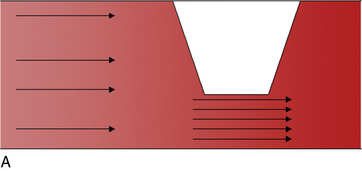
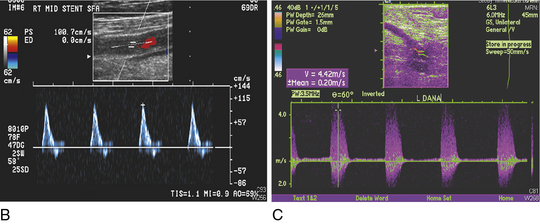
Applying the concepts of the Poiseuille law regarding movement of incompressible viscous fluids through a tube, the ratio of peak systolic velocity in the area of a stenosis is compared with the normal area of artery proximal to the stenosis. A ratio of 2 or greater is consistent with stenosis of 50% or more (Fig. 18-7). In one meta-analysis of seven studies, the sensitivity and specificity of duplex ultrasound to detect 50% or greater stenosis or occlusion were 88% and 96%, respectively, and to detect complete occlusion were 90% and 99%, respectively.32 In another meta-analysis of 14 studies, the sensitivity and specificity of duplex ultrasound with and without color-guided Doppler analysis to detect 50% or greater stenosis or occlusion was 93% and 95%, respectively.33 Duplex ultrasonography is less accurate at the site of calcified plaque because of the acoustic shadowing caused by the dense calcium. Serial stenoses are more difficult to diagnose because ultrasound diagnosis relies on comparing peak arterial velocities between adjacent segments, and there are altered hemodynamics between sequential stenoses. Single-center studies have found that ultrasound may be used alone in planning both percutaneous and surgical peripheral revascularization.34–37
Duplex ultrasonography is also used in the postoperative surveillance of arterial bypass grafts. A program of routine ultrasound surveillance is more likely to diagnose significant bypass graft stenoses than history, physical examination, or ABI. Clinical trials have evaluated the efficacy of ultrasound surveillance as a strategy to identify graft stenosis and prompt repair before graft occlusion occurs.38 One study randomized 156 patients to serial ultrasonography or ABI. Patients were referred for angiography and then corrective revascularization if 50% or greater stenosis was identified by ultrasound, or if the ABI decreased by 0.15 compared with the postoperative baseline.39 Assisted primary cumulative vein graft patency in the ultrasound group was 78% compared with 53% in the ABI group after 3 years. In other randomized studies, however, no benefit was found for ultrasound compared with ABI 1 year after surgery.40,41 The data for surveillance for synthetic bypass grafts39,42,43 and after angioplasty44 are less robust than for vein grafts.
Magnetic Resonance Angiography
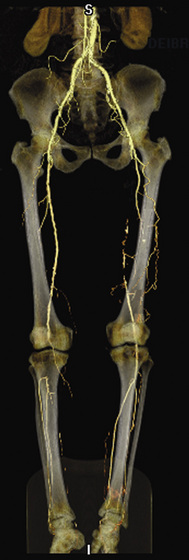
Magnetic resonance angiography (MRA) is an accurate imaging modality to diagnose PAD, visualize peripheral arteries, and determine the location of stenoses (see Chapter 13). Techniques used to image the arterial tree include black blood, phase contrast, time of flight (TOF), and contrast-enhanced MRA.45 The application of two magnetic pulses to suppress signal in the vessel lumen yields a dark appearance of flowing blood, with the vessel wall remaining white. Selective removal of blood from the image causes the lumen to appear black, and the technique is therefore called black blood. When the phase shift of moving electron spins in flowing blood is compared with surrounding stable tissue, blood volume and velocity can be measured to permit assessment of blood flow. Application of electrocardiographic gating while interrogating the flow-related enhancement of spins into a partially saturated area provides a time of flight angiogram.46,47 Limitations of a flow-based TOF MRA include lengthy acquisition types, turbulence, nonlinear vascular structures, and retrograde flow.48
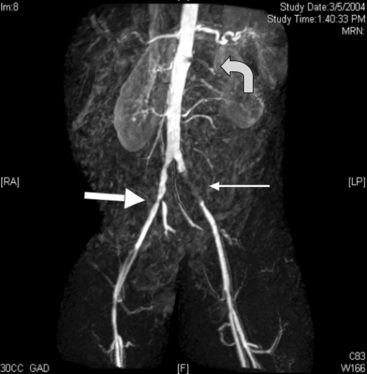
Most MRAs are performed using contrast, most commonly gadolinium. Contrast-enhanced MRA provides a high-resolution angiogram. Contrast-enhanced TOF MRA is useful as a noninvasive imaging test to define lower-extremity vascular anatomy. In a meta-analysis comparing contrast-enhanced MRA with TOF MRA, the contrast-enhanced study had a much greater diagnostic accuracy.48 Use of contrast has improved scan quality and efficiency and enhanced vessel visualization and identification, especially in distal vessels49 (Fig. 18-8). Magnetic resonance angiography can identify the presence of stenoses and reveal distal vessels suitable for bypass not demonstrated by contrast angiography.50,51 In a meta-analysis of 32 studies of contrast-enhanced MRA and intraarterial digital subtraction angiography (DSA), the pooled sensitivity of MRA was 95%, and specificity was 96%.52
One potential limitation is a tendency for MRA to overestimate lesion severity.53 Similar benefits and limitations exist in the imaging of bypass grafts. Magnetic resonance angiography has a sensitivity as high as 91% for identification of arterial bypass graft stenoses54 but overestimates lesion severity in up to 30% of stenoses.55,56 A sound strategy may involve use of MRA initially because of the noninvasive nature of the test and its superior identification of bypass vessels, reserving DSA for cases requiring greater definition.57 Technological advancement is rapid in MRA, improving detection and identification of arterial occlusive disease. As imaging protocols and techniques, such as three-dimensional (3D) MRA imaging, gain acceptance and are made commonly available, MRA may ultimately be used as a stand-alone evaluation prior to revascularization.58–60
Computed Tomographic Angiography
Computed tomographic angiography (CTA) has recently undergone rapid improvements in technology and imaging, allowing its entry into peripheral vascular imaging (see Chapter 14). Much of this advance results from development of multidetector-row CT scanners and improved resolution of arteries. Availability of higher resolution to scanners is particularly relevant for smaller and more distal arteries.
In meta-analyses of studies mostly using multidetector CT scanners, pooled sensitivity and specificity for detecting stenoses of 50% or greater in leg arteries were 91% to 92% and 91% to 93%, respectively.32,61 As scanner number increases, newer CT scanners should have even greater accuracy.
Computed tomography angiography is commonly presented using a maximal intensity projection (MIP) or with a volume rendering technique (Fig. 18-9). The MIP algorithm displays only the pixel with the highest intensity along a ray perpendicular to the plane of projection. This algorithm creates a two-dimensional (2D) projectional image similar in appearance to MRA or contrast angiography. Volume rendering applies shades of gray to pixels of varying density.62 Fourier transfer functions allow modification of the relative contribution of various pixel values. Volume rendering considers pixels that are only partially filled with contrast material. Arterial calcification limits imaging with both CT techniques. Optimal techniques used for postprocessing are being developed.63 As resolution improves, CTA may become a regular instrument in the diagnostic armamentarium because of its rapid study time (typically <1 minute) or because of the 75% reduction in ionizing radiation compared with angiography.
Contrast Angiography
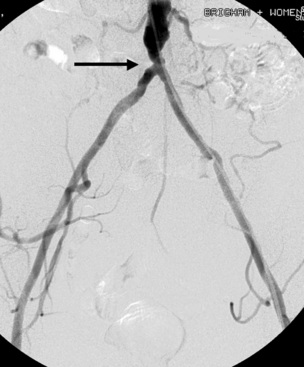
Contrast angiography is the most venerable and widely available method for imaging arterial anatomy (see Chapter 15). Angiography commonly serves as the standard for determining the sensitivity and specificity of newer techniques and is an excellent method to clarify arterial anatomical queries (Fig. 18-10). Technical improvements in equipment, including smaller catheters, image resolution, and digital subtraction, have enhanced the capability of angiography. Digital subtraction eliminates bony and soft-tissue shadows from the angiographic image, enhancing angiographic detail. Despite wide acceptance of angiography as a reliable method for defining arterial anatomy, its invasive nature, requirement for contrast, nephrotoxicity, risk of atheroembolism, and risk of pseudoaneurysms or arteriovenous fistula (AVF) continue to foster development of alternative angiographic methods.
Summary
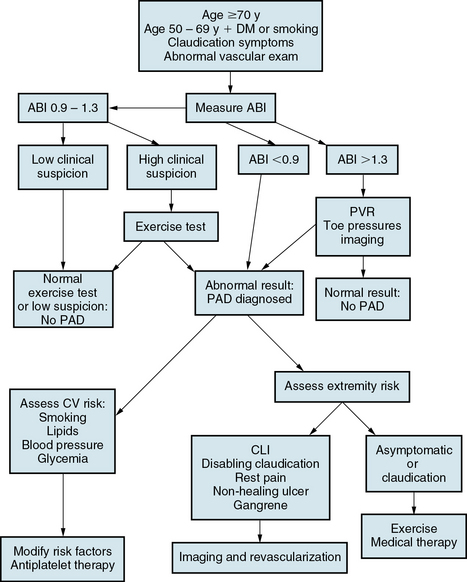
An algorithm for evaluating the patient with PAD is depicted in Figure 18-11. Diagnosis and evaluation of PAD is required in patients predisposed to develop PAD because of age or the presence of atherosclerotic risk factors and in patients whose history or examination are suggestive of PAD. An office evaluation should include measurement of ABI. An exercise test with measurement of the ABI after exercise is appropriate if resting ABI is normal, yet clinical suspicion remains high. Patients with noncompressible ankle vessels should be referred to a vascular laboratory for additional testing, including segmental pressure measurements, pulse-volume recordings, and/or duplex ultrasonography. Symptomatic patients, particularly those with CLI who are being treated for revascularization, should undergo anatomical imaging with CT, MRA, or conventional carotid angiography.
1 Pande R.L., Perlstein T.S., Beckman J.A., et al. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23.
2 Selvin E., Erlinger T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743.
3 Diehm C., Schuster A., Allenberg J.R., et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105.
4 Hirsch A.T., Criqui M.H., Treat-Jacobson D., et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324.
5 McDermott M.M., Greenland P., Liu K., et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606.
6 Rose G.A. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658.
7 Norgren L., Hiatt W.R., Dormandy J.A., et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–S67.
8 Regensteiner J.G., Steiner J.F., Panzer R.J., et al. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142.
9 McDermott M.M., Mehta S., Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159:387–392.
10 McDermott M.M., Ferrucci L., et al. The ankle-brachial index is associated with the magnitude of impaired walking endurance among men and women with peripheral arterial disease. Vasc Med. 2010;15:251–257.
11 McDermott M.M., Mehta S., Liu K., et al. Leg symptoms, the ankle-brachial index, and walking ability in patients with peripheral arterial disease. J Gen Intern Med. 1999;14:173–181.
12 Hirsch AT, Haskal ZJ, Hertzer NR, et al: ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease):endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Transatlantic Inter-Society Consensus; and Vascular Disease Foundation, Circulation 113:e463–e654, 2006.
13 Slovut D.P., Olin J.W. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862–1871.
14 Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. The Global Lower Extremity Amputation Study Group. Br J Surg. 2000;87:328–337.
15 Sigvant B., Wiberg-Hedman K., Bergqvist D., et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–1191.
16 Diabetes-related amputations of lower extremities in the Medicare population–Minnesota, 1993-1995. MMWR Morb Mortal Wkly Rep. 1998;47:649–652.
17 Jude E.B., Oyibo S.O., Chalmers N., et al. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433–1437.
18 Jonason T., Bergstrom R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221:253–260.
19 Lassila R., Lepantalo M. Cigarette smoking and the outcome after lower limb arterial surgery. Acta Chir Scand. 1988;154:635–640.
20 Khan N.A., Rahim S.A., Anand S.S., et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536–546.
21 Aboyans V: ABI scientific statement, Circulation In press.
22 McDermott M.M., Criqui M.H., Liu K., et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171.
23 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation;124:2020–2045, 2011.
24 Lijmer J.G., Hunink M.G., van den Dungen J.J., et al. Roc analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–398.
25 Leskinen Y., Salenius J.P., Lehtimaki T., et al. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis. 2002;40:472–479.
26 Ishimura E., Okuno S., Kitatani K., et al. Different risk factors for peripheral vascular calcification between diabetic and non-diabetic haemodialysis patients–importance of glycaemic control. Diabetologia. 2002;45:1446–1448.
27 Fowkes F.G., Murray G.D., Butcher I., et al. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208.
28 Vogt M.T., McKenna M., Anderson S.J., et al. The relationship between ankle-arm index and mortality in older men and women. J Am Geriatr Soc. 1993;41:523–530.
29 Sikkink C.J., van Asten W.N., van’t Hof M.A., et al. Decreased ankle/brachial indices in relation to morbidity and mortality in patients with peripheral arterial disease. Vasc Med. 1997;2:169–173.
30 McPhail I.R., Spittell P.C., Weston S.A., et al. Intermittent claudication: an objective office-based assessment. J Am Coll Cardiol. 2001;37:1381–1385.
31 Hiatt W.R., Hirsch A.T., Regensteiner J.G., et al. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92:614–621.
32 Collins R., Burch J., Cranny G., et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334:1257.
33 de Vries S.O., Hunink M.G., Polak J.F. Summary receiver operating characteristic curves as a technique for meta-analysis of the diagnostic performance of duplex ultrasonography in peripheral arterial disease. Acad Radiol. 1996;3:361–369.
34 Proia R.R., Walsh D.B., Nelson P.R., et al. Early results of infragenicular revascularization based solely on duplex arteriography. J Vasc Surg. 2001;33:1165–1170.
35 Ascher E., Mazzariol F., Hingorani A., et al. The use of duplex ultrasound arterial mapping as an alternative to conventional arteriography for primary and secondary infrapopliteal bypasses. Am J Surg. 1999;178:162–165.
36 Lofberg A.M., Karacagil S., Hellberg A., et al. The role of duplex scanning in the selection of patients with critical lower-limb ischemia for infrainguinal percutaneous transluminal angioplasty. Cardiovasc Intervent Radiol. 2001;24:229–232.
37 Mandolfino T., Canciglia A., D’Alfonso M., et al. Infrainguinal revascularization based on duplex ultrasound arterial mapping. Int Angiol. 2006;25:256–260.
38 Westerband A., Mills J.L., Kistler S., et al. Prospective validation of threshold criteria for intervention in infrainguinal vein grafts undergoing duplex surveillance. Ann Vasc Surg. 1997;11:44–48.
39 Lundell A., Lindblad B., Bergqvist D., et al. Femoropopliteal-crural graft patency is improved by an intensive surveillance program: A prospective randomized study. J Vasc Surg. 1995;21:26–33. discussion 33–24
40 Ihlberg L., Luther M., Alback A., et al. Does a completely accomplished duplex-based surveillance prevent vein-graft failure? Eur J Vasc Endovasc Surg. 1999;18:395–400.
41 Ihlberg L., Luther M., Tierala E., et al. The utility of duplex scanning in infrainguinal vein graft surveillance: results from a randomised controlled study. Eur J Vasc Endovasc Surg. 1998;16:19–27.
42 Tinder C.N., Chavanpun J.P., Bandyk D.F., et al. Efficacy of duplex ultrasound surveillance after infrainguinal vein bypass may be enhanced by identification of characteristics predictive of graft stenosis development. J Vasc Surg. 2008;48:613–618.
43 Brumberg R.S., Back M.R., Armstrong P.A., et al. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure. J Vasc Surg. 2007;46:1160–1166.
44 Connors G., Todoran T.M., Engelson B.A., et al. Percutaneous revascularization of long femoral artery lesions for claudication: patency over 2.5 years and impact of systematic surveillance. Catheter Cardiovasc Interv. 2011;77:1055–1062.
45 Tatli S., Lipton M.J., Davison B.D., et al. From the RSNA refresher courses: MR imaging of aortic and peripheral vascular disease. Radiographics. 2003:S59–S78. 23 Spec No
46 Steffens J.C., Link J., Schwarzenberg H., et al. Lower extremity occlusive disease: diagnostic imaging with a combination of cardiac-gated 2D phase-contrast and cardiac-gated 2D time-of-flight MRA. J Comput Assist Tomogr. 1999;23:7–12.
47 Quinn S.F., Sheley R.C., Semonsen K.G., et al. Aortic and lower-extremity arterial disease: evaluation with MR angiography versus conventional angiography. Radiology. 1998;206:693–701.
48 Nelemans P.J., Leiner T., de Vet H.C., et al. Peripheral arterial disease: meta-analysis of the diagnostic performance of MR angiography. Radiology. 2000;217:105–114.
49 Dellegrottaglie S., Sanz J., Macaluso F., et al. Technology insight: magnetic resonance angiography for the evaluation of patients with peripheral artery disease. Nat Clin Pract Cardiovasc Med. 2007;4:677–687.
50 Dorweiler B., Neufang A., Kreitner K.F., et al. Magnetic resonance angiography unmasks reliable target vessels for pedal bypass grafting in patients with diabetes mellitus. J Vasc Surg. 2002;35:766–772.
51 Kreitner K.F., Kalden P., Neufang A., et al. Diabetes and peripheral arterial occlusive disease: prospective comparison of contrast-enhanced three-dimensional MR angiography with conventional digital subtraction angiography. AJR Am J Roentgenol. 2000;174:171–179.
52 Menke J., Larsen J. Meta-analysis: accuracy of contrast-enhanced magnetic resonance angiography for assessing steno-occlusions in peripheral arterial disease. Ann Intern Med. 2010;153:325–334.
53 Winterer J.T., Schaefer O., Uhrmeister P., et al. Contrast enhanced MR angiography in the assessment of relevant stenoses in occlusive disease of the pelvic and lower limb arteries: diagnostic value of a two-step examination protocol in comparison to conventional DSA. Eur J Radiol. 2002;41:153–160.
54 Bendib K., Berthezene Y., Croisille P., et al. Assessment of complicated arterial bypass grafts: value of contrast-enhanced subtraction magnetic resonance angiography. J Vasc Surg. 1997;26:1036–1042.
55 Dorenbeck U., Seitz J., Volk M., et al. Evaluation of arterial bypass grafts of the pelvic and lower extremities with gadolinium-enhanced magnetic resonance angiography: comparison with digital subtraction angiography. Invest Radiol. 2002;37:60–64.
56 Loewe C., Cejna M., Schoder M., et al. Contrast material-enhanced, moving-table MR angiography versus digital subtraction angiography for surveillance of peripheral arterial bypass grafts. J Vasc Interv Radiol. 2003;14:1129–1137.
57 Brillet P.Y., Vayssairat M., Tassart M., et al. Gadolinium-enhanced MR angiography as first-line preoperative imaging in high-risk patients with lower limb ischemia. J Vasc Interv Radiol. 2003;14:1139–1145.
58 Cronberg C.N., Sjoberg S., Albrechtsson U., et al. Peripheral arterial disease. Contrast-enhanced 3D MR angiography of the lower leg and foot compared with conventional angiography. Acta Radiol. 2003;44:59–66.
59 Bezooijen R., van den Bosch H.C., Tielbeek A.V., et al. Peripheral arterial disease: sensitivity-encoded multiposition MR angiography compared with intraarterial angiography and conventional multiposition MR angiography. Radiology. 2004;231:263–271.
60 Steffens J.C., Schafer F.K., Oberscheid B., et al. Bolus-chasing contrast-enhanced 3D MRA of the lower extremity. Comparison with intraarterial DSA. Acta Radiol. 2003;44:185–192.
61 Heijenbrok-Kal M.H., Kock M.C., Hunink M.G. Lower extremity arterial disease: multidetector CT angiography meta-analysis. Radiology. 2007;245:433–439.
62 Lawler L.P., Fishman E.K. Multi-detector row CT of thoracic disease with emphasis on 3D volume rendering and CT angiography. Radiographics. 2001;21:1257–1273.
63 Becker C.R., Wintersperger B., Jakobs T.F. Multi-detector-row CT angiography of peripheral arteries. Semin Ultrasound CT MR. 2003;24:268–279.