Chapter 15 Perioperative and anesthesia management
1 INTRODUCTION
As the population in the United States becomes more obese every year, the incidence of obstructive sleep apnea/hypopnea syndrome (OSAHS) is also increasing. In the general population, the incidence of OSAHS is 2% in women and 4% in men.1 An increase in the body mass index (BMI) of one standard deviation increases the likelihood of co-existing OSAHS by a factor of 4.1 In the morbidly obese population, the incidence of OSA is 3% to 25% in women and 40% to 77% in men.2 Conversely, 90% of OSAHS subjects have a BMI greater than 28 kg/m2. Some subjects have severe (BMI=35 to 39 kg/m2) and even morbid (BMI=40 kg/m2) obesity.
The airway obstruction in OSAHS patients is due to a decrease in the upper airway muscle tone during sleep and airway narrowing due to the deposition of adipose tissue in pharyngeal structures. The structures that may increase in size due to deposition of fat are the uvula, tonsils, tonsillar pillars, tongue, aryepiglottic folds and the lateral pharyngeal walls (Fig. 15.1). The deposition of fat in the lateral pharyngeal walls not only narrows the airway, but also changes the shape of the pharynx. The normal shape of the pharynx with a long transverse (lateral) and a short anterior–posterior axis changes into an ellipsoid with a short transverse and a long anterior–posterior axis. This change in shape makes the action of the anterior pharyngeal dilator airway muscles (tensor palitini, genioglossus, and hyoid) less efficient (Fig. 15.2).3
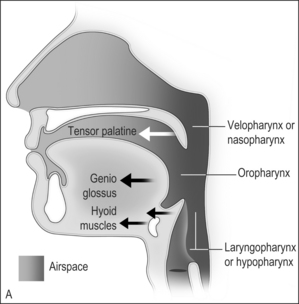
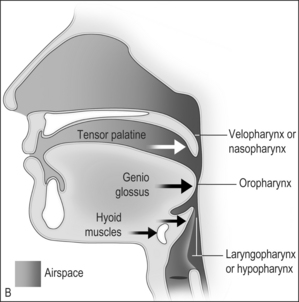
Fig. 15.1 A, The action of the most important dilator muscles of the upper airway. The tensor palatine, genioglossus, and hyoid muscles enlarge the nasopharynx, oropharynx, and the laryngopharynx, respectively. B, Collapse of the nasopharynx at the palatal level, the oropharynx at the glottic level, and the laryngopharynx at the epiglottic level. Modified from Benumof JL. Obstructive sleep apnea in the adult obese patient: implications for airway management. J Clin Anesth 2001;13(2):144–56, with permission.
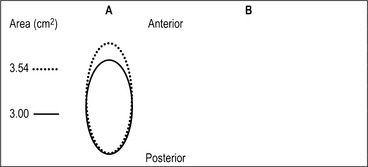

Fig. 15.2 The effects of a 5 mm change in the anteroposterior (AP) diameter of the airway on airway cross-sectional area is shown for two equally elliptical airways with different lateral/AP ratios. A, The lateral/AP ratio=0.5. B, The lateral/AP ratio=2. The lateral dimension of each ellipse was held constant. The solid line represents the starting area (3 cm2 in both ellipses), and the dotted line represents the area after a 5 mm increase in the AP diameter. The area change is greater in the ellipse with a more lateral orientation B, From Leiter JC. Upper airway shape. Is it important in the pathogenesis of obstructive sleep apnea? Am J Respir Crit Care Med 1996;153:894–8, with permission.
Rights were not granted to include this figure in electronic media. Please refer to the printed book.
Patients with diagnosed OSAHS may come to surgery for either OSAHS corrective surgery or for some other procedure. In addition, many patients coming for surgeries other than OSAHS correction may have undiagnosed OSAHS. Respiratory outcomes during the perioperative management of patients with OSAHS are a major concern for anesthesiologists. The inability to tracheally intubate the patient, respiratory obstruction soon after tracheal extubation at the end of the procedure, and severe respiratory depression and respiratory arrest after the administration of sedatives and narcotics are the biggest concerns.4
Recently, the American Society of Anesthesiologists (ASA) developed practice guidelines for the perioperative management of patients with OSAHS.5 These guidelines proposed a scoring system, which can be used to estimate whether a patient is at increased risk for perioperative complications from OSAHS (Table 15.1). The scoring system depends upon three factors: (1) the severity of OSAHS disease; (2) the invasiveness of the surgery and anesthesia; and (3) the requirement for postoperative opioids. The estimation of perioperative risk is based on the overall score; patients with an overall score of 5 or greater have an increased risk of perioperative complications.
Table 15.1 Scoring system for calculation of perioperative risk in patients with OSAHS‡
|
A. Severity of sleep apnea based on sleep study (or clinical indicators if sleep study not available). Point score ______ (0–3).*†
B. Invasiveness of the surgery and anesthesia. Point score ______ (0–3).
|
Estimation of perioperative risk is calculated as follows: Overall score=the score for A plus the greater of the score for either B or C.
* One point may be subtracted if a patient has been on CPAP or NIPPV before surgery and will be using his or her appliance consistently during the postoperative period.
† One point should be added if a patient with mild or moderate OSAHS also has a resting PaCO2 greater than 50 mm Hg.
‡ Patients with a score of 5 or 6 may be at significantly increased perioperative risk from OSAHS. Modified from Gross JB, Bachenberg KL, Benumof JL, et al. American Society of Anesthesiologists Task Force on Perioperative Management. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on perioperative management of patients with obstructive sleep apnea. Anesthesiology 2006;104(5):1081–93, with permission.
2 PREOPERATIVE ASSESSMENT OF THEPATIENT
Ideally, evaluation of the patient should take place in a preoperative clinic, several days before surgery. Unfortunately, many patients will not be seen by the anesthesiologist until the day of surgery. The evaluation should include a review of the current and previous medical records, an interview of the patient, and a physical examination.
A careful review of all medical records is very important. The preoperative diagnosis, exact surgical procedure being performed and proper consent for anesthesia and surgery should be noted. Reviewing old medical records can be particularly useful with regard to previous anesthetic history, which may reveal airway difficulties, an unusual response to anesthetic agents and the postoperative course. All co-existing medical conditions and treatments should be noted. In addition to the routine laboratory values, any work-up that has been done specific to OSAHS (such as polysomnographic testing, cephalometric measurements, cardiac or pulmon-ary function studies) should be checked. All consultations should be reviewed and if a specific question arises, the consultant in question should be contacted. A detailed history from the patient (and bedpartner if possible) should identify patients with undiagnosed OSAHS.
A thorough physical examination of the patient is essential. Many OSAHS patients have several co-existing diseases due to hypoxic episodes and obesity. Careful examination of the cardiovascular system should be done. Bradycardia occurs during apneic spells due to hypoxia and reverts to baseline during and after arousal. In about half of the patients, long sinus pauses, second-degree heart block and ventricular arrhythmias occur. When arterial oxygen saturation (SaO2) decreases below 60%, the severity of the bradycardia and the onset of arrhythmias markedly increase.6 Many patients develop both systemic and pulmonary hypertension. Systemic hypertension is present in half the patients due to repetitive increases in sympathetic tone during each hypoxic/hypercapnic arousal event.7 Pulmonary hypertension has several possible etiologies.
Special attention must be paid to the airway of the patient. In general, obese patients are more difficult to tracheally intubate.10 Since many OSAHS patients are obese, the same principles should be applied when securing the airway of an OSAHS patient. These patients have a large tongue and a thick neck. The probability of difficult tracheal intubation is 5% when the neck circumference is 40 cm whereas the probability increases to 35% when the neck circumference is 60 cm.11 The fact that the excess pharyngeal tissue that is deposited in the lateral walls of the pharynx may not be visible on routine examination is another cause for an unanticipated difficult tracheal intubation. During laryngoscopy, the commonly seen excessive adipose tissue in the interscapular region (‘buffalo hump’) causes malalignment of the oral, pharyngeal and tracheal axes. In addition, since these patients are more prone to aspiration of gastric contents, the Sellick maneuver (cricoid pressure) used during induction of anesthesia before tracheal intubation may also misalign the axes.12 These misalignments can interfere with the ‘line of sight’ and make visualization of the larynx more difficult.
Whether tracheal intubation should be performed with the patient awake or under general anesthesia must be individualized on the basis of a methodical, complete airway examination (Table 15.2). Thus, a multivariate airway risk index should be performed.13 This index identifies several criteria including:
Table 15.2 Components of the preoperative airway physical examination
| Airway examination component | Non-reassuring findings |
|---|---|
| Length of upper incisors | Relatively long |
| Relation of maxillary and mandibular incisors during normal jaw closure | Prominent ‘overbite’ (maxillary incisors anterior to mandibular incisors) |
| Relation of maxillary and mandibular incisors during voluntary protrusion of mandible | Patient cannot bring mandibular incisors anterior to (mandible in front of) maxillary incisors |
| Interincisor distance | Less than 3 cm |
| Visibility of uvula | Not visible when tongue is protruded with patient in sitting position (e.g. Mallampati class greater than II) |
| Shape of palate | Highly arched or very narrow |
| Compliance of mandibular space | Stiff, indurated, occupied by mass, or non-resilient |
| Thyromental distance | Less than three ordinary finger breadths |
| Length of neck | Short |
| Thickness of neck | Thick |
| Range of motion of head and neck | Patient cannot touch tip of chin to chest or cannot extend neck |
This table displays some findings of the airway physical examination that may suggest the presence of a difficult intubation. The decision to examine some or all of the airway components shown in this table depends on the clinical context and judgment of the practitioner. The table is not intended as a mandatory or exhaustive list of the components of an airway examination. The order of presentation in this table follows the ‘line of sight’ that occurs during conventional oral laryngoscopy.
Using rigid laryngoscopy, the multivariate risk index predicts the difficulty of laryngeal visualization better than a single criterion such as the Mallampati classification of the oropharyngeal view.
2.1 PREMEDICATION
Sedatives and narcotics have a propensity to exacerbate the sleep-related apneic episodes and may impair life-saving arousal in patients with OSAHS. Benzodiazepines and barbiturates preferentially decrease neural input to the upper airway dilating muscles, leading to airway obstruction.16 Even small doses of narcotics given intravenously or epidurally can cause severe airway obstruction and apnea.17,18 Administration of a combination of sedatives and narcotics can be disastrous. Anxiolytic drugs such as midazolam should only be administered when close monitoring of the patient by appropriate personnel is possible. This means that the drug should not be given until the patient is about to enter the operating room for surgery. Because of the increased incidence of aspiration of gastric contents, an antacid and/or metoclopramide should be given to decrease the gastric acidity and volume. Glycopyrrolate to reduce oral secretions and dexametasone to reduce airway edema and nausea and vomiting are generally administered.
2.2 MONITORING
In addition to the standard monitors (electrocardiogram, non-invasive blood pressure, pulse oximetry, end-tidal carbon dioxide), some patients may require an indwelling arterial catheter. In obese patients, the blood pressure cuff may not fit the upper arm properly, and thus may not read correctly. In addition, tongue advancement and mandibular osteotomy procedures may require hypotensive anesthesia where accurate blood pressure readings are essential. An indwelling arterial catheter also allows easy access to blood for arterial blood gases and other laboratory testing, intraoperatively and postoperatively. Very rarely, patients with advanced cardiac or pulmonary dysfunction may need a pulmonary artery catheter. If the procedure is very long and necessitates administration of large quantities of fluids, a Foley catheter should be considered. A depth of anesthesia monitor, when available, may facilitate rapid awakening of the patient at the end of the procedure. For all long procedures, some form of temperature monitor should be used.
2.3 POSITIONING OF THE PATIENT FOR SURGERY
Accommodating these large patients appropriately on the narrow operating tables is a common problem. If a wide operating table is not used, the arms may have to be placed on arm boards and positioned in such a way that the surgeon will have proper access to the surgical site, and at the same time the patient is protected from neurological damage.
Obese patients have increased oxygen consumption (VO2) and decreased lung volumes. Functional residual capacity (FRC) and expiratory reserve volume (ERV) are small and patients become desaturated faster during apnea.19 The supine position is the most common position for OSAHS surgery. In this position the abdominal contents and the diaphragm are pushed into the chest cavity, further decreasing the lung volumes and exaggerating the problem of desaturation.20 A ‘ramped’ position (Fig. 15.3), in which the upper body, neck and head are elevated to a point where an imaginary horizontal line can be drawn from the sternal notch to the external ear, not only improves the comfort of the patient but also improves the laryngeal view during intubation when compared to the ‘sniffing’ position.21 Once the endotracheal tube (ETT) is safely secured, some of the blankets can be removed before surgery commences.
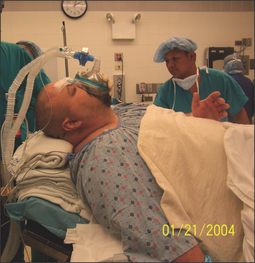
Fig. 15.3 The ‘ramped’ position, in which the upper body, neck and head are elevated to a point where an imaginary horizontal line can be drawn from the sternal notch to the external ear, not only improves the comfort of the patient but also improves the laryngeal view during intubation. Once the endotracheal tube (ETT) is safely secured, some of the blankets can be removed before surgery commences.
2.4 PREOXYGENATION OF THE PATIENT
Maximally preoxygenating obese, OSAHS patients is absolutely essential for many reasons, including a higher incidence of difficulty in securing the airway, not ventilating the patient during a rapid sequence induction before securing the airway, decreased lung volumes, and increased VO2, which lead to faster desaturation during apnea. In addition, these patients may be difficult to ventilate after induction agents are given since they can become obstructed very easily due to redundant soft tissue. Although the original ASA difficult airway algorithm made no mention of preoxygenation, an updated task force report includes facemask preoxygenation before initiating management of the difficult airway.22 The same ramped position (Fig. 15.3) that was mentioned earlier can also improve the preoxygenation.23 In obese patients with a large head, selecting the proper size facemask and maintaining a tight seal around the mask are very important to achieve a maximal inspired oxygen concentration. In normal patients, a minimum of 3 minutes of tidal volume breathing or eight deep breaths in 1 minute is necessary to sufficiently increase oxygen stores. In the obese OSAHS patient, a longer duration of preoxygenation (at least 5 minutes of tidal volume breathing) is necessary to increase the oxygen stores in the body. Oxygen insufflation into the pharynx via a small naso- or oropharyngeal catheter during laryngoscopy may further delay the onset of desaturation.24
3 INTRAOPERATIVE CARE
In the operating room, the anesthetic management ranges from essentially ‘routine’ to treacherous. As previously indicated, these cases require communication and discussion between the surgeon and the anesthesiologist, creating a team approach. Extra thought, extra planning, and extra care are indicated. There is a level of unpredictability associated with all phases of the perioperative period, particularly with (OSAHS) patients. Can the patient be mask ventilated? Should we even try? Can the patient’s trachea be intubated easily? Can the patient’s trachea be intubated with a laryngoscope? Experience shows that not all Mallampati Grade 1 airways14 are easily intubated, but also, not all Mallampati 3 or 4 airways require an awake or fiberoptic intubation.
At least two people should be present for the tracheal intubation of severe OSAHS patients, and at least one of them should be an experienced anesthesiologist. Familiarity with the ASA practice guidelines for management of the difficult airway,22 the difficult airway algorithm and the new practice guidelines for the management of patients with obstructive sleep apnea4 seem mandatory.
The importance of positioning, and in particular the ‘ramped’ position,21 has already been mentioned. Taking time to optimize the view is vital. Many anesthesiologists start the process in the prep-and-hold area, although others do it only in the operating room. The ‘sniffing’ position was the standard for many years, with up to 35 degrees flexion of the neck on the chest, up to 15 degrees extension of the facial plane and up to 85 degrees extension of the neck at the atlanto-occipital joint.26 Elevating the head even further, the ‘head-elevated’ laryngoscopy position, has been claimed27 to further improve the success rate of intubation. Many anesthesiologists, however, now believe that the ‘ramped’ position with blankets or other padding placed under the shoulders, head and neck is optimal. In some patients, however, no amount of position change improves ‘the view’.
Although succinylcholine seems to be the relaxant of choice for most patients with severe OSAHS, in some cases, where there is easy, comfortable mask ventilation after intravenous induction, some anesthesiologists may elect to give a non-depolarizing relaxant, usually rocuronium, for intubation. If the virtue of succinylcholine is its rapid onset and relatively rapid recovery, what is the virtue of rocuronium? Its slower onset necessitates longer mask ventilation with an inhalational anesthetic (sevoflurane). This can be advantageous in augmenting the relaxation, helping to guarantee amnesia, and raising the arterial oxygen tension (PaO2) even further. This, of course, assumes that the easy mask ventilation continues. A second benefit to the use of rocuronium is the prolonged relaxant effect, avoiding the problem of succinylcholine wearing off quickly, leading to less than optimal relaxation and failed intubation. The anesthesiologist must, of course, realize the ‘commitment’ he/she is making by using rocuronium, and having committed, not underdose. Relaxation with rocuronium also allows time for the use of the intubating LMA™ (LMA Fastrach™, LMA-North America, San Diego, CA) or for fiberoptic intubation, if necessary.
It is reasonable to have multiple laryngoscopes available. If possible, a Macintosh 3, Macintosh 4, and Miller and/or Phillips blades should be ready, each on its own handle to speed up the laryngoscopic process. The ETT should be styletted, with the stylet pushed down to the Murphy eye of the ETT, and the tip bent in a curve similar to a hockey stick. An Eschmann tube exchanger, sometimes called a gum elastic bougie, or a newer variation of this must also be within reach. An appropriately sized LMA or intubating LMA may also prove useful. Attempts at laryngoscopy must be done expeditiously and in a planned, orderly manner. The first attempt can be a longer and a more ‘exploratory’ look around. It is usually the anesthesiologist’s best attempt. If it fails, adjustments are made quickly. These adjustments may include changing the depth of anesthesia, the dose of muscle relaxant, the head and neck position, the type of laryngoscope blade, the lighting and the assistant. The anesthesiologist may attempt to optimize laryngeal view with external manipulations of the larynx and neck. Maneuvers such as BURP (backwards, upwards, rightward pressure) or OLM (optimal laryngeal manipulation) may make the larynx more visible.12 If necessary, the anesthesiologist should call for help from a colleague before a crisis arises. Once the airway is established, maintenance of anesthesia is routine.
3.1 EMERGENCE
Emergence after sleep apnea surgery is perhaps the most critical time for both the anesthesiologist and the patient. These patients, in particular, require extra vigilance because of their increased susceptibility to airway compromise and the deleterious effects of anesthetic medications given throughout the procedure.
Preparation for emergence from anesthesia and tracheal extubation begins when the surgeon approximates a time when the procedure will be completed. Usually, the volatile anesthetic is removed from the patient’s breathing circuit as the surgery comes to an end and the patient is kept anesthetized with nitrous oxide or intravenous propofol. There are several precautions the anesthesiologist should take before emergence from anesthesia and tracheal extubation. Some sleep apnea surgeries require manipulation of the ETT, so it is important to re-secure it after the surgery is completed. An oral airway should always be used to prevent obstruction by the tongue, which may or may not have been operated on. Some surgeons insert nasal trumpets that maintain a patent nasopharynx and may further assist with preventing airway obstruction. Although airway control is always of paramount importance to the anesthesiologist, OSAHS patients have an especially dynamic airway that requires extra support. Additional measures include insuring adequate reversal of all muscle relaxants, and adequate support staff to help control what is frequently a slow to awaken, agitated and aggressive postoperative patient. Tracheal extubation should never be rushed in OSAHS patients. When a patient tells you that it took six people to hold him down the last time he had surgery, believe him. One anesthesiologist and one nurse should not be expected to awaken a 130 kg patient who feels that he cannot breathe and must sit up or roll over.
The goal should be to awaken the patient with adequate spontaneous ventilation as soon as possible. The patient with OSAHS requires a cautious evaluation prior to tracheal extubation, including the demonstration of deliberate and appropriate responses to multiple commands. It is helpful to inform the patient in the preoperative area what they will be asked to do, and to confirm the name that they prefer to be called upon awakening. There are a number of ways to assess a postoperative patient and it is important to include several of these when considering extubation in OSAHS patients. The anesthesiologist’s goal is to determine the patient’s ability to protect their airway. More common methods include neck flexion or fist clenching for greater than 5 seconds, sustained tongue protrusion, and eye opening. Regardless of the technique used, the anesthesiologist looks for the ability of the patient to follow verbal commands repetitively; often the patient may raise his or her head for a very short time, but then falls back to sleep very quickly. ‘Do not interpret mindless reaching for the ETT as purposeful movement’30 The more difficult the tracheal intubation, the more patience is needed waiting for the tracheal extubation. The patient should be asked to nod if awake, nod if they want the ETT removed, attempt to head raise, etc. The return of rhythmic, spontaneous ventilation of good tidal volume is also sought. If there is any doubt, the anesthesiologist should leave the ETT in place.
Once the patient seems ready for extubation, the anesthesiologist should run through another last moment checklist. Check that the IV is running and has sufficient intravenous solution left in the bag. If not, flush it or re-establish it before proceeding or hang another bag. Make sure that succinylcholine is ready if needed, that the suction is still working, and that adequate help is present should trouble arise. Available airway devices on hand should include a nasopharyngeal airway, a new styletted ETT, and a tube exchanging device. The worst patients probably should have their tracheas extubated over either an Eschmann tube exchange catheter (rigid but solid) or a ventilating catheter (longer and hollow) (Cook Airway Exchange Catheter, Cook Medical Inc., Bloomington, IN) (Fig. 15.4). Both catheters, once placed into the trachea, are usually well tolerated provided that they are not pushed in too far. Each serves as an introducer to facilitate the potential re-insertion of an ETT, although neither can absolutely guarantee the success of that re-insertion attempt. The Cook catheter has the added advantage of being hollow, and having a 15 mm adaptor to allow for ventilation or oxygen insufflation of the lungs, should that be necessary. The amount of positive pressure that can be achieved, however, is limited due to the relatively small inner diameter of the Cook catheter.
Fig. 15.4 The difficult airway algorithm. From the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003;98(5):1269–77, with permission.
Rights were not granted to include this figure in electronic media. Please refer to the printed book.
When the patient is considered ready, the extubation procedure begins by moving the patient into a semi-upright position (approximately 45 degrees) to help move the tongue forward and the abdominal contents down and away from the diaphragm. After the throat is suctioned thoroughly, positive pressure is administered. The ETT is removed and 100% oxygen is given immediately via facemask, along with a jaw thrust. If a Cook catheter has been placed, the majority of patients are observed in the operating room for about 10 minutes and then extubated there. The worst cases may be transported to the PACU with the catheter in place and extubated later when they are fully awake. Some anesthesiologists believe that there is a role for doxapram (0.5–1.0 mg/kg) during emergence to stimulate ventilation and to help speed up awakening. The first minutes after tracheal extubation are crucial, as direct access to the airway has been lost, and postoperative edema and airway closure may ensue. Previously placed nasopharyngeal airway devices bypass the surgical site and decrease the patient’s work of breathing significantly. An oropharyngeal airway is not as well tolerated as a nasal, but may be used if needed. In addition, elevation of the head of the bed after surgery reduces soft tissue edema at the surgical sites and turbinate engorgement, thus improving the nasal airway diameter and airflow.
Two other immediate concerns after sleep apnea surgery (and tracheal extubation) are laryngospasm and obstruction secondary to local analgesic use and the presence of blood and other secretions. Although preoperative glycopyrrolate and judicious postoperative suctioning minimize the risk, laryngospasm may still occur. Treatment should include head extension, anterior displacement of the mandible, and positive airway pressure. If these maneuvers fail to stabilize the airway, direct laryngoscopy with tracheal reintubation is necessary; cricothyrotomy may be performed on rare occasions. Local anesthetics like bupivacaine and lidocaine are frequently used in sleep apnea surgeries and may exhibit unwanted side effects. For example, tongue ablation procedures require the injection of local anesthetic into the tongue base to diminish postoperative pain. However, when the operation is over, patients can experience difficulty with tongue proprioception, leading to feelings of obstruction and possible airway impediment. Minimizing the dose of local anesthetics, postoperative patient reassurance, and meticulous postoperative airway management are important.
4 POSTOPERATIVE CARE
The anesthesiologist’s primary concerns for the postoperative period center on the patency of the airway and the adequacy of ventilation and oxygenation. Potential edema, bleeding, or opioid-induced airway obstruction/hypoventilation are the factors that have led to a tendency to monitor these patients in the intensive care unit (ICU), but recent studies indicate ICU admissions may not be necessary for all patients. Significant complications generally emerge within the immediate postoperative period. Accordingly, a patient’s status should be evaluated frequently in the postanesthesia care unit and the level of postoperative care should be decided upon then. If in doubt, the conservative approach is to admit the patient to a surgical intensive care unit.
Airway edema is a major concern for patients undergoing surgical repair for OSAHS. Small airways compounded with surgical trauma or a difficult intubation put OSAHS patients at a higher risk for airway compromise. It is of paramount importance for both the anesthesiologist and the surgeon to make all attempts at reducing airway edema in patients undergoing OSAHS surgery. Preoperative and postoperative intravenous steroids have routinely been shown to be effective in the reduction of airway edema. Dexametasone (8–12 mg), which is administered just prior to surgery, should be repeated every 8 hours postoperatively (8 mg).
Adequate blood pressure control is very important in the management of a postoperative OSAHS patient. Hypertension is more commonly seen in OSAHS patients secondary to their heightened sympathetic drive. Consequently, elevated blood pressure leads to more bleeding and increased tissue swelling. Although induced hypotension is not currently used for OSA surgeries, the patient’s blood pressure should be managed prior to the procedure and controlled throughout the perioperative period. Another aspect of postoperative edema control is hemostasis. Current options for managing intraoperative bleeding are electrocautery, vessel ligature, thrombin on absorbable gelatin sponge (Gelfoam®, Pharmacia & Upjohn Co., Kalamazoo, MI), or a topical gelatin hemostatic matrix (Floseal, Baxter Bioscience, Fremont, CA). Finally, other means of controlling postoperative edema include elevating the head of the bed to improve venous return and tissue cooling with ice packs and ice chips.
Postoperative pain management for OSAHS patient is another topic with a debated and well-studied history. Ideally, the selected analgesic should have minimal effect on respiratory drive and airway muscle tone while supplying strong, adequate pain control. In addition, it is important to remember the sedatives and narcotics used throughout the case, because of their lingering analgesic and depressant effects on the patient. Unfortunately, the spectrum of pharmaceutical options for analgesia ranges from minimal pain relief with a low incidence of side effects, to good pain relief with a higher incidence of side effects. Non-steroidal anti-inflammatory drugs (NSAIDs) are usually inadequate for postoperative pain control, and standard narcotic dosing or patient-controlled analgesic infusions (PCAs) put the patient at increased risk of obstructive complications. One regimen found to be effective involves lower dosed intravenous narcotics for immediate pain control and oral hydrocodone or acetaminophen with codeine once the patient resumes eating.
1. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235.
2. van Boxem TJ, de Groot GH. Prevalence and severity of sleep disordered breathing in a group of morbidly obese patients. Neth J Med. 1999;54(5):202-206.
3. Leiter JC. Upper airway shape: is it important in the pathogenesis of obstructive sleep apnea? Am J Respir Crit Care Med. 1996;153(3):894-898.
4. Benumof JL. Obstructive sleep apnea in the adult obese patient: implications for airway management. J Clin Anesth. 2001;2(3):144-156.
5. Gross JB, Bachenberg KL, Benumof JL, et al. American Society of Anesthesiologists Task Force on Perioperative Management. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104(5):1081-1093. quiz 1117–8
6. Orr WC. Sleep apnea, hypoxemia, and cardiac arrhythmias [Editorial]. Chest. 1986;89(1):1-2.
7. Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: facts and theory. Am J Med. 1995;98(2):118-128.
8. Schafer H, Hasper E, Ewig S, Koehler U, Latzelsberger J, Tasci S, Luderitz B. Pulmonary haemodynamics in obstructive sleep apnoea: time course and associated factors. Eur Respir J. 1998;12(3):679-684.
9. Berman EJ, DiBenedetto RJ, Causey DE, Mims T, Conneff M, Goodman LS, Rollings RC. Right ventricular hypertrophy detected by echocardiography in patients with newly diagnosed obstructive sleep apnea. Chest. 1991;100(2):347-350.
10. Wilson ME, Spiegelhalter D, Robertson JA, Lesser P. Predicting difficult intubation. Br J Anaesth. 1988;61(2):211-216.
11. Brodsky JB, Lemmens HJ, Brock-Utne JG, Vierra M, Saidman LJ. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94(3):732-736.
12. Vanner RG, Clarke P, Moore WJ, Raftery S. The effect of cricoid pressure and neck support on the view at laryngoscopy. Anaesthesia. 1997;52(9):896-900.
13. el-Ganzouri AR, McCarthy RJ, Tuman KJ, Tanck EN, Ivankovich AD. Preoperative airway assessment: predictive value of a multivariate risk index. Anesth Analg. 1996;82(6):1197-1204.
14. Mallampati SR, Gatt SP, Gugino LD, Desai SP, Waraksa B, Freiberger D, Liu PL. A clinical sign to predict difficult tracheal intubation: a prospective study. 1985;32(4):429–34.
15. Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42(5):487-490.
16. Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131(1):41-45.
17. VanDercar DH, Martinez AP, De Lisser EA. Sleep apnea syndromes: a potential contraindication for patient-controlled analgesia. Anesthesiology. 1991;74(3):623-624.
18. Lamarche Y, Martin R, Reiher J, Blaise G. The sleep apnoea syndrome and epidural morphine. Can Anaesth Soc J. 1986;33(2):231-233.
19. Jense HG, Dubin SA, Silverstein PI, O’Leary-Escolas U. Effect of obesity on safe duration of apnea in anesthetized humans. Anesth Analg. 1991;72(1):89-93.
20. Ferretti A, Giampiccolo P, Cavalli A, Milic-Emili J, Tantucci C. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119(5):1401-1408.
21. Collins JS, Lemmens HJ, Brodsky JB, Brock-Utne JG, Levitan RM. Laryngoscopy and morbid obesity: a comparison of the ‘sniff’ and ‘ramped’ positions. Obes Surg. 2004;14(9):1171-1175.
22. American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98(5):1269-1277.
23. Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology. 2005;102(6):1110-1115. discussion 5A.
24. Teller LE, Alexander CM, Frumin MJ, Gross JB. Pharyngeal insufflation of oxygen prevents arterial desaturation during apnea. Anesthesiology. 1988;69(6):980-982.
25. Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39(11):1105-1111.
26. Benumof JL. Difficult laryngoscopy: obtaining the best view. Can J Anaesth. 1994;41(5 Pt 1):361-365.
27. Levitan RM, Mechem CC, Ochroch EA, Shofer FS, Hollander JE. Head-elevated laryngoscopy position: improving laryngeal exposure during laryngoscopy by increasing head elevation. Ann Emerg Med. 2003;41(3):322-330.
28. Benyamin RM, Wafai Y, Salem MR, Joseph NJ. Two-handed mask ventilation of the difficult airway by a single individual. Anesthesiology. 1998;88(4):1134.
29. El-Orbany MI, Joseph NJ, Salem MR. Tracheal intubating conditions and apnoea time after small-dose succinylcholine are not modified by the choice of induction agent. Br J Anaesth. 2005;95(5):710-714.
30. Benumof JL. Obesity, sleep apnea, the airway and anesthesia. Abstracted summary of Audio-Digest Anesthesiol. 2004;46(247):221.