Periodontal Considerations in the Evaluation and Treatment of Dentofacial Deformities
Teeth
General Information (Fig. 6-1)
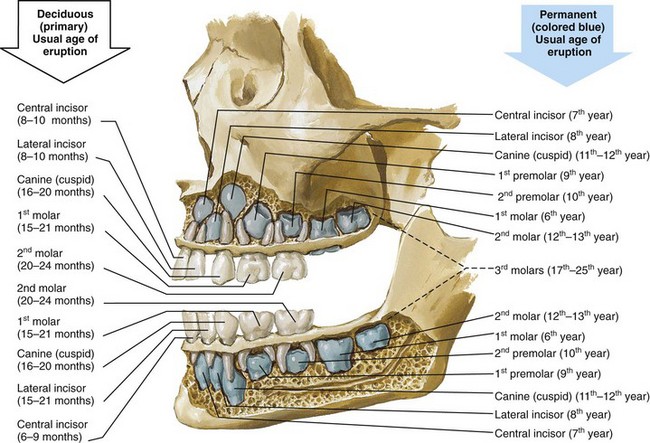
Figure 6-1 Illustration of a sagittal view of a maxilla and mandible with the outer cortex of bone removed to demonstrate a fully erupted deciduous (primary) dentition and the location of the developing permanent tooth buds. Also indicated on the illustration are the usual ages of dental eruption for each tooth of both the deciduous and the permanent dentitions. From www.netterimages.com. © Elsevier Inc. All rights reserved.
Descriptions of the Surfaces of the Teeth (Fig. 6-2)
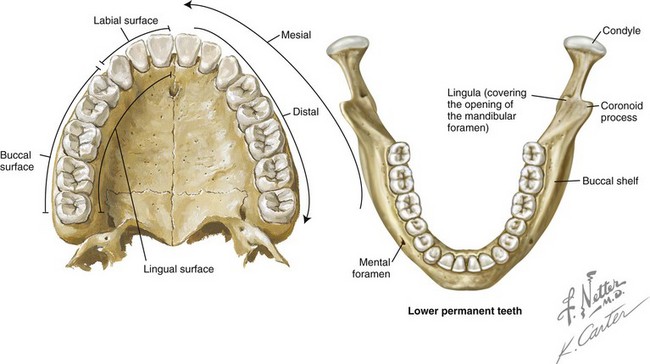
Figure 6-2 Illustrations of the fully erupted permanent dentition in the maxilla and mandible. The palate and the lingual view illustration are used to define terminology for the surfaces of a tooth. From www.netterimages.com. © Elsevier Inc. All rights reserved.
Labial: The surface of the anterior teeth that is closest to the lip
Buccal: The surface of the posterior teeth that is closest to the cheek
Facial: A synonym for labial or buccal
Lingual: Opposite the tongue in the mandibular arch and opposite the hard palate in the maxillary arch
Mesial: Closest to the midline of the dental arch
Distal: Farthest from the midline of the dental arch
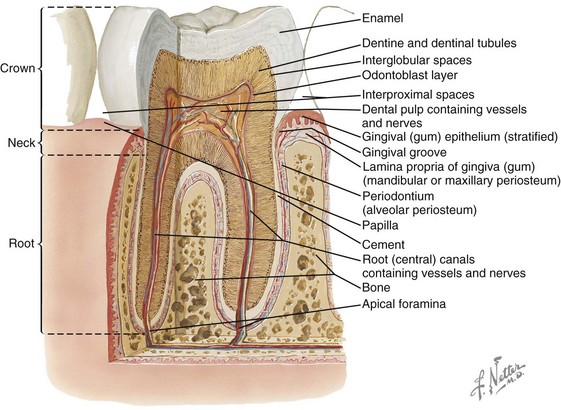
Basic Anatomy of a Tooth (Fig. 6-3)
Crown
Dentin
• The dentin is the hard tissue that underlies both the enamel and the cementum and that constitutes the major portion of the tooth. Dentin is a modification of osseous tissue. It is composed of a number of dental tubules (small, wavy, and branching tubes) that are located in a dense matrix.
• Dentin is 70% inorganic material by weight, 20% organic material, and 10% water.
The Tissues of the Periodontium
The periodontium includes the investing and supporting tissues of the teeth. It consists of two parts: the attachment apparatus (the cementum, the alveolar bone, and the intervening PDL) and the dentogingival unit (the gingival connective tissue that inserts into the supracrestal cementum and the sulcular and junctional epithelium as well as the latter’s attachment to enamel) (Fig. 6-4). The cementum covers the root surfaces. It serves as the attachment for the fibers of the PDL to the tooth as does the (cortical) alveolar bone of the alveolar socket. The periodontium is affected by the individual’s unique maxillofacial skeletal anatomy, by functional (occlusal) forces, and by degenerative cellular changes that occur with age. The greatest modifier of the periodontium is inflammatory disease.
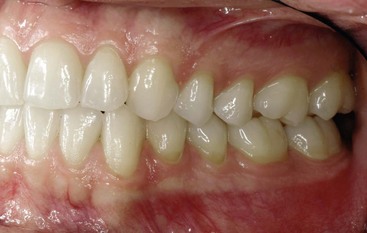
Figure 6-4 Intraoral view demonstrating the normal width of attached gingiva in the human permanent dentition. From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 2-3.
The oral epithelium consists of three zones (Figs. 6-5 and 6-6):
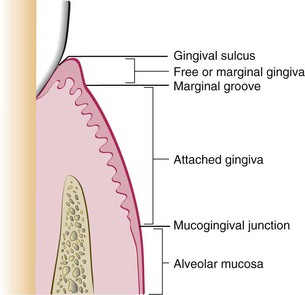
Figure 6-5 Diagram showing the anatomic landmarks of the gingiva. From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 2-2.
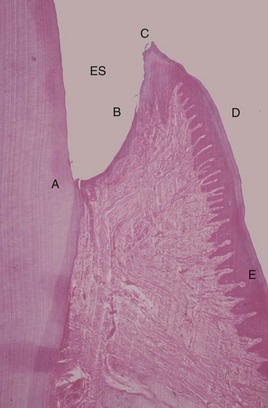
Figure 6-6 Histologic appearance of healthy gingiva. A photomicrograph of a demineralized tooth with the gingival tissues in situ (hematoxylin and eosin stain, low magnification). The amelocemental junction (A) and the enamel space (ES) are shown. Gingival health is characterized by the organization of the epithelium into distinct zones: the junctional epithelium (A to B), the sulcular epithelium (B to C), the free gingiva (C to D), and the attached gingiva (D to E). The gingival connective tissue is composed of densely packed, organized, and interlacing collagen bundles. There are a few scattered inflammatory cells, but there is no significant inflammatory cell infiltrate. From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 21-1.
1. Masticatory mucosa (keratinized tissue): This includes the gingiva, the hard palate, and the dorsal surface of the tongue. The gingiva is a fibrous investing tissue that immediately surrounds and is contiguous with its PDL and with the mucosal tissues of the mouth. Keratinized epithelium also covers the hard palate.
• Attached gingiva: This is the portion of the gingiva that is firm, dense, stippled, and tightly bound to the underlying periosteum, tooth, and bone.
• Free gingiva: This part of the gingiva surrounds the tooth and is not directly attached to the tooth surface.
• Marginal gingiva: This is most coronal portion of the gingiva. This term is often used to refer to the free gingiva that forms the wall of the gingival crevice in a normal, healthy mouth.
• Crevicular (sulcular) epithelium: This is the non-keratinized epithelium of the gingival crevice.
• Junctional epithelium: This involves a single layer or multiple layers of non-keratinized cells that adhere to the tooth surface at the base of the gingival crevice. This was formerly called the epithelial attachment.
2. Lining mucosa (non-keratinized tissue): This epithelium makes up the majority of the mucosa of the mouth and is the primary lining of the oral mucosa.
3. Specialized mucosa: This is found specifically in the regions of the taste buds on the dorsum of the tongue.
The gingiva is the part of the oral mucous membrane that covers the occlusal aspect of the alveolar processes of the jaws and that surrounds the necks of the teeth. The junctional epithelium and the connective tissue attachment have a characteristic dimension of 2 to 4 mm; this zone of tissue is called the biologic width. In general, an individual can only maintain health when the sulcus depth is no more than 3 to 4 mm. Greater depth provides a safe haven for bacteria that cannot be easily removed through normal oral hygiene maneuvers. A lower-than-average alveolar crest level is acceptable as long as it is stable (i.e., not progressive) and the periodontium is free of active disease. On the palatal surface of the teeth, the attached gingiva blends with the equally firm and highly keratinized palatal (masticatory) mucosa. The interdental gingiva occupies the gingival embrasure (i.e., the interproximal space beneath the area of tooth contact). It consists of the facial papilla, the lingual papilla, and the valley-like depression that connects the two in the interproximal contact area, which is called the col. In the absence of proximal tooth contact, the gingiva is firmly bound over the interdental bone; it forms a smooth, round surface without a triangular interdental papilla. The connective tissue of the gingiva is densely collagenous, and it contains collagen fiber bundles called gingival fibers (Fig. 6-7). When these fiber bundles insert into the tooth’s cementum, they are called Sharpey’s fibers, and they result in the mechanical attachment of the sulcular and supracrestal (i.e., crestal to the alveolar process) gingiva. These fibers are able to withstand masticatory forces without deflection or detachment from the tooth surface. The connective tissue attachments extend from just apical to the junctional epithelium of the sulcular (crevicular) gingiva to the supracrestal cementum of the root.
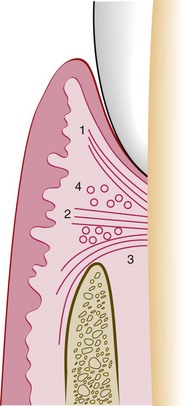
Figure 6-7 Diagram of the gingival dental fibers that extend from the cementum (1) to the crest of the gingiva, (2) to the outer surface, and (3) external to the periosteum of the labial plate. Circular fibers (4) are shown in cross-section. From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 2-20.
Aging and the Periodontium
The prevalence of periodontal disease with tissue destruction and the loss of teeth and tooth structure tends to increase with age.106,155,160,171 Another consequence of age is reduced tissue elasticity through the degeneration of the elastic fibers. Hormonal changes also occur, and these change the local tissue environment. Traditional thinking is that the degenerative gingival changes associated with aging may include recession, diminished keratinization, reduced stippling, decreased connective tissue cellularity, increased intercellular substances, and reduced oxygen consumption. In the PDL, degenerative aging effects are thought to include a decrease in elastic fibers and a decrease in vascularity and mitotic activity. If these effects occur, the ability of the alveolar bone to withstand occlusal forces is diminished. Frequent changes in tooth structure with age are seen, including occlusal wear with a loss of enamel substance that reduces cusp height and inclination. The degree of attrition is influenced by the masticatory musculature, the consistency of the food eaten, and occlusal factors and habits such as clenching and bruxism. A degree of continued tooth eruption usually occurs as teeth wear. As a result, the clinical crown may become longer, which creates further leverage on the bone with masticatory forces. An opposing factor is the fact that the clinical crowns are simultaneously reduced through attrition, often with an equilibrium or balance being present between the teeth and their bony support. The wear of the teeth along the proximal surfaces may also occur, which results in mesial migration. On average, proximal wear reduces the anteroposterior length of the dental arch by approximately 5 mm by the age of 40 years and by twice that by life’s end. When chronic periodontal disease is added to physiologic degenerative aging, the destructive response of the periodontium is exacerbated. There may be continued gingival recession, attrition, and the reduction of alveolar bone height as a result of a combination of these factors.31
Etiology of Periodontal Disease
Gingivitis and Periodontitis
Inflammation of the gingiva, which is also known as gingivitis, is the most common form of gingival disease (Figs. 6-8 through 6-12).1,19,177,183,216 This occurs as a result of local irritants, such as the toxins released by the microorganisms related to dental plaque and calculus. Foreign bodies (e.g., orthodontic appliances, irregular dental restorations) may also serve as local irritants as well as plaque traps. The inflammation caused by local irritants can result in ulcerative, necrotic, and proliferative changes in the gingival tissues.29,30,34,99,165,170,221 When there is deepening of the gingival sulcus, there can be injury to the supporting periodontal tissues; this may become an irreversible process. With continued inflammation, hyperplastic changes of the gingiva occur, and the crest of the gingival margin extends toward the crown. Inflammation causes a proliferation of and a change in the quality of the sulcus and the junctional epithelium such that their normal protective nature becomes dysfunctional. The sulcus becomes a pocket; ulceration through the epithelial barrier with exposure of the underlying connective tissue to the oral cavity is a frequent occurrence. The organisms and their toxins are then able to access the exposed connective tissue, which undergoes further pathologic changes.93,133,135,154,193 As the process continues, the epithelial junction may separate from the root, and the pocket will migrate downward. The epithelium of the lateral wall of the pocket proliferates with inflammatory tissue, which results in varying degrees of degeneration and necrosis. Intrabony periodontal pockets are said to be present when the base is apical to the level of the alveolar bone. The extension of inflammation from the margin of the gingiva into the supporting periodontal tissues marks the transition from gingivitis to periodontitis.6,40,41,107,117,175,200 The essential problem of periodontal disease is the destruction of alveolar bone with a loss of crestal height and PDL destruction. If periodontal disease is left untreated, it will lead to the loosening and loss of the teeth.
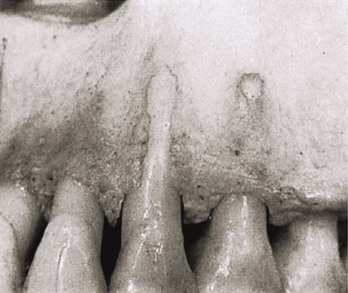
Figure 6-8 Photograph of the maxillary anterior dentoalveolar region. Dehiscence of the labial cortical plate of the canine and fenestration of the labial cortical plate of the first premolar are demonstrated. From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 2-60.
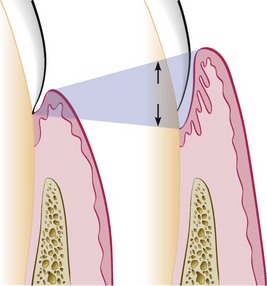
Figure 6-9 Illustration of pocket formation that indicates expansion in two directions (arrows) from the normal gingival sulcus (left) to the periodontal pocket (right). From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 13-1.
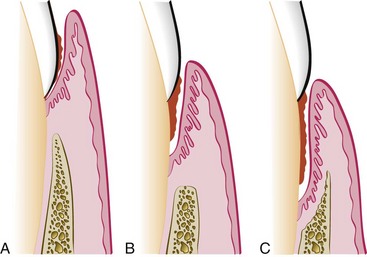
Figure 6-10 Different types of periodontal pockets. With a gingival pocket (A), there is no destruction of the supporting periodontal tissues. With a suprabony pocket (B), the base of the pocket is coronal to the level of the underlying bone, and bone loss is horizontal. With an intrabony pocket (C), the base of the pocket is apical to the level of the adjacent bone, and bone loss is vertical. From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 13-2.
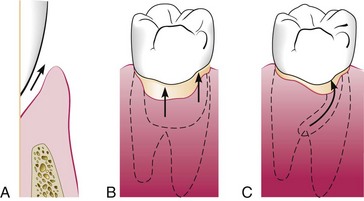
Figure 6-11 Classification of pockets according to involved tooth surfaces. A, Simple pocket. B, Compound pocket. C, Complex pocket. From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 13-3.
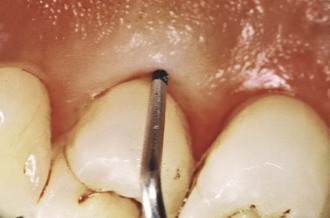
Figure 6-12 Probing of a deep periodontal pocket. The entire length of the periodontal probe has been inserted into the base of a pocket in the palatal surface of the first premolar. From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza’s clinical periodontology, ed 11, St. Louis, 2012, W.B. Saunders Company, Figure 13-4.
Trauma from Occlusion
Occlusal forces affect the condition and structure of the periodontium.53,54,59,104,129,134,160,181,191,204 To remain structurally and metabolically sound, the PDLs and the alveolar bone require the mechanical stimulation of occlusal forces. When occlusal forces exceed the adaptive capacity of the tissue, injury occurs. The injury that occurs to the periodontium is called trauma from occlusion, and it can be classified as either primary or secondary occlusal trauma. Primary occlusal trauma occurs when greater-than-normal occlusal forces are placed on teeth with a normal periodontal attachment apparatus (i.e., those that are periodontally stable). Secondary occlusal trauma occurs when normal occlusal forces are placed on teeth with compromised periodontal attachment (i.e., those with periodontal disease).
Effects of Orthodontic Appliances and Tooth Movement Forces
Tooth movement during orthodontic therapy is the result of controlled forces placed on the teeth and then transmitted to the PDL. Strong or heavy forces (i.e., forces that far exceed capillary blood pressure) result in the crushing of the PDL on the compression side of the tooth, with local ischemia and degeneration (i.e., hyalinization). Moderate forces that exceed capillary blood pressure result in the compression of the PDL with a delay in bone resorption and the movement of the tooth.89 Light continuous forces that are less than the capillary blood pressure result in only limited ischemia to the PDL, with gradual bone resorption on the compression side.174 The patient’s age is not a contraindication to orthodontic treatment per se. Interestingly, in the adult, the hyalinized (necrotic) zones are formed more readily on the pressure side of the orthodontically moving tooth; this will temporarily slow tooth movement.62 The hyalinized zone is soon eliminated with the reorganization of the tissues, first through the resorption of the marrow spaces (thus undermining resorption) and then through the repair of the PDL and finally of the alveolar bone.172 The anticipated regeneration of the PDL on the compression side and the formation of new bone on the tension side will likely be hampered by the presence of active inflammation in the periodontal tissues (i.e., periodontitis).169 This pathologic response is dependent on how long the PDL remains compromised. This is the reason why inflammation should be controlled through effective periodontal treatment before orthodontic tooth movement.*
Until the mid 1980s, heavy intermittent orthodontic forces were routinely used, and this required patient visits every 3 to 4 weeks.176 This allowed the hyalinized fibers to recover before another heavy orthodontic force was applied. Contemporary orthodontics involves the use of light, continuous force. This moves the teeth with less discomfort and more rapidly, and it also allows visits to be spaced at longer intervals.
In a patient with a periodontally compromised dentition and with a baseline loss of alveolar bone, the center of resistance of the involved teeth moves apically.141,157 The net effect is that the involved teeth are more prone to tipping rather than to bodily movement when orthodontic forces are applied. To achieve improvement in the periodontium, orthodontic treatment requires a combination of light controlled forces as well as the movement of teeth more completely into the alveolar housing. In the presence of active disease, orthodontic therapy should be postponed until effective periodontal treatment is accomplished. This approach to orthodontic tooth movement has been shown to improve any preexisting compromised periodontium.
Teeth that are already tipped outside of the cortical plate (e.g., proclined mandibular incisors in the individual with Class II malocclusion) and that are orthodontically uprighted into sound alveolar housing are likely to improve in overall periodontal health, even when the gingiva levels remain borderline. Animal studies indicate that, without the presence of plaque, orthodontic forces on the teeth do not in themselves induce gingivitis.67 In the presence of plaque, however, similar forces can cause angular bone defects and, with tipping or torquing movements, gingival attachment loss (i.e., recession) can occur.68–70 Clinical studies have demonstrated that, with adequate plaque control, even teeth with longstanding reduced periodontal support can undergo successful tooth movement without further compromise.63 In patients with no active periodontal disease and with good oral hygiene—and even in adults with reduced but healthy residual periodontium—physiologic orthodontic treatment causes no significant detrimental long-term effects on the periodontal attachment, including the bone levels. Physiologic tooth movement involves light forces and the movement of teeth into (not outside of) alveolar bone.37–39
In a cross-sectional study, radiographic crestal bone levels in adults (N = 104) who completed orthodontic treatment at least 10 years previously were shown to be no different than those of matched control subjects (N = 76).161 In a 2-year post orthodontic study, Trosello and colleagues compared adult women who had multi-banded orthodontic therapy (N = 30) with age-matched (non-orthodontically treated) controls (N = 30).203 It was found that the orthodontically treated patients had a higher prevalence of root resorption (17% versus 2%), although there was a lower prevalence of mucogingival defects (5% versus 12%). The root resorption differences were most common in the maxillary incisors, followed by the mandibular incisors. It appears that, in adults, when biologically sound orthodontic maneuvers are carried out, minimal detrimental effects on the health of the periodontium occur. In the short term, gingivitis and gingival hyperplasia may occur, but there is no attachment loss or irreversible effects. In the long term, when the teeth are moved into (not out of) the alveolar bone, mild root resorption (i.e., 1.0 to 1.5 mm) may be documented, but attachment loss (i.e., irreversible change) only occurs in areas of active periodontitis.
It is known that plaque is the primary etiologic factor of gingivitis. A patient’s inability to clean adequately around orthodontic devices (e.g., banded teeth, brackets, wires, springs, coils, elastics, plates) will promote plaque accumulation, which can lead to gingival inflammation. Before the extensive use of bonded brackets, overgrowth of anaerobes in the patient’s sulcus was typical.57 Fortunately, the common practice of placing numerous subgingival orthodontic bands in each quadrant has gone by the wayside. Nevertheless, a shift in the subgingival microflora to a more pathogenic population that is similar to what is seen in periodontal disease sites may occur with use of orthodontic devices.131 Even without banded bracket appliances, an active and diligent oral hygiene program for the patient that includes frequent periodic checkups by an appropriate dentist is required throughout orthodontic treatment.
Dental Restorations: Gingival Interface
The relationship between bacterial plaque accumulation and gingival inflammation has been documented since at least the 1960s.132 A patient’s susceptibility to gingival inflammation is not based solely on the mere quantity of dental plaque but also on the virulence of the resident plaque microorganisms. The bacterial composition of dental plaque is dynamic, and the pathogenicity of each specific organism is subject to change over time. It is a dental dictum that creating a confluent restorative–gingival interface is an important factor that allows patients’ oral hygiene efforts to reduce the accumulation of plaque and microorganisms and to decrease—if not eliminate—the resulting gingivitis. For this reason, emphasis should be placed on the marginal integrity of dental restorations; the coronal contour of the restoration; the embrasure and contact; and the marginal location of the restoration. The proper marginal location of a restoration relative to the alveolar bone is thought to be one of the most important parameters for achieving and maintaining long-term gingival health.92,124–126,139 Similarly, all patients who are undergoing dental therapy should be philosophically taught and technically trained to perform effective plaque-removal practices.
Assessing the Individual’s Biologic and Anatomic Risk Factors
Destruction of the periodontium is more likely to occur in the presence of known risk factors. Specific risk factors include 1) infection, 2) primary occlusal trauma, 3) iatrogenic causes, and 4) intrinsic biologic anatomic aspects. The known intrinsic (unique to the individual) biologic anatomic risk factors for periodontal disease may be referred to as the individual’s biotype (Figs. 6-13 through 6-29). These factors include the following:
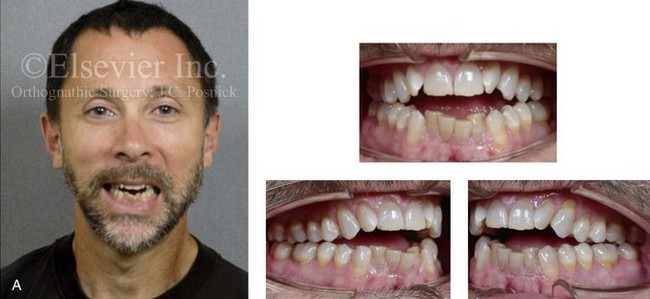
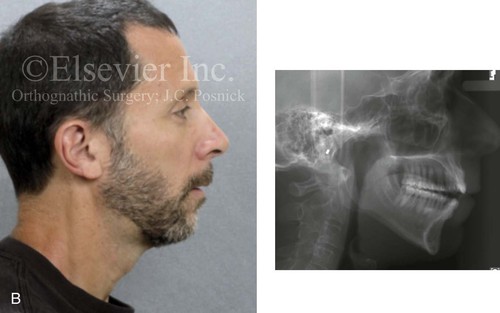
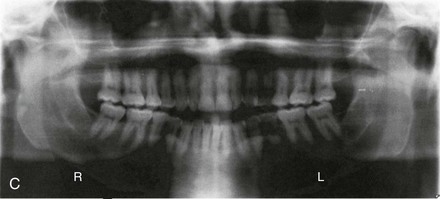
Figure 6-13 A 50-year-old man was referred by his general dentist to an orthodontist, who then referred the patient for surgical evaluation. The patient had a history of juvenile rheumatoid arthritis with temporomandibular joint involvement. There is condylar erosion with loss of posterior facial height with retrusion and clockwise rotation of the mandible, which resulted in a Class II anterior open-bite malocclusion. Adequate painless vertical mouth opening is present, and no symptomatic temporomandibular disorder is present. Over the years, there has been destruction of the dentition and the periodontal apparatus with a degree of labial bone loss and gingival recession. At referral to this surgeon, the patient had a history that documented heavy snoring, restless sleeping, and daytime somnolence. An attended polysomnogram confirmed a respiratory disturbance index of 10 events per hour. There is intranasal obstruction as a result of septal deviation and inferior turbinate enlargement, a retropositioned palate as a result of maxillary retrusion, and a retropositioned tongue as a result of mandibular retrusion. The soft palate and the tongue are normal with regard to function and size. The patient had previously undergone tonsillectomy. His biologic and anatomic risk factors for periodontal disease include inadequate alveolar bone (i.e., an inadequate alveolar bone/dental root ratio); nasal obstruction and mouth breathing with lip incompetence; and malocclusion with secondary trauma. A comprehensive approach was recommended to address the patient’s upper airway and breathing difficulties, his occlusion issues and his long-term dental health, and his facial aesthetics. A, Frontal facial and occlusal views before treatment. B, Profile facial view and lateral cephalometric radiograph before treatment. C, Panorex radiograph before treatment.
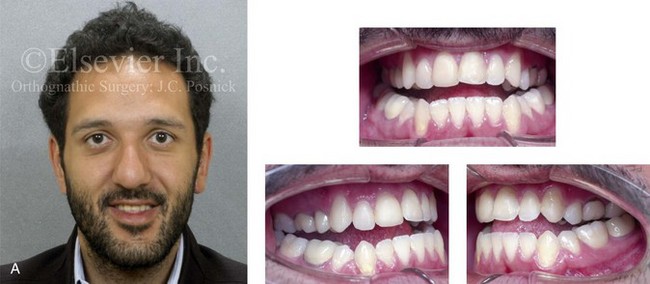
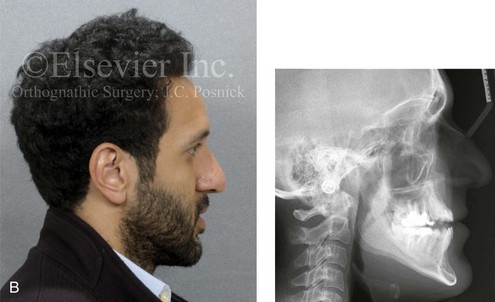
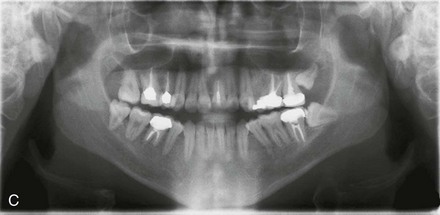
Figure 6-14 A 26-year-old college graduate arrived for surgical evaluation. Since the mixed dentition, he was recognized to have a developmental jaw deformity that was characterized by maxillary deficiency in combination with relative mandibular excess. Camouflage orthodontic treatment including four bicuspid extractions was carried out when he was between 12 and 16 years old. A residual Class III negative overjet with an anterior open-bite malocclusion remains. The patient had never been able to breathe well through his nose as a result of septal deviation and inferior turbinate enlargement. Secondary occlusal trauma resulted in the need for root canal therapy and crowns in all four posterior quadrants. There is labial bone loss and gingival recession. A comprehensive dental rehabilitative and reconstructive approach will require periodontal evaluation and treatment followed by orthodontic (dental) decompensation and then jaw and intranasal surgery. A, Frontal facial and occlusal views before treatment. B, Profile facial view and lateral cephalometric radiograph before treatment. C, Panorex radiograph before treatment.
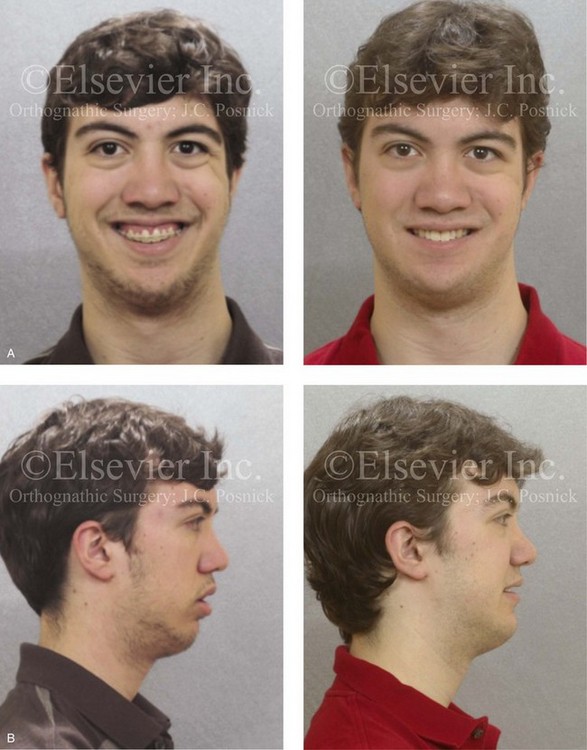
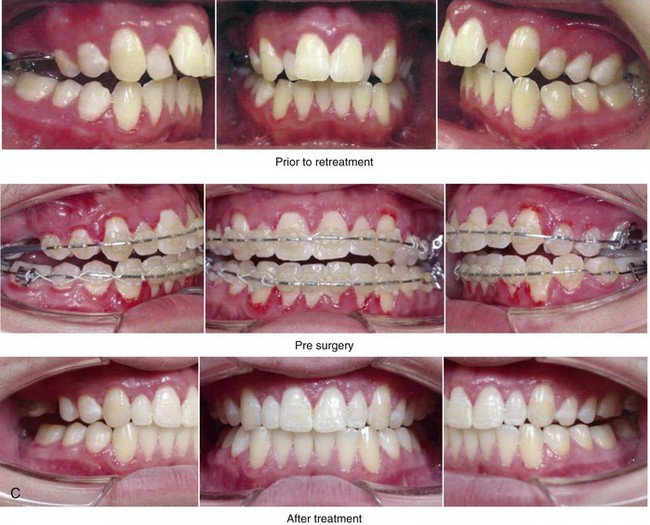
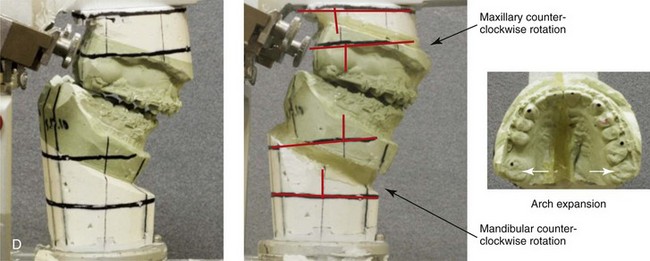
Figure 6-15 A 16-year-old boy was born with Stickler syndrome, which is a type II collagen mutation. At the time of his birth, the Pierre-Robin sequence was appreciated. The patient underwent repair of the cleft palate before he was 1 year old. He had positive eye findings and required the treatment of a retinal detachment during his teenage years; a small cataract is being followed. He arrived for the evaluation of a jaw deformity and malocclusion, which were characterized by maxillomandibular deficiency with anterior open-bite malocclusion. This was combined with chronic obstructive nasal breathing and a long face growth pattern. Attempted growth modification and camouflage orthodontics earlier in life were ineffective. There was generalized root deficiency throughout the maxillary and mandibular dentition, likely as a result of the collagenopathy. The patient agreed to an orthodontic and surgical approach. Further orthodontic (dental) decompensation was cautiously carried out as a result of the compromised periodontal apparatus. The procedures included maxillary Le Fort I osteotomy in segments (vertical intrusion, horizontal advancement, and arch expansion); bilateral sagittal split ramus osteotomies (horizontal advancement and limited counterclockwise rotation); osseous genioplasty (vertical shortening and horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views with smile before and after treatment. B, Profile views before and after treatment. C, Occlusal views before retreatment, with orthodontics in progress, and then after treatment. D, Articulated dental casts that indicate analytic model planning.
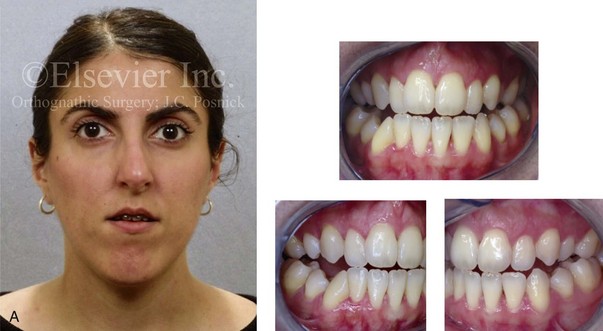
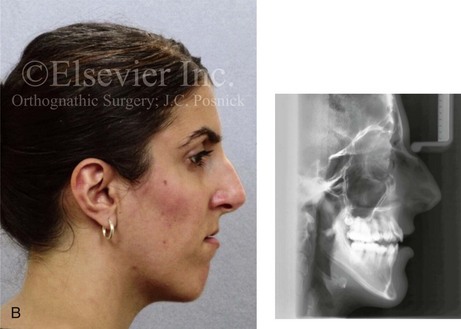
Figure 6-16 A woman in her late 20s was born with an isolated cleft palate. She underwent palate repair during infancy, and she later developed a jaw deformity with malocclusion. Attempts to neutralize the occlusion included years of growth modification and orthodontic mechanics with four bicuspid extractions between the ages of 11 and 17 years. The patient was left with generalized labial bone loss and gingival recession, especially of the lower anterior teeth. She presented to this surgeon as an adult with a lifelong history of obstructed nasal breathing. She also had a long face growth pattern that involved the maxilla, the mandible, and the chin and that included excess vertical height and horizontal retrusion of those areas. She underwent periodontic, prosthodontic, orthodontic, surgical, speech pathology, and ear, nose, and throat evaluations, among others. She received periodontal treatment and then orthodontic decompensation. Her surgery included a Le Fort I osteotomy (horizontal advancement and clockwise rotation); bilateral sagittal split ramus osteotomies (horizontal advancement and counterclockwise rotation); osseous genioplasty (vertical reduction and horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal floor recontouring (see Fig. 34-9). A, Frontal facial and occlusal views before retreatment. B, Profile view and lateral cephalometric radiograph before retreatment.
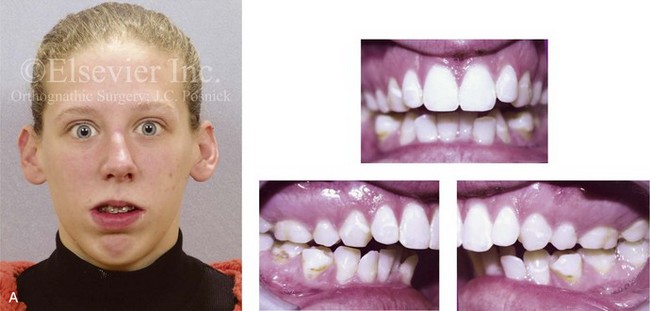
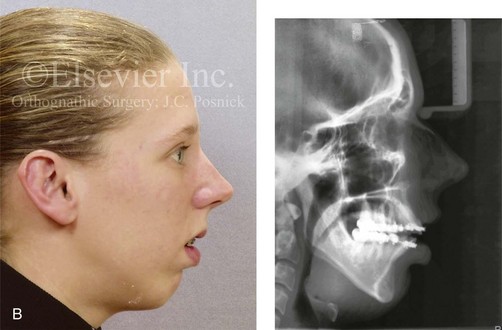
Figure 6-17 A 21-year-old college graduate arrived for surgical evaluation. The history and examination confirmed lifelong nasal obstruction, lip incompetence, and gummy smile. Further inspection confirmed a long face growth pattern with a Class II anterior open-bite malocclusion. The patient had been under an orthodontist’s care since the mixed dentition and throughout her high school years. Growth modification, rapid palatal expansion, and full bracketing with four bicuspid extractions introduced dental compensation but without successful closure of the open bite. Early periodontal deterioration is evident. She agreed to a comprehensive dental and facial rehabilitation approach. Periodontal evaluation and treatment followed by orthodontic (dental) decompensation and then jaw and intranasal surgery were carried out (see Figure 21-5). A, Frontal view in repose and occlusal views before retreatment. B, Profile view and lateral cephalometric radiograph before retreatment.
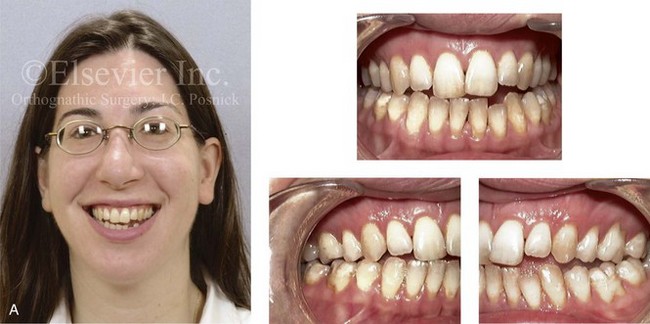
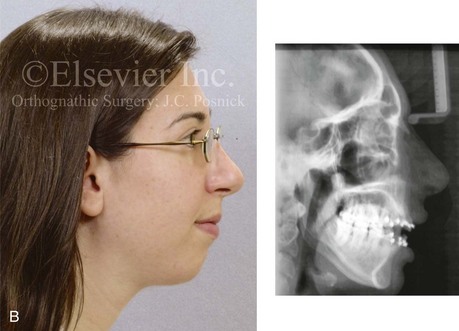
Figure 6-18 A woman in her mid 30s was referred by her orthodontist for surgical evaluation. She had a lifelong history of obstructed nasal breathing and a long face growth pattern. Throughout her mid childhood and teenage years, she had undergone attempted growth modification followed by orthodontic camouflage, which included four bicuspid extractions with an attempt to neutralize the occlusion. There is periodontal deterioration and dental relapse with residual malocclusion. The patient agreed to a comprehensive surgical and dental rehabilitative approach. Periodontal treatment was followed by orthodontic (dental) decompensation. Her surgery included maxillary Le Fort I osteotomy in segments (horizontal advancement, vertical shortening, counterclockwise rotation, arch expansion, and curve of Spee correction); bilateral sagittal split ramus osteotomies (horizontal advancement and counterclockwise rotation); osseous genioplasty (vertical reduction and horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal recontouring (see Fig. 25-8). A, Frontal facial and occlusal views before retreatment. B, Profile view and lateral cephalometric radiograph before retreatment.
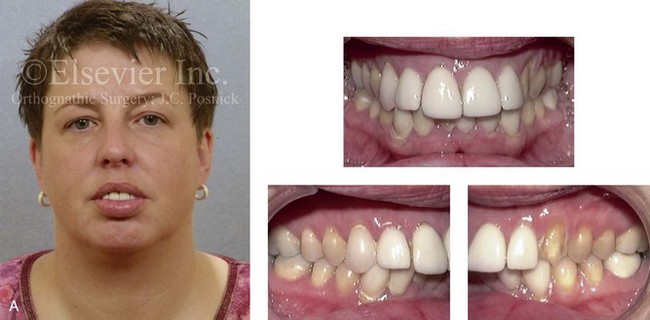
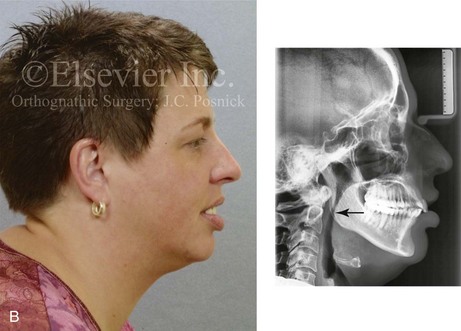
Figure 6-19 A woman in her late 40s with a long face growth pattern suffered deterioration of the dentition. The periodontal risk factors included inadequate attached gingiva, dental root crowding within limited alveolar bone support, nasal airway obstruction with forced mouth breathing, and lip incompetence that caused drying of the exposed gingiva. An attended polysomnogram confirmed obstructive sleep apnea. Unfavorable soft-tissue envelope distortions and premature facial aging were also discussed. She agreed to a comprehensive surgical and dental rehabilitative approach. Periodontal treatment was followed by restorative temporization and orthodontic alignment, including the extraction of the four first bicuspids. This was followed by surgery that included maxillary Le Fort I osteotomy in segments (vertical shortening, horizontal advancement, transverse widening, and limited clockwise rotation); bilateral sagittal split ramus osteotomies (horizontal advancement and limited counterclockwise rotation); osseous genioplasty (vertical shortening and horizontal advancement); an anterior approach to the soft tissues of the neck (cervical flap elevation, neck defatting, and vertical platysma muscle plication); and septoplasty, inferior turbinate reduction, and nasal floor recontouring (see Fig. 25-3). A, Frontal facial and occlusal views before treatment. B, Profile view and lateral cephalometric radiograph before treatment.
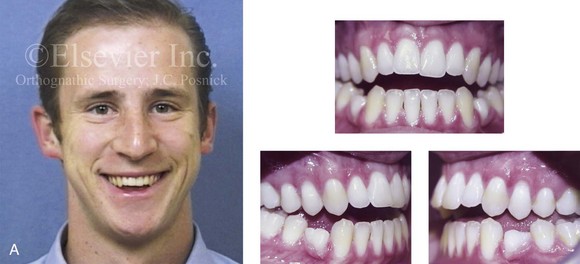
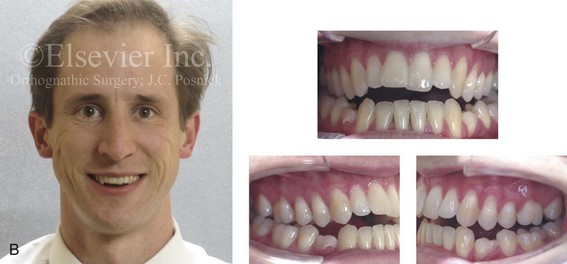
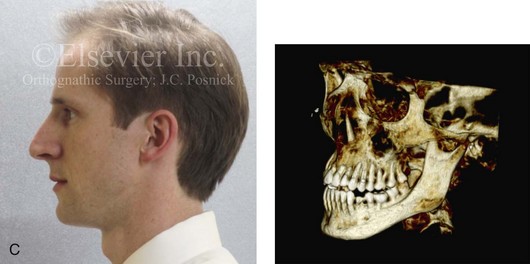
Figure 6-20 A 31-year-old man arrived for surgical evaluation. Since the mixed dentition, he was known to have a jaw deformity that was characterized as a maxillary deficiency with relative mandibular excess growth pattern. This results in an Angle Class III, negative overjet, anterior open bite, constricted arch-width malocclusion. The patient is congenitally missing the mandibular first premolars, and he has retained primaries. This surgeon had originally seen him 10 years earlier, when he was 21 years old. An orthodontic and surgical approach was recommended at that time. The patient then declined treatment, but, during the next 10 years, there was further labial bone loss and gingival recession of the mandibular anterior and maxillary posterior dentition. A comprehensive periodontal, orthodontic, and surgical approach was now recommended. The patient had generalized recession, localized minimal attached gingiva, and significant root exposure of teeth nos. 3, 4, 5, 12, 13, and 14. Subepithelial connective tissue grafting was recommended. This is to be followed by orthodontic decompensation and jaw surgery. The procedures are to include Le Fort I osteotomy in three segments (correction of the curve of Spee and the arch width) and bilateral sagittal split ramus osteotomies. A, Frontal view with smile and occlusal views at age 21. B, Frontal view with smile and occlusal views at age 31. C, Profile facial view and maxillofacial i-CAT image that indicate limited alveolar bone covering the roots of the teeth.
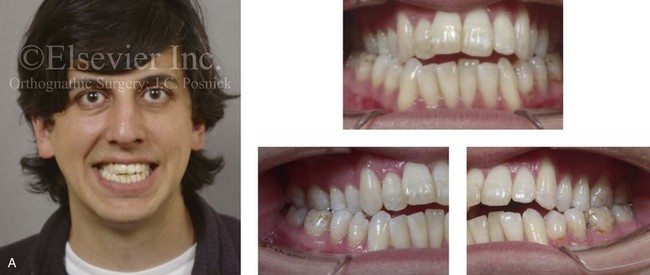
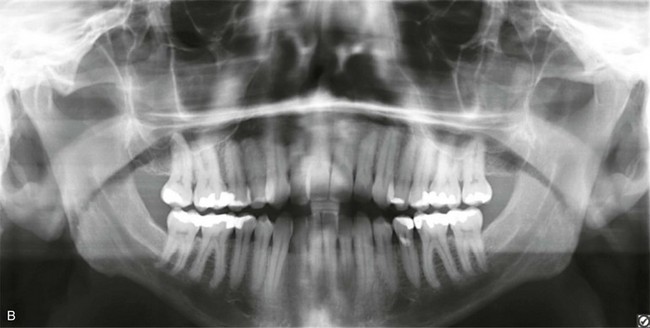
Figure 6-21 A 32-year-old man arrived for the evaluation of a longstanding jaw deformity with malocclusion, chronic obstructive nasal breathing, and periodontal issues. Since his early childhood years, he was known to have a maxillary deficiency with a relative mandibular excess growth pattern. The family elected a camouflage orthodontic approach in an attempt to neutralize the occlusion when the patient was 9 to 13 years old. He is a forced mouth breather with increased nasal airway resistance as a result of septal deviation and turbinate hypertrophy. His general dentist confirmed labial bone loss and gingival recession of the mandibular anterior and maxillary posterior dentition. He was referred to this surgeon, and a comprehensive orthognathic and dental approach was approved. Consultation with a periodontist, an orthodontist, and an ear, nose, and throat specialist was carried out. The patient was confirmed to have deviation of the septum and enlarged inferior turbinates. An attended polysomnogram is pending. The need for gingival grafting to attain improved root coverage and to generate a wider band of attachment was recommended for teeth nos. 3, 11, 14, 19, 20, 23 through 26, and 29 and 30. This will be followed by the orthodontic removal of dental compensation, including the extraction of a mandibular incisor to address the severe bone loss and gingival recession. Orthognathic and intranasal procedures to improve long-term dental health, to enhance facial aesthetics, and to open the upper airway will follow. A, Frontal facial and occlusal views before treatment. B, Panorex radiograph with treatment in progress.
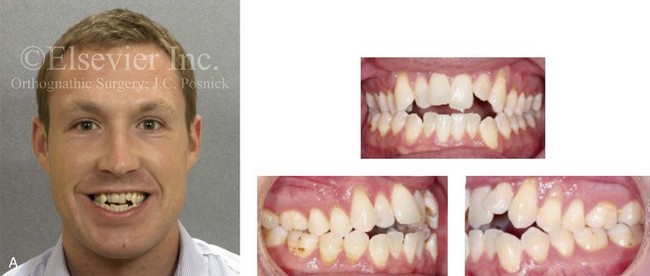
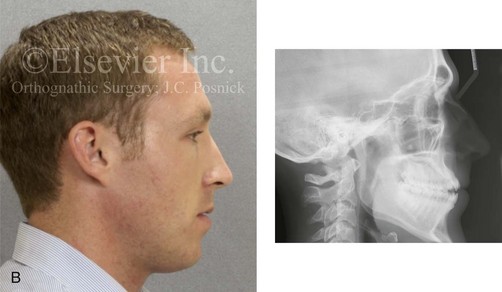
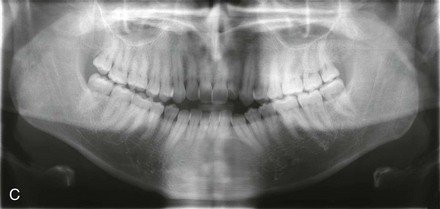
Figure 6-22 A 30-year-old man arrived for the evaluation of a longstanding jaw deformity with malocclusion, chronic obstructive nasal breathing, and awareness of periodontal issues. Since his early childhood years, he was known to have a jaw deformity with malocclusion that included an anterior open bite. When he was between 8 and 12 years old, he underwent orthodontic camouflage treatment in an attempt to close the open bite, including full bracketing and the use of heavy anterior elastics. By the time he graduated from high school, he was conscious of a significant recurrent anterior open bite with dental crowding. Through his college years, he was aware of gingival recession on the labial aspect of many of the anterior teeth; this was apparent on the maxilla more so than on the mandible. He was sent by his general dentist for an orthodontic evaluation and then to this surgeon for an opinion. He was referred for periodontal evaluation to confirm significant labial bone loss of the anterior dentition (on the maxilla more than the mandible) with gingival recession. A comprehensive approach was recommended, including gingival grafting and four bicuspid extractions with orthodontic retraction of the anterior dentition into solid alveolar bone. This would be followed by jaw reconstruction, finishing orthodontics, and periodontal surveillance. A, Frontal facial and occlusal views before retreatment. B, Profile facial view and lateral cephalometric radiograph before retreatment. C, Panorex radiograph before retreatment.
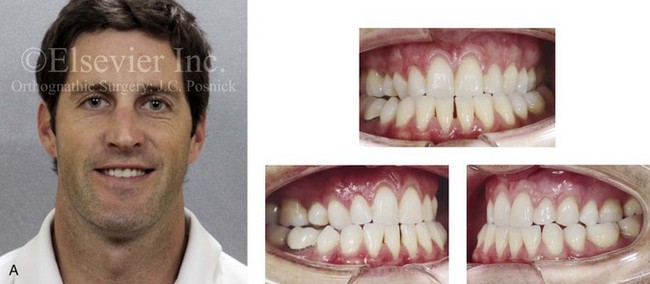
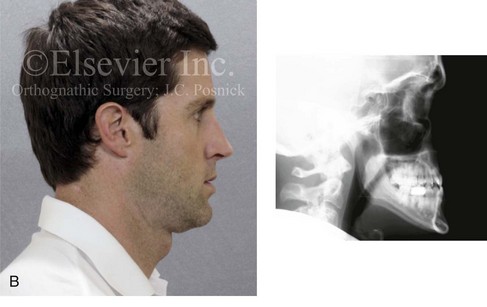
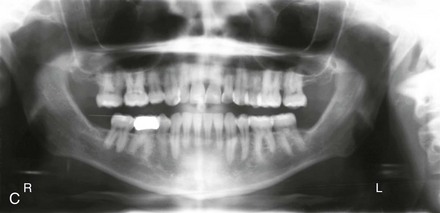
Figure 6-23 A 34-year-old man arrived for the evaluation of a longstanding jaw deformity with malocclusion, chronic obstructive nasal breathing, and periodontal issues. Since his early childhood years, he was known to have a maxillary deficiency with relative mandibular excess growth pattern. The family elected a camouflage orthodontic approach that included four bicuspid extractions and the introduction of dental compensation in an attempt to neutralize the occlusion when the patient was 10 to 15 years old. He is a forced mouth breather as a result of the increased nasal airway resistance. He underwent septoplasty when he was 20 years old, but this did not result in significant improvement. His general dentist confirmed labial bone loss and gingival recession of the mandibular anterior and maxillary posterior dentition. He was referred to this surgeon, and a comprehensive orthognathic and dental approach was recommended. Consultation with the periodontist, the orthodontist, and the ear, nose, and throat specialist was carried out. The patient was confirmed to have residual deviation of the septum and enlarged inferior turbinates, which explained the continued difficulty that he had breathing through his nose. A degree of root resorption of the anterior dentition was likely the result of previous orthodontic mechanics. The need for gingival grafting to attain as much root coverage as possible and to generate a wider band of attachment was recommended for teeth nos. 3, 11, 14, 19, 20, 23 through 26, and 29 and 30. This will be followed by the orthodontic removal of dental compensation and then orthognathic and intranasal procedures to improve the patient’s long-term dental health, to enhance his facial aesthetics, and to open his upper airway. A, Frontal facial and occlusal views before treatment. B, Profile facial view and lateral cephalometric radiograph before treatment. C, Panorex radiograph with treatment in progress.
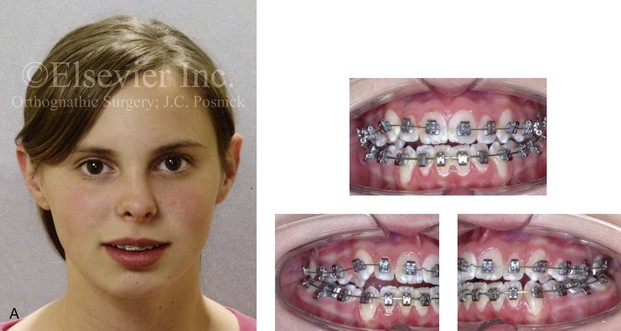
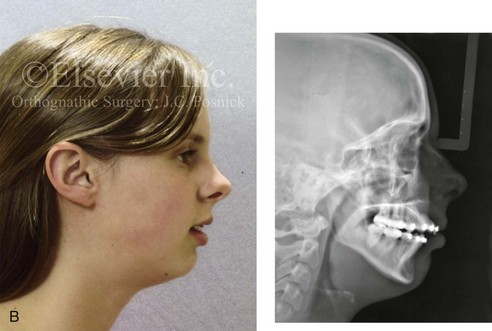
Figure 6-24 A 16-year-old girl with a primary mandibular deficiency growth pattern. She had undergone several years of growth modification followed by orthodontic mechanics that included four bicuspid extractions in an attempt to neutralize the occlusion. The patient was left with mandibular deficiency and malocclusion that included significant overjet, anterior open bite, and proclination of the maxillary incisors. Labial bone loss with gingival recession that affected the lower anterior dentition is evident. The patient was referred to this surgeon for a comprehensive orthodontic and surgical approach (see Fig. 19-11). A, Frontal and occlusal views in repose at the time of referral. B, Profile and lateral cephalometric radiograph views at the time of referral.
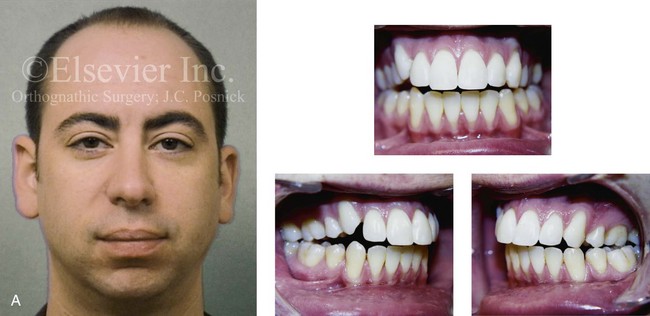
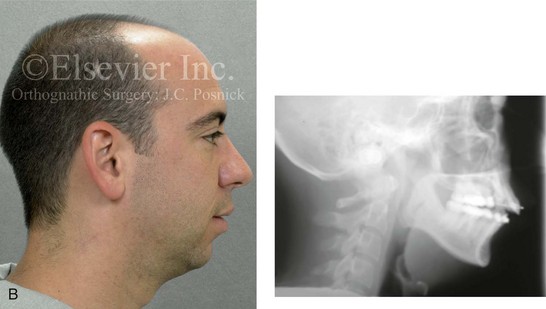
Figure 6-25 A man in his early 20s with a primary mandibular deficiency growth pattern. He underwent many years of growth modification followed by orthodontic camouflage maneuvers that included four bicuspid extractions in an attempt to neutralize the occlusion. Deterioration of the periodontium with labial bone loss and gingival recession along the mandibular anterior dentition are evident. A, Frontal facial and occlusal views before retreatment. B, Profile view and lateral cephalometric radiograph before retreatment.
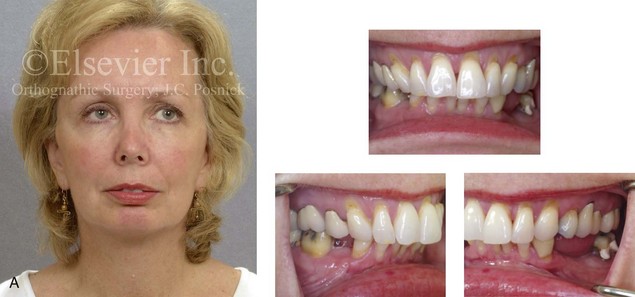
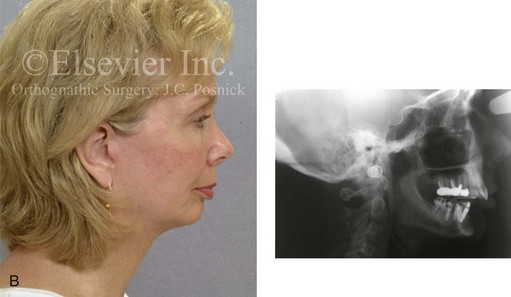
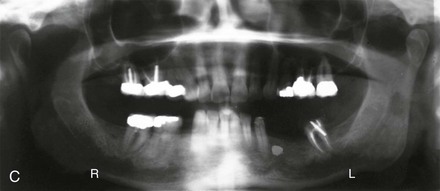
Figure 6-26 A woman in her early 50s was referred by a prosthodontist for surgical evaluation. There had been a gradual deterioration of the dentition, which was at least partially a result of the longstanding developmental Class II excess overjet deep-bite skeletal pattern. A head and neck evaluation confirmed a retrusive mandible, which also resulted in retroglossal airway obstruction. In addition, a desire for improved profile aesthetics was discussed. A comprehensive approach to dental rehabilitation, improvement of the upper airway, and facial rejuvenation and reconstruction was recommended. Coordinated endodontic, orthodontic, periodontic, prosthodontic, and surgical care was required. Periodontal treatment, extractions, dental implant placement, restorative temporization, and orthodontic alignment were carried out. These treatments were followed by surgery that included bilateral sagittal split ramus osteotomies (horizontal advancement and counterclockwise rotation); osseous genioplasty (vertical lengthening with interpositional grafting); and an anterior approach to the soft tissues of the neck (cervical flap elevation, neck defatting, and vertical platysma muscle plication). These procedure were followed by crown lengthening and then by final dental restorations (see Fig. 25-2). A, Frontal facial and occlusal views before treatment. B, Profile facial view and lateral cephalometric radiograph during treatment. C, Panorex radiograph before treatment.
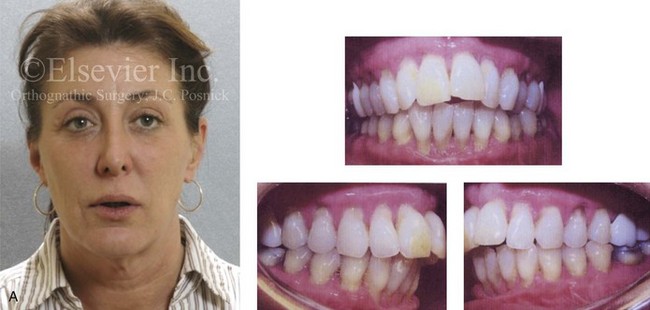
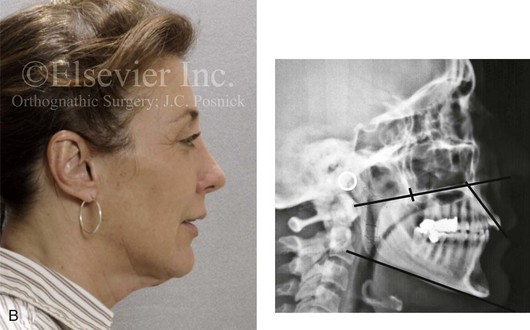
Figure 6-27 A 50-year-old woman was referred by her general dentist to an orthodontist and then for surgical evaluation. There was a mandibular deficiency and a constricted upper jaw growth pattern. This resulted in a Class II deep-bite excess overjet malocclusion. Over the years, there was destruction to the dentition and periodontal apparatus with generalized bone loss and gingival recession. The patient was now under the care of a restorative dentist and a periodontist. To resolve the malocclusion, maxillary expansion and mandibular advancement in combination with orthodontics were recommended. At referral to this surgeon, a history documented heavy snoring, restless sleeping, and daytime somnolence. An attended polysomnogram confirmed a respiratory disturbance index of 42.7 events per hour. There was intranasal obstruction caused by septal deviation and inferior turbinate enlargement, a retropositioned palate caused by maxillary retrusion, and a retropositioned tongue as a result of mandibular retrusion. The soft palate and the tongue were of normal size and function. There were minimum tonsil and adenoid structures. Continuous positive airway pressure was tried but not well tolerated. Surgery was agreed to in combination with orthodontics to improve the malocclusion and to open the airway. The procedures carried out included a maxillary Le Fort I osteotomy (horizontal advancement, vertical lengthening, and arch form correction); bilateral sagittal split osteotomies of the mandible (horizontal advancement); osseous genioplasty (horizontal advancement); septoplasty and inferior turbinate reduction; and an anterior approach to the neck (cervical flap elevation, neck defatting, and vertical platysma muscle plication). Subjectively, there was good relief of snoring and restless sleeping as well as the resolution of daytime fatigue. Six months after surgery, an attended polysomnogram confirmed the resolution of the obstructive sleep apnea with a respiratory disturbance index of 1.9 events per hour and minimum desaturation (see Fig. 26-8). A, Frontal facial and occlusal views before treatment. B, Profile facial view and lateral cephalometric radiograph before treatment.
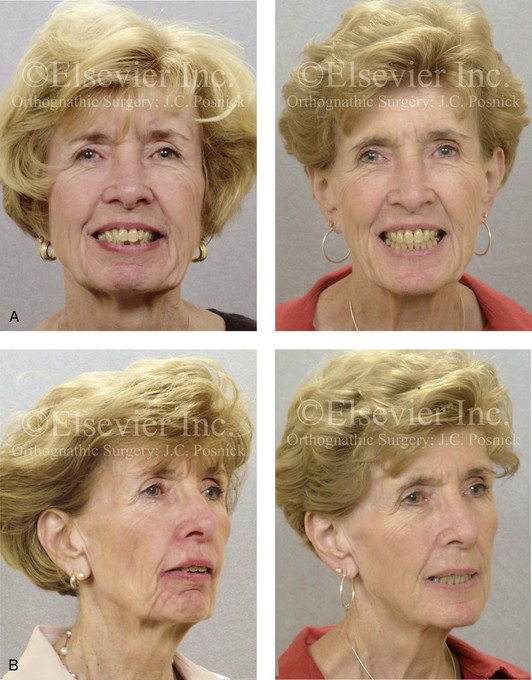
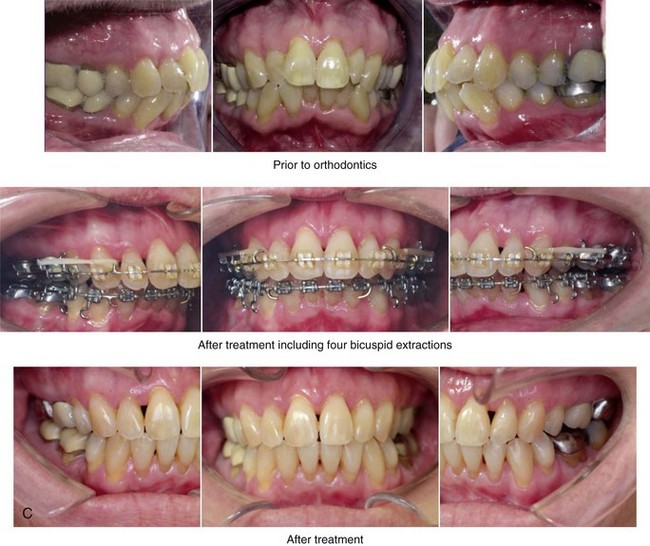
Figure 6-28 A 70-year-old woman arrived for surgical evaluation. She desired a stronger profile, less “loose” neck skin, and an improved smile. She initially requested a face lift and a chin implant. She was found to have a retrusive mandible and dental deterioration that was made worse by dental crowding and a deep-bite negative overjet malocclusion. She underwent orthodontics that included four bicuspid extractions and then surgery that included sagittal split ramus osteotomies (horizontal advancement) and osseous genioplasty (horizontal advancement) (see Fig. 25-10). A, Frontal views with smile before and after treatment. B, Oblique facial views before and after treatment. C, Occlusal views before treatment, with orthodontics in progress (including four bicuspid extractions), and after treatment.
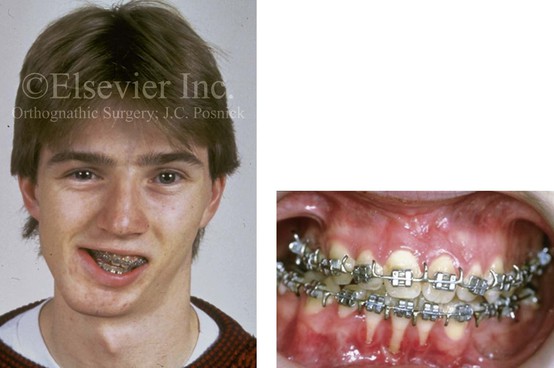
Figure 6-29 Frontal facial and occlusal views of a 16-year-old boy who had sustained a fracture of the right condyle of the mandible early during his childhood and who presented with the resulting facial asymmetry. He previously underwent several years of orthodontics to neutralize the occlusion. There was loss of labial bone along the anterior mandibular teeth. After surgical evaluation, the patient agreed to a comprehensive orthodontic and orthognathic approach. The procedures included a Le Fort I osteotomy, a left sagittal split ramus osteotomy, the reconstruction of the right condyle with a costochondral graft, and an osseous genioplasty (see Fig. 35-8). Note the recession along the labial aspects of the mandibular incisors before surgery. Gingival grafting is indicated. From Posnick JC: Management of facial fractures in children and adolescents. Ann Plast Surg 33:453, 1994.
• Inadequate attached gingiva surrounding the clinical crowns of the teeth
• Inadequate alveolar bone to house the roots of the full complement of teeth in each arch
• Inadequate nasal airflow with a forced mouth-breathing pattern, often in association with vertical (skeletal) excess and inadequate lip closure, with limited coverage of the incisors and the gingiva
• The effects of malocclusion associated with a jaw deformity
Effects of Inadequate Attached Gingiva
There are significant intrinsic biologic variations between humans with respect to the morphologic characteristics of the gingiva; this is known as the gingival biotype.52,73,138 The evaluation of the individual’s gingival biotype is important to orthodontic treatment planning, because thick and thin gingival biotypes are frequently associated with varied osseous patterns. These two tissue types are likely to respond very differently to similar orthodontic forces by demonstrating different patterns of osseous remodeling. With an understanding of the nature of the individual’s biologic risk factors (e.g., gingiva, alveolar bone, breathing pattern, degree of malocclusion), appropriate periodontal, orthodontic, and surgical preventative procedures and precautionary measures may be instituted to provide a more favorable tissue environment to minimize alveolar bone loss and gingival recession. Historically, Ochsenbein and Maynard discussed the importance of thick versus thin gingiva with regard to restorative treatment planning.150 In addition, in a group of patients reviewed by Olsson and colleagues, a thick periodontal biotype (85% of population) was found to be more prevalent than a thin periodontal biotype (15% of population).152 Thick gingival tissue is dense in appearance, with a fairly large zone (length) of attachment. The gingival topography is relatively flat, which is suggestive of a full underlying bony architecture. Thin gingival tissue tends to be delicate and almost translucent in appearance. The tissue is friable, with a minimal zone of attachment; this is suggestive of minimal bone over the labial roots of the teeth. The diametrically opposite thin and thick gingival biotypes will respond differently when subjected to inflammation, mechanical trauma, orthodontic forces, or surgical insults. Results from at least some clinical studies indicate that, as long as a tooth is being orthodontically moved with light forces and within or into (and not out of) the alveolar process, then the risk of harmful side effects on the marginal (gingival) soft tissues is minimal.212–215 In current clinical practice, pretreatment gingival augmentation (i.e., free gingival grafts, lateral pedicled flaps, and autogenous and allogenic connective tissue grafts) to improve the presenting thin keratinized gingiva—in combination with light orthodontic force—generally achieves objectives and limits recession and alveolar bone loss.
Effects of Inadequate Alveolar Bone (Alveolar Bone/Dental Root Ratio)
There will be inherent biologic variations among individuals with regard to the extent of alveolar housing as compared with the cumulative dental root volume of the compliment of teeth within each arch; this is known as the alveolar bone/dental root ratio.184,199 When the roots are crowded into limited available alveolar housing, the periodontal tissues are less able to withstand the routine inflammatory processes and the mechanical demands that they experience. In turn, the periodontium may recede (i.e., experience bone loss and gingival recession). To prevent this, either the space within the arch must be increased (e.g., rapid palatal expansion, surgically assisted rapid palatal expansion, segmental osteotomies) or extractions must be carried out as part of orthodontic treatment to limit the risk of periodontal deterioration.50,58,101,103,114,119,147,168,217 Orthodontic relief of dental crowding in the upper jaw by simply expanding the crown arch form in the presence of an inadequate alveolar arch form may align the dental crowns, but it will predictably result in cortical plate thinning, with the potential for dehiscence, fenestrations, loss of alveolar ridge height, and gingival recession (Fig. 6-8). Under these circumstances, successful dental uncrowding without extractions requires the initial expansion of the maxillary (skeletal) arch (e.g., rapid palatal expansion, surgically assisted rapid palatal expansion, segmental osteotomy).108 By following these principles, the achievement of improved arch form with the preservation of periodontal health should be possible.147
Effects of Nasal Obstruction and Mouth Breathing With Resulting Vertical Maxillary Excess, Lip Incompetence, and Inadequate Incisor/Gingiva Coverage
There is considerable variation among humans with respect to biologic respiratory patterns (i.e., mouth breathing versus nasal breathing). In the presence of significant nasal obstruction, a forced mouth-breathing pattern will occur and have the potential to cause detrimental effects on the periodontium.7,102,113,206 The existence of these two very different airway biotypes (i.e., nasal breathing and mouth breathing) and the secondary effects that may result should be recognized. Mouth breathing is an important etiologic factor for chronic gingivitis and chronic marginal periodontitis as well as for the formation of dental plaque and calculus. The presence of marginal gingivitis in individuals with mouth breathing was described in the literature by Coleyer in 1920. Warwick and Hastings (1933) also claimed that the surfaces of teeth and gingiva that were constantly exposed to air (i.e., in individuals who breathe habitually through the mouth) did not have the benefit of normal irrigation with saliva; these surfaces also did not benefit from the normal friction effects that result from the vestibular mucosa moving against the teeth and gingiva that would normally occur during lip closure.
Gulati and colleagues undertook a study to assess the effects of mouth breathing, lip seal, and upper lip coverage on the gingival health of children.98 School-aged children between the ages of 10 and 14 years (n = 240) were selected for the study. After clinical examination, they were divided into two major groups: mouth breathers and normal breathers. These two groups were further subdivided into six subgroups on the basis of lip-seal ability and upper incisor lip coverage. The results of the study indicate a gingival index that is higher in mouth breathers with lip incompetence than in normal breathers. Increased lip separation and decreased upper lip coverage (i.e., excess exposure of the maxillary incisors) were also associated with higher plaque and gingival indices.
Alexander completed clinical research with the intent of understanding habitual mouth breathing and its effect on gingival health.7–9 The study included 200 patients with a mean age of 30 years who were attending a clinic for routine dental treatment. Each of the subjects underwent systematic examinations to evaluate the presence of gingival inflammation, supragingival and subgingival calculus, and bacterial plaque. Each detailed examination was carried out under standardized conditions. Individuals were questioned carefully regarding the manner in which they usually breathe (i.e., mouth breathing versus nasal breathing). Mean gingival inflammation scores, calculus surface indices, and bacterial indices (with erythrosine disclosing solution) were calculated for each of their individual teeth. The study results confirm that habitual mouth breathing is highly associated with an increase in gingival inflammation and the prevalence of supragingival and subgingival calculus (see Figs. 6-15 through 6-22).
Fukuda and colleagues completed a study to evaluate the influence of mouth breathing on the periodontal tissues.76 Ten patients with chronic marginal periodontitis who were found to be habitual mouth breathers were included in the study, and they were evaluated for 30 days. During the experimental time frame, the study patients underwent taping of the lips to accomplish lip seal and to limit the mouth breathing option while sleeping. Oral examinations were completed and biopsy specimens of the gingiva were taken before and after nighttime lip tapings. The study indicates that, with satisfactory lip seal (i.e., lip taping), forced primary nasal breathing was achieved in these individuals while they were sleeping. This resulted in the following:
1. Halitosis and the uncomfortable feeling in the mouth upon awakening were decreased significantly.
2. The depth of the periodontal pockets and the labiolingual width of the gingiva were both decreased. The improvement was primarily seen on the labial side of the maxillary dentition.
3. The vacuolar degeneration of the epithelial cells was decreased as confirmed by histopathologic examination. However, inflammation of the connective tissue did not show improvement during the 30-day treatment time.
Effects of Malocclusion on Periodontal Health
Bollen completed a systematic review of the literature to answer the question, “Does a malocclusion affect periodontal health?”35 An electronic search included all publications from 1960 through 2006. A total of 25 studies that evaluated the association between a malocclusion and periodontal health were of sufficient quality for inclusion in the review.* Only 5 of these 25 studies adjusted for factors such as age, socioeconomic status, and oral hygiene. The total number of subjects included in the 25 studies was 35,300, and they had a mean age of 22 years (range, 3 to 60 years). Pertinent results of the review included the following:
1. Nineteen of the 25 studies found greater periodontal disease among subjects with greater malocclusion.
2. Six studies considered the interaction between malocclusion and “periodontopathies.” These studies found significantly greater periodontal problems among subjects with malocclusions as compared with subjects without malocclusions (P < .0001).
3. Two studies looked at the interaction between malocclusion and gingivitis. These studies found greater levels of gingivitis among subjects with malocclusions as compared with subjects without malocclusions (P < .0001).
The conclusions drawn by Bollen suggest that there is often a correlation between the presence of malocclusion and periodontal disease. However, the extent of this correlation, which is found in large population studies, should not be interpreted as demonstrating a clear cause and effect for any given individual.4,5
Potential Detrimental Effects of Orthodontic “Camouflage” Maneuvers on the Periodontium
Orthodontic camouflage maneuvers and dental restorative procedures carried out in an attempt to neutralize the malocclusion may be additive to the individual’s biologic risk factors with regard to his or her potential for progressive deterioration of the periodontium (see Figs. 6-16 through 6-25).
Long Face Growth Pattern
In the individual with a long face growth pattern, orthodontic camouflage maneuvers may attempt to correct maxillary arch–width deficiency via the buccal tipping of the posterior teeth (see Figs. 6-15 through Fig 6-19). If more than a few millimeters are required, this may result in the remodeling of the alveolar process with dehiscence and fenestration of the buccal cortex, followed by gingival recession.27,205,207,209
Maxillary Deficiency With Relative Mandibular Excess Growth Pattern
With a maxillary deficient and relative mandibular excess growth pattern, orthodontic camouflage maneuvers often attempt to correct upper arch–width deficiency via the buccal tipping of the posterior teeth (see Figs. 6-14 and 6-20 through 6-23). If more than a few millimeters are required, this may result in the remodeling of the alveolar process with dehiscence and fenestration of the buccal cortex, followed by gingival recession.27,205,207,209
Orthodontic mechanics that are carried out to manage the skeletal component of the negative overjet often result in the proclination of the maxillary incisors and retroclined mandibular anterior teeth. This approach may result in the remodeling of the alveolar process with dehiscence and fenestration of the mandibular labial cortex, followed by gingival recession. The remodeling of the alveolar process with a loss of maxillary labial cortex may also be seen.185,194,210
Primary Mandibular Deficiency Growth Pattern
In the individual who presents with a primary mandibular deficiency growth pattern, orthodontic camouflage maneuvers often attempt to uncrowd the mandibular arch by “flaring” the anterior dentition and to then decrease the overjet by proclining the incisors even further (see Figs. 6-24 through Fig. 6-28). This may result in the remodeling of the alveolar process with dehiscence and fenestrations of the labial cortex, followed by gingival recession.10–15,18,26,64,65,72,178,195,211
Another common error occurs when mandibular advancement surgery is planned to correct the excess overjet. Often an inter–arch-width discrepancy will be unmasked (i.e., in approximately 30% of cases). It is not uncommon for clinicians to then avoid maxillary surgery through the process of orthodontic buccal tipping the maxillary posterior teeth to relieve the crossbites. If more than a few millimeters are required, this may result in remodeling of the alveolar process with dehiscence and fenestration of the buccal cortex followed by gingival recession180 (see Figs. 6-24 through 6-28).
Establishing a Healthy Periodontium and Periodontal Therapeutics
Before the initiation of orthodontic treatment, the clinician should assess for adequate attached gingiva (i.e., the need for grafting); adequate alveolar bone (i.e., the need for extractions or alveolar expansion); caries and plaque control (i.e., the need for hygiene instruction, deep scaling, and root planing); the adequacy of dental restorations (i.e., the need for restorative dentistry); the adequacy of nasal breathing (i.e., the need to open the airway through intranasal surgery); and the adequacy of lip closure (i.e., the need for jaw surgery).16,17 When a jaw discrepancy is present (with or without combined nasal obstruction and lip incompetence), the orthodontic objectives should be modified to include the coordination of surgical treatment to achieve the desired end results.
A primary focus of periodontal therapeutics is to reduce inflammation by creating an environment that can be maintained as free of plaque as possible.2,3,22,87,136,159,164,166 After this is accomplished, the individual can be trained to perform the task of deplaquing the dentition and developing an effective habit of doing so at least once every 24 hours.179,186 Plaque-retaining traps will limit the individual’s ability to effectively deplaque the dentition.91 These traps include calculus, crowded teeth, malaligned teeth, restorative overhangs, overcontoured teeth, root anomalies, subgingival caries, and pocketing.97 Consultation with a periodontist is useful, because his or her skills include the ability to diagnose and remove plaque traps and also to effectively educate the affected individual with regard to how best to maintain clean teeth.130 Limiting plaque traps may also require orthodontic procedures to reposition dental crowns into more healthy relationships and to place roots more fully within the alveolar bone. This may be combined with procedures to surgically align the jaws (e.g., orthognathic correction) and to open the nasal airway (e.g., septoplasty, inferior turbinate reduction).48,51,71,74,75,78,88,90,94,140,158,162,167,168 Misguided orthodontic tooth movement carried out in isolation or in combination with surgery can result in injuries that includes the loss of alveolar crestal height, the dehiscence of or fenestrations through the labial and lingual plates, gingival recession, root resorption, tooth mobility, and inadequate occlusion (see Figs. 6-29 and 6-30).42,60,61,127,128,146,148,153
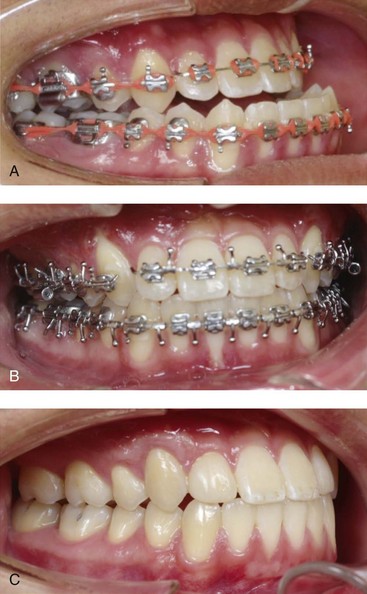
Figure 6-30 A 17-year-old high school student was referred by his orthodontist for surgical evaluation. The Class III negative overjet anterior open-bite malocclusion required retreatment with orthodontics, including maxillary bicuspid extractions and jaw surgery. With a lifelong history of nasal obstruction and consistent physical findings, intranasal procedures were also required. With orthodontic (dental) decompensation complete, the patient’s surgery included a maxillary Le Fort I osteotomy in segments (arch expansion, horizontal advancement, and vertical lengthening); sagittal split ramus osteotomies (limited corrections); osseous genioplasty (vertical reduction and minimal horizontal advancement); and septoplasty and inferior turbinate reduction. Occlusal views A, before retreatment, B, with orthodontics in progress, and C, after the completion of treatment. The patient required and underwent gingival grafting on the labial aspect of the right maxillary canine after the removal of the orthodontic appliances.
Adult Orthodontic Treatment
With aging, there tends to be progressive reduction of the periodontium, an increase in the root–tooth ratio, and a decrease in the resistance to spontaneous tooth migration. For these reasons, a reduced baseline periodontium may be present more frequently in adults than in adolescents. These periodontal findings must be taken into consideration before the initiation of an orthodontic program. With age, there may also be an increase in bone porosity (i.e., decreased density). The initial reaction of the PDL and the alveolar bone to orthodontic loading in the adult may be delayed. However, after tooth displacement gets under way, treatment can be carried out in the same way in the adult as it is in a teenager or a young adult. There is no known age limit to the orthodontic movement of teeth (see Fig. 6-28). By contrast, it is known that the precondition of the periodontium should be one of health and anatomic adequacy to mitigate the risks to the periodontium during orthodontic therapy. The presenting periodontium—whether normal or diminished—should be healthy when tooth movement is initiated.
References
1. Addy, M, Dummer, PMH, Griffiths, G, et al. Prevalence of plaque gingivitis and caries in 11-12-year-old children in South Wales. Community Dent Oral Epidemiol. 1986; 14:115–118.
2. Addy, M. Plaque control as a scientific basis for the prevention of caries. J R Soc Med. 1986; 79(Suppl):6–9.
3. Addy, M, Griffiths, G, Dummer, PMH, et al. The distribution of plaque and gingivitis and the influence of toothbrushing hand in a group of 11-12-year-old school children. J Clin Periodontol. 1987; 14:564–572.
4. Addy, M, Griffiths, GS, Duramer, PMH, et al. The association between tooth irregularity and plaque accumulation, gingivitis, and caries in 11-12-year-old children. Eur J Orthod. 1988; 10:76–83.
5. Ainamo, J. Relationship between malalignment of the teeth and periodontal disease. Scand J Dent Res. 1972; 80:104–110.
6. Albander, JM. A 6-year study on the pattern of periodontal disease progression. J Clin Periodontol. 1990; 17:467–471.
7. Alexander, AG. Habitual mouth breathing and its effect on gingival health. Paradontologie. 1970; 24:49–55.
8. Alexander, AG, Tipnis, AK. The effect of irregularity of the teeth and the degree of overbite and overjet on gingival health. Br Dent J. 1970; 128:539–544.
9. Alexander, AG. The effect of lack of function of teeth on gingival health, plaque and calculus accumulation. J Periodontol. 1970; 41:438.
10. Allais, D, Melsen, B. Does labial movement of lower incisors influence the level of the gingival margin? A case-control study of adult orthodontic patients. Eur J Orthod. 2003; 25(4):343–352.
11. Alstad, S, Zachrisson, BU. Longitudinal study of periodontal condition associated with orthodontic treatment in adolescents. Am J Orthod. 1979; 76:277–286.
12. Andlin-Sobocki, A, Bodin, L. Dimensional alterations of the gingiva related to changes of facial/lingual tooth position in permanent anterior teeth of children: a 2-year longitudinal study. J Clin Periodontol. 1993; 20:219–224.
13. Andlin-Sobocki, A, Persson, M. The association between spontaneous reversal of gingival recession in mandibular incisors and dentofacial changes in children: a 3-year longitudinal study. Eur J Orthod. 1994; 16:229–239.
14. Årtun, J, Osterberg, SK, Kokich, VG. Long-term effect of thin interdental alveolar bone on periodontal health after orthodontic treatment. J Periodontol. 1986; 57:341–346.
15. Årtun, J, Krogstad, O. Periodontal status of mandibular incisors following excessive proclination. A study in adults with surgically treated mandibular prognathism. Am J Orthod Dentofacial Orthop. 1987; 91:225–232.
16. Årtun, J, Osterberg, SK. Periodontal status of teeth facing extraction sites long-term after orthodontic treatment. J Periodontol. 1987; 58(1):24–29.
17. Årtun, J, Urbye, KS. The effect of orthodontic treatment on periodontal bone support in patients with advanced loss of marginal periodontium. Am J Orthod Dentofacial Orthop. 1988; 93(2):143–148.
18. Årtun, J, Grobety, D. Periodontal status of mandibular incisors after pronounced orthodontic advancement during adolescence: a follow-up evaluation. Am J Orthod Dentofacial Orthop. 2001; 119:2–10.
19. Asikainen, S, Jousimies-Somer, H, Kanervo, A, Summanen, P. Certain bacterial species and morphotypes in localized juvenile periodontitis and in matched controls. J Periodontol. 1987; 58:224–230.
20. Atherton, JD, Kerr, NW. Effect of orthodontic tooth movement upon the gingiva. Br Dent J. 1968; 124:555–560.
21. Atherton, JD. Gingival response to orthodontic tooth movement. Am J Orthod. 1970; 58:179–186.
22. Axelsson, P, Lindhe, J, Nystrom, B. On the prevention of caries and periodontal disease: results of a 15-year longitudinal study in adults. J Clin Periodontol. 1991; 18(3):182–189.
23. Badzian-Kobos, K, Stepien-Szczepanska, J, Strzelecka, E. Oral hygiene, condition of periodontium, and malocclusion in children aged 7-15 years from a region of mining and power production. Czas Stomatol. 1987; 40:309–315.
24. Baer, PN, Cocarro, PJ. Gingival enlargement coincident with orthodontic therapy. J Periodontol. 1964; 34:436–439.
25. Baker, DL, Seymour, GJ. The possible pathogenesis of gingival recession: a histological study of induced recession in the rat. J Clin Periodontol. 1976; 3:208–219.
26. Beagrie, CS, Thompson, GW, Basu, MK. Tooth position and anterior bone loss in skulls. Br Dent J. 1970; 129:471–474.
27. Beagrie, GS, James, GA. The association of posterior tooth irregularity and periodontal disease. Br Dent J. 1962; 113:239–243.
28. Behlfelt, K, Ericsson, L, Jacobson, L, Linder-Aronson, S. The occurrence of plaque and gingivitis and its relationship to tooth alignment within the dental arches. J Clin Periodontol. 1981; 8:329–337.
29. Bergström, J, Eliasson, S, Preber, H. Cigarette smoking and periodontal bone loss. J Periodontol. 1991; 62:242–246.
30. Bergström, J, Preber, H. Tobacco use as a risk factor. J Periodontol. 1994; 65:545–550.
31. Bernimoulin, JP, Curiloviec, Z. Gingival recession and tooth mobility. J Clin Periodontol. 1977; 4(2):107–114.
32. Bibb, CA. Occlusal evaluation and therapy in the management of periodontal disease. In: Newman MG, Takei HH, Carranza FA, eds. Carranza’s clinical periodontology. ed 9. Philadelphia: W. B. Saunders Company; 2002:698–701.
33. Bilimoria, KF. Malocclusion: its role in the causation of periodontal disease. J All India Dent Assoc. 1963; 35:293–300.
34. Bolin, A, Eklund, G, Frithiof, L, Lavstedt, S. The effect of changed smoking habits on marginal alveolar bone loss: a longitudinal study. Swed Dent J. 1993; 17(5):211–216.
35. Bollen, AM, Cunha-Cruz, J, Bakko, DW, et al. The effects of orthodontic therapy on periodontal health: a systematic review of controlled evidence. J Am Dent Assoc. 2008; 139(4):413–422.
36. Bondemark, L. Interdental bone changes after orthodontic treatment: a 5-year longitudinal study. Am J Orthod Dentofacial Orthop. 1998; 114(1):25–31.
37. Boyd, RL. Are your patients healthier periodontically because of orthodontic treatment? Bull Pac Coast Soc Orthod. 1984; 56:54–57.
38. Boyd, RL, Leggott, PJ, Quinn, RS, et al. Periodontal implications of orthodontic treatment in adults with reduced or normal periodontal tissues versus those of adolescents. Am J Orthod Dentofacial Orthop. 1989; 96(3):191–198.
39. Boyd, RL. Periodontal screening examination guide. In: American Association of Orthodontists risk management program. St. Louis: American Association of Orthodontists; 1992:13.
40. Bragd, L, Dahlen, G, Wikstrom, M, Slots, J. The capability of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius to indicate progressive periodontitis; a retrospective study. J Clin Periodontol. 1987; 14(2):95–99.
41. Brägger, U, Lang, NP. Significance of bone in periodontal disease. Semin Orthod. 1996; 2(1):32–38.
42. Brezniak, N, Wasserstein, A. Root resorption after orthodontic treatment: Part 2. Literature review. Am J Orthod Dentofac Orthop. 1993; 103:138–146.
43. Brown, IS. The effect of orthodontic therapy on certain types of periodontal defects: I. Clinical findings. J Periodontol. 1973; 44:742–756.
44. Buckley, LA. The relationship between malocclusion and periodontal disease. J Periodontol. 1972; 43:415–417.
45. Buckley, LA. The relationship between irregular teeth, plaque, calculus and gingival disease. Br Dent J. 1980; 148:67–69.
46. Buckley, LA. The relationship between malocclusion, gingival inflammation, plaque and calculus. J Periodontol. 1981; 52:35–40.
47. Burch, JG, Bagci, B, Sabulski, D, Landrum, C. Periodontal changes in furcations resulting from orthodontic uprighting of mandibular molars. Quintessence Int. 1992; 23:509–513.
48. Burke, JL, Provencher, RF, McKean, TW. Small segmental and unitooth ostectomies to correct dentoalveolar deformities. J Oral Surg. 1977; 35:453.
49. Burkett, LW. Effects of orthodontic treatment on the soft periodontal tissues. Am J Orthod. 1963; 49:671.
50. Carroll, WJ, Haug, RH, Bissada, NF, et al. The effects of the Le Fort I osteotomy on the periodontium. J Oral Maxillofac Surg. 1992; 50:128–132.
51. Clarke, NG, Hirsch, RS. Personal risk factors for generalized periodontitis. J Clin Periodontol. 1995; 22(2):136–145.
52. Coatoam, GW, Behrents, RG, Bissada, NF. The width of keratinized gingiva during orthodontic treatment: its significance and impact on periodontal status. J Periodontol. 1981; 52:307–313.
53. Cohen, W. A study of occlusal interferences in orthodontically treated occlusions and untreated normal occlusions. Am J Orthod. 1965; 51:647–689.
54. Cooper, MB, Landay, MA, Seltzer, S. The effect of excessive occlusal forces on the pulp. II. Heavier and longer term forces. J Periodontol. 1971; 42:353–359.
55. Davies, TM, Shaw, WC, Addy, M, Dummer, PH. The relationship of anterior overjet to plaque and gingivitis in children. Am J Orthod Dentofacial Orthop. 1988; 93:303–309.
56. Davies, TM, Shaw, WC, Worthington, HV, et al. The effect of orthodontic treatment on plaque and gingivitis. Am J Orthod Dentofacial Orthop. 1991; 99(2):155–161.
57. Diamanti-Kipioti, A, Gusberti, FA, Lang, NP. Clinical and microbiological effects of fixed orthodontic appliances. J Clin Periodontol. 1987; 14:326–333.
58. Diedrich, PR. Guided tissue regeneration associated with orthodontic therapy. Semin Orthod. 1996; 2(1):39–45.
59. Dominiak, M, Kalecinska, E, Krzyszton, E, et al. Correlation between temporomandibular dysfunction, disturbances of occlusion, and gingival recession in a group of youth students. Bull Group Int Rech Sci Stomatol Odontol. 2006; 47:40–46.
60. Dorfman, HS. Mucogingival changes resulting from mandibular incisor tooth movement. Am J Orthod. 1978; 74:286–297.
61. Dorfman, HS, Turvey, TA. Alterations in osseous crestal height following interdental osteotomies. Oral Surg Oral Med Oral Pathol. 1979; 48:120–125.
62. Driel, WD, Leeuwen, EJ, Hoff, JW, et al. Time-dependent mechanical behavior of the periodontal ligament. Proc Inst Mech Eng H. 2000; 214:497–504.
63. Eliasson, LA, Hugoson, A, Kurol, J, Siwe, H. The effects of orthodontic treatment on periodontal tissues in patients with reduced periodontal support. Eur J Orthod. 1982; 4(1):1–9.
64. Emslie, RD. The incisal relationship and periodontal disease. Paradontologie. 1958; 12:15.
65. Engelking, G, Zachrisson, BU. Effects of incisor repositioning on monkey periodontium after expansion through the cortical plate. Am J Orthod. 1982; 82:23–32.
66. Engström, C, Granström, G, Thilander, B. Effect of orthodontic force on periodontal tissue metabolism. A histologic and biochemical study in normal and hypocalcemic young rats. Am J Orthod Dentofacial Orthop. 1988; 93:486–495.
67. Ericsson, I, Thilander, B, Lindhe, J, Okamoto, H. The effect of orthodontic tilting movements on the periodontal tissues of infected and non-infected dentitions in dogs. J Clin Periodontol. 1977; 4:278–293.
68. Ericsson, I, Thilander, B, Lindhe, J. Periodontal condition after orthodontic tooth movements in the dog. Angle Orthod. 1978; 48:210–218.
69. Ericsson, I, Thilander, B. Orthodontic forces and recurrence of periodontal disease. Am J Orthodont. 1978; 74:41.
70. Ericsson, I, Lindhe, J. Recession in sites with inadequate width of the keratinized gingiva: an experimental study in the dog. J Clin Periodontol. 1984; 11(2):95–103.
71. Feliu, JL. Long-term benefits of orthodontic treatment on oral hygiene. Am J Orthod. 1982; 82(6):473–477.
72. Ferro, A, Aronna, P. Isolated gingival recession “without cause” localized to the lower incisors in Class II, 1 dental malocclusion: further studies. Arch Stomatol (Napoli). 1988; 29:1049–1062.
73. Forsberg, A. A clinical study of the periodontal tissues of the upper incisors in two age groups. Acta Odontologjca Scand Suppl. 1951; 9(8):1–105.
74. Foushee, DG, Moriarty, JD, Simpson, DM. Effects of mandibular orthognathic treatment on mucogingival tissue. J Periodontol. 1985; 56:727–733.
75. Fox, ME, Stephens, WF, Wolford, LM, Deeb, ME. Effects of interdental osteotomies on the periodontal and osseous supporting tissues. Int J Adult Orthodon Orthognath Surg. 1991; 6:39–46.
76. Fukuda, T, Yokomichi, K, Kato, H, Ishikawa, J. Studies on the influence of mouth breathing to the periodontal tissue. The effect of oral screen and lip-seal tape on the gingiva in mouth breathers. Nihon Shishubyo Gakkai Kaishi. 1975; 2:280.
77. Gardner, JH. A survey of malocclusions and some aetiological factors on 1,000 Sheffield school children. Dent Practit. 1956; 6:187–201.
78. Garib, DG, Henriques, JFC, Janson, G, et al. Rapid maxillary expansion—tooth-tissue-borne vs tooth-borne expanders: a CT evaluation of dentoskeletal effects. Angle Orthod. 2005; 75:548–557.
79. Geiger, AM. Occlusal studies in 188 consecutively treated cases of periodontal disease. Am J Orthod. 1962; 48:330–360.
80. Geiger, AM. Occlusion in periodontal disease. J Periodontol. 1965; 36:387–392.
81. Geiger, AM, Wasserman, BH, Thompson, RH, et al. Relationship of occlusion and periodontal disease: Part I. A system for evaluating periodontal status. J Periodontol. 1971; 42:371–378.
82. Geiger, AM, Wasserman, BH, Thompson, RH, Turgeon, LR. Relationship of occlusion and periodontal disease: Part V. Relation of classification of occlusion to periodontal status and gingival inflammation. J Periodontol. 1972; 43:554–560.
83. Geiger, AM, Wasserman, BH, Turgeon, LR. Relationship of occlusion and periodontal disease: Part VI. Relation of anterior overjet and overbite to periodontal destruction and gingival inflammation. J Periodontol. 1973; 44:150–157.
84. Geiger, AM, Wasserman, BH, Turgeon, LR. Relationship of occlusion and periodontal disease: Part VIII. Relationship of crowding and spacing to periodontal destruction and gingival inflammation. J Periodontol. 1974; 45:43–49.
85. Geiger, AM, Wasserman, BH. Relationship of occlusion and periodontal disease: Part IX. Incisor inclination and periodontal status. Angle Orthod. 1976; 46:99–110.
86. Geiger, AM, Wasserman, BH. Relationship of occlusion and periodontal disease: Part X. Relation of cross-bite to periodontal status. J Periodontol. 1977; 47:785–789.
87. Genco, RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996; 67(Suppl 10):1041–1049.
88. Goldberg, D, Turley, P. Orthodontic space closure of edentulous maxillary first molar area in adults. Int J Adult Orthodon Orthognath Surg. 1989; 4:255–266.
89. Goldie, RS, King, GJ. Root resorption and tooth movement in orthodontically treated, calcium-deficient, and lactating rats. Am J Orthod. 1984; 85:424–430.
90. Goldman, HM. The development of physiologic gingival contours by gingivoplasty. Oral Surg Oral Med Oral Pathol. 1950; 3:879–888.
91. Goldman, HM, Cohen, DW. Periodontal therapy, ed 4. St. Louis: The C. V. Mosby Company; 1968.
92. Gould, MSE, Picton, DC. The relationship between irregularities of the teeth and periodontal disease: a pilot study. Br Dent J. 1966; 121:20.
93. Graves, DT, Fine, D, Teng, YT, et al. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008; 35:89–105.
94. Greenbaum, KR, Zachrisson, BU. The effect of palatal expansion therapy on the periodontal supporting tissues. Am J Orthod. 1982; 81:12–21.
95. Grewe, JM, Chadha, JM, Hagan, D, Zermeno, JA. Oral hygiene and occlusal disharmony in Mexican-American children. J Periodontal Res. 1969; 4:189–192.
96. Griffiths, GS, Addy, M. Effects of malalignment of teeth in the anterior segments on plaque accumulation. J Clin Periodontol. 1981; 8:481–490.
97. Grossi, SG, Genco, RJ, Machtei, EE, et al. Assessment of risk for periodontal disease: risk indicators for alveolar bone loss. J Periodontol. 1995; 66(1):23–29.
98. Gulati, MS, Grewal, N, Kaur, A. A comparative study of effects of mouth breathing and normal breathing on gingival health in children. J Indian Soc Pedod Prev Dent. 1998; 16(3):72–83.
99. Haber, J. Cigarette smoking: a major risk factor for periodontitis. Compendium. 1994; 15(8):1004–1008.
100. Hamp, SE, Lundstrijm, F, Nyman, S. Periodontal conditions in adolescents subjected to multiband orthodontic treatment with controlled oral hygiene. Eur J Orthod. 1982; 4:77.
101. Handelman, CS. Nonsurgical rapid maxillary alveolar expansion in adults: a clinical evaluation. Angle Orthod. 1997; 67:291–305.
102. Hanson, ML, Andrianopoulos, MV. Tongue thrust, occlusion, and dental health in middle-aged subjects: a pilot study. Int J Orofacial Myology. 1987; 13:3–9.
103. Harry, MR, Sims, MR. Root resorption in bicuspid intrusion: a scanning electron microscope study. Angle Orthod. 1982; 52:235.
104. Heimlich, AC. Selective grinding as an aid to orthodontic therapy. Angle Orthod. 1951; 21:76–88.
105. Helm, S, Petersen, PE. Causal relation between malocclusion and periodontal health. Acta Odontol Scand. 1989; 47:223–228.
106. Henrikson, PA. Periodontal disease and calcium deficiency: an experimental study in the dog. Acta Odontol Scand. 1968; 26(Suppl 50):1–132.
107. Hollender, L, Rönnerman, A, Thilander, B. Root resorption, marginal bone support and clinical crown length in orthodontically treated patients. Eur J Orthod. 1980; 2:197–205.
108. Hom, BM, Turley, PK. The effects of space closure of the mandibular first molar area in adults. Am J Orthod Dentofacial Orthop. 1984; 85:457–469.
109. Horup, N, Melsen, B, Terp, S. Relationship between malocclusion and maintenance of teeth. Community Dent Oral Epidemiol. 1987; 15:74–78.
110. Ingervall, B, Jacobsson, U, Nyman, S. A clinical study of the relationship between crowding of teeth, plaque and gingival condition. J Clin Periodontol. 1977; 4:214–222.
111. Iyer, VS. Reaction of gingiva to orthodontic force: a clinical study. J Periodontol. 1962; 33:26–28.
112. Jacobson, L, Linder-Aronson, S. Crowding and gingivitis: a comparison between mouthbreathers and nosebreathers. Scand J Dent Res. 1972; 80:500–504.
113. Jacobson, L. Mouth breathing and gingivitis I. Gingival condition in children with epipharyngeal adenoids. J Periodontal Res. 1973; 3:269–277.
114. Jager, A, Polley, J, Mausberg, R. Effects of orthodontic expansion of the mandibular arch on the periodontal condition of the posterior teeth. Dtsch Zahnarztl Z. 1990; 45(2):113–115.
115. Janson, G, Bombonatti, R, Brandao, AG, et al. Comparative radiographic evaluation of the alveolar bone crest after orthodontic treatment. Am J Orthod Dentofacial Orthop. 2003; 124(2):157–164.
116. Janson, M. Gingival and periodontal relationships after orthodontic therapy: a study of class-II patients. Dtsch Zahnarztl Z. 1984; 39(3):254–256.
117. Jenkins, SM, Dummer, PM, Addy, M. Radiographic evaluation of early periodontal bone loss in adolescents: an overview. J Clin Periodontol. 1992; 19(6):363–366.
118. Kalamparov, K, Gantsev, GA, Ershov, VN. Relationship between dentomaxillary deformities and periodontal diseases in children. Stomatologiia (Mosk). 1972; 51:47–50.
119. Karring, T, Numan, S, Thilander, B, Magnusson, I. Bone regeneration in orthodontically produced alveolar bone dehiscences. J Periodont Res. 1982; 17:309–315.
120. Katz, RV. An epidemiologic study of the relationship between various states of occlusion and the pathological conditions of dental caries and periodontal disease. J Dent Res. 1978; 57:433–439.
121. Kessler, M. Interrelationships between orthodontic and periodontics. Am J Orthod. 1976; 70:154–177.
122. KIoehn, JS, Pfeiffer, JS. The effect of orthodontic treatment on the periodontium. Angle Orthodont. 1974; 44:127.
123. Klassman, B, Zacher, HW. Treatment of a periodontal defect resulting from improper tooth alignment and local factors. J Periodontol. 1969; 40:401.
124. Kois, JC. The restorative-periodontal interface: biological parameters. Periodontol. 1996; 2000(11):29–38.
125. Kokich, VG. Esthetics: the orthodontic-periodontic restorative connection. Semin Orthod. 1996; 2(1):21–30.
126. Kraus, BS, Jordan, RE, Abrams, L. Dental anatomy and occlusion. Baltimore: Williams & Wilkins Company; 1969.
127. Kwon, H-J, Pihlstrom, B, Waite, DE. Effects on the periodontium of vertical bone cutting for segmental osteotomy. J Oral Maxillofac Surg. 1985; 43:952–955.
128. Langford, SR, Sims, MR. Root surface resorption, repair, and periodontal attachment following rapid maxillary expansion in man. Am J Orthod. 1982; 81:108–115.
129. Lau, D, Grimths, GS, Shaw, WC. Reproducibility of an index for recording the alignment of individual teeth. Br J Orthod. 1984; 11:80–84.
130. Lee, H, Silness, J. Periodontal disease in pregnancy: I. Prevalence and severity. Acta Odontol Stand. 1963; 21:533–551.
131. Listgarten, MA, Hellden, K. Relative distribution of bacteria at clinically health and periodontally diseased sites in humans. J Clin Periodontol. 1978; 5:115–132.
132. Loe, H, Theilade, D, Jensen, SB. Experimental gingivitis in man. J Periodontol. 1965; 36:177–187.
133. Loesche, WJ. The role of spirochetes in periodontal disease. Adv Dent Res. 1988; 2:275–283.
134. Lundeen, HC, Gibbs, CH. The function of teeth. Gainesville, Fla: L and G Publishers; 2005.
135. Magnusson, I, Marks, RG, Clark, WB, et al. Clinical, microbiological and immunological characteristics of subjects with “refractory” periodontal disease. J Clin Periodontol. 1991; 18:291–299.
136. Marshall-Day, CD. The epidemiology of periodontal disease. J Periodontol. 1951; 22:13.
137. Massler, M, Sen Savara, BS. Relation of gingivitis to dental caries and malocclusion in children 14-17 years of age. J Periodontol. 1951; 22:87–96.
138. Maynard, JG, Ochsenbein, LD. Mucogingival problems, prevalence and therapy in children. J Periodontol. 1975; 46:543–552.
139. McCombie, F, Stothard, D. Relationships between gingivitis and other dental conditions. J Can Dent Assoc. 1964; 30:506–513.
140. Melsen, B, Agerbaek, N, Eriksen, J, Terps, S. New attachment through periodontal treatment and orthodontic intrusion. Am J Orthod Dentofacial Orthop. 1988; 94(2):104–116.
141. Melsen, B, Agerbaek, N, Makenstam, G. Intrusion of incisors in adult patients with marginal bone loss. Am J Orthod Dentofacial Orthop. 1989; 96:232–241.
142. Melsen, B. Biological reaction of alveolar bone to orthodontic tooth movement. Angle Orthod. 1999; 69:151–158.
143. Miller, J, Hobson, P. The relationship between malocclusion, oral cleanliness, gingival conditions and dental caries in school children. Br Dent J. 1961; 111:43–52.
144. Motegi, E, Nomura, M, Miyazaki, H, et al. Gingival recession in long-term post-orthodontic patients. J Dent Res. 2002; 81:A372.
145. Musialowicz, KJ. Relation between malocclusion and periodontal diseases in school children from urban and rural areas covered and not covered by planned stomatological care. Ann Acad Med Stetin. 1983; 29:371–385.
146. Newman, WG. Possible etiologic factors in external root resorption. Am J Orthod. 1975; 67:522.
147. Northway, WM, Meade, JB, Jr. Surgically assisted rapid maxillary expansion: a comparison of technique, response, and stability. Angle Orthod. 1997; 67:309–320.
148. Nyman, S, Karring, T, Bergenholtz, G. Bone regeneration in alveolar bone dehiscences produced by jiggling forces. J Periodontal Res. 1982; 17:316–322.
149. O’Leary, TJ, Badell, MC, Bloomer, RS. Occlusal characteristics and tooth mobility in periodontally healthy young males classified orthodontically. J Periodontol. 1975; 46:553–558.
150. Ochsenbein, C, Maynard, JG. The problem of attached gingiva in children. ASDC J Dent Child. 1974; 41:263–272.
151. Ogaard, B. Marginal bone support and tooth lengths in 19-year-olds following orthodontic treatment. Eur J Orthod. 1988; 10(3):180–186.
152. Olsson, M, Lindhe, J. Periodontal characteristics in individuals with varying forms of the upper central incisors. J Clin Periodontol. 1991; 118:78–82.
153. Omnell, ML, Tong, DC, Thomas, T. Periodontal complications following orthognathic surgery and genioplasty in 19-year-old: a case report. Int J Adult Orthodon Orthognath Surg. 1994; 9:133.
154. Paolantonio, M, Festa, F, di Placido, G, et al. Site-specific subgingival colonization by Actinobacillus actinomycetemcomitans in orthodontic patients. Am J Orthod Dentofacial Orthop. 1999; 115(4):423–428.
155. Papapanou, PN, Wennström, JL, Gröndahl, K. Periodontal status in relation to age and tooth type: a cross-sectional radiographic study. J Clin Periodontol. 1988; 15:463–478.
156. Parker, GR. Transseptal fibers and relapse following bodily retraction of teeth: a histologic study. Am J Orthod. 1972; 61:331–334.
157. Pearson, LE. Gingival heights of lower central incisors orthodontically treated and untreated. Angle Orthod. 1968; 38:337–339.
158. Pedrazzoli, V, Kilian, M, Karring, T, et al. Effect of surgical and nonsurgical periodontal treatment on periodontal status and subgingival microbiota. J Clin Periodontol. 1991; 18:598–604.
159. Pfeifer, JS. The reaction of alveolar bone to flap procedures in man. Periodontics. 1965; 3:135–140.
160. Pihlstrom, BL, Ramfjord, SP. Periodontal effect of nonfunction in monkeys. J Periodontol. 1971; 42:748.
161. Polson, AM. Long-term effect of orthodontic treatment on the periodontium. In: McNamara JA, Ribbens KA, eds. Orthodontic treatment and the periodontium. Monograph 15, Craniofacial Growth Series. Ann Arbor, Mich: Center for Human Growth and Development, The University of Michigan; 1984:89–100.
162. Pontoriero, R, Celenza, F, Ricci, G, Carvenale, G. Rapid extrusion with fiber resection: a combined orthodontic-periodontic treatment modality. Int J Periodontics Restorative Dent. 1987; 7:31–43.
163. Poulton, DR, Aaronson, SA. The relationship between occlusion and periodontal status. Am J Orthod. 1961; 47:690–699.
164. Preber, H, Bergström, J. The effect of non-surgical treatment on periodontal pockets in smokers and non-smokers. J Clin Periodontol. 1985; 13:319–323.
165. Preber, H, Bergström, J. The effect of cigarette smoking on periodontal healing following surgical therapy. J Clin Periodontol. 1990; 17:324–328.
166. Pritchard, JF. Gingivectomy, gingivoplasty, and osseous surgery. J Periodontol. 1961; 32:257–262.
167. Pritchard, JF. The effect of bicuspid extraction orthodontics on the periodontium. Findings in 100 consecutive cases. J Periodontol. 1975; 44:534–542.
168. Proffit, WR, Phillips, C, Fields, HW, Turvey, TA. The effect of orthognathic surgery on occlusal force. J Oral Maxillofac Surg. 1989; 47:457–463.
169. Reitan, K. Continuous bodily tooth movement and its histological significance. Acta Odontol Stand. 1947; 6:115.
170. Reitan, K. Tissue rearrangement during retention of orthodontically rotated teeth. Angle Orthod. 1959; 29:105–113.
171. Ren, Y, Maltha, JC, Von den Hoff, JW, Kuijpers-Jagtman, AM. Age effect on orthodontic tooth movement in rats. J Dent Res. 2003; 82:38–42.
172. Ren, Y, Maltha, JC, Kuijpers-Jagtman, AM. The rat as a model for orthodontic tooth movement: A critical review and a proposed solution. Eur J Orthod. 2004; 26:483–490.
173. Ribeiral, MBC, Bolognese, AM, Feres, EJ. Periodontal evaluation after orthodontic treatment. J Dent Res. 1999; 78(5):979.
174. Roberts, WE, Goodwin, WC, Jr., Heiner, SR. Cellular response to orthodontic force. Dent Clin North Am. 1981; 25:3–17.
175. Robertson, PB, Schultz, LD, Levy, BM. Occurrence and distribution of interdental gingival clefts following orthodontic movement into bicuspid extraction sites. J Periodontol. 1977; 48:232–235.
176. Roth, RH. Five-year clinical evaluation of the Andrews straight wire appliance. J Clin Orthod. 1976; 10:836–850.
177. Ruben, RP, Frankl, SN, Wallace, S. The histopathology of periodontal disease in children. J Periodontol. 1971; 42:473.
178. Ruf, S, Hansen, K, Pancherz, H. Does orthodontic proclination of lower incisors in children and adolescents cause gingival recession? Am J Orthod Dentofacial Orthop. 1998; 114:100–106.
179. Rugg-Gunn, AJ, MacGregor, IDM, Edgar, WM, Ferguson, MW. Toothbrushing behaviour in relation to plaque and gingivitis in adolescent school children. J Periodontal Res. 1979; 14:231–238.
180. Ryon, K. Effects of force, magnitude and direction of tooth movement on different alveolar bone types. Angle Orthod. 1964; 34:244.
181. Sadowsky, C, BeGole, EA. Long-term status of temporomandibular joint function and functional occlusion after orthodontic treatment. Am J Orthod. 1980; 78:201–212.
182. Sadowsky, C, BeGole, EA. Long-term effects of orthodontic treatment on periodontal health. Am J Orthod. 1981; 80(2):156–172.
183. Safkan-Seppala, B, Ainamo, J. Periodontal conditions in insulin-dependent diabetes mellitus. J Clin Periodontol. 1992; 19(1):24–29.
184. Salonen, LW, Frithiof, L, Wouters, FR, Hellden, LB. Marginal alveolar bone height in an adult Swedish population: a radiographic cross-sectional epidemiologic study. J Clin Periodontol. 1991; 18(4):223–232.
185. Sarikaya, S, Haydar, B, Ciger, S, Ariyurek, M. Changes in alveolar bone thickness due to retraction of anterior teeth. Am J Orthod Dentofacial Orthop. 2002; 122:15–26.
186. Schneider, HG, Brendel, AK. Oral hygiene study of children under orthodontic treatment. Stomatol DDR. 1981; 31:258–264.
187. Schneider, HG, Mauersberger, I, Bruchmann, M. Relation between periodontal diseases and malocclusions. Stomatol DDR. 1981; 31:494–500.
188. Sergl, HG, Krause, H. Studies on the effects of malocclusions on the periodontium. Dtsch Zahnarztl Z. 1973; 28:149–154.
189. Shaw, WC, Addy, M, Ray, C. Dental and social effects of malocclusion and effectiveness of orthodontic treatment: a review. Community Dent Oral Epidemiol. 1980; 8:36–45.
190. Shefter, GJ, McFall, WT, Jr. Occlusal relationships and periodontal status in human adults. J Periodontol. 1984; 55:368–374.
191. Shinberg, OE, Saakian, S, Zapashnik, EK. The functional periodontal overload in bite anomalies in adults. Stomatologiia (Mosk). 1991; 42–44.
192. Sjolien, T, Zachrisson, BW. Periodontal bone support and tooth length in orthodontically treated persons. Am J Orthod. 1973; 64:28–37.
193. Slots, J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977; 85:247–254.
194. Sperry, TP, Spiedel, TM, Isaacson, RJ, Worms, FW. The role of dental compensations in the orthodontic treatment of mandibular prognathism. Angle Orthod. 1977; 47:293–299.
195. Steiner, GG, Pearson, JK, Ainamo, J. Changes of the marginal periodontium as a result of labial tooth movement in monkeys. J Periodontol. 1981; 52:314–320.
196. Sten, IB. Tooth malpositions and periodontal pathosis: an evaluation of etiology and considerations in treatment. J Peridontol. 1958; 29:253.
197. Stuteville, OH. Injuries caused by orthodontic appliances and methods of preventing these injuries. J Am Dent Assoc. 1937; 24:1494–1507.
198. Tad, S, Zachrisson, BU. Longitudinal study of periodontal condition associated with orthodontic treatment in adolescents. Am J Orthod. 1979; 76:277–286.
199. Thilander, B, Nyman, S, Karring, T, Magnusson, I. Bone regeneration in alveolar bone dehiscences related to orthodontic tooth movements. Eur J Orthod. 1983; 5:105–114.
200. Thilander, B. Infrabony pockets and reduced alveolar bone height in relation to orthodontic therapy. Semin Orthod. 1996; 2(1):55–61.
201. Thompson, RH, Geiger, AM, Turgeon, LR. Relationship of occlusion and periodontal disease. Part III. Relation of periodontal status to general background characteristics. J Periodontol. 1972; 43:540–546.
202. Tirk, TM, Guzman, CA, Nalchajian, R. Periodontal tissue response to orthodontic treatment studied by panoramix. Angle Orthod. 1967; 37:94–103.
203. Trossello, VK, Gianelly, AA. Orthodontic treatment and periodontal status. J Periodontol. 1979; 50:665–671.
204. Vanarsdall, RL, Corn, H. Soft-tissue management of labially positioned unerupted teeth. Am J Orthod. 1977; 72(1):53–64.
205. Vanarsdall, RL, Jr. Periodontal/orthodontic interrelationships. In: Graber TM, Vanarsdall RL, eds. Orthodontics: current principle and techniques. ed 2. St. Louis: Mosby; 1994:712–749.
206. Wagaiyu, EG, Ashley, FP. Mouth breathing, lip seal and upper lip coverage and their relationship with gingival inflammation in 11-14 year-old schoolchildren. J Clin Periodontol. 1991; 18:698–702.
207. Wainwright, WM. Faciolingual tooth movement: influence on the root and cortical plate. Am J Orthod. 1973; 65:278.
208. Wasserman, BH, Geiger, AM, Turgeon, LR. Relationship of occlusion and periodontal disease: Part VII. Mobility. J Periodontol. 1973; 44:572–578.
209. Watson, WG. Expansion and fenestration or dehiscence. Am J Orthod. 1980; 77:330–332.
210. Wehrbein, H, Fuhrmann, RA, Diedrich, PR. Periodontal conditions after facial root tipping and palatal root torque of incisors. Am J Orthod Dentofacial Orthop. 1994; 106:455–462.
211. Wehrbein, H, Fuhrmann, RA, Diedrich, PR. Human histologic tissue response after long-term orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1995; 107(4):360–371.
212. Wennstrom, JL, Lindhe, J, Sinclair, F, Thilander, B. Some periodontal tissue reactions to orthodontic tooth movement in monkeys. J Clin Periodontol. 1987; 14(3):121–129.
213. Wennström, JL. The significance of the width and thickness of the gingiva in orthodontic treatment. Dtsch Zahnarztl Z. 1990; 45:136–141.
214. Wennström, JL, Lindskog-Stokand, B, Nyman, S, et al. Periodontal tissue response to orthodontic movement of teeth with infrabony pockets. Am J Orthod Dentofacial Orthop. 1993; 103:313–319.
215. Wennström, JL. Mucogingival considerations in orthodontic treatment. Semin Orthod. 1996; 2(1):46–54.
216. World Health Organization. Epidemiology, etiology and prevention of periodontal disease. Geneva: Report of WHO Scientific Group, Technical Report Series; 1978.
217. Yurkstas, A, Curby, W. Force analysis of prosthetic appliance during function. J Prosthet Dent. 1953; 3:82–87.
218. Zachrisson, BW, Alnaes, L. Periodontal condition in orthodontically treated and untreated children: 1. Loss of attachment, gingival pocket depth and clinical crown heights. Angle Orthod. 1973; 43:402–411.
219. Zachrisson, BW, Alnaes, L. Periodontal condition in orthodontically treated and untreated individuals. Angle Orthod. 1974; 44:48–55.
220. Zachrisson, S, Zachrisson, B. Gingival condition associated with orthodontic treatment. Angle Orthod. 1972; 42:26–34.
221. Zambon, JJ, Grossi, SG, Machtei, EE, et al. Cigarette smoking increases the risk for subgingival infection. J Periodontol. 1996; 67(Suppl 10):1050–1054.
*References 20, 21, 24, 25, 36, 43, 47, 49, 56, 66, 100, 111, 115, 116, 121, 122, 142, 144, 151, 156, 173, 182, 192, 197, 198, 202, 218–220
*References 23, 28, 32, 33, 44–46, 53, 55, 64, 65, 72, 77, 79–86, 95, 96, 105, 109, 110, 112, 118, 120, 123, 137, 143, 145, 149, 163, 187, 188–190, 196, 201, 208