Chapter 27 Perilymphatic Fistula
Iatrogenic poststapedectomy PLFs were first described in the mid-1960s. Steffen and colleagues1 reported findings of gross perilymph flow at the oval window in poststapedectomy patients with hearing loss, tinnitus, and vertigo. Fee2 reported three patients who presented with vertigo, fluctuating hearing loss, and tinnitus, who also had known or suspected recent head trauma. Intraoperative findings showed perilymph leak at the oval window. Repair of the leak resulted in significant improvement in symptoms.
In 1971, Goodhill3 coined the terms implosive (Valsalva-induced) and explosive (increased intracranial pressure) to describe pressure changes that can result in PLF. In reference to implosive, Goodhill stated that increased negative pressure from the tubotympanic region can be directed via the ossicles to the perilymphatic space. These external forces can result in a tear in the oval or round window membranes, driving air into the inner ear and displacing perilymph into the middle ear. The explosive route results from increased intracranial pressure possibly secondary to a severe blow to the head or abdomen. Increased intra-abdominal pressure transmits via the vertebral veins to increase the cerebrospinal pressure with resultant increase in intracranial pressure. Increased intracranial pressure can be transmitted to the inner ear via the cochlear aqueduct or internal auditory canal, potentially causing subsequent rupture, from inside out, of the oval or round window, or both.3
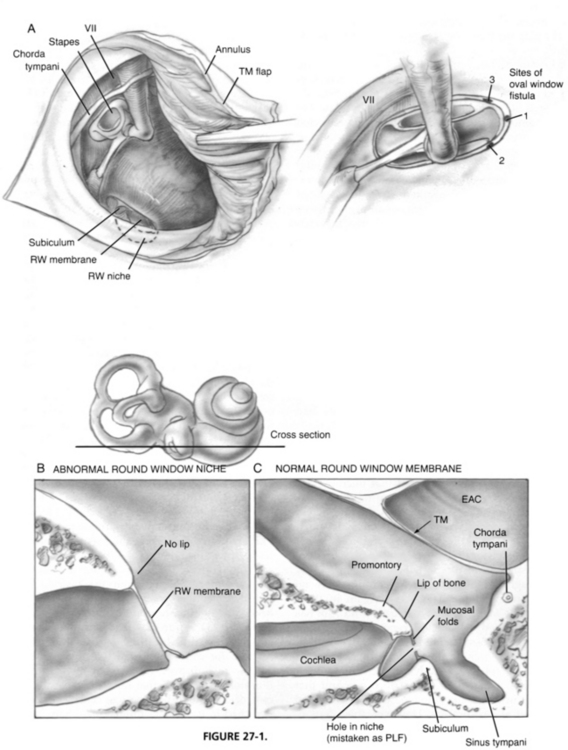
Homeostasis of the pressure differentials between endolymph and perilymph is maintained by patency of the endolymphatic duct and sac. If this pressure/volume balance is disrupted, as might occur in the case of a PLF, endolymphatic hydrops may result in damage to the hearing apparatus. Animal models with experimental PLF have shown hydrops, usually resolving within 3 weeks.4,5 The cochlear aqueduct provides an important communication between the subarachnoid and perilymphatic space. The only outlet from increased intracochlear pressure is via a tear of the oval or round window membranes. Tears of the oval window annular ligament occur most frequently anteroinferiorly, rarely superiorly around the stapes footplate. The round window membrane tears less frequently.6 Tears of the round window membrane have occurred when its position was 45 degrees to the promontory, and there was little or no overhanging promontory, allowing direct visualization of the round window membrane transtympanically (Fig. 27-1B).7,8
Microfissures of the otic capsule in the area of the oval window, round window niche, and posterior canal ampulla have been previously theorized to lead to PLF. This theory was based on temporal bone studies by Kohut and colleagues,9 who noted “loose fibrous composition in the fissula ante fenestra” in patients with PLF. A follow-up study by El Shazly and Linthicum10 showed that these microfissures are commonly present in temporal bones. No association between the presence of microfissures and sudden sensorineural hearing loss was found, and no evidence of PLF was noted.
Animal studies have tried to examine the pathophysiologic mechanism contributing to the hearing loss noted in PLF patients. Weisskopf and colleagues11 generated perforations in the round window membrane of guinea pigs. The resultant hearing loss recovered with time, and the authors concluded that perforations alone did not explain the degree of hearing loss present in PLF. Some research has shown pneumolabyrinth to cause sizable auditory threshold shifts, with removal of the air bubble from the cochlea resulting in resolution of hearing loss.12
Early stapedectomy procedures were more likely to result in PLF than methods practiced today. The entire stapes and footplate were often removed and replaced with a pointed polyethylene strut or wire loop prosthesis placed atop a thin piece of absorbable gelatin sponge (Gelfoam) or tissue seal. The wire could migrate, and the thin tissue membrane could rupture, both increasing chances of PLF. The current small fenestra, small piston, tissue seal techniques have dramatically reduced the incidence of PLF.13–17 Lesinski18 prospectively reviewed 19 cases of revision stapedectomy performed for symptoms of possible oval window fistula. The 14 of 19 patients who were noted to have an active PLF had undergone prior complete stapedectomy with migration of the prosthesis eccentrically, close to the edge of the oval window.
Invasive procedures of the stapes footplate, including the Fick or Cody tack procedure for Meniere’s disease (sacculotomy) were ultimately abandoned because of the high incidence of sensorineural hearing loss. Later exploration of many of these ears revealed a persistent PLF.19 Likewise, the 20% sensorineural hearing loss associated with cochleostomy or cochleosacculotomy, as it was originally described, was probably related to a persistent round window PLF.19
As awareness of PLFs increased, the number of diagnoses and surgical explorations for PLF did as well. Lower rates of positive exploration and of successful repair were being reported, however. In many cases of re-exploration for possible persistent PLF, prior operative records had described the presence of a hole in the round window membrane. Re-exploration findings showed a normal-appearing round window membrane deep in the niche (Fig. 27-1C). It is likely the prior surgeon had sealed the mucosal folds surrounding the round window niche.
Similarities in symptoms may have led to the misdiagnosis of Meniere’s disease or superior semicircular canal dehiscence as PLF. Attempts at identifying predictive tests, such as glycerin challenge testing or electrocochleography, to differentiate Meniere’s disease from PLF have been unsuccessful.20–23 The classic symptoms of Meniere’s disease are episodic vertigo, fluctuant hearing loss, tinnitus, and, in 80%, a sense of fullness in the involved ear. Patching of windows in these cases with no visible leak would give the same result as allowing time to pass. In Minor’s24 review of 65 patients with superior semicircular canal dehiscence, 54 (83%) had vestibular symptoms elicited by loud sounds, and 44 (67%) had pressure-induced (sneezing, coughing, and straining) symptoms. In addition, a 10 dB or greater conductive hearing loss was present in 70%. (See Chapter 42 for further discussion.)
Previous studies of children with sensorineural hearing loss of unknown etiology showed a rate of 6% to 16% positive PLFs on exploration.25–27 In the largest prospective series by Reilly25 of 244 children with sensorineural hearing loss, 17% underwent exploration, all of whom had prior abnormal computed tomography (CT) scan findings. Of the children who underwent exploration, 26% had active congenital PLF. A more recently published retrospective series reported 64% of ears with suspected PLF to have a leak confirmed visually.28 Middle ear malformations have been noted in 81% of PLF patients at the time of surgery, most commonly an anomalous stapes.29
PATIENT SELECTION
Patient presentation is variable with PLF. Symptoms have been reported to be present from days to decades. The typical patient with a PLF presents with a sudden onset of hearing loss or mild vertigo or dysequilibrium, or both hearing loss and vertigo, associated with a traumatic event. Trauma includes invasive middle or inner ear surgical procedures, abdominal or head blows, blast injuries, or severe changes in environmental pressure, particularly in the presence of an upper respiratory infection or an acute allergic attack. In some series, one third to one half of patients recall no potential triggering event.30,31
In cases of surgically proven PLF, patients present with some degree of vestibular and auditory disturbances. Patients reported vestibular complaints including episodic or positional vertigo, dysequilibrium, lightheadedness, and motion intolerance 46% to 91% of the time.31–35 A chief complaint of hearing loss was present in 28% to 93% of patients.31–35 Although only 28% reported noting hearing loss in the Iowa series, 54% had abnormal audiograms.32 Hearing losses are most commonly reported to be sudden or rapidly progressive; however, in some series they were also noted to be fluctuant, raising debate as to whether the disease was actually Meniere’s disease.
Tinnitus has been reported as a chief complaint in 25% to 76% of patients with PLFs, always in combination with vestibular or auditory symptoms or both.31,32,35,36 In some series, no patients reported tinnitus as a presenting symptom.34
PREOPERATIVE EVALUATION
Vestibular signs are commonly present in PLF. Positional nystagmus may have a very short or no latency and relatively long duration. Repeated testing shows nystagmus with minimal or no fatigue, no direction reversal when changing from inducing position to sitting position, and less violent than seen with benign paroxysmal positional vertigo. The nystagmus is rarely rotatory. From most to least frequent, the nystagmus may be horizontal, diagonal, or vertical.27,37 The direction of the nystagmus is of no diagnostic value in determining which ear is involved.
Hearing loss is typically sensorineural, without a specific pattern. Conductive hearing loss is rarely observed. This hearing loss has been attributed to a slipped stapes prosthesis or possible presence of air within the labyrinth. The speech reception threshold is usually worse than anticipated from the pure tone average, and the discrimination score is usually lower than expected.38
Most series report usage of a fistula test by applying positive or negative pressure to an intact tympanic membrane with a pneumatic otoscope. Resultant nystagmus indicates a positive test and is present only about 25% of the time.45 A sensation of dysequilibrium suggests a positive test. Modifications, such as administration of the test with the patient standing and eyes closed30,39 or on a moving posture platform (platform fistula test), have been used to try to improve test sensitivity. Electronystagmography has also been performed concurrently. Several studies noted no significant difference with electronystagmography-enhanced fistula testing.34,41 High positive exploration rates have been reported in cases using the moving platform fistula test; however, these findings have not been supported by others.42,43
The Quix test involves having the patient stand erect with feet together, eyes closed, and arms outstretched. The examiner looks for deviation of the arms to the side of the lesion. The result is positive in only about 20% of cases.44
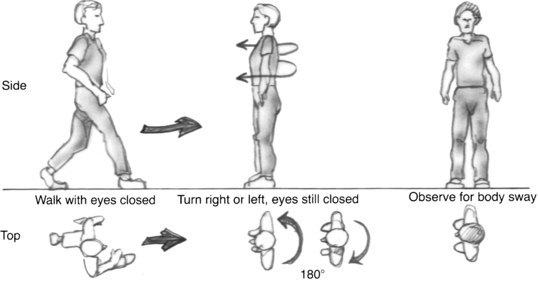
The eyes-closed-turning test has shown high sensitivity and specificity for PLF. It is performed by having the patient walk in a straight line with eyes closed. The examiner taps the subject’s shoulder, indicating to the patient to turn 180 degrees either right or left and stop in a position of attention with the eyes still closed (Fig. 27-2). A positive test is readily recognized by the patient’s swaying or having a tendency to lose balance when he or she has turned to the side of the lesion.45,46
Electrocochleography has been used in attempts at improving diagnostic ability; however, results have shown limited clinical utility. Because of pathophysiologic overlap with other conditions such as Meniere’s disease, electrocochleography lacks sensitivity in identifying PLF.21,47
Radiographic assessment has not proven to be helpful in diagnosis of PLF because adequate evaluation of the oval and round window regions is difficult. In patients with confirmed PLF, congenital anomalies of the middle or inner ear or both were able to be visualized on high-resolution CT only 50% of the time.48
Rigid and flexible endoscopy via myringotomy have been proposed to help in middle ear visualization before definitive exploration. Some authors report adequate visualization, whereas others have reported limited view of the round and oval window niches, often with mucosal adhesions obscuring the site.49–51 Negative findings were not followed up with formal exploration.
Visualization of clear fluid in the middle ear does not confirm presence of PLF. Injected local anesthetic pooling in the middle ear can confuse the picture. The volume of perilymph has been reported to be approximately 75 μL; visualization of such small volume can be difficult. Large fluid volume pooling in the middle ear raises the question of cerebrospinal fluid leak. Provocative testing, such as Trendelenburg position, Valsalva maneuver, and jugular vein compression, and application of mineral oil in the middle ear have been attempted; the usefulness of these techniques has not been proven.52,53
Numerous objective assays have been attempted to improve identification of perilymph in the middle ear. Technetium-labeled albumin was tried early on, but the albumin did not migrate into the perilymph. Intrathecal and intravenous fluorescein administration has not been shown to be useful given the potential neurologic complications and uncertainty of accurate perilymph labeling.54,55 Glucose measurement of approximately 100 mg/dL of middle ear fluid, in the absence of blood product, has been used to help identify perilymph. Silverstein56 reported on the use of indicator paper to identify protein concentration in middle ear fluid. The color change and correlation with known differing protein concentrations in perilymph, cerebrospinal fluid, and serum were used to determine presence of perilymph. Possible errors in identification could be due to local anesthetic dilution. Protein markers such as β2-transferrin57,58 and beta-trace protein,59 which are present in cerebrospinal fluid and perilymph, have also been investigated as possible diagnostic markers for PLF. Measurements of these markers in perilymph have been unreliable, owing to difficulty with collection methods and small sample volumes. In addition, processing time precludes use of these assays for intraoperative decision making.
SURGICAL PROCEDURE
Surgical Technique
The middle ear is opened at the notch of Rivinus by use of a Rosen needle. The chorda tympani nerve is identified, and the beginning of the annular ring is raised with the Rosen needle or drum elevator. The chorda tympani is gingerly dissected free of the tympanic membrane, and the posterior half of the tympanic membrane and ear canal flap are folded forward so that the middle ear may be inspected (see Fig. 27-1A).
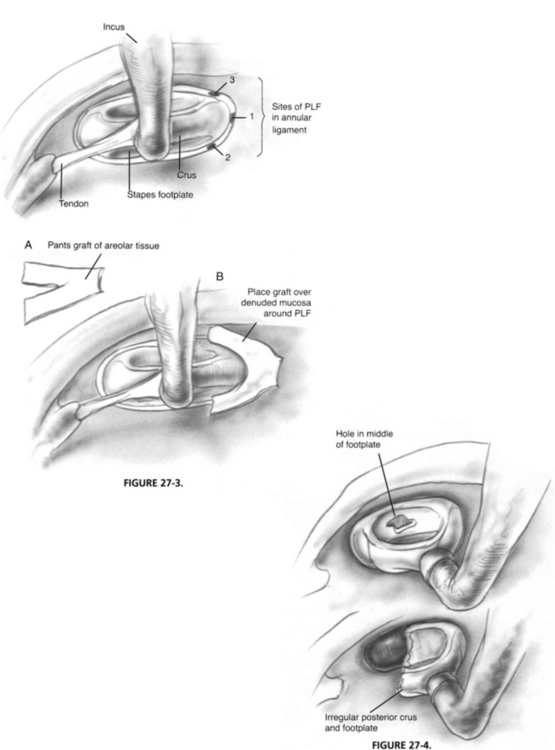
Most PLFs at the oval window are located directly anterior to the anterior crus or immediately below it; a few are superior to the anterior crus (Fig. 27-3). Generally, there is good visualization of this region. Leaks in this area are best repaired by teasing away the surface mucosa either with a straight pick or with a small right angle pick. A graft of adventitia is obtained from over the temporalis fascia. This graft is compressed and cut into the shape of a small set of trousers, approximately 3 mm long and 1.5 mm wide, and a 2 mm slit is created up the middle to form the pants’ legs (see Fig. 27-3A). The graft is draped around the anterior crus and packed in place with Gelfoam (see Fig. 27-3B) soaked in Ringer solution. Some authors have advocated adding fibrin glue to the graft for increased stability. Gelfoam is packed to the level of the tympanic membrane followed by a sheet of absorbable gelatin film (Gelfilm) to prevent adhesions to the tympanic membrane.
If there is a congenital defect of the stapes footplate, it may be present in the middle of the footplate, or may incorporate the entire posterior half of the footplate (Fig. 27-4). In these cases, the mucosa must be denuded completely around the footplate. With larger perforations, perichondrium from the tragus is used because it is thicker and easier to handle to effect a seal. The area between the crura is packed full with Gelfoam to hold the graft in place.
RESULTS
Most series report subjective improvements in vestibular and hearing symptoms, noting better response of vestibular disturbances to PLF surgery. After repair of PLF, 72% to 100% of patients report significant improvement of vertigo or dysequilibrium.31,32,34,40,60 Black and colleagues60 noted objective improvement in dynamic posturography after PLF surgery, with 12 of 32 patients having normal tests after PLF repair. Hearing outcomes after PLF surgery have not shown as much improvement. Hearing improvement has been reported in 13% to 49% of PLF repairs,32,34,40 and stabilization of hearing has been reported in 40% to 67%.33,35,60 Deterioration of hearing has been noted in 11% to 17% of PLF repairs.31,32,40
Negative exploration rates of 40% to 50% for suspected PLFs have been reported. Management of these suspected PLFs differ; some surgeons place grafts in the oval and round windows,34,35 whereas others avoid patching if no leak is visualized.
Fitzgerald and associates35 reported a negative exploration in 78% in their series of 197 patients. They patched the oval and round windows in all cases, and noted positive outcomes in 86% of their patients, leading them to define a proven PLF based on a positive surgical outcome, as opposed to positive intraoperative findings.
Hughes and colleagues61 surveyed 167 otologists in the United States, and noted 78% would place grafts on the oval and round windows at the time of exploration. Advocates of patching both windows in cases of negative exploration cite the possibility of minute62 or intermittent33 leaks. Overall improvement has been reported to range from 29% to 44% of negative explorations with prophylactic patching.33,34 The possibility of placebo effect of PLF surgery has also been considered.
Tissue allografts to patch the oval and round windows have included fat, fascia, perichondrium, and areolar tissue. Multiple authors recommend avoiding use of fat because it has been noted to have increased failure rate.32 Graft sites have also been covered with fibrin glue in hopes of improving postoperative outcome.31,35,63
RECURRENT PERILYMPHATIC FISTULA
Some patients have postoperative improvement with the balance disturbance and motion intolerance symptoms typical of PLF, but develop episodic whirling vertigo typical of Meniere’s disease. Potter and Conner64 raised the issue of possible endolymphatic hydrops secondary to membranous labyrinthine damage caused by PLF. Damage to the membranous labyrinth resulting in hydrops has been theorized to be a result of the initial trauma causing the PLF, the PLF leak itself causing stresses on the membranous labyrinth, or from trauma during surgical repair of the PLF.32 It is far more likely this group of patients had Meniere’s disease to begin with, not PLF.
Lollis and coworkers65 reported a retrospective review of a small series of patients with PLF refractory to otologic surgical management. For patients who showed improvement of their vertiginous symptoms, a ventriculoperitoneal shunt placement was performed. Subjective improvement in symptoms was reported in all patients so treated. No preoperative and postoperative objective measures were available for comparison in this study, however.
1. Steffen T.N., Sheehy J.L., House H.P. The slipped strut problem. Ann Otol Rhinol Laryngol. 1963;72:191-205.
2. Fee G.A. Traumatic perilymphatic fistulas. Arch Otolaryngol. 1968;88:477-480.
3. Goodhill V. Sudden deafness and round window rupture. Laryngoscope. 1971;81:1462-1474.
4. Nomura Y., Hara M., Funai H., Okuno T. Endolymphatic hydrops in perilymphatic fistula. Acta Otolaryngol. 1987;103:469-476.
5. Nomura Y., Hara M., Young Y.H., Okuno T. Inner ear morphology of experimental perilymphatic fistula. Am J Otol. 1992;13:32-37.
6. Singleton G.T. Perilymph fistula. In: Sharpe J.A., Barber H.O., editors. The Vestibulo-Ocular Reflex and Vertigo. New York: Raven Press, 1993.
7. Pullen F.W. Round window membrane rupture: A cause of sudden deafness. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:1444-1450.
8. Rybak L.P. “How I do it”—otology and neurotology. Laryngoscope. 1980;90:2049-2050.
9. Kohut R.I., Hinojosa R., Ryu J. Sudden-onset hearing loss in eleven consecutive cases: A temporal bone histopathologic study with identification of perilymphatic fistulae. Trans Am Otol Soc. 1989;77:81-88.
10. El Shazly M.A., Linthicum F.H. Microfissures of the temporal bone: Do they have any clinical significance? Am J Otol. 1991;12:169-171.
11. Weisskopf A., Murphy J.T., Merzenich M.M. Genesis of the round window rupture syndrome: Some experimental observations. Laryngoscope. 1978;88:389-397.
12. Nishioka I., Yanagihara N. Role of air bubbles in the perilymph as a cause of sudden deafness. Am J Otol. 1986;7:430-438.
13. Douek E. Perilymph fistula. J Laryngol Otol. 1975;89:123-130.
14. Goodhill V. Stapedectomy revision commandments: Posterior arch stapedioplasty. Trans Pac Coast Oto-ophthalmol Soc. 1974;55:35-59.
15. Harrison W.H., Shambaugh G.E., Derlacki E.L., et al. Perilymph fistula in stapes surgery. Laryngoscope. 1967;77:736-849.
16. Hemenway W.G., Hildyard V.H., Black F.O. Post stapedectomy perilymph fistulas in the Rocky Mountain area: The importance of nystagmography and audiometry in diagnosis and early tympanotomy in prognosis. Laryngoscope. 1968;78:1687-1715.
17. House H.P. The fistula problem in otosclerotic surgery. Laryngoscope. 1967;77:1410-1426.
18. Lesinski S.G. Causes of conductive hearing loss after stapedectomy or stapedotomy: A prospective study of 279 consecutive surgical revisions. Otol Neurotol. 2002;23:281-288.
19. Singleton G.T. Perilymph fistulas. Adv Otolaryngol Head Neck Surg. 1988;2:25-38.
20. Lehrer J.F., Poole D.C., Sigal B. Use of the glycerin test in the diagnosis of post-traumatic perilymphatic fistulas. Am J Otolaryngol. 1980;1:207-210.
21. Arenberg I.K., Ackley R.S., Ferraro J., Muchnik C. ECoG results in perilymphatic fistula: Clinical and experimental studies. Otolaryngol Head Neck Surg. 1988;99:435-443.
22. Meyerhoff W.L., Yellin M.W. Summating potential/action potential ratio in perilymph fistula. Otolaryngol Head Neck Surg. 1990;102:678-682.
23. Campbell K.C.M., Karker L.A., Abbas P.J. Interpretation of electrocochleography in Ménière’s disease and normal subjects. Ann Otol Rhinol Laryngol. 1992;101:496-500.
24. Minor L.B. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717-1727.
25. Reilly J.S. Congenital perilymphatic fistula: A prospective study in infants and children. Laryngoscope. 1989;99:393-397.
26. Parnes L.S., McCabe B.F. Perilymph fistula: An important cause of deafness and dizziness in children. Pediatrics. 1987;89:524-528.
27. Pappas D.G., Simpson L.C., Godwin G.H. Perilymphatic fistula in children with pre-existing sensorineural hearing loss. Laryngoscope. 1988;98:507-510.
28. Weber P.C., Bluestone C.D., Perez B. Outcome of hearing and vertigo after surgery for congenital perilymphatic fistula in children. Am J Otol. 2003;24:138-142.
29. Weber P.C., Perez B.A., Bluestone C.D. Congenital perilymphatic fistula and associated middle ear abnormalities. Laryngoscope. 1993;103:160-164.
30. Cole G.G. Validity of spontaneous perilymphatic fistula. Am J Otol. 1995;16:815-819.
31. Goto F., Ogawa K., Kunihiro T., et al. Perilymph fistula 45 case analysis. Auris Nasus Larynx. 2001;28:29-33.
32. Seltzer S., McCabe B.F. Perilymph fistula: The Iowa experience. Laryngoscope. 1986;96:37-49.
33. Shelton C., Simmons F.B. Perilymph fistula: The Stanford experience. Ann Otol Rhinol Laryngol. 1988;97:105-108.
34. Rizer F.M., House J.W. Perilymph fistulas: The House Ear Clinic experience. Otolaryngol Head Neck Surg. 1991;104:239-243.
35. Fizgerald D.C., Getson P., Brasseux C.O. Perilymphatic fistula: A Washington, DC experience. Ann Otol Rhinol Laryngol. 1997;106:830-837.
36. Glasscock M.E.III, Hart M.J., Rosdeutscher J.D., Bhansali S.A. Traumatic perilymphatic fistula: How long can symptoms persist? A follow-up report. Am J Otol. 1992;13:333-338.
37. Bluestone C.D. Otitis media and congenital perilymphatic fistula as a cause of sensorineural hearing loss in children. Pediatr Infect Dis J. 1988;7:S141-S145.
38. Singleton G.T. Correlation of clinical symptoms of vertigo and/or hearing loss with anatomic site of surgically confirmed PLF. In: Arenberg I.K., editor. Inner Ear Surgery. Proceedings of the Third International Symposium and Workshops on Surgery of the Inner Ear. New York, Kugler: Snowmass-Aspen, CO; 1990:395-397. 1991
39. Kohut R.I. Perilymph fistulas: Clinical criteria. Arch Otolaryngol Head Neck Surg. 1992;118:687-692.
40. Healy G.B., Strong M.S., Sampgna D. Ataxia, vertigo, and hearing loss: A result of rupture of inner ear window. Arch Otolaryngol. 1974;100:130-135.
41. Podoshin L., Fradis M., Ben-David J., et al. Perilymphatic fistula—the value of diagnostic tests. J Laryngol Otol. 1994;108:560-563.
42. Black F.O., Lilly D.J., Nashner L.M., et al. Quantitative diagnostic test for perilymph fistulas. Otolaryngol Head Neck Surg. 1987;96:125-134.
43. Meyerhoff W.L. Spontaneous perilymphatic fistula: Myth or fact. Am J Otol. 1993;14:478-481.
44. Hart C.W. The evaluation of vestibular function in healing and disease. In: Hoeber, editor. Otolaryngology. Hagerstown, MD: Harper & Row; 1972:1-63.
45. Singleton G.T., Karlan M.S., Post K.N., et al. Perilymph fistulas: Diagnostic criteria and therapy. Ann Otol Rhinol Laryngol. 1978;87:797-803.
46. Singleton G.T. Perilymph fistula. In: Sharpe J.A., Barber H.O., editors. The Vestibulo-Ocular Reflex and Vertigo. New York: Raven Press, 1993.
47. Campbell K.C., Parnes L. Electrocochleographic recordings in chronic and healed perilymphatic fistula. J Otolaryngol. 1992;21:213-217.
48. Weissman J.L., Weber P.C., Bluestone C.D. Congenital perilymphatic fistula: Computed tomography appearance of middle ear and inner ear anomalies. Otolaryngol Head Neck Surg. 1994;111:243-249.
49. Rosenberg S.I., Silverstein H., Willcox T.O., Gordon M.A. Endoscopy in otology and neurotology. Am J Otol. 1994;15:168-172.
50. Poe D.S., Bottrill I.D. Comparison of endoscopic and surgical explorations for perilymphatic fistulas. Am J Otol. 1994;15:735-738.
51. Pyykko I., Selmani Z., Ramsay H. Middle ear imaging in neurotological work-up. Acta Otolaryngol Suppl. 1995;520:273-276.
52. Althaus S.R. Long-term results of perilymph fistula repair. Laryngoscope. 1973;23:1502-1507.
53. Todd N.W., Jackson R.T. Oil-on-water: Proposed method for intraoperative identification of perilymphatic fistula. Am J Otol. 1995;16:539-542.
54. Poe D.S., Gadre A.K., Rebeiz E.E., Pankratov M.M. Intravenous fluorescein for detection of perilymphatic fistulas. Am J Otol. 1993;14:51-55.
55. Bojrab D.I., Bhansali S.A. Fluorescein use in the detection of perilymphatic fistula: A study in cats. Otolaryngol Head Neck Surg. 1993;108:348-355.
56. Silverstein H. Rapid protein test for perilymph fistula. Otolaryngol Head Neck Surg. 1991;105:422-426.
57. Levenson M.J., Desloge R.B., Parisier S.C. Beta-2 transferrin: Limitations of use as a clinical marker for perilymph. Laryngoscope. 1996;106:159-161.
58. Buchman C.A., Luxford W.M., Hirsch B.E., et al. Beta-2 transferrin assay in the identification of perilymph. Abstr Am Otol Soc. 1998;131:40.
59. Michel O., Petereit H., Klemm E., et al. First clinical experience with beta-trace protein (prostaglandin D synthase) as a marker for perilymphatic fistula. J Laryngol Otol. 2005;119:765-769.
60. Black F.O., Pesznecker S., Norton T., et al. Surgical management of perilymphatic fistulas: A Portland experience. Am J Otol. 1992;13:254-262.
61. Hughes G.B., Sismanis A., House J.W. Is there consensus in perilymph fistula management? Otolaryngol Head Neck Surg. 1990;102:111-117.
62. Kohut R.I., Waldorf R.A., Haenel J.L., Thompson J.N. Minute perilymph fistulas: Vertigo and Hennebert’s sign without hearing loss. Ann Otol Rhinol Laryngol. 1979;88:153-159.
63. Black F.O., Pesznecker S., Norton T., et al. Surgical management of perilymph fistulas: A new technique. Arch Otolaryngol. 1991;117:641-648.
64. Potter C.R., Conner G.H. Hydrops following perilymphatic fistula repair. Laryngoscope. 1983;93:810-812.
65. Lollis S.S., Dudley J.W., Phillips J.M., Roberts D.W. Ventriculoperitoneal shunt insertion for the treatment of refractory perilymphatic fistula. J Neurosurg. 2006;105:1-5.