18 Pericardiocentesis
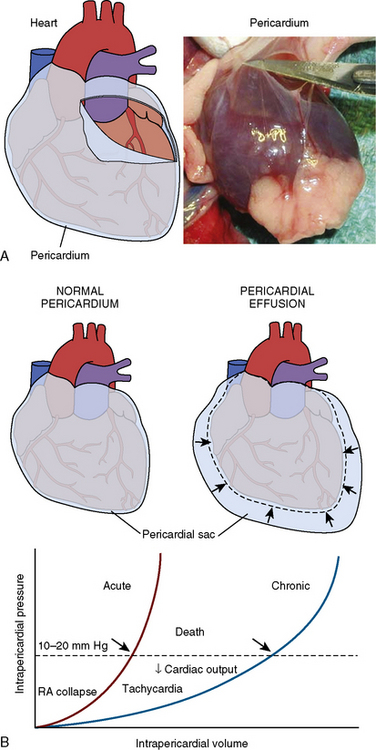
Cardiac tamponade is a life-threatening disorder that can result from any condition that causes a pericardial effusion. Although the most frequent cause is malignancy, tamponade may also occur from pericarditis (e.g., viral, uremic, inflammatory, or idiopathic), aortic dissection (with disruption of the aortic annulus), or ventricular rupture from myocardial infarction. In the cardiac catheterization laboratory, tamponade can result as a complication of a variety of invasive procedures and lead to rapid demise of the patient due to the swift accumulation of fluid in a poorly compliant pericardial space. Prompt recognition of the salient hemodynamic features and immediate pericardiocentesis are essential to the successful treatment of cardiac tamponade. The rate of pericardial fluid accumulation relative to the stiffness of the pericardium determines how quickly the clinical syndrome of tamponade will occur. Figure 18-1 shows a normal pericardial membrane and the mechanisms of pericardial tamponade.
Diagnosis of Tamponade
Echocardiography
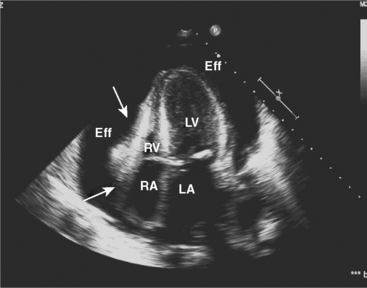
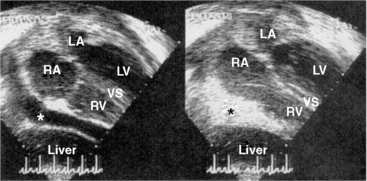
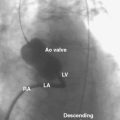
Two-dimensional and Doppler echocardiography are commonly used for diagnosing cardiac tamponade. Specific signs of tamponade include collapse of the right atrium and right ventricle, ventricular septal shifting with respiration, and plethora (enlargement) of the inferior vena cava (Fig. 18-2). Respiratory variation in the Doppler mitral inflow is a highly sensitive measure that occurs early in tamponade, and may precede changes in cardiac output, blood pressure, and other echocardiographic findings.
Hemodynamics
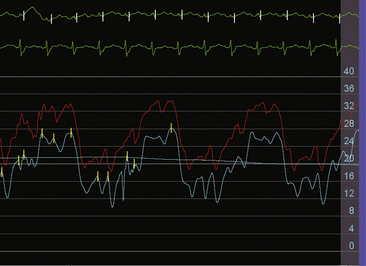
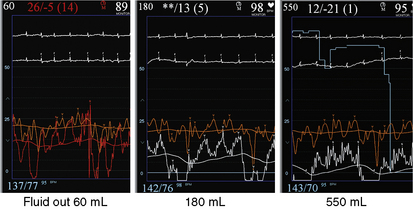
The invasive hemodynamic hallmarks of cardiac tamponade include pulsus paradoxus on the arterial tracing (Fig. 18-3) and prominent x descents and blunted y descents in the atrial pressure tracings (Fig. 18-4). Preservation of the x descent occurs because of the decrease in intracardiac volume during systolic ejection, which leads to a temporary reduction in intrapericardial and right atrial pressures (See Kern (2011) The Cardiac Catheterization Handbook 5th ed., Chapter 3 Hemodynamics). Elevated intrapericardial pressure impairs ventricular filling during the remainder of the cardiac cycle, resulting in blunting of the y descent. In patients with cardiac tamponade, the driving pressure to fill the left ventricle falls during inspiration. Consequently, there is a reduction in left ventricular filling and stroke volume, which manifests as a decrease in aortic pulse pressure during inspiration in a manner analogous to the bedside finding of pulsus paradoxus (see Fig. 18-3).
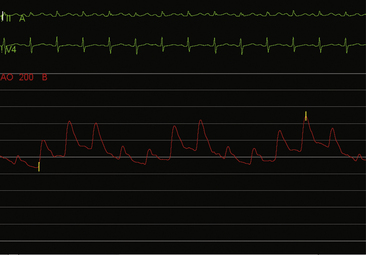
Figure 18-3 Arterial pressure in a patient with dyspnea at rest demonstrating pulsus paradoxus due to cardiac tamponade.
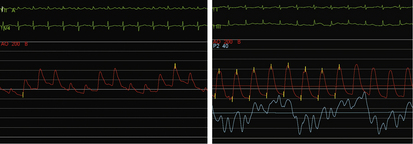
On relief of pericardial pressure and removal of the effusion, right atrial pressure and pericardial pressure fall usually to normal values if no residual pericardial disease is present (Fig. 18-5). However, in some cases, although pericardiocentesis empties the pericardial space and pericardial pressure falls to near zero, right atrial pressure may be unaffected, signifying the syndrome of effusive-constrictive pericardial disease (Fig. 18-6).
Technique for Pericardiocentesis
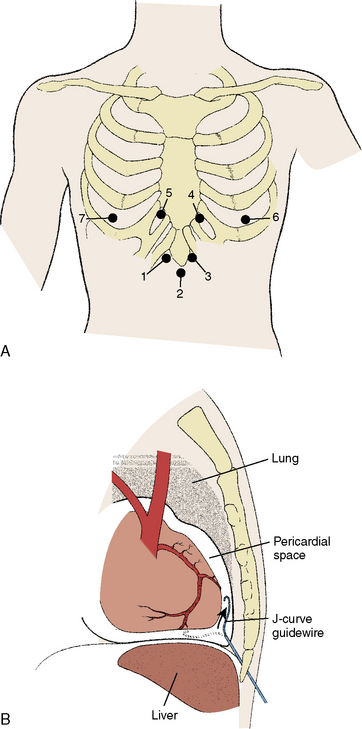
The basic technique of pericardiocentesis is discussed in Table 18-1 lists the equipment used for the procedure. For most patients, pericardiocentesis is performed with echocardiographic guidance. In some cases, additional use of hemodynamic monitoring through the pericardial needle adds important information during both puncture and after withdrawal of fluid to verify procedural findings. Certainly, in emergent situations where echocardiography is not immediately available, pericardiocentesis can be performed in a blinded or electrocardiogram (ECG)-guided fashion, usually from the subxiphoid approach. However, adjunctive echocardiography plays a significant role in the evaluation of patients with cardiac tamponade and will reduce the incidence of complications related to pericardiocentesis. Figure 18-7 shows several points of pericardial access.
| Sterile gloves, mask, and gown |
| Povidone-iodine solution or other skin antiseptic |
| Sterile transparent plastic drape |
| 20- or 25-gauge needle for local anesthesia administration |
| Local anesthesia (e.g., 1% lidocaine) |
| 18-gauge polytef-sheathed venous needles of varying lengths (5–8 cm) |
| Syringes (10 mL, 20 mL, and 50 mL) |
| 0.035-inch J-tipped guidewire |
| Scalpel (no. 11 blade) |
| 5F or 6F introducer sheath |
| 5F or 6F 65-cm pigtail catheter with multiple side holes |
| 4 × 4 inch gauze for dressing and ointment |
| 1-L vacuum bottle or comparable fluid receptacle |
| Labels for specimen collection |
| Sterile isotonic saline (for catheter flush) |
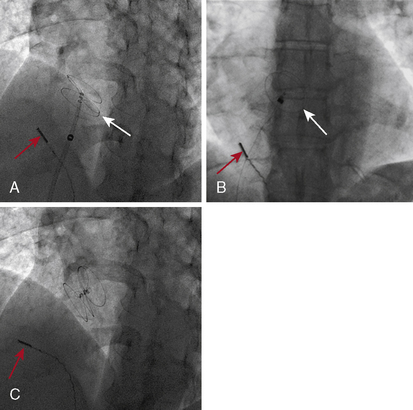
Site of entry. Echocardiography helps to determine the most appropriate site of entry and needle direction. Most frequently, the echocardiographic window that is closest to the effusion is selected. Common portals of entry are subxiphoid and apical, but other locations have included axillary, and left or right parasternal (see Fig. 18-7). Advantages of the subxiphoid approach are a lower risk of pneumothorax and laceration of internal mammary or intercostal arteries. For the subxiphoid approach, the needle must be angled clear below the bottom rib as it attaches to the inferolateral surface above the xiphoid process (typically one fingerbreadth inferior and lateral to the edge of the xiphoid).Punctures that are too high and near the recess at the xiphoid angle can pose challenges to delivering the needle under the rib. When using a parasternal approach, the needle should pass 1 cm lateral to the sternum to avoid injury to the internal mammary artery; the risk of pneumothorax increases with further lateral positioning. For intercostal approaches, the needle should pass superior to the rib margins to reduce the risk of injury to the neurovascular bundle. The angle of entry and direction should be transfixed in the operator’s mind. The site of entry can be marked with an indelible pen. The precordial or subxiphoid area is sterilized with antiseptic solution and covered with a sterile drape.
Needle insertion. Following local anesthesia, an 18-gauge, thin-walled polytef-sheathed needle is inserted at the entry site using the predetermined angulation. The needle is advanced with gentle aspiration into the pericardial space. Aggressive aspiration may cause tissue occlusion of the needle and inhibit detection of pericardial fluid. Once fluid has been obtained, the needle is advanced slightly further (~2 mm) to ensure placement of the sheath into the pericardial space. Figure 18-8 shows a pericardial needle and stopcock arrangement designed to check pericardial pressure and then aspirate pericardial fluid and discharge the fluid into a pericardial drainage bag. Alternately, a needle and sheath system can be used. Once the needle is in the pericardial space, the polytef sheath then is advanced over the needle, followed by withdrawal of the needle. The needle should not be re-advanced once it has been removed from the sheath.
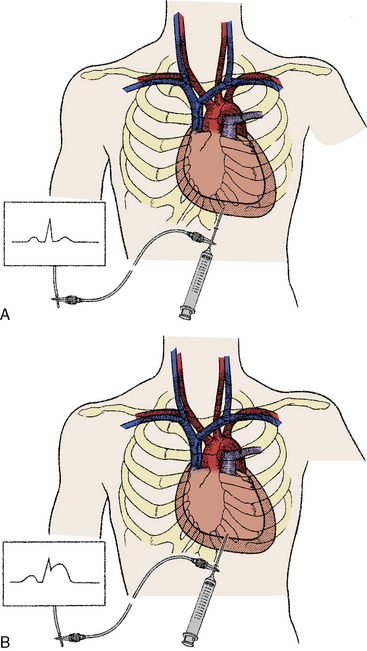
Confirmation of location. Agitated saline is injected into the sheath via a three-way stopcock with echocardiographic imaging (Fig. 18-9). If the agitated saline does not enhance the pericardial space, then repositioning of the needle by either withdrawal or another needle passage is performed. Radiographic contrast can also be administered under fluoroscopy. Small test injections should be given initially to exclude myocardial positioning, which is seen as myocardial staining. Contrast swirling will indicate a ventricular location, while pooling suggests intrapericardial positioning. Alternatively, the needle (before it is withdrawn) or the sheath can be connected to tubing connectors for pressure transduction. Intrapericardial pressure will be similar to the atrial pressure, while ventricular systolic pressure waveforms can immediately alert the operator to inadvertent ventricular perforation. For operators using an ECG-guided approach, the needle is connected to an alligator-tipped electrode. With myocardial contact, ST-segment elevation (i.e., injury current) will be detected that may not appear on other electrocardiographic leads (Fig. 18-10).
Appleton C.P., Hatle L.K., Popp R.L. Cardiac tamponade and pericardial effusion: respiratory variation in transvalvular flow velocities studied by Doppler echocardiography. J Am Coll Cardiol. 1988;11:1020–1030.
Atar S., Chiu J., Forrester J.S., et al. Bloody pericardial effusion in patients with cardiac tamponade: is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest. 1999;116:1564–1569.
Campbell P.T., Van Trigt P., Wall T.C., et al. Subxyphoid pericardiotomy in the diagnosis and management of large pericardial effusions associated with malignancy. Chest. 1992;101:938–943.
Duvernoy O., Borowiec J., Helmius G., et al. Complications of percutaneous pericardiocentesis under fluoroscopic guidance. Acta Radiol. 1992;33:309–313.
Galli M., Politi A., Pedretti F., et al. Percutaneous balloon pericardiotomy for malignant pericardial tamponade. Chest. 1995;108:1499–1501.
Holmes D.R., Nishimura, Fountain R., et al. Iatrogenic pericardial effusion and tamponade in the percutaneous intracardiac intervention era. JACC Cardiovasc Interv. 2009;2:705–717.
Isselbacher E.M., Cigarroa J.E., Eagle K.A. Cardiac tamponade complicating proximal aortic dissection: is pericardiocentesis harmful? Circulation. 1994;90:2375–2378.
Kopecky S.L., Callahan J.A., Tajik A.J., et al. Percutaneous pericardial catheter drainage: report of 42 consecutive cases. Am J Cardiol. 1986;58:633–635.
Little W.C., Freeman G.L. Pericardial disease. Circulation. 2006;113:1622–1632.
Maisch B., Seferović P.M., Ristić A.D., et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587–610.
Markiewicz W., Borovik R., Ecker S. Cardiac tamponade in medical patients: treatment and prognosis in the echocardiographic era. Am Heart J. 1986;111:1138–1142.
Meltser H., Kalaria V.G. Cardiac tamponade. Catheter Cardiovasc Interv. 2005;64:245–255.
Tsang T.S., Enriquez-Sarano M., Freeman W.K., et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429–436.
Tsang T.S., Freeman W.K., Barnes M.E., et al. Rescue echocardiographically guided pericardiocentesis for cardiac perforation complicating catheter-based procedures. The Mayo Clinic experience. J Am Coll Cardiol. 1998;32:1345–1350.
Tsang T.S., Freeman W.K., Sinak L.J., et al. Echocardiographically guided pericardiocentesis: evolution and state-of-the-art technique. Mayo Clin Proc. 1998;73:647–652.
Wolfe M.W., Edelman E.R. Transient systolic dysfunction after relief of cardiac tamponade. Ann Intern Med. 1993;119:42–44.
Ziskind A.A., Pearce A.C., Lemmon C.C., et al. Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: description of technique and report of the first 50 cases. J Am Coll Cardiol. 1993;21:1–5.