85 Pericardial Diseases
 Etiology and Classification of Pericardial Disease
Etiology and Classification of Pericardial Disease
The spectrum of pericardial diseases consists of congenital defects, pericarditis (dry, effusive, effusive-constrictive, constrictive), neoplasm, and cysts. The etiologic classification comprises infectious pericarditis, pericarditis in systemic autoimmune diseases, type 2 (auto) immune process, post-myocardial infarction syndrome, and autoreactive (chronic) pericarditis.1–3
 Pericardial Syndromes
Pericardial Syndromes
Congenital Defects of the Pericardium
Congenital defects of the pericardium occur in 1 in 10,000 autopsies. Pericardial absence can be partial left (70%), right (17%), or total bilateral (rare). Additional congenital abnormalities occur in approximately 30% of patients.4 Most patients with a total pericardial absence are asymptomatic. Homolateral cardiac displacement and augmented heart mobility impose an increased risk for traumatic aortic dissection.5 Partial left-side defects can be complicated by herniation and strangulation of the heart through the defect (chest pain, shortness of breath, syncope, or sudden death). Surgical pericardioplasty (Dacron, Gore-Tex, or bovine pericardium) is indicated for imminent strangulation.6
Acute Pericarditis
Acute pericarditis is dry, fibrinous, or effusive, independent of its etiology. Major symptoms are retrosternal or left pre-cordial chest pain (which radiates to the trapezius ridge, can be pleuritic or simulate ischemia, and varies with posture) and shortness of breath. A prodrome of fever, malaise, and myalgia is common, but elderly patients may not be febrile. The pericardial friction rub can be transient and monophasic, biphasic, or triphasic. Pleural effusion may be present. Heart rate is usually rapid and regular. Echocardiography is essential to detect effusion and concomitant heart or paracardial disease (Table 85-1).7–19
TABLE 85-1 Diagnostic Pathway and Sequence of Performance in Acute Pericarditis
| Diagnostic Measure | Characteristic Findings |
|---|---|
| Obligatory | |
| Auscultation | Pericardial rub (monophasic, biphasic, or triphasic) |
| ECG* | Stage I: anterior and inferior concave ST segment elevation. PR segment deviations opposite to P wave polarity |
| Early stage II: all ST junctions return to the baseline. PR segments deviated. | |
| Late stage II: T waves progressively flatten and invert | |
| Stage III: generalized T wave inversions in most or all leads | |
| Stage IV: ECG returns to prepericarditis state | |
| Echocardiography | Effusion types B to D (Horowitz) |
| Signs of tamponade | |
| Blood analyses | Erythrocyte sedimentation rate, C-reactive protein, lactate dehydrogenase, leukocytes (inflammation markers) |
| Troponin I†, CK-MB (markers of myocardial involvement) | |
| Chest radiograph | Ranging from normal to “water bottle” shape of the heart shadow |
| Performed primarily to reveal pulmonary or mediastinal pathology | |
| Mandatory in Tamponade, Optional in Large/Recurrent Effusions or if Previous Tests Inconclusive in Small Effusions | |
| Pericardiocentesis/drainage | Polymerase chain reaction and histochemistry for etiopathogenetic classification of infection or neoplasia |
| Optional or if Previous Tests Inconclusive | |
| CT | Effusions, pericardium, and epicardium |
| MRI | Effusions, pericardium, and epicardium |
| Pericardioscopy, pericardial/epicardial biopsy | Establishing the specific etiology |
* Typical lead involvement: I, II, aVL, aVF, and V3-V6. The ST segment is always depressed in aVR frequently in V1, and occasionally in V2. Stage IV may not occur, and there are permanent T wave inversions and flattenings. If ECG is first recorded in stage III, pericarditis cannot be differentiated by ECG from diffuse myocardial injury, “biventricular strain,” or myocarditis. ECG in early repolarization is very similar to stage I. Unlike stage I, this ECG does not acutely evolve and J-point elevations are usually accompanied by a slur, oscillation, or notch at the end of the QRS just before and including the J point (best seen with tall R and T waves—large in early repolarization pattern). Pericarditis is likely if in lead V6 the J point is greater than 25% of the height of the T wave apex (using the PR segment as a baseline).
† A cTnI rise was detectable in 38/118 patients (32.2%), more frequently in younger, male patients, with ST-segment elevation and pericardial effusion at presentation. An increase beyond 1.5 ng/mL was rare (7.6%), and associated with CK-MB elevation. cTnI increase was not a negative prognostic marker regarding the incidence of recurrences, constrictive pericarditis, cardiac tamponade, or residual left ventricular dysfunction (Imazio). Data from references 2, 3, and 7 to 19.
Hospitalization and symptomatic treatment is warranted. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the mainstay. Indomethacin should be avoided in elderly patients, owing to its effect on reducing flow in the coronaries. Ibuprofen (300 to 800 mg tid) is preferred for its rare side effects, favorable impact on coronary flow, and large dose range.7 Colchicine 0.5 mg at least twice daily for 3 months added to an NSAID or to aspirin reduced the recurrence rate impressively in the COPE trial20 even at the first episode of pericarditis or even as monotherapy in “idiopathic” effusions. It is well tolerated with fewer side effects than NSAIDs. Systemic corticosteroids should be restricted to connective tissue diseases and autoreactive or uremic pericarditis. Intrapericardial steroid application as long-acting crystalloid triamcinolone is effective for autoreactive effusions and avoids systemic side effects.2
Chronic Pericarditis
Chronic (>3 months) pericarditis includes effusive (inflammatory or hydropericardium in heart failure), adhesive, and constrictive forms.7 Symptoms are usually mild (chest pain, palpitations, fatigue), related to the degree of cardiac compression and pericardial inflammation. The detection of the curable causes (e.g., tuberculosis, toxoplasmosis, myxedema, viral, autoimmune, and systemic diseases) allows successful specific therapy. Symptomatic treatment and pericardiocentesis should be applied if indicated. For recurrences the etiology should be investigated intensely and if no specific therapy is effective, balloon pericardiotomy or pericardiectomy may be considered.22,23
Recurrent Pericarditis
The term recurrent pericarditis encompasses (1) the intermittent type (symptom-free intervals without therapy) and (2) the incessant type (discontinuation of anti-inflammatory therapy ensures a relapse). Massive pericardial effusion, overt tamponade, or constriction is rare. Symptomatic management relies on exercise restriction and the regimen used in acute pericarditis. Colchicine may be effective when NSAIDs and corticosteroids failed to prevent relapses.20,21,24,25 It should be considered first-choice treatment for recurrent pericarditis according to the CORE trial.21 Corticosteroids should be used only in patients with poor general condition or in frequent crises.7 A common mistake could be to use a dose too low to be effective or to taper the dose too rapidly. The recommended regimen is prednisone, 1 to 1.5 mg/kg, for at least 1 month. If patients do not respond adequately, azathioprine (75 to 100 mg/day) or cyclophosphamide can be added.26
Corticosteroids should be tapered over a 3-month period. Toward the end of the taper, introduce antiinflammatory treatment with colchicine (0.5 mg bid or tid) or an NSAID. Renewed treatment should continue for 3 to 6 months. Recently it was demonstrated in “idiopathic” pericarditis that previous corticoid treatment was even a risk factor for recurrence or chronicity. Therefore corticoids should be administered after definite exclusion of viral or bacterial infection of the pericardium. Pericardiectomy is indicated only in frequent and highly symptomatic recurrences resistant to medical treatment.27
Pericardial Effusion and Cardiac Tamponade
Pericardial effusion may appear as transudate (hydropericardium), exudate, pyopericardium, or hemopericardium. Large effusions are common with neoplastic, tuberculous, cholesterol, uremic, myxedema, and parasitoses pericarditis.28 Loculated effusions are more common when scarring has supervened (e.g., postsurgical, post trauma, purulent pericarditis). Effusions that develop slowly can be remarkably symptomatic, whereas rapidly accumulating smaller effusions can present as tamponade. Cardiac tamponade is the decompensated phase of cardiac compression caused by effusion accumulation and the increased intrapericardial pressure. Heart sounds are distant. Orthopnea, cough, and dysphagia, occasionally with episodes of unconsciousness, can be observed. Insidiously developing tamponade may present as the signs of its complications (renal failure, abdominal plethora, shock liver, worsening of glaucoma,29 and mesenteric ischemia). Tamponade without two or more inflammatory signs (typical pain, pericardial friction rub, fever, diffuse ST-segment elevation) is usually associated with a malignant effusion (likelihood ratio 2.9).30
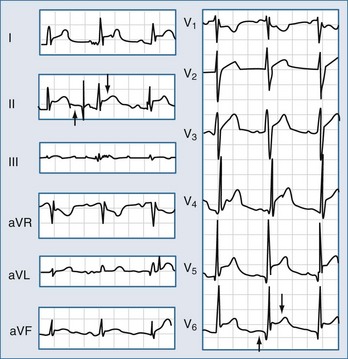
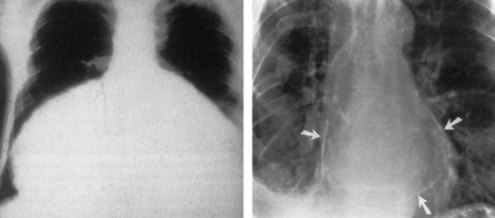
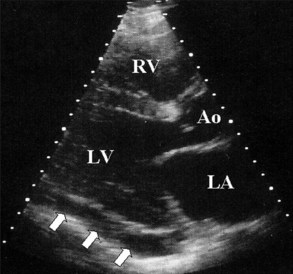
Electrocardiography demonstrates low QRS and T-wave voltages, PR-segment depression (Figure 85-1), ST-segment/T-wave changes, bundle branch block, and electrical alternans (rarely seen in the absence of tamponade).7 Microvoltage and electrical alternans are reversible after effusion drainage and resolution of the inflammatory process.19 In chest radiography large effusions are depicted as globular cardiomegaly with sharp margins (“water bottle” silhouette) (Figure 85-2).12 The size of effusions can be graded in echocardiography as (1) small (echo-free space in diastole < 10 mm), (2) moderate (10 to 20 mm) (Figure 85-3), (3) large (≥20 mm), or (4) very large (≥20 mm and compression of the heart). In large pericardial effusions, the heart may move freely within the pericardial cavity (“swinging heart”) inducing pseudoprolapse and pseudosystolic anterior motion of the mitral valve, paradoxical motion of the inter-ventricular septum, and midsystolic aortic valve closure (Table 85-2).31–41 Up to one third of patients with an asymptomatic large pericardial chronic effusion develop unexpected cardiac tamponade.22 Triggers for tamponade include hypovolemia, paroxysmal tachyarrhythmia, and intercurrent acute pericarditis.
| Clinical presentation | Elevated systemic venous pressure,* hypotension,† pulsus paradoxus,‡ tachycardia,§ dyspnea, or tachypnea with clear lungs |
| Precipitating factors | Drugs (cyclosporine, anticoagulants, thrombolytics), recent cardiac surgery, indwelling instrumentation, blunt chest trauma, malignancies, connective tissue disease, renal failure, septicemia|| |
| ECG | Can be normal or nonspecifically changed (ST-T wave), electrical alternans (QRS, rarely T), bradycardia (end stage), electromechanical dissociation (agonal phase) |
| Chest radiograph | Enlarged cardiac silhouette with clear lungs |
| M-mode/two-dimensional echocardiogram | Diastolic collapse of the anterior RV free wall,¶ RA collapse, LA and rarely LV collapse, increased LV diastolic wall thickness “pseudohypertrophy,” IVC dilatation (no collapse in inspiration),“swinging heart” |
| Doppler | Tricuspid flow increases and mitral flow decreases during inspiration (reverse in expiration) |
| Systolic and diastolic flows are reduced in systemic veins in expiration and reverse flow with atrial contraction is increased | |
| M-mode color Doppler | Large respiratory fluctuations in mitral/tricuspid flows |
| Cardiac catheterization | Confirmation of the diagnosis and quantification of the hemodynamic compromise |
| RA pressure is elevated (preserved systolic × descent and absent or diminished diastolic y descent) | |
| Intrapericardial pressure is also elevated and virtually identical to RA pressure (both pressures fall in inspiration) | |
| RV mid-diastolic pressure is elevated and equal to the RA and pericardial pressures (no dip-and-plateau configuration) | |
| Pulmonary artery diastolic pressure is slightly elevated and may correspond to the RV pressure | |
| Pulmonary capillary wedge pressure is also elevated and nearly equal to intrapericardial and right atrial pressure | |
| LV systolic and aortic pressures may be normal or reduced | |
| Documenting that pericardial aspiration is followed by hemodynamic improvement** | |
| Detection of coexisting hemodynamic abnormalities (LV failure, constriction, pulmonary hypertension) | |
| Detection of associated cardiovascular diseases (cardiomyopathy, coronary artery disease) | |
| RV/LV angiography | Atrial collapse and small hyperactive ventricular chambers |
| Coronary angiography | Coronary compression in diastole |
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; IVC, inferior vena cava.
* Jugular venous distention is less notable in hypovolemic patients or in “surgical tamponade.” An inspiratory increase or lack of fall of the pressure in the neck veins (Kussmaul sign), when verified with tamponade or after pericardial drainage, indicates effusive-constrictive disease.
† Heart rate is usually greater than 100 beats/min but may be lower in hypothyroidism and in uremic patients.
‡ Pulsus paradoxus is defined as a drop in systolic blood pressure greater than 10 mm Hg during inspiration, whereas diastolic blood pressure remains unchanged. It is easily detected by simply feeling the pulse, which diminishes significantly during inspiration. Clinically significant pulsus paradoxus is apparent when the patient is breathing normally. When this sign is present only in deep inspiration it should be interpreted with caution. The magnitude of pulsus paradoxus is evaluated by sphygmomanometry. If the pulsus paradoxus is present, the first Korotkoff sound is not heard equally well throughout the respiratory cycle, but only during expiration at a given blood pressure. The blood pressure cuff is therefore inflated above the patient’s systolic pressure. Then it is slowly deflated while the clinician observes the phase of respiration. During deflation, the first Korotkoff sound is intermittent. Correlation with the patient’s respiratory cycle identifies a point at which the sound is audible during expiration but disappears when the patient breathes in. As the cuff pressure drops farther, another point is reached when the first blood pressure sound is audible throughout the respiratory cycle. The difference in systolic pressure between these two points is the clinical measure of pulsus paradoxus. Pulsus paradoxus is absent in tamponade, complicating atrial septal defect, and in patients with significant aortic regurgitation.
§ Occasional patients are hypertensive, especially if they have preexisting hypertension.
|| Febrile tamponade may be misdiagnosed as septic shock.
¶ Right ventricular collapse can be absent in elevated right ventricular pressure and right ventricular hypertrophy or in right ventricular infarction.
** If after drainage of pericardial effusion intrapericardial pressure does not fall below atrial pressure, the effusive-constrictive disease should be considered.
Constrictive Pericarditis
Constrictive pericarditis is a rare but severely disabling consequence of the chronic inflammation of the pericardium, leading to an impaired filling of the ventricles and reduced ventricular function. Until recently, increased pericardial thickness has been considered an essential diagnostic feature of constrictive pericarditis. However, in the large surgical series from the Mayo Clinic constriction was present in 18% of the patients with normal pericardial thickness.42 Tuberculosis, mediastinal irradiation, and previous surgical procedures are frequent.43 Constrictive pericarditis may rarely develop only in the epicardial layer in patients with previously removed parietal pericardium.44 Transient constrictive pericarditis is an uncommon but important entity, because pericardiectomy is not indicated in these patients.45
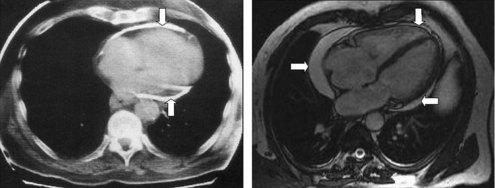
Patients complain about fatigue, peripheral edema, breathlessness, and abdominal swelling, which may be aggravated by a protein-losing enteropathy. In decompensated patients venous congestion, hepatomegaly, pleural effusions, and ascites may occur. Hemodynamic impairment can be additionally aggravated by a systolic dysfunction due to myocardial fibrosis or atrophy. Differential diagnosis has to include acute dilatation of the heart, pulmonary embolism, right ventricular infarction, pleural effusion, chronic obstructive lung diseases,46 and restrictive cardiomyopathy. The best way to distinguish constrictive pericarditis from restrictive cardiomyopathy is the analysis of respiratory changes with or without changes of preload by Doppler and/or tissue Doppler echocardiography,47 but physical findings, electrocardiogram (ECG), chest radiography (see Figure 85-2, Right), computed tomography (CT) (Figure 85-4, Left), magnetic resonance imaging (MRI) (see Figure 85-4, Right), hemodynamics, and endomyocardial biopsy may be helpful as well.7
Pericardiectomy is the only treatment for permanent constriction. The indications are based on clinical symptoms, echocardiography findings, CT/MRI, and heart catheterization. A primary installation of cardiopulmonary bypass (CPB) is not recommended (diffuse bleeding following systemic heparinization). Pericardiectomy for constrictive pericarditis has a mortality rate of 6% to 12%.48–51 The complete normalization of cardiac hemodynamics is reported in only 60% of patients.48,50 Major complications include acute perioperative cardiac insufficiency and ventricular wall rupture.52 Cardiac mortality and morbidity at pericardiectomy are mainly caused by the presurgically unrecognized presence of myocardial atrophy or myocardial fibrosis.43 Exclusion of patients with extensive myocardial fibrosis and/or atrophy reduced the mortality rate for pericardiectomy to 5%. Postoperative low cardiac output52 should be treated by fluid substitution and catecholamines, high doses of digitalis, and intra-aortic balloon pump in most severe cases. If the indication for surgery is established early, long-term survival after pericardiectomy corresponds to that of the general population.49,50 However, if severe clinical symptoms were present for a longer period before surgery, even a complete pericardiectomy may not achieve a total restitution.
Pericardial Cysts
Congenital pericardial cysts are uncommon; they may be unilocular or multilocular, with the diameter ranging from 1 to 5 cm.53 Inflammatory cysts comprise pseudocysts as well as encapsulated and loculated pericardial effusions, caused by rheumatic pericarditis, bacterial infection, particularly tuberculosis, trauma, and cardiac surgery. Most patients are asymptomatic and cysts are detected incidentally on chest radiographs as an oval, homogeneous radiodense lesion, usually at the right cardiophrenic angle.54 However, the patients can also present as chest discomfort, dyspnea, cough, or palpitations, owing to the compression of the heart. Echocardiography is useful, but additional imaging by CT (density readings) or MRI is often needed.55 The treatment of congenital and inflammatory cysts is percutaneous aspiration and ethanol sclerosis.56,57 If this is not feasible, video-assisted thoracotomy or surgical resection may be necessary. Echinococcal cysts usually originate from ruptured hydatid cysts in the liver and lungs. Their surgical excision is not recommended, instead percutaneous aspiration and instillation of ethanol or silver nitrate after pretreatment with albendazole (800 mg/day 4 weeks) is recommended.57
 Specific Forms of Pericarditis
Specific Forms of Pericarditis
Viral Pericarditis
Viral pericarditis is the most common infection of the pericardium. Inflammatory abnormalities are due to direct viral attack, the immune response (antiviral or anticardiac), or both.3,58 Early viral replication in pericardial and epimyocardial tissue elicits cellular and humoral immune responses against the virus and/or cardiac tissue. Deposits of IgM, IgG, and occasionally IgA can be found in the pericardium and myocardium for years.58 Various viruses can cause pericarditis (e.g., enteroviruses, echoviruses, adenoviruses, cytomegaloviruses, Epstein-Barr virus, herpes simplex, herpes humanus 6(HHV6), influenzaviruses, parvovirus B19(PVB19), hepatitis C, human immunodeficiency virus [HIV]), whereby in the last few years PVB19 and HHV6 have been increasing and entero-, echo- and adenoviruses have been decreasing as has also been observed in myocarditis. Attacks of enteroviral pericarditis follow the seasonal epidemics of coxsackievirus A+B and echovirus infections.59 Cytomegalovirus (CMV) pericarditis has an increased incidence in immunocompromised and HIV-infected hosts.60 Infectious mononucleosis may also present as pericarditis.
Treatment of viral pericarditis is directed to resolve symptoms (see acute pericarditis), prevent complications, and eradicate the virus. In patients with chronic or recurrent symptomatic pericardial effusion and confirmed viral infection the following specific treatment is under investigation61:
Pericardial manifestations of HIV infection can be due to infective, noninfective, and neoplastic diseases (Kaposi’s sarcoma and/or lymphoma). Infective (myo) pericarditis results from the local HIV infection and/or from other viral, bacterial (Staphylococcus aureus, Klebsiella pneumoniae, Mycobacterium avium, and M. tuberculosis), and fungal co-infections (Cryptococcus neoformans).62 In progressive disease the incidence of echocardiographically detected pericardial effusion may be up to 40%.63 Cardiac tamponade is rare.64 During treatment with retroviral compounds, lipodystrophy can develop (best demonstrated by MRI) with intense paracardial fat deposition leading to heart failure. Treatment is symptomatic, whereas in large effusions and cardiac tamponade pericardiocentesis is necessary. The use of corticosteroid therapy is contraindicated except in patients with secondary tuberculous pericarditis, as an adjunct to tuberculostatic treatment.65
Bacterial Pericarditis
Purulent pericarditis in adults is rare but always fatal if not treated.66–69 The mortality rate in treated patients is 40%, mostly due to cardiac tamponade, toxicity, and constriction. It is usually a complication of an infection originating elsewhere in the body, arising by contiguous spread or hematogenous dissemination.70 Predisposing conditions are pericardial effusion, immunosuppression, chronic diseases (e.g., alcohol abuse, rheumatoid arthritis), cardiac surgery, and chest trauma. The disease appears as an acute, fulminant infectious illness with short duration. Percutaneous pericardiocentesis must be promptly performed, and obtained pericardial fluid should undergo Gram, acid-fast, and fungal staining, followed by cultures of the pericardial and body fluids. Rinsing of the pericardial cavity, combined with effective systemic antibiotic therapy is mandatory (antistaphylococcal antibiotic plus aminoglycoside, followed by tailored antibiotic therapy according to pericardial fluid and blood cultures).67 Intrapericardial instillation of antibiotics (e.g., gentamicin) is useful but not sufficient. Frequent irrigation of the pericardial cavity with urokinase or streptokinase, using large catheters, may liquefy the purulent exudate,68,69 but open surgical drainage through subxiphoid pericardiotomy is preferable.66 Pericardiectomy is required in patients with dense adhesions, loculated and thick purulent effusion, recurrence of tamponade, persistent infection, and progression to constriction.67 Surgical mortality is up to 8%.
Tuberculous Pericarditis
In the past decade, tuberculous pericarditis in developed countries has been primarily seen in immunocompromised patients (acquired immunodeficiency syndrome [AIDS]).71 The mortality rate in untreated effusive tuberculous pericarditis approaches 85%. Pericardial constriction occurs in 30% to 50%.72,73
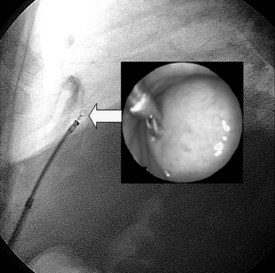
The clinical presentation is variable: acute pericarditis with or without effusion; cardiac tamponade; silent, often large pericardial effusion with a relapsing course; toxic symptoms with persistent fever; acute constrictive pericarditis; subacute constriction; effusive-constrictive or chronic constrictive pericarditis; and pericardial calcifications.3,74 The diagnosis is made by the identification of M. tuberculosis in the pericardial fluid or tissue and/or the presence of caseous granulomas in the pericardium.71 Importantly, PCR can identify DNA of M. tuberculosis rapidly from only 1 µl of pericardial fluid.75,76 Increased adenosine deaminase activity and interferon gamma concentration in pericardial effusion are also diagnostic, with a high sensitivity and specificity. Both pericardioscopy and pericardial biopsy have also improved the diagnostic accuracy for tuberculous pericarditis (Figure 85-5).15 Pericardial biopsy enables rapid diagnosis with better sensitivity than pericardiocentesis (100% vs. 33%).
Pericarditis in a patient with proven extracardiac tuberculosis is strongly suggestive of tuberculous etiology (several sputum cultures should be taken).77 The tuberculin skin test may be false negative in 25% to 33% of tests72 and false positive in 30% to 40% of patients.71 The more accurate enzyme-linked immunospot (ELISPOT) test detects T cells specific for M. tuberculosis antigen.78 Perimyocardial tuberculous involvement is also associated with high serum titers of antimyolemmal and antimyosin antibodies.79 The diagnostic yield of pericardiocentesis in tuberculous pericarditis ranges from 30% to 76% according to the methods applied for the analyses of pericardial effusion.72,75 Pericardial fluid demonstrates high specific gravity, high protein levels, and high white blood cell count (from 0.7 to 54 × 109/L).71
Various antituberculous drug combinations of different durations (6, 9, 12 months) have been applied.71,72,77,80–83 Prevention of constriction in chronic pericardial effusion of undetermined etiology by “ex iuvantibus” antitubercular treatment was not successful.80 The use of corticosteroids remains controversial.77,81–84 A meta-analysis of patients with effusive and constrictive tuberculous pericarditis82,83 suggested that tuberculostatic treatment combined with corticosteroids might be associated with fewer deaths and less frequent need for pericardiocentesis or pericardiectomy.77,85 If given, prednisone should be administered in relatively high doses (1 to 2 mg/kg/day) because rifampicin induces its liver metabolism.7 This dose is maintained for 5 to 7 days and progressively reduced in 6 to 8 weeks. If, in spite of combination therapy, constriction develops, pericardiectomy is indicated.
Pericarditis In Renal Failure
Renal failure is a common cause of pericardial disease producing large pericardial effusions in up to 20% of patients.86 Two forms have been described:
Most patients with uremic pericarditis respond rapidly to hemodialysis or peritoneal dialysis with resolution of chest pain and pericardial effusion. To avoid hemopericardium heparin-free hemodialysis should be used. Hypokalemia and hypophosphatemia should be prevented by supplementing the dialysis solution when appropriate.92 Intensified dialysis usually leads to resolution of the pericarditis within 1 to 2 weeks.93 Peritoneal dialysis, which does not require heparinization, may be therapeutic in pericarditis resistant to hemodialysis or if heparin-free hemodialysis cannot be performed. NSAIDs and systemic corticosteroids have limited success when intensive dialysis is ineffective.94 Cardiac tamponade and large chronic effusions resistant to dialysis must be treated with pericardiocentesis. Large, nonresolving symptomatic effusions should be treated with intrapericardial instillation of corticosteroids after pericardiocentesis or subxiphoid pericardiotomy (triamcinolone hexacetonide, 50 mg every 6 hours for 2 to 3 days).88,94 Pericardiectomy is indicated only in refractory, severely symptomatic patients owing to its potential morbidity and mortality. After renal transplantation, pericarditis has also been reported in 2.4% of patients.95 Uremia or infection (CMV) may be the causes.
Autoreactive Pericarditis and Pericarditis in Systemic Autoimmune Diseases
The diagnosis of autoreactive pericarditis is established using the following criteria2:
Pericarditis occurs in systemic autoimmune diseases: rheumatoid arthritis, systemic lupus erythematosus, progressive systemic sclerosis, polymyositis/dermatomyositis, mixed connective tissue disease, seronegative spondyloarthropathies, systemic and hypersensitivity vasculitides, Behçet’s syndrome, Wegener’s granulomatosis, and sarcoidosis.7 Intensified treatment of the underlying disease and symptomatic management is indicated.
The Post–Cardiac Injury Syndrome: Postpericardiotomy Syndrome
Post–cardiac injury syndrome develops within days to months after cardiac or pericardial injury or both.7,96,97 It resembles the post–myocardial infarction syndrome, both appearing to be variants of a common immunopathologic process. Pericardial effusion also occurs after orthotopic heart transplantation (21%). It is more frequent in patients receiving aminocaproic acid during the operation.98 Cardiac tamponade after open heart surgery is more common after valve surgery than coronary artery bypass grafting and may be related to the preoperative use of anticoagulants.99
Warfarin administration in patients with early postoperative pericardial effusion imposes the greatest risk, particularly in those who did not undergo pericardiocentesis and drainage of the effusion.100 Symptomatic treatment is as in acute pericarditis (NSAIDs or colchicine for several weeks or months,101 but has been questioned recently.102 If symptomatic treatment with NSAIDs or colchicines also reduces the effusion and not only symptoms is tested in the COPPS trial.103 Long-term (3 to 6 months) oral corticosteroids or preferably pericardiocentesis and intrapericardial instillation of triamcinolone (300 mg/m2) are therapeutic options in refractory forms. Redo surgery is rarely needed.
Postinfarction Pericarditis
Two forms of postinfarction pericarditis can be distinguished: an “early” form (pericarditis epistenocardiaca) and a “delayed” form (Dressler’s syndrome).104 Epistenocardiac pericarditis, caused by direct exudation, occurs in 5% to 20% of transmural myocardial infarctions but is clinically discovered rarely. Dressler’s syndrome occurs from 1 week to several months after clinical onset of myocardial infarction with symptoms and manifestations similar to the post-cardiac injury syndrome. It does not require transmural infarction105 and can also appear as an extension of epistenocardiaca pericarditis. Its incidence is 0.5% to 5%106 and is lower still in patients treated with thrombolytics (<0.5%)107 but more frequent in cases of pericardial bleeding after antithrombotic treatment.104,108 Of note, ECG changes are often overshadowed by myocardial infarction changes. Stage one ECG changes are uncommon and suggest “early” post-myocardial infarction syndrome, whereas failure to evolve or “resurrection” of previously inverted T waves strongly suggests myocardial infarction pericarditis.109,110 Postinfarction pericardial effusion greater than 10 mm is most frequently associated with hemopericardium, and two thirds of these patients may develop tamponade/free wall rupture.111 Urgent surgical treatment is lifesaving. If the immediate surgery is not available or contraindicated, pericardiocentesis and intrapericardial fibrin-glue instillation could be an alternative in subacute tamponade.111,112 Ibuprofen, which increases coronary flow, is the agent of choice.113 Aspirin, up to 650 mg every 4 hours for 2 to 5 days, has also been successfully applied. Corticosteroids can be used for refractory symptoms but may delay the healing after infarction.7
Traumatic Pericardial Effusion and Hemopericardium in Aortic Dissection
Direct pericardial injury can be induced by accidents or iatrogenic wounds.114–117 Iatrogenic tamponade occurs most frequently in percutaneous mitral valvuloplasty, during or after transseptal puncture, particularly if no biplane catheterization laboratory is available and a small left atrium is present. Whereas the puncture of the interatrial septum is asymptomatic, the passage of the free wall induces chest pain immediately. If high-pressure-containing structures are punctured, rapid deterioration occurs. However, if only the atrial wall is passed, the tamponade may be delayed for 4 to 6 hours. Rescue pericardiocentesis is successful in 95% to 100%, with a less than 1% mortality.118
Transection of the coronary artery and acute or subacute cardiac tamponade occur very rarely during percutaneous coronary interventions.119,120 A breakthrough in the treatment of coronary perforation has been the development of membrane-covered graft stents.121,122
During right ventricular endomyocardial biopsy the catheter may pass the myocardium, particularly when the bioptome has not been opened before reaching the endocardial border or it is directed to the right ventricular free wall instead of to the septum. Frank cardiac perforations are accompanied by sudden bradycardia and hypotension.123 A perforation rate of 0.3% to 5% was reported, leading to tamponade and circulatory collapse in less than half of the cases.123–125 The incidence of pericardial hemorrhage in left ventricular endomyocardial biopsy is lower (0.1% to 3.3%). Severe complications, leading to procedure-related mortality, were reported in only 0.05% in a worldwide survey of more than 6000 cases124 and in none of the 2537 patients in our center.125
Pacemaker leads penetrating the right ventricle or epicardial electrodes may cause pericarditis with tamponade, adhesions, or constriction.126–129 A right bundle branch block instead of a usually induced left bundle branch block is a clue.
Blunt chest trauma is the major risk of car accidents. The deceleration force can lead to myocardial contusion with intrapericardial hemorrhage, cardiac rupture, pericardial rupture, or herniation. Transesophageal echocardiography or immediate CT should be performed.130,132 Pericardial laceration and partial extrusion of the heart into the mediastinum and pleural space may also occur after injury.115
In dissection of the ascending aorta, pericardial effusion can be found in 17% to 45% of the patients and in 48% of the autopsy cases.130 In a clinical series of aortic dissection, pericardial tamponade was found by CT,132 MRI,133 or echocardiography134 in 17% to 33% of patients with type I dissection, 18% to 45% in type II dissection, and 6% in type III dissection.132 Pericardiocentesis is contraindicated, owing to the risk of intensified bleeding and extension of the dissection.135,136 Surgery should be performed immediately.
Neoplastic Pericarditis
Primary tumors of the pericardium are 40 times less common than metastatic ones.7 Mesothelioma, the most common of the primary tumors, is almost always incurable. The most common secondary malignant tumors are lung cancer, breast cancer, malignant melanoma, lymphomas, and leukemia. Effusions may be small or large with an imminent tamponade (frequent recurrences) or constriction. Tamponade may even be the initial sign of malignant disease.137 With small effusions most patients are asymptomatic. The onset of dyspnea, cough, chest pain, tachycardia, and jugular venous distention is observed when the volume of fluid exceeds 500 mL. Pulsus paradoxus, hypotension, cardiogenic shock, and paradoxical movement of the jugular venous pulse are important signs of cardiac tamponade. The diagnosis is based on the confirmation of the malignant infiltration within the pericardium by cytology or biopsy. Of note, in almost two thirds of the patients with documented malignancy pericardial effusion is caused by nonmalignant diseases (e.g., radiation pericarditis or opportunistic infections).138,139 The chest radiograph, CT, and MRI may reveal mediastinal widening, hilar masses, and pleural effusion.7 The analysis of pericardial fluid and pericardial or epicardial biopsy are essential for the confirmation of malignant pericardial disease.
Cardiac tamponade is an absolute indication for pericardiocentesis. In suspected neoplastic pericardial effusion without tamponade, systemic antineoplastic treatment as baseline therapy can prevent recurrences in up to 67% of cases.137 However, pericardial drainage is recommended in all patients with large effusions because of the high recurrence rate (40% to 70%).110–146 Prevention of recurrences may be achieved by intrapericardial instillation of sclerosing, cytotoxic agents, or immunomodulators. Intrapericardial treatment tailored to the type of the tumor indicates that administration of cisplatin is effective in secondary lung cancer, and intrapericardial instillation of thiotepa appears to be highly effective in breast cancer pericardial metastases.147–152 No patient showed signs of constrictive pericarditis. Tetracyclines as sclerosing agents also control the malignant pericardial effusion in around 85% of cases, but side effects and complications are quite frequent: fever (19%), chest pain (20%), and atrial arrhythmias (10%).137,145,146 Although intrapericardial administration of radionuclides has yielded very good results, it is not widely accepted because of the logistic problems connected with their radioactivity.153 Radiation therapy is very effective (93%) in controlling malignant pericardial effusion in patients with radiosensitive tumors such as lymphoma and leukemia. However, radiotherapy of the heart can cause myocarditis and pericarditis by itself.137
 Rare Forms of Pericardial Disease
Rare Forms of Pericardial Disease
Fungal pericarditis occurs mainly in immunocompromised patients or in the course of endemic, acquired fungal infections.154 It is due to endemic (Histoplasma, Coccidioides) or opportunistic fungi (Candida, Aspergillus, Blastomyces) and semifungi (Nocardia, Actinomyces).155–157 Diagnosis is obtained by staining and culturing pericardial fluid or tissue. Antifungal antibodies in serum are also helpful in establishing the diagnosis.3 Treatment with fluconazole, ketoconazole, itraconazole, amphotericin B, liposomal amphotericin B, or amphotericin B lipid complex is indicated. NSAIDs can support the treatment with antifungal drugs. Patients with histoplasmosis pericarditis do not need antifungal therapy but respond to NSAIDs given for 2 to 12 weeks. Sulfonamides are the drugs of choice for nocardiosis. Combination of three antibiotics including penicillin should be given for actinomycosis. Pericardiocentesis or surgical treatment is indicated for hemodynamic impairment. Pericardiectomy is indicated in fungal constrictive pericarditis.
Radiation pericarditis may begin already during exposure (very rare) or months and years later—with latency of up to 15 to 20 years. Its occurrence is influenced by the applied source, dose, fractionation, duration, radiation exposed volume, form of mantel field therapy, and the age of the patient.158 The effusion may be serous or hemorrhagic, later on with fibrinous adhesions or constriction, typically without tissue calcification. The symptoms may be masked by the underlying disease or the applied chemotherapy. Imaging should start with echocardiography, followed by cardiac CT or MRI if necessary. Pericarditis without tamponade may be treated conservatively but effusions respond favorably to intrapericardial triamcinolone instillation. Pericardiocentesis and fluid analysis can rule out neoplastic progression to the pericardium.159 Pericardial constriction occurs in up to 20% of patients, requiring pericardiectomy. The operative mortality is high (21%) and the postoperative 5-year survival is poor (1%), mostly owing to myocardial fibrosis.160
Chylopericardium refers to a communication between the pericardium and the thoracic duct, as a result of trauma or congenital anomalies, or as a complication of open-heart surgery,161 mediastinal lymphangiomas, lymphangiomatous hamartomas, lymphangiectasis, and obstruction or anomalies of the thoracic duct.162 Infection, tamponade, or constriction may aggravate the prognosis.163 The pericardial fluid is sterile, odorless, and opalescent with a milky white appearance and the microscopic finding of fat droplets. The chylous nature of the fluid is confirmed by its alkaline reaction, specific gravity between 1010 and 1021, Sudan III stain for fat, and the high concentrations of triglycerides (5 to 50 g/L) and protein (22 to 60 g/L).164,165 Enhanced CT, alone or combined with lymphography, can identify not only the location of the thoracic duct but also its lymphatic connection to the pericardium.166,167
Treatment depends on the etiology and the amount of chylous accumulation.168 Chylopericardium after thoracic or cardiac operation is preferably treated by pericardiocentesis and diet (medium-chain triglycerides).169,170 If further production of chylous effusion continues, surgical treatment is mandatory. When conservative treatment and pericardiocentesis fail, a pericardioperitoneal window is a reasonable option.171,172 Alternatively, when the course of the thoracic duct is precisely identified, its ligation and resection just above the diaphragm is the most effective treatment.173
Drug- and toxin-related pericarditis, tamponade, adhesions, fibrosis, or constriction may be induced by several drugs.7,174 Mechanisms include drug-induced lupus reactions, idiosyncrasy, “serum sickness,” foreign substance reactions, and immunopathy. Management is based on the discontinuation of the causative agent and symptomatic treatment.
Pericardial effusion in hypothyroidism occurs in 5% to 30% of patients.7 Fluid accumulates slowly and tamponade occurs rarely. In some cases, cholesterol pericarditis may be observed. The diagnosis is based on serum levels of thyroxine and thyroid-stimulating hormone. Bradycardia, low voltage of the QRS and T wave inversion or flattening in the ECG, cardiomegaly on the radiograph, and pericardial effusion on echocardiography, as well as a history of radiation-induced thyroid dysfunction, myopathy, ascites, pleural effusion, and uveal edema may be observed.175–179 Therapy with thyroid hormone decreases pericardial effusion.
Pericardial effusion and constriction in pregnancy may manifest as a minimal to moderate clinically silent hydropericardium by the third trimester. Cardiac compression is rare.180 ECG changes of acute pericarditis in pregnancy should be distinguished from the slight ST-segment depressions and T-wave changes seen in normal pregnancy.180,181 Occult constriction becomes manifest in pregnancy owing to the increased blood volume.181 Most pericardial disorders are managed as in nonpregnant women.182,183 Caution is necessary because high-dose aspirin may prematurely close the ductus arteriosus, and colchicine is contraindicated in pregnancy. Pericardiotomy and pericardiectomy can be safely performed if necessary and do not impose a risk for subsequent pregnancies.183,184
Fetal pericardial fluid can be detected by echocardiography after 20 weeks’ gestation and is normally 2 mm or less in depth. More fluid should raise questions of hydrops fetalis, Rh disease, neoplasia, hypoalbuminemia, immunopathy, or maternally transmitted mycoplasmal or other infections.185
Key Points
Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587-610.
First ESC guidelines for the diagnosis and treatment of pericardial diseases.
Maisch B, Ristic AD, Pankuweit S. Intrapericardial treatment of auto reactive pericardial effusion with triamcinolone: The way to avoid side effects of systemic corticosteroid therapy. Eur Heart J. 2002;23:1503-1508.
Maisch B, Ristic AD, Pankuweit S, et al. Neoplastic pericardial effusion: Efficacy and safety of intrapericardial treatment with cisplatin. Eur Heart J. 2002;23:1625-1631.
Maisch B, Ristic A, Seferovic PM, Tsang TS. Interventional pericardiology. Springer; 2011.
Seferovic PM, Ristic AD, Maksimovic R, et al. Diagnostic value of pericardial biopsy: Improvement with extensive sampling enabled by pericardioscopy. Circulation. 2003;107:978-983.
1 Maisch B, Ristic AD. The classification of pericardial disease in the age of modern medicine. Curr Cardiol Rep. 2002;4:13-21.
2 Maisch B, Ristic AD, Pankuweit S. Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone: The way to avoid side effects of systemic corticosteroid therapy. Eur Heart J. 2002;23:1503-1508.
3 Spodick DH. Infectious pericarditis. In: The Pericardium: A Comprehensive Textbook. New York: Marcel Dekker; 1997:260-290.
4 Cottrill CM, Tamaren J, Hall B. Sternal defects associated with congenital pericardial and cardiac defects. Cardiol Young. 1998;8:100-104.
5 Meunier JP, Lopez S, Teboul J, et al. Total pericardial defect: Risk factor for traumatic aortic type A dissection. Ann Thorac Surg. 2002;74:266.
6 Loebe M, Meskhishvili V, Weng Y, et al. Use of polytetrafluoroethylene surgical membrane as a pericardial substitute in the correction of congenital heart defects. Tex Heart Inst J. 1993;20:213-217.
7 Spodick DH. Pericardial diseases. In: Braunwald E, Zippes DP, Libby P, editors. Heart Disease. 6th ed. Philadelphia: WB Saunders; 2001:1823-1876.
8 Maisch B, Bethge C, Drude L, et al. Pericardioscopy and epicardial biopsy: New diagnostic tools in pericardial and perimyocardial diseases. Eur Heart J. 1994;15(Suppl C):68-73.
9 Levine MJ, Lorell BH, Diver DJ, et al. Implications of echocardiographically assisted diagnosis of pericardial tamponade in contemporary medical patients: Detection before hemodynamic embarrassment. J Am Coll Cardiol. 1991;17:59-65.
10 Chuttani K, Pandian NG, Mohanty PK, et al. Left ventricular diastolic collapse: An echocardiographic sign of regional cardiac tamponade. Circulation. 1991;83:1999-2006.
11 Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111:1213-1221.
12 Eisenberg MJ, Dunn MM, Kanth N, et al. Diagnostic value of chest radiography for pericardial effusion. J Am Coll Cardiol. 1993;22:588-593.
13 Tsang TS, Enriquez-Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429-436.
14 Chiles C, Woodard PK, Gutierrez FR, et al. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics. 2001;21:439-449.
15 Nugue O, Millaire A, Porte H, et al. Pericardioscopy in the etiologic diagnosis of pericardial effusion in 141 consecutive patients. Circulation. 1996;94:1635-1641.
16 Seferovic PM, Ristic AD, Maksimovic R, et al. Diagnostic value of pericardial biopsy: improvement with extensive sampling enabled by pericardioscopy. Circulation. 2003;107:978-983.
17 Bonnefoy E, Godon P, Kirkorian G, et al. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21:832-836.
18 Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol. 2001;87:1326-1328.
19 Bruch C, Schmermund A, Dagres N, et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: Reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol. 2001;38:219-226.
20 Imazio M, Bobbio M, Cecchi E, et al. Results of the COlchicine for acute PEricaditis(COPE) Trial. Circulation. 2005;112:2012-2016.
21 Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: A decade of experience. Circulation. 1998;97:2183-2185.
22 Sagrista-Sauleda J, Angel J, Permanyer-Miralda G, et al. Long-term follow-up of idiopathic chronic pericardial effusion. N Engl J Med. 1999;341:2054-2059.
23 Ziskind AA, Pearce AC, Lemmon CC, et al. Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: Description of technique and report of the first 50 cases. J Am Coll Cardiol. 1993;21:1-5.
24 Guindo J, Rodriguez de la Serna A, Ramie J, et al. Recurrent pericarditis—relief with colchicine. Circulation. 1990;82:1117-1120.
25 Imazio M, Bobbioo M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE(COlchicine for Recurrent pericarditis) trial. Arch Intern Med. 2005;165:1098-1991.
26 Asplen CH, Levine HD. Azathioprine therapy of steroid-responsive pericarditis. Am Heart J. 1970;80:109-111.
27 Miller JI, Mansour KA, Hatcher CR. Pericardiectomy: Current indication, concept, and results in a university center. Ann Thorac Surg. 1982;84:40-45.
28 Merce J, Sagrista-Sauleda J, Permanyer-Miralda G, et al. Should pericardial drainage be performed routinely in patients who have a large pericardial effusion without tamponade? Am J Med. 1998;105:106-109.
29 Erdol C, Erdol H, Celik S, et al. Idiopathic chronic pericarditis associated with ocular hypertension: Probably an unknown combination. Int J Cardiol. 2003;87:293-295.
30 Sagrista-Sauleda J, Merce J, Permanyer-Miralda G, et al. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000;109:95-101.
31 D’Cruz IA, Cohen HC, Prabhu R, et al. Diagnosis of cardiac tamponade by echocardiography: Changes in mitral valve motion and ventricular dimensions, with special reference to paradoxical pulse. Circulation. 1975;52:460-465.
32 Reydel B, Spodick DH. Frequency and significance of chamber collapses during cardiac tamponade. Am Heart J. 1990;119:1160-1163.
33 Kochar GS, Jacobs LE, Kotler MN. Right atrial compression in postoperative cardiac patients: Detection by transesophageal echocardiography. J Am Coll Cardiol. 1990;16:511-516.
34 Torelli J, Marwick TH, Salcedo EE. Left atrial tamponade: Diagnosis by transesophageal echocardiography. J Am Soc Echocardiogr. 1991;4:413-414.
35 Fresman B, Schwinger ME, Charney R, et al. Isolated collapse of left-sided heart chambers in cardiac tamponade: Demonstration by two-dimensional echocardiography. Am Heart J. 1991;121:613-616.
36 Di Segni E, Feinberg MS, Sheinowitz M, et al. LV pseudohypertrophy in cardiac tamponade: An echocardiographic study in canine model. J Am Coll Cardiol. 1993;21:1286-1294.
37 Feigenbaum H, Zaky A, Grabham L. Cardiac motion in patients with pericardial effusion: A study using ultrasound cardiography. Circulation. 1966;34:611-619.
38 Bansal RC, Chandrasekaram K. Role of echocardiography in Doppler techniques in evaluation of pericardial effusion. Echocardiography. 1989;6:313-316.
39 Saxena RK, D’Crus IA, Zitaker M. Color flow Doppler observations on mitral valve flow in tamponade. Echocardiography. 1991;8:517-521.
40 Singh S, Wann LS, Schuchard GH, et al. Right ventricular and right atrial collapse in patients with cardiac tamponade—a combined echocardiographic and hemodynamic study. Circulation. 1984;70:966-971.
41 Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587-610.
42 Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
43 Rienmuller R, Gurgan M, Erdmann E, et al. CT and MR evaluation of pericardial constriction: A new diagnostic and therapeutic concept. J Thorac Imaging. 1993;8:108-121.
44 Byrne JG, Karavas AN, Colson YL, et al. Cardiac decortication (epicardiectomy) for occult constrictive cardiac physiology after left extrapleural pneumonectomy. Chest. 2002;122:2256-2259.
45 Ly QH, Sauve C, Lalonde G. Acute pericarditis with transient constriction. Can J Cardiol. 2001;17:973-976.
46 Oh JK, Seward JB, Tajik AJ. The Echo Manual, 2nd ed. Philadelphia: Lippincott; 1999. p. 181-94
47 Rajagopalan N, Garcia MJ, Rodriguez L, et al. Comparison of new Doppler echocardiographic methods to differentiate constrictive pericardial heart disease and restrictive cardiomyopathy. Am J Cardiol. 2001;87:86-94.
48 DeValeria PA, Baumgartner WA, Casale AS, et al. Current indications, risks, and outcome after pericardiectomy. Ann Thorac Surg. 1991;52:219-224.
49 Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: Evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386.
50 Senni M, Redfield MM, Ling LH, et al. Left ventricular systolic and diastolic function after pericardiectomy in patients with constrictive pericarditis: Doppler echocardiographic findings and correlation with clinical status. J Am Coll Cardiol. 1999;33:1182-1188.
51 Ufuk Y, Kestelli M, Yilik L, et al. Recent surgical experience in chronic constrictive pericarditis. Texas Heart Inst J. 2003;30:27-30.
52 Sunday R, Robinson LA, Bosek V. Low cardiac output complicating pericardiectomy for pericardial tamponade. Ann Thorac Surg. 1999;67:228-231.
53 Satur CM, Hsin MK, Dussek JE. Giant pericardial cysts. Ann Thorac Surg. 1996;61:208-210.
54 Borges AC, Gellert K, Dietel M, et al. Acute right-sided heart failure due to hemorrhage into a pericardial cyst. Ann Thorac Surg. 1997;63:845-847.
55 Wang ZJ, Reddy GP, Gotway MB, et al. CT and MRI imaging of pericardial disease. Radiographics. 2003;23(Spec No):S167-S180.
56 Kinoshita Y, Shimada T, Murakami Y, et al. Ethanol sclerosis can be a safe and useful treatment for pericardial cyst. Clin Cardiol. 1996;19:833-835.
57 Simeunovic D, Seferovic PM, Ristic AD, et al. Pericardial cysts: Incidence, clinical presentations and treatment. Maksimovic R, Ristic AD (assoc eds). In: Seferovic PM, Spodick DH, Maisch B, editors. Pericardiology: Contemporary Answers to Continuing Challenges. Belgrade: Science; 2000:203-212.
58 Maisch B, Outzen H, Roth D, et al. Prognostic determinants in conventionally treated myocarditis and perimyocarditis: Focus on antimyolemmal antibodies. Eur Heart J. 1991;12:81-87.
59 Saatci U, Ozen S, Ceyhan M, Secmeer G. Cytomegalovirus disease in a renal transplant recipient manifesting with pericarditis. Int Urol Nephrol. 1993;25:617-619.
60 Campbell P, Li J, Wall T, et al. Cytomegalovirus pericarditis: A case series and review of the literature. Am J Med Sci. 1995;309:229-234.
61 Maisch B, Ristic AD, Seferovic PM. New directions in diagnosis and treatment of pericardial disease: An update by the Taskforce on pericardial disease of the World Heart Federation. Herz. 2000;25:769-780.
62 DeCastro S, Migliau G, Silvestri A, et al. Heart involvement in AIDS: A prospective study during various stages of the disease. Eur Heart J. 1992;13:1452-1459.
63 Chen Y, Brennessel D, Walters J, et al. Human immunodeficiency virus-associated pericardial effusion: Report of 40 cases and review of literature. Am Heart J. 1999;137:516-521.
64 Silva-Cardoso J, Moura B, Martins L, et al. Pericardial involvement in human immunodeficiency virus infection. Chest. 1999;115:418-422.
65 Hakim JG, Ternouth I, Mushangi E, et al. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart. 2000;84:183-188.
66 Sagrista-Sauleda J, Barrabes JA, Permanyer-Miralda G, et al. Purulent pericarditis: Review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993;22:1661-1665.
67 Goodman LJ. Purulent pericarditis. Curr Treat Options Cardiovasc Med. 2000;2:343-350.
68 Defouilloy C, Meyer G, Slama M, et al. Intrapericardial fibrinolysis: A useful treatment in the management of purulent pericarditis. Intensive Care Med. 1997;23:117-118.
69 Ustunsoy H, Celkan MA, Sivrikoz MC, et al. Intrapericardial fibrinolytic therapy in purulent pericarditis. Eur J Cardiothorac Surg. 2002;22:373-376.
70 Keersmaekers T, Elshot SR, Sergeant PT. Primary bacterial pericarditis. Acta Cardiol. 2002;57:387-389.
71 Fowler NO. Tuberculous pericarditis. JAMA. 1991;266:99-103.
72 Sagrista-Sauleda J, Permanyer-Miralda G, Soler-Soler J. Tuberculous pericarditis: Ten year experience with a prospective protocol for diagnosis and treatment. J Am Coll Cardiol. 1988;11:724-728.
73 Long R, Younes M, Patton N, et al. Tuberculous pericarditis: Long-term outcome in patients who received medical therapy alone. Am Heart J. 1989;117:1133-1139.
74 Permanyer-Miralda G, Sagrista-Sauleda J, Soler-Soler J. Primary acute pericardial disease: A prospective series of 231 consecutive patients. Am J Cardiol. 1985;56:623-630.
75 Godfrey-Faussett P. Molecular diagnosis of tuberculosis: The need for new diagnostic tools. Thorax. 1995;50:709-711.
76 Seino Y, Ikeda U, Kawaguchi K, et al. Tuberculosis pericarditis presumably diagnosed by polymerized chain reaction analysis. Am Heart J. 1993;126:249-251.
77 Strang JI, Kakaza HH, Gibson DG, et al. Controlled clinical trial of complete open surgical drainage and of prednisolone in treatment of tuberculous pericardial effusion in Transkei. Lancet. 1988;2:759-764.
78 Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell–based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168-1173.
79 Maisch B, Maisch S, Kochsiek K. Immune reactions in tuberculous and chronic constrictive pericarditis. Am J Cardiol. 1982;50:1007-1013.
80 Dwivedi SK, Rastogi P, Saran RK, et al. Antitubercular treatment does not prevent constriction in chronic pericardial effusion of undetermined etiology: A randomized trial. Indian Heart J. 1997;49:411-414.
81 Senderovitz T, Viskum K. Corticosteroids and tuberculosis. Respir Med. 1994;88:561-565.
82 Mayosi BM, Ntsekhe M, Volmink JA, et al. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev 2002:CD000526.
83 Ntsekhe M, Wiysonge C, Volmink JA, et al. Adjuvant corticosteroids for tuberculous pericarditis: Promising, but not proven. Q J Med. 2003;96:593-599.
84 Alzeer AM, Fitzgerald JM. Corticosteroids and tuberculosis: Risks and use as adjunct therapy. Tuberc Lung Dis. 1993;74:6-11.
85 Strang JI. Rapid resolution of tuberculous pericardial effusion with high dose prednisone and antituberculous drugs. J Infect. 1994;28:251-254.
86 Colombo A, Olson HG, Egan J, et al. Etiology and prognostic implications of a large pericardial effusion in men. Clin Cardiol. 1988;11:389-394.
87 Rostand SG, Rutsky EA. Pericarditis in end-stage renal disease. Cardiol Clin. 1990;8:701-706.
88 Rutsky EA. Treatment of uremic pericarditis and pericardial effusion. Am J Kidney Dis. 1987;10:2-7.
89 Lundin AP. Recurrent uremic pericarditis: A marker of inadequate dialysis. Semin Dial. 1990;3:5-9.
90 Tarng DC, Huang TP. Uraemic pericarditis: A reversible inflammatory state of resistance to recombinant human erythropoietin in haemodialysis patients. Nephrol Dial Transplant. 1997;12:1051-1057.
91 Gunukula SR, Spodick DH. Pericardial disease in renal patients. Semin Nephrol. 2001;21:52-57.
92 Emelife-Obi C, Chow MT, Qamar-Rohail H, et al. Use of a phosphorus-enriched hemodialysate to prevent hypophosphatemia in a patient with renal failure-related pericarditis. Clin Nephrol. 1998;50:131-136.
93 Connors JP, Kleiger RE, Shaw RC, et al. The indications for pericardiectomy in the uremic pericardial effusion. Surgery. 80, 1976. 689–674
94 Wood JE, Mahnensmith RL. Pericarditis associated with renal failure: Evolution and management. Semin Dial. 2001;14:61-66.
95 Sever MS, Steinmuller DR, Hayes JM, et al. Pericarditis following renal transplantation. Transplantation. 1991;51:1229-1234.
96 Maisch B, Berg PA, Kochsiek K. Clinical significance of immunopathological findings in patients with post-pericardiotomy syndrome: I. Relevance of antibody pattern. Clin Exp Immunol. 1979;38:189-197.
97 Maisch B, Schuff-Werner P, Berg PA, et al. Clinical significance of immunopathological findings in patients with post-pericardiotomy syndrome: II. The significance of serum inhibition and rosette inhibitory factors. Clin Exp Immunol. 1979;38:198-203.
98 Quin JA, Tauriainen MP, Huber LM, et al. Predictors of pericardial effusion after orthotopic heart transplantation. J Thorac Cardiovasc Surg. 2002;124:979-983.
99 Kuvin JT, Harati NA, Pandian NG, et al. Postoperative cardiac tamponade in the modern surgical era. Ann Thorac Surg. 2002;74:1148-1153.
100 Matsuyama K, Matsumoto M, Sugita T, et al. Clinical characteristics of patients with constrictive pericarditis after coronary bypass surgery. Jpn Circ J. 2001;65:480-482.
101 Horneffer PJ, Miller RH, Pearson TA, et al. The effective treatment of postpericardiotomy syndrome after cardiac operations: A randomized placebo-controlled trial. J Thorac Cardiovasc Surg. 1990;100:292-296.
102 Meurin P, Tabet JY, Thabut G, et al. Nonsteroidal anti-inflammatory drug treatment for postoperative pericardial effusion: a multicenter randomized, double-blind trial. Ann Intern Med. 2010 Feb 2;152(3):137-143.
103 Imazio M, Cecchi E, Demichelis B, et al. COPPS Investigators. Rationale and design of the COPPS trial: a randomised, placebo-controlled, multicentre study on the use of colchicine for the primary prevention of postpericardiotomy syndrome. J Cardiovasc Med (Hagerstown). 2007;8(12):1044-1048.
104 Sugiura T, Takehana K, Hatada K, et al. Pericardial effusion after primary percutaneous transluminal coronary angioplasty in first Q-wave acute myocardial infarction. Am J Cardiol. 1998;81:1090-1093.
105 Spodick DH. Post-myocardial infarction syndrome (Dressler’s syndrome). ACC Curr J Rev. 1995;4:35-37.
106 Lichstein E. The changing spectrum of post-myocardial infarction pericarditis. Int Cardiol. 1983;4:234-237.
107 Shahar A, Hod H, Barabash GM, et al. Disappearance of a syndrome: Dressler’s syndrome in the era of thrombolysis. Cardiology. 1994;85:255-258.
108 Nagahama Y, Sugiura T, Takehana K, et al. The role of infarction-associated pericarditis on the occurrence of atrial fibrillation. Eur Heart J. 1998;19:287-292.
109 Oliva PB, Hammill SC, Edwards WD. Electro cardiographic diagnosis of postinfarction regional pericarditis: Ancillary observations regarding the effect of reperfusion on the rapidity and amplitude of T wave inversion after acute myocardial infarction. Circulation. 1993;88:896-904.
110 Oliva PB, Hammill SC, Talano JV. T wave changes consistent with epicardial involvement in acute myocardial infarction: Observations in patients with a postinfarction pericardial effusion without clinically recognized postinfarction pericarditis. J Am Coll Cardiol. 1994;24:1073-1077.
111 Figueras J, Juncal A, Carballo J, et al. Nature and progression of pericardial effusion in patients with a first myocardial infarction: Relationship to age and free wall rupture. Am Heart J. 2002;144:251-258.
112 Joho S, Asanoi H, Sakabe M, et al. Long-term usefulness of percutaneous intrapericardial fibrin-glue fixation therapy for oozing type of left ventricular free wall rupture: A case report. Circ J. 2002;66:705-706.
113 Spodick DH. Safety of ibuprofen for acute myocardial infarction pericarditis. Am J Cardiol. 1986;57:896.
114 Nagy KK, Lohmann C, Kim DO, Barrett J. Role of echocardiography in the diagnosis of occult penetrating cardiac injury. J Trauma. 1995;38:859-862.
115 Buckman RF, Buckman PD. Vertical deceleration trauma: principles of management. Surg Clin North Am. 1991;71:331-340.
116 Asensio JA, Berne JD, Demetriades D, et al. Penetrating cardiac injuries: A prospective study of variables predicting outcomes. J Am Coll Surg. 1998;186:24-34.
117 Narins CR, Cunningham MJ, Delehantry JM, et al. Nonhemorrhagic cardiac tamponade after penetrating chest trauma. Am Heart J. 1996;132:197-198.
118 Tsang TS, Freeman WK, Barnes ME, et al. Rescue echocardiographically guided pericardiocentesis for cardiac perforation complicating catheter-based procedures. The Mayo Clinic experience. J Am Coll Cardiol. 1998;32:1345-1350.
119 Jungbluth A, Diiber C, Rumpelt HJ, et al. Koronararterienmorphologie nach perkutaner transluminaler Koronarangioplasatie (PTCA) mit Hämoperikard. Z Kardiol. 1988;77:125-129.
120 Liu F, Erbel R, Haude M, Ge J. Coronary arterial perforation: Prediction, diagnosis, management, and prevention. In: Ellis SG, Holmes DR, editors. Strategic Approaches in Coronary Intervention. 2nd ed. Philadelphia: Lippincott; 2000:501-514.
121 Welge D, Haude M, von Birgelen C, et al. Versorgung einer Koronarperforation nach perkutaner Ballonangioplastie mit einem neuen Membranstent. Z Kardiol. 1998;87:948-953.
122 von Birgelen C, Haude M, Herrmann J, et al. Early clinical experience with the implantation of a novel synthetic coronary stent graft. Cathet Cardiovasc Intervent. 1999;47:496-503.
123 Levine MJ, Bairn DS. Endomyocardial biopsy. In: Grossmann W, Bairn DS, editors. Cardiac Catheterization, Angiography and Interventions. Philadelphia: Lea & Febiger; 1991:383-395.
124 Sekiguchi M, Take M. World survey of catheter biopsy of the heart. In: Sekiguchi M, Olsen EGJ, editors. Cardiomyopathy Clinical, Pathological, and Theoretical Aspects. Baltimore: University Park Press; 1980:217-225.
125 Maisch B. Myokardbiopsien und Perikardioskopien. In: Hess OM, Simon RWR, editors. Herzkatheter: Einzatz in Diagnostik und Therapie. Berlin: Springer; 2000:302-349.
126 Kiviniemi MS, Pirnes MA, Eranen HJ, et al. Complications related to permanent pacemaker therapy. Pacing Clin Electrophysiol. 1999;22:711-720.
127 Matsuura Y, Yamashina H, Higo M, Fujii T. Analysis of complications of permanent transvenous implantable cardiac pacemaker related to operative and postoperative management in 717 consecutive patients. Hiroshima J Med Sci. 1990;39:131-137.
128 Spindler M, Burrows G, Kowallik P, et al. Postpericardiotomy syndrome and cardiac tamponade as a late complication after pacemaker implantation. Pacing Clin Electrophysiol. 2001;24(9 Pt 1):1433-1434.
129 Elinav E, Leibowitz D. Constrictive pericarditis complicating endovascular pacemaker implantation. Pacing Clin Electrophysiol. 2002;25:376-377.
130 Chirillo F, Totis O, Cavarzerani A, et al. Usefulness of transthoracic and transesophageal echocardiography in recognition and management of cardiovascular injuries after blunt chest trauma. Heart. 1996;75:301-306.
131 Erbel R. Diseases of the aorta. Heart. 2001;86:227-234.
132 Hausmann D, Gulba D, Bargheer K, et al. Successful thrombolysis of an aortic arch thrombus in a patient after mesenteric embolism. N Engl J Med. 1992;327:500-501.
133 Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328:1-9.
134 Erbel R, Engberding R, Daniel W, et al. Echocardiography in diagnosis of aortic dissection. Lancet. 1989;1:457-461.
135 Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642-1681.
136 Mellwig KP, Vogt J, Schmidt HK, et al. Acute aortic dissection (Stanford A) with pericardial tamponade—extension of the dissection after emergency pericardial puncture. Z Kardiol. 1998;87:482-486.
137 Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. JAMA. 1994;272:59-64.
138 Millaire A, Wurtz A, de Groote P, et al. Malignant pericardial effusions: Usefulness of pericardioscopy. Am Heart J. 1992;124:1030-1034.
139 Porte HL, Janecki-Delebecq TJ, Finzi L, et al. Pericardioscopy for primary management of pericardial effusion in cancer patients. Eur J Cardiothorac Surg. 1999;16:287-291.
140 Tomkowski W, Szturmowicz M, Fijalkowska A, et al. New approaches to the management and treatment of malignant pericardial effusion. Support Care Cancer. 1997;5:64-66.
141 Tsang TSM, Seward JB, Barnes ME. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin Proc. 2000;75:248-253.
142 Susini G, Pepi M, Sisillo E, et al. Percutaneous pericardiocentesis versus subxiphoid pericardiotomy in cardiac tamponade due to postoperative pericardial effusion. J Cardiothorac Vase Anesthes. 1993;7:178-183.
143 Fagan SM, Chan KI. Pericardiocentesis. Blind no more!. Chest. 1999;116:275-276.
144 Soler-Soler J, Merce J, Sagrista-Sauleda J. Should pericardial drainage be performed routinely in patients who have a large pericardial effusion without tamponade? Am J Med. 1998;105:106-109.
145 DeCamp MM, Mentzer SJ, Swanson SJ, et al. Malignant effusive disease of pleura and pericardium. Chest. 1997;112(Suppl):291-295.
146 Zwischenberger JB, Sanker AB, Lee R. Malignant pericardial effusion. In: Pass HJ, Mitchell JB, Johnson DH, et al, editors. Lung Cancer. Principles and Practice. Philadelphia: Lippincott Wiliams & Wilkins; 2000:1038-1046.
147 Bishiniotis TS, Antoniadou S, Katseas G, et al. Malignant cardiac tamponade in women with breast cancer treated by pericardiocentesis and intrapericardial administration of triethylenethiophosphoramide (thiotepa). Am J Cardiol. 2000;86:362-364.
148 Colleoni M, Martinelli G, Beretta F, et al. Intracavitary chemotherapy with thiotepa in malignant pericardial effusion: An active and well tolerated regimen. J Clin Oncol. 1998;16:2371-2376.
149 Girardi LN, Ginsberg RJ, Burt ME. Pericardiocentesis and intrapericardial sclerosis: Effective therapy for malignant pericardial effusion. Ann Thorac Surg. 1997;64:1422-1428.
150 Maisch B, Pankuweit S, Brilla C, et al. Intrapericardial treatment of inflammatory and neoplastic pericarditis guided by pericardioscopy and epicardial biopsy—results from a pilot study. Clin Cardiol. 1999;22(Suppl 1):117-122.
151 Maisch B, Ristic AD, Pankuweit S, et al. Neoplastic pericardial effusion: Efficacy and safety of intrapericardial treatment with cisplatin. Eur Heart J. 2002;23:1625-1631.
152 Tomkowski WZ, Wisniewska J, Szturmowicz M, et al. Evaluation of intrapericardial cisplatin administration in cases with recurrent malignant pericardial effusion and cardiac tamponade. Support Care Cancer. 2004;12:53-57.
153 Dempke W, Firusian N. Treatment of malignant pericardial effusion with 32 P-colloid. Br J Cancer. 1999;80:1955-1957.
154 Canver CC, Patel AK, Kosolcharoen P, et al. Fungal purulent constrictive pericarditis in heart transplant patient. Ann Thorac Surg. 1998;65:1792-1794.
155 Cishek MB, Yost B, Schaefer S. Cardiac aspergillosis presenting as myocardial infarction. Clin Cardiol. 1996;19:824-827.
156 Wheat J. Histoplasmosis: Experience during outbreaks in Indianapolis and review of the literature. Medicine. 1997;76:339-354.
157 Rabinovici R, Szewczyk D, Ovadia P, et al. Candida pericarditis: Clinical profile and treatment. Ann Thorac Surg. 1997;63:1200-1204.
158 Kumar PP. Pericardial injury from mediastinal irradiation. J Natl Med Assoc. 1980;72:591-594.
159 Maisch B, Ristic A, Pankuweit S. Evaluation and management of pericardial effusion in patients with neoplastic disease. Prog Cardiovasc Dis. 2010. (in press)
160 Karram T, Rinkevitch D, Markiewiczß W. Poor outcome in radiation-induced constrictive pericarditis. Int J Radiât Oncol Biol Phys. 1993;25:329-331.
161 Kentsch M, Dôring V, Rodemerk U, et al. Primary chylopericardium—stepwise diagnosis and therapy of a differential diagnostically important illness. Z Kardiol. 1997;86:417-422.
162 Denfield SW, Rodriguez A, Miller-Hance WC, et al. Management of postoperative chylopericardium in childhood. Am J Cardiol. 1989;63:1416-1418.
163 Morishita Y, Taira A, Fuori A, et al. Constrictive pericarditis secondary to primary chylopericardium. Am Heart J. 1985;109:373-375.
164 Akamatsu H, Amano J, Sakamoto T, Suzuki A. Primary chylopericardium. Ann Thorac Surg. 1994;58:262-266.
165 Bendayan P, Glock Y, Galinier M, et al. Idiopathic chylopericardium: Apropos of a new case: Review of the literature. Arch Mal Coeur Vaiss. 1991;84:127-130.
166 Svedjeholm R, Jansson K, Olin C. Primary idiopathic chylopericardium—a case report and review of the literature. Eur J Cardiothorac Surg. 1997;11:387-390.
167 Kannagi T, Osakada G, Wakabayashi A, et al. Primary chylopericardium. Chest. 1982;81:105-108.
168 Chan BB, Murphy MC, Rodgers BM. Management of chylopericardium. J Pediatr Surg. 1990;25:1185-1189.
169 Crosby IK, Crouch J, Reed WA. Chylopericardium and chylothorax. J Thorac Cardiovasc Surg. 1973;65:935-939.
170 Martinez GJ, Marco E, Marin F, et al. Chylopericardium after acute pericarditis. Rev Esp Cardiol. 1996;49:226-228.
171 Scholten C, Staudacher M, Girsch W, et al. A novel therapeutic strategy for the management of idiopathic chylopericardium and chylothorax. Surgery. 1998;123:369-370.
172 Groves LK, Effler DB. Primary chylopericardium. N Engl J Med. 1954;250:520-523.
173 Furrer M, Hopf M, Ris HB. Isolated primary chylopericardium: Treatment by thoracoscopic thoracic duct ligation and pericardial fenestration. J Thorac Cardiovasc Surg. 1996;112:1120-1121.
174 Spodick DH. Drug- and toxin-related pericardial disease. In: The Pericardium: A Comprehensive Textbook. New York: Marcel Dekker; 1997:411-416.
175 Tarbell NJ, Thomson L, Mauch P. Thoracic irradiation in Hodgkin’s disease: Disease control and long-term complications. Int J Radiat Oncol Biol Phys. 1990;18:275-281.
176 Zimmerman J, Yahalom J, Bar-On H. Clinical spectrum of pericardial effusion as the presenting feature of hypothyroidism. Am Heart J. 1983;106:770-771.
177 Kerber RE, Sherman B. Echocardiographic evaluation of pericardial effusion in myxedema: Incidence and biochemical and clinical correlations. Circulation. 1975;52:823-827.
178 Hardisty CA, Naik RD, Munro DS. Pericardial effusion in hypothyroidism. Clin Endocrinol. 1980;13:349-354.
179 Parving HH, Hansen JM, Nielsen SV, et al. Mechanism of edema formation in myxedema-increased protein extravasation and relatively slow lymphatic drainage. N Engl J Med. 1981;301:460-465.
180 Enein M, Aziz A, Zima A, et al. Echocardiography of the pericardium in pregnancy. Obstet Gynecol. 1987;69:851-855.
181 Oakley CM. Pericardial disease. In: Heart Disease in Pregnancy. London: BMJ; 1997:226-236.
182 Maisch B, Ristic AD. Practical aspects of the management of pericardial disease. Heart. 2003;89:1096-1103.
183 Ristic AD, Seferovic PM, Ljubic A, et al. Pericardial disease in pregnancy. Herz. 2003;28:209-215.
184 Richardson PM, Le Roux BT, Rogers NM, et al. Pericardiectomy in pregnancy. Thorax. 1970;25:627-630.
185 Tollens T, Casselman F, Devlieger H, et al. Fetal cardiac tamponade due to an intrapericardial teratoma. Ann Thorac Surg. 1998;66:59-60.