Chapter 29 Percutaneous Transforaminal Lumbar Interbody Stabilization
Percutaneous endoscopic transforaminal discectomy has been an effective alternative to microdiscectomy for patients with soft lumbar disc herniations [1–5]. However, it is ineffective in the presence of lumbar segmental instability due to degenerative disc disease. Percutaneous transforaminal lumbar interbody stabilization (PTLIS) is a new surgical method for treating lumbar segmental instability due to degenerative disc disease and low-grade degenerative spondylolisthesis.
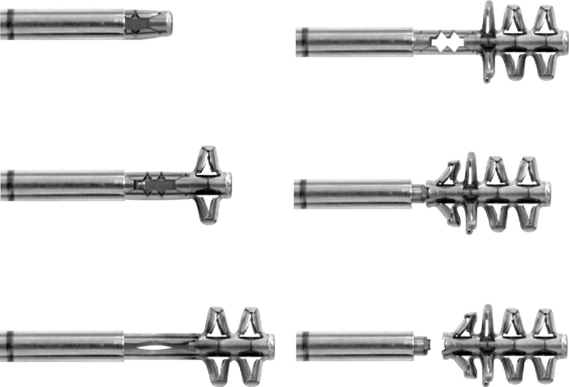
The specially designed B-Twin expandable holder (Disc-O-Tech Medical Technologies Ltd., Herzliya, Israel), which is made of titanium, is used as an interbody spacer to achieve stability without open discectomy and fusion [6–11]. In cases of failure, this minimally invasive procedure does not impede conventional surgical approaches.
Instrumentation
Prior to surgery, the required implant diameter is determined through the use of lateral and anteroposterior radiographs and computed tomography or magnetic resonance imaging. The intervertebral space is measured, and 10% to 20% is added to determine the appropriate expanded diameter of the device (Table 29.1). This diameter determines the implant to be selected.
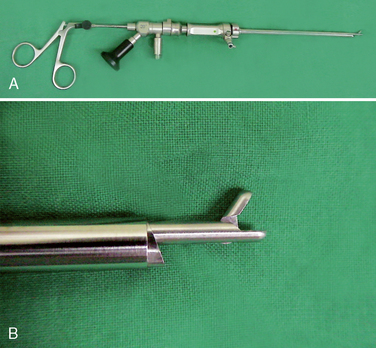
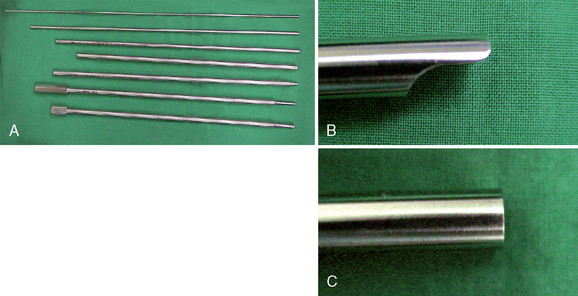
The length of the intervertebral space (the anteroposterior borders of the vertebral body) should be at least 28 mm for the use of the trapezoid implants. Verification of implant diameter and length is also performed during surgery, as detailed in the later Figures 29-1 to 29-8 illustrate the instrumentation for PTLIS.
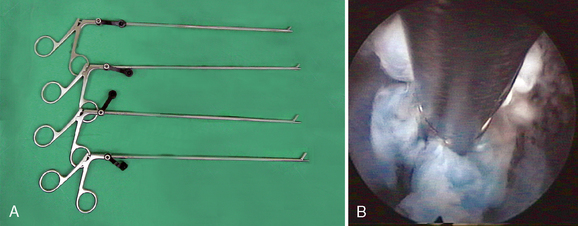
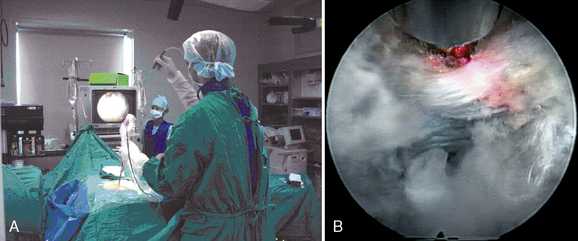
Figure 29–6 Microforceps (A) and the endoscopic view of the disc fragment which is grabbed by microforcep (B).
Anesthesia
 Communication between the surgeon and the patient is necessary during the procedure. The operation is typically conducted with the use of local anesthesia and analgesia via neuroleptics so that the surgeon may know immediately of any changes in symptoms and signs. General anesthesia may be used in the few patients who wish it or cannot tolerate the position. With general anesthesia, however, the urgent need for conversion to open surgery may not be detected early.
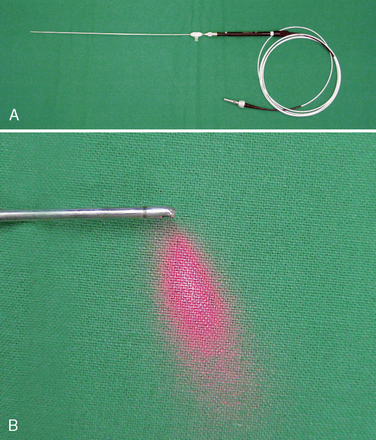
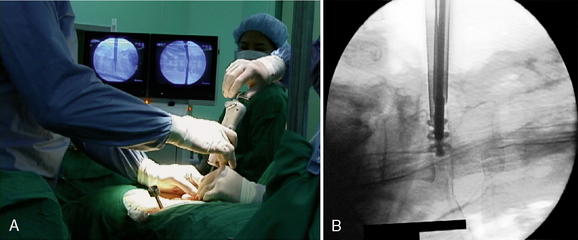
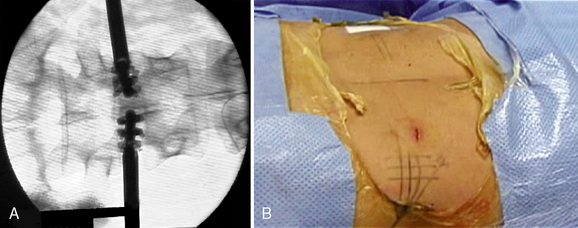
Communication between the surgeon and the patient is necessary during the procedure. The operation is typically conducted with the use of local anesthesia and analgesia via neuroleptics so that the surgeon may know immediately of any changes in symptoms and signs. General anesthesia may be used in the few patients who wish it or cannot tolerate the position. With general anesthesia, however, the urgent need for conversion to open surgery may not be detected early. A solution of 1% lidocaine is usually used to infiltrate the skin and subcutaneous tissue (Fig. 29-9). To minimize the thickening of underlying tissues and to allow a minute palpation of the spinal axis, we prefer to not infiltrate more deeply with lidocaine.
A solution of 1% lidocaine is usually used to infiltrate the skin and subcutaneous tissue (Fig. 29-9). To minimize the thickening of underlying tissues and to allow a minute palpation of the spinal axis, we prefer to not infiltrate more deeply with lidocaine.Procedure
Postprocedural management
 Patients should be observed for 3 hours to detect possible complications. All patients are permitted to go home on the day of surgery.
Patients should be observed for 3 hours to detect possible complications. All patients are permitted to go home on the day of surgery. Mild physical therapy may be helpful for fast recovery within 2 weeks postoperatively. If the pain and discomfort remain, complementary epidural injection of steroids with lidocaine may be added so that no further open procedure is necessary.
Mild physical therapy may be helpful for fast recovery within 2 weeks postoperatively. If the pain and discomfort remain, complementary epidural injection of steroids with lidocaine may be added so that no further open procedure is necessary. Four to 6 weeks postoperatively, twice-weekly rehabilitation exercises for back muscle strengthening and improvement of range of motion are recommended for 3 months.
Four to 6 weeks postoperatively, twice-weekly rehabilitation exercises for back muscle strengthening and improvement of range of motion are recommended for 3 months.CASE STUDY 29.1 L5-S1 Instability Stabilized By B-Twin Expandable Holder
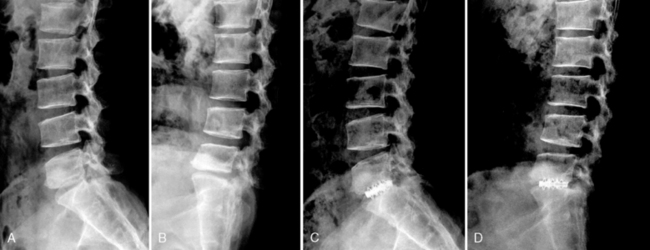
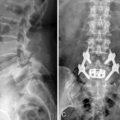
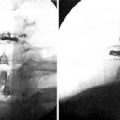
A 35-year-old man with suffered from low back pain and pain in both legs for 8 months. Preoperative radiographs demonstrated L5-S1 instability (Fig. 29-15 A and B). He underwent percutaneous endoscopic lumbar discectomy and stabilization using the B-Twin expandable holder. Postoperative radiographs showed improvement of the patient’s lordosis (Fig. 29-15C and D).
CASE STUDY 29.2 L4-L5 Instability Stabilized By B-Twin Expandable Holder
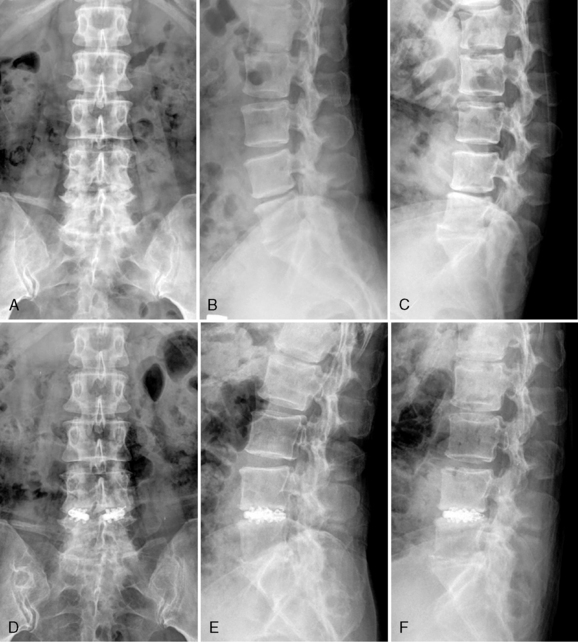
A 52-year-old woman with had suffered from low back pain and pain in both legs for 5 years. Preoperative radiographs demonstrated L4-L5 instability (Fig. 29-16A to C). The patient underwent percutaneous endoscopic lumbar discectomy and stabilization using the B-Twin expandable holder. Postoperative radiographs demonstrated improvement of lordosis without laminectomy (Fig. 29-16D to F).
1 Lee S.H., Gastambide D. Perkutane endoskopische diskotomie der halswirbelsaule. In: Pfeil J., Siebert W., Janousek A., et al, editors. Minimal-Invasive Verfahren in der Orthopadie und Traumatologie. New York: Springer; 2000:41-61.
2 Lee H.Y., Ahn Y., Kim D.Y., et al. Percutaneous ventral decompression for L4-L5 degenerative spondylolisthesis in medically compromised elderly patients: technical case report. Neurosurgery. 2004;55:435.
3 Ahn Y., Lee S.H., Park W.M., et al. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine. 2004;29:E326-E332.
4 Lee S.C., Kim S.K., Lee S.H., Shin S.W. New surgical technique of percutaneous endoscopic lumbar discectomy for migrated disc herniation. Joint Dis Relat Surg. 2005;16:102-110.
5 Choi G., Lee S.H., Raiturker P.P., et al. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery. 58, 2006. ONS–59–ONS–68, discussion ONS–59–ONS–68.
6 Choi W.C., Park C.W., Lee S.H. Percutaneous endoscopic lumbar interbody fusion. J Minim Invasive Spinal Tech. 2002;2:6-7.
7 Ahn Y., Lee S.H. Percutaneous endoscopic cervical fusion with expandable holder: case report of initial technique. J Minim Invasive Spinal Tech. 2002;2:8-9.
8 Lee S.H., Choi B.K., Choi S. Update on percutaneous cervical stabilization. J Minim Invasive Tech. 2004;4:22-32.
9 Lee S.H., Ahn Y. Percutaneous endoscopic cervical diskectomy and stabilization. In: Kim D., Fessler R., editors. Endoscopic Spine Surgery and Instrumentation. New York: Thieme; 2005:59-65.
10 Lee S.H., Choi B.K., Choi W.C., Choi S.M. Percutaneous cervical stabilization. In: Savitz M.H., Chiu J.C., Rauschning W., et al, editors. The Practice of Minimally Invasive Spinal Technique. 2005 ed. New York: AAMISS Press; 2005:540-542.
11 Choi W.C., Lee S.H., Ahn Y., et al. Percutaneous cervical stabilization using the WSH cervical B-Twin. Joint Dis Relat Surg. 2005;16:82-87.