Chapter 28 Percutaneous management of aortic and peripheral vascular disease
 From a technical standpoint, carotid stenting is less invasive and avoids the potential complications of a neck dissection that surgery involves.
From a technical standpoint, carotid stenting is less invasive and avoids the potential complications of a neck dissection that surgery involves. Currently, the decision to intervene with carotid disease is based primarily on two important clinical trials, NASCET and ACAS.
Currently, the decision to intervene with carotid disease is based primarily on two important clinical trials, NASCET and ACAS. A percutaneous approach to carotid revascularization appears to offer many advantages over CEA in select individuals.
A percutaneous approach to carotid revascularization appears to offer many advantages over CEA in select individuals. Revascularization for atherosclerotic renal artery stenosis is primarily for the improvement of blood pressure control.
Revascularization for atherosclerotic renal artery stenosis is primarily for the improvement of blood pressure control. Percutaneous revascularization of renal arterial stenosis has become an acceptable alternative to traditional surgical therapy.
Percutaneous revascularization of renal arterial stenosis has become an acceptable alternative to traditional surgical therapy.INTRODUCTION
Currently, a wide variety of balloons and stents are being utilized to treat aortic, iliac, femoral, renal, and carotid disease. The development of embolic protection devices has further allowed the advancement and application of these technologies. As a result, complication rates have decreased and have arguably allowed percutaneous therapy to become the preferred revascularization strategy. Randomized clinical trials continue to evolve and its results will ultimately dictate the superiority of a percutaneous rather than surgical approach. In this chapter, we will examine the current applications of percutaneous endovascular peripheral intervention and its potential to treat patients with severe disabling vascular disease.
CAROTID ANGIOPLASTY AND STENTING
Currently, the decision to intervene with carotid disease is based primarily on two important clinical trials, NASCET and ACAS. NASCET (North American Symptomatic Carotid Endarterectomy Trial) was a prospective, multi-center randomized trial which compared carotid endarterectomy (CEA) to best medical therapy.36 In this relatively low risk symptomatic cohort, the two-year outcome for CEA with respect to freedom from ipsilateral stroke or death was 92%. This is in contrast with the medical management arm, who fared much worse with a 72% freedom from the above clinical endpoints. Although there were some shortcomings of this trial, it did provide significant insight on the treatment of carotid disease. The second pivotal trial was ACAS, the Asymptomatic Carotid Atherosclerotic Study.36 Unlike the patient population in NASCET, these individuals were asymptomatic and found to have carotid disease based on duplex imaging. At five years, there was a statistically significant reduction in the incidence of ipsilateral stroke with CEA vs. medical management, 4.7% vs. 9.4% respectively.
Indications for carotid angioplasty and stenting continue to evolve as more clinical trial data becomes available. Various medical, neurological, and angiographic risk factors for carotid surgical revascularization have been identified based on previous CEA trials. These are listed in Table 28.1.38 Identification of these risk factors is crucial before proceeding with revascularization. In addition, based on early experience with carotid stenting, a number of contraindications have been recognized. These are summarized in Table 28.2.38 With improvements in catheter-based technique and equipment, the above mentioned criteria will continue to evolve. Operator experience should also influence the types of patients considered appropriate for a percutaneous approach.
TABLE 28.2 CONTRAINDICATIONS TO STENTING
|
• Pedunculated thrombus at the lesion site. This type of thrombus is best seen using 15 frame per second cine imaging.
|
Although many of the above mentioned risk factors have been shown to increase both surgical and percutaneous complication rates, there are situations which overwhelmingly favor a percutaneous approach. These may include post-CEA restenosis, discrete stenosis in patients with prior neck radiation or radical dissection, discrete proximal or ostial common carotid lesions, and discrete lesions in the distal internal carotid or involving high bifurcations.40 It is in these patients which percutaneous carotid intervention will hopefully find its niche and improve revascularization outcomes.
Once the diagnostic study is completed and the location of the lesion has been identified, the Vitek catheter is advanced over the 0.038 glide wire into the ipslateral external carotid artery. A stiff 0.038 Amplatz wire is then exchanged for the glide wire and Vitek catheter is removed. Alternatively, a 0.014 Tad wire can also be utilized for this step. The stiffer wire provides adequate support for the insertion of a 7 French-90 cm sheath, which is placed in the common carotid artery. The dilator and wire are removed and anticoagulation is administered prior to inserting the 0.014 wire/EPD into the internal carotid artery. Similar to coronary intervention, an activated clotting time (ACT) of 200–250 seconds is recommended. At this time, a 0.014 coronary guide wire/filter device is advanced across the area of interest. Various wires and embolic protection are available for carotid intervention. The selection of the appropriate system will depend on the type of lesion as well as the operator’s familiarity with the equipment. The most commonly utilized filter wire at this time is the Accunet device (Cordis). The tip of the wire is usually placed at the base of the skull, and the filter is ideally deployed 2 cm distal to the stenosis.
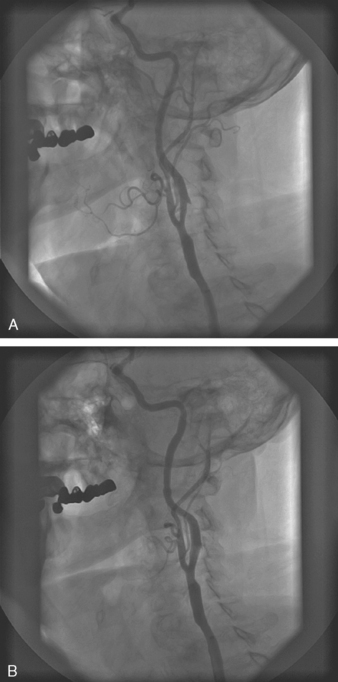
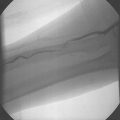
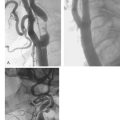
Pre-dilatation is subsequently performed with a 4.0 mm balloon unless the stenosis is pre-occlusive or the artery is occluded. In these cases, a step-wise pre-dilatation strategy is advised with the initial placement of a 2 mm balloon. Short, repeat inflations are performed with careful monitoring of the heart rate, blood pressure, and patient symptomatology. Once adequate pre-dilation has been accomplished, the balloon is removed and a self-expanding stent is loaded onto the wire. Self-expanding stents are utilized much more frequently than balloon-expandable stents for a number of reasons. One, with balloon-expandable stents, the stent has to be differentially expanded to accommodate to the size of the common carotid artery, bifurcation, and internal carotid artery. Therefore, more than one balloon has to be delivered for optimal dilatation and stent expansion. Secondly, the balloon can rupture while deploying the stent and there may be difficulty in advancing the balloon-stent assembly through the guiding sheath. Finally, balloon-expandable stents tend to occlude the external carotid artery. There have been a few reports of external compression with the balloon expandable stents. For these reasons, self-expanding stents are recommended for carotid intervention in a majority of cases. The final step to successful carotid stenting is post-dilatation. A 5–6 mm balloon is utilized depending upon the size of the internal carotid artery. Once this has been performed, the retrieval sheath of the EPD is advanced over the filter, and the wire and filter are removed as one unit together. Final angiographic images are obtained through the common carotid sheath and the procedure is complete. A carotid angiogram both pre-intervention and post-intervention is demonstrated in Fig. 28.1.
Careful post-procedural monitoring of these patients is vital since they are prone to a number of possible complications. These include puncture-site bleeding issues, transient brady-arrhythmias, hypotension, and neurologic sequelae from thrombotic and athero-embolic material. Early recognition of these potential adverse outcomes of carotid revascularization is crucial. Vasopressor and vagolytic agents are often utilized for post-procedural hemodynamic alterations. These hemodynamic changes are often secondary to the mechanical pressure on the carotid baro-receptors. This effect can result in hypotension up to 24–48 hours after the procedure depending upon the sensitivity of the baro-receptors. Usually there are no long-term clinical ramifications if this is treated in a prudent manner. Rarer complications such as carotid dissection, carotid perforation, and cerebral hemorrhage can obviously be fatal. The technical aspects of neurovascular rescue to treat these scenarios are beyond the scope of this chapter. However, as procedure-dedicated equipment continues to evolve and operator experience improves, it is anticipated that these complications will be infrequently encountered.
As alluded to above, the indications and technical aspects of a percutaneous approach to carotid revascularization have been evolving over the past decade. Just as NASCET and ACAS sought to establish the superiority of surgery over medical therapy for the treatment of carotid disease, percutaneous carotid intervention had to prove its safety and efficacy as well. Initially, the CAVATAS (Carotid and Vertebral Artery Transluminal Angioplasty Study) trial was conducted in Great Britain.41 This was a prospective, randomized, controlled trial comparing CEA vs. carotid angioplasty in higher risk, symptomatic patients with high-grade carotid stenoses. Approximately two-thirds of the patients in the percutaneous arm were treated with carotid angioplasty without stenting. Despite sub-optimal equipment and operator experience in the percutaneous arm, both early and late outcomes were similar in the two groups. The 30-day incidence of major stroke and death was approximately 5% and the incidence for all strokes (disabling and non-disabling) was 11% in both arms.
The advent of EPD and improved stent design lead to the first randomized, prospective, multi-center trial for comparison of carotid stenting with protection vs. CEA. The SAPPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy) trial was conducted in 2002 and randomized a total of 307 patients.42 Eligible patients could be either asymptomatic with >80% stenoses by ultrasound or symptomatic with >50% stenosis and one high-risk factor (see Table 28.3). The primary endpoint was the incidence of MACE, including death, stroke, or MI within 30 days of the procedure. At 30 days, SAPPHIRE reported a composite endpoint of 5.8% and 12.6% for carotid stenting with embolic protection and CEA, respectively. In addition, complication rates were similar among the two groups of patients with respect to TIA and major bleeding. There was a statistically significant difference in cranial nerve injury, 0% in the stenting arm and 5.3% in the CEA arm. One-year results presented in 2003 demonstrated a MACE rate of 11.9% and 19.9% for carotid stenting and CEA respectively. SAPPHIRE was a landmark trial because it was the first randomized study to compare carotid stenting with distal embolic protection with carotid surgery in high-risk patients. Its results prove the hypothesis that among patients with severe carotid artery stenosis and coexisting conditions, carotid stenting is not inferior to carotid endarterectomy.
TABLE 28.3 FDA-APPROVED AND INVESTIGATIONAL ENDOGRAFTS
A percutaneous approach to carotid revascularization appears to offer many advantages over CEA in select individuals. As with all novel modalities in cardiovascular intervention, rigorous testing and clinical trial outcomes will be vital before it is widely accepted. However, initial clinical experience appears very promising at this time. Improvements in catheter, stent, and embolic protection design will likely lead to better outcomes with a reduction in complications. Obviously, formal training centers need to be established as well and incorporated in interventional cardiovascular programs. In parallel with the advancements made in coronary intervention, carotid stenting represents the natural progression to a successful, less-invasive technique for revascularization.
ABDOMINAL AORTIC ANEURSYMS AND ENDOLUMINAL TREATMENT
Approximately 95% of AAAs are infra-renal in origin. Detection of an AAA during clinical examination has only moderate sensitivity at best. Therefore, if clinical suspicion is high, an abdominal ultrasound is warranted. If a patient is obese or if the ultrasound is suboptimal, other non-invasive studies such as MRI/MRA or CT can be performed with excellent sensitivity. Once the diagnosis is made, the decision whether to intervene is based upon the size and the rate of enlargement of the aneurysm. It is well accepted that a diameter of >5 cm is an indication for intervention. This size is based upon surveillance studies which have indicated that AAAs 5.5 cm and 6.5 cm have annual rupture rates of 11% and 26% respectively.43 If an aneurysm is discovered measuring 3–4 cm, serial examinations with ultrasound are recommended every six months to monitor the rate of enlargement.
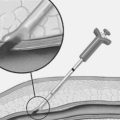
Historically, the treatment for AAAs has been surgical in nature. However, open surgical repair carries a considerable risk especially in patients with a host of cardiovascular and pulmonary co-morbidities. Consequently, many of these patients are turned down for open surgical repair. In a series of 109 patients who were refused AAA repair, death due to aneurysm rupture occurred in 36% of patients with AAA 5.5–5.9 cm and >50% in AAA 6.0–7.0 cm.44 In patients with multiple risk factors for a poor surgical outcome, endoluminal grafting has gained substantial popularity in the last few years. The development of this technique to exclude the aneurysm by placement of an endoluminal graft began in 1991 by Parodi and collegues.45 An illustration of a typical endoluminal graft is shown in Fig. 28.2. The procedure is performed under local anesthesia and involves the placement of a stent-anchored Dacron prosthetic graft in a retrograde femoral cannulation. A number of aortic endografts have been developed in the United States and are listed in Table 28.3.46
Various clinical and angiographic criteria are required for approval of an aortic endoluminal graft. Clinical criteria include the following: (1) the risks of an open repair are unacceptable; and (2) The risk of aneurysm rupture is high. These scenarios are listed in Table 4.46 Anatomic criteria are also very important and must be evaluated carefully prior to proceeding with an endoluminal repair. These are listed in Table 28.5.46 Once these criteria have been met, the decision to perform an endoluminal vs. surgical repair will depend on the expected peri-operative mortality and life-expectancy of the patient. If the peri-operative mortality is less than 5% and the life expectancy is >5 years, a conventional surgical approach is advised. On the other hand, if the perioperative mortality is >5% and the life expectancy is short secondary to other co-morbid conditions, an endoluminal repair should be considered.
TABLE 28.5 ANATOMIC CONSIDERATIONS OF ENDOLUMINAL GRAFT REPAIR
Although a step-wise technical description of performing an endoluminal repair is beyond the scope of this chapter, one should be familiar with the potential adverse outcomes of these devices. Most importantly, the presence of an endoleak should be identified after endoluminal repair. An endoleak is defined as intraaneurysm flow around an endovascular graft. There are four types of endoleaks which are listed in Table 28.6.46 Endoleaks can be diagnosed non-invasively with ultrasound, CT, and MRI, or invasively with angiography. Endoleaks can lead to rupture of an aneurysm because of their persistent pressure on the vessel wall. A variety of structural changes of the endograft can lead to the development of an endoleak. These include: suture breaks between the stent and the graft, fracture of the hooks used to anchor the proximal end of the endograft to the aortic wall, circular and longitudinal stent wire separation, separation of the connection between wire loops, graft fatigue, device migration, component separation, and endograft or vessel thrombosis.47 The decision to intervene once an endoleak has been discovered depends upon the type of endoleak as well as the size of the aneurysm. Another potential adverse outcome is the development of endotension. This refers to the expansion of the aneurysm in the absence of an obvious endoleak. One of the leading causes of endotension is transmission of pressure through a sealed or thrombosed endoleak.
TABLE 28.4 RISK FACTORS FOR ANEURYSM RUPTURE
Although numerous advancements have been made in the development of endoluminal grafts, there has been variable results reported when compared to traditional surgical repair. Up until recently, a variety of prospective, cohort, controlled studies have been performed.48,49,50 However, in the last year, early results were released for two randomized trials comparing endoluminal and open surgical repair. EVAR-1 (Endovascular Aneurysm Repair Trial 1) and DREAM (Dutch Randomized Endovascular Aneurysm Management Trial) have both reported lower operative mortality rates after endovascular repair as compared to open repair.51,52 In EVAR-1, the in-hospital mortality was 6% for open repair and 1.6% for endoluminal repair, while in DREAM, the in-hospital mortality was 4.6% and 1.2% for open repair and endoluminal repair, respectively. These trials concluded that in patients who are candidates for both techniques, endovascular repair is preferable to open repair. Further randomized data with long-term follow up will be required.
The diagnosis and subsequent treatment of AAAs are vital because of the high morbidity and mortality associated with it. Endovascular repair has made dramatic advancements in the past few years with regard to device integrity and durability. Early comparisons with the traditional open surgical repair have shown promise as an alternative treatment in a majority of patients. Long-term clinical results will ultimately dictate the popularity of this technique. Incorporating endoluminal repair procedures into training programs for interventional cardiologists is important for understanding how to diagnose and treat patients with AAAs/AAA repairs. As with all cardiovascular therapies, the goal is to continue to improve efficacy while striving for more non-invasive modalities to treat patients.
RENAL ANGIOPLASTY AND STENTING
Secondary hypertension represents approximately 5% of the overall hypertensive population. Atherosclerotic renal artery stenosis accounts for the majority of patients with secondary hypertension.21 Although this appears to represent a small number of individuals, its prevalence is much higher among patients with co-existing coronary and peripheral arterial disease. Approximately 15–18% of patients undergoing cardiac catheterization for suspected coronary artery disease have been found to have reno-vascular stenosis.22,23 Furthermore, the incidence increases even more in patients with aneurysmal or occlusive peripheral vascular disease.24
Revascularization for atherosclerotic renal artery stenosis is primarily for the improvement of blood pressure control. Both immediate and long-term effects on hypertension have been demonstrated in various studies.25,25 This has lead to a reduction in the number of anti-hypertensive agents required for optimal blood pressure control. This is beneficial in many regards including a smaller number of drug interactions and a limited exposure to pharmacological side effects. Secondly, renal arterial revascularization can potentially result in the preservation of renal function as well as a slowing in the progression of overt renal failure.27 Even patients with end stage renal disease on hemo-dialysis who have stenotic renal arteries may experience a reversal in renal function.28 Finally, patients with refractory congestive heart failure and angina may indirectly benefit.29 Improved blood pressure control with afterload reduction may result in both improvements in systolic and diastolic performance.
If non-invasive imaging is suggestive of reno-vascular stenosis, a referral for diagnostic angiography is justified. Diagnostic renal angiography is most commonly performed from a femoral arterial access site. A variety of catheters are available for selective cannulation of the renal ostia. These include a Judkins’ right configuration, an internal mammary, and a cobra catheter. A 6 French catheter is usually sufficient to engage the renal ostia without causing trauma to the aorta which may lead to potential cholesterol embolization. The renal ostia are usually located approximately 1–2 cm below the takeoff of the superior mesenteric artery. The left renal artery often has a higher origin than the right. In addition, multiple renal arteries can be present in up to 30% of patients. One of the most common anomalies include two separate arteries to the renal hilum. Atherosclerotic renal artery stenosis most often occurs at the ostium or proximal portion of the renal artery. This is in contrast to fibromuscular dysplasia which more commonly involves the distal segment of the artery.
Percutaneous renal revascularization has been shown to be an acceptable therapy for renovascular stenosis. Balloon angioplasty alone can achieve adequate short-term angiographic results; however, the incidence of restenosis is unacceptably high in many instances.30 This is especially true for aorto-ostial lesions which are prone to vascular recoil. For this reason, endovascular stents are frequently utilized despite optimal immediate results with balloon angioplasty alone. Similar to coronary arterial stents, renal arterial stents are able to scaffold the arterial wall and prevent elastic recoil. Early clinical results demonstrate superior angiographic and hemodynamic outcomes with stenting vs. balloon angioplasty alone.31,32 Primary success rates have been reported between 95–100% while restenosis rates vary widely between 1.6–39%.33,34 As mentioned earlier, one of the primary indications for renal arterial revascularization is improvement in blood pressure control. Various studies have reported promising results. White et al. reported a statistically significant improvement in blood pressure control with a reduction in the number of anti-hypertensive medications from 2.6 +/− 1.0 to 2.0 +/− 0.9 (p<0.001).35 In patients who underwent follow-up angiography, a restenosis rate of 18.8% was demonstrated. The only procedural variable which was associated with angiographic restenosis was the immediate post-stent minimum lumen diameter (MLD). Other studies have demonstrated benefit for unstable angina as well as congestive heart failure symptoms.32 However, there has not been a clear benefit in improvement in creatinine clearance. Their still remains debate as to whether revascularization can result in normalization of renal function in patients with chronic renal insufficiency. It does appear that the rate of progression to renal failure can be slowed with revascularization. Additional multi-center, randomized trials will be needed to address some of these issues.
AORTO-ILIAC ANGIOPLASTY AND STENTING
Vascular access for aorto-iliac angiography is usually obtained from the ipslateral femoral artery. However, a contralateral retrograde femoral approach may be necessary if a contra-indication exists for ipslateral access. Likewise, in rare instances, an upper extremity arterial cannulation may be required. In any case, a standard Seldinger technique is employed, with the initial placement of a 6 French vascular sheath. A 0.035 Wholey wire or hydrophilic glide wire is utilized for crossing aorto-iliac disease, with subsequent placement of a pigtail catheter in the abdominal aorta. Diagnostic angiography is performed with visualization of occlusive disease and distal runoff of the lower extremities. An antero-posterior (AP) projection is usually standard, however, caudal angulation may be beneficial in some aorto-iliac disease. A roadmap is obtained in preparation for possible aorto-iliac intervention.
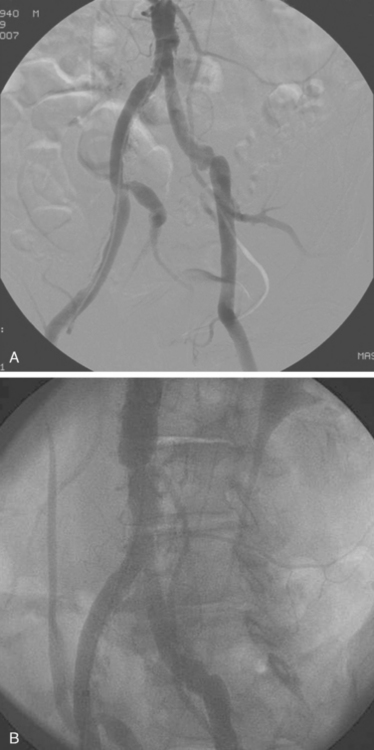
Early experience with intervention of aorto-iliac disease involved PTA only in a majority of cases. An example of a stenotic lesion in the iliac artery is shown in Fig. 28.3. Ideal characteristics of iliac lesions for PTA included a stenotic, noncalcified, discrete (<3 cm) lesion with >2 patent run-off vessels.1 In contrast, relative contraindications for a percutaneous approach involved total occlusions, long lesions (>5 cm), bilateral disease and the presence of an aneurysm. If the above criteria were met for a lesion, accurate assessment of vessel size and length was subsequently made with quantitative angiography or IVUS. Pre-treatment with Aspirin and intra-procedural Heparin was the standard pharmacological regimen utilized. After balloon dilation, a pressure gradient was obtained. In the case of a >5 mmHg pressure gradient or a residual stenosis of >30%, stenting or repeat higher-pressure balloon inflation would be performed.
Similar to coronary intervention, the long-term patency of iliac vessel angioplasty is dependent upon both clinical and anatomical variables.2 Favorable criteria include non-diabetic, male patients with discrete non-occlusive stenoses with good distal run-off. In contrast, restenosis tends to be higher in diabetic, female patients with diffuse and lengthy occlusive lesions with poor distal run-off.
Prior to the routine placement of iliac stents as a preferred strategy for revascularization, early clinical trials examined the efficacy of PTA vs. medical and surgical therapy.3 Patient enrollment criteria in these early trials included lifestyle-limiting or progressive claudication, ischemic pain at rest, non-healing ischemic ulcerations and gangrene. After randomized clinical trial data established the superiority of iliac PTA over medical therapy, investigators began to compare this percutaneous approach with traditional surgical therapy.3 Wilson et al. conducted a randomized trial comparing PTA vs. bypass surgery for 157 iliac lesions.4 No significant differences were found for the clinical endpoints of death, amputations, or loss of patency at three years. In addition, measurement of the ABI at three years demonstrated no difference in the two treatment arms. In parallel with the advancements made in coronary intervention, the availability of stents dramatically affected the results of iliac intervention. Bosch et al. published a meta-analysis of over 2000 patients undergoing PTA and stent placement for aorto-iliac occlusive disease.5 Compared with PTA only, iliac stenting had a higher procedural success rate and a 43% reduction in late (four year) failures. Other randomized trials have studied PTA with provisional stenting (stent placement for unsatisfactory balloon angioplasty results) vs. primary iliac stenting.6,7,8 Results have demonstrated that there is a significantly improved technical success rate as well as reduction in the pressure gradient with primary stenting. In addition, if provisional stenting was implemented in the PTA arm, the outcomes were similar to the primary stenting arm. Again indicating the overall improved clinical and angiographic outcome with iliac stenting. Additional clinical trials have yielded similar results. Richter et al. demonstrated a four-year patency rate of 92% and 74% for iliac stenting and PTA, respectively.9
FEMORAL ARTERY ANGIOPLASTY AND STENTING
Femoral arterial disease, like aorto-iliac pathology, can present with a wide variety of symptoms. Often times, patients may be asymptomatic especially with the development of an adequate collateral circulation. If symptoms of intermittent claudication develop, the progression to critical limb ischemia is approximately 5% per year.10 The risk of revascularization, whether it be surgical or percutaneous, is 11% at five years and 14% at ten years in these patients.11 Therefore, as with all types of peripheral arterial disease, lifestyle modification should be implemented first. Only in the presence of ischemic rest pain, disabling claudication, or ischemic tissue loss should revascularization be considered as a first option.
Prior to the availability of endovascular treatment, surgical bypass was the primary means of revascularization. Historically, patency rates of above the knee femoropopliteal bypass were 80% at one year and approximately 50% at five years.12 Because of the need for less invasive means to treat these patients, percutaneous therapies for femoral arterial stenotic and occlusive disease began to emerge in the early to mid-1990s. Early experience with PTA of femoral arterial disease demonstrated patency rates comparable to traditional surgical revascularization.13,14
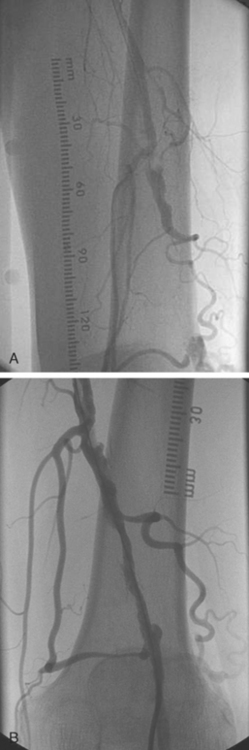
Similar to aorto-iliac disease, ideal characteristics for PTA of femoral disease included a stenotic, short, and non-calcified lesion. In parallel with coronary intervention, long and chronically occluded lesions had poor short and long term patency rates.15,16 A chronic total occlusion in the SFA is shown in Fig. 28.4. Initially, ‘debulking’ modalities such as rotational and directional atherectomy were employed. Unfortunately, these strategies did not prove to be beneficial.17,18
The advent of stenting for femoral disease was thought to eliminate some of the shortcomings of PTA alone. Initial indications for stenting included the presence of a significant residual stenosis (>30%) after PTA, an arterial dissection, and the incidence of restenosis. The types of stents available for utilization in the femoral artery included a self-expanding and a balloon expandable stent. Advantages and disadvantages of each of these were mentioned previously.
Despite its theoretical advantage over PTA alone, there does not appear to be overwhelming data to support the use of stents in the femoral artery. Thus far, little randomized clinical data exists to support the routine practice of stenting over PTA. A small study was conducted which randomized 51 patients to PTA vs. stenting in the femoral artery.19 At one year of follow up, no statistically significant difference was found with regard to patency between the two arms. Other observational data to date yield similar results.20 Obviously, larger randomized clinical trials are needed to justify the routine utilization of stents in the femoral artery.
With the improvements in technology that has emerged in coronary intervention, there has been parallel advancements in peripheral therapies. Novel devices, such as the Fox Hollow atherectomy device, has shown some initial success in treating de novo and in-stent restenotic lesions. Thermo-cooling catheters, namely the Polar-catheter (Boston Scientific) have also shown some initial promise in the femoral and even popliteal arterial circulation. As with all new devices, randomized data will be needed to justify the costs of these techniques. However, there has been encouraging results with these devices in a number of individuals. Currently, percutaneous femoral arterial intervention provides an alternative therapy for revascularization in many patients. The optimal endovascular strategy, both from a pharmacologic and mechanical standpoint, will continue to evolve with further operator experience and clinical trial results.
1 Heuser RR. Peripheral Vascular Stenting for Cardiologists. Martin Duntz Ltd., 1999;47. Chapter 5
2 Johnston KW. Balloon Angioplasty: Predictive factors for long term success. Semin Vasc Surg. 1989;3:117-122.
3 Whyman MR, Kerracher EMG, Gillespie IN, et al. Randomized Controlled Trial of Percutaneous Transluminal Angioplasy for Intermittant Claudiaction. Eur J Vasc Surg. 1996;12:167-172.
4 Wilson SE, Wolf GL, Cross AP. Percutaneous transluminal angioplasty versus operation for peripheral arteriosclerosis: report of a prospective randomized trial in a selected group of patients. J Vasc Surg. 1989;9:1-9.
5 Bosch JL, Hunink MGM. Meta-analysis of the results of percutaneous transluminal angioplasty and stent placement for aortoiliac occlusive disease. Radiology. 1997;204:87-96.
6 Henry M, Amor M, Thevenof G, et al. Palmaz stent placement in iliac and femoropoplitieal arteries: primary and secondary patency in 310 patients with 2–4 year follow up. Radiology. 1995;197:167-174.
7 Vorwerk D, Gunther RW, Schumann K, Wendt G. Aortic and iliac stenosis: follow-up results of stent placement after insufficient balloon angioplasty in 118 cases. Radiology. 1996;198:45-48.
8 Tetteroo E, Haaring C, Van der Graaf Y, et al. Intraarterial pressure gradients after randomized angioplasty or stenting of iliac artery lesions. Dutch Iliac Stent Trial Study Group. Cardiovasc Intervent Radiol. 1996;19:411-417.
9 Richter GM, Noeldige G, Roeren T, et al. First long-term results of a randomized mulitcenter trial: iliac balloon-expandable stent placement versus regular percutaneous transluminal angioplasty. In: Lierman D, editor. State of the Art and Future Developments. Morin Heights, Canada: Polyscience; 1995:30-35.
10 Hertzer NR. The natural history of peripheral vascular disease. Implications for its management. Circulation. 1991;83(Suppl):112-119.
11 Cox GS, Hertzer NR, Young JR, et al. Nonoperative treatment of superficial femoral artery disease: long-term follow-up. J Vasc Surg. 1993;17:172-181.
12 Michaels JA. Choice of material for above-knee femoropopliteal bypass graft. Br J Surg. 1989;76:7-14.
13 Holm J, Arfridsson B, Jivegard L, et al. Chronic lower limb ischemia. A prospective randomized controlled study comparing the 1-year results of vascular surgery and percutaneous transluminal angioplasty (PTA). Eur J Vasc Surg. 1991;5:517-522.
14 Yucel EK. Femoropopliteal angioplasty: Can we predict success with duplex sonography? AJR AM J Roentgenol. 1994;162:184-186.
15 Matsi PJ, Manninen HI, Soder HK, et al. Percutaneous transluminal angioplasty in femoral artery occlusions: primary and long-term results in 107 claudicant patients using femoral and popliteal catherization techniques. Clin Radiol. 1995;50:237-244.
16 Capek P, McLean GK, Berkowitz HD. Femoropopliteal angioplasty. Factors influencing long-term success. Circulation. 1991;83(Suppl):I700. II80
17 Belli AM, Murphy GJ, Bolia A. Recanalization using a low speed rotational device (ROTACS) in total occlusions of the femoropopliteal artery. Clin Radiol. 1994;49:304-306.
18 Vroegindeweij D, Tielbeck AV, Buth J, et al. Directional atherectomy versus balloon angioplasty in segmental femoropopliteal artery disease: two-year follow-up with color-flow Doppler scanning. J Vasc Surg. 1995;21:255-268.
19 Vroegindeweij D, Vos LD, Tielbeek AV, et al. Balloon angioplasty combined with primary stenting versus balloon angioplasty alone in femoropopliteal obstructions: a comparative randomized study. Cardiovasc Intervent Radiol. 1997;20:420-425.
20 Do-daiy-Do, Triller J, Walpoth BH, et al. A comparison study of self-expandable stents versus balloon angioplasty alone in femoropopliteal artery occlusions. Cadiovasc Intervent Radiol. 1992;15:301-312.
21 Simon N, Franklin SS, Bleifer KH, Maxwell MH. Clinical characteristics of renovascular HTN. JAMA. 1972;220:1209-1218.
22 Olin JW, Melia M, Young JR, et al. Prevalence of atherosclerosis renal artery stenosis in patients with atherosclerosis elsewhere. Am J Med. 1990;88:46N-51N.
23 Harding MB, Smith LR, Hinmelstein SI, et al. Renal artery stenosis: prevalence and associated risk factors in patients undergoing routine cardiac catherization. J Am Soc Nephrol. 1992;2:1608-1616.
24 Valentine RJ, Clagett GP, Miller GL, et al. The coronary risk of unsuspected renal artery stenosis. J Vasc Surg. 1993;18:433-440.
25 Derkx F, Schalekamp M. Renal artery stenosis and HTN. Lancet. 1994;344:237-239.
26 Losinno F, Zuccala A, Busato F, Zucchelli P. Renal artery angioplasty for renovascular HTN and preservation of renal function: long-term angiographic and clinical F/V. Am J Radiol. 1994;162:853-857.
27 Harden PN, Macleod MJ, Rodiger RSC, et al. Effect of renal artery stenting on progression of renovascular renal failure. Lancet. 1997;349:1133-1136.
28 Novick AC, Pohl MA, Schreiber M, et al. Revascuarization for preservation of renal function in patients with atherosclerotic renovascular disease. J Urol. 1983;129:907-912.
29 Khosla S, White CJ, Collins TJ, et al. Effects of renal artery stent implantation in patients with renovascular HTN. Presenting with USA or CHF. Am J Cardiol. 1997;80:363-366.
30 Weibull H, Bergquist D, Jonsson K, et al. Long-term results after percutaneous transluminal angioplasty of atherosclerotic renal artery stenosis: The importance of intensive follow-up. Eur J Vasc Surg. 1991;5:291-301.
31 Isles CG, Robertson S, Hill D. Management of renovascular disease: A review of renal artery stenting in ten studies. QJ Med. 1999;92:159-167.
32 Khosla S, White CJ, Collins TJ, et al. Effects of renal artery stenting implantation in patients with renovascular HTN presenting with USA or CHF. Am J Cardiol. 1997;80:363-366.
33 Van de Ven PJG, Kaatee R, Beutler JJ, et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: A randomized trial. Lancet. 1999;353:282-286.
34 Dorros G, Jaff M, Jain A, et al. Follow-up of primary Palmaz-Schatz stent placement for atherosclerotic renal artery stenosis. Am J Cardiol. 1995;75:105i-1055.
35 White CJ, Ramee SR, Collins TJ, et al. Renal artery stent placement: utility in difficult lesions for balloon angioplasty. J A Cell Cardiol. 1997;30:1445-1450.
36 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade carotid stenosis. New Eng J Med. 1991;325:445-453.
37 Executive Committee for the Asymptomatic Carotid Atherosclerotic Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;274:1421-1428.
38 Heuser RR. Periperal Vascular Stenting for Cardiologists. Martin Dunitz Ltd, 1999;71. Chapter 7
39 Heuser RR. Periperal Vascular Stenting for Cardiologists. Martin Dunitz Ltd., 1999;73. Chapter 7
40 Heuser RR. Periperal Vascular Stenting for Cardiologists. Martin Dunitz Ltd., 1999;77. Chapter 7
41 CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery transluminal Angioplasty study (CAVATAS): a randomized study. Lancet. 2001;357:1729-1737.
42 SAPPHIRE (Stenting and Angioplasty with Protection in Patients of High Risk for Endarterectomy) Investigators. NEJM. 2001;351:1493-1501.
43 The UK Small Aneurysm Trial Participants. Mortality results for randomized controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet. 1998;352:1649-1655.
44 Conway KP, Byrne J, Townsend M, et al. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagy; revisited? J Vasc Surg. 2001;33:757-762.
45 Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491-499.
46 Kamineni R, Heuser RR. A review of endoluminal treatment. Journal of Interventional Cardiology. 2004;17:437-445.
47 Beebe HG. Late failures of devices used for endovascular treatment of abdominal aortic aneurysm: What have we learned and what is the task for the future? New York: Thieme Medical Publishers, 2001.
48 Zarins CK, White RA, Schwarten D, et al. Aneurx Stnet graft versus open surgical repair of abdominal aortic aneurysms: Multicenter prospective clinical trial. J Vasc Surg. 1999;29:292-305.
49 May J, White GH, Waugh R, et al. Improved survival of endoluminal repair with second-generation prostheses compared with open repair in the treatment of abdominal aortic aneurysms: A 5-year concurrent comparison using life table method. J Vasc Surg. 2001;33:21-26.
50 Cohnert TV, Oelert G, Wahlers T, et al. Matched-pair analysis of conventional versus endoluminal AAA treatment outcomes during the initial phase of an aortic endografting program. J Endovasc Ther. 2000;7:94-100.
51 EVAR-1 Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomized controlled trial. Lancet. 2004 Sep 4;364(9437):843-848.
52 DREAM Investigators. A Randomized Trial Comparing Conventional and Endovascular Repair of Abdominal Aortic Aneurysms. N Engl J Med. 2004;351:1607-1618.