Chapter 25 Percutaneous coronary intervention in saphenous vein graft disease
 Long term outcome of CABG is limited by occlusive atherothrombotic disease of vein grafts in up to 50% of patients after ten years.
Long term outcome of CABG is limited by occlusive atherothrombotic disease of vein grafts in up to 50% of patients after ten years. Stent placement in vein grafts is associated with a high (up to 20%) risk of distal embolisation and consequent complications.
Stent placement in vein grafts is associated with a high (up to 20%) risk of distal embolisation and consequent complications. Routine use of embolic protection devices significantly reduces procedural complications during vein graft PCI and where anatomy permits, their use is mandatory.
Routine use of embolic protection devices significantly reduces procedural complications during vein graft PCI and where anatomy permits, their use is mandatory. Three types of embolic protection devices are available: distal occlusion, proximal occlusion and distal filter. The type to use is determined by lesion location in the vein graft.
Three types of embolic protection devices are available: distal occlusion, proximal occlusion and distal filter. The type to use is determined by lesion location in the vein graft. The routine use of glycoprotein IIb/IIIa inhibitors is not recommended for vein graft PCI. Some evidence suggests benefit in patients in whom a distal filter device is employed.
The routine use of glycoprotein IIb/IIIa inhibitors is not recommended for vein graft PCI. Some evidence suggests benefit in patients in whom a distal filter device is employed.INTRODUCTION
Since first implantation of coronary grafts in 1967 the success of venous conduits in relieving myocardial ischaemia has been limited by their temporal attrition. Although internal mammary grafts have unsurpassed long term patency, utilisation is limited by scarcity. The use of gastroepiploic and radial arteries has been shown to be less than optimal.1 Consequently, in spite of declining surgical revascularization rates worldwide, the use of venous grafts seems unlikely to diminish and treatment of symptomatic patients with degenerated venous grafts will remain a problem for interventional cardiologists.
Vein graft disease progression and its timing
Three interlinked pathological processes – thrombosis, neointimal hyperplasia and atherosclerosis, which are temporally distinct, contribute to vein graft disease.2,3 Awareness of the processes and their timing may help in optimising outcome from intervention.
Atherosclerosis is the main pathological process that contributes to vein graft occlusion one year and beyond after surgery.2 The predisposing factors and the basic process of atheroma development are similar to those documented in native coronary arteries. However, there are a few distinct topographic, temporal, and histological differences in the pathology of vein graft disease.
The distinct morphological difference seen in vein grafts compared to native coronary arteries is that vein graft atheroma is diffuse, concentric, and friable with poorly developed or absent fibrous cap with little calcification. Intravascular ultrasound studies reveal the absence of focal compensatory enlargement (‘Glagov’s law’) of diseased vein graft segments.3,4 Late graft thrombosis triggers recurrent ischaemia and is frequently seen in old degenerating grafts with advanced atherosclerotic disease.5
REVASCULARISATION FOR VEIN GRAFT DISEASE
Redo surgery
Recurrence of anginal symptoms is seen in up to 20% of patients within one year following surgical revascularisation followed by 4% of patients annually during the next 5 years, 19% at 10 years and 31% at 12 years.6
There are limited data comparing percutaneous revascularisation to repeat surgery in patients with angina recurrence. Repeat surgery achieves complete revascularisation in 92% of patients compared to 38% in the percutaneous intervention group. However, in-hospital complications are more common in surgical patients: death (7.3 % vs. 0.3%), Q-wave MI (6.1% vs. 0.9%), and low output syndromes (24% vs. 9%). Both procedures result in equal survival rates at one and six years with equal relief of angina but repeat intervention and/or revascularisation is more frequent in the percutaneous group (64% vs. 8%) at six years.7,8
Given the high morbidity and mortality of re-operation reduced symptomatic benefit as compared to first bypass surgery,8,9 percutaneous revascularisation should be the preferred first route for post surgery angina recurrence.
Percutaneous coronary intervention
Balloon angioplasty for vein graft disease has moderate procedural success limited by high periprocedural complication rate, high incidence of restenosis (35%) and high repeat revascularisation rate.10,11 Clinical trials show BMS to be superior to balloon angioplasty in vein graft lesions. The use of stents is associated with a high procedural success, superior clinical outcome, and reduced TLR. However, as compared to native vessels the restenosis rate remains high12,13 coupled with higher periprocedural complications.
The significant problems associated with vein graft intervention are:
The use of DES in native coronary arteries has shown promising results with significant reduction in restenosis rates.14,15 Limited data is available on the long term efficacy of DES in patients with vein graft disease. Most studies confirm a high procedural success rate but there is conflicting data on how DES would affect the clinical outcome. Two nonrandomized trials have shown a trend towards a reduction in MACE at six to nine months follow up.16,17 The use of DES was associated with a lower restenosis rate (10% vs. 26.7%, P=0.03) and TVR (4.9% vs. 23.1%, P <0.01) at six to nine months. However, one study showed similar clinical outcome at 30 days, 60 days and 1 year.18
Diffuse SVG disease
Unlike focal graft lesions, diffusely degenerative graft disease with large ulcerated fragile plaques with associated thrombus is associated with a low procedural success, higher complication rates and low long term patency rate.19
Endoluminal reconstruction by stenting of diffuse lesions (‘full metal jacket’) is a possible option and clinical trials reveal a high procedural success for stenting of diffuse lesions. However, it has been associated with a high incidence of death (11.1%) or myocardial infarction (9.4%) and frequent need for repeat angioplasty.20
EMBOLIC PROTECTION DEVICES
The first recorded use of these devices was during carotid stenting21 and subsequent studies confirmed their efficacy in reducing the incidence of periprocedural embolic stroke and death. The high risk of periprocedural myocardial infarction and MACE during vein graft intervention led to exploration of embolic protection devices in this setting.
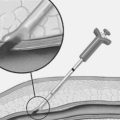
Distal occlusion devices
An alternative device is the TriActiv system (Kensey Nash). In contrast to the GuardWire, this device utilises gas to fill the balloon and thus allows rapid inflation and deflation times. In addition there is an active flush and extraction system. In a randomised controlled trial, the TriActiv system was found to be non inferior to the GuardWire and Filter EZ systems.22
Distal filtration devices
The major advantage with the filtration devices is flow preservation. One potential disadvantage is that the filter has pores of between 80 to 150 μm and, therefore, may allow debris smaller than 80μm to pass through. However, studies comparing to occlusive devices have failed to show a difference in MACE even though electron microscopy of material aspirated with the GuardWire reveal 50% of particles to be <100μm.23
Proximal occlusion devices
The Proxis (Velocimed, Maple Grove, Minnesota) is the only one of its kind licensed for use at the time of writing. Where the landing zone is inadequate, this type of device is the only one suitable for use. The Proxis is compatible with any guidewire. A sealing balloon is deployed proximal to the lesion and this occludes antegrade flow. Intervention is then performed in an environment of stasis created before the passage of any device whereas with the other products described above, prior stenosis dilation may be necessary before crossing with the device. The obvious advantage of the proximal occlusion device is that any and most importantly, all debris dislodged in the dilation process can be aspirated. The device is 7 F compatible with a maximum balloon size of 5 mm. Predilation balloons and stents are delivered through the Proxis system, deployed and then debris aspirated before balloon deflation and restoration of antegrade flow.
Preliminary data from a non-randomized trial of 40 patients, demonstrated successful deployment in 95%.24 The reported MACE rate of 5% was much lower than that achieved with distal devices but data comparing the two are awaited.
Limitations of embolic protection devices
Clinical studies
The first such device evaluated in saphenous vein grafts with low thrombus burden was the GuardWire. A registry of 105 patients confirmed safety and reduction of MACE when compared to historical controls and led to the larger, randomized controlled, SAFER study.25 This study excluded patients with acute myocardial infarction or severe left ventricular dysfunction but did include patients with a higher thrombus burden than that in the SAFE registry.
The first registry evaluating FilterWire safety and efficacy excluded high risk patients with acute myocardial infarction or severe left ventricular dysfunction.26 Confirmation of safety and efficacy led to direct comparison with the GuardWire (FIRE study) in patients with low thrombus burden and good pre procedural flow. Primary end-point analysis confirmed similar rates of successful deployment, angiographic and biomarker release and 30-day MACE.23 Subgroup analysis revealed superiority of the FilterWire in smaller vessels and eccentric lesions.
The proximal occlusion device (Proxis) has been shown to be safe and effective. The first clinical trial with this device, involving 600 patients (PROXIMAL trial) confirmed the Proxis device to be at least as good as the distal devices. Although the trial design was complex, direct comparison with the distal devices revealed a non-significant reduction in MACE (6.2% vs 11.2%, P = 0.89).27
THROMBECTOMY DEVICES
The benefit of thrombectomy on clinical end points is unproven. In the X-Sizer AMI registry there was no difference in TIMI flow grade 3.28 There may, however, be situations where the thrombus burden is so large as to preclude the use of embolic protection devices. In these rare instances the X-Sizer system (EndiCOR Medical, San Clemente, California) or the AngioJet device (Possis, Minneapolis, Minnesota) are available for use, although trial evidence of benefit is lacking.
GP IIB/IIIA RECEPTOR INHIBITORS IN VEIN GRAFT INTERVENTION
Even with appropriate use of embolic protection devices, acute complication rates after degenerative vein graft PCI may exceed 10%. This is most often secondary to ‘no-reflow’ resulting in Q or non Q-wave myocardial infarction. Adjunctive glycoprotein IIb/IIIa inhibitors have proven efficacy in patients with ACS undergoing PCI to native coronary arteries. Data supporting use in vein graft PCI, however, is lacking. Pooling results from several randomized trials29–34 suggested worse 30-day outcomes. More recently, a differential effect of GP IIb/IIIa agents has been reported.35 The authors reported on a pre specified subgroup analysis of the FIRE trial, where 651 patients undergoing vein graft PCI and stent insertion were randomized to the filter wire or distal occlusion embolic protection devices. Patients randomised to the distal occlusion device revealed a marked increase in MACE when assigned to GP IIb/IIIa inhibitors (16% vs. 6.3%; P=0.007). No difference was seen in patients in whom the filter device was used. Although the use of GP IIb/IIIa inhibitors was not randomly assigned, the results are nevertheless surprising given the central role of platelet aggregation in distal platelet embolisation. These findings underscore the recognition that pathophysiology of distal embolisation in vein graft PCI is multifactoral with involvement among others, of plaque embolisation and vasospasm. The difference between the two devices is most likely explained by the fact that the filter wire allows antegrade flow of small particulate matter and the reduced platelet aggregation and deposition with GP IIb/IIIa use translates to reduced MACE. During balloon occlusion use of these agents is unlikely to have a beneficial effect as embolic retrieval is facilitated by occlusion and aspiration of all particulate matter.
PTFE COVERED STENT
However, three major randomised trials revealed no additional benefit in respect to restenosis and cumulative MACE when compared to BMS.36–38 Use in vein graft interventions, therefore, cannot be recommended except in emergency situations to seal perforations.
1 Khot UN, Friedman DT, Pettersson G, et al. Radial artery bypass grafts having an increased occurrence of angiograghically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation. 2004;109:2086-2091.
2 Motwani JG, Topol EJ. Aortocoronary Saphenous Vein Graft Disease. Pathogenesis, Predisposition, and Prevention. Circulation. 1998;97:916-931.
3 Kalan JM, Roberts WC. Morphologic findings in saphenous veins used as coronary arterial bypass conduits for longer than 1 year: necropsy analysis of 53 patients, 123 saphenous veins, and 1865 five-millimeter segments of veins. Am Heart J. 1990;119:1164-1184.
4 Nishioka T, Luo H, Berglund H, et al. Absence of Focal Compensatory Enlargement or Constriction in diseased human coronary saphenous vein bypass grafts: an intravascular ultrasound study. Circulation. 1996;93:683-690.
5 Peykar S, Angillillo J, Bass TA, et al. Saphenous vein graft disease. Minerva Cardioangiol. 2004;52:379-390.
6 Weintraub WS, Jones EL, Craver JM, et al. Frequency of repeat coronary bypass or coronary angioplasty after coronary artery bypass surgery using saphenous venous grafts. Am J Cardiol. 1994;73:103-112.
7 Stephan WJ, O’Keefe JH, Piehler JM, et al. Coronary angioplasty versus repeat coronary artery bypass grafting for patients with previous bypass surgery. J Am Coll Cardiol. 1996;28:1140-1146.
8 Yau TM, Borger MA, Weisel RD, et al. The changing pattern of reoperative coronary surgery. J Thorac Cardiovasc Surg. 2000;120:156-163.
9 Schmuziger M, Christenson JT, Maurice J, et al. Reoperative myocardial revascularization: an analysis of 458 reoperations and 2645 single operations. Cardiovasc Surg. 1994;2:623-629.
10 Plokker HW, Meester BH, Serruys PW. The Dutch experience in percutaneous transluminal angioplasty of narrowed saphenous veins used for aortocoronary arterial bypass. Am J Cardiol. 1991;67:361-366.
11 de Feyter PJ, van Suylen RJ, de Jaegere PP, et al. Balloon angioplasty for the treatment of lesions in saphenous vein bypass grafts. J Am Coll Cardiol. 1993;21:1539-1549.
12 Fenton SH, Fischman DL, Savage MP, et al. Long-term angiographic and clinical outcome after implantation of balloon-expandable stents in aortocoronary saphenous vein grafts. Am J Cardiol. 1994;74:1187-1191.
13 Savage MP, Douglas JS, Fischman DL, et al. Stent placement compared with balloon angioplasty for obstructed coronary bypass grafts. Saphenous Vein de novo Trial Investigators. N Engl J Med. 1997;337:740-747.
14 Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents is patients with stenosis in a native coronary artery. N Eng J Med. 2003;349:1315-1323.
15 Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788-794.
16 Lei GE, Iakovou I, Sangiorgi GM, et al. Treatment of saphenous vein graft lesions with drug-eluting stents. Immediate and midterm outcome. J Am Coll Cardiol. 2005;45:989-994.
17 Lee MS, Shah AP, Aragon J, et al. Drug-eluting stenting is superior to bare metal stenting in saphenous vein grafts. Catheter Cardiovasc Interv. 2005 Dec;66:507-511.
18 Chu WW, Rha SW, Kuchulakanti PK, et al. Efficacy of sirolimus-eluting stents compared with bare metal stents for saphenous vein graft intervention. Am J Cardiol. 2006;97:34-37.
19 Ellis SG, Brener SJ, DeLuca S, et al. Late myocardial ischemic events after saphenous vein graft intervention-importance of initially “nonsignificant” vein graft lesions. Am J Cardiol. 1997;79:1460-1464.
20 Choussat R, Black AJR, Bossi I, et al. Long-term clinical outcome after endoluminal reconstruction of diffusely degenerated saphenous vein grafts with less-shortening wallstents. J Am Coll Cardiol. 2000;36:387-394.
21 Theron J, Courtheoux P, Alachkar F, et al. New triple coaxial catheter system for carotid angioplasty with cerebral protection. AJNR Am J Neuroradiol. 1990;11:869-874.
22 Carrozza JP, Mumma M, Breall JA, et al. Randomized evaluation of the TriActive balloon-protection flush and extraction system for the treatment of saphenous vein graft disease. J Am Coll Cardiol. 2005;46:1677-1683.
23 Stone GW, Rogers C, Hermiller J, et al. Randomized Comparison of Distal Protection With a Filter-Based Catheter and a Balloon Occlusion and Aspiration System During Percutaneous Intervention of Diseased Saphenous Vein Aorto-Coronary Bypass Grafts. Circulation. 2003;108:548-553.
24 Sievert H, Wahr D, Schuler G, et al. Effectiveness and safety of the Proxis system in demonstrating retrograde coronary blood flow during proximal occlusion and in capturing embolic material. Am J Cardiol. 2004;94:1134-1139.
25 Baim DS, Wahr D, George B, et al. Randomized Trial of a Distal Embolic Protection Device During Percutaneous Intervention of Saphenous Vein Aorto-Coronary Bypass Grafts. Circulation. 2002;105:1285-1290.
26 Stone GW, Rogers C, Ramee S, et al. Distal filter protection during saphenous vein graft stenting: technical and clinical correlates of efficacy. J Am Coll Cardiol. 2002;40:1882-1888.
27 White J. TCT 2005 Late-Breaking Trials Promise to Influence Practice Patterns. Journal of Interventional Cardiology. 2006;19(1):113-116.
28 Napodano M, Pasquetto G, Sacca S, et al. Intracoronary thrombectomy improves myocardial reperfusion in patients undergoing direct angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2003;15(8):1395-1402. 42
29 Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigators. N Engl J Med. 1994;330:956-961.
30 Platelet glycopoten IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. N Engl J Med. 1997;336:1689-1696.
31 Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. The EPISTENT Investigators. Evaluation of platelet IIb/IIIa inhibitors for stenting. Lancet. 1998;352:87-92.
32 Randomised placebo-controlled trial of effect of eptifbatide on complications of percutaneous coronary interventions: IMPACT-II. Integrilin to minimize platelet aggregation and coronary thrombosis-II. Lancet. 1997;349:1422-1428.
33 Inhibition of platelet glycoprotein IIb/IIIa with eptifbatide in patients with acute coronary syngrome. The PURSUIT Trial Investigators. Platelet glycoprotein IIb/IIIa in unstable angina: Receptor suppression using Integrilin therapy. N Engl J Med. 1998;339:436-443.
34 Jonas M, Stone GW, Mehran R, et al. Platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment during saphenous vein graft stenting: differential effects after randomization to occlusion or filter-based embolic protection. Eur Heart J. 2006;27(8):920-928.
35 Laarman GJ, Kiemeneij F, Mueller R, et al. Feasibility, safety, and preliminary efficacy of a novel ePTFE-covered self-expanding stent in saphenous vein graft lesions: the Symbiot II trial. Catheter Cardiovasc Interv. 2005;64(3):361-368.
36 Mahmud E, Pezeshki B, Salami A, et al. Highlights of the 2004 Transcatheter Cardiovascular Therapeutics (TCT) annual meeting: Clinical implications. J Am Coll Cardiol. 2005;45:796-801.
37 Stankovic G, Colombo A, Presbitero P, et al. The Randomized Evaluation of polytetrafluoroethylene COVERed stent in Saphenous vein grafts (RECOVERS) Trial. Circulation. 2003;108:37.
38 Schachinger V, Hamm CW, Munzel T, et al. A randomized trial of Polytetrafluoroethylene-Membrane-Covered stents compared with conventional stents in aortocoronary saphenous vein grafts. J Am Coll Cardiol. 2003;42:1360-1369.