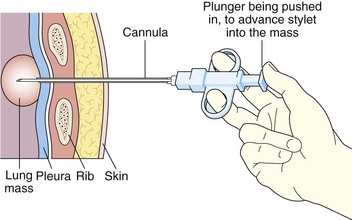
Chapter 13 Percutaneous Biopsy Procedures
Techniques and Indications
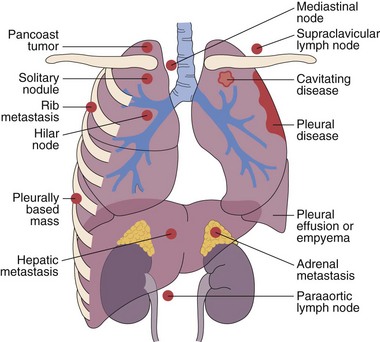
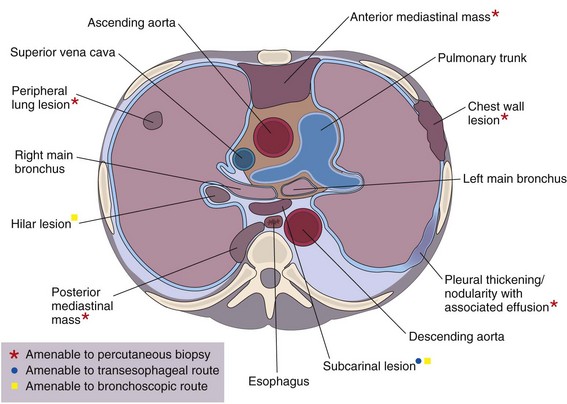
This chapter describes the techniques and equipment available to obtain diagnostic samples from intrathoracic and selected extrathoracic lesions by the percutaneous route. The common lesions sampled and their locations are listed in Figure 13-1. The most frequent methods of sampling intrathoracic lesions, depending on their site, are summarized in Figure 13-2. The techniques and indications are different from those required to diagnose more diffuse intrapulmonary disease. The latter, which includes conditions such as idiopathic pulmonary fibrosis, requires a transbronchial lung biopsy or an open lung biopsy through a mini-thoracotomy or video-assisted thoracoscopy (VATS) procedure.
Methods of Tissue Sampling
Imaging Techniques
Integrated Positron Emission Tomography–Computed Tomography
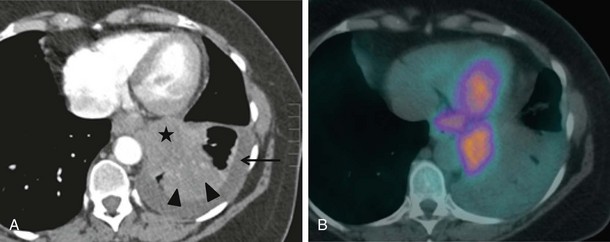
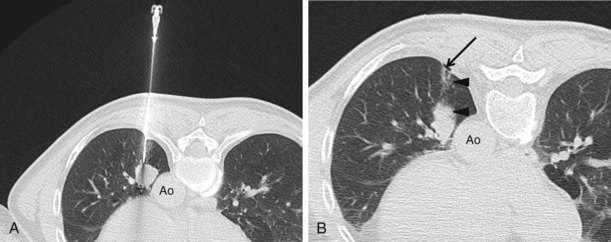
CT combined with PET allows accurate anatomic localization of FDG-avid foci. This technique is particularly useful in isolating active foci surrounded by benign changes, such as within thickened pleura, or in separating tumor from collapse, allowing greater precision in positioning the biopsy needle (Figure 13-3).
Needles
The two main modes of sampling are fine needle aspiration (FNA) for cytologic study and core biopsy for histopathologic examination. Both methods retrieve samples that are suitable for culture. Although the sensitivity of FNA is improved by having a cytologist present to ensure that an adequate sample is obtained, the diagnostic yield in benign disease remains low (20% to 50%) compared with that for core biopsy (70%) (Greif et al., 1999). In malignant disease, the techniques are analogous, with a sensitivity of 90% to 95% (Klein et al., 1996). The clinical requirement for cores of tissue for immunohistochemical analysis has led to a reduction in the use of FNA, and when feasible, cores are always obtained.
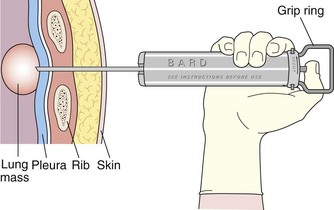
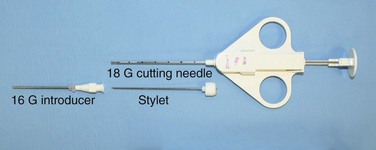
Core biopsy specimens are obtained using cutting needles, which are larger in diameter (14 to 18 gauge) and are available in different lengths (6-, 9-, and 15-cm lengths are commonly used). These needles are more sturdy, allowing greater control in placement. Cutting needles are typically mounted on a spring-loaded mechanism. Older designs such as the Bard Biopty biopsy system, when triggered, simultaneously fire an inner notched stylet and an outer cutting cannula. The handle of the Bard system is bulky but reusable (Figure 13-4). Newer, lighter designs include the Cook Quickcore and Bauer Temno devices, which allow the inner notched stylet to be advanced and secured within the lesion before the cutting cannula is activated. The throw can be increased from 1 to 2 cm to obtain better core samples (Figure 13-5). The Cook device seems to have a slightly sharper needle tip, which in practice can be advanced through tougher tissues.
Historically, it was believed that use of larger cutting needles carried higher complication rates than those associated with fine needles. A 2002 study of practice based in the United Kingdom analyzed data from 5444 lung biopsy and FNA procedures and found no difference between the two methods, a conclusion supported by other studies (Richardson et al., 2002).
With transpulmonary biopsy, the risk of pneumothorax is more closely related to the number of pleural passes. The introduction of a coaxial system has transformed lung biopsies. A single pleural pass is made with a thin-walled introducer needle (usually 16 gauge). A smaller cutting needle (usually 18 gauge with a 1- or 2-cm throw) is inserted through the introducer and multiple cores are taken without repuncturing the pleura. The process is quicker, simpler, and safer than attempting multiple pleural passes, leading to a reduction in the complication rate with an improved diagnostic yield (Figure 13-6).
Percutaneous Biopsy of Intrapulmonary Lesions
Indications
The role of percutaneous lung biopsy in diagnosing malignant disease is well established, with a sensitivity of 90% to 95%. Its main application is in patients with inoperable lung cancer, when sputum cytology and bronchoscopy are nondiagnostic, to provide a means of establishing cell type before chemotherapy and radiotherapy. In the past, both FNA and core biopsy have been comparable in diagnosing and distinguishing small cell lung cancer (SCLC) from non-SCLC (NSCLC), providing oncologists with sufficient information to make choices regarding appropriate chemotherapy regimens. New targeted chemotherapy and biologic agents (e.g., the epidermal growth factor receptor [EGFR] inhibitor drugs) have proven prognostic benefit with particular histologic subtypes. This has meant that core sampling is essential to allow routine performance of detailed tissue analysis. FNA can be adequate to differentiate between subtypes of NCSLC, but it remains less accurate overall than core biopsy. The latter allows sufficient material to be obtained for both accurate histologic subtyping and molecular testing (e.g., to determine EGFR mutation status) (Barnes et al., 2010).
Biopsy also is widely used to exclude lung metastases in patients with potentially operable lung tumors, and in those with extrathoracic malignancies (Figure 13-7).
Contraindications
The contraindications to percutaneous lung biopsy are largely relative and are summarized in Table 13-1. In general, patients need to be able to cooperate, including lying still in the desired position, resisting excessive coughing, and controlling breathing. In patients with very poor lung function, biopsy of a lesion is still possible, provided that the lesion is peripheral and a carefully considered route is identified (usually with CT) that does not traverse lung parenchyma. In performing the biopsy, care is taken to avoid creating a pneumothorax, which could be life-threatening. Before the procedure, a coagulation screen is performed according to local protocol, and bleeding diatheses are corrected, when appropriate, to keep within safe guidelines. Biopsy of parenchymal hydatid lesions has been documented, but an increased and probably unacceptable risk of anaphylactic reaction has been documented in patients with such lesions.
| Type of Contraindication | Comment |
|---|---|
| Relative | Inability of patient to cooperate: uncontrollable cough, inability to lie prone or supine |
| Poor lung function/chronic obstructive pulmonary disease (FEV1 <40% of predicted normal or multiple bullae) | |
| Pneumonectomy | |
| Bleeding disorder | |
| Pulmonary hypertension | |
| Pulmonary fibrosis | |
| Small nodules (<5 mm in diameter) | |
| Hydatid disease (associated with risk of anaphylactic reaction) | |
| Absolute | Arteriovenous malformation with high pulmonary artery pressure |
FEV1, forced expiratory volume in 1 second.
Procedure
Practical Aspects
Radiopaque surface skin markers are placed 0.5 to 1.0 cm apart over the region of interest, and a limited CT scan covering the area is performed with the patient in gentle respiration (Figure 13-8). Once the target lesion is identified, the safest percutaneous route to the lesion is mapped along the axial and sagittal axes, using the CT slice number and surface markers, respectively, as a guide. A cross is drawn on the patient’s skin to mark the corresponding entry site and a sterile field is created. Lidocaine 1% (10 to 15 mL) or 2% (5 mL) is infiltrated down to the pleura, with care taken not to puncture the visceral pleura or lung. The patient is warned that it is not always possible to anesthetize the pleura fully, because of the risk of pneumothorax from the anesthetic needle.
Complications
A risk-benefit analysis should always be made before any procedure, particularly in high-risk groups (Table 13-2).
| Category | Complication/Cause |
|---|---|
| Early complications | Pneumothorax: 5-50% |
| Hemoptysis: 5-10% | |
| Hemorrhage: 10-40% | |
| Air embolism: rare | |
| Late complications | Tumor seeding: extremely rare |
| Empyema | |
| Bronchopleural fistula | |
| Increased risk of pneumothorax | Common associations:
Less common associations: |
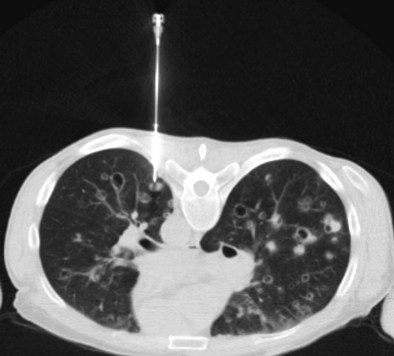
The most common complication after lung biopsy is pneumothorax (Figure 13-9). Most pneumothoraces are small (less than 2 cm on chest film) and are managed conservatively. Those requiring intervention usually are detected early: 88% immediately after biopsy and the remainder after 1 hour. A delayed presentation is rare. Intervention can involve aspiration by way of a three-way tap or drainage through a one-way (Heimlich) valve; drainage is performed at a frequency of 0 to 17%. Positioning the patient with the puncture site dependent can reduce the pneumothorax rate and should be considered in patients at high risk. Surprisingly, use of a cutting needle does not increase the incidence of pneumothorax over that with fine needle aspiration, the pneumothorax rate being in the range of 3% to 42%. Tension pneumothorax is rare but may be seen in patients with emphysema. It develops within minutes and constitutes a medical emergency.
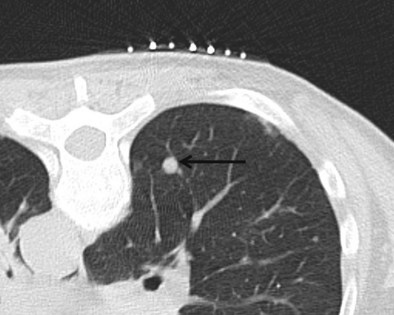
Minor perilesional hemorrhage is common (see Figure 13-9); however, actual hemoptysis occurs in only 4% to 5% of biopsy procedures (Richardson et al., 2002) and is more common in patients with pulmonary hypertension. It often follows a bout of coughing and can be extremely frightening for the patient. Supportive measures are initiated with the patient positioned biopsy side down. Treatment is rarely required, because spontaneous recovery is rapid. Massive hemoptysis rarely occurs and can be fatal.
Although rare, a systemic arterial air embolism is a serious and increasingly recognized complication of interventional lung procedures, and its effects can be clinically subtle (Hiraki et al., 2007). It occurs when air enters into the pulmonary venous circulation, is pumped through the left side of the heart, and subsequently enters the cerebral and coronary arteries, which become occluded. Air may enter the pulmonary veins by two main processes. It can be drawn in from the atmosphere, through the introducer needle on deep inspiration, when the hub is temporarily exposed in between biopsies. Entry of air through this route is avoided by promptly covering the hub with the thumb each time the cutting needle or stylet is withdrawn, and by asking the patient to hold the breath during the process. Alternatively, air can enter the pulmonary veins through the airways along the needle tract during a bout of coughing. To prevent this from occurring, needles should be removed during uncontrollable coughing. Rapid diagnosis and treatment of a pulmonary venous air embolism are essential. The patient should be placed in the right lateral decubitus position, and 100% oxygen should be administered while awaiting transfer to a hyperbaric oxygen chamber if available. A CT scan of the head and chest should be performed to confirm the diagnosis.
Reported mortality rates for transpulmonary biopsy range from 0.07% to 0.15% (Richardson et al., 2002; Tomiyama et al., 2006). Death may result from cardiac arrest, systemic arterial air embolism, tension pneumothorax, or hemorrhage.
Ultrasound-Guided Biopsy: General Principles
Practical Aspects
Depending on the size of the lesion, a cutting needle with a 1- or 2-cm throw is selected. Adjacent lymph nodes can be lined up and sampled simultaneously, enabling the 2-cm throw to be used more often, which is preferable, because it provides a better specimen (Figure 13-10). After a small skin incision is made, the biopsy needle is introduced and carefully observed as it is advanced toward and into the lesion. With the tip of the needle secured well within the lesion (which often requires a firm nudge to pierce its capsule or wall), the whole apparatus can be flattened out, so that the inner stylet can be advanced through but not out of the lesion. Once the stylet is optimally positioned, the biopsy specimen is taken by pushing the trigger, after the patient is warned to expect a clicking sound. The needle is then swiftly withdrawn, and pressure is applied to the skin to control any bleeding; the process is repeated as appropriate after the first sample is retrieved.
Percutaneous Sampling of Extrapulmonary Intrathoracic Tissues
Mediastinal Nodes and Masses
Method
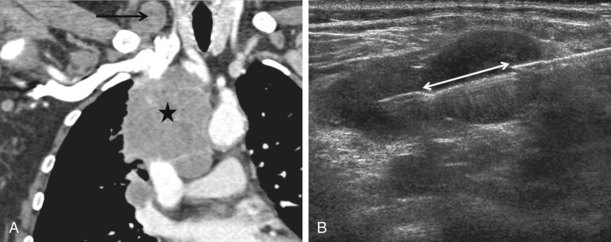
With large mediastinal masses abutting the chest wall, a biopsy route that avoids the lung parenchyma is readily available (Figure 13-11). With smaller lesions, a safer route may be achieved with use of a transpleural approach through an existing effusion or pneumothorax. Saline can be injected into the pleural space where there is no effusion, or directly into the mediastinum, providing an extrapleural route. Placing the patient in the lateral decubitus position to shift the mediastinum also can help to create an extrapulmonary route. These alternative approaches are associated with reduced incidence of complications.
Pleural and Chest Wall Disease
Method for Targeted Pleural and Chest Wall Biopsies
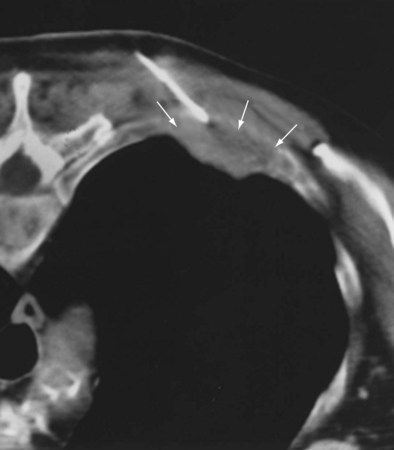
Targeted pleural and chest wall biopsies are indicated to sample a discrete mass and are usually performed under ultrasound guidance (Figure 13-12). If visualization is difficult with use of ultrasound imaging, or if the lesion is small, with insufficient pleural fluid, biopsy is performed under CT guidance. The optimum route is one that runs along the main axis of the pathologic process (i.e., an oblique tract), allowing more of the lesion to be sampled with less risk of pneumothorax (Figure 13-13).
Percutaneous Sampling of Extrathoracic Lesions
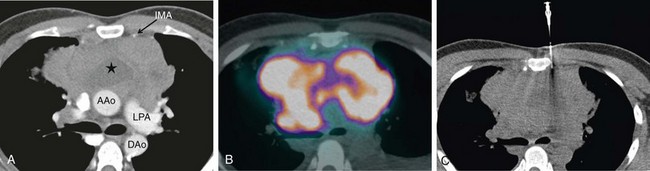
During staging of thoracic malignancies, lesions may be identified in the liver, adrenal glands, lymph nodes or bones. If appearances suggest metastases (e.g., increased FDG avidity on PET imaging), it is essential to perform a biopsy to confirm inoperability (Figure 13-14). If multiple sites are involved, several factors are considered in deciding which tissue to sample—for example, the extent of the disease, the likelihood of obtaining a diagnostic sample safely, and how accessible the site is with a minimally invasive approach.
Liver
Method
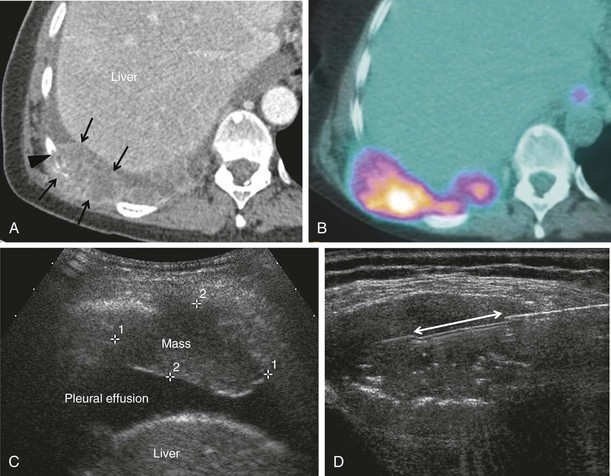
Hepatic lesions usually are biopsied under ultrasound guidance, where they are easily imaged and rapidly sampled (Figure 13-15). Owing to the risk of bleeding and the potential need for intervention, preoperative preparation of the patient includes fasting for 6 hours before the procedure.
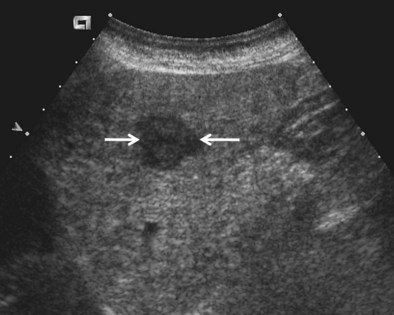
Figure 13-15 Ultrasound image showing a hypoechoic liver metastasis (arrows), which can be targeted for biopsy.
With strict adherence to aseptic technique, local anesthetic is infiltrated down to the liver capsule, which has a rich neurovascular supply. The tip of the anesthetic needle is kept in full view to avoid breaching the capsule, because this can increase the risk of bleeding. A small skin incision is then made, and a larger cutting needle is introduced (usually 18 gauge); radiologists at our institution favor the traditional Bard Biopty biopsy system, which obtains a 1-cm core. The biopsy sample is taken with the patient in arrested respiration to limit movement. If insufficient tissue is obtained with a single core, the biopsy can be repeated. However, careful risk-benefit analysis should be made, owing to the increased risk of hemorrhage associated with multiple passes (Grant et al., 1999). In practice, no more than two cores are usually taken. Although multiple cores are avoided, the coaxial system can still be useful, as hemostatic agents (such as Gelfoam sponge) can be inserted down the introducer needle as it is withdrawn, helping to control bleeding along the biopsy tract.
Aftercare
A study of more than 9000 liver biopsies showed that the risk of hemorrhage not only is related to the number of passes made but also is closely associated with the presence of malignancy and the age of the patient (McGill et al., 1990). Given these risk factors, in the context of malignant liver disease, patients undergoing targeted biopsy usually are admitted overnight for careful observation.
A retrospective study of 68,276 percutaneous liver biopsy procedures (performed over a decade) demonstrated that the majority of complications occur early on (61% in the first 2 hours), with most detected within a day (96% within 24 hours). Death was uncommon (with 9 deaths reported per 100,000 procedures) but occurred in patients with either underlying malignant disease or cirrhosis and was due to intraperitoneal hemorrhage, with signs of bleeding seen within 6 hours (Piccininio et al., 1986). After 24 hours, if clinical status remains stable and pain has subsided, the patient can be discharged home with advice to return if any delayed symptoms are experienced.
Adrenal Glands
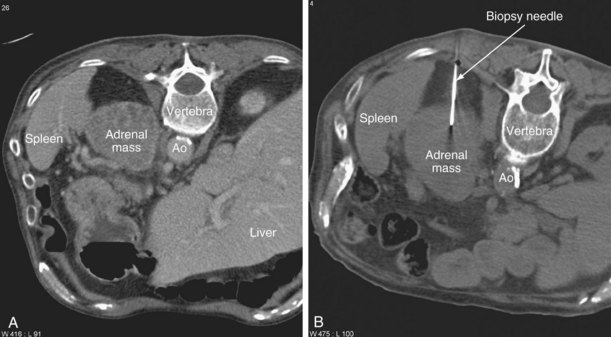
Biopsy of adrenal lesions is usually performed under CT guidance (Figure 13-16). It may be necessary to traverse the pulmonary or hepatic parenchyma to enter the adrenal mass. If the mass is on the side opposite the primary lung carcinoma, the pulmonary parenchymal route should be avoided, because a pneumothorax could delay surgery or result in significant respiratory compromise. A coaxial system is invaluable, allowing several aspirates or cores to be obtained with a single pass of the introducer needle.
Lymph Nodes
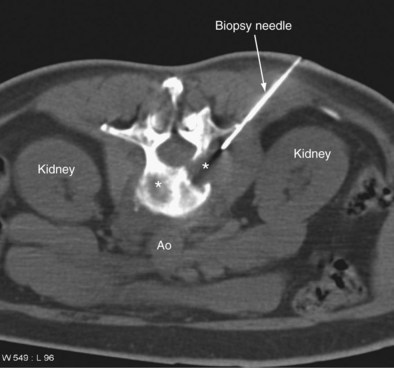
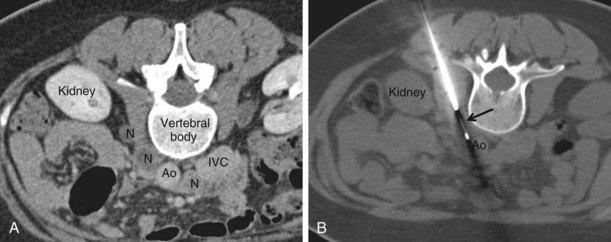
Superficial nodes, including supraclavicular and axillary, are amenable to ultrasound-guided biopsy, which is a simple and safe procedure. Biopsy of retroperitoneal masses (including lymph nodes) requires CT guidance (Figure 13-17). Core biopsy is preferable to FNA. A 16 or 18 gauge cutting needle is used, depending on the site.
Pleural Drainage
Technique
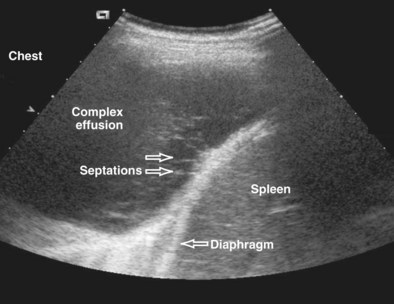
Ultrasound imaging is the easiest and quickest way to confirm the presence and volume of pleural fluid and also demonstrates the presence of echogenic material, septa, and multiple locules, which may require multiple drainage tubes (Figure 13-18).
Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology. 2001;219:510–514.
Arakawa H, Nakajima Y, Kurihara Y, et al. CT-guided transthoracic needle biopsy: a comparison between automated biopsy gun and fine needle aspiration. Clin Radiol. 1996;51:503–506.
Barnes D, Souza C, Entwisle J. The role of percutaneous biopsy in the evolving diagnosis and treatment of lung cancer. Clin Radiol. 2010;65:951–952.
Fultz PJ, Harrow AR, Elvey SP, et al. Sonographically guided biopsy of supraclavicular lymph nodes: a simple alternative to lung biopsy and other more invasive procedures. AJR Am J Roentgenol. 2003;180:1403–1409.
Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):108S–130S.
Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. Gut. 1999;45:IV1–IV11.
Greif J, Marmor S, Schwarz Y, et al. Percutaneous core needle biopsy versus fine needle aspiration in diagnosing benign lung lesions. Acta Cytol. 1999;43:756–760.
Gupta S, Seaberg K, Wallace MJ, et al. Imaging-guided percutaneous biopsy of mediastinal lesions: different approaches and anatomic considerations. Radiographics. 2005;25:763–786.
Hare SS, Gupta A, Goncalves AT, et al. Systemic arterial air embolism after percutaneous lung biopsy. Clin Radiol. 2011;66:589–596.
Henry M, Arnold T, Harvey J. BTS guidelines for management of spontaneous pneumothorax. Thorax. 2003;58:ii39.
Hiraki T, Fujiwara H, Sakurai J, et al. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest. 2007;132:684–690.
Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology. 1996;198:715–720.
Lacasse Y, Wong E, Guyatt G, Cook D. Transthoracic needle aspiration biopsy for the diagnosis of localised pulmonary lesions: a meta-analysis. Thorax. 1999;54:884–893.
Lucidarme O, Howarth N, Finet J-F, Grenier PA. Intrapulmonary lesions: percutaneous automated biopsy with a detachable, 18-gauge, co-axial cutting needle. Radiology. 1998;207:759–765.
McGill DB, Rakela J, Zinsmeister AR, et al. A 21-year experience with major haemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396–1400.
Piccininio F, Sagnelli E, Pasquale G, et al. Complications following percutaneous liver biopsy. J Hepatol. 1986;2:165–173.
Richardson CM, Pointon KS, Manhire AR, Macfarlane JT. Percutaneous lung biopsies: a survey of UK practice based on 5444 biopsies. Br J Radiol. 2002;75:731–735.
Tokuda Y, Matsushima D, Stein GH, Miyagi S. Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: a meta-analysis. Chest. 2006;129:783–790.
Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complications based on 9783 biopsies in Japan. Eur J Radiol. 2006;59:60–64.
Westcott JL, Rao N, Colley DP. Transthoracic needle biopsy of small pulmonary nodules. Radiology. 1997;202:97–103.