Chapter 55 Penile Cancer
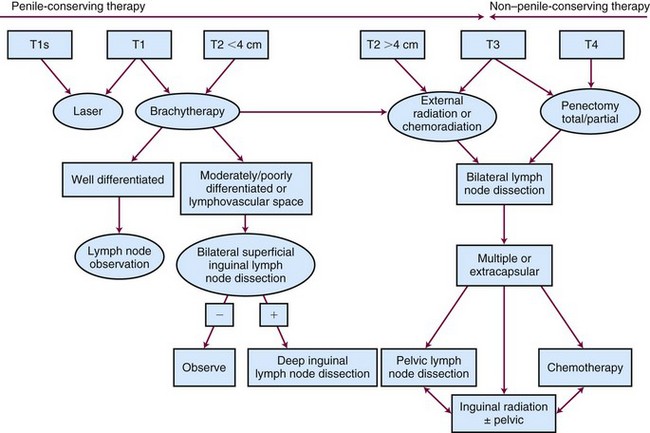
Early-stage, well-differentiated cancers of the penis can be effectively managed with local therapy, and attention should be paid to preservation of penile function and morphology. Primary surgical management is effective but can be associated with considerable psychosexual morbidity. Even partial penectomy can have a profound effect on sexual health and self-image.1 Suicide or attempted suicide after partial penectomy has been reported.2,3 EBRT or interstitial RT is an organ-sparing alternative that preserves penile morphology and function without compromising disease control or survival in selected patients. Unfortunately, many urologists do not routinely offer this penis-conserving option.4 Quality of life and sexual health after surgery is infrequently studied or reported.5 Sexuality and expectations should be discussed with the patient and partner when deciding on primary management. Advanced or poorly differentiated tumors require a multimodality approach.
Etiology And Epidemiology
Carcinoma of the penis is rare, with an estimated incidence of 1 case per 100,000 men in North America and Europe, where it accounts for 0.4% to 0.6% of cancers. Higher incidences are seen in parts of Asia, Africa, and South America, where it represents up to 10% of malignancies in male patients.6 The peak incidence is in the sixth decade in developed countries but earlier where the incidence is higher.
Case-control studies have identified important risk factors (odds ratio [OR] >10) to be phimosis, chronic inflammatory conditions such as lichen sclerosis, treatment with psoralens and ultraviolet A photochemotherapy (PUVA), smoking (dose-dependent association), and a history of genital condylomata (threefold to fivefold increase in risk). Neonatal circumcision is associated with a threefold decrease in risk of penile carcinoma. Circumcised men with a history of human papillomavirus (HPV) infection remain at increased risk7; and, in some series, up to 20% of patients have been circumcised neonatally. HPV types 16 and 18 have been identified in about 50% of invasive penile cancers.8,9
Prevention And Early Detection
Infant circumcision is highly effective in prevention of this disease, but it is no longer recommended on these grounds alone. The emphasis is instead on education, the promotion of good hygiene for the normally retractile foreskin, and surgical correction of phimosis. Men should be made aware of the association between certain HPV subtypes, venereal warts,7 and cancer, as well as the premalignant nature of conditions such as lichen sclerosis, which may precede the diagnosis of cancer by many years.
Biologic Characteristics And Molecular Biology
The overall incidence of HPV in penile carcinoma is 40% to 45%, as detected by polymerase chain reaction (PCR) amplification of DNA. HPV-16 and HPV-18 are the most frequent subtypes detected.10,11 The frequency of HPV detection depends on the histopathologic subtype of penile cancer. HPV association is seen in 80% to 100% of basaloid and warty penile cancers but in only approximately 35% of verrucous or squamous cell carcinomas. The difference in prevalence of HPV in these two groups suggests different pathogenesis. HPV presence in a tumor does not appear to confer a worse prognosis.
TP53 positivity is found in 41% to 75% of invasive penile cancers.12,13 In multivariate analysis, TP53 positivity and lymphatic embolization are predictive of lymph node metastases.12,14
Pathology And Pathways Of Spread
Premalignant lesions are associated with invasive cancers in 20% to 30% of cases. Intraepithelial neoplasia such as bowenoid papulosis, Bowen disease, and erythroplasia of Queryat15 are precursor lesions of warty and basaloid penile cancers. Lichen sclerosis (balanitis xerotica obliterans) is associated with non-HPV variants of penile carcinoma.16,17 Condylomata, Buschke-Löwenstein disease, Kaposi sarcoma, and leukoplakia are also associated with penile cancer.
Clinical Manifestations, Patient Evaluation, And Staging
General Approach
Because of the rarity of carcinoma of the penis, reported series often span several decades. Three different staging systems are encountered in a review of the literature of the past two decades18–20 (Table 55-1).
| Stage | Description |
|---|---|
| Jackson Staging System* | |
| 1 | Tumor limited to the glans or prepuce |
| 2 | Tumor extending into the shaft or corpora but without node involvement |
| 3 | Tumor confined to the shaft but with malignant but operable lymph nodes |
| 4 | Invasion beyond the shaft with inoperable lymph nodes or distant metastases |
| TNM (UICC, 1978)† | |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤ 2 cm |
| T2 | Tumor > 2 cm and ≤ 5 cm |
| T3 | Tumor > 5 cm or deep invasion including urethra |
| T4 | Tumor invades adjacent structures |
| N1 | Metastases in unilateral inguinal lymph nodes |
| N2 | Metastases in bilateral inguinal lymph nodes |
| N3 | Fixed inguinal lymph nodes |
| TNM (UICC, 1987-2002)‡ | |
| T1 | Tumor in subepithelial connective tissue |
| T2 | Tumor in corpus spongiosum/cavernosum |
| T3 | Tumor in urethra/prostate |
| T4 | Tumor in other adjacent structures |
| N1 | Tumor in one superficial inguinal lymph node |
| N2 | Tumor in multiple/bilateral superficial inguinal lymph nodes |
| N3 | Tumor in deep inguinal or pelvic lymph nodes |
UICC, International Union Against Cancer (Union Internationale Contre le Cancer).
* From Jackson S: The treatment of carcinoma of the penis, Br J Surg 53:33-35, 1966.
† From Harmer M: Penis (ICD-0187). In TNM Classification of Malignant Tumours, ed 3, Berlin, 1978, Springer-Verlag, pp 126-128.
‡ From Hermanek PS, Sobin LH: Penis (ICD-0187). In Hermanek PS, Sobin LH, editors: TNM Classification of Malignant Tumours, ed 4, Berlin, 1987, Springer-Verlag, pp 130-132.
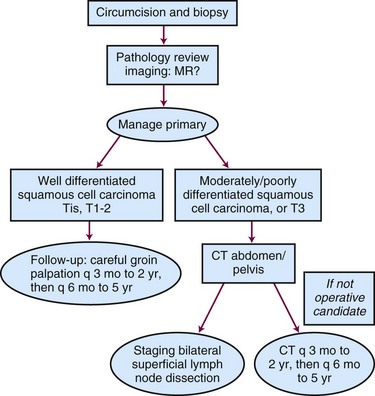
Clinical staging of carcinoma of the penis is very subjective, and it may be difficult to distinguish T1 (i.e., invasion of subepithelial connective tissue) from T2 (i.e., invasion of corpus spongiosum or cavernosum). For this reason, techniques that treat less than the full thickness of the penis must be restricted to very carefully selected cases. MRI may be helpful in cases that are difficult to stage clinically, with a positive predictive value for invasion of the corpora cavernosa of 75% (6 of 8 patients).21 When combined with artificial erection, MRI-guided staging showed good agreement with ultimate pathologic stage.22,23 Any patient who presents with phimosis and chronic discharge, bleeding, balanitis, or swelling in the region of the coronal sulcus or glans under an unretractable foreskin should have a dorsal slit of the foreskin to allow inspection of the glans and should preferably have a full circumcision. Any suspicious lesions should be sampled. Figure 55-1 shows the diagnostic algorithm for a clinically node-negative patient.
Lymph Node Assessment: The N0 Patient
Management of the patient with clinically negative groins remains controversial. Although the gold standard treatment for invasive penile cancer has been amputation and bilateral groin dissection,12 many RT series have advocated a “wait and see” policy, with no systematic staging investigations such as CT or fine-needle aspiration cytology.3,24–27 Because only about 20% of clinically negative nodes have micrometastases, staging lymph node dissection is not warranted for all patients. Furthermore, inguinal node dissection may be complicated in one third of cases by infection, skin flap necrosis, deep vein thrombosis, or severe leg edema.28,29,30
Nodal status is, however, the strongest predictive factor for OS.3,31,32 Lymph node dissection may be curative for men with microscopic regional spread. Several surgical series have identified that therapeutic node dissection confers an inferior survival compared with prophylactic node dissection.33 McDougal34 found a 5-year survival after inguinal node dissection of 92% for clinically N0 patients, compared with 33% for those with clinically involved nodes. Modified inguinal lymphadenectomy, sparing the saphenous vein and limiting the dissection laterally, distally, and proximally, may reduce morbidity.28
Stage and histopathologic factors should be used to identify patients at high risk for microscopic regional spread to selectively offer surgical staging of lymph nodes. Grade is predictive for regional failure, which occurs with 30% of well-differentiated and 81% of moderately to poorly differentiated tumors.29 T stage and lymphovascular invasion are also predictive for nodal relapse.31 McDougal34 reported that patients with clinically negative nodes but with poorly differentiated cancers or invasion of the corpora had a 78% rate of node positivity after inguinal dissection, compared with 4% for moderately or well-differentiated tumors and no invasion of the corpora.
The difficulty with applying this information to decision making in RT is that the primary tumor is not available for complete histopathologic examination. Invasion of the corpora may be underappreciated clinically. Diagnostic biopsies are often superficial and unreliable in determining the depth of invasion, the presence of lymphovascular invasion, or the ultimate tumor grade.35 However, if the biopsy shows the presence of lymphovascular invasion or moderate to poor differentiation, surgical assessment of regional lymph nodes is warranted.36 The specificity and sensitivity of CT are not sufficiently reliable for staging,37 although ultrasound-guided fine-needle aspiration cytology is a valuable tool to evaluate suspicious inguinal nodes.38
Dynamic sentinel lymph node mapping using a gamma probe after intradermal injection of technetium-99m around the primary tumor is becoming more widely available in the detection of early lymph node involvement.37 False-negative rates have fallen to 2% to 11% in the recent literature.39,40,41,42 The use of single proton emission computed tomography may decrease the risk of false-negative results from tumor blockage and rerouting of lymphatics,43 but superficial inguinal lymph node dissection remains the gold standard.
In summary, for patients with clinically negative nodes and a favorable primary tumor (Tis, Ta G1 to G2, T1 G1), observation of the inguinal regions is appropriate. For G2 to G3 or T2 disease, either a staging modified inguinal lymph node dissection or dynamic sentinel lymph node biopsy is recommended.44
Primary Therapy
Surgery
Circumcision is the first step in all cases. Small tumors that are limited to the prepuce can be treated by circumcision alone. Penis-conserving surgical techniques such as laser or Mohs surgery may be suitable for selected tumors. Mohs surgery45 for carcinoma in situ or very superficial tumors involves excision of tissue in successive layers with complete microscopic scanning of each horizontally cut layer to identify any tumor outgrowths that may extend beyond the visible or palpable lesion. Successive layers are removed until margins are histologically clear.
Laser surgery using carbon dioxide or neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers has been reported to provide a superior functional and cosmetic result compared with standard surgical techniques for selected premalignant and malignant lesions.46,47,48,49 Most tumors appropriate for this modality are Tis or T1, the recurrence rate being unacceptable for T2 disease.46 Windahl and Andersson47 reported a 19% local recurrence rate for 67 patients, although 50% of failures could be re-treated with laser. Extended, careful follow-up is required because only 57% of local recurrences occur within the first 2 years, 30% between 6 and 10 years, and 15% after 10 years. Whether these late failures represent true local recurrences or new primary tumors is unknown.
Selected cases, especially those involving only skin, can be treated by wide excision, but local wedge excision is associated with a high recurrence rate. Glansectomy and reconstruction with a split-thickness skin graft preserves a greater length of penile shaft than traditional partial penectomy.50,51 Total or partial penectomy is indicated for larger or more invasive tumors. Traditionally, an adequate resection margin is considered to be 2 cm, but closer margins of 10 mm or less may be acceptable if tumor-free on frozen section.52 Partial penectomy is possible when the tumor involves the glans or the distal shaft, and the penile remnant will allow the patient to direct the urinary stream. Total amputation is indicated for larger or proximal tumors and requires a perineal urethrostomy.
Radiation Therapy
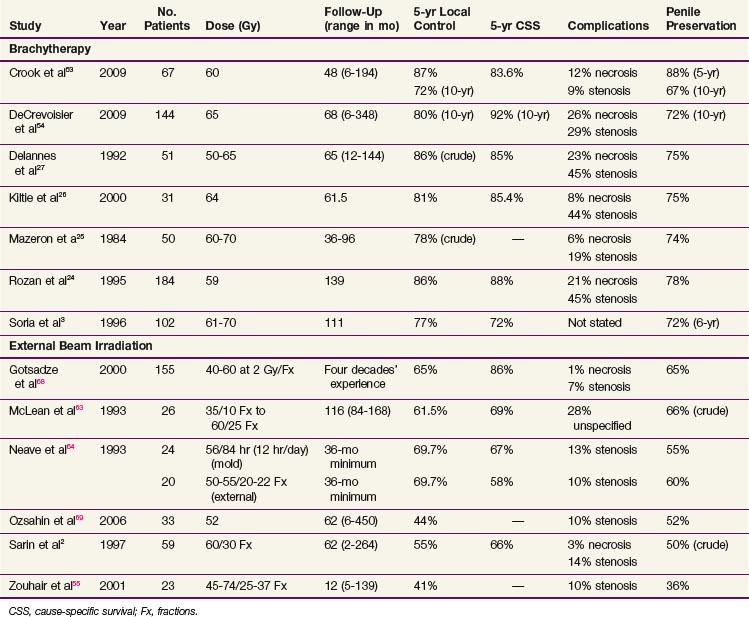
Radical RT in the form of brachytherapy or EBRT is effective in achieving local control in a high percentage of patients (Table 55-2). Often, series span several decades, during which time treatment techniques and dose prescriptions evolve. Staging systems have also undergone significant modifications (see Table 55-1).
Interstitial brachytherapy yields a 5-year local tumor control rate of 77% to 87%, with penile preservation rates at 5 years ranging from 72% to 88%. For EBRT, local control rates are less favorable, 41% to 70% at 5 years. The increased need for surgical salvage decreases penile preservation rates to 36% to 66%. Careful extended follow-up is recommended because local failures can occur several years after treatment. Crook and colleagues53 found that although five of eight local failures in a series of 67 men occurred in the first 2 years, the remaining three occurred at 4.5, 7, and 8 years. Similarly, Mazeron and associates25 reported that 18% of local failures occurred between years 5 and 8, and de Crevoisier and colleagues54 reported that 20% of the local failures occurred after 8 years. This late recurrence pattern is strikingly similar to that seen after penile-conserving laser treatment47 and necessitates careful long-term follow-up because the majority can be successfully salvaged.
Surgery for Salvage
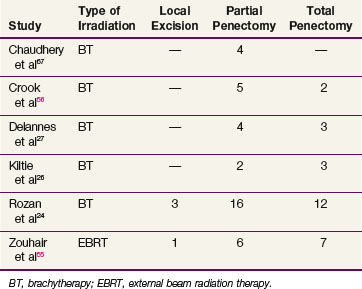
Surgery for salvage is successful in more than 80% of failures.24,55 Local excision is rarely appropriate, with procedures equally divided between total and partial penectomy24,26,27,55 (Table 55-3). The choice depends on penile length and the proportion of the shaft irradiated by the primary treatment. The localized nature of brachytherapy should result in less radical salvage operations.
Patient Selection for Radiation Therapy
Several prognostic factors have been identified for penile preservation. For EBRT, a total dose of less than 60 Gy, a protracted treatment time of more than 45 days, and a daily fraction size of less than 2 Gy are all associated with an increased risk of local failure.2,55
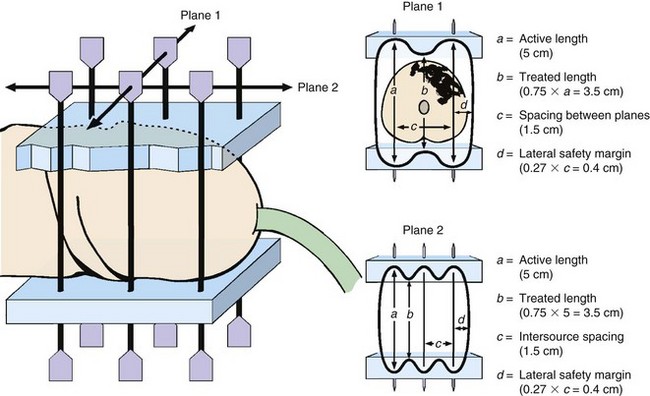
For brachytherapy, the volume of tumor and depth of invasion are predictive of local control. The ideal tumor for brachytherapy should be less than 4 cm in its maximum diameter, with less than 1 cm of invasion.3,25,26,56 Local failure rates of 50% to 60% for tumors larger than 4 cm in diameter have been reported, compared with 14% to 30% for those less than 4 cm. Use of more than six brachytherapy needles and tumor volumes greater than 8 mL are also associated with an increased risk of failure.3,24,26 Crook and colleagues57 found that wider needle spacing was associated with a decreased risk of failure (p = .006). Wider spacing results in a wider lateral margin, ensuring more generous margins around the tumor (Fig. 55-2).
Histopathology does not appear to be a factor in local control. Most penile cancers are well-differentiated squamous cell carcinomas. A more favorable outcome for verrucous cancers has been reported, but most series do not specify this pathology subtype. Moderate to poor differentiation influences OS and the risk of nodal involvement58 but does not preclude a penile-conserving approach.
Locally Advanced Disease And Palliation
Node-Positive Disease
Involved nodes should be resected if possible. As in squamous cancers of other sites that drain to the inguinal regions, patients with pathologic findings of multiple positive nodes or extracapsular spread should be offered postoperative RT.58,59 If dissection of deep pelvic nodes is negative, treatment can be limited to the involved groin. A direct anterior electron field of suitable energy to deliver a dose of 45 to 50 Gy over 5 weeks to the depth of the nodes is appropriate. If the status of the pelvic nodes is unknown, they should be included in the treatment volume.
Unresectable nodes are rarely controlled by RT but can be palliated. A combined approach beginning with systemic chemotherapy should be considered if the patient’s general condition permits an aggressive approach. The choice of agents is not well established. Intra-arterial chemotherapy for locally advanced or recurrent penile cancer using cisplatin, methotrexate, and bleomycin (CMB regimen)60 or CMB plus 5-fluorouracil and mitomycin C has shown promise.60 The Southwest Oncology Group has reported a trial using the CMB regimen in locally advanced or metastatic penile cancer in 40 men, with a response rate of 32.5%.61 An ongoing trial of neoadjuvant paclitaxel, ifosfamide, and cisplatin at the M.D. Anderson Cancer Center has reported a response rate of 55% in the first 20 patients.62 Surgery or RT, or both, can be used after a response to chemotherapy. Concurrent chemoradiation should be evaluated in view of improved local control when compared with either EBRT alone (cervical cancer) or sequential chemoradiation (head and neck cancer) in other disease sites.
Irradiation Techniques AND tolerance
External Beam Irradiation
EBRT has several advantages in that it is widely available, delivers a reliably homogeneous dose, and does not require the specific expertise of brachytherapy. Well-localized carcinoma in situ (Tis) may be treated effectively using 125-kV orthovoltage beams or 9-MeV electrons with suitable bolus, using fractionation schemes commonly employed for skin cancer such as 35 Gy given in 10 fractions over 2 weeks63; however, most such lesions would be treated by a penile-conserving surgical technique. Most lesions referred for RT will require irradiation to the full thickness of the penis with a full dose to the skin surface. Fraction sizes less than 2 Gy are suboptimal,2 and fraction sizes larger than 2 Gy may be associated with increased long-term sequelae.64 Doses range from 60 Gy in 25 fractions over 5 weeks to 74 Gy in 37 fractions over 7.5 weeks, avoiding interruptions due to acute reactions (e.g., edema, pain, desquamation). An easily reproducible setup over the 6-week period that is comfortable for the patient despite increasing local reaction and is easily verified by technologists is essential.
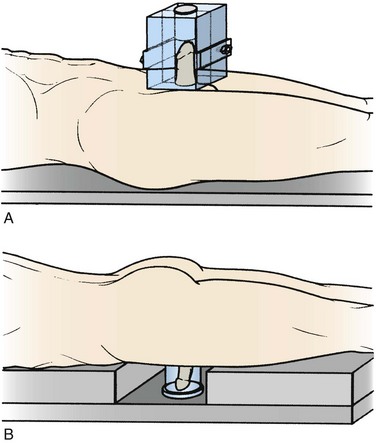
A 10 × 10-cm to 10 × 15-cm wax block can be constructed in two halves with a central cylindrical chamber.55,63 The patient is positioned supine on the treatment couch, and the penis is supported in a vertical position, encased in the wax block. Tissue-equivalent material must be placed in the distal portion of the cylindrical chamber to maintain full dose to the glans. Parallel-opposed beams of cobalt 60 or 4- to 6-MV photons treat the entire length of the penis (Fig. 55-3A).This technique may become increasingly uncomfortable for the patient as treatment progresses. Penile swelling may require modification of the wax block. Verification of penile position within the wax is impossible, but catheterization throughout the 6 weeks of treatment may prevent the penis from “slumping” inside the wax.
Perspex is a good alternative to wax and provides full buildup to the skin surface while allowing treatment by parallel-opposed beams.2 Preconstructed blocks in a range of sizes of the central cylindrical chamber can be sterilized for reuse. Penile swelling can be accommodated easily by choosing the next larger size, and penile position within the transparent block can be easily verified visually.
An alternative approach has been described with the patient positioned prone and the penis suspended in a water bath (see Fig. 55-3B). This approach may not, however, be applicable to all types of body habitus.65
Brachytherapy
Molds
Unlike interstitial brachytherapy, a mold is not invasive and does not require anesthesia.64 The acute reaction develops after the treatment is finished. A surface dose of 55 to 60 Gy is prescribed, with a central axis dose of 46 to 50 Gy over 84 hours (12 hr/day) and may be delivered using a Perspex tube or silicon monomer.64
Interstitial Brachytherapy
There is broad experience with interstitial brachytherapy for T1 or T2 penile carcinoma, with reports from many European countries and from Canada. Implants can be performed with the patient under general or local anesthesia. The Paris system of dosimetry66 is applicable to manually afterloaded implants and remote afterloading pulse-dose-rate systems, although for the latter some optimization can be introduced.
Penile brachytherapy is generally performed as a volume implant delivering full-thickness irradiation, although well-selected, very superficial tumors may be treated with a single-plane implant. A video of penile brachytherapy is available on the Expert Consult version![]() of this chapter. For volume implants, two or three parallel planes of sources are inserted, with two or three needles in each plane. Equal intersource and interplane spacing ranges from 12 to 18 mm. The distribution, spacing, and total number of needles depend on tumor size. Planes are usually oriented with the needles passing from the dorsal to the ventral surface of the glans, but a left-to-right orientation may be acceptable depending on tumor location. Visual verification of coverage can be readily accomplished. Needle placement should be planned such that the prescription isodose will cover 10 mm beyond visible or palpable tumor. Care must be taken to adhere to the rules of the Paris system66 and to be aware of the relationship of the treated volume to the needles such that placement ensures that the entire tumor and desired margin are within the high-dose volume (Fig. 55-4). Specific considerations of needle placement for meatal tumors or unilateral tumors have been described.53
of this chapter. For volume implants, two or three parallel planes of sources are inserted, with two or three needles in each plane. Equal intersource and interplane spacing ranges from 12 to 18 mm. The distribution, spacing, and total number of needles depend on tumor size. Planes are usually oriented with the needles passing from the dorsal to the ventral surface of the glans, but a left-to-right orientation may be acceptable depending on tumor location. Visual verification of coverage can be readily accomplished. Needle placement should be planned such that the prescription isodose will cover 10 mm beyond visible or palpable tumor. Care must be taken to adhere to the rules of the Paris system66 and to be aware of the relationship of the treated volume to the needles such that placement ensures that the entire tumor and desired margin are within the high-dose volume (Fig. 55-4). Specific considerations of needle placement for meatal tumors or unilateral tumors have been described.53
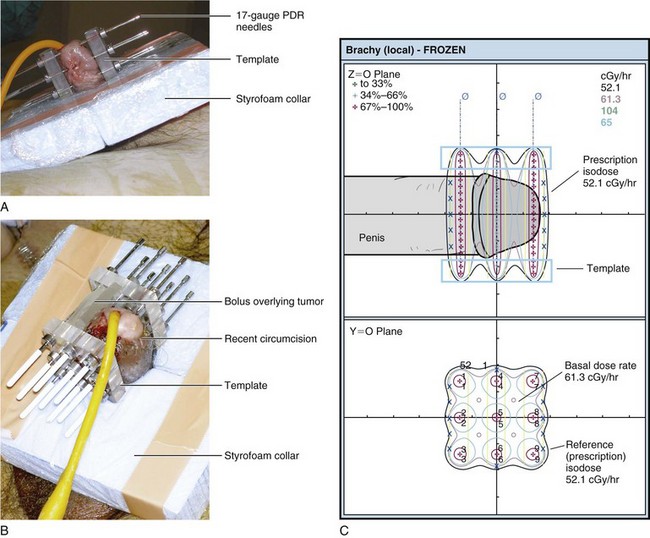
Figure 55-4 Interstitial brachytherapy according to the Paris system of dosimetry.66 A, Six-needle, two-plane implant. B, Nine-needle, three-plane implant. C, Dose distribution for B. According to the Paris system, dose rate minima in the central plane (61.3 cGy/hr in this case) are the basal dose rate. The prescription isodose is 85% of the basal dose rate (52.1 cGy/hr in this case).
With the increasing availability of high-dose-rate (HDR) brachytherapy it is very likely that this modality will be applied to penile brachytherapy. At present there is very little evidence on which to base recommendations for fractionation. A recent experience with 10 patients over 8 years using twice-daily fractions of 3 Gy to deliver 54 Gy over 9 days has been reported.67 These researchers did not observe any necrosis but very small volume implants were used and the majority were single plane. Limited personal experience suggests that 3.75 Gy × 12 over 6 days for a total dose of 45 Gy may be effective for a two-plane volume implant. CT planning is required with delineation of the gross tumor volume using fine wire. It is recommended to keep the V150 less than 20%. Further published experience and longer follow-up is required before any definitive recommendations can be made regarding optimal fractionation for HDR brachytherapy in the management of penile carcinoma.
Tolerance: Acute and Chronic Reactions
Late Reactions
The two most common and significant late complications of RT for penile cancer are soft tissue necrosis and meatal stenosis. Either can occur with EBRT or brachytherapy (see Table 55-2).
Soft Tissue Necrosis
Soft tissue necrosis is reported in 0% to 23% of patients and is more common after brachytherapy than after EBRT. Necrosis is the most common reason for amputation of a tumor-free penis. The risk of necrosis increases with increasing dose over 60 Gy, stage T3 tumors, larger implant volume (>30 mL; p = .01), and a greater number of needles (p = .04) or implant planes (>2; p = .001).24,25,58
The peak time for soft tissue ulceration is 7 to 18 months after brachytherapy, but it can occur much later. Causative factors include trauma and cold exposure. Areas of ulceration should be treated conservatively, with attention to good hygiene, antibiotics, and topical corticosteroid or vitamin E creams. Biopsy should be avoided unless there is suspicion of concomitant tumor. A deeper or painful area of necrosis may respond well to hyperbaric oxygen treatment.58 If such treatment is available, it should be tried before resorting to amputation.
Urethral Stenosis
Urethral or meatal stenosis is reported in 10% to 45% of patients and tends to occur later in the follow-up period, but usually before 3 years. It appears to be dose related for EBRT and may be more common with fraction sizes greater than 2 Gy. For brachytherapy, Rozan and colleagues reported that the only factor predictive of urethral stenosis was the number of needle planes used in the implant (p = .001, if two planes).24 When a three-plane implant is indicated because of tumor size or morphology, central plane needles will be close to the urethra.53 A pulse-dose-rate remote afterloader allows optimization of dwell times to limit the urethral dose.
Sexual Function
There is no systematic study available in the literature dealing with sexual function after RT for penile cancer. The impact on sexual function often is not assessed. Crook and colleagues58 found that in a series of 49 men treated with brachytherapy, 22 of 27 who were potent at baseline reported satisfactory potency at last follow-up. Delannes and associates27 observed that apart from 1 patient who developed painful erections due to sclerosis, “sexual function did not appear to be altered by the implant.” Sarin and colleagues2 provided details for only 14 of the 59 patients, commenting that 12 were normal and 2 had minor impairment. Mazeron and associates25 stated that for noninfiltrating or moderately infiltrating tumors less than 4 cm in diameter, “sexual function remained the same as before treatment.”
If necessary, dose to the testes can be limited by incorporation of a layer of 2 mm of lead into the Styrofoam collar around the penis. Thermoluminescent dosimeter measurements for pulse-dose-rate penile brachytherapy then indicate a total dose to the anterior testis of 55 cGy and 26 cGy to the posterior testis during a 60-Gy treatment course.58 No data are available on postbrachytherapy sperm counts.
Treatment Algorithm, Controversies, And Challenges
Our preferred treatment algorithm is shown in Figure 55-5. For Tis, T1, and T2 tumors less than 4 cm in diameter, a penile-conserving approach as primary management is warranted. Laser surgery can provide satisfactory cosmesis, function, and local tumor control, especially for Tis or small T1 lesions, with the option of re-treating local recurrences with further laser surgery. The RT of choice is interstitial brachytherapy, which results in penile preservation in 70% of cases at 10 years. If brachytherapy expertise is not available, EBRT can prevent amputation in 50% to 60% of patients. For more locally advanced lesions (T2 >4 cm, T3, ? T4), concurrent chemoradiation should be evaluated. With any penis-conserving approach, protracted follow-up is necessary because local failure can occur many years later and surgical salvage can be curative.
1 Opjordsmoen S, Waehre H, Aass N, et al. Sexuality in patients treated for penile cancer. Patients’ experience and doctors’ judgement. Br J Urol. 1994;73:554-560.
2 Sarin R, Norman AR, Steel GG, et al. Treatment results and prognostic factors in 101 men treated for squamous carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1997;38:713-722.
3 Soria JC, Fizazi K, Piron D, et al. Squamous cell carcinoma of the penis. Multivariate analysis of prognostic factors and natural history in monocentric study with a conservative policy. Ann Oncol. 1997;8:1089-1098.
4 Harden SV, Tan LT. Treatment of localized carcinoma of the penis. A survey of current practice in the UK. Clin Oncol (R Coll Radiol). 2001;13:284-287.
5 Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
10 Picconi MA, Eijan AM, Distefano AL, et al. Human papillomavirus (HPV) DNA in penile carcinomas in Argentina. Analysis of primary tumors and lymph nodes. J Med Virol. 2000;61:65-69.
11 Bezerra AL, Lopes A, Santiago G, et al. Human papillomavirus as a prognostic factor in carcinoma of the penis. Analysis of 82 patients treated with amputation and bilateral lymphadenectomy. Cancer. 2001;91:2315-2321.
12 Lopes A, Bezerra AL, Pinto CA, et al. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma. Analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol. 2002;168:81-86.
13 Yanagawa N, Osakabe M, Hayashi M, et al. Detection of HPV-DNA, p53 alterations, and methylation in penile squamous cell carcinoma in Japanese men. Pathol Int. 2008;58:477-482.
21 Lont AP, Besnard AP, Gallee MP, et al. A comparison of physical examination and imaging in determining the extent of primary penile carcinoma. BJU Int. 2003;91:493-495.
22 Scardino E, Villa G, Bonomo G, et al. Magnetic resonance imaging combined with artificial erection for local staging of penile cancer. Urology. 2004;63:1158-1162.
23 Petralia G, Villa G, Scardino E, et al. Local staging of penile cancer using magnetic resonance imaging with pharmacologically induced penile erection. Radiol Med. 2008;113:517-528.
24 Rozan R, Albuisson E, Giraud B, et al. Interstitial brachytherapy for penile carcinoma: A multicentric survey (259 patients). Radiother Oncol. 1995;36:83-93.
25 Mazeron JJ, Langlois D, Lobo PA, et al. Interstitial radiation therapy for carcinoma of the penis using iridium 192 wires: The Henri Mondor experience (1970-1979). Int J Radiat Oncol Biol Phys. 1984;10:1891-1895.
26 Kiltie AE, Elwell C, Close HJ, et al. Iridium-192 implantation for node-negative carcinoma of the penis. The Cookridge Hospital experience. Clin Oncol (R Coll Radiol). 2000;12:25-31.
27 Delannes M, Malavaud B, Douchez J, et al. Iridium-192 interstitial therapy for squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1992;24:479-483.
30 Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma. The M.D. Anderson Cancer Center experience. J Urol. 2002;167:1638-1642.
31 Slaton JW, Morgenstern N, Levy DA, et al. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer. Independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J Urol. 2001;165:1138-1142.
32 Horenblas S, van Tinteren H. Squamous cell carcinoma of the penis. IV. Prognostic factors of survival: Analysis of tumor, nodes and metastasis classification system. J Urol. 1994;151:1239-1243.
35 Velazquez EF, Barreto JE, Rodriguez I, et al. Limitations in the interpretation of biopsies in patients with penile squamous cell carcinoma. Int J Surg Pathol. 2004;12:139-146.
40 Crawshaw JW, Hadway P, Hoffland D, et al. Sentinel lymph node biopsy using dynamic lymphoscintigraphy combined with ultrasound-guided fine needle aspiration in penile carcinoma. Br J Radiol. 2009;82:41-48.
42 Jensen JB, Jensen KM, Ulhoi BP, et al. Sentinel lymph-node biopsy in patients with squamous cell carcinoma of the penis. BJU Int. 2009;103:1199-1203.
43 Leijte JA, van der Ploeg IM, Valdes Olmos RA, et al. Visualization of tumor blockage and rerouting of lymphatic drainage in penile cancer patients by use of SPECT/CT. J Nucl Med. 2009;50:364-367.
44 Solsona E, Algaba F, Horenblas S, et al. European Association of Urology guidelines on penile cancer. European Urology. 2004;46:1-8.
45 Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am. 1992;19:291-304.
46 Meijer RP, Boon TA, van Venrooij GE, et al. Long-term follow-up after laser therapy for penile carcinoma. Urology. 2007;69:759-762.
47 Windahl T, Andersson SO. Combined laser treatment for penile carcinoma. Results after long-term followup. J Urol. 2003;169:2118-2121.
51 Palminteri E, Berdondini E, Lazzeri M, et al. Resurfacing and reconstruction of the glans penis. Eur Urol. 2007;52:893-898.
52 Hoffman MA, Renshaw AA, Loughlin KR. Squamous cell carcinoma of the penis and microscopic pathologic margins. How much margin is needed for local cure? Cancer. 1999;85:1565.
53 Crook J, Jezioranski J, Cygler JE. Penile brachytherapy. Technical aspects and postimplant issues. Brachytherapy. 2010;9:151-158.
54 de Crevoisier R, Slimane K, Sanfilippo N, et al. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N− or NX). Int J Radiat Oncol Biol Phys. 2009;74:1150-1156.
57 Crook J, Ma C, Grimard L. Radiation therapy in the management of the primary penile tumor. An update. World J Urol. 2009;27:189.
62 Pagliaro LC, Crook J. Multimodality therapy in penile cancer. When and which treatments? World J Urol. 2009;27:221-225.
67 Petera J, Sirák I, Kašaová L, et al. High dose rate brachytherapy for penile cancer—a first experience. Brachytherapy. 2011;10:136-140.
1 Opjordsmoen S, Waehre H, Aass N, et al. Sexuality in patients treated for penile cancer. Patients’ experience and doctors’ judgement. Br J Urol. 1994;73:554-560.
2 Sarin R, Norman AR, Steel GG, et al. Treatment results and prognostic factors in 101 men treated for squamous carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1997;38:713-722.
3 Soria JC, Fizazi K, Piron D, et al. Squamous cell carcinoma of the penis. Multivariate analysis of prognostic factors and natural history in monocentric study with a conservative policy. Ann Oncol. 1997;8:1089-1098.
4 Harden SV, Tan LT. Treatment of localized carcinoma of the penis. A survey of current practice in the UK. Clin Oncol (R Coll Radiol). 2001;13:284-287.
5 Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
6 Rippentrop JM, Joslyn SA, Konety BR. Squamous cell carcinoma of the penis. Evaluation of data from the Surveillance, Epidemiology, and End Results program. Cancer. 2004;101:1357-1363.
7 Saibishkumar EP, Crook J, Sweet J. Neonatal circumcision and invasive squamous cell carcinoma of the penis. A report of 3 cases and a review of the literature. Can Urol Assoc J. 2008;2:39-42.
8 Dillner J, von Krogh G, Horenblas S, et al. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol Suppl. 2000;205:189-193.
9 McCance DJ, Kalache A, Ashdown K, et al. Human papillomavirus types 16 and 18 in carcinomas of the penis from Brazil. Int J Cancer. 1986;37:55-59.
10 Picconi MA, Eijan AM, Distefano AL, et al. Human papillomavirus (HPV) DNA in penile carcinomas in Argentina. Analysis of primary tumors and lymph nodes. J Med Virol. 2000;61:65-69.
11 Bezerra AL, Lopes A, Santiago G, et al. Human papillomavirus as a prognostic factor in carcinoma of the penis. Analysis of 82 patients treated with amputation and bilateral lymphadenectomy. Cancer. 2001;91:2315-2321.
12 Lopes A, Bezerra AL, Pinto CA, et al. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma. Analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol. 2002;168:81-86.
13 Yanagawa N, Osakabe M, Hayashi M, et al. Detection of HPV-DNA, p53 alterations, and methylation in penile squamous cell carcinoma in Japanese men. Pathol Int. 2008;58:477-482.
14 Zhu Y, Zhou XY, Yao XD, et al. The prognostic significance of p53, ki-67, epithelial cadherin and matrix metalloproteinase-9 in penile squamous cell carcinoma treated with surgery. BJU Int. 2007;100:204-208.
15 Gross G, Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med Microbiol Immunol (Berl). 2004;193:35-44.
16 Perceau G, Derancourt C, Clavel C, et al. Lichen sclerosus is frequently present in penile squamous cell carcinomas but is not always associated with oncogenic human papillomavirus. Br J Dermatol. 2003;148:934-938.
17 Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis. Frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453.
18 Jackson S. The treatment of carcinoma of the penis. Br J Surg. 1966;53:33-35.
19 FromHarmer M. Penis (ICD-0187). In TNM Classification of Malignant Tumours, ed 3, Geneva: Springer-Verlag; 1978:126-128.
20 Sobin LH, et al. Penis (ICD-0 C60). TNM Classification of Malignant Tumours, ed 6. John Wiley & Sons, New York, 2002..
21 Lont AP, Besnard AP, Gallee MP, et al. A comparison of physical examination and imaging in determining the extent of primary penile carcinoma. BJU Int. 2003;91:493-495.
22 Scardino E, Villa G, Bonomo G, et al. Magnetic resonance imaging combined with artificial erection for local staging of penile cancer. Urology. 2004;63:1158-1162.
23 Petralia G, Villa G, Scardino E, et al. Local staging of penile cancer using magnetic resonance imaging with pharmacologically induced penile erection. Radiol Med. 2008;113:517-528.
24 Rozan R, Albuisson E, Giraud B, et al. Interstitial brachytherapy for penile carcinoma. A multicentric survey (259 patients). Radiother Oncol. 1995;36:83-93.
25 Mazeron JJ, Langlois D, Lobo PA, et al. Interstitial radiation therapy for carcinoma of the penis using iridium 192 wires. The Henri Mondor experience (1970-1979). Int J Radiat Oncol Biol Phys. 1984;10:1891-1895.
26 Kiltie AE, Elwell C, Close HJ, et al. Iridium-192 implantation for node-negative carcinoma of the penis. The Cookridge Hospital experience. Clin Oncol (R Coll Radiol). 2000;12:25-31.
27 Delannes M, Malavaud B, Douchez J, et al. Iridium-192 interstitial therapy for squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1992;24:479-483.
28 Parra RO. Accurate staging of carcinoma of the penis in men with nonpalpable inguinal lymph nodes by modified inguinal lymphadenectomy. J Urol. 1996;155:560-563.
29 Theodorescu D, Russo P, Zhang ZF, et al. Outcomes of initial surveillance of invasive squamous cell carcinoma of the penis and negative nodes. J Urol. 1996;155:1626-1631.
30 Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma. The M.D. Anderson Cancer Center experience. J Urol. 2002;167:1638-1642.
31 Slaton JW, Morgenstern N, Levy DA, et al. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer. Independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J Urol. 2001;165:1138-1142.
32 Horenblas S, van Tinteren H. Squamous cell carcinoma of the penis. IV. Prognostic factors of survival. Analysis of tumor, nodes and metastasis classification system. J Urol. 1994;151:1239-1243.
33 Ornellas AA, Seixas AL, Marota A, et al. Surgical treatment of invasive squamous cell carcinoma of the penis. Retrospective analysis of 350 cases. J Urol. 1994;151:1244-1249.
34 McDougal WS. Carcinoma of the penis. Improved survival by early regional lymphadenectomy based on the histological grade and depth of invasion of the primary lesion. J Urol. 1995;154:1364-1366.
35 Velazquez EF, Barreto JE, Rodriguez I, et al. Limitations in the interpretation of biopsies in patients with penile squamous cell carcinoma. Int J Surg Pathol. 2004;12:139-146.
36 Lopes A, Hidalgo GS, Kowalski LP, et al. Prognostic factors in carcinoma of the penis. Multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J Urol. 1996;156:1637-1642.
37 Horenblas S, van Tinteren H, Delemarre JF, et al. Squamous cell carcinoma of the penis. II. Treatment of the primary tumor. J Urol. 1992;147:1533-1538.
38 Kochhar R, Taylor B, Sangar V. Imaging in primary penile cancer. Current status and future directions. Eur Radiol. 2010;20:36-47.
39 Tanis PJ, Lont AP, Meinhardt W, et al. Dynamic sentinel node biopsy for penile cancer. Reliability of a staging technique. J Urol. 2002;168:76-80.
40 Crawshaw JW, Hadway P, Hoffland D, et al. Sentinel lymph node biopsy using dynamic lymphoscintigraphy combined with ultrasound-guided fine needle aspiration in penile carcinoma. Br J Radiol. 2009;82:41-48.
41 Hadway P, Smith Y, Corbishley C, et al. Evaluation of dynamic lymphoscintigraphy and sentinel lymph-node biopsy for detecting occult metastases in patients with penile squamous cell carcinoma. BJU Int. 2007;100:561-565.
42 Jensen JB, Jensen KM, Ulhoi BP, et al. Sentinel lymph-node biopsy in patients with squamous cell carcinoma of the penis. BJU Int. 2009;103:1199-1203.
43 Leijte JA, van der Ploeg IM, Valdes Olmos RA, et al. Visualization of tumor blockage and rerouting of lymphatic drainage in penile cancer patients by use of SPECT/CT. J Nucl Med. 2009;50:364-367.
44 Solsona E, Algaba F, Horenblas S, et al. European Association of Urology guidelines on penile cancer. European Urology. 2004;46:1-8.
45 Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am. 1992;19:291-304.
46 Meijer RP, Boon TA, van Venrooij GE, et al. Long-term follow-up after laser therapy for penile carcinoma. Urology. 2007;69:759-762.
47 Windahl T, Andersson SO. Combined laser treatment for penile carcinoma. Results after long-term followup. J Urol. 2003;169:2118-2121.
48 Schlenker B, Gratzke C, Seitz M, et al. Fluorescence-guided laser therapy for penile carcinoma and precancerous lesions. Long-term follow-up. Urol Oncol. 2009. Nov 26 [Epub ahead of print]
49 Frimberger D, Hungerhuber E, Zaak D, et al. Penile carcinoma. Is Nd:YAG laser therapy radical enough? J Urol. 2002;168:2418-2421. discussion 2421
50 Smith Y, Hadway P, Biedrzycki O, et al. Reconstructive surgery for invasive squamous carcinoma of the glans penis. Eur Urol. 2007;52:1179-1185.
51 Palminteri E, Berdondini E, Lazzeri M, et al. Resurfacing and reconstruction of the glans penis. Eur Urol. 2007;52:893-898.
52 Hoffman MA, Renshaw AA, Loughlin KR. Squamous cell carcinoma of the penis and microscopic pathologic margins. How much margin is needed for local cure? Cancer. 1999;85:1565.
53 Crook J, Jezioranski J, Cygler JE. Penile brachytherapy. Technical aspects and postimplant issues. Brachytherapy. 2010;9:151-158.
54 de Crevoisier R, Slimane K, Sanfilippo N, et al. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N− or NX). Int J Radiat Oncol Biol Phys. 2009;74:1150-1156.
55 Zouhair A, Coucke PA, Jeanneret W, et al. Radiation therapy alone or combined surgery and radiation therapy in squamous-cell carcinoma of the penis? Eur J Cancer. 2001;37:198-203.
56 Crook J, Grimard L, Tsihlias J, et al. Interstitial brachytherapy for penile cancer. An alternative to amputation. J Urol. 2002;167:506-511.
57 Crook J, Ma C, Grimard L. Radiation therapy in the management of the primary penile tumor. An update. World J Urol. 2009;27:189.
58 Crook JM, Jezioranski J, Grimard L, et al. Penile brachytherapy. Results for 49 patients. Int J Radiat Oncol Biol Phys. 2005;62:460-467.
59 Katz A, Eifel PJ, Jhingran A, et al. The role of radiation therapy in preventing regional recurrences of invasive squamous cell carcinoma of the vulva. Int J Radiat Oncol Biol Phys. 2003;57:409-418.
60 Huang XY, Kubota Y, Nakada T, et al. Intra-arterial infusion chemotherapy for penile carcinoma with deep inguinal lymph node metastasis. Urol Int. 1999;62:245-248.
61 Haas GP, Blumenstein BA, Gagliano RG, et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis. A Southwest Oncology Group study. J Urol. 1999;161:1823-1825.
62 Pagliaro LC, Crook J. Multimodality therapy in penile cancer. When and which treatments? World J Urol. 2009;27:221-225.
63 McLean M, Akl AM, Warde P, et al. The results of primary radiation therapy in the management of squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1993;25:623-628.
64 Neave F, Neal AJ, Hoskin PJ, et al. Carcinoma of the penis. A retrospective review of treatment with iridium mould and external beam irradiation. Clin Oncol (R Coll Radiol). 1993;5:207-210.
65 Vujovic O, Grant JB, Stewart D. Carcinoma of the penis. A treatment technique with external beam radiation. Annals RCPSC. 2001;34:495-497.
66 Pierquin B, Chassagne D, Wilson F. Modern Brachytherapy. New York: Masson; 1987.
67 Petera J, Sirák I, Kašaová L, et al. High dose rate brachytherapy for penile cancer—a first experience. Brachytherapy. 2011;10:136-140.
68 Gotsadze D, Matveev B, Zak B, et al. Is conservative organ-sparing treatment of penile carcinoma justified? Eur Urol. 2000;38:306-312.
69 Ozsahin M, Jichlinski P, Weber DC, et al. Treatment of penile carcinoma. To cut or not to cut? Int J Radiat Oncol Biol Phys. 2006;66:674-679.