Pediatric Respiratory Emergencies
Disease of the Lungs
Pneumonia
Epidemiology
The causative organisms also vary with the age of the child. Because the organism is not definitively identified in most pneumonia cases, it is difficult to determine the true incidence of the specific etiologic agents.1 Overall, it has been estimated that viral agents cause 60 to 90% of pneumonias.2 Viral agents are more common in younger children. Bacteria predominate in neonates but are a less frequent causative agent in toddlers and older children. Outside of the neonatal period, the incidence of bacterial agents is stable throughout different age groups.2 Mixed viral and bacterial infections or concomitant bacterial infections may occur in one third of pneumonias.2,3 Chlamydia trachomatis is a unique cause of pneumonia in infants 3 to 19 weeks old. Bordetella pertussis classically occurs in children younger than 1 year but also occurs in older children and adolescents.4,5 Mycoplasma pneumoniae is one of the most common causes of pneumonia among children older than 5 years and may play a role in younger children.6,7 Chlamydia pneumoniae is more often seen in children older than 5 years but also may cause infection in younger children.7,8
Among bacteria, group B streptococci and gram-negative bacilli predominate in neonates. Ureaplasma urealyticum and Listeria monocytogenes may cause illness in infants younger than 3 months.1 Streptococcus pneumoniae is the leading bacterial cause of pneumonia in all age groups beyond the newborn period; Haemophilus influenzae and Staphylococcus aureus are less common etiologic agents, most often seen in the first years of life. The incidence of H. influenzae type b disease has decreased by 90% since the onset of immunization of infants and young children.9 The heptavalent pneumococcal conjugate vaccine Prevnar (Lederle Laboratories/Wyeth-Ayerst Pharmaceuticals, Wayne, N.J.) was licensed by the U.S. Food and Drug Administration in 2000 and is recommended as a primary series at 2, 4, and 6 months of age, with a fourth booster dose given at 12 to 15 months of age. Clinical trials suggested 85% protection against serotype-specific cases of bacteremic pneumonia.10 Studies have also shown a decrease in carriage rates of the serotypes included in the daycare setting.11 Pneumococcal polysaccharide vaccines have been recommended for children older than 2 years at high risk for pneumococcal disease since 1985, but they are not immunogenic in younger patients. Pneumococcal vaccine has also been shown to provide some protection against viral pneumonia. One study found a 31% reduction in the incidence of pneumonia associated with seven respiratory viruses in hospitalized children. This may be because viral pneumonia in hospitalized children is often associated with concurrent pneumococcal infection.12 Other, less common bacterial agents include group A streptococci, Neisseria meningitidis, and anaerobic bacteria, the last being particularly common in the setting of aspiration pneumonia. Unusual causes of pneumonia include Pseudomonas aeruginosa, Legionella pneumophila, Pneumocystis jiroveci, and rickettsial infections. The incidence of Mycobacterium tuberculosis is increasing in the United States, particularly in urban and low-income areas and among nonwhite racial or ethnic groups. Infants and adolescents are at highest risk in the United States.
Principles of Disease
Bacterial pneumonia and mycoplasmal infections are transmitted most often person to person by droplet aspiration. Asymptomatic upper airway colonization often occurs in children and may spread infection to other children.13 Less commonly, bacterial pneumonia also may result from hematogenous spread from a distant focus or during primary bacteremia. Viral agents that cause pneumonia proliferate in the upper respiratory tract and spread contiguously to involve the lower respiratory tract. Viruses such as varicella, CMV, herpes simplex, Epstein-Barr, measles, and rubella also may infect the lung through hematogenous spread.
Clinical Features
History.: Clinical symptoms and signs of pneumonia in pediatric patients vary with the age of the patient, specific pathogen, and severity of the disease. Infants younger than 3 months generally have respiratory symptoms, such as tachypnea, cough, retractions, and grunting, but may show only nonlocalizing symptoms, such as isolated fever or hypothermia, vomiting, poor feeding, irritability, and lethargy. Toddlers with S. pneumoniae infection may have nonspecific symptoms, such as high fever and lethargy, without respiratory symptoms. In general, with increasing age, signs and symptoms in children become more specific and are manifestations of generalized infection, lower respiratory tract disease, and associated extrapulmonary disease, although pneumonia in any child may have only a few or subtle manifestations. General symptoms related to the infection include fever and chills, headache, rigors, and malaise. Symptoms of lower respiratory tract disease may include cough and wheezing. Pleural irritation may cause chest, abdominal, or neck pain or result in neck stiffness. Vomiting or poor oral intake can be seen with pneumonia of any cause and may imply more severe disease or dehydration.
Physical Examination.: Physical examination of a child with suspected pneumonia should begin by noting the general appearance and breathing pattern. Vital signs, including oxygen saturation, also should be evaluated on arrival. Important findings include toxicity, level of alertness and interaction, color, and state of hydration and perfusion. Fever most often is present with bacterial pneumonia but may be low grade or absent in neonates and patients with nonbacterial disease. Cardiovascular parameters may indicate dehydration or, rarely, shock. Tachypnea, although not universal, is the most sensitive indicator of pneumonia and may be the only manifestation in a young child.14 The World Health Organization has published guidelines for the clinical diagnosis of pneumonia in developing countries and cites tachypnea and retractions as indicators of lower respiratory disease. Tachypnea is defined by the World Health Organization as a respiratory rate of more than 50 breaths/minute in infants younger than 1 year and more than 40 breaths/minute in children older than 1 year.15 Other manifestations of lower airway disease may include cough, wheezing, nasal flaring, retractions, grunting, and use of accessory muscles. The characteristics of the cough may aid in the diagnosis; a staccato and paroxysmal cough in an infant may indicate pneumonia caused by C. trachomatis or B. pertussis. A hacking quality is often present with Mycoplasma infection. Auscultatory examination of an older child may reveal rales, wheezing, and diminished breath sounds, often with dullness to percussion and associated decreased fremitus. Although these findings may be present, the clinical findings are much less consistent in a younger child; rales may be masked by poor inspiratory effort or noisy upper airway sounds.
Specific Disorders
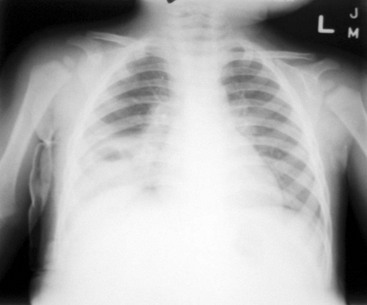
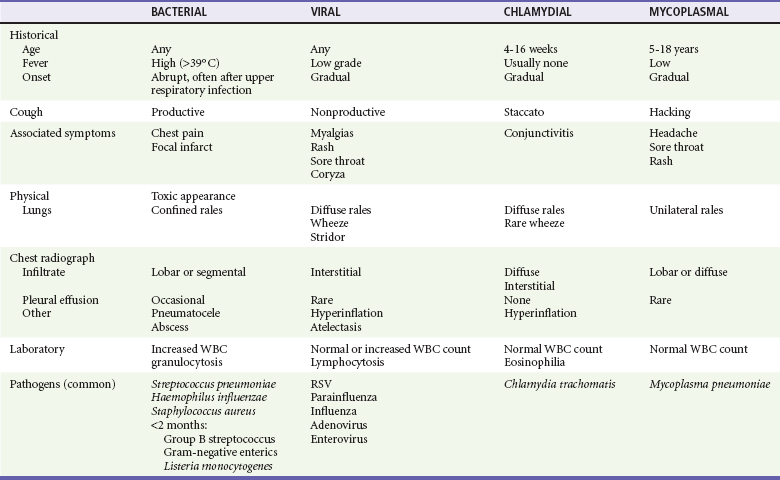
Perspective.: S. pneumoniae is one of the most frequently seen bacterial agents causing pneumonia in children.12 Whereas any child can acquire S. pneumoniae, children at increased risk for development of infection from S. pneumoniae are children with immune deficiency, chronic renal disease, or functional or anatomic asplenia and Native Americans.10 S. aureus pneumonia, although less common, tends to cause a more severe pneumonia; more than 70% of all cases occur in the first year of life.16 Children with foreign body aspiration, immunosuppression, or skin infections may be at increased risk for S. aureus pneumonia. Progression of the disease is rapid, and empyema (90%), pneumatocele (50%), pneumothorax (25%), and bacteremia are common complications (Fig. 170-1). In contrast to classically described methicillin-resistant S. aureus, which is typically broadly resistant and nosocomially acquired, this agent is resistant to fewer antibiotics.17
Before widespread immunization, H. influenzae was the second most common bacterial cause of pneumonia. However, its incidence has decreased by 90% since the onset of effective immunization, and it is rarely seen presently.9 H. influenzae previously was considered a disease of younger children, but most cases now occur in older children.18 Although it is often clinically indistinguishable from S. pneumoniae pneumonia, H. influenzae pneumonia has a higher incidence of associated pleural effusions (25-75%) and bacteremia (75-95%).19
Although it is still uncommon, the incidence of group A streptococcal pneumonia may have increased since the 1980s. One study reported an increase in the incidence from 0.16 per 100,000 in 1992 to 0.35 per 100,000 in 1999 in Canada, whereas another study suggested stable rates since the 1980s.19,20 Group A streptococcal pneumonia may occur sporadically and may be a complication of varicella.19 It is typically a severe illness with abrupt onset, rapid progression to toxicity, and high fatality rate (30-60% fatality rate reported in a study of all ages).19
Clinical Features.: Bacterial pneumonia beyond the neonatal period generally has a sudden onset, and fever is almost universal (temperature often above 39° C). Patients may or may not have a cough and often appear relatively toxic with tachypnea disproportionate to the fever. Confined rales or wheezes and localized decreased or tubular breath sounds commonly occur in older children, although the physical examination in a younger child may be completely unrevealing.
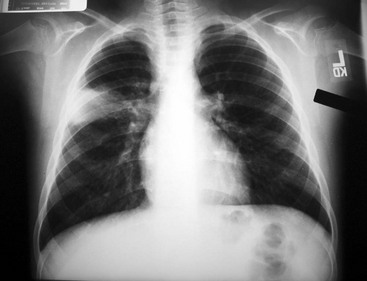
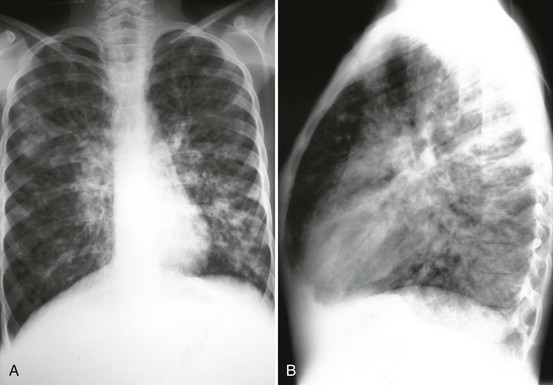
Diagnostic Considerations.: Patients with pneumococcal infections often have a preceding dramatic presentation with high white blood cell (WBC) counts with associated pleural effusion and bacteremia in 10 to 30% of children.21 Radiographic findings may show an alveolar infiltrate in a patchy or consolidated lobar (Fig. 170-2) or subsegmental distribution, although patients with bacterial pneumonia may have an interstitial infiltrate.22 Bilateral involvement, pleural effusion, pneumatocele, and pneumothorax may occur with more severe disease. Although the WBC count may be normal with bacterial pneumonia, leukocytosis often occurs, sometimes exceeding 20,000/mm3. Uncomplicated bacterial pneumonia often has a rapid response to institution of appropriate antibiotics; a stagnant or worsening clinical picture should prompt further investigation.
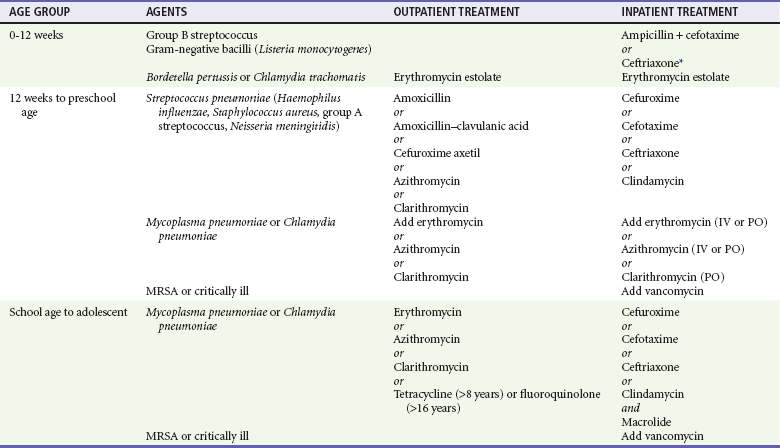
Management.: Of particular concern is the emergence of resistance to penicillin and cephalosporins. At this time, it seems that the outcome of otherwise healthy children with pneumonia secondary to resistant pneumococcus may not differ significantly from the outcome of children with pneumonia secondary to penicillin-sensitive pneumococcus.23 Pneumonia caused by S. pneumoniae may be complicated by empyema, pleural effusion, lung abscess, or necrotizing pneumonia. Since the 1990s, there seems to have been an increase in the incidence of such complications; one study noted an increase from 22.6% in 1994 to 53% in 1999 in children hospitalized with pneumococcal pneumonia.56 It does not seem that this increased incidence is related to intermediate resistant organisms. It is unclear if highly resistant organisms play a role.56 High-dose amoxicillin is recommended for initial outpatient treatment of suspected pneumococcal pneumonia in children younger than 4 years; a standard dose may be used in older children who are immunocompetent (Table 170-1). Children who appear well can be treated with oral antibiotics. In some cases, a dose of intravenous antibiotics followed by an oral prescription may be beneficial. One study reported decreased admission rates for children with S. pneumoniae given an initial intravenous dose of antibiotics followed by oral antibiotics.56
Viral Pneumonia
Most viral pneumonias resolve without specific therapy. Because of the likelihood of bacterial superinfection and the difficulty in differentiating between bacterial and viral pneumonia, antibiotics should be considered in a more severely ill child. Potential complications include dehydration, local progression of the disease, bronchiolitis obliterans, and apnea (most commonly in the first 3 months of life).25
Mycoplasmal Pneumonia
Mycoplasmal pneumonia accounts for 10 to 20% of all pneumonias and has traditionally been thought to occur most commonly in 5- to 18-year-olds. It is now clear that it also may play a significant role in younger children but is still rare in infants younger than 1 year.25 The onset is classically gradual and insidious, but some patients also may present with abrupt onset of symptoms similar to its bacterial counterpart.4 Prodromal symptoms include fever, headache, and malaise, followed several days later by a nonproductive, hacking cough. Patients also may present with pertussis-like illness. Other symptoms of infection may include hoarseness, sore throat, and chest pain; coryza is unusual. Children with mycoplasmal pneumonia generally appear nontoxic. Patients may have rales; wheezing occurs less often. Pharyngitis, cervical lymphadenopathy, conjunctivitis, and otitis media may occur occasionally. Bullous myringitis, although rare, is believed to be indicative of Mycoplasma.25 Rash is present in 10% of patients and may be urticarial, erythema multiforme, maculopapular, or vesicular.26 The course may be complicated by pneumatocele, pleural effusion, pneumothorax, or bronchiectasis. Mycoplasma, typically thought to be a benign and self-limited infection, has been shown to play a significant role in exacerbation of asthma and may cause chronic pulmonary structural abnormalities.4
Laboratory diagnosis is problematic. Although it has been used in the past, bedside cold agglutination testing is a poor indication of infection, especially in patients younger than 12 years, and is rarely used today.25 Infection often is diagnosed clinically and treated empirically. Diagnosis may be confirmed with acute and convalescent antibody titers; however, patients may take 4 to 6 weeks to seroconvert, and some patients may fail to mount an immune response.4,26 Culture is not routinely available; polymerase chain reaction diagnosis at this time is available only from research laboratories.4 Complications are varied but unusual and include hemolytic anemia, myopericarditis, neurologic disease (meningoencephalitis, Guillain-Barré syndrome, transverse myelitis, cranial neuropathy), rhabdomyolysis, arthritis, and rash.
Chlamydial Pneumonia
C. trachomatis is a common sexually transmitted organism causing cervical infection in 2 to 30% of pregnant women.8 It can be transmitted from the genital tract of infected mothers to their newborn infants, resulting in conjunctivitis in 22 to 44% and pneumonia in 5 to 20%.8 An infant with pneumonia caused by C. trachomatis presents at 3 to 19 weeks of age after colonization with the organism at birth. The illness usually begins with nasal congestion followed by cough. In half of the cases, conjunctivitis precedes the onset of respiratory symptoms. The infant is often afebrile and alert but tachypneic, with a repetitive staccato cough that may interfere with feeding or sleeping. It can resemble the paroxysms of pertussis and occasionally precipitates episodes of alarming respiratory distress. Mild retractions and diffuse inspiratory crepitant rales are noted on chest examination; expiratory wheezing is usually absent or minimal. Middle ear abnormalities are present in half of the cases.
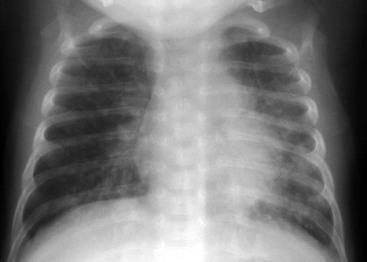
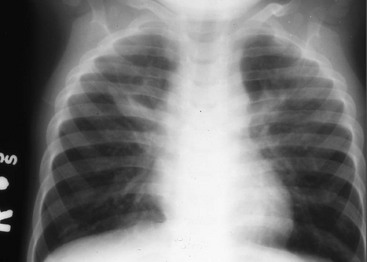
The radiograph usually shows hyperinflation with bilateral and symmetrical diffuse interstitial infiltrates (Fig. 170-3). The total WBC count is usually normal but often with an eosinophilia of more than 400/mm3.8 Definitive diagnosis is made by isolation of the organism in the tissue culture specimen; diagnostic tests based on polymerase chain reaction are more sensitive than fluorescent antibody stain or tissue culture but may not be as specific. Although it is often a mild illness, chlamydial pneumonia may be complicated by apnea and hypoxemia. Treatment with erythromycin may shorten the course; however, the disease tends to be protracted, with cough and tachypnea often requiring weeks to clear despite the administration of antibiotics.
C. pneumoniae is a species of Chlamydia that is antigenically, genetically, and morphologically distinct from other Chlamydia species. C. pneumoniae infection is readily transmitted from person to person. C. pneumoniae may play a role in respiratory tract infections in infants and young children and may cause mild illness or asymptomatic infection in children and adults. Like Mycoplasma, C. pneumoniae may play a much greater role in pediatric pneumonia than was previously thought. It also commonly may be present as a mixed bacterial infection.4 C. pneumoniae has been reported to cause sore throat, fever, headache, pertussis-like cough, pneumonia, and influenza-like illness.27 Outbreaks have been reported in schools, daycare centers, military camps, adolescents, and families.28 Infection with C. pneumoniae can trigger acute episodes of wheezing in children with asthma.
Pertussis
Pertussis, or whooping cough, is a respiratory tract infection seen most commonly in infants younger than 6 months (38% of cases are in children younger than 6 months, and 71% of cases are in children younger than 5 years). The incidence of pertussis increased in the 1980s and 1990s, especially in adolescents and adults, despite high immunization rates.28
The disease is characterized by three clinical stages: catarrhal stage, paroxysmal stage, and convalescent stage.12 In infants, the disease begins with mild upper respiratory tract symptoms and cough; this catarrhal stage usually lasts 1 to 2 weeks. The disease progresses to severe paroxysms of a staccato cough, followed by post-tussive emesis, and may be accompanied by periods of cyanosis and apnea in infants younger than 6 months.6 Classic whoop is rare, occurring in only 6% of patients, and is generally seen in children older than 2 to 3 years. Fever is often absent, and the examination findings are remarkably normal between paroxysms. The paroxysmal stage lasts 2 to 4 weeks and is followed by a convalescent stage, during which symptoms gradually wane. The duration of the illness in more complicated cases may be 6 to 10 weeks.6
Immunization is only 80% effective in providing immunity after three doses. Pertussis still must be considered in an immunized infant, although the illness may be mild.12 The WBC count is usually elevated, exceeding 15,000/mm3 and occasionally 40,000/mm3, with a marked lymphocytosis, although this finding may not be present in infants younger than 3 to 6 months. The chest radiograph may show a “shaggy” right-sided heart border or have clear lung fields. The organism is most easily recovered in the catarrhal or early paroxysmal stages and is rarely found after the fourth week of illness.12 B. pertussis can be cultured from nasopharyngeal secretions. Cultures may be negative during the first week or after the fourth week of illness in immunized patients or patients treated with antibiotics.12 Direct fluorescent antibody staining may be used, but this test has low specificity and variable sensitivity.12 Diagnosis made by direct fluorescent antibody should be confirmed by culture.
Polymerase chain reaction is overall more sensitive and specific. Pertussis is a particularly severe disease in the first year of life; complications are common and include apneic episodes, seizures, secondary bacterial pneumonia, encephalopathy, and death. Pertussis has been increasing in incidence among immunized children and young adults who have waning immunity.5 Adults are believed to be a significant source for the disease within the community. The illness in these patients does not follow the classic stages as described here. These patients have a mild but prolonged course. A dry cough is the predominant symptom, often lasting 3 weeks or more. Because of the risk of apnea, all children younger than 6 months with presumed pertussis should be observed in the hospital for monitoring and supportive care and should be treated with erythromycin. Other macrolides and trimethoprim-sulfamethoxazole are possible alternatives.12 Antimicrobials have no effect on the disease progression after the paroxysmal stage is established but may be beneficial because they limit the spread of organisms. Vaccination of health care workers and the adult population in general with Tdap has been shown to decrease rates of pertussis in infants.8
Diagnostic Strategies
Not every child thought to have pneumonia requires laboratory or radiographic evaluation. A well-appearing child with cough and rales may be diagnosed clinically and treated with antibiotics as an outpatient. A child who appears ill or in whom the diagnosis is unclear requires further evaluation. Young children with high fever and leukocytosis may have an occult pneumonia. A chest radiograph should be considered in these patients even in the absence of respiratory findings.29
Laboratory Studies
Further laboratory studies are obtained only as warranted to help identify the disease process and potential complications. The leukocyte count may be useful in differentiating causes of pneumonia; peripheral WBC counts greater than 15,000/mm3, with predominance of mature and immature granulocytes, suggest bacterial infection; pneumococcal pneumonia typically produces the highest WBC counts.30 Marked leukocytosis and toxic granulation of WBCs also may help identify a child at risk for bacteremia and its potential complications. Normal to elevated WBC counts with lymphocytosis may be seen in viral infections, and eosinophilia suggests chlamydial disease. Increased WBC counts with extreme lymphocytosis typically are associated with pertussis, but this hematologic finding may be absent in infants younger than 6 months. Blood cultures may grow pathogens in only 1 to 10% of cases of bacterial pneumonia. Unfortunately, the contamination rate at most hospitals for cultures is in this range as well. In a well-appearing child with an uncomplicated pneumonia, blood culture is unlikely to be helpful.1,31 When a blood culture is positive, however, it identifies the specific pathogen, and blood culture should be considered in an ill patient in whom hospitalization with bacterial pneumonia is likely. Sputum cultures may be useful in adolescents but are technically difficult and not useful in younger children.32,33
Patients with pleural effusions should have lateral decubitus radiographs to assess effusion size and loculation. Computed tomography scan is useful to provide greater detail of effusions and lung abnormalities in critically ill children with complicated pneumonia.34 Routine computed tomography to establish the diagnosis is not recommended. Patients with significant effusions should have thoracentesis for diagnostic and therapeutic purposes. Although parapneumonic effusions are most suggestive of bacterial infection, they also occur with mycoplasmal and occasionally with viral infections.35 The fluid should be sent for Gram’s stain and culture (anaerobic and aerobic bacterial), cell count and differential, total protein level, pH, and glucose concentration. Interpretation of pleural fluid in children follows adult guidelines (see Chapters 76 and 77). Cultures for rare pathogens may be considered if initial assessment is not diagnostic. Bronchoscopy with bronchoalveolar lavage may be useful in a severely ill child.
Nasopharyngeal viral cultures, antigen detection for specific viral or bacterial agents, and serum antibodies for specific agents may be helpful in determining certain etiologic agents. Although most pediatric patients with tuberculosis do not have pulmonary symptoms, skin testing for tuberculosis should be considered for patients with lobar pneumonia, pulmonary effusions, or hilar adenopathy, especially in immunocompromised children or children who have recently immigrated from less developed countries. Acid-fast bacilli often may be shown on an early morning gastric aspirate.36 Sputum specimens generally are not helpful in a child younger than 8 years because of contamination by organisms of the upper respiratory tract.
Radiology
A chest radiograph may be obtained to confirm or to rule out an infiltrate. Rarely, a dehydrated child with pneumonia may have a normal study, and once the child is rehydrated, the infiltrate becomes readily apparent. The radiograph also may provide clues to the underlying disease process. Although great variability exists, bacterial pathogens classically produce alveolar infiltrates in a lobar distribution but may produce diffuse interstitial infiltrates.37 Viral and chlamydial infections tend to appear as diffuse interstitial infiltrates, commonly with hyperinflation and atelectasis. Chest radiographs also identify multilobar disease, pleural effusions, pneumatoceles, and pneumothorax (Fig. 170-4). Hilar adenopathy may indicate tuberculosis or malignant neoplasm.
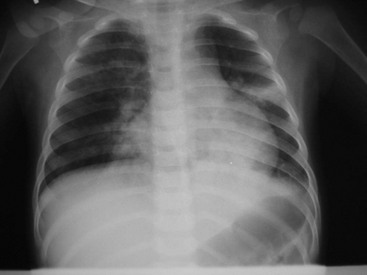
Figure 170-4 Radiograph shows multilobar pneumonia in a child with respiratory distress. (Courtesy Marianne Gausche-Hill, MD.)
Children without comorbid conditions, who are without fever, unilateral wheezing, or tachypnea, are unlikely to have pneumonia and a chest radiograph is unnecessary. Further, a Cochrane review demonstrated that for non–ill-appearing children with fewer than 14 days of symptoms and clinical signs of pneumonia, chest radiography does not reduce subsequent hospitalization rates or duration of symptoms.38,39 Routine chest radiography is not beneficial in these ambulatory children older than 2 months with acute lower respiratory infections.39
Differential Considerations
The major conditions to be differentiated in children with pneumonia include bacterial pneumonias amenable to conventional antibiotics, viral disease, other unusual infectious causes (mycobacterial, protozoal, fungal), and noninfectious pathologic conditions (Box 170-1). Certain features help differentiate the common infectious causes (Table 170-2). As detailed previously, each disease entity has certain classic historical, clinical, and laboratory findings. At the extremes of presentation, these diseases can be distinguished easily; however, the broad spectrum of illness for each process may make accurate diagnosis in an individual patient difficult. No specific feature reliably differentiates patients with bacterial infection from children with nonbacterial pneumonia. Because of limitations of reliably detecting bacterial pneumonia by culture technique, one should strongly consider a bacterial cause in a child with a temperature higher than 39° C, clinical toxicity, lobar infiltrate, or pleural effusions. Although it is often difficult to make a precise etiologic diagnosis, consideration of host factors, epidemiology, and the clinical picture with judicious use of laboratory tests generally points the clinician toward the likely diagnosis and allows effective patient management.
Complications
Several complications of pneumonia result from local and systemic effects of the infection. Pleural effusions or empyemas accumulate most often with bacterial pathogens (notably S. pneumoniae, H. influenzae, and S. aureus) but are occasionally associated with mycoplasmal, viral, and tuberculous pneumonia. An increase in the occurrence of such complications seemed to have occurred in the 1990s for reasons unknown.40 Similarly, lung abscess, pneumatocele, and pneumothorax are local complications primarily seen with bacterial disease, particularly with S. aureus. Extensive pulmonary involvement of any cause may lead occasionally to hypoxia and progressive respiratory failure with multiple organ failure. Apnea without other symptoms is seen most often in viral, chlamydial, and pertussis infections in infants younger than 3 months. The most common systemic complication of pneumonia is dehydration, which is a result of decreased intake from malaise and excessive respiratory effort and increased losses of fluid caused by vomiting, fever, and tachypnea. Another systemic complication of bacterial disease is the development of additional infectious foci resulting from bacteremia (e.g., meningitis, epiglottitis, pericarditis, septic arthritis, and soft tissue infections). Viral and bacterial pneumonias are rarely associated with meningitis, encephalitis, arthritis, rhabdomyolysis, and hemolytic anemia.
Management and Disposition
Treatment of pneumonia in a pediatric patient consists of appropriate antimicrobial use and supportive therapy (see Table 170-1). Because of the difficulty in identifying an etiologic agent, antibiotic choice is generally empirical. The three most important factors in directing management are the patient’s age, likely pathogen, and degree of illness. An infant younger than 2 months with pneumonia should usually be admitted to the hospital. This age group is immunologically immature, and signs of sepsis may be subtle. Blood, urine, and cerebrospinal fluid cultures generally are indicated before initiation of antibiotics. Ampicillin plus an aminoglycoside or ampicillin and a third-generation cephalosporin would be appropriate initial antibiotic choices for an infant younger than 1 month (although ceftriaxone is contraindicated in infants younger than 1 month).
Infants 2 to 3 Months of Age
In an infant 2 to 3 months old, blood and urine cultures are appropriate. The decision to perform a lumbar puncture depends on clinical suspicion of central nervous system infection. Ampicillin and a third-generation cephalosporin are reasonable for an infant 1 to 3 months old. If C. trachomatis or B. pertussis is suspected, the infant also should be treated with erythromycin after appropriate diagnostic samples have been obtained; other macrolides or sulfonamides (trimethoprim-sulfamethoxazole) may be an alternative in older infants and children.12 Supportive therapy in this age group consists of fever control and intravenous hydration. A trial of inhaled beta-agonists or racemic epinephrine may be indicated if the patient is wheezing or in significant respiratory distress; supplemental oxygen and respiratory support should be provided as needed. All infants should be monitored with continuous pulse oximetry because apnea and respiratory failure may be precipitous.
Infants and Children Older Than 3 Months
If a viral agent is suspected and the child is not ill, antibiotics should be withheld. Uncomplicated bacterial pneumonia in immunocompetent children is safe to treat as an outpatient disease. A well-appearing infant or preschool-age child with isolated pneumonia may be treated with oral antibiotics on an outpatient basis. In an infant beyond the neonatal period or a preschool-age child, amoxicillin and amoxicillin–clavulanic acid are considered appropriate first-line agents. Cefuroxime axetil or high-dose amoxicillin (80-100 mg/kg/day) may be used when resistant S. pneumoniae is suspected. Azithromycin is considered an appropriate first-line oral agent in this age group by some researchers.1,33 One study of the treatment of pediatric community-acquired pneumonia showed no difference in clinical cure rate when azithromycin, erythromycin estolate, and amoxicillin–clavulanic acid were compared.41 A macrolide is the first-line antibiotic choice in a school-age child or adolescent, in whom M. pneumoniae and C. pneumoniae are more common. Every child with bacterial pneumonia treated as an outpatient should be reevaluated within 24 to 48 hours. Patients who are not afebrile, clinically improved, and well hydrated should be evaluated at this time for possible admission to the hospital for parenteral antibiotic therapy. For patients in whom signs of bacteremia are present, a single dose of intramuscular ceftriaxone followed by outpatient oral therapy is recommended. In these cases, close follow-up monitoring is crucial.
For a patient who is admitted to the hospital, intravenous therapy with cefuroxime, cefotaxime, or ceftriaxone is appropriate. Addition of a macrolide should be considered if M. pneumoniae is a possible etiologic agent. Vancomycin should be added if methicillin-resistant S. aureus is suspected.13,23 If an organism has been identified, an antibiotic with a more specific spectrum might be indicated.
Since the 1980s, S. pneumoniae has been developing increasing resistance to penicillin and other antibiotics; in general, this has not been clinically relevant because higher doses overcome the relative resistance. Clinical outcomes with intermediate resistance levels seem to be unchanged.42 These patients still respond to penicillin and the cephalosporins at higher doses. If the patient does not respond clinically after a trial of these agents or if the patient is critically ill, use of vancomycin with or without rifampin is recommended.43
Long-term management of a child with pneumonia should include a clinical reevaluation 2 to 3 weeks after diagnosis. If the child had a prompt response to therapy and is entirely well at follow-up evaluation with a normal physical examination, a repeated radiograph is unnecessary.44 If the child has undergone a complicated disease course (e.g., pleural effusion), has residual symptoms, or manifests any abnormality on examination or if the illness is not the child’s first episode of pneumonia, a chest radiograph should be obtained to ensure complete resolution of the disease. The follow-up radiograph should be performed 6 to 8 weeks after diagnosis.
Other Respiratory Emergencies
Perspective
CF is an autosomal recessive disease caused by a mutation in the CF transmembrane conductance regulator (CTFR) gene. In whites, approximately 1 in 25 is a carrier, and the disease has an incidence of 1 in 2500 births.44 The disease also is present (in decreasing incidence) in Hispanics, Native Americans, African Americans, and Asians. Progressive lung disease and infection account for most of the morbidity and nearly all of the mortality in CF. Defects in chloride transport across the airway epithelium result in reduced ciliary clearance of thickened mucus, decreased antimicrobial effect of the airway surface, increased bacterial adherence, and innate secretion of inflammatory cytokines. All of these factors result in a unique sensitivity to bacterial infection of the airway.45,46
Diagnostic Strategies
Chest radiographic findings of CF include emphysema, peribronchial thickening, bronchiectasis, and focal infiltration, which may be linear or nodular in nature (Fig. 170-5). Crucial to the effective treatment of pulmonary infections in CF patients is the identification of pathogens involved, usually through sputum culture. Early childhood pneumonias in patients with CF are predominantly caused by S. aureus and H. influenzae.
Management
With the emergence of methicillin-resistant S. aureus, careful attention should be paid to antibiotic coverage. Patients receiving antistaphylococcal prophylaxis may be at increased risk for pseudomonal infections.44 There is emerging evidence that CF patients also are at increased risk for hospitalization with nonbacterial pathogens.47
By 18 years of age, 80% of patients are colonized with P. aeruginosa. When colonization is established, it is generally considered permanent.48 Acute infective exacerbations generally are managed by oral and intravenous antimicrobial drugs, typically a penicillin (e.g., ticarcillin or piperacillin) or ceftazidime combined with an aminoglycoside for purposes of synergy.5 If a patient’s previous sputum culture result is available, care should be taken to ensure coverage of the last known bacterial pathogens. Resistant strains may benefit from imipenem or meropenem. Patients routinely are hospitalized for 10 to 14 days for the course of therapy.44
Burkholderia cepacia, a significant pathogen in CF patients, has been associated with an accelerated decline in clinical status and increased mortality. In general, antimicrobial coverage is similar to that for Pseudomonas. Resistance is common, however, and the existence of differing colonization and resistance patterns of patients with CF necessitates respiratory isolation from other susceptible individuals.49
Clearance of thick mucoid secretions is vital for treatment. Patients may respond favorably to bronchodilator therapy and to mucolytics, such as inhaled N-acetylcysteine, in the acute setting.50 Chest physiotherapy often is provided by a high-frequency oscillator device. A flutter valve or a positive expiratory pressure mask also may be of assistance for improved mucoid clearance. Short-term control of inflammation may be obtained by inhaled corticosteroids.51
Chronic Lung Disease
Chronic lung disease (CLD) of infancy, also called bronchopulmonary dysplasia, is extremely common in premature infants and affects 40% of children with a birth weight of less than 1000 g.52 The severity of disease is related to several factors, including degree of prematurity, use of peripartum steroids, damage incurred by ventilation in the neonatal period, and nutritional status. Infants with CLD have greatly increased rates of hospitalization because of respiratory illness in the first year of life, approaching 65% in infants born weighing less than 1000 g.53
Immunizations are vital to the prevention of pneumonia in patients with CLD. All infants 6 to 23 months old should receive the influenza vaccine during the appropriate season. The heptavalent pneumococcal vaccine and H. influenzae type b vaccine are especially vital for prevention of bacterial pneumonia.54 In addition, monthly prophylaxis against RSV is administered to carefully selected patients with the monoclonal immunoglobulin palivizumab, which reduces the incidence of RSV disease and risk of subsequent hospitalization.12,55
Patients with CLD have increased airway resistance, decreased lung compliance, and obstructive lung disease. Pneumonia in patients with CLD may be complicated by a reactive airway component. If complicated by pneumonia, radiographs show marked hyperinflation and infiltrates (Fig. 170-6). Inhaled bronchodilators may be efficacious, although these medications may worsen air exchange in patients with concomitant airway malacia. Hypoxia and hypercarbia are common, despite an increased respiratory effort. Patients with severe CLD may be receiving long-term diuretic therapy to improve lung mechanics; care should be taken not to confuse pneumonia with cor pulmonale, which is especially prevalent in younger infants with chronic supplemental oxygen requirements.52
References
1. Pelton, SI, Hammerschlag, MR. Overcoming current obstacles in the management of bacterial community-acquired pneumonia in ambulatory children. Clin Pediatr (Phila). 2005;44:1.
2. Juven, T, et al. Etiology of community acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;10:293.
3. Korppi, M. Community-acquired pneumonia in children: Issues in optimizing antibacterial treatment. Paediatr Drugs. 2003;12:821.
4. Yaari, E, et al. Clinical manifestations of Bordetella pertussis infection in immunized children and young adults. Chest. 1999;115:1254.
5. Mink, CA, et al. Outbreak of pertussis in a fully immunized adolescent and adult population. Arch Pediatr Adolesc Med. 1994;148:153.
6. Principi, N, Esposito, S. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis. 2001;1:334.
7. Principi, N, et al. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001;32:1281.
8. Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: Use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. MMWR Recomm Rep. 2006;55:17.
9. Hinman, AR, Orenstein, WA, Schuchat, A, Centers for Disease Control and Prevention. Vaccine-preventable diseases, immunizations, and MMWR—1961-2011. MMWR Surveill Summ. 2011;60(Suppl 4):49–57.
10. Overturf, GD. American Academy of Pediatrics Committee on Infectious Disease: Technical report: Prevention of pneumococcal infections, including the use of pneumococcal conjugate and polysaccharide vaccines and antibiotic prophylaxis. Pediatrics. 2000;106:367.
11. Dagan, R, et al. Effect of a noncovalent conjugate vaccine on carriage of antibiotic-resistant Streptococcus pneumoniae in day-care centers. Pediatr Infect Dis. 2003;22:532.
12. Madhi, SA, Klugman, KP, The Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813.
13. Bradley, JS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76.
14. Mani, CS, Murray, DL. Acute pneumonia and its complications. In Long SS, Pickering LK, Prober CG, eds.: Principles and Practice of Pediatric Infectious Diseases, 3rd ed, New York: Churchill Livingstone, 2008.
15. The WHO Young Infants Study Group. Serious infections in young infants in developing countries: Rationale for a multicenter study. Pediatr Infect Dis J. 1999;18(Suppl):4.
16. Jain, A, Ben-Ami, T, Daum, RS. Staphylococcal infections in children: Part 2. Pediatr Rev. 1999;20:219.
17. Marcinak, J, Frank, A. Treatment of community-acquired methicillin-resistant Staphylococcus aureus in children. Curr Opin Infect Dis. 2003;16:265.
18. Melville, CAS, Flaurenson, JF, Augrow, JA. Invasive disease due to Haemophilus influenzae infection [letter]. BMJ. 1994;309:58.
19. Muller, M, et al. Clinical and epidemiologic features of group A streptococcal pneumonia in Ontario, Canada. Arch Intern Med. 2003;163:467.
20. O’Brien, K, Beall, B, Barrett, N. Epidemiology of invasive group A streptococcus disease in the United States, 1995-1999. Clin Infect Dis. 2002;35:268.
21. Todd, JK. Pneumonia. In: Behrman RE, ed. Nelson Textbook of Pediatrics. 16th ed. Philadelphia: WB Saunders; 2000:1432.
22. Rudan, I, Boschi-Pinto, C, Biloglav, Z, Mulholland, K, Campbell, H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408.
23. Gomez-Barreto, D, et al. Clinical outcome of invasive infections in children caused by highly penicillin-resistant Streptococcus pneumoniae compared with infections caused by penicillin-susceptible strains. Arch Med Res. 2000;31:592.
24. Reference deleted in proofs.
25. Cimolai, N. Mycoplasma pneumoniae respiratory infection. Pediatr Rev. 1998;19:327.
26. Waris, ME, et al. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J Clin Biol. 1998;36:3155.
27. Hagiwara, K, et al. An epidemic of pertussis-like illness caused by Chlamydia pneumoniae. Pediatr Infect Dis J. 1999;18:271.
28. Vitek, C, et al. Increase in deaths from pertussis among young infants in the United States in the 1990’s. Pediatr Infect Dis J. 2003;22:628.
29. Bachur, R, Perry, H, Harper, MB. Occult pneumonias: Empiric chest radiographs in febrile children with leukocytosis. Ann Emerg Med. 1999;33:166.
30. Lin, CJ, et al. Radiographic, clinical, and prognostic features of complicated and uncomplicated community-acquired lobar pneumonia in children. J Microbiol Immunol Infect. 2006;39:489.
31. Shah, SS, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30:6.
32. Bradley, J. Management of community-acquired pediatric pneumonia in an era of increasing antibiotic resistance and conjugate vaccines. Pediatr Infect Dis J. 2002;21:592.
33. McCracken, G. Diagnosis and management of pneumonia in children. Pediatr Infect Dis J. 2000;19:924.
34. Hodina, M, et al. Imaging of cavitary necrosis in complicated childhood pneumonia. Eur Radiol. 2002;12:391.
35. Givan, DC, Eigen, H. Disease of the pleura: Common pleural effusions in children. Clin Chest Med. 1998;19:363.
36. Starke, JR. Pediatric tuberculosis: Time for a new approach. Tuberculosis. 2003;83:208.
37. Virkki, R, et al. Differentiation of bacterial and viral pneumonia in children. Thorax. 2002;57:438.
38. Swingler, GH, Hussey, GD, Zwarenstein, M. Randomised controlled trial of clinical outcome after chest radiograph in ambulatory acute lower-respiratory infection in children. Lancet. 1998;351:404.
39. Swingler, GH, Zwarenstein, M. Chest radiograph in acute respiratory infections. Cochrane Database Syst Rev. (1):2008.
40. Byington, C, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: Risk factors and microbiological associations. Clin Infect Dis. 2002;34:434–440.
41. Wubbel, L, et al. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J. 1998;18:98.
42. Kaplan, S, et al. Outcome of invasive infections outside the central nervous system caused by Streptococcus pneumoniae isolates nonsusceptible to ceftriaxone in children treated with beta-lactam antibiotics. Pediatr Infect Dis J. 2001;20:392.
43. American Academy of Pediatrics Committee on Infectious Diseases. Therapy for children with invasive pneumococcal infections. Pediatrics. 1997;99:289.
44. Brennan, AL, Geddes, DM. Cystic fibrosis. Curr Opin Infect Dis. 2002;15:175.
45. Conese, M, Assael, BM. Bacterial infections and inflammation in the lungs of cystic fibrosis patients. Pediatr Infect Dis J. 2001;20:207.
46. Hanutcu, R, Woo, MS. Advanced cystic fibrosis lung disease in children. Curr Opin Pulm Med. 2001;7:448.
47. Meissner, HC. Selected populations at increased risk from respiratory syncytial virus infection. Pediatr Infect Dis J. 2003;22(Suppl):40.
48. Rajan, S, Saiman, L. Pulmonary infections in patients with cystic fibrosis. Semin Respir Infect. 2002;17:47.
49. Garau, J, Gomez, L. Pseudomonas aeruginosa pneumonia. Curr Opin Infect Dis. 2003;16:135.
50. Ramsey, BW. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335:179.
51. van der Schans, C, et al. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis (Cochrane Review). In: The Cochrane Library, Issue 1. Chichester, UK: John Wiley & Sons; 2004.
52. Marshall, DD. Primary care follow-up of the neonatal intensive care unit graduate. Clin Fam Pract. 2003;5:243.
53. Davis, PG, et al. Evaluating “old” definitions for the “new” bronchopulmonary dysplasia. J Pediatr. 2002;140:555.
54. Saari, TN. Immunization of preterm and low birth weight infants. Pediatrics. 2003;112:193.
55. Welliver, RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143(Suppl):112.
56. Chumpa, A, Bachur, RG, Harper, MB. Bacteremia-associated pneumococcal pneumonia and the benefit of initial parenteral antimicrobial therapy. Pedia Inf Disease J. 1999;18:12.