18 Pediatric Pacing and Defibrillator Use
Permanent cardiac pacemakers have been used in children for 60 years.1 Technologic advances have increased pediatric use through customized pacemaker design and a smaller, longer-lasting generator. Although many aspects of pediatric pacing are similar to adult pacing, children are not only physically smaller than adults, but also have different underlying cardiac diseases. Life circumstances differ, and pediatric patients face longer lifetime therapy. Therefore, differences exist not only in selection of the optimal pacing system, but also in implantation techniques, programming considerations, and follow-up methods.
 Midwest Pediatric Pacemaker Registry
Midwest Pediatric Pacemaker Registry
Because the number of patients with congenital heart disease requiring pacemakers is small, a large study at one center is lacking. Therefore, conclusions are based on limited experience susceptible to statistical inaccuracies. To address this problem, the Midwest Pediatric Cardiology Society formed the Midwest Pediatric Pacemaker Registry (MPPR) in 1980. Member institutions submit data on patient demographics, pacing indication, associated structural cardiac disease, type of generator/electrode and threshold data at implantation, and device explantation data (Table 18-1). No long-term follow-up data are provided. Annual reports are presented to promote data submission and validity, address concerns about the types of data collected and the methods used, and ensure uniformity among participating institutions.
TABLE 18-1 Midwest Pediatric Pacemaker Registry: Data Collection*
| Information | Data Collected |
|---|---|
| Patient |
RMS, Mean spontaneous waveform amplitude.
* Data are collected on all new patients entered in the Registry, all generators implanted and explanted, and all electrodes implanted, explanted, or invasively tested.
 Indications for Permanent Pacemaker Implantation
Indications for Permanent Pacemaker Implantation
Sinus Node Dysfunction
Most of these patients have undergone cardiac surgery many years earlier, usually involving extensive atrial procedures. The most common procedure is the atrial switch operation for transposition of the great arteries.2 The likelihood of these patients needing permanent pacing increases with time since surgery.3 Even though the use of this procedure for simple dextrotransposition of the great arteries is now rare, its use is increasing in more complex disease, such as levotransposition of the great arteries combined with the arterial switch procedure (“double switch”) to place the morphologic right ventricle in the pulmonary circuit and the morphologic left ventricle in the systemic circuit.
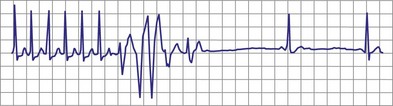
With increased use of the Fontan procedure, or right-sided heart bypass, the incidence of sinus node dysfunction is increasing. The indications are similar to those for congenital complete heart block. In addition, the presence of tachyarrhythmias, with the subsequent risk for prolonged asystole after acute termination of the tachycardia, is also an indication for pacemaker implantation (Fig. 18-1). In patients with sinus node dysfunction after cardiac surgery, our practice is to recommend pacemaker implantation in all patients with a sleeping heart rate of less than 30 beats per minute (bpm) even in the absence of symptoms, a decreasing exercise tolerance with inadequate heart rate increase with exercise (see later discussion), or sinus pauses for longer than 3 to 4 seconds. The need for medications known to affect atrioventricular (AV) conduction for the control of tachyarrhythmias in these patients would also necessitate pacemaker placement.3
Surgically Induced Heart Block
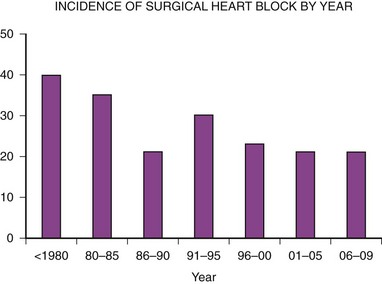
The second major indication for pacemaker implantation in children is surgically induced heart block, which classically has accounted for 30% to 40% of children undergoing pacemaker implantation.4–9 MPPR data show that the indication for initial pacemaker placement was surgically induced heart block in an average of 35% of patients before 2000 (Fig. 18-2). The percentage varies from year to year and reached a low of 20% in 2001-2005. There has been no definite downward trend since 2000, with the percentage remaining near 20%, although the underlying structural cardiac diseases in patients with surgically induced heart block have changed dramatically. Since 2000, surgical heart block has accounted for 15% to 25% of initial implantations. Most recent data from our center show that the incidence is 22% over the last 5 years. Most children acquiring surgical heart block in the last 5 years have had complex disease and have undergone complex surgical repairs. The surgical procedure resulting in the greatest incidence of heart block is the repair of atrioventricular septal defect, which has accounted for 17% of patients with surgical heart block since 1988. Table 18-2 lists the other common diagnoses associated with surgical heart block in the recent era.
TABLE 18-2 Most Prevalent Structural Cardiac Defects Associated with Surgically Induced Complete Heart Block*
| Defect | Percentage of Cases |
|---|---|
| Atrioventricular septal defects | 17 |
| Isolated ventricular septal defect | 14 |
| Dextrotransposition of the great arteries | 12 |
| Levotransposition of the great arteries | 12 |
| Tetralogy of Fallot | 7 |
| Aortic valve replacement | 3 |
* The most common structural cardiac lesions associated with surgically induced heart block at the time of complete repair for children undergoing initial implantation since 1988.
Data from Midwest Pediatric Pacemaker Registry.
Surgical heart block can develop at the initial cardiac repair or later. In addition, the heart block acquired at repair may be temporary, with return of reliable AV conduction. For this reason, our current practice is to implant only temporary pacing electrodes at the initial surgery and to defer permanent pacemaker implantation for 10 to 14 days in the hope of a return of AV conduction. However, ventricular escape rhythms are unstable, and no child with a permanent, surgically acquired complete heart block is discharged without a permanent pacemaker. Even in the hospital, all children are supported with an external pacemaker through temporary pacing wires placed at surgery until consistent AV conduction returns or a permanent pacemaker is inserted. Monitoring should consist of both electrocardiographic (ECG) monitoring and non-ECG monitoring, such as arterial pressure measurements or pulse oximetry. Many ECG monitors detect the pacing artifact and do not recognize the lack of capture with subsequent bradycardia or asystole. This is avoided by the use of a non-ECG method of detecting cardiac ejection, such as pulse oximetry.10
Congenital Complete Heart Block
The next most common indication for pacemaker implantation is congenital complete heart block. The cause of congenital heart block varies; an autoimmune mechanism is often implicated, with clinical or laboratory evidence of connective tissue disease in the mother.11 All mothers of infants with congenital heart block should have antibody determinations performed. Even children born to such mothers but with intact conduction at birth must be monitored for the development of heart block over time. Treatment of antibody-positive mothers with steroids during pregnancy has not been shown to alter outcomes or reverse the development of heart block. Congenital heart block is also associated with specific forms of structural disease, particularly those involving abnormalities of the AV junction, such as levotransposition of the great arteries with AV discordance and atrial situs ambiguus.12 It is common for fetal heart block to “develop” in utero with intact conduction present in the young fetus and heart block developing at 20 to 30 weeks of gestation.
Data from the MPPR indicate that 10% to 25% of patients have congenital heart block as the primary indication for permanent pacing. Most recent data from our center show that 17% of patients receiving pacemakers have congenital heart block. The age at which the pacing system is implanted varies, ranging from a few hours to more than 20 years. Most children with associated structural cardiac disease who need pacing before 1 year of age have congestive heart failure (CHF) requiring an increased heart rate for adequate therapy. The mortality rate in such children is also high, with 43% dying by 2 years of age.13
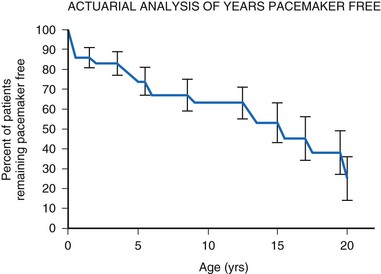
For children with structurally normal hearts and congenital heart block, the incidence of pacemaker implantation is lower in younger children but increases with age, associated with a gradually decreasing ventricular rate.14 The gradual and steady increase in the need for permanent pacing continues with advancing age, reaching 75% by age 20 years (Fig. 18-3). The need for permanent pacing results from the development of syncope, CHF, or increasing ventricular ectopy, often associated with prolongation of the corrected QT interval (QTc). Death is rare in children with no structural cardiac disease (only 5% by age 20) but can occur suddenly.
Current recommendations call for the implantation of a permanent pacemaker system whenever CHF is present. In addition, implantation is recommended if the average heart rate is less than 50 bpm in the awake child and 55 bpm in the infant, if there is a history of a syncopal or presyncopal event, if significant ventricular ectopy is present, or if there is exercise intolerance.15,16 However, symptoms of exercise intolerance can be difficult to elicit. Many children deny such symptoms, as do their parents, when in fact their exercise tolerance would be improved with permanent pacing. Many parents return after pacemaker implantation to relate that the activity level of their child has greatly increased. They are amazed at this change, because they did not believe that the child was significantly hindered before pacemaker implantation. Exercise testing is often useful as an indicator of the child’s exercise capabilities compared with those of a normal child. The physician should also periodically assess the child for increasing cardiac size by chest radiography and for decreasing cardiac function by echocardiography. The presence of either of these conditions should be considered an indication for permanent pacemaker placement.
Some children with congenital complete heart block develop a tachydysrhythmia, specifically ventricular tachycardia (VT), which can be controlled only with permanent pacing.17 The maintenance of a minimal heart rate often suppresses the tendency toward ventricular ectopy, particularly during exercise. The development of tachyarrhythmias with the stress of exercise, even in children with otherwise asymptomatic disease, necessitates pacemaker implantation.
Other Indications
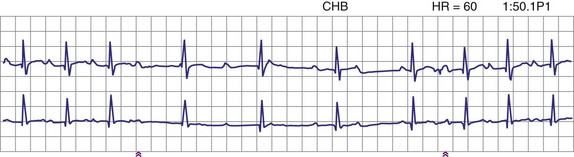
Patients with long QT syndrome and uncontrollable VT may also benefit from pacemaker placement, as well as those with intermittent complete heart block18 (Fig. 18-4). A chronic increase in heart rate shortens the QT interval and decreases the occurrence of VT. The combined use of pacing and an ICD may be even more efficacious, particularly with the advent of the dual-chamber ICD.
Other indications for pacemaker placement reported in the MPPR include the need for control of atrial tachyarrhythmias unresponsive to pharmacologic therapy, second-degree heart block associated with symptoms, and concern about a sudden loss of AV conduction in patients receiving certain antiarrhythmic therapies known to interfere with AV conduction. Although such indications are rare, the clinician should not restrict pacemaker use to those children with complete heart block. First-degree heart block and trifascicular block with no documented loss of AV conduction are not considered indications for pacemaker implantation.19
A relatively controversial indication for pacemaker placement is symptomatic hypertrophic obstructive cardiomyopathy with significant outflow tract obstruction. Although pacemaker placement is not effective in all children with this disorder, both hemodynamic and symptomatic improvements have been observed,20 with decreases in gradient and measures of diastolic performance. Generators used for this indication must allow programming of relatively short AV intervals and rate-adaptive AV intervals to maximize the QRS width and degree of preexcitation. Younger patients with more rapid heart rates may present insurmountable difficulties, and other therapies are probably indicated initially. When pacing is employed in this setting a dual-chamber ICD should be used (see later).
 Selection of the Appropriate Pacemaker System
Selection of the Appropriate Pacemaker System
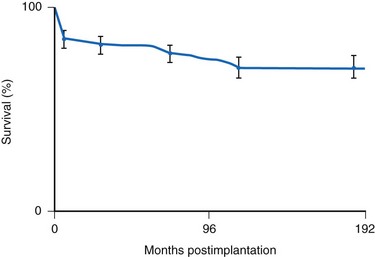
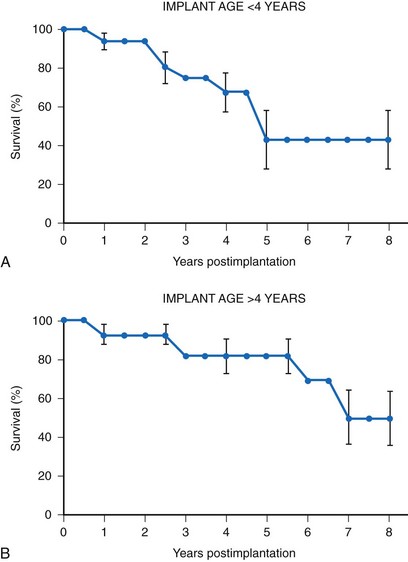
Many factors must be considered in the selection of the most appropriate pacemaker generator and electrode system. Unlike that in the adult patient, the 5-year patient survival rate after pacemaker implantation in children exceeds 70% (Fig. 18-5), and death is usually related to the underlying structural heart defect.8,21 Therefore, pacing may be needed for more than 50 years in the average child. This affects pacing choices, because the number of replacement generators and electrodes may be high. The average longevity of currently available pulse generators is only 5 years when all children are grouped together (Fig. 18-6). However, when children are divided into those younger and those older than 4 years at generator implantation, longevity is much different (Fig. 18-7). The generator half-life is 5 years for children younger than 4 at implantation and increases to 7 years for the older children. This is presumably a result of the higher heart rates present in younger patients, when the device is used in dual-chamber mode to track the atrial rate, and the higher programmed lower rates used in younger children. Initially, epicardial electrodes used in the younger children contributed to higher current drain. With newer epicardial electrodes, this difference has largely disappeared.
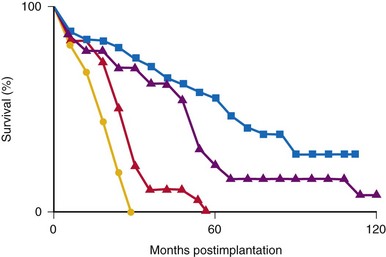
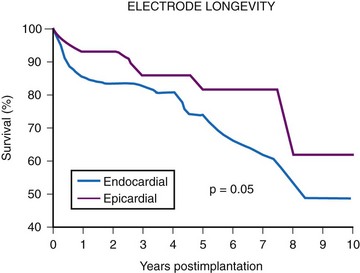
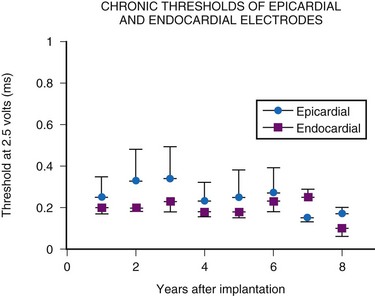
The average epicardial electrode lasts 7 years.22 With improvements in epicardial electrode design, it is hoped that this will increase. Although the average endocardial electrode’s longevity in children is significantly increased, it is still only slightly more than 10 years23 (Fig. 18-8). For the child undergoing an initial implantation at age 1 year, a minimum of nine electrode changes and 17 generator changes can be expected. The multiple procedures that will be needed and the effects of one on subsequent procedures must be considered.
Generator Mode Selection
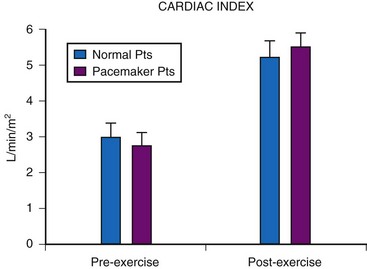
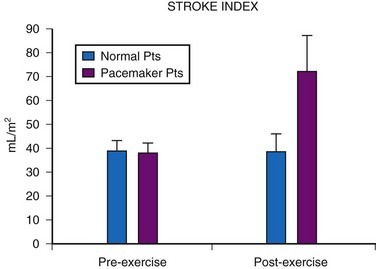
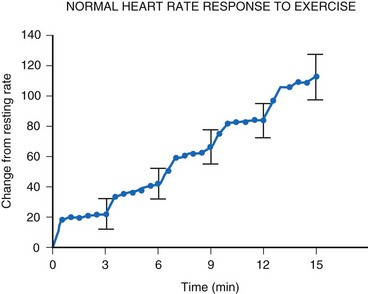
Although cardiac output increases with exercise, even during fixed-rate pacing (Fig. 18-9), this results from a large increase in stroke volume (Fig. 18-10), with presumed increased wall stress and potentially increased myocardial work compared with the same change in cardiac output, when a heart rate increase is possible.24 However, enhanced exercise tolerance is achieved when rate-variable pacing is used.25 This suggests an advantage to rate-variable pacing in the child who is expected to lead an active life. The rate-responsive mode should always be used unless the patient can demonstrate an adequate intrinsic rate response to exercise by exercise testing or ambulatory electrocardiogram.
Single-chamber pacing in either the atrium or the ventricle has been advocated for the treatment of sick sinus syndrome.15 When atrial pacing is chosen, the presence of normal AV node function must be established by provocative electrophysiologic testing before implantation, especially in the postsurgical patient, because AV nodal disease can accompany SA nodal disease and may not be apparent in the resting, nonprovoked state. AAI(R) pacing has the advantage of preserving the normal ventricular activation sequence with potentially better cardiovascular function. In addition, some evidence in animals points toward the long-term development of myocardial changes when an abnormal pattern of myocardial activation is present.26 A comparison of cardiac myocyte changes in ventricular free wall pacing versus high septal pacing near the bundle of His, which has a narrower QRS morphology, is striking; the clinical implication of these changes is unknown.27
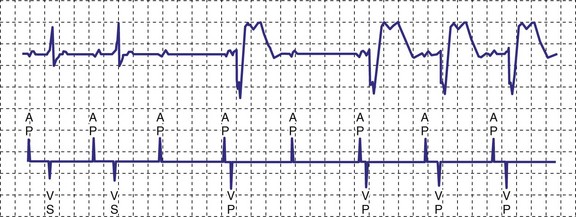
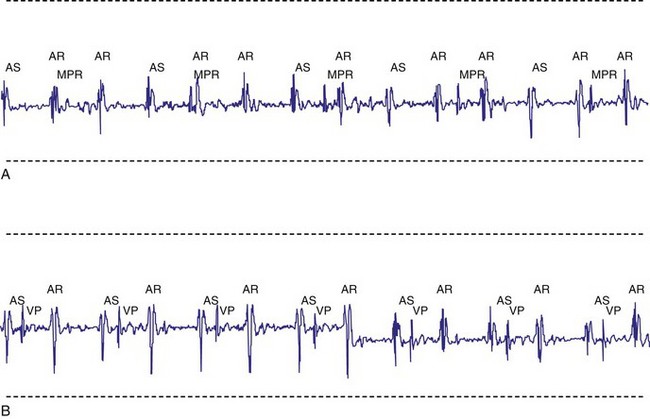
A newer pacing mode, managed ventricular pacing (MVP; Medtronic) has great utility in a patient with predominantly sinus node dysfunction but also with intermittent lack of AV conduction.28 In this mode, pacing is AAIR, but the device monitors for ventricular activity between two consecutive P waves without regard to timing. If two consecutive P waves occur without an intervening R wave, a ventricular pace is generated. If this occurs twice within four P waves, the device shifts mode to DDD(R). After a predetermined time, the ventricular output is inhibited, and the device searches for an R wave between two P waves. If one occurs, the mode reverts to AAIR; if not, DDDR mode remains in force (Fig. 18-11).
Single-chamber ventricular pacing, or VVI(R), allows the use of more stable electrode systems and, in the rate-responsive mode, still allows rate variability to be maintained in the ambulatory child. The importance of atrial systole in the maintenance of cardiac output is debatable and varies from child to child. Because most children have good myocardial function, the atrial contribution is probably minimal. The cardiac output increase with exercise is improved in children with DDD versus VVI pacing. It is unclear, however, whether this increase is the result of atrial synchrony or rate variability. Pacemaker syndrome from VVIR pacing is uncommon but can occur, especially over time. In one series, 19 of 33 patients developed symptoms suggestive of pacemaker syndrome over time (median, 11 years) that resolved with upgrading to dual-chamber pacing.29 The major factor to be considered in such a choice is the difficulty in placing an adequate atrial electrode. If prior surgery or underlying structural disease precludes atrial electrode placement, single-chamber pacing is an acceptable alternative. However, dual-chamber pacing should be considered for all patients, with single-chamber pacing used only if contraindications to dual-chamber pacing exist, as discussed later. Even in patients with sinus node dysfunction, dual-chamber pacing should be considered, particularly if AV nodal function is suspect.
We now consider dual-chamber pacing to be the mode of choice in children. We use single-chamber pacing only if a contraindication to dual-chamber pacing exists, as well as in some small infants who will need cardiac surgery in the immediate future, with an upgrade to dual-chamber pacing performed at that time. The major contraindications to dual-chamber pacing are (1) persistent atrial tachyarrhythmias; (2) changing AV nodal status, making numerous programming changes necessary; and (3) inability to place reliable atrial and ventricular electrodes. An example of the third contraindication is the small child in whom endocardial pacing is preferred, but the presence of two electrodes in the superior vena cava might present a high risk for thrombosis. Another example is the child requiring epicardial electrode placement in whom atrial electrode placement would necessitate a greatly enhanced surgical procedure. One must remember that rate sensors do not function in nonambulatory infants. Therefore, in young infants, single-chamber pacing becomes fixed-rate pacing. The generator’s size and functionality are no longer considerations, because dual- and single-chamber pacemakers are comparable in both regards. Table 18-3 lists the most common contraindications to dual-chamber pacing.
TABLE 18-3 Major Contraindications to Dual-Chamber Pacing in Children
| Contraindication | Causes |
|---|---|
| Inability to place both an atrial and a ventricular electrode |
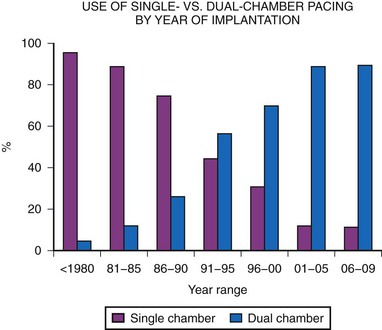
Dual-chamber pacing in children has previously been underused. MPPR data show a significant increase in dual-chamber pacing, with 43% of generators implanted in 1991 to 1992 using the DDD or DDDR mode, increasing to a majority (68%) of implantations by 1995 to 1996, and with a further increase, to more than 80%, since 2000. This is in marked contrast to the years before 1985, when fewer than 10% of generators were in the DDD mode (Fig. 18-12). This increase in dual-chamber pacing has been the result of improvements in atrial epicardial electrodes, increased experience with endocardial pacing in children, the smaller size of dual-chamber generators, and a better understanding of the benefits of dual-chamber pacing.
Generator Features
General Characteristics
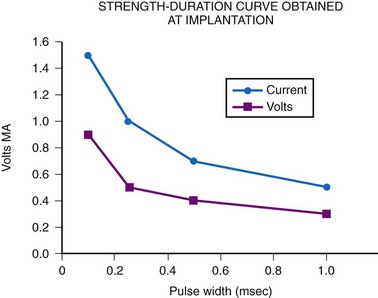
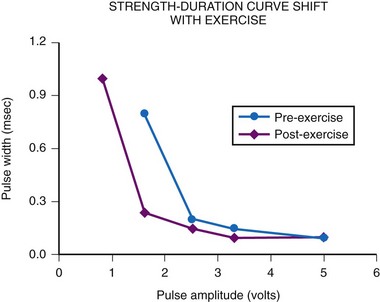
The most important consideration is related to the range of energy output available, which includes both the pulse width and the pulse amplitude programmability. Although most pacemakers are programmed to have 2.5 or 5 V of amplitude, the presence of other amplitudes is of key importance. Specifically, when a generator is used with an epicardial electrode, high-output features are mandatory. Although few children require long-term pacing at output greater than 5 V, many have an initial threshold rise and temporarily require such high output. Even with endocardial implants, acute increases in threshold can occur, and the ability to increase the pacemaker amplitude to values greater than 5 V may avert the necessity for emergency electrode replacement. In addition, threshold testing at multiple low-pulse amplitudes allows a more accurate determination of the characteristics of the strength-duration curve. This testing is mandatory to determine the lowest, but still safe, pulse amplitude and width settings. The strength-duration curve characteristics are not constant, varying not only with time but also in relation to activity and time of day.30 Such changes are discussed more fully in the consideration of appropriate follow-up. Knowing where the steep part of the strength-duration curve begins is crucial for appropriate programming; the clinician wants to ensure an adequate safety margin while minimizing the energy output, to maximize generator longevity. The ability to determine thresholds at a multitude of pulse amplitudes is a necessity.
Newer pacemakers now can automatically determine the voltage threshold, either on a beat-to-beat basis or at predetermined intervals throughout the day, and then adjust the pulse amplitude within a predetermined range to minimize energy drain and potentially increase generator longevity. This is accomplished by looking for an evoked potential after the test pulse, within a predetermined window of time indicating myocardial depolarization. Initially, these pacemakers required special low-polarization electrodes to distinguish true evoked potentials from electrode polarization. Newer designs have improved this discrimination, and now this feature has been expanded to function with most types of electrodes. Some devices still require bipolar electrode systems; some do not. Both endocardial electrodes31 and epicardial electrodes32,33 have been employed with newer devices. Once the voltage threshold has been determined, the amplitude is adjusted to a predetermined value above the threshold. Threshold data are saved in the pacemaker, and later interrogation can provide the clinician with the threshold trend over time. Such a feature has been extended to the atrium as well as the ventricle in some devices. Even if the clinician is reticent to allow automatic changes to the output parameters, use of the feature in a “monitor only” mode can provide information as to the long-term changes in threshold and the potential need for programming changes. This feature has the potential to increase generator longevity and decrease the number of pacemaker replacements needed.
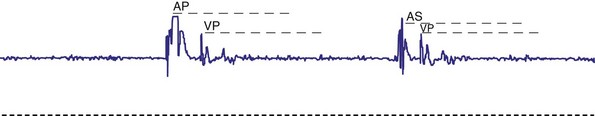
Another parameter often overlooked is the refractory period. In single-chamber pacing, this is often fixed to an arbitrary value of 325 msec, without much thought about whether this value is appropriate. For the ventricular channel, the refractory period must be of sufficient duration to prevent inappropriate T-wave sensing but not prevent sensing of spontaneous ventricular depolarizations. Measurement of the pace or sensing point to well beyond the T wave using the intracardiac electrogram (EGM) is straightforward (Fig. 18-13). In healthy children, the QT interval decreases with an increasing heart rate. When rate-variable pacing is used, the ventricular refractory period may be appropriate at rest but too long during exercise. Ideally, the period should vary with the pacing rate. Therefore, this value must be long enough to prevent T-wave sensing at the resting heart rate and short enough not to limit appropriate sensing at the upper pacing rate. Children with complete heart block may have spontaneous ventricular beats during the stress of exercise, which must be appropriately sensed. Appropriate programming is discussed later. Again, the wider the range of available refractory periods, the more universally applicable the pacemaker generator is to the entire pediatric population.
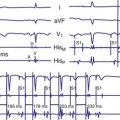
For AAIR pacing, the refractory period must be long enough to prevent sensing of ventricular events, but again, must not be too limiting in terms of the upper rate. Recording of the intracardiac EGM shows the extent to which ventricular events are sensed by the pacemaker and the minimum value to which the atrial refractory period may be safely programmed (Fig. 18-14).
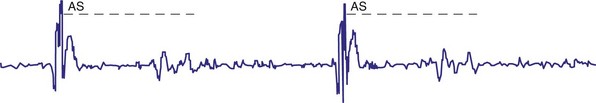
Figure 18-14 Intracardiac atrial electrogram recorded from a patient with AAI pacing. The refractory period (“dashed” line) must be long enough to prevent sensing of ventricular events by the atrial sensing (AS) electrode. Note the marked increase in refractory period required compared with VVI pacing (see Fig. 18-13).
Rate-Responsive Pacing
For the single-chamber rate-responsive pacemaker, appropriate settings to mimic the pediatric response to exercise are mandatory. During exercise, the healthy child’s heart rate increases linearly with the increasing intensity of the exercise34 (Fig. 18-15). When healthy children are exercised using the Bruce treadmill protocol, the heart rate increases an average of 20 bpm with each increase in exercise stage. This continues throughout the course of the exercise. After an abrupt increase in exercise intensity (from stage I to II), the heart rate shows a sudden rapid increase, reaching a plateau value. The child’s pacemaker should increase its rate in an appropriate manner with increasing exercise intensity—and this must occur quickly, reaching a plateau value rapidly for that level of intensity. Therefore, not only must the heart rate increase to an appropriate degree, but also must increase in an appropriate time frame to mimic the normal physiologic response to exercise in the pediatric patient.
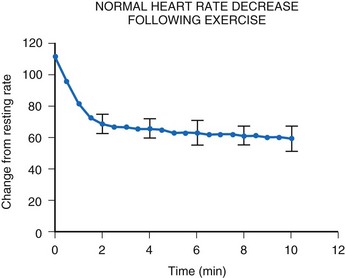
After termination of exercise, the heart rate decreases exponentially (Fig. 18-16). Although an initial rapid drop occurs, the heart rate does not reach resting levels for at least 10 minutes. Inappropriate rapid declines in heart rate after exercise termination may not meet the body’s metabolic demands and may result in inadequate cardiac output and a syncopal episode.
Numerous types of sensors have been used, the most common being activity (as a function of body vibration), blood temperature, and minute ventilation. Body vibration sensing is the most useful in children because it does not require special electrodes and is much the same in the child as in the adult. Blood temperature and minute-ventilation sensing35 have been used in children, but to a lesser degree.
Summary
Traditional factors in pediatric pacing, such as generator longevity and size, are now less important in the selection of a generator. All generators are much smaller than previous models, and yet longevity has not been sacrificed, a result of improved circuit efficiency. The difference in size among single-chamber, dual-chamber, and rate-responsive pacemakers is often undetectable. Pediatric patients generally have long life expectancy, and the ability to have a highly programmable pacemaker implanted to meet changing metabolic demands of growth, age, or patient choices is indispensable. The difference in cost between a highly programmable unit and one with fewer features is minimal, particularly when the cost is spread over the life of the pacemaker. Box 18-1 summarizes the features that must be considered for the appropriate selection of a generator. The choice of a pacemaker should be based solely on the features it possesses and its ability to meet the demands of the patient.
Pacing Electrode Selection
Endocardial versus Epicardial Pacing
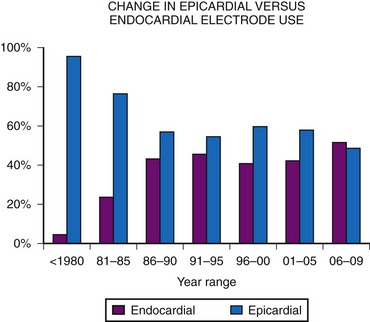
Initially, almost all electrode systems implanted in children were epicardial, because of the large size of the endocardial electrodes and pacing generators. The development of smaller electrodes and generators has changed this, although children still undergo epicardial lead placement as a result of small patient size or other factors that do not allow placement of an endocardial electrode system. MPPR data show a gradual increase in endocardial electrode use, but almost one half of all patients still receive epicardial electrodes (Fig. 18-17). Our basic approach is to assume that all children should undergo the placement of an endocardial system; we then evaluate the child for factors that do not allow endocardial electrode use. The major factors to be considered, in addition to patient size, are (1) venous access to the ventricle, (2) presence of intracardiac right-to-left shunting, (3) increased pulmonary vascular resistance, (4) right-sided prosthetic valves, and (5) severe right ventricular (RV) dysfunction or fibrosis causing either increased risk of thrombus formation or an inability to stimulate the endocardium.
Initially, it was believed that children weighing less than 15 kg (33 lb) and those younger than 4 years should not undergo placement of endocardial electrodes.36 This was based on the belief that the subclavian vein and superior vena cava (SVC) were too small, leading to a high risk for thrombosis with vessel occlusion, and that the large size of the generators made implantation in the subclavicular area impractical. With increased experience and smaller generator and electrode sizes, however, many centers now routinely implant endocardial lead systems in children weighing less than 15 kg.9,37–39 The lower range for weight is not yet known. From a technical standpoint, children as small as 3 kg (6.6 lb) can undergo endocardial electrode placement, but the follow-up of such children is too limited to know whether this is in their best interest. A study of 39 patients weighing 2.3 to 10 kg showed that 23% had some pacing system problem related to endocardial electrode placement.39 Such problems included skin necrosis over the generator, subclavian vein thrombosis, and endocarditis on the electrode. Another 23% required electrode extraction. All except one patient had received a single-chamber device.
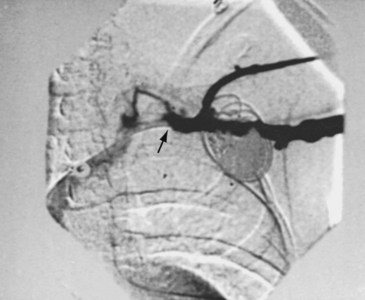
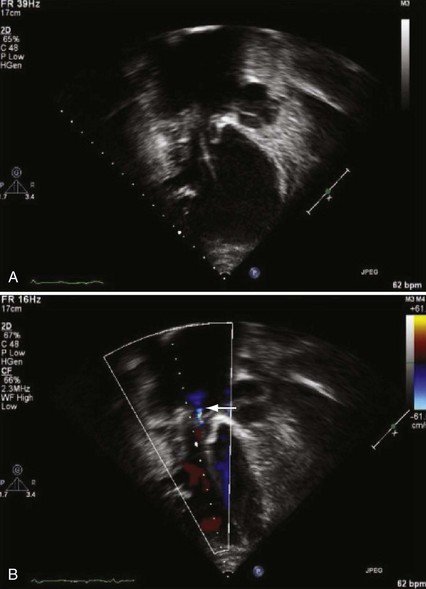
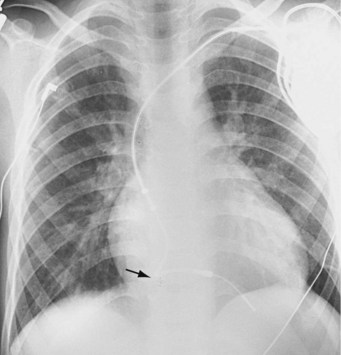
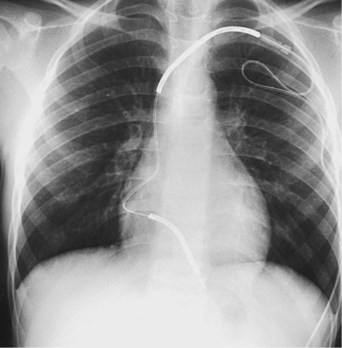
The risk for vessel thrombosis appears to be less than once believed, at least in the short term.40 Although SVC thrombosis has been reported,41,42 the true incidence is unknown, because noninvasive methods of detecting thrombosis are not sensitive and angiography is not routinely done unless thrombosis is suspected clinically (Fig. 18-18). Lead displacement secondary to growth remains a concern, although techniques have been proposed to deal with this problem.9,38 The placement of large electrode loops within the atrium was proposed to allow for growth, but these loops may not be as effective as originally believed because they can fibrose to the cardiac wall, precluding uncoiling with growth.
The major objection to endocardial electrode use in the small child is related to long-term problems. Because young children can be expected to require numerous electrodes over their lifetime, many more than in adult patients, the clinician must consider how many electrodes can be left in place before problems with vessel obstruction or tricuspid valve dysfunction occur, as well as the difficulty of extraction of old electrodes. Although now more widely used,43,44 lead extraction still represents a significant problem in children, with the potential for damage to the cardiac structures, mainly the tricuspid valve. To commit a child to potentially numerous lead extractions is still a concern. In our institution, current guidelines call for the placement of dual-chamber endocardial systems in children weighing 15 kg or more and the placement of single-chamber endocardial systems, if single-chamber pacing is appropriate, in children heavier than 8 kg (17.5 lb). These guidelines may change as electrode development continues and as data on the long-term follow-up of children with endocardially placed electrodes become available.
The next factor that must be considered is the presence of intracardiac shunting. Electrodes are potential sources of small particulate matter, with the risk for subsequent embolization until endothelialization occurs.45 This does not tend to be a problem when particulate matter goes only to the lungs, where it is filtered out of the circulation and eventually absorbed, except in the presence of preexisting elevated pulmonary vascular resistance (PVR) or after a Fontan procedure. In the presence of right-to-left shunting, however, the potential for systemic embolization is great. The general recommendation is to avoid such electrodes in patients with documented right-to-left shunting.36 This also must be considered in patients with the potential for right-to-left shunting, even if their net intracardiac shunt is left to right. Children with atrial and ventricular septal defects can show right-to-left shunting in the setting of elevated RV pressure, even with a net left-to-right shunt.46,47 The specific hemodynamic situation of the individual child must be considered before endocardial electrode implantation is performed.
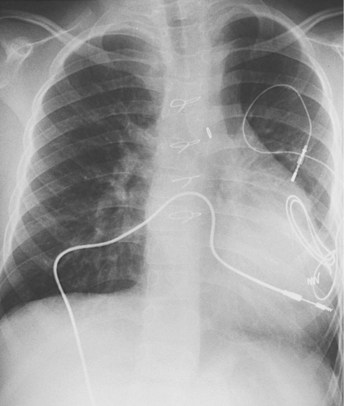
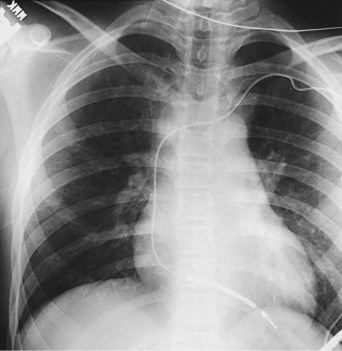
The presence of a mechanical tricuspid valve prosthesis negates the ability to use an endocardial pacing system. There have been isolated reports of endocardial electrode placements at open-heart surgery through the perivalvular area.48 This requires cardiopulmonary bypass and can be done only at valve placement. This technique cannot be used in the usual transvenous implantation; and it prevents lead extraction should that become necessary, except during repeat open-heart surgery. In cases of a heterograft valve rather than a mechanical one, the new, smaller transvenous leads have been placed without interfering with valve function (Fig. 18-19). Placement of a ventricular lead through the coronary sinus in the patient with an artifical tricuspid valve has been reported.49
The patient who has undergone a Fontan procedure presents a somewhat unique situation. As mentioned previously, controversy surrounds the best approach for electrode placement in these children. If ventricular pacing is required, the epicardial approach is the only one available. However, many of these patients have intact AV conduction, require pacing only for sinus node dysfunction, and are best served by atrial-only pacing. The original Fontan procedure connected the right atrial appendage to the pulmonary artery, leaving the entire right atrial chamber available for electrode placement. The more common techniques at present either create a tunnel along the lateral right atrial wall from the inferior vena cava to the pulmonary artery or use an extracardiac prosthetic conduit. Often, a communication exists from the system to the pulmonary atrial chamber, resulting in a right-to-left shunt. Transvenous pacing has been performed in such patients despite anticoagulation,50 but electrode placement can be difficult because of the small-system venous chamber, and right-to-left embolization with neurologic sequelae has been reported.50 For these reasons, our approach has been to avoid the transvenous approach unless the epicardial approach is not possible because of extensive fibrosis. Pacing in this group of patients can be extremely challenging regardless of the approach chosen.
In summary, endocardial pacing is generally preferable because of the ease of implantation and the improved longevity of the electrode. Long-term thresholds are as stable as epicardial electrodes and tend to be lower (Fig. 18-20). This permits lower-output programming of the pacing generator, enhancing its longevity. However, endocardial electrode use is contraindicated in many situations (Box 18-2). As such, epicardial electrodes still play a significant role in pediatric pacing.
Unipolar versus Bipolar Pacing
For endocardial pacing systems, the choice between unipolar and bipolar pacing becomes more controversial. Again, MPPR data show that most endocardial systems are bipolar (Fig. 18-21). It was initially believed that unipolar pacing was preferable because of the smaller size of the unipolar pacing electrode.51 With improved electrode design, however, this size difference has become negligible. Electrode body diameter is important in determining the risk for venous thrombosis, currently with minimal difference in size. For example, the Model 4057M unipolar screw-in electrode (Medtronic) has a body diameter of 2.2 mm and the 4058M bipolar version, 2.4 mm. Such minimal difference is also seen in passive-fixation electrodes, such as the Model 4023 unipolar steroid-eluting electrode, which has a body diameter of 1.2 mm, compared with 1.9 mm for the bipolar version (Model 4024). Newer electrodes have continued this trend toward small bipolar electrodes; the Medtronic SelectSecure has a lead body diameter of 1.4 mm (4.1 French). In all cases, diameter of the electrode head is smaller for a unipolar electrode, which may make insertion easier, although once implanted, size of the head is of little consequence.
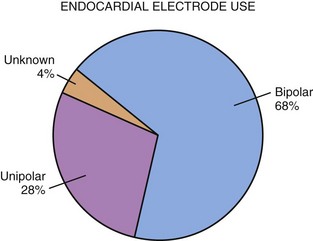
Figure 18-21 More than two thirds of endocardial electrode systems use bipolar electrodes.
(Data from Midwest Pediatric Pacemaker Registry.)
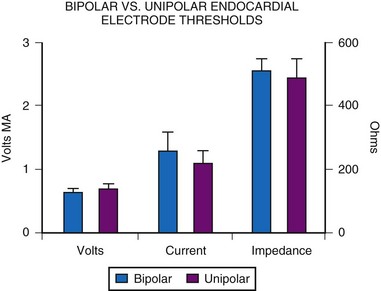
An MPPR comparison of acute implantation characteristics between unipolar and bipolar electrodes of similar design showed no significant difference for threshold values of voltage, current, or resistance (Fig. 18-22). However, some believe that long-term thresholds are improved in unipolar pacing.51 This may result from the smaller size of the head and its lower weight, which creates less tension on the endocardial surface, particularly with active-fixation electrodes, and therefore results in less tip fibrosis. However, this issue appears to affect only active-fixation leads and has not been reported with passive-fixation tined electrodes.
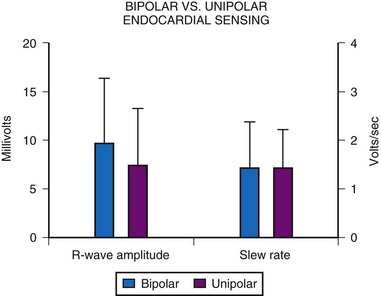
Although sensing using a bipolar electrode was initially thought to be inferior to unipolar sensing because of the proximity of the two electrodes, MPPR data show this is not the case. Acute-implantation R-wave amplitudes and slew rates show no significant difference between unipolar and bipolar electrodes (Fig. 18-23). However, unipolar sensing is more affected by myopotentials and is more prone to oversensing and inappropriate pacemaker inhibition, affecting an estimated 31% to 93% of patients, as discussed later. Bipolar sensing is rarely affected by such myopotential inhibition because of the closer proximity of the electrodes. The degree to which such inappropriate inhibition is seen depends on the location of the generator, the provocative tests used, and the generator model implanted. All series, however, report a significant incidence of this problem, which can be a particular concern in active children.
Epicardial Electrode Types
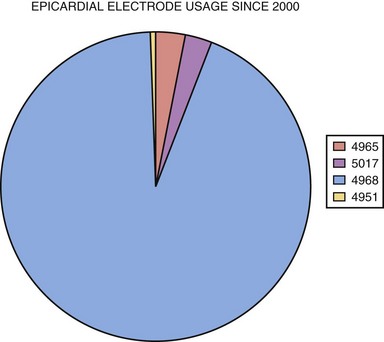
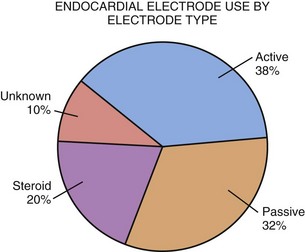
Compared with endocardial electrodes, relatively few epicardial electrode types are available. Previously, all electrodes were intramyocardial, with this portion either a corkscrew coil or a single wire with barbed end (fishhook).52–54 A true epicardial electrode with steroid-eluting capabilities is sutured to the epicardial surface and requires direct contact of the electrode with excitable myocardium55–58 (see later discussion). Based on MPPR data, since 2000 the most widely used electrode types are the bipolar true epicardial (>90% of implants), unipolar true epicardial, screw-in corkscrew, and barbed fishhook, used exclusively in the atrium (Fig. 18-24). The choice of electrode depends on the chamber to be paced and the preference of the implanting physician.
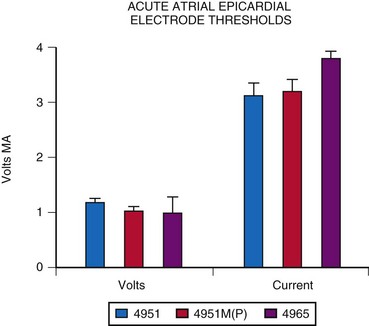
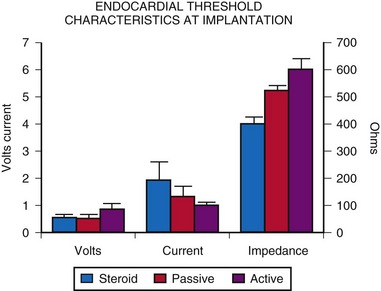
Atrial epicardial implantation requires an electrode that sits on the epicardial surface or has only shallow penetration into the atrial myocardium. Should the electrode extend through the chamber wall into the atrial cavity, a low-resistance circuit is established through the blood pool, with an inability to pace reliably. Few data can be found on the longevity or thresholds of atrial epicardial electrodes, partly because of the constant change in electrode design, making long-term comparisons difficult. The most widely used atrial electrode is the fishhook or stab-in electrode, because it does not penetrate deeply into the myocardium, compared with the screw-in or corkscrew type. The Medtronic Model 4951M(P), which has a platinized coating, has resulted in slightly improved thresholds at acute implantation (Fig. 18-25). The average threshold at implantation is about 1.05 V and 3.3 mA, measured at a pulse width of 0.5 msec. The average acute electrode impedance is 320 Ω.
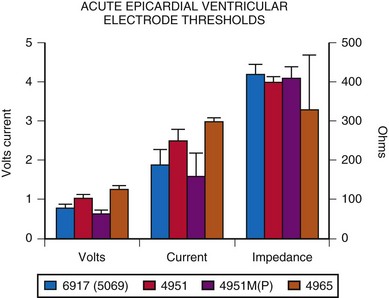
More diversity exists in the choice of the electrode for ventricular pacing. A comparison of the original nonplatinized fishhook electrode (Medtronic Model 4951) with the screw-in electrode (Model 6917 or 5069) reveals essentially no difference in acute implantation thresholds. However, implantation thresholds for the currently available platinized fishhook electrode, 4951M(P), are slightly improved (Fig. 18-26). Both steroid-eluting electrodes tend to have slightly higher thresholds. Lead survival appears to be longer for the screw-in electrode than for the fishhook type, with 5-year lead survival rates of about 84% and 65%, respectively.55 Most failures were caused by exit block. Other studies, however, have found little difference in longevity between these two types of electrodes.53 Some of this interstudy discrepancy is believed to be related to different surgical modes of implantation for the fishhook electrode. Whether or not the lead was stabilized by being sutured to the myocardium was variable. Lack of such stabilization may cause more electrode movement in the myocardium and therefore increased fibrosis and risk for exit block develpoment54 (see Implantation Techniques).
A key advance in epicardial pacing, as mentioned earlier, is the steroid-eluting electrode.55–58 This electrode consists of a platinized flat electrode that sits atop the epicardial surface, with a dexamethasone-impregnated silicone plug to allow elution of the drug onto the area of myocardium being stimulated. About 1 mg of dexamethasone is present within the electrode. Both a unipolar version (Model 4965) and a bipolar version (4968) of this electrode are available (Medtronic). This electrode is affixed to the epicardial surface by sutures, with the active portion of the electrode not extending into the myocardium as with other epicardial electrodes. This is both advantageous and disadvantageous, depending on the patient. For patients whose epicardial surfaces are relatively nonfibrotic, this appears to be an advantage, because there is less myocardial injury to provoke subsequent fibrosis. However, for patients with extremely fibrotic epicardial surfaces, as often occurs after multiple open-heart surgical procedures, these electrodes are difficult to use. In such patients, the surgeon must either find an area of myocardium with limited epicardial fibrotic reaction or strip away a fibrotic layer to expose the myocardium, which simply leads to further fibrosis.
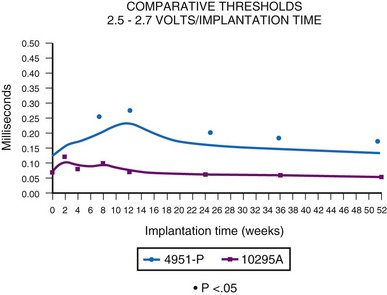
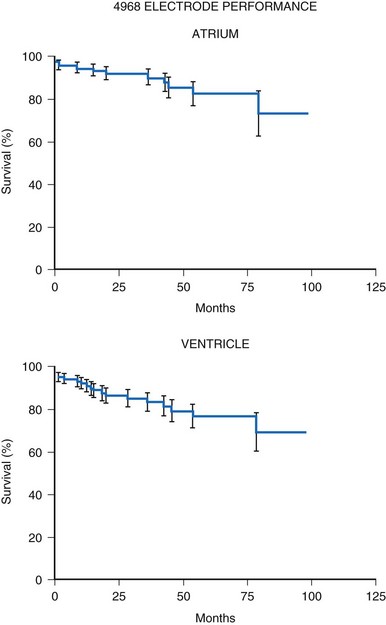
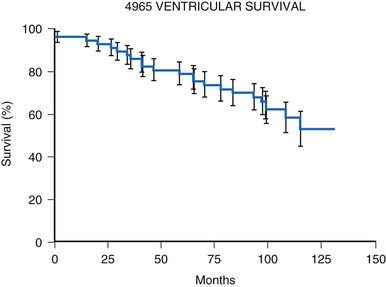
Initial experience with this steroid-eluting electrode is encouraging. Although acute thresholds appear to be comparable with those of other epicardial electrodes (see Fig. 18-26), the threshold rise is less over the first several months after implantation (Fig. 18-27).57 Midterm survival is also encouraging. For the Model 4968 lead placed on either the ventricle or the atrium, longevity is 85% at 75 months (Fig. 18-28). For the unipolar version (Model 4965), longevity is 75% at 75 months and 55% at 115 months for ventricular usage (Fig. 18-29).
Endocardial Electrode Types
As in the adult population, several general types of endocardial electrodes are in pediatric use: passive-fixation non-steroid-eluting electrodes, passive-fixation steroid-eluting electrodes, and active-fixation electrodes—both non-steroid-eluting and developed steroid-eluting models. However, the vast majority of electrodes used currently are steroid eluting. The most common endocardial electrode type in children continues to be the active-fixation screw-in electrode (Fig. 18-30). The use of this electrode has been advocated because of its better fixation qualities, given the tremendous range of anatomic variations in children with congenital heart disease. For implantation within the morphologic left ventricle in a child who has undergone an atrial switch repair for transposition of the great arteries, or in a child with ventricular inversion, the active-fixation electrode is almost universally used. Also, in a child whose right ventricle is greatly dilated, such that it is difficult to wedge the electrode into the trabecular recesses of the right ventricle, active-fixation electrodes are preferable. Alternate site RV pacing also requires an active-fixation electrode.
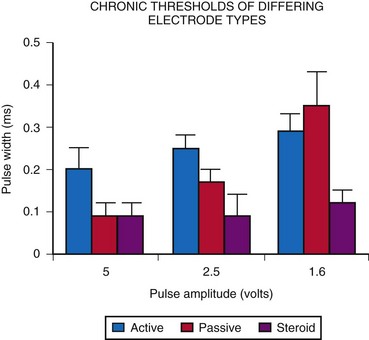
A comparison of acute threshold data shows that active-fixation and passive-fixation electrodes have similar thresholds (Fig. 18-31). Both non-steroid-eluting passive-fixation electrodes and steroid-eluting passive-fixation electrodes have lower acute thresholds and do not differ from each other. The presence of the steroid does not influence the acute implantation threshold, although with increasing electrode age, this is no longer the case. Follow-up data indicate significantly lower electrode thresholds for the steroid-eluting electrode compared with other types.58 A comparison of steroid-eluting and non-steroid-eluting electrodes showed no difference in chronic thresholds at 5-V pulse amplitude but did show differences at 2.5 and 1.6 V (Fig. 18-32). Therefore, it appears that the strength-duration curve is shifted leftward for the steroid-eluting electrodes.
Such changes affect generator programming. In one study, only 33% of generators that used non-steroid-eluting electrodes could be programmed to 2.5 V of pulse amplitude, compared with 77% of generators that used steroid-eluting electrodes.58 The remainder required a pulse amplitude of 5 V or greater. Therefore, the use of steroid-eluting passive-fixation electrodes allowed chronically lower output settings for the pulse generator, thereby increasing generator longevity. The follow-up averaged 3.3 years (median, 3.6 years). There are insufficient data to compare the steroid-eluting active-fixation electrodes with the older types.
A smaller bipolar active-fixation electrode introduced in 2005 (Medtronic Model 3830) differs in many ways from other leads. It has an isodiametric design with diameter of 4.1 French and a fixed helix. With no central lumen, the electrode must be introduced through a guiding catheter (see Implantation Techniques). This makes the lead much smaller and thus more advantageous in children. It is steroid eluting, but instead of a steroid plug behind the helix, the steroid actually coats the helix. This electrode is particularly useful in patients with venous narrowing and should decrease the incidence of postimplant venous occlusion. Early data show excellent acute thresholds and low dislodgement rates comparable to stylet-placed leads.59 The catheter delivery system makes this an excellent choice for children with complex anatomy and alternate-site pacing.
Single-lead VDD systems have been employed in children. These systems obviate the need for two electrodes yet maintain AV synchrony.60 However, a major problem is lack of atrial pacing capabilities when sinus node function is inadequate. Also, the spacing of the atrial electrodes from the electrode tip often is too great for use in children. The electrode must be buckled so that the atrial dipole is within the ventricle (Fig. 18-33), which could lead to ventricular electrode dislodgement. These problems, together with the increased complexity of the electrode construction, with a concomitant potential for increased electrode failure, combine to limit the usefulness of this approach in children, especially smaller children. Also, these are rather large leads, and with the development of smaller leads, utility of these leads is minimal.
 Implantation Techniques
Implantation Techniques
Epicardial Implantation Techniques
The subxiphoid approach requires the smallest incision. Through the same incision, both electrode implantation on the epicardial surface of the heart and pacemaker implantation in the abdomen can be accomplished.61–63 This approach has been used for both ventricular and atrial epicardial electrode implantation. The disadvantage of the subxiphoid approach is that it exposes only a limited ventricular and atrial surface, and in a patient who has extensive epicardial fibrosis from prior cardiac surgery, finding a suitable location for implantation of the electrode can be challenging. Such an approach requires a 6- to 7-cm skin incision from the xiphoid tip to a point superior to the umbilicus. The dissection is continued as deep as believed necessary to provide adequate tissue between the pacemaker and the skin surface. Depending on the depth of subcutaneous tissue, the pacemaker can be implanted above the rectus muscle, below the rectus muscle but above the peritoneum, or in some circumstances, intraperitoneally, housed in a Silastic pouch sutured to the underside of the peritoneum and anchored to the rectus fascia.62 The depth to which the incision is carried should be governed solely by the tissue available to cover the pacemaker. When only minimal tissue is present over the pacemaker, besides an unsightly bulge, the skin is more susceptible to trauma, with a resultant risk of pacemaker erosion and infection.
A left thoracotomy approach is often used in the child who has had cardiac surgery and thus is at high risk for significant epicardial fibrosis.64 This approach affords an increased myocardial surface from which to choose an appropriate pacing site. The generator can be implanted in the abdomen, in the subclavicular region, or in rare settings, intrathoracically. However, for implantation of a dual-chamber system, the left thoracotomy approach affords less exposure of the atrium and requires left atrial pacing. Although this has been accomplished, it introduces complexity into the programming, because the postpace AV interval must be prolonged sufficiently to afford adequate time for RV filling, because of the time necessary for left-to-right atrial excitation spread. The postsense AV interval must be short to avoid an excessive AV interval. For this reason, we believe that right atrial pacing is preferable, but we use left atrial pacing if right atrial pacing is not possible. When the thoracotomy approach is used with abdominal placement of the generator, the electrode must be passed subcostally to a pocket created in the abdomen through a separate incision. The electrode must not be passed over a rib and must be tunneled from the thoracic cavity to the abdomen as medially as possible, to minimize the risk for traumatic electrode fracture.
A gentle loop of the electrode should be left in the thoracic cavity to allow for some growth, but excessive loops should be avoided. Cases of entrapment of vascular structures by pacemaker electrodes have been reported.65,66 The extra length of the electrode is easily coiled and placed within the generator pocket; even this may allow for some growth, because the electrode wire slowly uncoils with growth of the patient.
The placement of unused electrodes is not recommended. It was once believed that the placement of an unused electrode would provide a “spare” electrode that could be used if the primary electrode failed, thus precluding the need for a second thoracotomy. It was found, however, that if the primary electrode failed, the redundant electrode likely was also unusable.67 Therefore, the extra electrode provided no benefit to the child.
Endocardial Implantation Techniques
Whether the electrode is introduced into the vascular system by a direct subclavian vein puncture,37,68,69 a cephalic vein cutdown procedure,9,38 or axillary vein puncture should be guided by the experience of the implanting physician. In our institution, direct vein puncture is used exclusively. Our approach is to enter the subclavian or axillary vein percutaneously and introduce a guide wire. An incision is then made laterally either along the deltopectoral groove or in the axilla,70 if the axillary vein approach is chosen. For the more common subclavian vein approach, the dissection is then carried down to the pectoral muscle. At this point, the depth of subcutaneous tissue is examined to determine whether tissue is sufficient to cover the generator. In many children, this tissue is inadequate, and placement of the generator above the pectoral muscle results in a significant pacemaker bulge as well as an increased risk for pacemaker erosion and trauma to the tissue covering the pacemaker.69 This is also psychologically important, because many children are self-conscious about a prominent bulge. If the tissue is believed to be inadequate, the dissection is carried through the pectoral, using blunt dissection to separate the muscle fibers, and a pocket is created in the subpectoral region.
For the axillary approach, the pocket is always created under the pectoral muscle.71 After a pocket adequate to accommodate the pacemaker is created and the guidewire is incorporated into the pocket, a sheath and dilator are introduced into the subclavian vein over the previously positioned guidewire. The electrode is then passed into the right atrium, together with a new guidewire. The sheath is then removed. Retention of the guidewire allows for the introduction of a second electrode, in the case of a dual-chamber implant, or the option to remove the prior electrode and replace it without having to repuncture the subclavian vein. Some prefer to perform two separate vein punctures for introduction of two guidewires. This has the advantage of separating the entry sites of the electrodes such that manipulation of one is less likely to affect the other. Also, having two smaller holes in the vein rather than one larger hole may decrease bleeding. After the electrodes have been positioned and tested, any retained guidewire can be removed. The electrodes are then connected to the generator and placed in the pocket.
Another implantation approach is a hybrid of the epicardial and endocardial methods previously described. In some patients, transvenous implantation would be preferable but there is no venous access; the electrode can be inserted into the atrium through an incision in the atrial wall, then advanced into the ventricle across the AV valve.70 The atrial incision is closed with a purse-string suture after the electrode has been positioned in the ventricle. This provides the advantages of endocardial pacing with improved thresholds when venous access to the ventricle is not available. We have employed this approach in children with unacceptable epicardial electrode thresholds but with no venous access (Fig. 18-34). This can be especially useful after a right-sided heart bypass (Fontan) procedure, even though anticoagulation is necessary because of implantation within the systemic ventricle.45
The optimal site within the right ventricle for electrode placement has been questioned. Initially, the RV apex was used because it affords a stable site. However, the alteration of excitation sequence may lead to altered hemodynamics.72,73 Studies suggest that apical pacing may induce long-term changes in both RV and LV performance. Midseptal pacing may be more hemodynamically beneficial, but it is more difficult to achieve a stable electrode position with this approach.73 Use of the Medtronic 3830 using the guiding catheter makes midseptal positioning easier to obtain.
Acute Electrode Evaluation
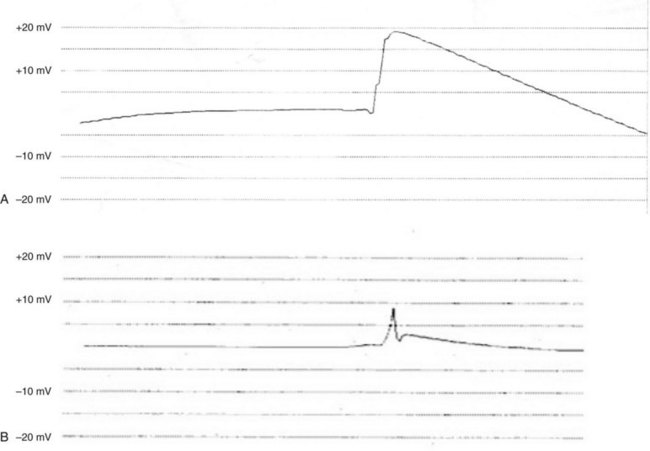
After placement of the electrode, its electrical characteristics must be determined. For active-fixation or intramyocardial electrodes, 15 minutes should be allowed after placement of the electrode before threshold testing, to permit acute myocardial changes caused by electrode entry into the myocardium to subside. All changes do not subside in this period, but this short delay is warranted. Our initial approach is to measure the electrode’s impedance and, if possible, the intrinsic EGM amplitudes. The impedance should be between 200 and 600 Ω for an epicardially placed electrode (values up to 1200 Ω can be acceptable with bipolar Medtronic 4968 electrode) and between 300 and 700 Ω for an endocardially placed electrode. The amplitude of the EGM should be sufficient to allow appropriate sensing by the generator being implanted. Care must be taken to avoid measurement of injury current rather than true signal amplitude (Fig. 18-35). Also, pacing thresholds can be adversely affected in the presence of injury current, and all measurements should be postponed until it resolves. The minimally acceptable spontaneous signal amplitude varies, depending on the specific generator.
If these measurements are found to be acceptable, threshold testing is performed. It is our general practice to set a given pulse width and then determine the minimum pulse amplitude necessary to maintain capture. Multiple threshold determinations at differing pulse widths are performed to define the strength-duration curve adequately. It is important to determine the shape of the strength-duration curve to ensure that the minimum pulse width necessary to pace at any pulse amplitude and the minimum amplitude necessary to pace at any pulse width are sufficiently removed from the proposed generator settings, to allow for some movement in the strength-duration curve without risking loss of capture (Fig. 18-36). Data from the MPPR suggest that the ventricular threshold of a new electrode at a 0.5-msec pulse width is less than 1 V, and for the atrium, less than 1.5 V. Such threshold guidelines apply to both endocardial and epicardial electrodes.
Before the pulse generator is connected to the electrodes, it is advisable to pace the ventricle at the maximal output of the pacing system analyzer. In children, because the diaphragm is close to the electrode, diaphragmatic pacing can occur. If diaphragmatic pacing occurs at the maximal pacing system analyzer output, the electrode must be moved. This is particularly relevant for children with transposition of the great arteries, in whom a ventricular electrode is positioned within the left ventricle. Positioning of the electrode tip at the LV apex puts it close to the diaphragm, and there is a high incidence of diaphragmatic pacing. To prevent this, we position the electrode on the midposterior free wall at the approximate level of the papillary muscles (Fig. 18-37). This affords acceptable thresholds with a minimal risk for diaphragmatic pacing. This problem is more often seen in endocardial pacing than in epicardial pacing, but it can occur in either setting. Diaphragmatic stimulation can be seen with atrial pacing as a result of phrenic nerve stimulation. Thus, high-output atrial pacing must also be performed.
 Follow-Up Methods for the Child with a Pacemaker
Follow-Up Methods for the Child with a Pacemaker
Pacemaker Clinic Visit
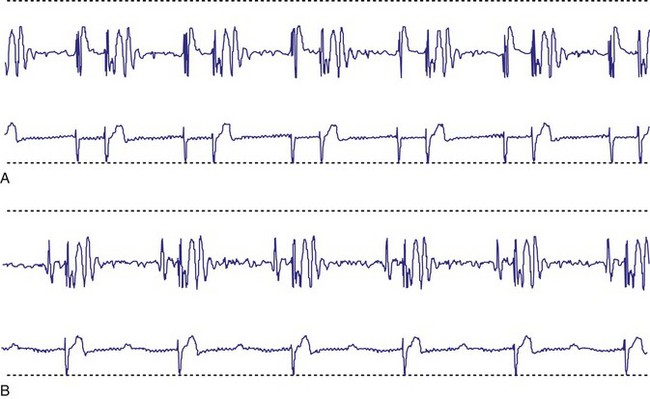
Interrogation of the pacemaker’s diagnostic counters is then performed to assess the extent of pacemaker use and the rate variability the patient is experiencing. Information provided by diagnostic counters is variable, depending on the pacemaker model. The most useful data are those collected over many months to assess degree of the pacemaker’s rate variability and the patient’s dependence on the pacemaker. This information is useful in assessing the appropriateness of the LRL in DDD pacing, the degree of AV conduction, and sinus node function (Fig. 18-38).
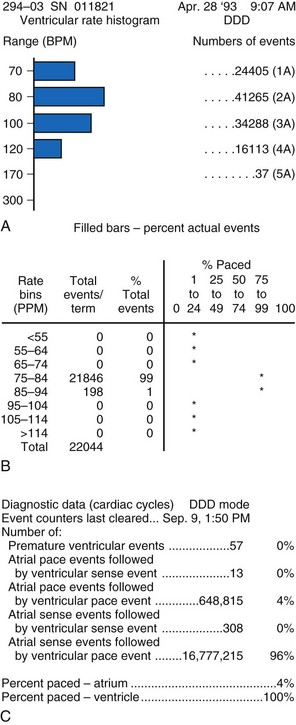
Figure 18-38 Examples of data obtained from generator diagnostic counters showing the number of beats occurring in each rate range. A, Patient had acceptable heart rate variability with predominantly atrial pacing and ventricular sensing (intact AV conduction). B, Patient had minimal heart rate variability; reprogramming to a rate-responsive mode was required. C, Information about the types of atrial and ventricular beats occurring. This patient had mostly atrial and ventricular sensing. There was less than 20% atrial pacing, indicating an appropriate lower rate limit. The MVP feature has been enabled to promote AV conduction and minimize ventricular pacing (see Fig 18-11).
Intracardiac Electrogram Determinations
Intracardiac EGMs should be obtained whenever possible. Electrograms are obtained both simultaneously with a surface electrocardiogram and with telemetered annotation markers that indicate the point during the EGM at which sensing or pacing occurs and the beginning and length of each refractory period. The relationship of programmed refractory periods to the waveform is shown. This is particularly useful for the atrial channel, to ascertain the appropriateness of the programmed settings. For example, if the atrial electrode has been implanted in the left atrium, a determination of the appropriate AV interval can be difficult because the spontaneous P wave on the body surface significantly precedes the time at which atrial sensing occurs (Fig. 18-39). This results in a prolonged PR interval, as determined from the surface ECG recording, which may raise concerns about appropriate generator function.
Recording the annotation markers with the intracardiac EGM also permits minimization of refractory periods, to maximize the multiblock rate but not risk oversensing of ventricular depolarization or repolarization by the atrial channel (Fig. 18-40). This is an even greater concern in young children who experience high atrial rates and are at risk for multiblock, with a subsequent sudden decrease in the ventricular rate and a concomitant decrease in cardiac output if TARP is too long (see later discussion on results of exercise testing).
The intracardiac EGM is also useful in the diagnosis of atrial dysrhythmias. In the active child, presence of a high pace rate may not be unusual or may suggest an atrial tachycardia. An example of such a situation is in the presence of atrial flutter (Fig. 18-41). In the first example in the figure, the rapid atrial rate was apparent on the body surface ECG recording, and the ventricular pacing was at the URL. In the second example, the body surface ECG recording showed an apparently regular rhythm at a rate below URL; atrial EGM showed atrial flutter, with every other atrial beat in the refractory period.
Exercise Testing
Exercise testing should be an integral part of all pacemaker follow-up in children old enough to undergo treadmill testing. Such testing often pinpoints inappropriate programming, manifested by inappropriate performance. In one series, 43% of patients had clinically significant inappropriate programming while exercising that was not apparent on routine pacemaker testing.74–75 Of these patients, 75% showed an inadequate heart rate increase with the stress of exercising. Although most were paced in the VVIR mode, one patient in the DDD mode was shown to have an inadequate exercise response indicative of chronotropic incompetence. All such patients underwent reprogramming with subsequent appropriate heart rate increases. Other patients displayed spontaneous beats at maximal exercise, causing an automatic extension of the PVARP and acute multiblock, with an acute decline in the heart rate (Fig. 18-42). The development of multiblock was also seen with an acute decline in the heart rate from an inappropriately long TARP. The multiblock rate was only slightly above the URL, causing an abrupt decline in heart rate (Fig. 18-43). If decreasing the AV interval or the PVARP cannot adequately shorten TARP, use of the DDDR or VDDR mode may be helpful. When the multiblock rate is reached during either of these pacing modes, the ventricular rate falls to the sensor rate rather than the inappropriately sensed atrial rate, providing a smaller decline in the heart rate. Loss of capture at the maximal heart rate was also observed, even though there had been 100% capture at rest and programming of the pacemaker was believed to be appropriate, based on resting threshold testing (Fig. 18-44).
Changes in pacing thresholds do occur and can worsen, as the previous example shows, or can improve.26,76 For fixed-rate pacing, thresholds decline after exercise, so resting thresholds are not necessarily indicative of those present during the stress of exercise (Fig. 18-45). However, these findings may not be applicable to the patient whose pacemaker is in the rate-responsive mode, as the example in the patient who developed loss of capture at maximal exercise showed. This is a potentially serious problem that would not have been apparent had evaluation been performed only while the patient was at rest.
Changes in P-wave amplitude were also documented at maximal exercise.77–79 A decrease in P-wave amplitude can result in loss of atrial sensing; in the DDD mode this can cause an immediate drop to the LRL of the pacemaker. The result can be syncope caused by a sudden decrease in cardiac output. Although DDDR pacing can minimize the magnitude of this heart rate drop, the physician should not rely on this mechanism, because the sensor rate may not be the same as the atrial rate, especially during activities such as swimming or cycling. Myopotential sensing by either channel can have similar effects.80,81 This is more evident in unipolar systems, but can occur in bipolar systems.
Ambulatory Electrocardiographic Monitoring
Ambulatory ECG monitoring is also essential in the appropriate follow-up of pediatric patients with pacemakers. This is particularly true for patients who are unable to exercise, because it may be the only method to evaluate high-rate performance. In many series, 24-hour ambulatory ECG monitoring was the only means by which pacemaker malfunction was detected.82–84 Such monitoring is particularly valuable in evaluating atrial and ventricular sensing problems and intermittent lack of capture. This is important because myocardial characteristics and thus appropriate pacemaker programming may vary, depending on the activity state and time of day.30 Interactions between the patient’s intrinsic rhythm and the pacemaker are also more completely evaluated by ambulatory monitoring, potentially resulting in more optimal programming.
Remote and Transtelephonic Pacemaker Monitoring
Transtelephonic pacemaker monitoring also plays an important role in pacemaker follow-up and has undergone major changes. Previously, transtelephonic monitoring consisted of only a real-time electrocardiogram with and without magnet application. This did provide some information, but was often difficult to perform in the uncooperative child. No stored data or trend data were available. Newer remote systems permit complete pacemaker interrogation of current and stored information, together with real-time EGMs and electrode threshold data performed automatically by the pacemaker in the ventricle,84 and more recently in the atrium.85 If interrogation is interrupted during data retrieval, it is halted until reception is again established, and retrieval resumes. This is particularly helpful in the young, uncooperative patient. When interrogation is complete, the data are transmitted in digital form to a central computer, eliminating the need for simultaneous data retrieval and telephone transmission. An example of such a system is the Medtronic CareLink system. Similar remote systems include Merlin.net (St. Jude Medical) and Biotronik Home Monitoring Service and also allow pacemaker follow-up. Benefits of such a system for early determination of potential problems have recently been evaluated.86 Other systems, such as Latitude (Boston Scientific), allow remote follow-up of ICD devices but not pacemakers.
Transtelephonic monitoring is cost-effective, decreasing the number of outpatient visits needed, and also provides a method to address parent/child concerns about proper pacemaker function.87 The capability of the child or the parent to detect pacemaker malfunction is very limited.88 Use of transtelephonic ECG monitoring not only can confirm pacemaker malfunction but also can reassure the parent and child of proper function.
Early Follow-Up Period
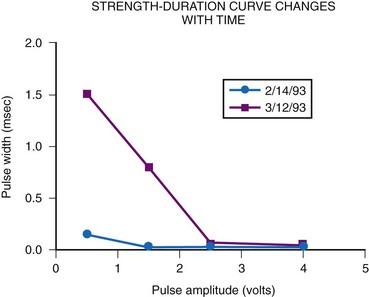
Follow-up during the crucial early period must be frequent and thorough. This is especially true after a new electrode has been implanted. Our current protocol includes both pacemaker interrogation and noninvasive threshold determination at multiple pulse amplitudes performed at 1 day, 6 weeks, and 12 weeks after implantation. During this period, thresholds often vary, and the generator’s output may also need to be reprogrammed to maintain reliable pacing. Exit block, in particular, is common during this period, whereas little to no exit block is noted beyond 3 months.87 In another series, there appeared to be a slight increase in threshold after 3 months, but this occurred in only 24% of patients.88 It is important to determine thresholds at multiple pulse amplitudes, because the strength-duration curve can move in a horizontal direction, with thresholds at the higher outputs unchanging and thresholds at lower outputs showing marked increases (Fig. 18-46).
 Antitachycardia Devices in Children with Congenital Heart Disease
Antitachycardia Devices in Children with Congenital Heart Disease
Implantable Defibrillator Use
The use of ICDs in children had been limited by many factors, including uncertainty of the indications for use, device size, and the difficulty initially of implanting the epicardial electrodes. Randomized studies are lacking in patients with structural heart disease to determine those at highest risk who would receive the greatest benefit from ICD placement. With additional experience in the adult population and smaller devices and electrodes, both epicardial and endocardial, the pediatric use of ICDs has increased. Studies include a large multicenter experience with initial use of ICDs in children age 1.9 to 20 years,89 a single-center experience with 11 children age 4 to 16 years,90 use of nonthoracotomy electrode systems in 17 children age 12 to 20 years,91 and a larger single-center experience with 27 patients.91
Patient Selection
The only current class I indication for ICD placement is for secondary prevention in patients who have sustained an aborted sudden cardiac death (aSCD) episode not amenable to surgery or ablation. In all the series mentioned, all patients had experienced a syncopal or sudden cardiac death (SCD) episode documented to be a ventricular arrhythmia or had an inducible ventricular arrhythmia at electrophysiologic study after their syncopal or SCD event. Silka et al.89 found that 76% of children were survivors of an SCD episode, whereas 10% had experienced a syncopal episode with inducible sustained ventricular tachycardia; the remainder had drug-refractory VT. The underlying diagnoses were hypertrophic cardiomyopathy, long QT syndrome (LQTS), dilated cardiomyopathy, or repaired congenital heart disease. Fewer than one-half the children had depressed ventricular function. In general, any child who has survived an SCD episode or has a condition that can result in SCD should be considered for ICD implantation. Medical therapy should always be employed, but one must decide whether medical therapy alone will completely eliminate the likelihood of a repeat episode. By example, the patient with LQTS who has experienced an aSCD episode should be treated medically and should also undergo ICD placement. Conversely, a child with congenital complete heart block and a documented episode of ventricular fibrillation (VF) may require only permanent pacing.
Although cardiomyopathies and primary electrical diseases constitute the most common diagnoses requiring ICD placement, children who have undergone repair of congenital heart disease are beginning to present with malignant ventricular arrhythmias. Patients with tetralogy of Fallot, aortic stenosis, and transposition of the great arteries after atrial switch repair have been identified as higher-risk patients.89 In our experience, 15% of all patients receiving ICDs had undergone prior repair of tetralogy of Fallot. Children with this diagnosis accounted for 22% of patients in one report.92 Any child who has undergone a ventriculotomy or repair of a coronary artery abnormality is at risk for ventricular arrhythmias.
There are fewer data to support guidelines for which pediatric patients would benefit from an ICD for primary prevention. The most recent guidelines list implantation for high-risk patients without syncope as a class IIb indication (class IIb defined as probably useful but less well established by evidence).19 Patients with inherited arrhythmias such as the malignant types of LQTS, short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia (CPVT) are often considered for ICD placement for primary prevention. Minimal data support ICD use in patients based solely on QRS duration. In tetralogy of Fallot, QRS duration is caused mostly by RV enlargement, and risk of VF is unknown. Our practice has been to place an ICD for episodes of documented VT or VF, syncope with an unknown etiology, or inducible VT at electrophysiologic testing. Placement for primary prevention is rare in our hands.
Implantation Approaches
Two decisions affect ICD implantation: epicardial versus endocardial electrode placement and subclavicular versus abdominal device placement. Each can be made independently of the other. Initially, only epicardial electrode placement was used in children because of the large size of the transvenous electrode and the inappropriate shocking coil spacing, or the need to place a second electrode. Even with transvenous electrode improvements (discussed later), epicardial placement remains the preferred method for smaller children. The smallest child to receive an endocardial system in the study of Kron et al.91 weighed 32 kg, and the smallest in Hamilton et al.90 weighed 27 kg. In our center, the smallest child weighed 15 kg. Historically, we used epicardial patches in children who weigh less than 15 kg or in whom there is no venous access to the ventricle.
When used, patches are usually placed to maximize the distance between them and to maximize exposure of the myocardium to the defibrillation energy. Patches are sutured to the pericardium rather than the epicardial surface, to minimize growth effects and patch distortion (Fig. 18-47). With time, distortion can develop, but it does not necessarily have an adverse effect on the defibrillatory thresholds. The leads are then tunneled to the abdomen, and the pocket is created, as for implantation of an epicardial pacemaker system. In one case of a child with absent abdominal musculature, the device was placed intrathoracically.
In some patients, even the small patch electrodes may be too large. Recently, we have begun to use coils placed in the posterior mediastinal space medially and along the lateral LV wall laterally or posteriorly behind the ventricular mass. Defibrillatory energy is delivered between these electrodes, traversing the heart in an anterolateral direction (Fig. 18-48). Such an arrangement has resulted in acceptable defibrillatory thresholds, using a multiple coil or a single coil and an active can.93–96 Such systems have shorter longevity than traditional transvenous systems but still afford adequate defibrillation.96
With the development of smaller-diameter transvenous electrodes and use of the device can as the second defibrillation electrode, a transvenous system can be used in most children. The currently available electrodes, both single and dual coil, can be introduced with a 7F to 9F introducer depending on the type of electrode used. However, the intercoil spacing in the dual-coil electrode is usually too large to be used in children. Positioning the second coil properly requires buckling of the electrode within the right atrium, potentially dislodging the electrode tip (Fig. 18-49). Use of the single-coil electrode eliminates this problem. Even with electrode buckling, the SVC coil remains close to the clavicle, placing it at risk for fracture. In addition, the second coil may make extraction more difficult. The second coil may also increase the risk of an extraction procedure later, because it may cause more fibrosis around the SVC coil in an area that is narrow and potentially at the innominate vein–SVC junction, where the lead makes a sharp bend, unlike in the larger child or adult, where the coil seats entirely in the SVC.
Use of a single-coil electrode requires that the device can serves as the second electrode; alternatively, a second intravascular coil or a subcutaneous array can be used as the second electrode, if initial defibrillatory thresholds are not satisfactory. Initially, use of the active can was restricted to implantation of the device under the left clavicle. However, we have implanted the device in a left upper quadrant abdominal pocket, with the single-coil, bipolar-pacing, steroid-eluting electrode introduced into the left subclavian vein and tunneled subcutaneously to the device pocket97 (Fig. 18-50). This technique is similar to the method proposed by Molina et al.98 for transvenous pacemaker implantation in small patients. Defibrillatory thresholds have been less than 10 J in all cases, even though the electrode area is reduced.99 No patient has required an additional electrode (subcutaneous array or second transvenous electrode).
Programming Considerations
The issues of appropriate pediatric programming are similar to those in the adult patient. The major concern is the higher sinus rates that can occur in a child. Initially, β-blockers were employed to reduce the maximal sinus rate. However, this approach often resulted in significant exercise intolerance. Although β-blockers may be indicated to treat the underlying disease (e.g., LQTS), their use solely to reduce the maximal sinus rate is no longer necessary. Current devices can now be programmed to very high rates as a criterion of VT and can also employ other markers, such as increased QRS duration or altered QRS morphology and sudden onset. For most children, however, use of high rate alone is usually sufficient to detect fibrillation. Also, many children have wide QRS complexes as a result of prior cardiac surgery, making the QRS morphology a difficult criterion to use. In practice, we program the device to a detection cycle interval that is 50 msec greater than the documented fibrillation cycle interval, consistent with the approach of Hamilton et al.90 QRS duration is used only if cycle interval alone has been unable to prevent inappropriate therapy delivery.
Follow-Up Procedures
After each therapy delivery, the child is seen in the clinic and the device interrogated, or a remote transmission is sent to evaluate the event and to ensure the therapy was appropriate. This is particularly crucial because inappropriate therapies are not rare. Up to 50% of patients have been found to receive at least one inappropriate therapy within 4 years.100 Also, the medical therapy is reevaluated to ensure that it does not require alteration. Finally, the psychological aspects of the child’s reaction to the therapy are evaluated, and support is offered as needed. Assessing the need for long-term support is extremely important, as discussed later.
Antitachycardia Pacing
Pace termination of atrial arrhythmias is often used in patients with congenital heart disease.101 This has become possible with the development of a dual-chamber antitachycardia pacemaker with a rate response suitable for use in some patients. Although it does not have defibrillator capabilities and therefore is not suitable for pace termination of VTs, the device has proved useful for treatment of atrial flutter, particularly in postoperative patients, in whom atrial flutter is often seen but is difficult to treat with medication or ablative techniques. The Medtronic AT500 has been used in a series of patients with congenital heart disease and atrial tachycardias.101 The most recent version (Medtronic EnRhythm) has improved functionality and allows enhanced remote follow-up; it successfully terminated the tachycardia in 54% of episodes. Success is influenced by many factors, including proximity of the electrode to the reentrant circuit, atrial size, and the potential for multiple reentrant circuits. It does require placement of both atrial and ventricular electrodes, even if only atrial pacing is anticipated, because ventricular sensing is needed to prevent pacing in 1 : 1 AV rhythms. In addition, these electrodes must be bipolar in configuration, and close electrode spacing is required to minimize far-field oversensing, especially of the atrial electrode.
 Cardiac Resynchronization Therapy in Patients with Structural Cardiac Disease
Cardiac Resynchronization Therapy in Patients with Structural Cardiac Disease
Cardiac resynchronization therapy (CRT) has the potential to improve function in the patient with structural heart disease. The major issues with the recent use of CRT in this patient population are similar to those in the adult population, including the approach to measure dyssynchrony and to assess improvement.102 However, unique issues exist. Most children being considered for CRT are already paced; they rarely have left bundle branch block unless being paced from the right ventricle, often have right bundle branch block (RBBB), have often had ventricular surgery, may have a systemic right ventricle rather than systemic left ventricle, or only one ventricle, and have multiple anatomic variants, making group comparisons difficult. Indications also continue to vary among patients, making evaluation of efficacy difficult. Therefore, implantation criteria developed for patients with ischemic or even nonischemic disease and LV conduction delays have little relevance. Guidelines for this patient population comparable to adult patients do not exist.
Results of initial series on the long-term benefits of biventricular pacing for LV function appear promising.103–106 However, nonresponders continue to be seen in all series, ranging from 10% to 15%, even though available series are small and selection criteria varied. Patients with univentricular hearts in particular appear to have a high number of nonresponders.104 Patients with RV failure and RBBB pose unique problems, with variable results.104,106,107 Dubin et al.107 found an acute improvement in RV dP/dT with RV pacing in patients with intact AV conduction, suggesting a possible role for improved RV function with CRT or multisite pacing. Also, the question of CRT versus combined CRT/ICD remains unanswered.104
Preimplant assessment remains crucial. In our institution, this consists of assessment of the degree of heart failure, including ejection fraction, and assessment of mechanical dyssynchrony using electrocardiographic techniques.108,109 Use of regional measures of velocity and strain by speckle tracking techniques quantitatively measures dyssynchrony by time to maximal velocity or strain of multiple wall segments and calculation of the average time and standard deviation (SD) for each segment about the mean (Fig. 18-51). An SD above 35 msec is considered significant dyssynchrony. This method also allows for identification of the site of the latest mechanical activation to use in determining the optimal LV lead position. This is simplified by epicardial placement over transvenous placement by allowing easier access to a much larger portion of the left ventricle. LV pacing through a coronary sinus electrode in small children can be problematic because of the small size of the coronary sinus, anatomic variation (especially presence of left SVC draining to sinus), and possible long-term need for such therapy. Given the coronary sinus electrodes currently available, most LV pacing may need to be accomplished through the epicardial route. Intraoperative or body surface ECG mapping has also been proposed to indentify the site of latest LV activation to determine the optimal site for LV pacing.110 This does narrow the QRS width, but further work is required to see if this has a long-term beneficial effect on LV function.
Acute use of multisite pacing in the postoperative patient has also been investigated as a means to improve cardiac function acutely. Zimmerman et al.111 placed temporary pacing leads on the atrium, midanterior RV free wall (ground or positive pole of ventricular pacing), diaphragmatic surface of right ventricle, and distal RV outflow tract. The latter two leads were connected to the negative ventricular pacing pole. With such pacing, blood pressure improved compared with no pacing, and cardiac index improved as well. Although patient numbers were small, the authors concluded that multisite pacing improved RV synchrony and cardiac performance. Pacing sites were chosen specifically to address RV dyssynchrony because many patients had RBBB.
Proper programming parameters are largely unknown for CRT in patients with structural cardiac disease. Acute adjustment with measurement of select hemodynamic parameters may be of some help, but long-term benefits may not be predicted by acute measurements. Although it is anticipated that CRT will prove beneficial, much work remains before it becomes an established therapy for this patient population.112
 Prophylactic Medication
Prophylactic Medication
Significant controversy has surrounded the need for prophylactic antibiotic agents in the prevention of subacute bacterial endocarditis in children with pacemakers. The current recommendations of the American Heart Association do not suggest the use of antibiotics in children with pacemakers.113 However, this is influenced by the underlying structural disease that may require such therapy. Prophylactic anticoagulation has not proved useful in children with endocardial pacing systems. Only children with chronic atrial flutter or atrial fibrillation are routinely anticoagulated. We believe that the risk to the active child from chronic anticoagulation is significant, and that the benefit is minimal.
 Psychosocial Adjustments
Psychosocial Adjustments
Children with Pacemakers
Comprehensive care of the child with a pacemaker requires awareness of the psychosocial issues faced by children and families. Although there are issues specific to having a pacemaker, such as dependence on a mechanical device and the certainty of periodic surgeries throughout life, the overall challenges are similar to those faced by children with other types of chronic illness. Treatment goals include facilitation of positive child and family adjustments and prevention of avoidable negative psychosocial outcomes. Important illness-related tasks for parents of children with pacemakers include participating in the treatment plan, preserving emotional well-being for themselves and their children, and preparing for an uncertain future.114 Children who are at high risk for emotional or behavioral adjustment problems may benefit from early identification and intervention.115
Because they require lifelong device therapy, these children, by definition, have a chronic illness.116 Because of the underlying disease and permanent need for a device, the children and their families are chronically subjected to stressful experiences, including repeated surgeries, body image changes, and exposure to the possibility of death.117 Although disease severity varies, most patients can anticipate many fruitful adult years, and many are anticipated to have a normal or nearly normal life span.
When examining psychosocial outcomes in this population, no significant differences were found between children with pacemakers and comparison children on standardized measures of trait anxiety, self-competence, and self-esteem.118 Children with pacemakers were more external in their locus of control orientation than healthy controls, but not significantly different from children with comparable heart disease who did not have a pacemaker.119 Interview data suggested that comparison children were likely to report negative stereotypes about children with pacemakers. However, most chronically ill children make positive adjustments, although an increased risk for psychosocial problems suggests that up to 30% of children with chronic illness demonstrate secondary psychosocial impairment by 15 years of age.120,121
The risk for psychosocial problems in the pediatric pacemaker population can be understood as a response to chronic stress that challenges the adaptive capacities of children and families.122,123 Consistent with this model, negative adjustments are likely to occur when stress exceeds the child’s adaptive capacities. Certainly, there is wide variation in children’s adaptations, and some children are able to make positive adaptations despite extremely stressful circumstances.124,125
Many of the factors linked with children’s adjustments reflect a continuum, with one end representing a protective factor and the other a risk factor. Families who are cohesive and supportive are associated with positive adaptations in children, whereas family functioning that is disengaged and rigid is associated with emotional and behavioral problems in the children.126
Disease-related biomedical factors anticipated to affect children’s adjustments include diagnosis (disease severity, prognosis, treatment), functional impairment, cognitive abilities, and visibility (physical changes).127,128 For children with a chronic illness, diagnosis and disease severity are less important predictors of adjustment than other factors.127 However, the diagnosis of heart disease during childhood may pose particular challenges, for reasons not well understood.129 Biomedical factors that may negatively influence psychosocial outcomes for children with heart disease include early mother-infant attachment problems, neuropsychological changes, learning disabilities, and exclusion from competitive sports.130–132
Inherent childhood factors that may affect adjustment include temperament, developmental stage, and competency.127 For example, an academically gifted child may experience opportunities for self-esteem, socialization, and social recognition that ameliorate the negative effects of having a chronic illness, whereas the child with a learning disability who has a chronic illness is at increased risk for serious adjustment problems.133
Social-environmental factors linked with children’s adjustments include family environment, maternal psychological functioning, family economic resources, and children’s peer relationships. Adaptive, cohesive families who communicate effectively support positive adjustments for children.134,135 Maternal adjustment may play a particularly important role in children’s adjustment,136,137 and persistent maternal distress (e.g., symptoms of anxiety or depression) warrants intervention.127 Economic resources appear to be powerful moderators of children’s adjustment to stressful life circumstances.125,138,139 The quality of children’s friendships is also important, because positive peer relationships are linked with prosocial behavior, academic achievement, stress resistance, and later, adolescent competence.140–142 Conversely, negative child peer relationships are associated with academic failure, aggressive behavior,139,143 and general psychopathology, which persists during adulthood.144 Social isolation and feelings of loneliness are potential sources of emotional distress for chronically ill children and family members.135
Coping methods are the processes used by children and families to accomplish disease-related tasks, maintain normal function, and minimize family disruption. Adaptive parental coping strategies include maintaining family integrity through cooperation and maintenance of an optimistic outlook; maintaining social support, self-esteem, and psychological stability; and understanding the medical situation through communication with other parents and health care providers.145 Coping is a dynamic process that may promote or hinder positive adjustments. Coping methods can be categorized as problem focused or emotion focused; in general, both methods are used by individuals responding to stress.146 Problem-focused coping methods involve actions taken to address a stressful situation, whereas emotion-focused methods are those coping methods that change the emotional reaction to an event, such as altering the perception of the experience, blunting, or denial. The different coping strategies employed by fathers and mothers may be a source of distress in the marriage.147 Children’s coping strategies reflect their developmental stage, and problem-focused strategies precede emotion-focused strategies during childhood.148
Provider-Family Interactions
Communication with providers about the child’s prognosis, appropriate activities, and restrictions is necessary for parents and children to adjust to life with a pacemaker. At initial implantation, many parents are unfamiliar with the use of pacemakers in children and may assume that the child “won’t live long, because only old people get pacemakers.” Conversations with the child and family about what the pacemaker means for the child’s future are important, because emotional well-being is predicated on a hopeful outlook—one that permits belief in a personal and meaningful future.148
An important functional outcome for children with pacemakers is successful integration into the school community. Although there is realistic concern about negative stereotyping by school personnel, some data suggest that parents believe school personnel should be informed about their child’s illness, and that health professionals are the most appropriate people to provide this education.149 To facilitate school integration, we have used a variety of interventions, including participation in parent-teacher meetings at the child’s school, visiting the child’s classroom, and making pacemaker models and other materials available to the child for school presentations.
Children with Implantable Cardioverter-Defibrillators
Minimal data are available regarding the adjustments of children and adolescents after ICD implantation. In nine patients age 13 to 49 years, Vitale and Funk150 reported difficulty sleeping, low energy, and interruptions in planned activities. Problems reported for older patients after ICD implantation include increased anxiety levels, excessive anger, psychological disturbances, sexual abstinence, and restricted physical and social activities.151,152 Some studies emphasize that most older adults appear to make positive adjustments after ICD implantation.153
It is known that repeated unpredictable aversive stimuli, in addition to producing fear and avoidance behavior, can have long-term debilitating effects.154 Many of the experiments done on the effects of aversive conditioning actually used unpredictable electrical shocks as the source of the stimuli. Maier and Seligman155 proposed that, in such circumstances, people may develop the expectation that their behavior has little effect on their environment, and this expectation may generalize to a wide range of situations. This behavior mode, referred to as learned helplessness, has been linked with human depression.
Complicating the situation for patients with tachyarrhythmias is the catecholamine release that occurs secondary to anxiety. The increased heart rate is often perceived as the possible onset of the arrhythmia, leading to increased anxiety and feelings of loss of control. Relaxation under these conditions can present a challenge for young people. Potential therapies, in addition to counseling, include relaxation methods and biofeedback.156
Support groups have been helpful in promoting positive adjustments to chronic illness in family members.157 Factors associated with support group participation that may be therapeutic for patients and family members include sharing of information, instilling of hope, universality, altruism, and interpersonal learning. Although this is an effective intervention, the use of support groups by this population thus far appears limited. Professionals working with these patients report that many younger patients do not participate in ongoing ICD patient support groups, because the groups do not address issues of personal concern, such as work, intimate relations, childbirth, school, friends, and dating. Also, the relatively small numbers of young defibrillator patients in any one geographic area have limited the ability to initiate support groups specifically for this population.
1 Moquin PM, Vaysse J, Durand M, et al. Implantation d’un stimulateur interne pour correction d’un bloc auriculo-ventriculoventriculaire chirurgical chez une enfant de 7 ans. Arch Mal Coeur Vaiss. 1962;55:241.
2 Gillette PC, Wampler DG, Shannon C, Oth D. Use of cardiac pacing after the Mustard operation for transposition of the great arteries. J Am Coll Cardiol. 1986;7:138.
3 El-Said G, Rosenberg HS, Mullins LE, et al. Dysrhythmias after Mustard’s operation for transposition of the great arteries. Am J Cardiol. 1972;30:526.
4 Shearn RPN, Flemming WH. Fourteen years of implanted pacemakers in children. Ann Thorac Surg. 1978;25:144.
5 Simon AB, Dick MII, Stern AM, et al. Ventricular pacing in children. Pacing Clin Electrophysiol. 1982;5:836.
6 Walkens JJS. Cardiac pacemakers in infants and children. Pediatr Cardiol. 1982;3:337.
7 Ector H, Dhooghe G, Daenen W, et al. Pacing in children. Br Heart J. 1985;53:541.
8 Serwer GA, Mericle JM. Evaluation of pacemaker pulse generator and patient longevity in patients aged 1 day to 20 years. Am J Cardiol. 1987;59:824.
9 Walsh CA, McAlister HF, Anders CA, et al. Pacemaker implantation in children: a 21-year experience. Pacing Clin Electrophysiol. 1988;11:1940.
10 Brownlee JR, Serwer GA, Dick MII, et al. Failure of electrocardiographic monitoring to detect cardiac arrest in patients with pacemakers. Am J Dis Child. 1989;143:105.
11 McCue CM, Mantakas ME, Tingelstad JB, Ruddy S. Congenital heart block in newborns of mothers with connective tissue disease. Circulation. 1977;56:82.
12 Kangos JJ, Griffiths SP, Blumenthal S. Congenital complete heart block: a classification and experience with 18 patients. Am J Cardiol. 1967;20:632.
13 Dorostkar P, Serwer GA, LeRoy S, Dick MII. Long-term course of children and young adults with congenital complete heart block. J Am Coll Cardiol. 1993;21:295A.
14 Michaelsson M, Riesenfeld T, Jonzon A. Natural history of congenital complete atrioventricular block. Pacing Clin Electrophysiol. 1997;20:2098.
15 Kugler JD, Danford DA. Pacemakers in children: an update. Am Heart J. 1989;117:665.
16 Karpawich PP, Gillette PC, Garrison A, et al. Congenital complete atrioventricular block: clinical and electrophysiologic predictors of need for pacemaker insertion. Am J Cardiol. 1981;48:1098.
17 Winkler RB, Freed MD, Nadas AS. Exercise-induced ventricular ectopy in children and young adults with complete heart block. Am Heart J. 1980;99:87.
18 Eldor M, Griffin JC, Abbott VA, et al. Permanent cardiac pacing in patients with long QT syndrome. J Am Coll Cardiol. 1987;3:600.
19 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008;117:e350.
20 Rishi F, Hulse LE, Auld DO, et al. Effects of dual-chamber pacing for pediatric patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 1997;29:734.
21 McGrath LB, Gonzalez-Lavin L, Morse DP, Levett JM. Pacemaker system failure and other events in children with surgically induced heart block. Pacing Clin Electrophysiol. 1988;11:1182.
22 Serwer GA, Mericle JM, Armstrong BE. Epicardial ventricular pacemaker electrode longevity in children. Am J Cardiol. 1988;61:104.
23 Serwer GA, Uzark K, Dick MII. Endocardial pacing electrode longevity in children. J Am Coll Cardiol. 1990;15:212A.
24 Serwer G, Dick MII, Eakin B. Cardiac output response to treadmill exercise in children with fixed-rate pacemakers. Circulation. 1991;84:II-514.
25 Karpawich PP, Perry BL, Farooki ZQ, et al. Pacing in children and young adults in nonsurgical atrioventricular block: comparison of single-rate ventricular and dual-chamber modes. Am Heart J. 1987;113:316.
26 Karpawich PP, Justice CD, Cavitt DL, Chang CH. Developmental sequelae of fixed-rate ventricular pacing in the immature canine heart: an electrophysiologic, hemodynamic, and histopathologic evaluation. Am Heart J. 1990;119:1077.
27 Karpawich PP, Rabah R, Haas JE. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol. 1999;22:1372.
28 Kaltman JR, Ro PS, Zimmerman F, et al. Managed ventricular pacing in pediatric patients and patients with congenital heart disease. Am J Cardiol. 2008;102:875.
29 Horenstein MS, Karpawich PP. Pacemaker syndrome in the young: do children need dual chamber as the initial pacing mode? Pacing Clin Electrophysiol. 2004;27:600.
30 Preston TA, Fletcher RD, Luechesi BR, et al. Changes in myocardial threshold: physiologic and pharmacologic factors in patients with implanted pacemakers. Am Heart J. 1967;74:235.
31 Lau C, Cameron DA, Nishimura SC, et al. A cardiac evoked response algorithm providing threshold tracking: a North American multicenter study. Pacing Clin Electrophysiol. 2000;23:953.
32 Cohen MI, Buck K, Tanel RE, et al. Capture management efficacy in children and young adults with endocardial and unipolar epicardial systems. Europace. 2004;6:248.
33 Bauersfeld U, Nowak B, Molinari L, et al. Low-energy epicardial pacing in children: the benefit of autocapture. Ann Thorac Surg. 1999;68:1380.
34 Serwer GA, Uzark K, Beekman R, Dick MII. Optimal programming of rate altering parameters in children with rate-responsive pacemakers using graded treadmill exercise testing. Pacing Clin Electrophysiol. 1990;13:541.
35 Yabek SM, Wernly J, Check TW, et al. Rate-adaptive cardiac pacing in children using a minute ventilation biosensor. Pacing Clin Electrophysiol. 1990;13:2108.
36 Gillette PC, Shannon C, Blair H, et al. Transvenous pacing in pediatric patients. Am Heart J. 1983;105:843.
37 Ward DE, Jones S, Shinebourne EA. Long-term transvenous pacing in children weighing ten kilograms or less. Int J Cardiol. 1982;15:112.
38 Spotnitz HM. Transvenous pacing in infants and children with congenital heart disease. Ann Thorac Surg. 1990;49:495.
39 Kammeraad JAE, Rosenthal E, Bostock J, et al. Endocardial pacemaker implantation in infants weighing ≤10 kilograms. Pacing Clin Electrophysiol. 2004;27:1466.
40 Gillette PC, Zeigler V, Bradhaus GB, Kinsella P. Pediatric transvenous pacing: a concern for venous thrombosis? Pacing Clin Electrophysiol. 1988;11:1935.
41 Yakvevich V, Alogen D, Papo J, Vidne BA. Fibrotic stenosis of the SVC with widespread thrombotic occlusion of its major tributaries. J Thorac Cardiovasc Surg. 1983;85:632.
42 Sunder SK, Ekong EA, Sevalingram K, Kumar A. SVC thrombosis due to pacing electrodes. Am Heart J. 1992;123:790.
43 Wilkoff BL, Byrd CL, Love CJ, et al. Pacemaker lead extraction with the laser sheath: results of the Pacing Lead Extraction with the Excimer Sheath (PLEXES) trial. J Am Coll Cardiol. 1999;33:1671.
44 Kantharia BK, Kutalek SP. Extraction of pacemaker and implantable cardioverter-defibrillator leads. Curr Opin Cardiol. 1999;14:44.
45 Sharifi M, Sorkin R, Lakier JB. Left heart pacing and cardioembolic stroke. Pacing Clin Electrophysiol. 1994;17:1691.
46 Levin AR, Spach MS, Boineau JP, et al. Atrial pressure-flow dynamics in atrial septal defects (secundum type). Circulation. 1968;37:476.
47 Serwer GA, Armstrong BE, Anderson PAW, et al. Use of contrast echocardiography for evaluation of right ventricular hemodynamics in the presence of ventricular septal defects. Circulation. 1978;58:327.
48 Westerman CR, Van Revanter SH. Surgical management of difficult pacing problems in patients with congenital heart disease. J Cardiovasc Surg. 1987;2:351.
49 Scheffer M, van Gelder B. Transvenous implantation of a ventricular pacing lead in a patient with an artificial tricuspid valve prosthesis. Neth Heart J. 2003;11:359.
50 Shah MJ, Nehgme R, Carboni M, Murphy JD. Endocardial atrial pacing lead implantation and midterm follow-up in young patients with sinus node dysfunction after the Fontan procedure. Pacing Clin Electrophysiol. 2004;27:949.
51 Moak JP, Friedman RA, Motfat D, et al. Dual-chamber pacing in children: the optimal lead configuration-unipolar or bipolar? J Am Coll Cardiol. 1991;17:208A.
52 Michalik RE, Williams WH, Zorn-Chelten S, Hotches CR. Experience with a new epimyocardial pacing lead in children. Pacing Clin Electrophysiol. 1984;7:83.
53 Kugler J, Minsour W, Blodgett C, et al. Comparison of two myoepicardial pacemaker leads: follow-up in 80 children, adolescents, and young adults. Pacing Clin Electrophysiol. 1988;11:2216.
54 DeLeon SY, Ilbawi MN, Becker CL, et al. Exit block in pediatric cardiac pacing: comparison of the suture-type and fishhook epicardial electrodes. Thorac Cardiovasc Surg. 1990;99:905.
55 Hamilton R, Gow R, Bahoric B, et al. Steroid-eluting epicardial leads in pediatrics: improved epicardial thresholds in the first year. Pacing Clin Electrophysiol. 1991;14:2066.
56 Karpawich PP, Harkini M, Arciniegas E, Cavitt DL. Improved chronic epicardial pacing in children: steroid contribution to porous platinized electrodes. Pacing Clin Electrophysiol. 1992;15:1151.
57 Johns JA, Fuh FA, Burger JD, Hammon JWJr. Steroid-eluting epicardial pacing leads in pediatric patients: encouraging early results. J Am Coll Cardiol. 1992;20:395.
58 Serwer GA, Dorostkar PC, LeRoy S, Dick MII. Comparison of chronic thresholds between differing endocardial electrode types in children. Circulation. 1992;86:1-43.
59 Chakrabarti S, Morgan GJ, Kenny D, et al. Initial experience of pacing with a lumenless lead system in patients with congenital heart disease. PACE. 2009;32:1428.
60 Rosenthal E, Bostock J. VDD pacing in children with congenital complete heart block: advantages of a single pass lead. Pacing Clin Electrophysiol. 1997;20:2102.
61 Robertson JM, Hillel L. A new technique for permanent pacemaker implantation in infants and children. Ann Thorac Surg. 1987;44:209.
62 Ulicny KSJr, Detterbeck FC, Starek PJK, Wilcox BR. Conjoined subrectus pocket for permanent pacemaker placement in the neonate. Soc Thorac Surg. 1992;53:1130.
63 Ott DA, Gillette PC, Cooley DA. Atrial pacing via the subxiphoid approach. Tex Heart Inst J. 1982;9:149.
64 Lawrie GM, Seale JP, Morris GCJr, et al. Results of epicardial pacing by the left subcostal approach. Ann Thorac Surg. 1979;28:561.
65 Brenner JI, Gaines S, Cordier J, et al. Cardiac strangulation: two-dimensional echo recognition of a rare complication of epicardial pacemaker therapy. Am J Cardiol. 1988;61:654.
66 Perry JC, Nihill MR, Ludomirsky A, et al. The pulmonary artery lasso: epicardial pacing lead causing right ventricular outflow obstruction. Pacing Clin Electrophysiol. 1991;14:1018.
67 Serwer GA, Dick MII, Uzark K, et al. The value of redundant ventricular epicardial electrode placement in children. Pacing Clin Electrophysiol. 1988;9:531.
68 Hoyer MH, Beerman LB, Ettedgui JA, et al. Transatrial lead placement for endocardial pacing in children. Ann Thorac Surg. 1994;58:97.
69 Hayes DC, Holmes DRJr, Maloney JD, et al. Permanent endo-cardial pacing in pediatric patients. J Thorac Cardiovasc Surg. 1983;85:618.
70 Gillette PC, Edgerton J, Kratz J, Zeigler V. The subpectoral pocket: the preferred implant site for pediatric pacemakers. Pacing Clin Electrophysiol. 1991;14:1089.
71 Lee JC, Shannon K, Boyle NG, et al. Evaluation of safety and efficacy of pacemaker and defibrillator implantation by axillary incision in pediatric patients. Pacing Clin Electrophysiol. 2004;27:304-307.
72 Karpawich PP. Chronic right ventricular pacing and cardiac performance: the pediatric perspective. Pacing Clin Electrophysiol. 2004;27:884.
73 Karpawich PP, Horenstein MS, Webster P. Site-specific right ventricular implant pacing to optimize paced left ventricular function in the young without and without congenital heart disease. Pacing Clin Electrophysiol. 2002;25:566.
74 Serwer GA, Dorostkar PC, LeRoy S, et al. Evaluation of rate-variable pacemaker function at maximal exertion in children and young adults. Pacing Clin Electrophysiol. 1993;16:899.
75 Serwer GA, Kodali R, Eakin B, et al. Changes in pacemaker threshold with exercise in children and young adults. J Am Coll Cardiol. 1991;17:207A.
76 Gillette PC, Zinner A, Kratz J, et al. Atrial tracking (synchronous) pacing in a pediatric and young adult population. J Am Coll Cardiol. 1987;9:811.
77 Frohlig G, Blank W, Schwerdt H, et al. Atrial sensing performance of AV universal pacemakers during exercise. Pacing Clin Electrophysiol. 1988;11:47.
78 Ross BA, Zeigler V, Zinner A, et al. The effect of exercise on the atrial electrogram voltage in young patients. Pacing Clin Electrophysiol. 1991;14:2092.
79 Bricker JT, Barison A, Traveek MS, et al. The use of exercise testing in children to evaluate abnormalities of pacemaker function not apparent at rest. Pacing Clin Electrophysiol. 1985;8:656.
80 Jain P, Kaul U, Wasir HS. Myopotential inhibition of unipolar demand pacemakers: utility of provocative manoeuvres in assessment and management. Int J Cardiol. 1992;34:33.
81 Strathmore NF, Mond HG. Noninvasive monitoring and testing of pacemaker function. Pacing Clin Electrophysiol. 1987;10:1359.
82 Kerstjens-Frederikse MWS, Bink-Boelkens MTE, de Jongste MJL, Homan van der Heide JN. Permanent cardiac pacing in children: morbidity and efficacy of follow-up. Int J Cardiol. 1991;33:207.
83 Jarosik DL, Redd RM, Buckingham TA, et al. Utility of ambulatory electrocardiography in detecting pacemaker dysfunction in the early postimplantation period. Am J Cardiol. 1987;60:1030.
84 Cohen MI, Buck K, Tanel RE, et al. Capture management efficacy in children and young adults with endocardial and unipolar epicardial systems. Europace. 2004;6:248.
85 Sperzel J, Milasinovic G, Smith TW, et al. Automatic measurement of atrial pacing thresholds in dual-chamber pacemakers: clinical experience with atrial capture management. Heart Rhythm. 2005;2:1203.
86 Crossley GH, Chen J, Choucair W, et al. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol. 2009;54:2012.
87 Vincent JA, Cavitt DL, Karpawich PP. Diagnostic and cost effectiveness of telemonitoring the pediatric pacemaker patient. Pediatr Cardiol. 1997;18:86.
88 Shepard RB, Kam J, Colvin EC, et al. Pacing threshold spikes months and years after implant. Pacing Clin Electrophysiol. 1991;14:1835.
89 Silka MJ, Kron J, Dunnigan A, Dick MII. Sudden cardiac death and the use of cardioverter-defibrillators in pediatric patients. Circulation. 1993;87:800.
90 Hamilton RM, Dorian P, Gow RM, Williams WG. Five-year experience with implantable defibrillators in children. Am J Cardiol. 1996;77:524.
91 Kron J, Silka MJ, Ohm O-J, et al. Preliminary experience with nonthoracotomy implantable cardioverter-defibrillators in young patients. Pacing Clin Electrophysiol. 1994;17:26.
92 Stefanelli CB, Bradley DJ, Leroy S, et al. Implantable cardioverter-defibrillator therapy for life-threatening arrhythmias in young patients. J Interv Card Electrophysiol. 2002;6:235.
93 Berul CI, Triedman JK, Forbess J, et al. Minimally invasive cardioverter-defibrillator implantation for children: an animal model and pediatric case report. Pacing Clin Electrophysiol. 2001;24:1789.
94 Luedemann M, Hund K, Stertmann W, et al. Implantable cardioverter-defibrillator in a child using a single subcutaneous array lead and an abdominal active can. Pacing Clin Electrophysiol. 2004;27:117.
95 Stephenson EA, Batra AS, Knilans TK, et al. A multicenter experience with novel implantable cardioverter-defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol. 2006;17:41.
96 Radbill AE, Triedman JK, Berul CI, et al. System survival of nontransvenous implantable cardioverter-defibrillators compared to transvenous cardioverter-defibrillators in pediatric and congenital heart disease patients. Heart Rhythm. 2009;7:193.
97 Fischbach PS, Law IH, Dick MII, et al. Use of a single-coil transvenous electrode with an abdominally placed implantable cardioverter-defibrillator in children. Pacing Clin Electrophysiol. 2000;23:884.
98 Molina LE, Dunnigan AC, Crosson JE. Implantation of transvenous pacemakers in infants and small children. Ann Thorac Surg. 1995;59:689.
99 Halperin BD, Reynolds B, Fain ES, et al. The effect of electrode size on transvenous defibrillation energy requirements: a prospective evaluation. Pacing Clin Electrophysiol. 1997;20:893.
100 Korte T, Koditz H, Niehaus M, et al. High incidence of appropriate and inappropriate ICD therapies in children and adolescents with implantable cardiovertor-defibrillators. Pacing Clin Electrophysiol. 2004;27:924.
101 Stephanson EA, Casavant D, Tuzi J, et al. ATTEST Investigators. Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol. 2003;92:871.
102 Janousek J. Cardiac resynchronization in congenital heart disease. Heart. 2009;95:940.
103 Strieper M, Karpawich P, Frias P, et al. Initial experience with cardiac resynchronization therapy for ventricular dysfunction in young patients with surgically operated congenital heart disease. Am J Cardiol. 2004;94:1352.
104 Dubin AM, Janousek J, Rhee E, et al. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol. 2005;46:2277.
105 Janousek J, Vojtovic P, Hucin B, et al. Resynchronization pacing is a useful adjunct to the management of acute heart failure after surgery for congenital heart defects. Am J Cardiol. 2001;88:145.
106 Cecchin F, Frangini PA, Brown DW, et al. Cardiac resynchronization therapy (and multisite pacing) in pediatrics and congenital heart disease: five years experience in a single institution. J Cardiovasc Electrophysiol. 2009;20:58.
107 Dubin AM, Feinstein JA, Reddy M, et al. Electrical resynchronization: a novel therapy for the failing right ventricle. Circulation. 2003;107:2287.
108 Vannan MA, Pedrizzetti G, Li P, et al. Effect of cardiac resynchronization therapy on longitudinal and circumferential left ventricular mechanics by velocity vector imaging: description and initial clinical application of a novel method using high-frame rate B-mode echocardiographic images. Echocardiography. 2002;2:8265.
109 Freidberg MK, Silverman NH, Dubin AM, Rosenthal DN. Mechanical dysschrony in children with systolic dysfunction secondary to cardiomyopathy: a Doppler tissue and vector velocity imaging study. J Am Soc Echocardiogr. 2007;20:756.
110 Silva JNA, Ghosh S, Bowman TM, et al. Cardiac resynchronization therapy in pediatric congenital heart disease: insights from noninvasive electrocardiographic imaging. Heart Rhythm. 2009;6:1178.
111 Zimmerman FJ, Starr JP, Koenig PR, et al. Acute hemodynamic benefit of multisite ventricular pacing after congenital heart surgery. Ann Thorac Surg. 2003;75:1775.
112 Serwer GA. Cardiac resynchronization therapy in the young patient: current status and future directions. J Cardiovasc Electrophysiol. 2006;17:1072.
113 Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation. 2007;116:1736.
114 Moos RH, Tsu UD. The crisis of physical illness: an overview. In: Moos RH, editor. Coping with Physical Illness. New York: Plenum; 1977:3.
115 Satin W, LaGreca AM, Zigo MA, Skyler JS. Diabetes in adolescence: effects of a multifamily group intervention and parent simulation of diabetes. J Pediatr Psychol. 1989;14:259.
116 Eiser C. Growing Up with a Chronic Disease. London: Jessica Kingsley; 1993.
117 Gamble WJ, Owens JP. Pacemaker therapy for conduction defects in the pediatric population. In: Roberts N, Gelband H, editors. Cardiac Arrhythmias in the Neonate, Infant, and Child. New York: Appleton-Century-Crofts; 1977:469.
118 Alpern D, Uzark K, Dick MII. Psychosocial responses of children to cardiac pacemakers. J Pediatr. 1989;114:494.
119 Wallander JL, Thompson RJ. Psychosocial adjustment of children with chronic physical conditions. In: Roberts MC, editor. Handbook of Pediatric Psychology. ed 2. New York: Guilford Press; 1995:124.
120 Pless IB, Roghmann KJ. Chronic illness and its consequences: observations based on three epidemiologic surveys. J Pediatr. 1971;79:351.
121 Rolland J. Chronic illness and the life cycle: a conceptual framework. Fam Proc. 1987;26:203.
122 Wallander JL, Vami JW. Adjustment in children with chronic physical disorders: Programmatic research on a disability-stress-coping model. In: LaGreca AM, Siegal L, Wallander JL, Walker CE, editors. Stress and Coping with Pediatric Conditions. New York: Guilford Press; 1992:279.
123 Garmezy N, Tellegen A. Studies of stress-resistant children: methods, variables, and preliminary findings. In: Morrison F, Lord C, Keating D, editors. Advances in Applied Developmental Psychology, vol 1. New York: Academic Press; 1984:231.
124 Werner EE, Smith RS. Vulnerable but Invincible: a Longitudinal Study of Resilient Children and Youth. New York: McGraw-Hill; 1982.
125 Wallander JL, Varni JW, Babani L, et al. Family resources as resistance factors for psychological maladjustment in chronically ill and handicapped children. J Pediatr Psychol. 1989;14:157.
126 Thompson R, Gustafson K. Adaptations to Chronic Childhood Illness. Washington, DC: American Psychological Association; 1996.
127 Stein RE, Jessop DJ. A noncategorical approach to chronic childhood illness. Public Health Rep. 1982;97:354.
128 Lavigne JV, Faier-Routman J. Psychological adjustment to pediatric physical disorders: a meta-analytic review. J Pediatr Psychol. 1992;17:133.
129 Goldberg S, Simmons RJ, Newman J, et al. Congenital heart disease, parental stress, and infant-mother relationships. J Pediatr. 1991;119:661.
130 Miller G, Tesman JR, Ramer JC, et al. Outcome after open-heart surgery in infants and children. J Child Neurol. 1996;11:49.
131 Wright M, Nolan T. Impact of cyanotic heart disease on school performance. Arch Dis Child. 1994;71:64.
132 Gutgesell HP, Gessner IH, Better VL, et al. Recreational and occupational recommendations for young patients with heart disease. Circulation. 1986;74:1195A.
133 Werner ME, Smith R. Kauai’s Children Come of Age. Honolulu: University of Hawaii Press; 1982.
134 Hamlett KW, Pellegrini DS, Katz KS. Childhood chronic illness as a family stressor. J Pediatr Psychol. 1992;17:33.
135 Blechman EA, Delamater AM. Family communication and type 1 diabetes: a window on the social environment of chronically ill children. In: Cole RE, Reiss D, editors. How Do Families Cope with Chronic Illness?. Hillsdale, NJ: Lawrence Erlbaum, 1993.
136 Demaso D, Campis L, Wypij D, et al. The impact of maternal perceptions and medical severity on the adjustment of children with congenital heart disease. J Pediatr Psychol. 1991;16:137.
137 Lyons-Ruth K, Zoll D, Conell D, Grunebaum HV. The depressed mother and her one year old infant: environment, interaction, attachment and infant development. In: Field T, Tronick E, editors. Maternal Depression and Infant Disturbance. San Francisco: Jossey-Bass; 1986:61.
138 Masten AS, Garmezy N, Tellegen A, et al. Competence and stress in school children: the moderating effects of individual and family qualities. J Child Psychol Psychiatry. 1988;29:745.
139 Green KD, Forehand R, Beck SJ, Vosk B. An assessment of the relationship among measures of children’s social competence and children’s academic achievement. Child Dev. 1980;51:1149.
140 Masten AS, Morison P, Pellegrini DS. A revised class play method of peer assessment. Dev Psychol. 1985;21:523.
141 Morison P, Masten AS. Peer reputation in middle childhood as a predictor of adaptation in adolescence: a seven year follow-up. Child Dev. 1991;62:991.
142 Pelham WE, Milich R. Peer relationships in children with hyperactivity/attention deficit disorder. J Learn Disabil. 1984;17:560.
143 Cowen EL, Pederson A, Babigian H, et al. Long-term follow-up of early detected vulnerable children. J Consult Clin Psychol. 1973;41:438.
144 McCubbin HI, McCubbin MA, Patterson JM, et al. CHIP: Coping-Health Inventory for Parents. An assessment of parental coping patterns in the care of the chronically ill child. J Marr Fam. 1983;45:359.
145 Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984.
146 Affleck G, Tennen H, Rowe J. Mother, fathers, and the crisis of newborn intensive care. Infant Mental Health. 1990;11:12.
147 Compas BE, Worsham NL, Ey S. Conceptual and developmental issues in children’s coping with stress. In: LaGreca AM, Siegal LJ, Wallander JL, Walker CE, editors. Stress and Coping in Child Health. New York: Guilford Press; 1992:7.
148 Yarcheski A, Scoloveno MA, Mahon N. Social support and well-being in adolescents: the mediating role of hopefulness. Nurs Res. 1994;43:288.
149 Andrews SG. Informing schools about children’s chronic illness. Pediatrics. 1991;88:306.
150 Vitale MB, Funk M. Quality of life in younger persons with an implantable cardioverter-defibrillator. Dimens Crit Care Nurs. 1995;14:100.
151 Cooper DK, Luceri RM, Thurer RJ, et al. The impact of the automatic implantable cardioverter-defibrillator on quality of life. Clin Prog Electrophysiol Pacing. 1986;4:306.
152 Kuiper R, Nyamathi A. Stressors and coping strategies of patients with automatic implantable cardioverter-defibrillators. J Cardiovasc Nurs. 1991;5:65.
153 Luderitz B, Jung W, Deister A, Manz M. Patient acceptance of implantable cardioverter-defibrillator devices: changing attitudes. Am Heart J. 1994;127:1179.
154 Mazur JE. Learning and Behavior. Upper Saddle River, NJ: Prentice Hall; 1998. p 187
155 Maier SF, Seligman M. Learned helplessness: theory and evidence. J Exp Psychol Gen. 1976;105:3.
156 Stoyva JM, Carlson JG. A coping/rest model of relaxation and stress management. In: Goldberger L, Breznitz S, editors. Handbook of Stress: Theoretical and Clinical Aspects. New York: Free Press; 1993:724.
157 Teplitz L, Egenes KJ, Brask L. Life after sudden death: the development of a support group for automatic implantable cardioverter-defibrillator patients. J Cardiovasc Nurs. 1990;4:20.