Pediatric Orthopedic Trauma
Musculoskeletal trauma is the most common medical emergency in children.1 In children ages 1 to 14 years, accidents are the leading cause of death.2 However, not all orthopedic injuries sustained by children are life threatening. The chance of a child sustaining a fracture before age 16 is 42% for boys and 27% for girls.3 It has been estimated that between 1–2% of children present with a fracture each year.4 As participation in sports and other recreational activities increases, the number of fractures is likely to increase. A study in 2006 looking at the impact of trauma on an urban pediatric orthopedic practice demonstrated that fracture management (both operative and nonoperative) accounted for approximately one-third of the total work-related relative value units. The most common fracture-related operations were performed on the elbow (23%), tibia (12%), femur (9.8%), forearm (5.5%), and the distal aspect of the radius (5%).5 The same practice reported that trauma care comprised 44% of their operative volume in 2006 and 2007.6
Patient gender, age, climate, time of day, and social situation in the home have been shown to impact the frequency of orthopedic injuries. In children, boys sustain fractures at 2.7 times the rate of girls.7 However, as girls become involved in more athletic events, this margin may narrow. It has been shown that fracture location varies with chronologic age, a finding that is probably due to a combination of the anatomic maturation of the child and the age-specific activities of childhood.3 Several authors have shown that fractures are more common during the summer months when children are out of school.4,7,8 It has also been shown that there is also a strong association between sunshine and fractures, and a negative association between rain and fractures.9 Likewise, two studies have proven that the afternoon is the most frequent time for fractures to occur.10,11 This correlates with the time of peak activity for children. Injuries in the home during the late afternoon and evening account for more than 83% of all injuries to children.12 Moreover, the overall incidence of fractures occurring at home increases with the age of the child.4,13 In a Swedish study, fracture incidence was correlated with the degree of social handicaps such as welfare or alcoholism in the family.14 Similarly, a study from Manitoba elicited the social situation at home as a major influence of children’s injuries.15
No discussion of pediatric orthopedic trauma is complete that does not include a discussion of nonaccidental trauma. The incidence of physical abuse to children is estimated to be 4.9 per 1,000. Of those abused, 1 of every 1,000 will ultimately die as a result.16 Early recognition and reporting is essential because children who return home after hospitalization with unrecognized abuse have a 25% risk of serious injury and a 5% risk of death.17 Children at highest risk for abuse are first-born children, premature infants, stepchildren, and handicapped children.18 Most cases of child abuse involve children younger than 3 years of age. Any child presenting with fractures, particularly if they involve the long bones, should be viewed with circumspection as to cause (Table 18-1).19,20
TABLE 18-1
Specificity of Musculoskeletal Radiologic Findings in Nonaccidental Trauma
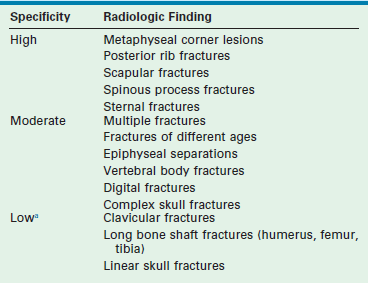
aLow specificity, but these findings are commonly seen in nonaccidental trauma.
Adapted from O’Connor JF, Cohen J. Dating fractures. In: Kleinman PK, editor. Diagnostic Imaging of Child Abuse. Baltimore: Williams & Wilkins, 1987. p. 6.
Pathophysiology
In the immature skeleton, longitudinal and appositional growth takes place through the physes (growth plates) that are located at the ends of the long bones, in the endplates of the vertebral bodies, or at the periphery of the round bones in the feet and hands. Thus, the physis is essential for normal skeletal growth, but is also the weakest portion of the bone in children. It is estimated that approximately 30% of fractures of the long bones include an injury to the physis.21–23 Most fractures that involve the growth plates heal without consequence. However, some injuries can result in permanent damage with significant sequelae such as angular deformity or complete cessation of growth.
The ends of every long bone consist of an epiphysis (near the joint), physis, and metaphysis (area of newly formed bone). At the time of skeletal maturity, the physis closes, which means there is no more longitudinal growth. Fracture healing in children is rapid and the potential for remodeling is great due to the growth potential and dynamism of the immature skeleton. These characteristics allow for nonoperative treatment of some fractures in children that demand operative treatment in skeletally mature patients. Remodeling of fractures predictably occurs in the plane of primary motion of the adjacent joint (usually flexion/extension) and, to a lesser degree, in the coronal plane (varus and valgus deformities). Remodeling is virtually nonexistent in the transverse plane with rotational malalignment.24
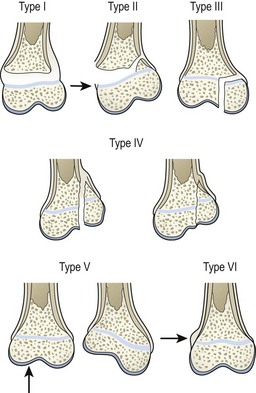
Physeal fractures are classified to predict outcome and guide treatment. Currently, most orthopedic surgeons use the Salter Harris classification (Fig. 18-1).25 Classic teaching states that type I and II injuries heal without growth abnormalities if reduced appropriately. However, some reports dispute some of this dogma.26–28 Types III and IV injuries usually occur in older children and require anatomic realignment via open reduction to restore congruity of the joint to minimize the risk of arthritis. Reduction also restores continuity of the physis to decrease the risk of growth disturbance. Type V are crush injuries that are not usually recognized at presentation, but have a high risk of growth arrest.29
Complex Injuries
Children sustain injuries that are different from adults due to their size and activities. A common example is a pedestrian struck by a car. An adult will frequently sustain an injury to the tibia or knee from the car’s bumper. However, the same mechanism will result in a fracture of the femur or pelvis in conjunction with a chest or head injury in a small child.30 Motor vehicle accidents (MVAs) are the most common cause of multiple injuries to children, both as occupants and pedestrians.30,31
Open fractures are considered one of the true orthopedic emergencies in children.32 These injuries usually result from high-energy mechanisms and are often seen in the setting of multiple trauma. Open fractures in children and adults are classified according to the system of Gustilo–Anderson (Table 18-2).33–35 The four goals of treatment of open fractures are: prevention of infection, bony union, prevention of malunion, and return to function of limb and patient.32,36 To attain these goals, open fractures must be treated by early irrigation and debridement along with broad-spectrum antibiotics. 33–35 Kindsfater and colleagues found that early treatment of tibial shaft fractures in children resulted in fewer cases of osteomyelitis when compared to those treated later.37 As a counterpoint, data from our institution demonstrated no difference in infection or nonunion rates with delayed debridement in 390 open fractures of the lower extremities in adults.38 Additionally, in a study of 554 pediatric open fractures, there was no difference in infection rates when debridement was within 6 hours of injury as compared to seven to 24 hours.39 There is no consensus on the effect of delayed operative treatment of open fractures in regards to rates of infection and need for secondary surgical procedures to promote bone healing.32,39–45 Our practice is to debride open fractures within 24 hours of presentation and more urgently if there is severe contamination.
TABLE 18-2
Severity Classification for Open Fractures
| Grade | Description |
| I | Wound <1 cm |
| II | Transitional wound (1–10 cm) |
| III | Wound >10 cm |
| IIIA | Extensive soft tissue injury |
| IIIB | Reconstructive soft tissue injury |
| IIIC | Vascular injury |
From Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: A new classification of type III open fractures. J Trauma 1984;24:747–96; Gustilo RB, Anderson T. Prevention of infection in the treatment of 1025 open fractures of long bones: Retrospective and prospective analyses. J Bone Joint Surg Am 1976;50:453–58.
Push and riding lawn mowers produce complex wounds and open fractures in children with an annual incidence of approximately 11 per 100,000.46 These injuries frequently require serial debridement, internal or external fixation, and reconstruction of soft tissue defects. Unfortunately, amputation is often needed.
Fractures of the Lower Extremity
Due to the high energy required, fractures of the pelvis and proximal femur are rare but serious injuries in children. Approximately two-thirds of patients with pelvic fractures have associated injuries, and approximately one-third have residual, long-term morbidity.47,48 Pelvic fractures rank second to head injuries in terms of complications, including life-threatening visceral injuries. The mortality rate of pelvic fractures is between 9–18%.47 Children with multiple injuries should be checked carefully to exclude fractures of the pelvis. Some common findings of fractures of the pelvis are the presence of a hematoma beneath the inguinal ligament (Desot sign); decreased distance between the greater trochanter and anterior superior iliac spine on the affected side in lateral compression injuries (Roux sign); the presence of a bony prominence or hematoma on rectal exam (Earl sign). An anteroposterior pelvis radiograph is usually sufficient as the initial screening study, although increasingly these injuries are diagnosed by computed tomography (CT) as part of the initial trauma evaluation.49 Most pediatric pelvic fractures, even those in which the pelvic ring is disrupted, can be treated nonoperatively with good outcomes.48
Fractures of the femoral neck are serious injuries that typically require operative treatment.50–59 Osteonecrosis caused by disruption of the blood supply to the femoral epiphysis is a dreaded complication of this fracture that occurs in up to 75% of children after this injury.50–55,59–62 The risk of developing osteonecrosis correlates with a higher anatomic location of the fracture in the femoral neck, extent of displacement, and delay in reducing the fracture. Accordingly, fractures and dislocations of the proximal femur are orthopedic emergencies. They require immediate anatomic reduction, which may be achieved by closed or open techniques, and internal fixation (Fig. 18-2).52,56,59,63–66
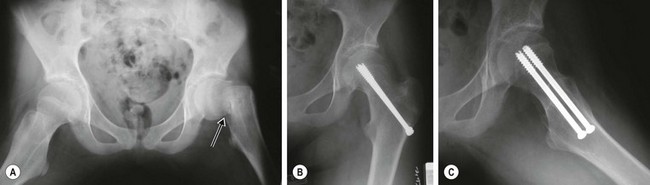
FIGURE 18-2 (A) Anteroposterior radiograph of the pelvis of a 12-year-old female injured from a fall showing a displaced transcervical fracture of the left femoral neck (arrow). The fracture was treated emergently by closed reduction and internal fixation with two cannulated screws. (B,C) Radiographs one year later show healing of the fracture and no evidence of osteonecrosis.
Femoral shaft fractures are common injuries in children. The incidence and mechanism of these fractures varies with patient age and gender. Child abuse accounts for up to 67% of femur fractures in children younger than age 1, but only 11% of fractures in children between ages 1 and 2 years old.67–69 Classic teaching states that spiral fractures in preambulatory children are pathognomonic for abuse. However, studies have demonstrated that any fracture pattern can occur as the result of abuse.68 Falls are the leading cause of femur fractures in children age 2 to 3 years, and MVAs are the most common cause in older children.67 Although bleeding following a femur fracture can be fairly extensive, transfusion in isolated, closed injuries is rarely needed. Therefore, other causes of blood loss must be considered if there is hemodynamic instability or a falling hematocrit at 24 hours after injury in a patient with a femur fracture, especially in the setting of multiple trauma.70
Treatment of femur fractures also varies with age.69 Younger children (<4 to 5 years) are usually treated nonoperatively by closed reduction and immediate spica cast immobilization.71–74 Older children (4 to 10 years) are managed with flexible nails75–82 or plates.83–85 Adolescents (>10 years or >100 pounds) may be treated as adults with solid, reamed, femoral nails, which should be introduced through the tip of the greater trochanter rather than through the piriformis fossa to avoid injury to the vascular supply to the femoral head (Fig. 18-3). A recent review of rigid nails for older children and adolescents noted no cases of osteonecrosis with nail entry via the lateral aspect of the greater trochanter.86 In contrast to adults, the timing of femur fracture stabilization in children, even in the setting of multiple trauma, does not appear to have an effect on the development of pulmonary complications.87 The implications are that operation can be deferred until the general medical condition of the child permits, with the caveat that expeditious stabilization of femur (as well as other long bone) fracture(s) will facilitate mobilization and nursing care in the overall management of the child.
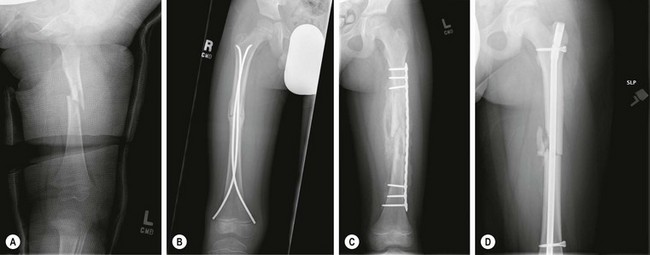
FIGURE 18-3 Four methods for treatment of femoral shaft fractures in children and adolescents are shown: (A) spica cast in a 24-month-old; (B) flexible intramedullary nails in a 7-year-old; (C) submuscular plating in an 8-year-old with severe head injury; (D) rigid locked intramedullary nail in an 11-year-old.
Knee injuries in children differ from those in adults. In children, the cartilage of the physes, apophyses, menisci, and articular surface are weaker than the knee ligaments and are thus more prone to injury.88 Therefore, fractures about the knee occur more commonly than ligamentous injuries in skeletally immature individuals.89 The distal femoral physis is the largest and fastest growing physis. It is often injured as a result of a direct blow and is a common injury in American football players. Most fractures are Salter Harris type I or II injuries. These fractures can usually be treated by closed reduction and percutaneous, cross-pin stabilization. Fractures extending into the articular surface (type III and IV injuries) require open reduction and internal fixation if displacement of the articular surface is greater than 2 mm. Because of the size of this growth plate, its complex, undulating anatomy, and the forces required for displacement, fractures of the distal femoral physis, even type I and II injuries, may result in permanent growth disturbance in up to 50% of cases.90 All of these fractures should be followed for a minimum of 1 year to evaluate for sequelae of growth arrest.
Proximal tibial physeal injuries are uncommon due to the reinforcement provided by the knee joint capsular attachments and collateral ligaments. Vascular compromise of the lower leg due to popliteal artery injury is possible, particularly with extension-type injuries in which the proximal portion of the tibial metaphysis is displaced posteriorly. Such injuries tent the popliteal artery at the level of the physis and proximal to the trifurcation, where it is relatively tethered by the peroneal branch as it courses thru the fascia entering the anterior compartment of the leg (Fig. 18-4). Close attention to the vascular examination of the lower extremity is critical following injuries to the proximal tibia. Intra-articular knee injuries typically present with a hemarthrosis and include patellar fractures or dislocations, tibial spine/plateau fractures, osteochondral fractures, and ligamentous/meniscal injuries. These injuries are typically not emergencies and can be splinted with delayed definitive treatment.
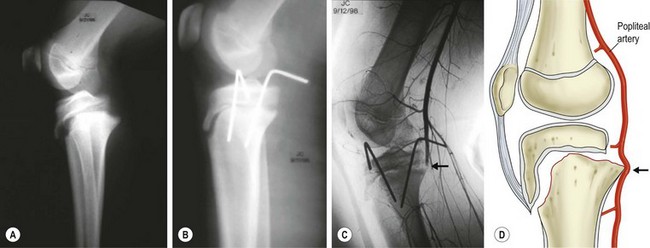
FIGURE 18-4 (A) Anteroposterior and lateral radiographs of a 13-year-old male showing a Salter Harris type I fracture of the proximal tibial physis with posterior displacement of the distal fragment following an extension-type injury. (B) Distal pulses were diminished prior to and following closed reduction and stabilization of the fracture. (C) Arteriogram shows occlusion of the popliteal artery (arrow) at the level of the fracture. Vascular repair with an interposition graft was performed successfully. (D) Drawing shows the relationship of the popliteal artery to the proximal tibial physis and mechanism of vascular injury (arrow) in this fracture. (D, Adapted from Zionts LE: Fractures and Dislocations about the Knee. In Green NE, Swiontkowski MF [eds]: Skeletal Trauma in Children, 3rd Edition. Philadelphia, Saunders, 2003. 3:460)
Nonphyseal fractures of the tibia and fibula are among the most common injuries involving the lower extremity in children.91,92 Fortunately, most of these injuries are low energy and can be treated nonoperatively. However, one must always be cognizant of the possibility of compartment syndrome following closed or open fractures of the tibial shaft.93 Indications for operative treatment of tibial shaft fractures include: open fractures, neurovascular injury, impending compartment syndrome, unacceptable alignment following closed reduction, and fractures occurring in the setting of multiple trauma.
Ankle fractures are typically caused by indirect, torsional forces. Injuries to the distal tibial and fibular physes account for 25% to 38% of all children’s physeal injuries.94,95 Sports injuries account for up to 60% of physeal fractures about the ankle.96 Nonoperative management has historically been the preferred approach, except for intra-articular fractures and those unable to be adequately reduced by closed techniques. Newer data suggests improved results with open reduction of distal tibial physeal injuries.26 CT is very useful in defining the pathoanatomy of fractures with intra-articular involvement or unusual patterns, and is useful in making management decisions and in preoperative planning.97 Foot fractures are uncommon and most can be treated nonoperatively with immobilization and restricted weight bearing. More complex problems that require operative intervention include fractures of the talar neck and calcaneus, fractures or dislocations of the tarsometarsal (Lisfranc) joint, open fractures, and lawn mower injuries.97
Spine Injuries
Cervical Spine Injuries
Cervical spine injuries in children are relatively uncommon but potentially catastrophic. Accurate diagnosis requires an awareness of the injury patterns, anatomic characteristics, and radiographic variants of the immature cervical spine.98 These injuries account for approximately 1% of all pediatric fractures and only 2% of all spine fractures.99–101 Pediatric cervical spine injuries are fundamentally different from their adult counterparts due to the anatomic characteristics of the immature spine and, and to a lesser extent, the differences in the mechanisms of injury.102 The cervical spine in children is inherently mobile because of the presence of generalized laxity of the interspinous ligaments and joint capsules, underdeveloped neck musculature, thick cartilaginous end plates, incomplete vertebral ossification (wedge-shaped vertebral bodies), and shallow-angled facet joints, particularly between the occiput and C4.102
In infants and young children, injuries to the upper cervical spine (above C3) predominate because the head is disproportionately large and creates a large bending moment in the upper cervical spine. In an 11-year experience with 122 pediatric neck injuries, none of the 21 patients age 8 years or less had evidence of injury below C3.103 Also, multiple level spinal injuries are common, occurring in approximately 25% of children with cervical spine fractures.103–106 Spinal cord injury without radiographic abnormality (SCIWORA) occurs more frequently in children than in adults.100,102 After age 8 to 10 years, the anatomical and biomechanical characteristics of the cervical spine are more like an adult, and injuries to the cervical spine are much more likely to occur in the subaxial region (below C3). Evaluation and treatment of these injuries is essentially the same as in an adult.98,100,102,107
Mechanisms of injury vary somewhat with age. In neonates, birth trauma is the most common cause of cervical spine injury and occult spinal cord injury has been demonstrated at necropsy in 30% to 50% of stillborns. Excessive distraction and/or hyperextension of the cervical spine are thought to be the most common mechanisms of injury, and may be associated with abnormal intrauterine position (transverse lie) or a difficult cephalic or breech delivery.108,109 In infants and young children, nonaccidental trauma is a significant cause of injury to the cervical spine. Avulsion fractures of the spinous processes, fractures of the pars or pedicles (most commonly C2), or compression fractures of multiple vertebral bodies are the most common patterns of injury and are thought to result from severe shaking or battering.110,111 These injuries may be associated with other signs of nonaccidental trauma including fractures of the skull, rib, or long bones, and superficial ecchymoses. In older children (up to about age 10), the most common causes are pedestrian-MVAs and falls. In children over 10 years of age, the most common etiologies are passenger-related MVAs, sports-related injuries, and diving accidents.
Appropriate methods of immobilizing children for transport and proper clinical and radiographic evaluation are crucial to avoid detrimental outcomes. The goal of immobilization during transport of the injured child with potential spine trauma is to avoid excessive angulation of the spinal column so as to avoid causing or exacerbating spinal cord injury. Immobilization of children less than 8 years of age on a standard spine board during emergency transportation will cause excessive flexion of the cervical spine due to the disproportionately large diameter of the head relative to the torso. It has been recommended that the child’s spine board be modified by building up the area under the torso with padding to allow the head to fall back slightly or cutting out the area under the occiput to recess the skull (Fig. 18-5).112 In addition to proper spine-board immobilization, an appropriately fitting cervical collar is necessary to achieve neutral alignment of the cervical spine after injury.113
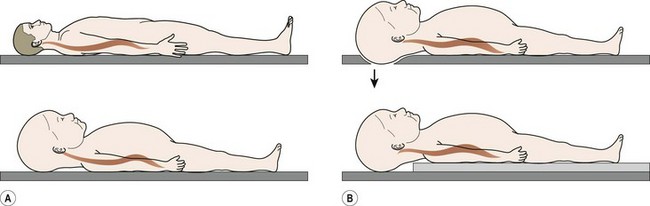
FIGURE 18-5 (A) Drawings of an adult and a child on a normal spine board contrasting the differences in position of the head and neck during emergency transport. Because of the disproportionate head-to-body ratio in children, the child’s cervical spine is flexed. (B) Two methods of modifying the traditional spine board for pediatric patient transport are shown. In the upper illustration, a cutout in the board allows the occiput to be recessed. In the lower illustration, the area under the thorax is built up with padding. Both methods effectively allow the head to translate posteriorly, creating more normal alignment of the cervical spine. (Adapted from Herzenberg JE, Hensiger RN, Dedrick DK. Emergency transport and positioning of young children who have an injury to the cervical spine. J Bone Joint Surg Am 1989;71:15–21.)
Clinical evaluation of a child suspected of having an injury to the cervical spine is often hampered by an inability to obtain an accurate history and the unreliability of the physical examination.98,100,107,114–116 Historically, overt or occult injury to the cervical spine is more likely to occur as a result of falls from a height of more than four feet, pedestrian or cyclist MVAs, and unrestrained occupant MVAs. Head or facial trauma, altered mental status and/or loss of consciousness are also risk factors. Neck pain, guarding, and torticollis are the most reliable signs of an injury to the cervical spine in children. Extremity weakness, sensory changes, bowel and bladder dysfunction, and, less frequently, headaches, seizures, syncope, and respiratory distress are signs of injury to the spinal cord.98,99,104,117–126 When these conditions present, the cervical spine should be immobilized until imaging studies can be completed and the spine cleared.98
Radiographic evaluation of the cervical spine in children is hampered by the presence of normal anatomic variants that can be mistaken for trauma. Synchondroses and incompletely ossified, wedged-shaped vertebral bodies can simulate fractures.127–130 Anterior angulation of the odontoid is a normal variant in approximately 5% of children and may be mistaken for a Salter Harris type I fracture of the dens. Physiologic subluxation of C2 on C3 or C3 on C4 of up to 3 mm is a normal variant (pseudosubluxation) in about 40% of children younger than age 8 years and is often misinterpreted as pathologic instability.129,131 Focal kyphosis of the mid-cervical spine is a normal variant in about 15% of children under age 16 years that can also be misinterpreted as pathologic.
Initial radiographic evaluation should include cross-table lateral and anteroposterior radiographs. On the lateral view, it is essential to see the C7–T1 disc space. Oblique radiographs provide detail of the pedicles and facet joints.127 Open mouth odontoid radiographs are technically difficult to perform and rarely helpful.132 CT is a much better way to image the upper cervical spine and also provides excellent definition of known fractures, confirmation of suspicious areas, and excellent visualization of the cervicothoracic level, which can be difficult to adequately image on plain radiography. CT has been shown to be more efficacious than conventional images in evaluating the cervical spine in adult and pediatric trauma and to lower institutional costs and complications in urban trauma centers.124,133,134 Magnetic resonance imaging (MRI) is the preferred study to evaluate the spinal cord and soft tissue structures including ligaments, cartilage, and intervertebral discs.
Once a cervical collar has been placed on a child or the neck immobilized, either at the scene of an accident or in the emergency department, formal clearance of the cervical spine is necessary before immobilization is discontinued.115 In general, the cervical spine may be cleared based on clinical examination alone if the child is awake, alert, and cooperative; if there are no signs of cervical injury; and if the mechanism of injury is not consistent with cervical trauma.98,115 For children under age 8 to 10 years who are obtunded or otherwise unable to be examined, and all those with a profile suggestive of injury to the cervical spine, clearance may be based on a five-view cervical spine radiographic series, consisting of anteroposterior, lateral, open mouth odontoid, and two oblique views, and a CT of the axial region of the spine, from occiput to C2. In a study at our institution in which this protocol was followed, eight of 112 children were diagnosed with cervical spine injuries. Two of six children with bony injuries (33%) were diagnosed only by CT scan. No injuries were missed and cervical immobilization was discontinued in a timely fashion.135 The rationale for CT include the predisposition for injuries to occur in the upper cervical region in children younger than 8 years old and the technical difficulty imaging this area with plain radiographs.98 In a subsequent study from our institution, helical CT was shown to be have higher specificity, sensitivity and negative predictive value than conventional radiographs in evaluating the cervical spine in children with blunt trauma.134
Others have advocated for the definitive role of MRI, particularly in identifying soft tissue injury.136–138 In a study of 79 children, MRI revealed injuries in 15 patients with normal radiographs and excluded injuries suspected on plain radiographs and CT scans in 7 and 2 patients, respectively. In 25 obtunded or uncooperative children, MRI demonstrated three with significant injuries.136
Halo vest immobilization is being used with increasing frequency in children with cervical spine injuries. It affords superior immobilization to a rigid cervical collar and is easier to apply and more versatile than a Minerva cast. It permits access for skin and wound care while avoiding the skin problems (maceration, ulceration) typically associated with both hard collars and casts. However, complication rates up to 70% have been reported with use of halo vests in children.139 Pin site infections are the most common problems, but skull perforation, cerebrospinal fluid leaks, and brain abscesses have also been described.107,139 In children younger than age 6 years, a CT scan of the skull to measure calvarial thickness can be helpful in determining optimal sites for pin placement.140 In children older than 6 years, the standard adult halo construct utilizing four pins (two anterolaterally, two posterolaterally) inserted at standard torques of 6–8 inch-pounds generally works well (Fig. 18-6A). In younger children, more pins (up to 12) placed with lower insertional torques (2–4 inch-pounds) have been advocated (Fig. 18-6B) .139,140 Standard pediatric halo rings fit most children, but infants and toddlers may require custom rings. Although standard pediatric halo vests are available, custom vests or body casts generally provide better immobilization.141
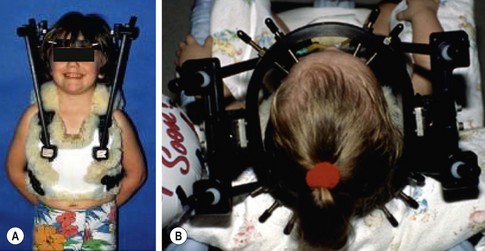
FIGURE 18-6 (A) A 6-year-old child immobilized in standard halo construct with four pins (two anterolateral, two posterolateral) inserted at torques of 6 to 8 inch-pounds. (B) A 3-year-old child with halo ring with ten pins inserted at low torque, in contrast to the usual four-pin configuration used in older children and adults. (Adapted from Weiser ER, Mencio GA. Pediatric cervical spine injuries: Assessment and treatment. Semin Spine Surg 2001;13:142–51.)
The possibility of SCIWORA should be considered in children, particularly in those younger than 8 years old. SCIWORA is defined as spinal cord injury in a patient in whom there is no visible fracture on plain radiographs or CT scan.142–145 MRI may be diagnostic in demonstrating spinal cord edema or hemorrhage, soft tissue or ligamentous injury, or apophyseal or disc disruption, but is completely normal in approximately 25% of cases. SCIWORA is the cause of paralysis in approximately 20–30% of children with injuries of the spinal cord. Potential mechanisms of SCIWORA include hyperextension of the cervical spine, which can cause compression of the spinal cord by the ligamentum flavum, followed by flexion, which can cause longitudinal traction. Other mechanisms include transient subluxation without gross failure or unrecognized cartilaginous end plate failure (Salter Harris type I fracture).
Regardless of the specific mechanism, injury to the spinal cord occurs because of the variable elasticity of the elements of the spinal column in children.146 Experimentally, it has been shown that the bone, cartilage and soft tissue in the spinal column can stretch about two inches without disruption but that the spinal cord ruptures after one-quarter inch.109,145,147 Spinal cord injury occurs when deformation of the musculoskeletal structures of the spinal column exceeds the physiologic limits of the spinal cord.146 Injury may be complete or incomplete and may occur at more than one level.148 Partial spinal cord syndromes reported in SCIWORA include Brown–Sequard, anterior, and central cord syndromes, as well as mixed patterns of injury.142–144,146
Prognosis following SCIWORA is correlated to MRI findings, if any are present, and to the severity of neurological injury.142–144,149 Effective management demands careful evaluation of the cervical spine to exclude osseous or cartilaginous injury or mechanical instability. In addition, immobilization with a rigid cervical collar for two to three months has been recommended to prevent recurrent injury.142–145 However, the need for prolonged immobilization in the absence of radiographic or MRI evidence of instability has been challenged. In a 34-year review of SCIWORA at a single institution, recurrent injury was uncommon and of uncertain etiology. Immobilization did not prevent recurrent symptoms or improve outcomes. A full recovery occurred in all cases of recurrent SCIWORA.149 Surgery is occasionally necessary for unstable injury patterns. The prevalence of scoliosis following infantile quadriplegia is over 90%. Therefore, long-term follow-up is necessary to monitor for vertebral column deformity. Administration of high-dose corticosteroids within the first eight hours of spinal cord injury has been shown to improve the chances of neurologic recovery in adults.150–153 According to guidelines from the third National Acute Spinal Cord Injury Study (NASCIS), when treatment is initiated within 3 hours of injury, methylprednisolone should be administered as an intravenous bolus of 30 mg/kg over 15 minutes followed by a continuous infusion of 5.4 mg/kg/h over the next 23 hours. Also, if treatment cannot be started until more than 3 to 8 hours after injury, the corticosteroids should be given for 48 hours. After 8 hours, there is no benefit and corticosteroids should not be administered.152,153 As the effect on younger children (<13 years old) is not known, this protocol is not universally followed at all institutions.
Thoracic, Lumbar, and Sacral Fractures
Thoracic, lumbar and sacral fractures are also relatively uncommon in children. MVAs or falls causes the majority of these injuries. Child abuse should be considered in younger children.120,154–158 The most common injuries are compression fractures and flexion–distraction injuries. Compression fractures are caused by a combination of hyperflexion and axial compression. Because the disc in children is stronger than cancellous bone, the vertebral body is the first structure to fail. It is common for children to sustain multiple compression fractures. Compression rarely exceeds more than 20% of the vertebral body. In the multiply injured patient, CT has become the preferred imaging modality to diagnose and characterize these fractures.49,159 These fractures are managed conservatively with rest, analgesics and bracing.158Flexion/distraction injuries (seat-belt injuries) occur in the upper lumbar spine in children wearing a lap belt.160–168 With sudden deceleration, the seat belt slides up on the abdomen where it provides an axis about which the spine rotates. As a result, the torso is forcibly flexed and the spinal column fails in tension, resulting primarily in disruption of the posterior column with variable patterns of extension into the middle and anterior columns (Chance fracture). These injuries may be missed on axial CT because of the transverse plane of orientation of the fracture. Widening of the interspinous distance on a lateral radiograph or CT with sagittal reconstruction are the most helpful studies in diagnosing this fracture (Fig. 18-7). Approximately two-thirds of patients have injury to a hollow viscus, a solid organ injury, or even injury to the abdominal aorta. These injuries often result in greater morbidity than the spine fracture and may be life-threatening, particularly if unrecognized initially.165,169,170 Fortunately, neurologic injury is unusual. Lap belt injuries with mostly bony involvement and kyphosis less than 20° can be treated with hyperextension casting. Those with posterior ligamentous disruption and significant intra-abdominal injury require surgical stabilization with compression instrumentation and posterior arthrodesis.
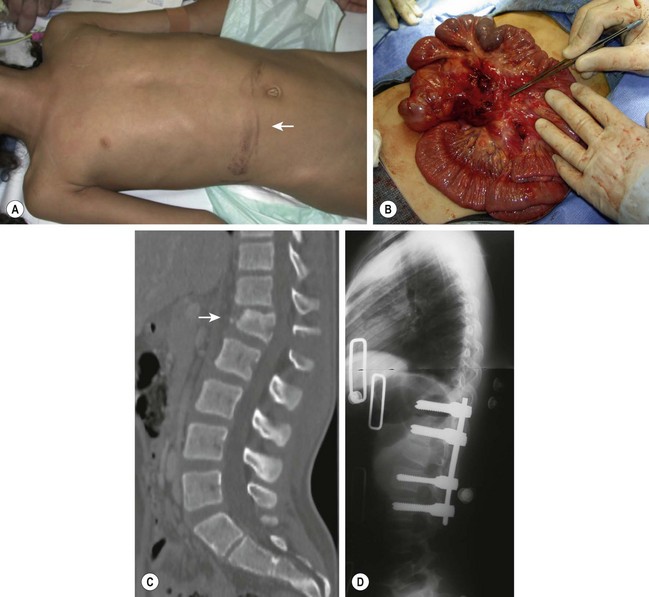
FIGURE 18-7 (A) A 12-year-old female involved in a motor vehicle accident with ecchymosis (arrow) in the lower abdomen caused by the lap belt portion of a three-point restraint. (B) This child had a laceration of the mesentery discovered at laparotomy and a (C,D) flexion–distraction fracture of L1 (arrow) with disruption of all three columns of the spine that required operative stabilization.
Fracture–dislocations of the spine are unstable injuries that usually occur at the thoracolumbar junction and often are associated with neurologic deficits. These are rare injuries in children and require surgical stabilization and fusion. Burst fractures are also rare injuries in children that result from axial compression, and typically occur at the thoracolumbar junction or in the lumbar spine.171 The need for operative treatment is determined by the stability of the fracture and the presence of neurologic deficits. Fractures of the sacrum are usually associated with pelvic fractures. Fractures that involve the sacral foramina or central sacral canal are associated with neurologic deficits in 28% and 50% of patients, respectively. Decompression of the sacral nerve root(s) and stabilization of the sacral fracture may be necessary to improve neurologic function. In most instances, however, fractures of the sacrum may be treated nonoperatively.
Fractures of the Upper Extremity
Fractures of the clavicle are common injuries. Clavicle shaft fractures in children are usually uncomplicated and require little, if any, treatment other than sling immobilization for comfort. Enthusiasm for internal fixation of clavicle fractures in adults has increased due to studies that have documented greater rates of nonunion, symptomatic malunion, and residual shoulder disability with nonoperative management.172–176 In 2007, a prospective randomized trial of 132 patients with clavicle fractures aged 16 to 60 years demonstrated that internal fixation produced better outcome scores, earlier union, reduced rate of nonunions, and no malunions in comparison to nonoperative management.177 Internal fixation of adolescent clavicle fractures remains controversial, and accepted indications include open fractures and skin compromise. Relative indications in older adolescents include multiple trauma, floating shoulder injuries, comminuted fractures, and shortened fractures. Distal clavicular fractures in the immature child may mimic acromioclavicular separation. The periosteal sleeve of the distal clavicle remains intact with the coracoclavicular ligaments attached.23,178–180 This fracture heals rapidly and requires no treatment other than sling immobilization for comfort.
Injuries to the medial end of the clavicle are rare but potentially problematic from the standpoint of recognition and neurovascular complications. The physis of the medial clavicle is the last to close, and frequently not until after age 21.23,180,181 The so-called ‘dislocation of the sternoclavicular joint’ is almost always a type I physeal fracture in children and adolescents. This injury may occur as a result of direct or indirect trauma to the shoulder. Pain and swelling is localized to the sternoclavicular joint and the shoulder is usually held forward. Although uncommon, compression of the mediastinal structures is the most devastating complication of this injury. Without treatment, the most frequent problem associated with persistent posterior displacement is dysphagia.
Diagnosis of the injury requires awareness of the injury and a CT to confirm the diagnosis. Radiographs may show the posterior dislocation, but it is most clearly seen on CT (Fig. 18-8). Closed reduction may be attempted under general anesthesia, however recurrent displacement is common.180,182–184 When open reduction is necessary, internal fixation with large, nonabsorbable suture through the thick periosteal sleeve is usually sufficient. Smooth pins should be avoided because of the risk of migration. Open reduction should be performed in conjunction with a general, vascular or thoracic surgeon assisting or available in the event of unrecognized or iatrogenic injury of the great vessels.
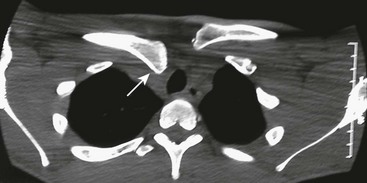
FIGURE 18-8 A 16-year-old male injured his right clavicle when he was checked into the board while playing hockey. He complained of difficulty swallowing. Anteroposterior radiograph of the right clavicle appeared normal. CT scan with thin cuts through the sternoclavicular joint shows posterior displacement (arrow) of the medial end of the clavicle.
Fractures about the proximal humerus can usually be treated nonoperatively. In all age groups, the tremendous arc of motion in the shoulder joint allows a fairly large margin for fracture alignment. In the younger child, the rapid growth of the proximal humeral physis, which accounts for about 80% of the length of the bone, contributes to rapid and predictable remodeling of all but the most angulated fractures. In these children, no treatment other than immobilization for comfort is necessary.185,186 Markedly displaced fractures in the teenager, however, may not remodel because there is not sufficient remaining growth.186–188 Most injuries in this age group are Salter Harris type II fractures, which are usually not stable in addition to being significantly angulated and displaced. These fractures need to be reduced and usually require fixation with percutaneous Steinmann pins or cannulated screws.189,190
Fractures about the elbow in children can be difficult to diagnose because the anatomy of the immature elbow is confusing due to the presence of numerous centers of ossification. Knowledge of the sequence of appearance and maturation of the secondary ossification centers is mandatory. A comparison radiograph of the contralateral elbow is often helpful in correctly identifying the nature of the injury.191
The most common fracture about the distal humerus in the child is a supracondylar fracture. These fractures are classified according to the amount of displacement. Type III fractures are the most severe, with both fragments completely displaced. The injury usually occurs from a fall on the outstretched hand. In children who are ligamentously lax, the elbow will hyperextend and shear off the distal portion of the humerus through the olecranon fossa.192
The major problems with this injury are swelling and nerve and/or vascular injury. In the past, it has been thought that this fracture should be treated immediately. However, we now recognize that this fracture does not need immediate operative stabilization unless there are other extenuating circumstances, such as vascular injury, compartment syndrome, or an open wound. Currently, the general policy is to delay treatment until the next day. Initially, the elbow is splinted in less than 90° of flexion with a loose bandage over a posterior splint.193,194 These fractures can usually be reduced by closed manipulation and stabilized by percutaneous pins (Fig. 18-9).
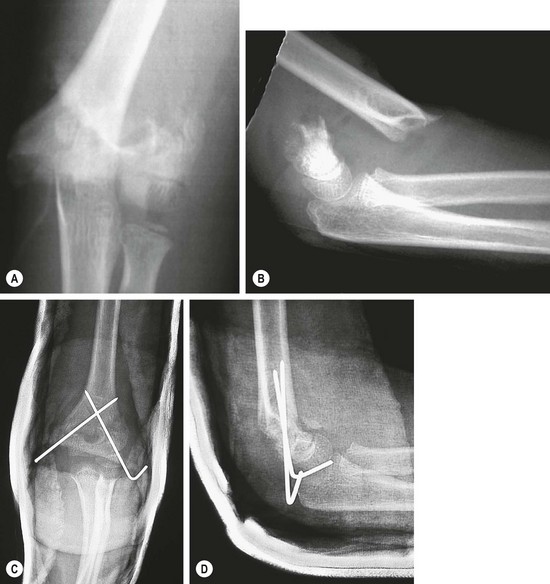
FIGURE 18-9 A 6-year-old fell while horseback riding and landed on his outstretched left arm. (A) Anteroposterior and (B) lateral radiographs show a completely displaced (type III) supracondylar humerus fracture. Neurovascular status of the extremity was intact. (C,D) The child was treated with closed reduction and percutaneous fixation with smooth Steinmann pins.
There has long been a controversy as to the treatment of the pulseless extremity in patients who have sustained a supracondylar fracture. Absence of the pulse with this fracture is not uncommon. It is believed that the absence of the pulse may either be the result of vascular spasm and/or direct vascular injury. However, the collateral circulation about the elbow is so rich that the circulation to the forearm and hand usually remains normal. Treatment of the vascular injury has been debated for decades in the orthopedic and vascular surgery literature. The current practice is observation as long as circulation to the hand and forearm is clinically normal.192,193,195 It has been shown by follow-up studies that vein grafts of vascular injuries will frequently clot because of the excellent collateral circulation about the elbow.196 The only true indication for vascular exploration is the pulseless, ischemic extremity which is a true surgical emergency. In this instance, the fracture should be reduced and stabilized with crossed pins before vascular repair.197,198
Compartment syndrome is a feared complication that is uncommon in the modern era. Stabilizing the fracture with internal fixation avoids the need to immobilize the elbow in hyperflexion, which has been shown to increase the risk of vascular compression and forearm compartment swelling.192
The signs of compartment syndrome are well known, but the main one is pain that is out of proportion to the fracture itself. Once this fracture is stabilized, the child should be comfortable and not have significant pain. Passive extension of the fingers should be possible to a neutral position. If not, it suggests the need for investigation of a compartment syndrome by removal of the splint, palpation of the forearm compartment, and pressure measurements, if necessary. If the pressures are elevated, then fasciotomy should be urgently performed.
Salter Harris type I fractures of the distal humerus are less common than other injuries about the elbow and are frequently misdiagnosed. In very young children, this fracture often occurs as the result of nonaccidental trauma and should trigger investigation into the possibility of abuse.199 This fracture may also occur in newborns as a result of birth trauma.192 Unfortunately, especially in instances of nonaccidental trauma, the child is commonly seen a significant time after this injury has already begun to heal, and manipulation of the fracture is either not possible or ill advised (Fig. 18-10).
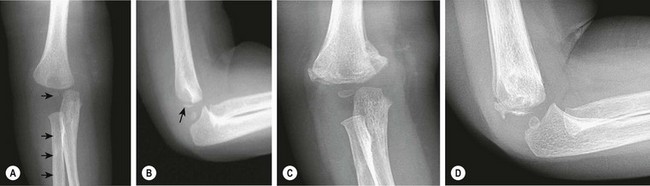
FIGURE 18-10 An 11-month-old infant, ultimately determined to have been the victim of abuse, presented with a swollen arm. (A) On the anteroposterior radiograph of the elbow, the capitellum, the proximal radius, and the ulna are displaced from their normal positions relative to the distal humerus (arrows), consistent with a fracture-separation of the distal humeral epiphysis. In an elbow dislocation, the radius and ulna are displaced relative to the distal humerus but the capitellum is not displaced from its normal position in the distal humerus. (B) The lateral radiograph of the elbow shows a small metaphyseal fragment (arrow), also consistent with a fracture of the distal humeral physis and not with dislocation of the elbow. This fracture was treated with cast immobilization. (C,D) Note the exuberant fracture callus 3 weeks after the injury.
Fractures of the lateral condyle of the humerus must also be distinguished from the Salter Harris type I fractures of the distal humeral physis (Fig. 18-11). If the condylar fracture is displaced at all, it requires open reduction and pin fixation because the risk of nonunion is high when it is treated nonoperatively. Nonunion will result in a progressive valgus deformity of the elbow with an ulnar nerve palsy.200–202
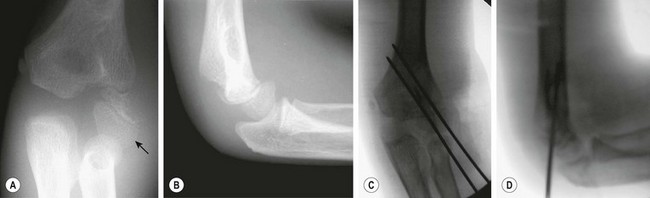
FIGURE 18-11 A 7-year-old child fell from a bunk bed, resulting in a fracture of the lateral condyle of the humerus. (A) Anteroposterior and (B) lateral radiographs show displacement of the capitellum (arrow), whereas the radius and ulna remain aligned with the humerus. Even if they were minimally displaced, the risk of joint incongruity and nonunion is high after nonoperative treatment of this fracture. (C) Anteroposterior and (D) lateral radiographs of the elbow after open reduction and pin fixation show anatomic alignment.
Forearm and wrist fractures have traditionally been treated with closed manipulation and casting. Our preference is to perform closed reduction of these fractures in the emergency department with conscious sedation.203,204 The use of a portable fluoroscopy unit is essential, both to guide reduction of the fracture and to confirm alignment following immobilization. Use of a mini-C-arm improves reduction quality, decreases need for repeat reduction, and results in less overall radiation exposure.205 After manipulation of the fracture, the extremity is immobilized in a sugar tong splint that will allow for swelling. When swelling is no longer a concern, the sugar tong splint is incorporated into a long-arm, fiberglass cast. In general, the child should be seen weekly for the first three weeks to be certain that the alignment of the fracture is maintained. After 3 weeks, a short arm cast is applied for another 3 weeks after which progressive use and motion is started.
Fractures of the shaft of both bones of the forearm have a limited range of acceptable residual deformity to avoid loss of motion, particularly in children older than the age of 8 or 9 years. It may be difficult to maintain reduction of these fractures in a cast or splint. For this reason, fractures of the shafts of the radius and ulna are being treated by internal fixation using flexible titanium nails with increasing frequency.172,173,176,206–214 These fractures can be reduced by closed manipulation and stabilized by percutaneous insertion of titanium or stainless steel intramedullary nails, although open reduction may be required approximately in 29–44% of closed fractures (Fig. 18-12).172,173,176

FIGURE 18-12 (A) Anteroposterior and (B) lateral radiographs show grade 1 open forearm fractures in an 8-year-old. (C,D) Following debridement, the fracture was stabilized with flexible titanium nails inserted via 1 cm incisions, distally in the radius and proximally in the ulna, and advanced across the fracture under fluoroscopy.
References
1. Smith, MD, Burrington, JD, Woolf, AD. Injuries in children sustained in free falls: An analysis of 66 cases. J Trauma. 1975; 15:987.
2. Starfield, B. Childhood Morbidity: Comparisons, Clusters, and Trends. Pediatrics. 1991; 88:519.
3. Landin, LA. Fracture patterns in children. Acta Orthop Scand. 1983; 54(Suppl. 202):1.
4. Worlock, P, Stower, M. Fracture patterns in Nottingham children. J Pediatr Orthop. 1986; 6:656.
5. Ward, WT, Rihn, JA. The impact of trauma in an urban pediatric orthopaedic practice. J Bone Joint Surg Am. 2006; 88:2759–2764.
6. Tuason, D, Hohl, JB, Levicoff, E, et al. Urban pediatric orthopaedic surgical practice audit: Implications for the future of this subspecialty. J Bone Joint Surg Am. 2009; 91:2992–2998.
7. Cheng, JC, Shen, WY. Limb fracture pattern in different pediatric age groups: A study of 3350 children. J Orthop Trauma. 1993; 7:15.
8. Reed, MH. Fractures and dislocations of the extremities in children. J Trauma. 1977; 17:351.
9. Masterson, E, Borton, D, Foster, BK. Victims of our climate. Injury. 1993; 24:247.
10. Shank LP, Bagg RJ, Wagnon J, eds. Etiology of pediatric fractures: The fatigue factors in children’s fractures. Indianapolis: National Conference of Pediatric Trauma, 1992.
11. Westfelt, JARN. Environmental factors in childhood accidents: A prospective study in Goteborg, Sweden. Acta Paediatr Scand. (Suppl. 291):1982.
12. Izant, RJ, Hubay, CA. The annual injury of 15,000,000 children: A limited study of childhood accidental injury and death. J Trauma. 1966; 6:65.
13. Ong, ME, Ooi, SB, Manning, PG. A review of 2,517 childhood injuries seen in a Singapore emergency department in 1999–mechanisms and injury prevention suggestions. Singapore Med J. 2003; 44:12–19.
14. Wilkins, KE. The Incidence of Fractures in Children. In: Rockwood CE, Jr., Wilkins KE, Beaty JH, eds. Fractures in Children. Philadelphia: Lippincott-Raven; 1996:1–17.
15. Brownell, M, Friesen, D, Mayer, T. Childhood injury rates in Manitoba: socioeconomic influences. Can J Public Health. 2002; 93(Suppl 2):S50–S56.
16. Johnson, CF. Inflicted injury versus accidental injury. Pediatr Clin North Am. 1990; 37:791–814.
17. Schmitt, BD, Gray, JD, Britton, HL. Child Abuse. In: Green M, Haggerty RJ, eds. Ambulatory. Pediatrics III: W.B. Saunders, 1984.
18. Akbarnia, BA, Akbarnia, NO. The role of the orthopedist in child abuse and neglect. Orthop Clin North Am. 1976; 7:733–742.
19. Galleno, H, Oppenheim, WL. The battered child syndrome revisited. Clin Orthop. 1982; 62:11–19.
20. O’Connor, JF, Cohen, J. Dating Fractures. In: Kleinman PK, ed. Diagnostic Imaging of Child Abuse. Baltimore: Williams & Wilkins; 1987:6.
21. Mann, DC, Rajmatra, S. Distribution of physeal and non-physeal fractures of long bones in children aged 0 to 16 years. J Pediatr Orthop. 1990; 10:713.
22. Marcus, RE, Mills, MF, Thompson, GH. Multiple injury in children. JBJS. 1983; 65:1290.
23. Ogden, J. Skeletal Injury in the Child, 2nd ed. Philadelphia: Lea & Feibeger; 1990.
24. Ogden, J. Complications of Fractures. Philadelphia: J.B. Lippincott; 1995.
25. Salter, RB, Harris, WR. Injuries involving the epiphyseal plate. JBJS Am. 1963; 45:587.
26. Barmada, A, Gaynor, T, Mubarak, S. Premature physeal closure following distal tibia physeal fractures: A new radiographic predictor. J Pediatr Orthop. 2003; 23:733–739.
27. Kling, TFJ, Bright, RW, Hensinger, RN. Distal tibial physeal fractures in children that may require open reduction. J Pediatr Orthop. 1984; 66:647–657.
28. Spiegel, PG, Mast, JW, Cooperman, DR, et al. Epiphyseal fractures of the distal ends of the tibia and fibula. A retrospective study of two hundred and thirty-seven cases in children. JBJS Am. 1978; 60:1046–1050.
29. Mendez, AA, Bartal, E, Grillot, MB, et al. Compression (Salter Harris type V) physeal fracture: An experimental model in the rat. J Pediatr Orthop. 1992; 12:29.
30. Wilber, JH, Thompson, GH. The Multiply Injured Child. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 3rd ed. Philadelphia: Saunders; 2003:73–101.
31. Morrison, A, Stone, DH, Redpath, A, et al. Childhood injury mortality in Scotland, 1981-95. Health Bull (Edinb). 1999; 57:241–246.
32. Stewart, DG, Jr., Kay, RM, Skaggs, DL. Open fractures in children. Principles of evaluation and management. J Bone Joint Surg Am. 2005; 87:2784–2798.
33. Gustillo, R, Mendoza, R, Williams, D. Problems in the management of type III (severe) open fractures: A new classification of Type III open fractures. J Trauma. 1984; 24:747–796.
34. Gustilo, RB, Anderson, JT. Prevention of infection in treatment of 1025 open fractures of long bones: Retrospective and prospective analysis. JBJS Am. 1976; 58:453–458.
35. Gustilo, RB, Merkow, RL, Templeman, D. Current Concepts Review: The Management of Open Fractures. JBJS Am. 1990; 72:299–304.
36. Chapman, MW. The use of immediate internal fixation in open fractures. Orthop Clin North Am. 1980; 11:579–591.
37. Kindsfater, K, Jonassen, EA. Osteomyelitis in grade II and III open tibia fractures with late debridement. J Orthop Trauma. 1995; 9:121–127.
38. Rohmiller MT, Kusuma S, Blanchard GM, et al, eds. Management of Open Fractures of the Lower Extremity: Does Time to Debridement and Primary Wound Closure Really Matter? Toronto, Ontario, Canada: OTA, 2002.
39. Skaggs, DL, Friend, L, Alman, B, et al. The effect of surgical delay on acute infection following 554 open fractures in children. J Bone Joint Surg Am. 2005; 87:8–12.
40. Charalambous, CP, Siddique, I, Zenios, M, et al. Early versus delayed surgical treatment of open tibial fractures: Effect on the rates of infection and need of secondary surgical procedures to promote bone union. Injury. 2005; 36:656–661.
41. Iobst, CA, Tidwell, MA, King, WF. Nonoperative management of pediatric type I open fractures. J Pediatr Orthop. 2005; 25:513–517.
42. Khatod, M, Botte, MJ, Hoyt, DB, et al. Outcomes in open tibia fractures: relationship between delay in treatment and infection. J Trauma. 2003; 55:949–954.
43. Skaggs, DL, Kautz, SM, Kay, RM, et al. Effect of delay of surgical treatment on rate of infection in open fractures in children. J Pediatr Orthop. 2000; 20:19–22.
44. Spencer, J, Smith, A, Woods, D. The effect of time delay on infection in open long-bone fractures: a 5-year prospective audit from a district general hospital. Ann R Coll Surg Engl. 2004; 86:108–112.
45. Yang, EC, Eisler, J, Treatment of isolated type I open fractures: is emergent operative debridement necessary? Clin Orthop Relat Res 2003;(410):289–294.
46. Vollman, D, Smith, GA. Epidemiology of lawn-mower-related injuries to children in the United States, 1990-2004. Pediatrics. 2006; 118:e273–e278.
47. Demetriades, D, Karaiskakis, M, Velmahos, GC, et al. Pelvic fractures in pediatric and adult trauma patients: Are they different injuries? J Trauma. 2003; 54:1146–1151.
48. Grisoni, N, Connor, S, Marsh, E, et al. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002; 16:458–463.
49. Stewart, BG, Rhea, JT, Sheridan, RL, et al. Is the screening portable pelvis film clinically useful in multiple trauma patients who will be examined by abdominopelvic CT? Experience with 397 patients. Emerg Radiol. 2002; 9:266–271.
50. Azouz, EM, Karamitsos, C. Types and complications of femoral neck fractures in children. Pediatr Radiol. 1993; 23:415–420.
51. Bagatur, AE, Zorer, G. Complications associated with surgically treated hip fractures in children. J Pediatr Orthop B. 2002; 11:219–228.
52. Canale, ST. Fractures of the hip in children and adolescents. Orthop Clin North Am. 1990; 21:341–352.
53. Maeda, S, Kita, A, Fujii, G, et al. Avascular necrosis associated with fractures of the femoral neck in children: Histological evaluation of core biopsies of the femoral head. Injury. 2003; 34:283–286.
54. Moon, ES, Mehlman, CT. Risk factors for avascular necrosis after femoral neck fractures in children: 25 Cincinnati cases and meta-analysis of 360 cases. J Orthop Trauma. 2006; 20:323–329.
55. Morsy, HA. Complications of fracture of the neck of the femur in children. A long-term follow-up study. Injury. 2001; 32:45–51.
56. Ng, GP, Cole, WG. Effect of early hip decompression on the frequency of avascular necrosis in children with fractures of the neck of the femur. Injury. 1996; 26:419–421.
57. Pape, H, Kretteck, C, Friedrich, A, et al. Long-term outcome in children with fractures of the proximal femur after high energy trauma. J Trauma. 1999; 46:58–64.
58. Ratliff, A. Fractures of the neck of the femur in children. J Bone Joint Surg Br. 1962; 44:528–554.
59. Shrader, MW, Jacofsky, DJ, Stans, AA, et al. Femoral neck fractures in pediatric patients: 30 years’ experience at a level 1 trauma center. Clin Orthop Relat Res. 2007; 454:169–173.
60. Mirdad, T. Fractures of the neck of femur in children: An experience at the Aseer Central Hospital, Abha, Saudi Arabia. Injury. 2002; 33:823–827.
61. Theruvil, B, Kapoor, V. Avascular necrosis associated with fractures of the femoral neck in children: Histological evaluation of core biopsies of the femoral head. Injury. 2005; 36:230–231.
62. Togrul, E, Bayram, H, Gulsen, M, et al. Fractures of the femoral neck in children: Long-term follow-up in 62 hip fractures. Injury. 2005; 36:123–130.
63. Dhammi, IK, Singh, S. Displaced femoral neck fracture in children and adolescents: Closed versus open reduction–a preliminary study. J Orthop Sci. 2005; 10:173–179.
64. Flynn, JM, Wong, KL. Displaced fractures of the hip in children. Management by early operation and immobilization in a hip spica cast. J Bone Joint Surg Br. 2002; 84:108–112.
65. Forster, N, Ramseier, L. Undisplaced femoral neck fractures in children have a high risk of secondary displacement. J Pediatr Orthop. 2006; 15:131–133.
66. Song, KS, Kim, YS, Sohn, SW, et al. Arthrotomy and open reduction of the displaced fracture of the femoral neck in children. J Pediatr Orthop B. 2001; 10:205–210.
67. Nork, SE, Bellig, GJ, Woll, JP, et al, Overgrowth and outcome after femoral shaft fracture in children younger than 2 years. Clin Orthop 1998;(357):186–191.
68. Scherl, SA, Miller, L, Lively, N, et al, Accidental and nonaccidental femur fractures in children. Clin Orthop 2000;(376):96–105.
69. Solga, P. Pediatric femur fractures: Treatment in the year 2007. Med Health R I. 2007; 90:122–126.
70. Lynch, JM, Gardner, MJ, Gains, B. Hemodynamic significance of pediatric femur fractures. J Pediatr Surg. 1996; 31:1358–1361.
71. Cassinelli, EH, Young, B, Vogt, M, et al. Spica cast application in the emergency room for select pediatric femur fractures. J Orthop Trauma. 2005; 19:709–716.
72. Czertak, DJ, Hennrikus, WL. The treatment of pediatric femur fractures with early 90-90 spica casting. J Pediatr Orthop. 1999; 19:229–232.
73. Hughes, BF, Sponseller, PD, Thompson, JD. Pediatric femur fractures: effects of spica cast treatment on family and community. J Pediatr Orthop. 1995; 15:457–460.
74. Infante, AF, Jr., Albert, MC, Jennings, WB, et al, Immediate hip spica casting for femur fractures in pediatric patients. A review of 175 patients. Clin Orthop Relat Res 2000;(376):106–112.
75. Flynn, JM, Hresko, T, Reynolds, RA, et al. Titanium elastic nails for pediatric femur fractures: A multicenter study of early results with analysis of complications. J Pediatr Orthop. 2001; 21:4–8.
76. Flynn, JM, Luedtke, L, Ganley, TJ, et al. Titanium elastic nails for pediatric femur fractures: Lessons from the learning curve. Am J Orthop. 2002; 31:71–74.
77. Heinrich, SD, Drvaric, D, Darr, K, et al. Stabilization of pediatric diaphyseal femur fractures with flexible intramedullary nails (a technique paper). J Orthop Trauma. 1992; 6:452–459.
78. Ho, CA, Skaggs, DL, Tang, CW, et al. Use of flexible intramedullary nails in pediatric femur fractures. J Pediatr Orthop. 2006; 26:497–504.
79. Kraus, R, Schiefer, U, Schafer, C, et al. Elastic stable intramedullary nailing in pediatric femur and lower leg shaft fractures: Intraoperative radiation load. J Pediatr Orthop. 2008; 28:14–16.
80. Lee, SS, Mahar, AT, Newton, PO. Ender nail fixation of pediatric femur fractures: A biomechanical analysis. J Pediatr Orthop. 2001; 21:442–445.
81. Mehlman, CT, Nemeth, NM, Glos, DL. Antegrade versus retrograde titanium elastic nail fixation of pediatric distal-third femoral shaft fractures: A mechanical study. J Orthop Trauma. 2006; 20:608–612.
82. Sink, EL, Gralla, J, Repine, M. Complications of pediatric femur fractures treated with titanium elastic nails: A comparison of fracture types. J Pediatr Orthop. 2005; 25:577–580.
83. Hedequist, D, Bishop, J, Hresko, T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008; 28:6–9.
84. Hedequist, DJ, Sink, E. Technical aspects of bridge plating for pediatric femur fractures. J Orthop Trauma. 2005; 19:276–279.
85. Kanlic, EM, Anglen, JO, Smith, DG, et al, Advantages of submuscular bridge plating for complex pediatric femur fractures. Clin Orthop Relat Res 2004;(426):244–251.
86. MacNeil, JA, Francis, A, El-Hawary, R. A systematic review of rigid, locked, intramedullary nail insertion sites and avascular necrosis of the femoral head in the skeletally immature. J Pediatr Orthop. 2011; 31:377–380.
87. Hedequist, D, Starr, AJ, Wilson, P, et al. Early versus delayed stabilization of pediatric femur fractures: Analysis of 387 patients. J Orthop Trauma. 1999; 13:490–493.
88. Zobel, MS, Borrello, JA, Siegel, MJ, et al. Pediatric knee MR imaging: pattern of injuries in the immature skeleton. Radiology. 1994; 190:397–401.
89. Close, BJ, Strouse, PJ. MR of physeal fractures of the adolescent knee. Pediatr Radiol. 2000; 30:756–762.
90. Riseborough, EJ, Barrett, IR, Shapiro, F. Growth disturbances following distal femora physeal fracture-separation. JBJS Am. 1983; 65:885–893.
91. Karholm, J, Hansson, LI, Svensonn, K. Incidence of tibio-fibular shaft and ankle fractures in children. J Pediatr Orthop. 1982; 2:386–392.
92. Shannak, AO. Tibial fractures in children: Follow-up study. J Pediatr Orthop. 1988; 8:306–310.
93. Hope, PG, Cole, WG. Open fractures of the tibia in children. J Bone Joint Surg [Br]. 1992; 74:546–553.
94. Hynes, D, O’Brien, T. Growth disturbance lines after injury to the distal tibial physis. J Bone Joint Surg [Br]. 1988; 70:231.
95. Rogers, LF. The radiography of epiphyseal injuries. Radiology. 1970; 96:289.
96. Goldberg, VM, Aadalen, R. Distal tibial epiphyseal injuries: The role of athletics in fifty-three cases. Am J Sports Med. 1978; 6:263.
97. Vanhoenacke, FM, Bernaerts, A, Gielen, J, et al. Trauma of the pediatric ankle and foot. J Bone Joint Surg [Br]. 2002; 85:212–218.
98. Weiser, ER, Mencio, GA. Pediatric cervical spine injuries: Assessment and treatment. Semin Spine Surg. 2001; 13:142–151.
99. Eleraky, M, Theodore, N, Adams, M, et al. Pediatric cervical spine injuries: Report of 102 cases and review of the literature. J Neurosurg. 2000; 92(1 Suppl):12–17.
100. Jones, E, Haid, R. Injuries to the pediatric subaxial cervical spine. W.B. Saunders Company; 1991.
101. McGrory, B, Klassen, R, Chao, E, et al. Acute fractures and dislocations of the cervical spine in children and adolescents. J Bone Joint Surg [Am]. 1993; 75:988–995.
102. Givens, T, Polley, K, Smith, G, et al. Pediatric cervical spine injury: A three-year experience. J Trauma. 1996; 41:310–314.
103. Hill, S, Miller, C, Kosnik, E. Pediatric neck injuries: A clinical study. J Neurosurg. 1984; 60:700.
104. Brown, RL, Brunn, MA, Garcia, VF. Cervical spine injuries in children: A review of 103 patients treated consecutively at a level 1 pediatric trauma center. J Pediatr Surg. 2001; 36:1107–1114.
105. Hadden, W, Gillepsie, W. Multiple level injuries of the cervical spine. Injury. 1985; 16:628–633.
106. Heilman, CB, Riesenburger, RI. Simultaneous noncontiguous cervical spine injuries in a pediatric patient. Neurosurg. 2001; 49:1017–1020.
107. Jones, E, Hensinger, R. Injuries of the cervical spine. In: Rockwood W, Beaty, eds. Fractures in Children. Philadelphia: Lippincott-Raven; 1996:1024–1062.
108. Bresnam, J, Adams, F. Neonatal spinal cord transection secondary to intrauterine neck hyperextension in breech presentation; 84. Fatal Neonat Med, 1971.
109. Leventhal, H. Birth injuries of the spinal cord. J Pediatr Orthop. 1960; 56:447–453.
110. Caffey, J. The whiplash shaken infant syndrome. Pediatrics. 1974; 54:396.
111. Swischuck, L. Spinal cord trauma in the battered child syndrome. Radiology. 1977; 92:733.
112. Herzenberg, J, Hensiger, R, Dedrick, D, et al. Emergency transport and positioning of young children who have an injury to the cervical spine. J Bone Joint Surg [Am]. 1989; 71:15.
113. Curran, C, Dietrich, A, Bowman, M, et al. Pediatric cervical spine immobilization: Achieving neutral position? J Trauma. 1995; 39:729–732.
114. Jaffe, DM, Binns, H, Radkowski, MA, et al. Developing a clinical algorithm for early management of cervical spine injury in child trauma victims. Ann Emerg Med. 1987; 16:270–276.
115. Lee, SL, Sena, M, Greenholz, SK, et al. A multidisciplinary approach to the development of a cervical spine clearance protocol: process, rationale, and initial results. J Pediatr Surg. 2003; 38:358–362.
116. Viccellio, P, Simon, H, Pressman, BD, et al. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001; 108:E20.
117. Anderson, RC, Kan, P, Hansen, KW, et al. Cervical spine clearance after trauma in children. Neurosurg Focus. 2006; 20:E3.
118. Avellino, AM, Mann, FA, Grady, MS, et al. The misdiagnosis of acute cervical spine injuries and fractures in infants and children: The 12-year experience of a level I pediatric and adult trauma center. Childs Nerv Syst. 2005; 21:122–127.
119. Bayless, P, Ray, VG. Incidence of cervical spine injuries in association with blunt head trauma. Am J Emerg Med. 1989; 7:139–142.
120. d’Amato, C, Pediatric spinal trauma: Injuries in very young children. Clin Orthop Relat Res 2005;(432):34–40.
121. Davis, J, Phreaner, D, Hoyt, D, et al. The etiology of missed cervical spine injuries. J Trauma. 1993; 34:342–346.
122. Evans, D, Bethem, D. Cervical spine injuries in children. J Pediatr Orthop. 1989; 9:563–568.
123. Finch, G, Barnes, M. Major cervical spine injuries in children and adolescents. J Pediatr Orthop. 1998; 18:811–814.
124. Grogan, EL, Morris, JA, Jr., Dittus, RS, et al. Cervical spine evaluation in urban trauma centers: Lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005; 200:160–165.
125. Lewis, VL, Manson, PN, Morgan, RF. Facial injuries associated with cervical fractures: Recognition patterns and management. J Trauma. 1985; 25:90–93.
126. Patel, JC, Tepas, JJ, 3rd., Mollitt, DL, et al. Pediatric cervical spine injuries: Defining the disease. J Pediatr Surg. 2001; 36:373–376.
127. Lally, KP, Senac, M, Hardin, WD, Jr., et al. Utility of the cervical spine radiograph in pediatric trauma. Am J Surg. 1989; 158:540–541.
128. Smith, T, Skinner, S, Shonnard, N. Persistent synchondrosis of the second cervical vertebra simulating a hangman’s fracture in a child. J Bone Joint Surg [Am]. 1993; 75:892–893.
129. Swischuck, L. Anterior displacement of C2 in children. Physiologic or pathologic? Radiology. 1977; 122(Suppl 2):759–763.
130. Swischuck, L, Swischuck, P, SD, J. Wedging of C3 in infants and children: Usually a normal finding not a fracture. Radiology. 1993; 188:523–526.
131. Cattell, H, Filtzer, D. Pseudosubluxation and other normal variations of the cervical spine in children. J Bone Joint Surg [Am]. 1965; 47:1295–1309.
132. Buhs, C, Cullen, M, Klein, M, et al. The pediatric trauma C-spine: is the ‘odontoid’ view necessary. J Pediatr Surg. 2000; 35:994–997.
133. Adelgais, KM, Grossman, DC, Langer, SG, et al. Use of helical computed tomography for imaging the pediatric cervical spine. Acad Emerg Med. 2004; 11:228–236.
134. Carlan D, Bradbury T, Green N, Mencio GA, eds. The efficacy of helical CT versus conventional radiography of the cervical spine in pediatric trauma. Albuquerque, NM: Pediatric Orthopaedic Society of North America, Annual Meeting, 2008.
135. Hartley, W, Mencio, G, Green, N. Clinical and radiographic algorithm for acute management of pediatric cervical spine trauma. St. Louis, Missouri: Scoliosis Research Society, 32nd Annual Meeting; 1997.
136. Dormans J, ed. The role of MRI in the assessment of pediatric cervical spine injuries in Evaluation and Management of Pediatric Spine Trauma. Orlando, Florida: American Academy of Orthopaedic Surgeons, 67th Annual Meeting Instructional Course Lecture 321, 2000.
137. Flynn, JM, Closkey, RF, Mahboubi, S, et al. Role of magnetic resonance imaging in the assessment of pediatric cervical spine injuries. J Pediatr Orthop. 2002; 22:573–577.
138. Frank, JB, Lim, CK, Flynn, JM, et al. The Efficacy of Magnetic Resonance Imaging in Pediatric Cervical Spine Clearance. Spine. 2002; 27:1176–1179.
139. Dormans, J, Criscitiello, A, Drummond, D, et al. Complications in children managed with immobilization in a halo vest. J Bone Joint Surg [Am]. 1995; 77:1370–1373.
140. Letts, M, Kaylor, D, Gouw, G. A biomechanical analysis of halo fixation in children. J Bone Joint Surg [Am]. 1988; 70B:277–279.
141. Mubarak, S, Camp, J, Vueltich, W, et al. Halo application in the infant. J Pediatr Orthop. 1989; 9:612.
142. Pang, D. Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurg. 2004; 55:1325–1342.
143. Pang, D, Pollack, I. Spinal cord injury without radiographic abnormality in children–the SCIWORA syndrome. J Trauma. 1989; 29:654–664.
144. Pang, D, Wilberger, J. Spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1982; 57:114–129.
145. Sullivan, A. Fractures of the Spine in Children. In: Green N, Swiontowski M, eds. Skeletal Trauma in Children. 2nd ed. Philadelphia: Saunders; 2003:344–371.
146. Kriss, V, Kriss, T. SCIWORA (Spinal Cord Injury Without Radiographic Abnormality) in infants and children. Clin Pediatr. 1996; 35:119–124.
147. Copley, L, Dormans, J. Pediatric cervical spine problems: Developmental evaluation and congenital anomalies. J Am Acad Orthop Surg. 1998; 6:204–214.
148. Pollina, J, Li, V. Tandem spinal cord injuries without radiographic abnormalities in a young child. Pediatr Neurosurg. 1999; 30:263–266.
149. Bosch, PP, Vogt, MT, Ward, WT. Pediatric spinal cord injury without radiographic abnormality (SCIWORA): The absence of occult instability and lack of indication for bracing. Spine (Phila Pa 1976). 2002; 27:2788–2800.
150. Bracken, M, Shepard, M, Collins, W, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury: Results of the second national spinal cord injury study. New England J Med. 1990; 322:1405–1411.
151. Bracken, MB, Shepard, MJ. Treatment of acute spinal cord injury with methylprednisolone: Results of a multicenter randomized clinical trial. J Neurtrauma. 1991; 8(Suppl):47–50.
152. Bracken, MB, Shepard, MJ, Holford, TR. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: Results of the third national acute spinal cord injury randomized controlled trial- National Acute Spinal Cord Injury Study. J Am Med Assoc. 1997; 277:1597–1604.
153. Bracken, MB, Shepard, MJ, Holford, TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow-up: Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. J Neurosurg. 1998; 1998:699–706.
154. Cirak, B, Ziegfeld, S, Knight, V, et al. Spinal injuries in children. J Pediatr Surg. 2004; 39:607–612.
155. Clark, CR, White, AA. Fractures of the dens. J Bone Joint Surg. 1985; 67:1340.
156. Diamond, P, Hansen, CM, Christofersen, MR. Child abuse presenting as a thoracolumbar spinal fracture dislocation: a case report. Pediatr Emerg Care. 1994; 10:83–86.
157. Reynolds, R. Pediatric spinal injury. Curr Opin Pediatr. 2000; 12:67–71.
158. Santiago, R, Guenther, E, Carroll, K, et al. The clinical presentation of pediatric thoracolumbar fractures. J Trauma. 2006; 60:187–192.
159. Hauser, CJ, Visvikis, G, Hinrichs, C, et al. Prospective validation of computed tomographic screening of the thoracolumbar spine in trauma. J Trauma. 2003; 55:228–235.
160. Akbarnia, BA. Pediatric spine fractures. Orthop Clin North Am. 1999; 30:521–536.
161. Banerian, KG, Wang, AM, Samberg, LC, et al. Association of vertebral end plate fracture with pediatric lumbar intervertebral disk herniation: value of CT and MR imaging. Radiology. 1990; 177:763–765.
162. Greenwald, TA, Mann, DC. Pediatric seatbelt injuries: Diagnosis and treatment of lumbar flexion-distraction injuries. Paraplegia. 1994; 32:743–751.
163. Griffet, J, Bastiani-Griffet, F, El-Hayek, T, et al. Management of seat-belt syndrome in children. Gravity of 2-point seat-belt. Eur J Pediatr Surg. 2002; 12:63–66.
164. Johnson, DL, Falci, S. The diagnosis and treatment of pediatric lumbar spine injuries caused by rear seat lap belts. Neurosurgery. 1990; 26:434–441.
165. Newman, KD, Bowman, LM, Eichelberger, MR, et al. The lap belt complex: intestinal and lumbar spine injury in children. J Trauma. 1990; 30:1133–1138.
166. Raney, EM, Bennett, JT. Pediatric Chance fracture. Spine. 1992; 17:1522–1524.
167. Reid, AB, Letts, RM, Black, GB. Pediatric Chance fractures: Association with intra-abdominal injuries and seatbelt use. J Trauma. 1990; 30:384–391.
168. Smith, MD, 2nd., Camp, E, 3rd., James, H, Kelley, HG. Pediatric seat belt injuries. Am Surg. 1997; 63:294–298.
169. Choit, RL, Tredwell, SJ, Leblanc, JG, et al. Abdominal aortic injuries associated with chance fractures in pediatric patients. J Pediatr Surg. 2006; 41:1184–1190.
170. Letts, M, Davidson, D, Fleuriau-Chateau, P, et al. Seat belt fracture with late development of an enterocolic fistula in a child. A case report. Spine. 1999; 24:1151–1155.
171. Lalonde, F, Letts, M, Yang, JP, et al. An analysis of burst fractures of the spine in adolescents. Am J Orthop. 2001; 30:115–120.
172. Hill, JM, McGuire, MH, Crosby, LA. Closed treatment of displaced middle-third fractures of the clavicle gives poor results. J Bone Joint Surg Br. 1997; 79:537–539.
173. Lazarides, S, Zafiropoulos, G. Conservative treatment of fractures at the middle third of the clavicle: the relevance of shortening and clinical outcome. J Shoulder Elbow Surg. 2006; 15:191–194.
174. McKee, MD, Pedersen, EM, Jones, C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006; 88:35–40.
175. Wick, M, Muller, EJ, Kollig, E, et al. Midshaft fractures of the clavicle with a shortening of more than 2 cm predispose to nonunion. Arch Orthop Trauma Surg. 2001; 121:207–211.
176. Zlowodzki, M, Zelle, BA, Cole, PA, et al. Treatment of acute midshaft clavicle fractures: systematic review of 2144 fractures: on behalf of the Evidence-Based Orthopaedic Trauma Working Group. J Orthop Trauma. 2005; 19:504–507.
177. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007; 89:1–10.
178. Golthamer, C. Duplication of the clavicle (“os claviculare”). Radiology. 1957; 68:576–578.
179. Twigg, HL. Duplication of the clavicle. Skeletal Radiol. 1981; 6:281–283.
180. Webb, LX, Mooney, JF. Fractures and dislocations about the shoulder. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia: Saunders; 2003:322–344.
181. Gray, H. Anatomy of the Human Body. Philadelphia: Lea and Febiger; 1985.
182. Groh, GI, Wirth, MA, Rockwood, CA, Jr. Treatment of traumatic posterior sternoclavicular dislocations. J Shoulder Elbow Surg. 2011; 20:107–113.
183. Laffosse, JM, Espie, A, Bonnevialle, N, et al. Posterior dislocation of the sternoclavicular joint and epiphyseal disruption of the medial clavicle with posterior displacement in sports participants. J Bone Joint Surg Br. 2010; 92:103–109.
184. Waters, PM, Bae, DS, Kadiyala, RK. Short-term outcomes after surgical treatment of traumatic posterior sternoclavicular fracture-dislocations in children and adolescents. J Pediatr Orthop. 2003; 23:464–469.
185. Baxter, MP, Wiley, JJ. Fractures of the proximal humeral epiphysis: Their influence on humeral growth. J Bone Joint Surg [Br]. 1986; 68:570–573.
186. Beaty, JH. Fractures of the proximal humerus and shaft in children. Instr Course Lect. 1992; 41:369–372.
187. Dameron, TB, Reibel, DB. Fractures involving the proximal humeral epiphyseal plate. J Bone Joint Surg [Am]. 1969; 51:289–297.
188. Smith, FM. Fracture-separation of the proximal humeral epiphysis. Am J Surg. 1956; 91:627–635.
189. Beebe, A, Bell, DF. Management of severely displaced fractures of the proximal humerus in children. Tech Orthop. 1989; 4:1–4.
190. Loder, RT. Pediatric polytrauma. Orthopaedic care and hospital course. J Orthop Trauma. 1987; 1:48–54.
191. Haraldsson, S. On osteochondritis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand Suppl. 38, 1959.
192. Green, NE. Fractures and dislocations about the elbow. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia: Saunders; 2003:257–322.
193. Green, NE. Overnight delay in the reduction of supracondylar fractures of the humerus in children. J Bone Joint Surg [Am]. 2001; 93:321–322.
194. Mehlman, CT, Strub, WM, Roy, DR. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J Bone Joint Surg [Am]. 2001; 83:323–327.
195. Sabharwal, S, Tredwell, SJ, Beauchamp, RD. Management of pulseless pink hand in pediatric supracondylar fractures of the humerus. J Pediatr Orthop. 1997; 17:303–310.
196. Sabharwal, S. Role of Ilizarov external fixator in the management of proximal/distal metadiaphyseal pediatric femur fractures. J Orthop Trauma. 2005; 19:563–569.
197. Copley, LA, Dormans, JP, Davidson, RS. Vascular injuries and their sequelae in pediatric supracondylar humerus fractures: Toward a goal of prevention. J Pediatr Orthop. 1996; 16:99–103.
198. Schoenecker, PL, Delgado, E, Rotman, M. Pulseless arm in association with totally displaced supracondylar fracture. J Orthop Trauma. 1996; 10:410–415.
199. DeLee, JC, Wilkins, KE, Rogers, LF. Fracture separation of the distal humerus epiphysis. J Bone Joint Surg [Am]. 1980; 62:46–51.
200. Jakob, R, Fowles, JV, Rang, M. Observations concerning fractures of the lateral condyle in children. J Bone Joint Surg Br. 1975; 57:430–436.
201. Jeffrey, C. Nonunion of epiphysis of the lateral condyle of the humerus. J Bone Joint Surg Am. 1958; 40:396–405.
202. Rutherford, A. Fractures of the lateral humeral condyle in children. J Bone Joint Surg Am. 1995; 67:851–856.
203. McCarty, EC, Mencio, GA, Green, NE. Ketamine sedation for the reduction of children’s fractures in the emergency department. J Bone Joint Surg Am. 2000; 82:912–918.
204. McCarty, EC, Mencio, GA, Green, NE. Anesthesia and analgesia for the ambulatory management of fractures in children. J Am Acad Orthop Surg. 1999; 2:81–91.
205. Lee, MC, Stone, NE, 3rd., Ritting, AW, et al. Mini-C-arm fluoroscopy for emergency-department reduction of pediatric forearm fractures. J Bone Joint Surg Am. 2011; 93:1442–1447.
206. Agarwal, A. Treatment of pediatric both bone forearm fractures: a comparison of operative techniques. J Pediatr Orthop. 2007; 27:480–481.
207. Flynn, JM, Waters, PM. Single bone fixation of both bone forearm fractures. J Pediatr Orthop. 1996; 16:655–659.
208. Garg, NK, Ballal, MS, Malek, IA, et al. Use of elastic stable intramedullary nailing for treating unstable forearm fractures in children. J Trauma. 2008; 65:109–115.
209. Griffet, J, FeHayek, T, Baby, M. Intramedullary nailing of forearm fractures in children. J Pediatr Orthop Br. 1999; 8:88–89.
210. Kanellopoulos, AD, Yiannakopoulos, CK, Soucacos, PN. Flexible intramedullary nailing of pediatric unstable forearm fractures. Am J Orthop. 2005; 34:420–424.
211. Lascombes, P, Haumont, T, Journeau, P. Use and abuse of flexible intramedullary nailing in children and adolescents. J Pediatr Orthop. 2006; 26:827–834.
212. Lee, S, Nicol, RO, Stott, NS, Intramedullary fixation for pediatric unstable forearm fractures. Clin Orthop Relat Res 2002;(402):245–250.
213. Luhmann, SJ, Gordon, JE, Schoenecker, PL. Intramedullary fixation of unstable both bone forearm fractures in children. J Pediatr Orthop. 1998; 18:451–456.
214. Muensterer, OJ, Regauer, MP. Closed reduction of forearm refractures with flexible intramedullary nails in situ. J Bone Joint Surg Am. 2003; 85-A:2152–2155.