CHAPTER 26 Pediatric Kyphosis
Scheuermann Disease and Congenital Deformity
The normal adult spine has four curves in the sagittal plane. In utero and at birth, there are two primary kyphotic curves in the thoracic spine and sacrococcygeal region. The lordotic curves in the cervical and lumbar spine are compensatory curves and develop as a child holds his or her head upright and begins to stand and walk.1 Lordotic curves are considered secondary and compensate for the degree of kyphosis in the primary curves to allow a balanced spine in the sagittal plane.
There is great variability in defining the normal range of thoracic kyphosis. As measured by the Cobb method, the normal range of thoracic kyphosis is 20 to 45 degrees.2,3 Normal kyphosis increases with age and is slightly greater in women.3 Thoracic kyphosis is measured on a lateral radiograph using the Cobb method from the superior endplate of T2 to T5 depending on visibility to the inferior endplate of T12. The thoracolumbar junction is normally neutral or slightly lordotic (0 to 10 degrees of lordosis). Any degree of kyphosis at the thoracolumbar junction is considered abnormal. Lumbar lordosis is measured from the superior endplate of L1 to the superior endplate of S1, and normal values are 40 to 65 degrees.
The importance of achieving a neutral sagittal balance has been emphasized in recent years in the evaluation and treatment of various kyphotic deformities. The C7 plumb line should fall through the posterosuperior corner of the L5-S1 disc space. If it falls anterior to this point, there is positive sagittal balance, and if it falls posterior to this point, there is negative sagittal balance. Jackson and McManus4 reported values in asymptomatic adults with a mean sagittal vertical axis offset of 0.5 cm (± 2.5 cm SD). According to these data, offset greater than 2.5 cm anteriorly or posteriorly is considered beyond the normal range. A positive sagittal balance is poorly tolerated because intradiscal pressures increase in the lumbar spine, and the posterior spinal musculature is placed at a mechanical disadvantage leading to back pain.
Scheuermann Disease
In 1921, the Danish radiologist Scheuermann described a pathologic condition and distinguished it from passively correctable postural humpback when he noted the development of painful fixed kyphosis in 105 children.5 Scheuermann likened the entity to the femoral head abnormality described by Calvé and Perthes and named it osteochondritis deformans juvenilis dorsi. Several terms have been used in the past to describe this entity, including kyphosis dorsalis juvenilis, but Scheuermann disease and Scheuermann kyphosis are the most common.6
Scheuermann disease is the most common cause of severe thoracic kyphosis in adolescents with reported prevalence of 1% to 8%.7–9 The prevalence is approximately equal in boys and girls. Approximately one third of patients have concomitant scoliosis, which is usually mild.10
In 1964, Sorensen11 defined the radiographic criteria that have now become widely accepted for diagnosing Scheuermann disease: anterior vertebral wedging greater than 5 degrees on three or more consecutive vertebrae at the apex of the curve. Associated radiographic findings include endplate irregularities and Schmorl nodes (herniation of disc into vertebral endplates). Schmorl nodes are not specific to Scheuermann disease and can be found in various conditions. Scheuermann disease is typically diagnosed at age 10 to 12 years. Sorenson’s criteria are typically not present in patients younger than age 10 because the ring apophysis has not ossified before this age.
There are two curve patterns in Scheuermann disease. The thoracic type is most common, and its apex is located between T7 and T9. The thoracolumbar type has also been referred to as “atypical” Scheuermann disease; its apex is located between T10 and T12, and it is more likely to become symptomatic in adult life.10 Vertebral endplate changes, Schmorl nodes, and disc space narrowing are much more common in the thoracolumbar form of Scheuermann disease. Sorenson’s criteria (three consecutive wedged vertebrae) are unnecessary to diagnose the thoracolumbar form of Scheuermann disease.
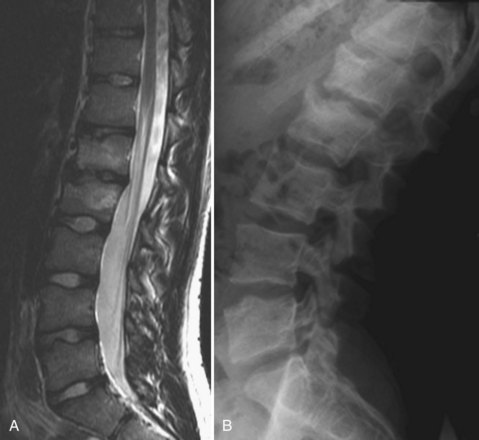
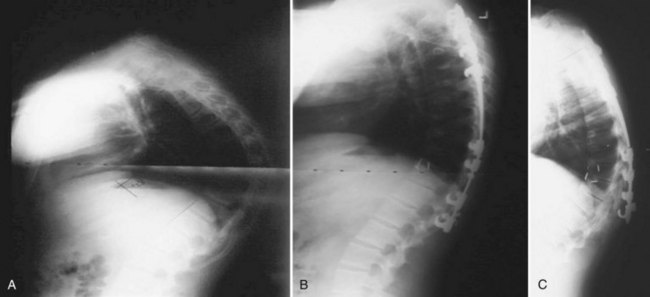
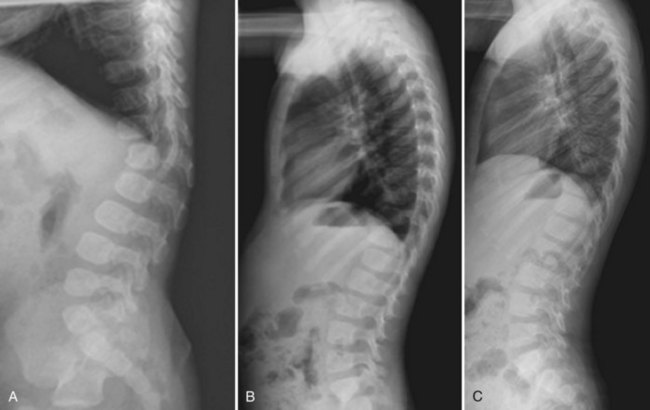
Lumbar Scheuermann disease is a distinct entity in which significant degenerative changes are present in the lumbar spine (typically L1-4) without vertebral wedging or significant kyphotic deformity (Fig. 26–1). Schmorl nodes and endplate irregularities are common. Lumbar Scheuermann disease is more common in males, especially laborers who engage in heavy lifting activites.
Etiology
Although the etiology of Scheuermann disease is unknown, several theories exist. Scheuermann’s initial description suggested that the condition results from avascular necrosis of the vertebral ring apophyses, which leads to a premature growth arrest with resultant wedging of the anterior portion of the vertebral bodies, and mentioned that it resembled Legg-Calvé-Perthes disease of the hip.5,6 Schmorl and Junghans12 hypothesized that herniation of disc material into the vertebral body endplates occurred as a result of inherent weakening of the cartilaginous endplate, with resultant damage to the endplate causing growth disturbance and kyphosis. Schmorl nodes are not specific to kyphotic deformities, however, and are found in normal spines.
Genetic factors have also been proposed. An autosomal dominant inheritance pattern with incomplete penetrance and variable expression in families with Scheuermann disease has been described.13–15 Three cases of Scheuermann kyphosis in monozygotic twins have been reported in the English literature,11,16,17 supporting the genetic etiology hypothesis.
Other etiologies of the deformity have been attributed to defective endplates, upright posture, juvenile osteoporosis, increased release of growth hormone, defective formation of collagen fibrils with subsequent weakening of vertebral endplates, strenuous manual labor, trauma, vitamin A deficiency, epiphysitis, poliomyelitis, prolonged sitting, and osteochondrosis.18–23 Mechanical factors have also been proposed to play a role in pathogenesis, owing to partial reversal of vertebral wedging with brace treatment and thickening of the anterior longitudinal ligament.10,24
Clinical Evaluation
The clinical evaluation begins with a complete history and physical examination. The deformity is often attributed to poor posture in an adolescent, delaying diagnosis and treatment.10 One should inquire about the onset of the deformity and the location of pain. If the patient has pain, it is usually mild and aggravated by prolonged sitting or exercise and typically is near the apex of the kyphotic deformity.10 Spondylolysis and spondylolisthesis have been noted with an increased incidence in patients with Scheuermann disease and can be a source of low back pain.25 There is a 50% incidence of spondylolysis in Scheuermann kyphosis,25 presumably resulting from increased stress on the pars interarticularis in the lower lumbar spine as a result of hyperlordosis. With significant deformity, the erector spinal musculature is placed at a mechanical disadvantage, which may also contribute to pain that is common with this condition.
Increased lumbar lordosis is often noted in these patients as compensation for the kyphotic deformity to maintain overall sagittal balance. It is important to assess coronal and sagittal balance. Lowe and Kasten26 studied the sagittal contour of 24 patients with Scheuermann kyphosis and found that most patients with Scheuermann kyphosis have negative balance before surgery and have slightly more negative balance after surgery. Lumbar hyperlordosis was reduced from an average of 75 degrees before surgery to 55 degrees after surgery.
Tightness and contracture of pectoral and hamstring muscles is common.27 One should look for cutaneous lesions, foot deformities, or muscle contractures. These may signal an underlying neurologic problem. A complete neurologic examination should be performed. Although neurologic findings are rare in Scheuermann disease, spinal cord compression has been reported.18,28–31 Causes of cord compression include thoracic disc herniation, dural cysts, or severe kyphosis.
Imaging Studies
Routine radiographs include standing posteroanterior and lateral 36-inch films. Sorenson’s criteria (three consecutive wedged vertebrae of ≥5 degrees) are used as diagnostic criteria. On the lateral view, the fists are placed in the supraclavicular fossa to visualize the upper thoracic spine better. Arm position has a tendency to displace the C7 plumb line. Patients with Scheuermann kyphosis typically have normal or negative sagittal balance, however. It is important to be consistent with the methods used when obtaining x-rays in the preoperative and postoperative periods. A supine hyperextension bolster lateral view is useful in assessing the flexibility of the deformity and can help differentiate it from postural kyphosis.32 Other radiographic abnormalities that may be present, such as spondylolysis, scoliosis, disc space narrowing, and endplate irregularities, should be noted.
All patients with a rapidly progressive kyphosis, neurologic abnormalities, or any evidence of congenital kyphosis should undergo magnetic resonance imaging (MRI). One report showed transient paraparesis owing to thoracic spinal stenosis and recommended preoperative MRI in patients undergoing surgical correction.33 Thoracic disc herniations are known to occur with increased frequency in patients with Scheuermann disease.34,35 The issue of whether all surgical patients should undergo MRI preoperatively is controversial; the authors’ practice is to obtain MRI in all patients before surgery to rule out stenosis, disc herniation, or other pathologies.
Natural History
Several early studies suggested an ominous natural history for Scheuermann disease, with significant back pain, embarrassment about physical appearance, interference with social functioning, and cardiopulmonary failure.11,36–40 Ponte and colleagues36 showed in their series that all curves greater than 45 degrees progressed during the adolescent growth spurt and continued to increase after age 30. Sorensen11 reported a 50% incidence of pain during the adolescent growth spurt, and a high rate of pain was noted in patients with kyphosis greater than 60 degrees.37 Other studies noted the often unremitting and incapacitating nature of the pain, progressive nature of the deformity, risk of cardiopulmonary failure, and unacceptable appearance of the deformity in untreated adults.38–40 Aggressive surgical treatment was recommended to prevent these problems in the future.40
In 1993, Murray and colleagues18 provided the first long-term follow-up study on the natural history of untreated Scheuermann disease in patients with an average age of 53 years and average kyphosis of 71 degrees. These investigators found that 64% of patients (compared with 15% of controls) reported back pain. The proportion of patients who had pain that interfered with their daily lives was not significantly different, however, from control subjects. Their data suggested that although patients with Scheuermann disease may have some functional limitations, they do not have major interference with their lives. Murray and colleagues18 found that their patients adapted reasonably well to this condition and recommended that surgical treatment should be carefully reviewed. Other reports have also suggested the generally benign natural history of this condition.41–43
The natural history is more favorable when the deformity is in the thoracic spine, rather than the thoracolumbar spine. Back pain is much more common in the latter. It is believed that thoracolumbar kyphosis has a higher incidence of progression because of the lack of support provided by the surrounding rib cage.44 When a thoracolumbar kyphosis exceeds 50 to 55 degrees, the deformity is readily apparent, especially in thin patients, and pain is common.44 The thoracolumbar spine is typically neutral to slightly lordotic, and any degree of kyphosis may be clinically apparent. In adults, degenerative disc disease is frequently seen at the apical segments of the kyphosis and may be a source of back pain.45,46
Although progression of deformity can be rapid during the adolescent growth spurt, it is unknown whether the deformity would progress after skeletal maturity is reached. Thoracic curves greater than 80 degrees and thoracolumbar curves greater than 55 to 60 degrees may be at risk of progression after skeletal maturity, although the true incidence of progression is unknown.10,44 If nonsurgical treatment is chosen for curves of this magnitude, periodic follow-up is recommended into adulthood.
Pulmonary failure secondary to severe deformity is very rare. Murray and colleagues18 found that lung volume, lung mechanics, and diffusing capacity were not significantly affected and were normal in patients with curves less than 100 degrees. They found that as the deformity reaches 100 degrees, restrictive lung disease occurs more often when the curve apex is between T1 and T8.
Nonsurgical Treatment
The use of the Milwaukee brace for the treatment of scoliosis was first reported by Blount and colleagues in 1958.47 In 1959, Moe began treating Scheuermann kyphosis with the Milwaukee brace.38 In 75 patients who completed treatment, the kyphosis improved by 40%, and the vertebral wedging improved by 42%. Bradford and colleagues38 reported factors that limited the amount of correction included kyphosis greater than 65 degrees, skeletal maturity, and vertebral wedging averaging more than 10 degrees.
Sachs and colleagues32 found that curves less than 74 degrees in skeletally immature patients can be successfully treated in a Milwaukee brace. They reported 120 patients with Scheuermann disease who were treated with the Milwaukee brace with more than 5 years of follow-up; 76 patients had improvement in kyphosis, 10 patients had no change in kyphosis, and 24 patients had worsening of deformity compared with initial studies. One third of patients with an initial kyphosis of 75 degrees or greater subsequently underwent surgery. Results showed that treatment with the Milwaukee brace consistently improved kyphosis by approximately 50% during the active phase of treatment, but some loss of correction occurred over time. The final result showed improvement in 69% of patients.
Gutowski and Renshaw48 reported 75 patients treated with either a Milwaukee or Boston brace. For compliant patients, the average improvement in kyphosis was 27% in the Boston orthosis group and 35% in the Milwaukee orthosis group, despite the fact that patients in the former group were younger and had smaller, more flexible curves. Compliance with orthosis wearing was twice as likely with the Boston orthosis (61% compliance vs. 29% compliance for Milwaukee orthosis). Results in patients who wore their orthoses at least 16 hours per day were equal to results in patients with 23 hours of daily wear. The Boston brace provided satisfactory correction in curves less than 70 degrees and had better compliance. For larger curves, a Milwaukee brace was recommended.
Ponte and colleagues36 reported on 1043 patients treated with casts for 8 to 16 months, followed by Milwaukee brace and physical therapy until skeletal maturity. Patients had a mean initial curve of 57 degrees and at 3-year follow-up had a mean 62% wedge improvement and 40% curve correction.
If bracing is chosen, physical therapy should be initiated for trunk stabilization and postural exercises and to stretch the pectoral and hamstring muscles. The brace should be worn for 23 hours a day. To get a meaningful correction with brace treatment in Scheuermann disease, correction of the vertebral wedging deformity by bone remodeling is necessary. In contrast to scoliosis, in which bracing does not correct the curve, bracing in Scheuermann disease may help achieve some curve correction. After 12 to 18 months of bracing, partial reversal of anterior wedging of vertebral bodies is often noted.10 Loss of correction can occur, however, after discontinuation of the brace. Montgomery and Hall24 reported an average loss of correction of 15 degrees in 21 patients 18 months or more after they stopped wearing the brace. Brace treatment initially leads to some correction by opening the disc spaces, which close down again unless sufficient time is allowed to reverse the wedging of the vertebral bodies.
Indications for Surgery
Neurologic compromise is an indication for surgery, but it rarely occurs in Scheuermann disease. Cord compression may be due to disc herniation at the apex, severe kyphosis, and extradural cysts.34 Similarly, pulmonary compromise is rare and does not occur until the curve is greater than 100 degrees.7,18,40,43
Surgical Treatment
The surgical options for Scheuermann disease include posterior-only correction and fusion, combined anterior and posterior fusion, and anterior-only procedures. Historically, the surgical treatment of Scheuermann disease consisted of apical anterior release and fusion followed by posterior fusion. This approach originated from work by Bradford and colleagues49 in 1975, in which they found an unacceptably high rate of correction loss after posterior-only fusion with Harrington compression instrumentation. These investigators reported a 9% pseudarthrosis rate and 23% risk of instrumentation complications. In 1980, work from the same institution showed solid fusion and good maintenance of correction with anterior release and fusion followed by posterior compression instrumentation in 24 patients.39
The efficacy of combined anterior release and posterior fusion is well documented in the literature.39,50–52 Anterior release traditionally has been recommended for patients with severe, rigid deformities that do not correct to less than 50 degrees on a hyperextension lateral radiograph. With use of modern pedicle screw instrumentation, the indications for performing an anterior release are less common, however, and even severe deformities are being successfully treated with a posterior-only approach.
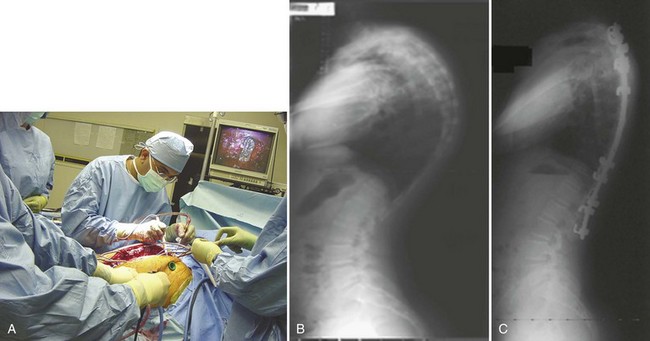
The anterior release can be performed through an open thoracotomy or thoracoabdominal approach depending on the location of the apex of the deformity or can be performed through a video-assisted thoracoscopic surgery approach.53,54 The senior author has achieved excellent results with simultaneous video-assisted thoracoscopic surgery release and posterior fusion and instrumentation in the prone position (Fig. 26–2). The levels requiring anterior release typically include six to eight segments centered around the apex of the deformity. The anterior longitudinal ligament and entire disc are removed, and the space is packed with fibular structural allograft bone. In very severe deformities, an intervening period of halo-femoral traction may be considered between the anterior and posterior procedures.
Anterior release and fusion ensures that correction is reliably achieved and solid fusion is obtained. There is, however, the obvious morbidity of the additional operating time and reported complications including hemothorax, pneumothorax, and pulmonary embolism.7,50,55 There may be potential negative effects on pulmonary function, which may not return to baseline even at 2 years postoperatively.56–58 Although anterior surgery has benefits in achieving correction of severe deformities, the additional surgery is not without consequence.
Over the last 3 decades, several studies have shown good results with posterior-only fusion and instrumentation with various constructs.37,55,59–62 Speck and Chopin37 found posterior-only fusion to be adequate in skeletally immature patients, but that combined anterior and posterior surgery is needed for skeletally mature patients.
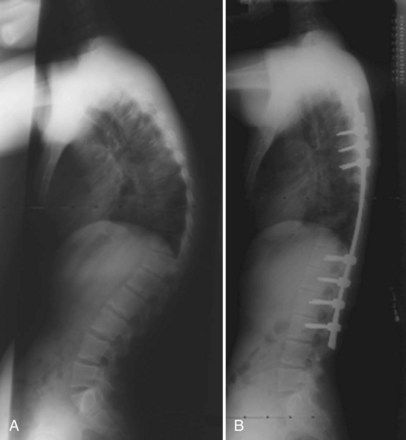
In 1984, Ponte and colleagues63 described shortening of the posterior column by employing multiple osteotomies and posterior compression instrumentation and fusion. The Ponte osteotomy is similar to the Smith-Peterson osteotomy except that it is performed at multiple levels in the thoracic spine (Figs. 26-3 and 26-4). Smith-Peterson’s original osteotomy was in the lumbar spine, at one level, in patients with rheumatoid arthritis.64 After Ponte’s description in 1984,65 this procedure did not gain popularity for many years, especially among North American surgeons, until pedicle screw instrumentation became popular in the thoracic spine. As pedicle screws became more widely used and the ability to obtain strong, three-column fixation in the spine became possible, several studies have documented successful results with posterior-only treatment of more severe deformities.66,67
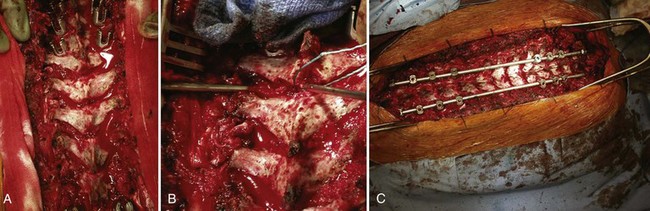
FIGURE 26–4 A-C, Multilevel Ponte osteotomies performed on the patient shown in Figure 26–3. Removal of ligamentum flavum and superior and inferior facets out through neural foramen allows shortening of posterior column and correction of kyphosis.
Lee and colleagues67 compared posterior-only fusion (18 patients) with combined anterior and posterior fusion (21 patients) and found better correction and fewer complications in the posterior-only group at a mean 2-year follow-up. The anterior and posterior group did not have Ponte osteotomies, and hybrid hook and screw constructs were used, whereas the posterior-only group had Ponte osteotomies (in 67%) and had all pedicle screw constructs.
Geck and colleagues66 obtained good correction in 17 patients employing multiple osteotomies and pedicle screw fixation, averaging 9.3 degrees of correction per osteotomy across the apex. There is growing evidence that anterior release may be unnecessary to obtain satisfactory correction of even severe deformities if multilevel segmental osteotomies are performed in conjunction with pedicle screw fixation.66–68 Hosman and colleagues68 suggested that posterior-only correction is often adequate for correcting up to a 100-degree kyphosis to a physiologic range of 40 to 50 degrees without an anterior release.
Surgical Principle
Degree of Deformity Correction
Surgical treatment of Scheuermann kyphosis should aim to correct the thoracic kyphosis to the high-normal range of thoracic kyphosis (40 to 50 degrees). Overcorrection of a kyphotic deformity can lead to neurologic complications, postoperative sagittal malalignment, and proximal junctional kyphosis. The last condition may occur as a result of forces being transferred to the proximal junction after an aggressive corrective maneuver.10,51,68 Lowe and Kasten26 recommended that no more than 50% of the preoperative kyphosis be corrected and that the final kyphosis should never be less than 40 degrees. These authors also found that the negative sagittal balance is worsened postoperatively, and this may predispose patients to junctional kyphosis.26
Selection of Fusion Levels
Proper selection of fusion levels is crucial to avoid complications related to junctional decompensation. The proximal extent of the fusion should be the proximal end vertebra in the measured kyphotic deformity.7,26,55,69 There has been considerable debate regarding the optimal distal extent of the fusion. Traditionally, it has been suggested to extend the distal fusion level to the first lordotic disc beyond the end vertebra to minimize risk of junctional kyphosis.26,60 Some authors have advocated the inclusion of L1,20 including the inferior neutral vertebra, whereas others have advocated including L2 in the fusion. Poolman and colleagues70 advocated the inclusion of the second lordotic disc below the kyphotic deformity. More recently, Cho and colleagues71 emphasized the importance of the sagittal stable vertebra, defined as the most proximal vertebra touched by the posterior sacral vertical line. The posterior sacral vertical line is a line drawn vertically from the posterior-superior corner of the sacrum on the lateral upright radiograph. Cho and colleagues71 found that distal junctional problems were more common when the fusion level was to the first lordotic vertebra rather than down to the sagittal stable vertebra. They recommended extending the distal end of the fusion to the vertebra that touches the posterior sacral vertical line.
Complications
The most worrisome complication of surgical treatment of Scheuermann disease is neurologic deficit. The 1999 Morbidity and Mortality Report of the Scoliosis Research Society reported the risk of neurologic injury during surgery for Scheuermann disease to be 1 per 700 cases. Surgical treatment of kyphotic deformities is associated with a higher incidence of neurologic complications than treatment of coronal deformities because the spinal cord is lengthened anteriorly after correction, which may compromise its anterior vascular supply.72
Neurologic complications during deformity surgery can be caused by direct trauma to the neural elements (during instrumentation or osteotomies); stretch injury to the spinal cord during corrective maneuvers; or a vascular insult to the spinal cord, whether secondary to stretch or disruption of vascular supply or secondary to hypotension.73–75 A preexisting spinal canal stenosis may increase the risk of neurologic injury during correction (Tribus33), and although controversial, the authors believe that preoperative MRI should be obtained to rule out any compressive lesions. Overcorrection of a kyphotic deformity is hazardous to the neural elements because the spinal cord is lengthened, and no more than a 50% correction (from preoperative kyphosis) should be attempted.
Distraction has been shown to induce spinal cord injury in feline studies by a reduction in cord perfusion.76,77 Cantilever forces used during corrective maneuvers increase the length of the anterior column, which may have the same effect as distraction. Caution should be used during correction, which should ideally be a gentle combination of cantilever forces and compressive forces to shorten the posterior column.
Junctional Kyphosis
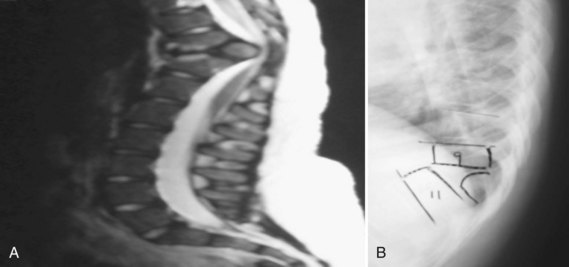
Proximal junctional kyphosis is defined as kyphosis measured from one segment cephalad to the upper end instrumented vertebra to the proximal instrumented vertebra, with an abnormal value defined as 10 degrees or greater (Fig. 26–5).78 Similarly, distal junctional kyphosis is defined as kyphosis measured from one segment caudal to the end instrumented vertebra to the distal instrumented vertebra, with abnormal value again being 10 degrees or more of kyphosis (Fig. 26–6).78 In a retrospective multicenter review of 78 patients with Scheuermann kyphosis treated surgically, there was a 32% incidence of proximal junctional kyphosis and 5% incidence of distal junctional kyphosis.78 The investigators found that proximal junctional kyphosis was related to stopping the fusion caudal to the proximal end vertebra and is influenced by pelvic incidence. Distal junctional kyphosis was always associated with fusion cephalad to the sagittal stable vertebra. Despite the high rate of proximal junctional kyphosis, the problem was clinically problematic or required reoperation in only 5.1% of cases.
Lowe and Kasten26 reported a radiographic rate of proximal junctional kyphosis of 30% ranging from 12 to 49 degrees and of distal junctional kyphosis of 28% ranging from 10 to 30 degrees. In their study, proximal junctional kyphosis was related to greater than 50% correction of the curve magnitude in 5 of 10 patients who developed proximal junctional kyphosis. Proximal junctional kyphosis was also related to fusing short of (caudal to) the proximal Cobb end vertebrae by one or two levels. The authors recommended correcting kyphosis to no less than 40 degrees.
Although earlier studies reported a high incidence of pseudarthrosis with a posterior-only procedure,39,49 the use of modern instrumentation and techniques has resulted in a low rate of pseudarthrosis in adolescents. The tensile forces applied to a kyphotic deformity likely increase the incidence of pseudarthrosis in patients with Scheuermann disease as opposed to patients with idiopathic scoliosis, but this is unproven. Thorough preparation of the fusion bed with decortication and use of allograft bone is recommended. The authors do not use iliac crest autograft, but it is a viable option if other risk factors for pseudarthrosis exist.
Other reported complications include pulmonary embolus, pleural effusion, persistent back pain, instrumentation failure, and superior mesenteric artery syndrome.8,26,49,52 Daniels and colleagues79 reported a case of acute celiac artery occlusion resulting in necrosis of the stomach after combined anterior and posterior fusion, with 50% correction of the curve.
Congenital Kyphosis
The first description of congenital kyphosis in the English language was by Greig80 in 1916 when he reported a 2-year-old child with a posterior hemivertebra. In 1932, Van Schrick81 differentiated failure of vertebral body formation versus failure of segmentation as the cause of congenital kyphosis in four patients.
By definition, a vertebral anomaly is present at birth, but the clinical deformity may not manifest until much later. Congenital kyphosis develops prenatally as a result of growth deficits of the centrum occurring during the late stages of chondrification and ossification, leading to hypoplasia or aplasia of the vertebral body.82,83 The defect is thought to be due to inadequate vascularization of the vertebral body during fetal development. Most vertebral malformations occur between days 20 and 30 of fetal development.84
Congenital kyphosis is less common than congenital scoliosis, but it is associated with a higher risk of neurologic compromise and progression of deformity if untreated.85 Congenital kyphosis and kyphoscoliosis are not separate entities but rather a spectrum of spinal deformities caused by vertebral anomalies. If one side of the vertebra is involved more than the other, concomitant scoliosis may develop. In most cases, the deformity involves the coronal plane to some extent.
Classification
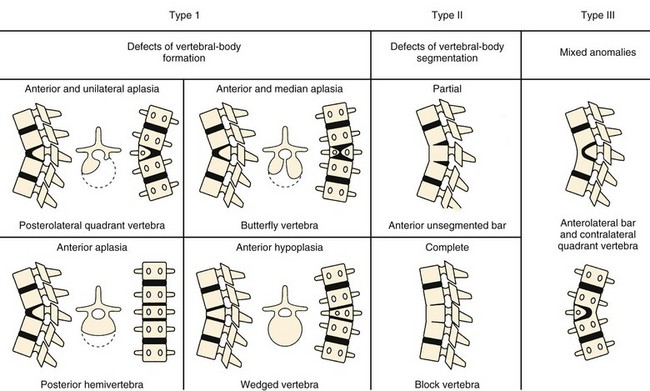
Van Schrick81 was the first to classify this condition into failure of vertebral body formation and failure of segmentation. Winter and colleagues86 described three types in 1973 based on their classic review of 130 patients with kyphotic deformity of the spine owing to congenital vertebral anomalies. They classified the deformities as type I, congenital failure of vertebral body formation; type II, congenital failure of vertebral body segmentation; and type III, mixed failure of formation and segmentation. Type I was the most common with most deformities in the thoracolumbar region followed by the upper thoracic region and the least deformities occurring in the lumbar spine. Failure of segmentation was symmetrical and produced pure kyphosis. Failure of vertebral body formation was often asymmetrical producing scoliosis and kyphosis. In the series by McMaster and Singh,87 65% of patients had anterior failure of vertebral body formation; 20% had segmentation defects, 10% had mixed anomalies, and 5% could not be classified (Fig. 26–7).
Type I deformity is due to partial failure of vascularization of the cartilaginous centrum of the vertebral body.87 These deformities are the most common type. The absence of two growth plates anteriorly with continued growth of posterior elements results in a sharp angular kyphosis and can lead to spinal cord compression. The prognosis is considerably worse than the unsegmented type (type II). The natural history involves relentless progression if untreated.
McMaster and Singh87 further classified type I deformities into four patterns of malformations: posterolateral quadrant vertebra (35%), posterior hemivertebra (7%), butterfly vertebra (13%), and anterior wedged vertebrae (5%). If the failure of formation is purely anterior, a pure kyphosis results. More commonly, the defect is anterolateral with a posterior corner hemivertebra (posterolateral quadrant type), resulting in kyphoscoliosis. McMaster and Singh87 noted that the posterolateral quadrant vertebra has the worst prognosis, progresses relentlessly, and has a high rate of spinal cord compression. This anomaly is due to a complete failure of formation of the anterolateral portion of the vertebral body, leaving a posterolateral fragment of bone of varying size attached to one pedicle and the neural arch.87 Defects of formation are treated surgically because of the high risk of progression and potential neurologic complications if left untreated.
Dubousset88 classified type I deformities into two types: a well-aligned spinal canal and a dislocated canal. Shapiro and Herring89 and Zeller and colleagues90 used the terms congenital vertebral displacement (Shapiro and Herring) and congenital dislocated spine (Zeller and colleagues) to describe deformities in which anterior and posterior elements were abnormal and there was posterior displacement of anomalous vertebrae. Zeller and colleagues90 described the “step-off” sign as the loss of continuity of the posterior cortex of adjacent vertebral bodies as a half of a congenital dislocated spine.
Patients with type II deformity have a better prognosis in terms of rate of progression of deformity and neurologic complications. This deformity is secondary to bony metaplasia in the anterior portion of the anulus fibrosus, resulting in an anterior unsegmented bar.87 There is no longitudinal growth, but posterior growth continues resulting in a kyphotic deformity. The rate of progression is slow, and spinal cord compression does not occur.87 McMaster and Singh87 subdivided defects of vertebral body segmentation into partial (anterior unsegmented bar) or complete (block vertebrae) failure of segmentation.
Imaging Studies
In younger children, vertebral anomalies may be difficult to appreciate on plain films because of incomplete ossification. Computed tomography (CT) scans with three-dimensional reconstructions are useful for precisely defining the nature of the deformity. Flexion and extension lateral radiographs are useful to assess flexibility of the deformity. MRI is recommended preoperatively in all cases to rule out intraspinal anomalies, which can be present in 5% to 37% of patients with congenital kyphosis and scoliosis.91,92 MRI is also useful in identifying cord compression or cord signal changes that may result from the angular kyphosis.
Natural History
The natural history of congenital kyphosis depends on the type of deformity, age of the patient and amount of growth remaining, and location of the deformity. Winter and colleagues86 found type I deformities to have the worst prognosis and to progress rapidly, followed by type III and type II. McMaster and Singh87 found the most rapid progression to occur in type III deformities, followed by type I and II. The posterolateral quadrant vertebra has the worst prognosis.
Kyphosis resulting from defects of formation progresses an average of 7 degrees per year and is most rapid during the adolescent growth spurt.86 Progression is most likely to occur during rapid periods of growth (birth to 3 years and adolescent growth spurt). Type II deformities progress an average of 5 degrees per year.93 Progression rate is slower in type II deformities because bony bar formation of the anterior disc occurs later in childhood, and the growth discrepancy between the posterior and anterior elements is not as great as type I deformities.
Neurologic involvement and spinal cord compression occur primarily with anterior failure of vertebral body formation (type I or III) because of the acute angular kyphosis over a short segment. The rate of neurologic involvement has been estimated to be around 18%.87 This rate is likely to be much higher if all patients were untreated. Neurologic compromise is most common during the adolescent growth spurt when progression is most rapid. The greatest risk of spinal cord compression occurs when the apex is at the mid-thoracic region (T4-9) because this is a vascular watershed area. The onset of cord compression can occur at any age, but is most common during the adolescent growth spurt. All patients who develop neurologic deficit progress to paraplegia if left untreated.87 Progressive neurologic deficit should be immediately treated surgically.
Type II deformities (failure of segmentation) produce a more rounded kyphosis compared with the angular gibbous deformity that occurs with failure of formation.86,93 These deformities occur most often in the lower thoracic and thoracolumbar region. They are less likely to progress and rarely cause neurologic compromise because of the more gradual kyphosis that is spread out over several segments (as opposed to the sharp angular kyphosis of type I deformities).
Treatment
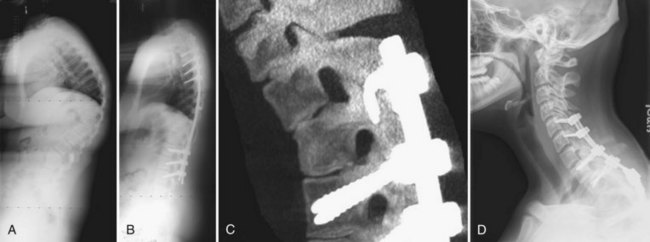
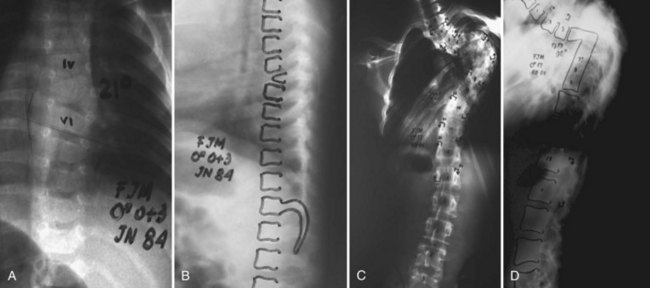
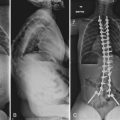
Nonoperative treatment in congenital kyphosis primarily consists of diligent observation to document progression of the deformity (Fig. 26–8). Bracing and casting is believed to be ineffective in congenital kyphosis.86,94 Progression can be insidious, and radiographs may show small degrees of progression over months that may be deemed insignificant. However, over a 2- to 3-year time frame, the progression may be much more significant. At each follow-up evaluation, current radiographs should be compared with the initial radiographs to document progression more precisely. In all cases of congenital kyphoscoliosis, especially with type I deformities, it is crucial to obtain a posteroanterior radiograph and a lateral radiograph at each visit because the scoliosis may appear to be stable but the lateral view may show significant progression of the kyphosis owing to a hemivertebra. The authors refer to such a hemivertebra as a “snake in the grass” (Fig. 26–9).
The importance of observation was highlighted by a more recent report by Campos and colleagues,95 who described seven patients with early thoracolumbar kyphosis associated with single lumbar vertebral hypoplasia, which appeared similar to a failure of formation (Fig. 26–10). All patients had spontaneous correction of alignment over time. In all seven patients, the anomaly was limited to one lumbar level (L1 or L2), and the defect was limited to the superior portion of the anterior half of the vertebral body without posterior malformations. The investigators recommended a brief period of observation to document progression versus spontaneous correction. If progression was documented or if the deformity persisted at age 3 years, further workup was recommended.
Surgical Treatment
The guiding principle of operative treatment of congenital kyphosis is early fusion to prevent severe deformity and to allow for some correction with growth.85 If curves with a bad prognosis are recognized at an early age, simple prophylactic surgical treatment can prevent progression and neurologic complications. The surgical treatment of congenital kyphosis can be challenging, and many factors must be considered when deciding on the operative plan, such as type of vertebral anomaly, patient age and amount of growth remaining, severity of deformity, and presence or absence of spinal cord compression or neurologic deficits or both.85
All operations should be performed with spinal cord monitoring, including motor evoked potentials, somatosensory evoked potentials, and electromyography monitoring. Traction is contraindicated because of the high incidence of neurologic complications.86,96 Because of the rigidity of the kyphosis at the apex, traction primarily causes correction of the ends of the curve, which are more flexible. This correction lengthens the spine and pulls the spinal cord against the unforgiving apical bone, which leads to more compression of the cord and resulting neurologic complications.
The treatment of type I deformities is almost always surgical because of the high risk of progression and neurologic sequelae if deformities are left untreated. The presence of a posterolateral quadrant vertebra mandates early prophylactic surgical treatment before the child is 5 years of age and before the kyphosis is 50 degrees.87 Traditionally, this treatment was best achieved by a posterior growth arrest procedure, which is essentially a posterior (convex) hemiepiphysiodesis. More recently, hemivertebra excision performed through an all-posterior approach has been used for these deformities, and this is discussed later in the chapter. In situ fusion can be done with or without instrumentation, extending one level above to one level below the abnormal vertebra. If the deformity is less than 50 to 55 degrees, and there is growth potential anteriorly, creating a posterior tether allows some gradual correction in the presence of continuing anterior growth. This also eliminates the risk of spinal cord compression.
Although cases reported earlier did not use instrumentation, pseudarthrosis rates were relatively high, and re-exploration of the fusion mass was routinely recommended for this reason. Earlier reports recommended prolonged bed rest (up to 6 months) after surgery. The use of spinal instrumentation, specifically pedicle screw instrumentation, has been shown to be safe and effective in very young patients with congenital spinal deformities97,98 and improves the fusion rate, making re-exploration and prolonged bed rest unnecessary. The authors prefer to use allograft bone because fusion rates tend to be high in young patients. The fundamental principles of fusion surgery, including facet excision and thorough decortication, must be adhered to for optimal results. Postoperatively, the authors immobilize very young children in a brace given their inability to restrict their activities.
The surgical treatment of older patients (>5 years old) with more severe deformities (>55 to 60 degrees) has traditionally been a combined anterior and posterior fusion. Several studies have documented the inefficacy of posterior fusion alone for these patients,85,99,100 and a combined anterior and posterior fusion with or without instrumentation has been recommended.24,86,96 Winter and colleagues86 made this recommendation in a review of 94 patients in whom progressive congenital kyphosis had been treated after the age of 5 years. Some degree of vertebral resection is necessary to obtain satisfactory correction. McMaster and Singh85 recommended anterior strut grafting and instrumented posterior fusion because of the substantial rate of pseudarthrosis and deformity progression with posterior fusion alone. Large curves place the posterior fusion at a mechanical disadvantage in the absence of anterior fusion, leading to a high pseudarthrosis rate. Older patients (>5 years old) lack sufficient spinal growth to produce an appreciable correction. Other studies have corroborated these findings that posterior fusion alone, with or without instrumentation, is insufficient for a type I or type III kyphotic deformity greater than 50 degrees.24,96 McMaster and Singh85 recommended fusing the entire deformity in older patients with large curves (longer fusion than in younger patients with smaller curves).
An alternative to early in situ posterior fusion for a hemivertebra is complete resection of the hemivertebra. Traditionally, hemivertebra excisions have been done through combined anterior and posterior exposures. More recent reports have shown this procedure to be safe and effective through a posterior-only approach,101–103 and this is the authors’ preference. Shono and colleagues104 reported on one-stage posterior hemivertebra resection and fusion and instrumentation in 12 patients 8 to 24 years old with kyphoscoliosis. Satisfactory correction of the scoliosis (from 49 degrees to 18 degrees) and the kyphosis (from 40 degrees to 17 degrees) was obtained, without neurologic complications or pseudarthrosis. Ruf and Harms97,101 described a similar approach in children 15 months old, mostly in congenital scoliosis, with successful outcomes. The location of a hemivertebra in congenital kyphosis or kyphoscoliosis (at the apex of kyphotic deformity) makes it amenable to resection from a posterior approach with visualization of the spinal cord during correction. This approach allows for correction and short segment fusion without concern regarding continued growth of the anterior column that may lead to future progression or crankshaft phenomenon.
A type II kyphosis does not require immediate surgical treatment, unless the deformity is already sufficiently severe to require correction, or the curve is shown to be progressive under observation.85 If a type II deformity is detected early and has been noted to be progressive, but the curve is not severe and is within acceptable limits, a posterior fusion alone (one level above to one level below the congenital kyphosis) is sufficient to prevent further progression.24,86,93,96 These deformities have no potential for anterior growth because of ossification of anterior disc spaces. For more severe curves in which substantial correction is necessary, traditionally an anterior approach has been recommended to perform osteotomies of the unsegmented areas, discectomies and fusion, and posterior fusion and instrumentation.93 All patients who undergo surgical treatment should be observed to skeletal maturity because adding-on of additional vertebrae above and below the fused segment can occur during the adolescent growth spurt.
Complications
Pseudarthrosis and Progressive Deformity
Techniques to obtain a solid fusion more reliably include combined anterior and posterior fusion, use of instrumentation, proper facet decortication, and use of autogenous bone graft. With use of modern spinal instrumentation and proper technique, pseudarthrosis rates are low in young children even with a posterior-only fusion.98,105 Pseudarthrosis typically leads to progression of the deformity, and if any progression is noted in the postoperative period, re-exploration of the fusion mass is recommended. For severe curves (>55 to 60 degrees), the most reliable means of avoiding these complications is by performing a combined anterior and posterior fusion.
Neurologic Complications
The rate of neurologic complications is higher in the surgical treatment of congenital kyphosis than other spinal deformities, especially in older patients with large deformities.96 As mentioned previously, traction is contraindicated in the treatment of congenital kyphosis. Neurologic complications are more likely to occur if attempts are made to obtain maximum correction of the curve with use of instrumentation. Partial correction is preferable, and the goal is to obtain a balanced spine in the coronal and sagittal planes, rather than maximal correction. Spinal cord monitoring is mandatory, and the use of the wake-up test is recommended if the patient can cooperate.
Pearls
Pitfalls
Key Points
1 Winter RB, Moe JH. The results of spinal arthrodesis of congenital spinal deformities in patients younger than five years old. J Bone Joint Surg. 1982;64-A:419-432.
2 Winter RB, Moe JH, Lonstein JE. The surgical treatment of congenital kyphosis: A review of 94 patients age 5 years or older with 2 years or more follow-up in 77 patients. Spine. 1985;10:224-231.
3 Murray PM, Weinstein SL, Spratt KF. The natural history and long-term follow-up of Scheuermann’s kyphosis. J Bone Joint Surg Am. 1993;75:236-248.
This was the first long-term follow-up study on the natural history of Scheuermann disease.
4 McMaster MJ, Singh H. Natural history of congenital kyphosis and kyphoscoliosis. A study of one hundred and twelve patients. J Bone Joint Surg Am. 1999;81:1367-1383.
5 Lowe TG. Scheuermann’s disease. Orthop Clin North Am. 1999;30:475-487.
This review article discusses the natural history, diagnosis, and treatment of Scheuermann disease.
6 Campos MA, Fernandes P, Dolan LA, Weinstein SL. Infantile thoracolumbar kyphosis secondary to lumbar hypoplasia. J Bone Joint Surg Am. 2008;90:1726-1729.
1 Bernhardt M. Normal spinal anatomy: Normal sagittal plane alignment. 2nd ed. Bridwell KH, DeWald RL, editors. The Textbook of Spinal Surgery, vol 1. Philadelphia: Lippincott-Raven. 1997:185-191.
2 Bernhardt M, Bridwell K. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spine and thoracolumbar junction. Spine. 1989;14:717-721.
3 Fon GT, Pitt MJ, Thies ACJr. Thoracic kyphosis: Range in normal subjects. AJR Am J Roentgenol. 1980;134:979-983.
4 Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size: A prospective controlled clinical study. Spine. 1994;19:1611-1618.
5 Scheuermann HW. Kyphosis douselis juveniles. Orthop Chir. 1921;41:305.
6 Scheuermann HW. Kyphosis juveniles (Scheuermann’s kaukheit). Clin Orthop Relat Res. 1977;128:5-7.
7 Wenger DR, Frick SL. Scheuermann kyphosis. Spine. 1999;24:2630-2639.
8 Tribus CB. Scheuermann’s kyphosis in adolescents and adults: Diagnosis and management. J Am Acad Orthop Surg. 1998;6:36-43.
9 Scoles PV, Latimer BM, DiGiovanni BF, et al. Vertebral alterations in Scheuermann’s kyphosis. Spine. 1991;16:509-515.
10 Lowe TG. Scheuermann’s disease. Orthop Clin North Am. 1999;30:475-487.
11 Sorensen KH. Scheuermann’s Juvenile Kyphosis: Clinical Appearances, Radiography, Aetiology, and Prognosis. Copenhagen: Enjar Munksgaard Forlag; 1964.
12 Schmorl G, Junghans H. Die Gesunde und Kranle Wirbel-seule in Roentgenbild. Leipzig: Thieme Verlag; 1932.
13 Findlay A, Conner AN, Conner JM. Dominant inheritance of Scheuermann’s juvenile kyphosis. J Med Genet. 1989;26:400-403.
14 McKenzie L, Sillence D. Familial Scheuermann disease: A genetic and linkage study. J Med Genet. 1992;29:41-45.
15 Nielsen OG, Pilgaard P. Two hereditary spinal diseases producing kyphosis during adolescence. Acta Paediatr Scand. 1987;76:133-136.
16 Carr AJ. Idiopathic thoracic kyphosis in identical twins. J Bone Joint Surg Br. 1990;72:144.
17 Graat HCA, Van Rhijn LW, Schrander-Stumpel CTRM, et al. Classical Scheuermann disease in male monozygotic twins. Spine. 2002;27:E485-E487.
18 Murray PM, Weinstein SL, Spratt KF. The natural history and long-term follow-up of Scheuermann’s kyphosis. J Bone Joint Surg Am. 1993;75:236-248.
19 Ippolito E, Ponseti IV. Juvenile kyphosis: Histological and histochemical studies. J Bone Joint Surg Am. 1981;63:175.
20 Ascani E, La Rosa G. Scheuermann kyphosis. In: Weinstein SL, editor. The Pediatric Spine: Principles and Practice. New York: Raven Press; 1994:557-584.
21 Lambrinudi L. Adolescent and senile kyphosis. BMJ. 1934;2:800.
22 Bradford DS, Daher YH. Vascularized rib grafts for stabilization of kyphosis. J Bone Joint Surg Br. 1986;68:357.
23 Lopez RA, Burke SW, Levine DB, et al. Osteoporosis in Scheuermann’s disease. Spine. 1988;13:1099.
24 Montgomery SP, Hall JE. Congenital kyphosis. Spine. 1982;4:360-364.
25 Ogilvie JW, Sherman J. Spondylolysis in Scheuermann’s disease. Spine. 1987;12:251-253.
26 Lowe TG, Kasten MD. An analysis of sagittal curves and balance after Cotrel-Dubousset instrumentation for kyphosis secondary to Scheuermann disease: A review of 32 patients. Spine. 1994;19:1680-1685.
27 Somhegyi A, Ratko I. Hamstring tightness and Scheuermann’s disease (commentary). Am J Phys Med Rehab. 1993;72:44.
28 Bhojraj SY, Dandawate AV. Progressive cord compression secondary to thoracic disc lesions in Scheuermann’s kyphosis managed by posterolateral decompression, interbody fusion and pedicular fixation: A new approach to management of a rare clinical entity. Eur Spine J. 1994;3:66.
29 Klein DM, Weiss RL, Allen JE. Scheuermann’s dorsal kyphosis and spinal cord compression: Case report. Neurosurgery. 1986;18:628.
30 Lesoin F, Leys D, Rousseaux M, et al. Thoracic disk herniation and Scheuermann’s disease. Eur Neurol. 1987;26:145.
31 Yablon JD, Kasdon DL, Levine H. Thoracic cord compression in Scheuermann’s disease. Spine. 1988;13:896.
32 Sachs B, Bradford DS, Winter R, et al. Scheuermann kyphosis: Follow-up of Milwaukee brace treatment. J Bone Joint Surg Am. 1987;69:50-57.
33 Tribus CB. Transient paraparesis: A complication of the surgical management of Scheuermann’s kyphosis secondary to thoracic stenosis. Spine. 2001;26:1086-1089.
34 Bradford DS, Garcia A. Neurological complications in Scheuermann’s disease: A case report and review of the literature. J Bone Joint Surg Am. 1969;51:567-572.
35 Chiu KY, Luk KD. Cord compression caused by multiple disc herniations and intraspinal cyst in Scheuermann’s disease. Spine. 1995;20:1075-1079.
36 Ponte A, Gebbia F, Eliseo F. Non-operative treatment of adolescent hyperkyphosis. Orthop Trans. 1985;9:108.
37 Speck GR, Chopin DC. The surgical treatment of Scheuermann’s kyphosis. J Bone Joint Surg Br. 1986;68:189-193.
38 Bradford DS, Moe JH, Montalvo FJ, et al. Scheuermann’s kyphosis and roundback deformity: Results of Milwaukee brace treatment. J Bone Joint Surg Am. 1974;56:740-758.
39 Bradford DS, Ahmed KB, Moe JH, et al. The surgical management of patients with Scheuermann’s disease: A review of twenty-four cases managed by combined anterior and posterior spine fusion. J Bone Joint Surg Am. 1980;62:705-712.
40 Bradford DS. Vertebral osteochondrosis (Scheuermann’s kyphosis). Clin Orthop Relat Res. 1981;158:83-90.
41 Travaglini F, Conte M. Cifosi 25 anni. Progress in patologia vertebrate. In: Goggia A, editor. Le cifosi, vol 5. Bologna: Goggia; 1982:163.
42 Travaglini F, Conte M. Untreated kyphosis: 25 years later. In: Gaggi A, editor. Kyphosis. Bologna: Italian Scoliosis Research Group; 1984:21.
43 Lowe TG. Scheuermann disease. J Bone Joint Surg Am. 1990;72:940-945.
44 Lowe TG. Kyphosis of the thoracic and thoracolumbar spine in the pediatric patient: Surgical treatment. Instr Course Lect Pediatrics. 2006:189-196.
45 Harreby M, Neergaard K, Hesselsoe G, et al. Are radiologic changes in the thoracic and lumbar spine of adolescence risk factors for low back pain in adults? A 25 year prospective cohort study of 640 school children. Spine. 1995;20:2298-2302.
46 Paajaanen H, Alanen A, Erkintalo M, et al. Disc degeneration in Scheuermann disease. Skeletal Radiol. 1989;18:523-526.
47 Blount WP, Schmidt AC, Bidwell RG. Making the Milwaukee brace. J Bone Joint Surg Am. 1958;40:526-528.
48 Gutowski WT, Renshaw TS. Orthotic results in adolescent kyphosis. Spine. 1988;13:485-489.
49 Bradford DS, Moe JH, Montalvo FJ, et al. Scheuermann’s kyphosis: Results of surgical treatment by posterior spine arthrodesis in twenty-two patients. J Bone Joint Surg Am. 1975;57:439-448.
50 Herndon WA, Emans JB, Micheli LJ, et al. Combined anterior and posterior fusion for Scheuermann’s kyphosis. Spine. 1981;6:125-130.
51 Lowe TG. Double L-rod instrumentation in the treatment of severe kyphosis secondary to Scheuermann’s disease. Spine. 1987;12:336-341.
52 Lim M, Green DW, Billinghurst JE, et al. Scheuermann kyphosis: Safe and effective surgical treatment using multisegmental instrumentation. Spine. 2004;29:1789-1794.
53 Herrera-Soto JA, Parikh SN, Al-Sayyad MJ, et al. Experience with combined video-assisted thoracoscopic surgery (VATS) anterior spinal release and posterior spinal fusion in Scheuermann’s kyphosis. Spine. 2005;30:2176-2181.
54 Yang C, Askin G, Yang SH. Combined thoracoscopic anterior spinal release and posterior correction for Scheuermann’s kyphosis [in Chinese]. Zhonghua Wai Ke Za Zhi. 2004;42:1293-1295.
55 Papagelopoulos PJ, Klassen RA, Peterson HA, et al. Surgical treatment of Scheuermann disease with segmental compression instrumentation. Clin Orthop Relat Res. 2001:139-149.
56 Vedantam R, Lenke LG, Bridwell KH, et al. A prospective evaluation of pulmonary function in patients with adolescent idiopathic scoliosis relative to the surgical approach used for spinal arthrodesis. Spine. 2000;25:82-90.
57 Graham EJ, Lenke LG, Lowe TG, et al. Prospective pulmonary function evaluation following open thoracotomy for anterior spinal fusion in adolescent idiopathic scoliosis. Spine. 2000;25:2319-2325.
58 Wong CA, Cole AA, Watson L, et al. Pulmonary function before and after anterior spinal surgery in adult idiopathic scoliosis. Thorax. 1996;51:534-536.
59 Taylor TC, Wenger DR, Stephen J, et al. Surgical management of thoracic kyphosis in adolescents. J Bone Joint Surg Am. 1979;61:496-503.
60 Sturm PF, Dobson JC, Armstrong GW. The surgical management of Scheuermann’s disease. Spine. 1993;18:685-691.
61 Otsuka NY, Hall JE, Mah JY. Posterior fusion for Scheuermann kyphosis. Clin Orthop Relat Res. 1990:134-139.
62 Johnston CE, Elerson E, Dagher G. Correction of adolescent hyperkyphosis with posterior-only threaded rod compression instrumentation: Is anterior spinal fusion still necessary? Spine. 2005;30:1528-1534.
63 Ponte A, Vero B, Siccardi GL. Surgical treatment of Scheuermann’s hyperkyphosis. In: Winter RB, editor. Progress in Spinal Pathology: Kyphosis. Bologna: Aulo Gaggi; 1984:75-80.
64 Smith-Peterson MN, Larson CB, Aufrank OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. J Bone Joint Surg Am. 1945;27:1-11.
65 Ponte A. Posterior column shortening for Scheuermann’s kyphosis. In: Haher TR, Merola AA, editors. Surgical Techniques for the Spine. New York: Thieme Verlag; 2003:107-113.
66 Geck MJ, Macagno A, Ponte A, et al. The Ponte procedure: Posterior only treatment of Scheuermann’s kyphosis using segmental posterior shortening and pedicle screw instrumentation. J Spinal Disord Tech. 2007;20:586-593.
67 Lee SS, Lenke LG, Kuklo TR, et al. Comparison of Scheuermann kyphosis correction by posterior-only thoracic pedicle screw fixation versus combined anterior/posterior fusion. Spine. 2006;31:2316-2321.
68 Hosman AJ, de Kleuver M, Anderson PG, et al. Analysis of the sagittal plane after surgical management for Scheuermann’s disease: A view on overcorrection and the use of an anterior release. Spine. 2002;27:167-175.
69 de Jonge T, Ilés T, Bellyei A. Surgical correction of Scheuermann kyphosis. Int Orthop. 2001;25:70-73.
70 Poolman RW, Been HD, Ubags LH. Clinical outcome and radiographic results after operative treatment of Scheuermann disease. Eur Spine J. 2002;11:561-569.
71 Cho KJ, Lenke LG, Bridwell KH, et al. Selection of the optimal distal fusion level in posterior instrumentation and fusion for thoracic hyperkyphosis: The sagittal stable vertebra concept. Spine. 2009;34:765-770.
72 Cheh G, Lenke LG, Padberg AM, et al. Loss of spinal cord monitoring signals in children during thoracic kyphosis correction with spinal osteotomy: Why does it occur and what should you do? Spine. 2008;33:1093-1099.
73 Wilburg G, Thompson GH, Shaffer JW, et al. Postoperative neurological deficits in segmented spinal instrumentation. J Bone Joint Surg Am. 1984;66:1178-1188.
74 Winter B. Spine update: Neurologic safety in spinal deformity surgery. Spine. 1997;22:1527-1533.
75 Othman Z, Lenke LG, Bolon SM, et al. Hypotension-induced loss of intraoperative monitoring data during surgical correction of Scheuermann kyphosis: A case report. Spine. 2004;29:E258-E265.
76 Dolan EJ, Transfeldt EE, Tator CH, et al. The effect of spinal distraction on regional spinal cord blood flow in cats. J Neurosurg. 1980;53:756-764.
77 Yeoman PM, Gibson MJ, Hutchinson A, et al. Influence of induced hypotension and spinal distraction on feline spinal somatosensory evoked potentials. Br J Anaesth. 1989;63:315-320.
78 Lonner BS, Newton P, Betz R, et al. Operative management of Scheuermann’s kyphosis in 78 patients: Radiographic outcomes, complications, and technique. Spine. 2007;32:2644-2652.
79 Daniels AH, Jurgensmeier D, McKee J, et al. Acute celiac artery compression syndrome after surgical correction of Scheuermann kyphosis. Spine. 2009;34:E149-E152.
80 Greig DM. Congenital kyphosis. Edinburgh Medical Journal. February 1916.
81 Van Schrick F. Die angeborene Kyphose. Z Orthop Chir. 1932;56:238-259.
82 Tsou PM. Embryology of congenital kyphosis. Clin Orthop. 1977;128:18-25.
83 Tsou PM, Yau A, Hodgson AR. Embryogenesis and prenatal development of congenital vertebral anomalies and their classification. Clin Orthop. 1980;152:211-231.
84 Rivard CH, Narbaitz R, Uhthoff HK. Congenital vertebral malformations: Time of induction in human and mouse embryo. Orthop Rev. 1979;8:135.
85 McMaster MJ, Singh H. Surgical management of congenital kyphosis and kyphoscoliosis. Spine. 2001;26:2146-2155.
86 Winter RB, Moe JH, Wang JF. Congenital kyphosis: Its natural history and treatment as observed in a study of one hundred and thirty patients. J Bone Joint Surg Am. 1973;55:223-256.
87 McMaster MJ, Singh H. Natural history of congenital kyphosis and kyphoscoliosis: A study of one hundred and twelve patients. J Bone Joint Surg Am. 1999;81:1367-1383.
88 Dubousset J. Congenital kyphosis and lordosis. In: Weinstein SL, editor. The Pediatric Spine. New York: Raven Press; 1994:245-258.
89 Shapiro J, Herring J. Congenital vertebral displacement. J Bone Joint Surg Am. 1993;75:656-662.
90 Zeller RD, Ghanem I, Dubousset J. The congenital dislocated spine. Spine. 1996;21:1235-1240.
91 Basu PS, Elsebaie H, Noordeen ChM. Congenital spinal deformity: A comprehensive assessment at presentation. Spine. 2002;27:2255-2259.
92 Suh S-W, Sarwark JF, Vora A, et al. Evaluating congenital spine deformities for intraspinal anomalies with magnetic resonance imaging. J Pediatr Orthop. 2001;21:5525-5531.
93 Mayfield JK, Winter RB, Bradford DS, et al. Congenital kyphosis due to defects of anterior segmentation. J Bone Joint Surg Am. 1980;62:1291-1301.
94 James JI. Kyphoscoliosis. J Bone Joint Surg Br. 1955;37:414-426.
95 Campos MA, Fernandes P, Dolan LA, et al. Infantile thoracolumbar kyphosis secondary to lumbar hypoplasia. J Bone Joint Surg Am. 2008;90:1726-1729.
96 Winter RB, Moe JH, Lonstein JE. The surgical treatment of congenital kyphosis: A review of 94 patients age 5 years or older with 2 years or more follow-up in 77 patients. Spine. 1985;10:224-231.
97 Ruf M, Harms J. Pedicle screws in 1- and 2-year-old children: Technique, complications, and effect on further growth. Spine. 2002;27:E460-E466.
98 Hedequist DJ, Hall JE, Mans JB. The safety and efficacy of spinal instrumentation in children with congenital spine deformities. Spine. 2004;29:2081-2086.
99 Kim HW, Weinstein SL. Atypical congenital kyphosis: Report of two cases with long-term follow-up. J Bone Joint Surg Br. 1998;80:25-30.
100 Winter RB, Moe JH. The results of spinal arthrodesis of congenital spinal deformities in patients younger than five years old. J Bone Joint Surg Am. 1982;64:419-432.
101 Ruf M, Harms J. Posterior hemivertebra resection with transpedicular instrumentation: Early correction in children aged 1 to 6 years. Spine. 2003;28:2132-2138.
102 Nakamura H, Matsuda H, Konishi S, et al. Single-stage excision of hemivertebrae via the posterior approach alone for congenital spine deformity: Follow-up period longer than ten years. Spine. 2002;27:110-115.
103 Suk SI, Kim JH, Kim WJ. Posterior vertebral column resection for severe spinal deformities. Spine. 2002;27:2374-2382.
104 Shono Y, Abumi K, Kaneda K. One-stage posterior hemivertebra resection and correction using segmental posterior instrumentation. Spine. 2001;26:752-757.
105 Kim YJ, Otsuka NY, Flynn JM. Surgical treatment of congenital kyphosis. Spine. 2001;26:2251-2257.