Chapter 15 Pediatric Eye Diseases
Introduction
Because of its lack of invasiveness and its painless nature, ultrasonography, especially B-scan, has taken on a very important role in the evaluation of a number of ocular disorders of children. This is especially true for peripheral retinal lesions for which scleral depression or contact lens examination is impossible without anesthesia. Furthermore, a number of causes of a white pupillary reflex in children (leukocoria) can be differentiated on the basis of ophthalmoscopy with additional information from ultrasonography. Ultrasonography also provides important information on orbital tumors and correlates with histopathological features.1
Technique
Individuals performing ultrasound examinations on children quickly learn that a combination of gentleness and playful firmness are essential to obtaining the child’s cooperation and achieving the desired goal of a reliable examination. B-scans are generally performed through the closed eyelids in children (Chapter 3). This does not result in a significant loss of quality or information for the conditions the test is utilized for. A-scans or ultrasound biomicroscopy on the other hand can only be performed by corneal contact or using a water immersion adaptor. They require excellent cooperation, otherwise they can be done under anesthesia (Chapter 4). A-scans are most commonly done in preparation for cataract surgery, or less frequently to determine axial length for the diagnosis of microphthalmia or nanophthalmos.
Clinical conditions
Orbit
Hemangiomas and lymphangiomas
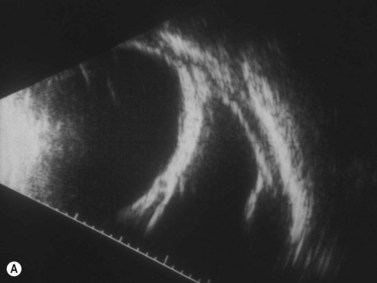
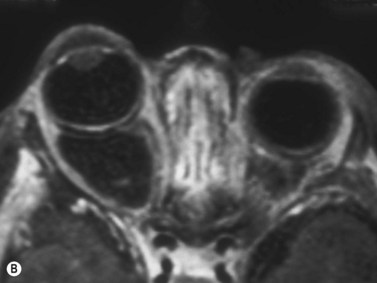
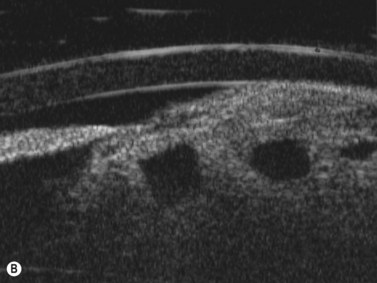
Hemangiomas are the most common orbital tumors in children. Lymphangiomas are less common. Hemangiomas are composed of vascular channels with a proliferation of capillary vascular endothelial cells; some have a cavernous component with larger venous spaces. Hemangiomas generally run a course of increase in size in the first year of life, followed by a regression over a few years. Lymphangiomas tend to remain stationary with episodic exacerbation secondary to hemorrhage or inflammation. These tumors do not invade structures but grow around them and push them. The orbit may be enlarged. While MRI is generally obtained in all cases, B-scan is helpful in the diagnosis as it shows a specific pattern of vascular spaces (Figure 15.1).2
Orbital cysts
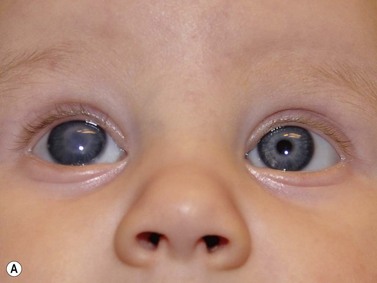
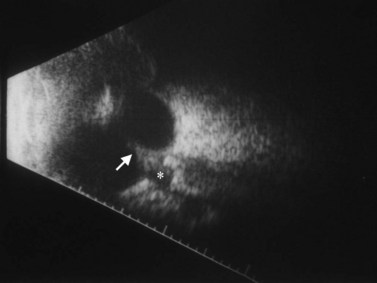
Cystic lesions of the orbit in children include microphthalmia with cyst, congenital cystic eye, and cysts associated with teratomas. Extension of encephaloceles into the orbit can also mimic a primary orbital cystic lesion. The associated clinical findings give away the diagnosis. In microphthalmia with cyst, the eye is reduced in size and may have a coloboma (Figure 15.2). The eye itself may be very small and the cyst may constitute the main orbital content. In such cases there usually is a bulge in the lower lid with small eye pushed superiorly. Congenital cystic eye is extremely rare;3 There is no lens and the globe is replaced by a large cyst. Teratomas are present at birth and are composed of a variety of tissues derived from two or more of the three embryonic layers.4 The tumor can be small and retrobulbar or may be extremely large and push the eye forward while enlarging the orbit significantly. Such birth defects can be detected during prenatal ophthalmic ultrasonography (Chapter 14).
Rhabdomyosarcoma
Rhabdomyosarcomas are rapidly enlarging orbital tumors of striated muscle origin that can be fatal if not diagnosed and treated early.5 Patients present with proptosis, eyelid swelling or ptosis. Several histopathological varieties exist including: alveolar, embryonal and mixed. Treatment consists of total excision followed by a combination of chemotherapy and radiotherapy. Survival is excellent in cases where the diagnosis is made promptly after the onset of signs and symptoms. Mortality was 3% in one series of 33 patients.6 MRI remains the main imaging modality.
Anterior segment
Peters’ anomaly
In this heterogeneous group of malformations, there is a central corneal opacity with variable degree of iridolenticulocorneal adhesions. The abnormality results from failure of separation of the lens vesicle from the posterior aspect of the cornea. A central defect in the corneal endothelium and Descemet’s membrane is pathognomonic (Figure 15.3). Two-thirds of cases are bilateral. A cataract may be present and posterior pole malformations such as persistent hyperplastic primary vitreous (PHPV) and colobomas of the disk and retina are common. Ultrasound biomicroscopy and B-scan are helpful in determining the extent of anterior segment involvement, especially in cases where corneal opacification is severe.7
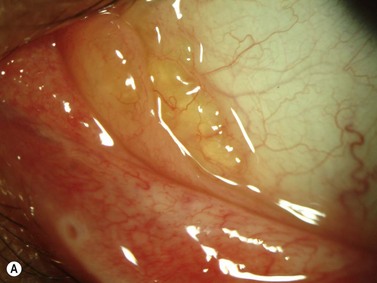
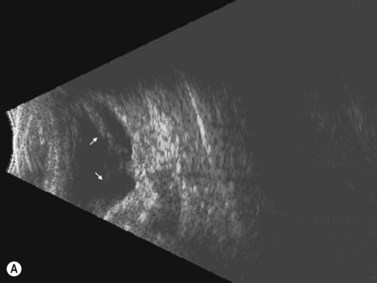
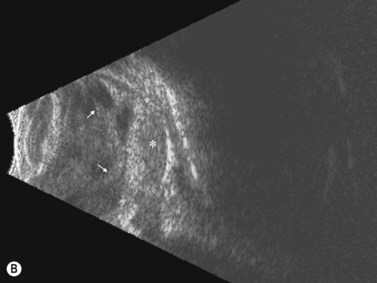
Limbal dermoid
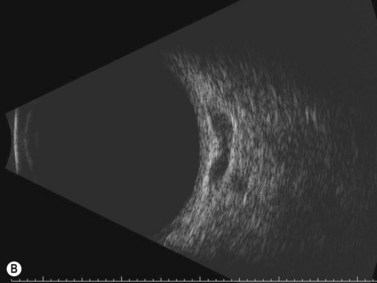
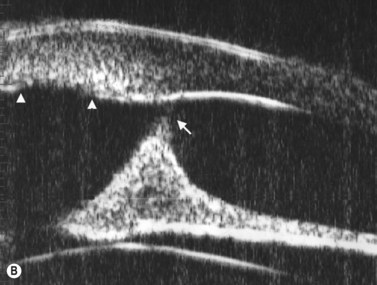
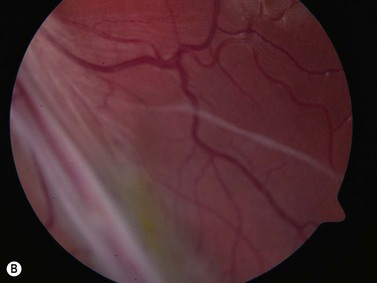
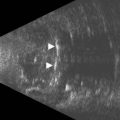
These congenital lesions are characteristically located at the limbus inferotemporally and vary in size and depth of corneal involvement. They can occur in the context of Goldenhar syndrome in which they are associated with preauricular skin tags and first branchial arch defects. Ultrasound biomicroscopy can be used to determine the depth of corneal involvement and assist in the planning of surgery (Figure 15.4).
Posterior segment
Persistence of the fetal vasculature (PFV)
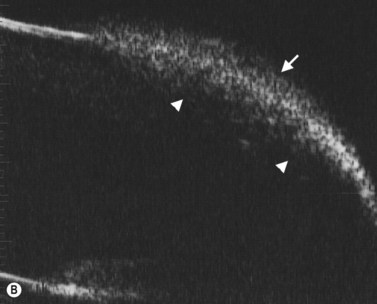
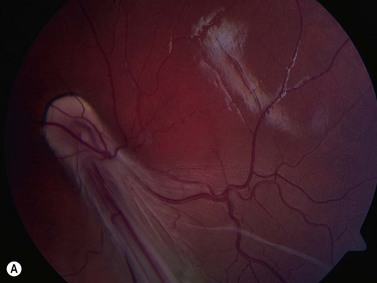
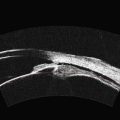
In normal eyes, the primary embryonic vitreous regresses throughout gestation and is replaced by the secondary vitreous, which is translucent and fairly acellular. In some instances, this process does not take place properly and there are remnants of the primary vitreous and of the fetal hyaloid vasculature.8 The eye can be smaller than normal and the remaining primary vitreal tissues are augmented by the formation of fibrous and vascular membranes, most commonly located in the posterior lens capsule. The presence of blood vessels in the posterior lens capsule is pathognomonic of PFV. The contraction of this retrolenticular tissue results in pulling on the ciliary processes and their elongation towards the center of the posterior lens capsule. Remnants of the hyaloid vessels can be present (Figure 15.5). In the less common posterior form of PHPV, the abnormal tissue overlies the area of the nerve head and can fuse with it, distort it and elevate it and the surrounding retina in a tent-like structure. A retinal fold can also occur in cases of posterior PHPV and can run from the disk area to the fundus periphery (Figure 15.6).
Congenital retinal detachment
Congenital retinal detachments are quite rare. The underlying causes include some forms of PHPV, an underlying coloboma or morning glory disk anomaly, X-linked juvenile retinoschisis, Stickler syndrome, or congenital retinal non-attachment in Norrie disease. The individual ultrasonographic findings depend on the underlying cause, but all share the characteristic features of a detached retina (Figure 15.7).
Retinopathy of prematurity (ROP)
ROP results from a reactive proliferation of abnormal blood vessels in the peripheral fundus of premature babies in response to incomplete vascularization of the peripheral retina and exposure to oxygen. Several stages are recognized.9 The retina can separate from traction in the peripheral fundus by fronds of neovascularization in stage IV. The retina is thrown into a funnel that can be open (stage IVa) or closed (stage IVb). Ultrasonography plays a critical role in revealing the individual pattern of detachment and assists in guiding surgical repair (Chapter 11).
Shaken baby
Intraretinal and retrohyaloid hemorrhages are typical of non-accidental injuries in infants.10 A characteristic pattern of posterior hyaloid separation with hemorrhages has been described (Chapter 10). Retinal detachment and subluxation of the crystalline lens can also occur in severe cases. Bleeding can take place in the optic nerve sheath and can be detected by A-scan.
Optic nerve malformations
Morning glory disk anomaly
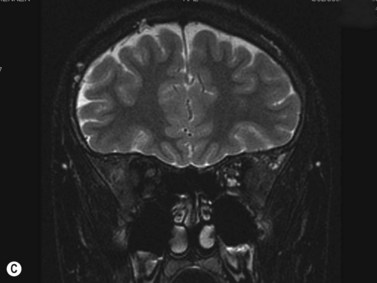
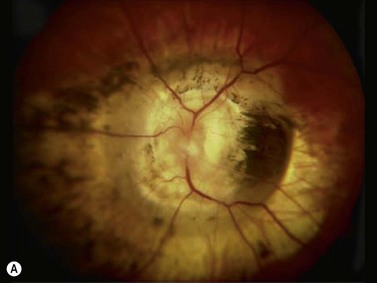
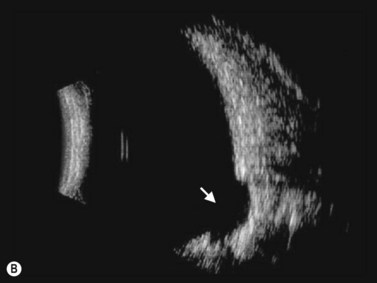
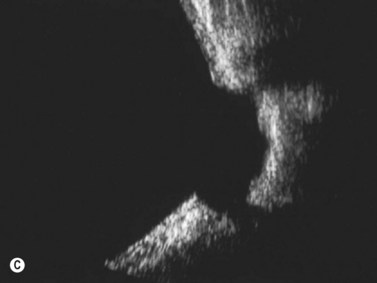
This is a malformation of the optic nerve head characterized by a large scleral opening, radially-oriented retinal blood vessels, a central tuft of fibrous tissue and a variable ring of pigmentation around the nerve head.11 Most cases are unilateral. Some are associated with a basal encephalocele, and up to 40% are associated with intracranial vascular abnormalities such as moyamoya disease. Up to one-third of cases develop exudative retinal detachments. B-scan shows a large opening in the sclera at the optic nerve head with a cone-shaped extrusion of ocular contents that is continuous with the optic nerve. In most patients with morning glory disc anomaly (MGDA) ophthalmoscopy is sufficient for the diagnosis. MRI and MR angiography are obtained to look for associated encephaloceles or moyamoya carotid vascular abnormalities.
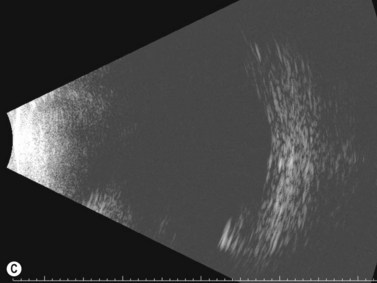
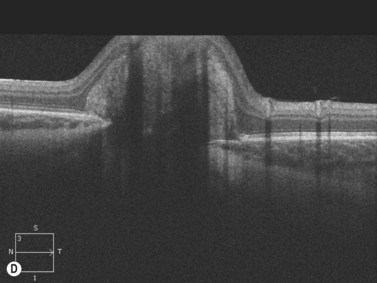
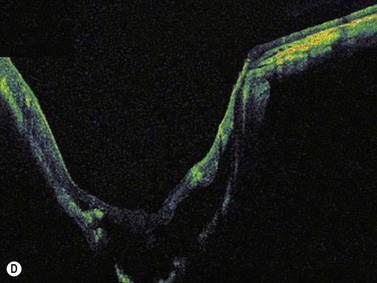
Harasymowycz et al reported B-scan ultrasonographic features in 10 patients.12 Excavation of the optic nerve was present in all cases. Other frequent findings included calcification, “overhang sign” (retinal tissue overhanging the posterior staphyloma), central glial tuft and microphthalmos.12 Spectral domain optical coherence tomography findings offering greater resolution have been recently published (Figure 15.8).13
Coloboma
Colobomas result from a failure of the embryonic fissure to close and are characteristically located in the inferior-nasal part of the fundus. They may involve or be confined to the optic nerve head. The etiology of colobomas is varied and includes genetic as well as environmental factors.14 The sclera in the area of the coloboma can be ectatic and this abnormality is evident on B-scan ultrasonography (Chapter 13).
Tumors
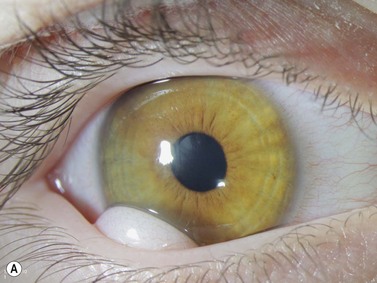
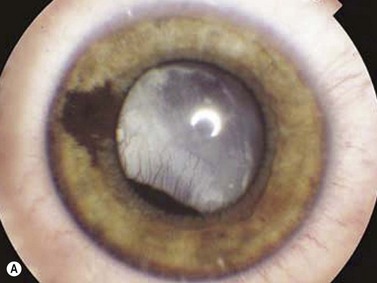
Intraocular medulloepithelioma is an embryonal neoplasm of the ciliary epithelium. It may contain cartilage, skeletal muscle, and brain tissue (teratoid medulloepithelioma).15,16 Medulloepithelioma typically presents during the first decade of life with poor vision, pain, leukocoria, and iris vascularization associated with a mass or cyst appearing behind the pupillary area (Figure 15.9).15–17 Children with neovascularization of the iris of unknown cause should be evaluated to exclude underlying medulloepithelioma.18 Detailed descriptions and examples of other tumors in children are covered elsewhere (Chapter 11).
1 Hasenfratz G. Orbital tumours – the importance of standardized echography. Acta Ophthalmol Suppl. 1992;204:82-86.
2 Haik BG, Jakobiec FA, Ellsworth RM, et al. Capillary hemangioma of the lids and orbit: an analysis of the clinical features and therapeutic results in 101 cases. Ophthalmology. 1979;86(5):760-792.
3 Hayashi N, Repka MX, Ueno H, et al. Congenital cystic eye: report of two cases and review of the literature. Surv Ophthalmol. 1999;44(2):173-179.
4 Mamalis N, Garland PE, Argyle JC, et al. Congenital orbital teratoma: a review and report of two cases. Surv Ophthalmol. 1985;30(1):41-46.
5 Shields JA, Shields CL. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol. 2003;48(1):39-57.
6 Shields CL, Shields JA, Honavar SG, et al. Primary ophthalmic rhabdomyosarcoma in 33 patients. Trans Am Ophthalmol Soc. 2001;99:133-142. discussion 142–3
7 Nischal KK, Naor J, Jay V, et al. Clinicopathological correlation of congenital corneal opacification using ultrasound biomicroscopy. Br J Ophthalmol. 2002;86(1):62-69.
8 Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1997;124(5):587-626.
9 Stout AU, Stout JT. Retinopathy of prematurity. Pediatr Clin North Am. 2003;50(1):77-87. vi
10 Levin AV. Retinal hemorrhage in abusive head trauma. Pediatrics. 2010;126(5):961-970.
11 Lee BJ, Traboulsi EI. Update on the morning glory disc anomaly. Ophthal Genet. 2008;29(2):47-52.
12 Harasymowycz P, Chevrette L, Decarie JC, et al. Morning glory syndrome: clinical, computerized tomographic, and ultrasonographic findings. J Pediatr Ophthalmol Strabismus. 2005;42(5):290-295.
13 Cennamo G, de Crecchio G, Iaccarino G, et al. Evaluation of morning glory syndrome with spectral optical coherence tomography and echography. Ophthalmology. 2010;117(6):1269-1273.
14 Gregory-Evans CY, Williams MJ, Halford S, et al. Ocular coloboma: a reassessment in the age of molecular neuroscience. J Med Genet. 2004;41(12):881-891.
15 Broughton WL, Zimmerman LE. A clinicopathologic study of 56 cases of intraocular medulloepitheliomas. Am J Ophthalmol. 1978;85(3):407-418.
16 Shields JA, Eagle RCJr, Shields CL, et al. Congenital neoplasms of the nonpigmented ciliary epithelium (medulloepithelioma). Ophthalmology. 1996;103(12):1998-2006.
17 Shields JA, Eagle RCJr, Shields CL, et al. Pigmented medulloepithelioma of the ciliary body. Arch Ophthalmol. 2002;120(2):207-210.
18 Singh A, Singh AD, Shields CL, et al. Iris neovascularization in children as a manifestation of underlying medulloepithelioma. J Pediatr Ophthalmol Strabismus. 2001;38(4):224-228.