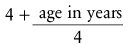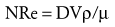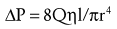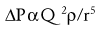51 Pediatric Equipment
Heating and Cooling Systems
The operating room (OR), which is comparable to a large infant incubator, provides the physical environment for the conduct of anesthesia. Readily controlled heating and cooling systems are crucial for thermal stability of the child, that is, control of the external environment. Neonates and small infants require a warmer room temperature than adults; they should be considered poikilothermic, particularly if they are preterm, critically ill, or stressed. With approximately 40% of heat loss by radiation and 35% by convection, heating the OR temperature warms the walls, which decreases radiation heat loss and warms the air, which decreases convection heat loss (see Chapters 35 and 36). Exposure to a cool room temperature during induction of anesthesia and surgical preparation may cause significant thermal stress, particularly in the neonate and young infant. Mild to moderate hypothermia may cause acidosis and apnea in infants, alter the pharmacokinetics of medications, cause difficulty in antagonizing the neuromuscular blockade, and increase oxygen (O2) consumption with shivering.1 Once the child is prepared and draped, the OR temperature may be reduced to a more comfortable level.
Warming Devices
Radiant Warmers
Overhead radiant heating units with servomechanism temperature control were useful to maintain the temperature of small neonates and infants in the past, but these have been replaced with other strategies. Given the risk of skin burn, the servomechanism control sensor must be applied to the warmed skin and should not measure body core temperature.1 A maximum skin temperature of 98.6° F (37° C) should preclude surface burns, although the risk persists in poorly perfused skin. The radiant warmer may be used during induction and surgical preparation; it may be used again as the drapes are removed. If infrared light bulbs are used, care must be taken to ensure that the heat lamp is an appropriate distance from the child to avoid thermal injury2; note that the red cast from the light may make evaluation of a child’s color difficult.
Warming Blankets
Circulating water mattresses help maintain normothermia in children with body surface areas of 0.5 m2 or less (approximately 10 kg).3 For most children, conductive heat loss accounts for less than 5% of total body heat loss; therefore these devices have limited usefulness in maintaining thermal homeostasis and virtually no benefit for children weighing more than 10 kg. To avoid surface burns, the fluid temperature should never exceed 102.2° F (39° C) and must be monitored4; several layers of material should be interposed between the child and the warming blanket to avoid direct contact. In many institutions these have been replaced by forced air warming mattresses.
Forced Warm Air Devices
The most useful device for maintaining thermal homeostasis is the warm air mattress that can be wrapped around the head and upper or lower torso.5–9 These devices rely on the combination of convection with warm air and plastic wrap that also serves to reduce evaporative heat losses. They are the single most effective means for warming children, even when only a portion of the body is covered.5,10 One report suggests that simple delivery of warm air beneath bed sheets without the use of a plastic blanket can be effective,10 but significant thermal injuries have been reported without the use of a licensed blanket or when used in children with poor skin perfusion.6 Therefore unlicensed use of these devices is not recommended. In extreme cases of hypothermia (cardiac surgery or liver transplant), a blanket can be placed both beneath and on top of the child to speed the warming process. Two concerns have been raised regarding these devices. First, concern has been expressed that these devices may incubate infections and distribute them into the OR. The standard design and design changes in the hardware may, unbeknownst to the operator, increase the infectious risk from some warmers.7–9 Concern also exists that under laminar airflow conditions for joint replacement, these devices increase the infectious risk.11 Also, the cost of these disposable air mattresses may be a considerable expense.
Passive Heat and Moisturizer Exchangers, “Artificial Nose”
Heat moisture exchangers are effective in preserving body heat, but ineffective to raise the child’s body temperature.12–14 In infants and children, these devices add humidity to the circuit15 and may also function as filters for infectious pathogens and reduce the transmission of infectious agents between children.16,17 These devices may increase the resistance to breathing during spontaneous ventilation and completely obstruct the circuit with prolonged use.18–20
Fluid and Blood Warmers
The effectiveness of standard fluid warmers depends on the time that the intravenous fluids or blood products are in contact with the warmer. Excessive heating may cause hemolysis of red blood cells.21 With slow intravenous fluid therapy, the fluid warmer does not contribute significant heat transfer because heat is lost along the intravenous tubing between the warmer and the child, before the fluid enters the child’s body. Warming intravenous fluid during maintenance therapy is virtually useless for temperature regulation.
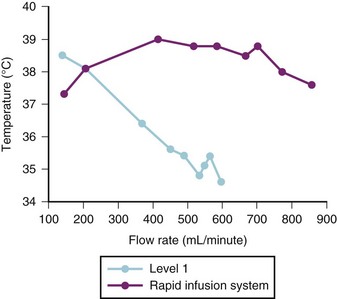
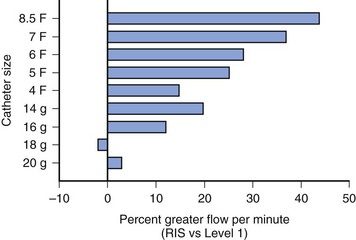
Blood warmers vary from simple coiled tubing in a warming bath to the more sophisticated rapid transfusion devices. Rapid transfusion systems are vitally important in any institution that manages trauma patients or performs major surgical procedures. The new generation of warming devices is far superior to waterbath warmers. They use countercurrent heat exchange or microwave technology as a means of rapidly warming fluid along the length of the column of fluid. The choice of device depends on the size of the child, the anticipated rate of blood loss, and cost. The length of the intravenous tubing and the diameter of the intravenous catheter also limit the rate of rapid infusion. An example of these devices with the lowest capacity is the Hot Line (Smiths Medical ASD, Rockland, Mass.), which is extremely effective at rates up to 75 mL/min.22 The manufacturer’s data suggest that approximately 90 mL/min of refrigerated blood products can be warmed to 95° F (35° C) with this device. Another device that has a low volume (6 mL priming volume) and is inserted within the intravenous system just before the intravenous catheter is the Belmont Buddy fluid warmer (Belmont Instrument Corp., Billerica, Mass.). The manufacturer claims a warming capacity of cold fluids to 100.4° F (38° C) at flow rates as low as “keep vein open” (KVO) and as great as 100 mL/min.23 This device should be inserted close to the intravenous catheter because it may help maintain the child’s temperature by minimizing heat loss along the length of the intravenous tubing. A more complex and larger capacity system is the Level 1 System 1000 (Smiths Medical ASD, Rockland, Mass.), which uses countercurrent warming technology combined with the capacity to infuse fluid, blood, or plasma in bags under pressure. Because this device infuses bags of fluid under pressure, it is important to eliminate all air from the bags to avoid the possibility of air embolization. This system is capable of warming fluids at rates up to approximately 500 mL/min.24 The Level 1 device is capable of warming blood starting at 41° F (5° C) to 42.8° F (6° C) to at least 91.4° F (33° C) at rates as high as 250 mL/min.25 The manufacturer’s data suggest that up to 600 mL/min of red cells diluted to a hematocrit of 60% can be delivered through a 14-gauge intravenous catheter and 750 mL/min through an 8.5F introducer sheath. The addition of an air detection device with automatic flow interruption has improved safety.26 Another high-capacity system is the Belmont FMS warmer (Belmont Instrument Corp, Billerica, Mass.) uses microwave warming and is capable of fluid delivery at rates of 10 to 750 mL/min; this device has two air detectors and an in-line pressure detector. One study compared the flow rates and warming capabilities of the Level 1 system with the Rapid Infusion System (no longer manufactured but equivalent to two Belmont FMS systems); both devices were comparable when used with 18- or 20-gauge intravenous catheters in terms of warming and flow rates. However, for any catheter larger than 18 gauge, the Rapid Infusion System was superior for both flow and fluid warming, particularly for flows greater than 200 mL/min (Figs. 51-1 and 51-2).27,28 A similar comparison has been made with the Level 1 and the Belmont FMS systems, with a similar difference in terms of better warming capacity during high flows.29
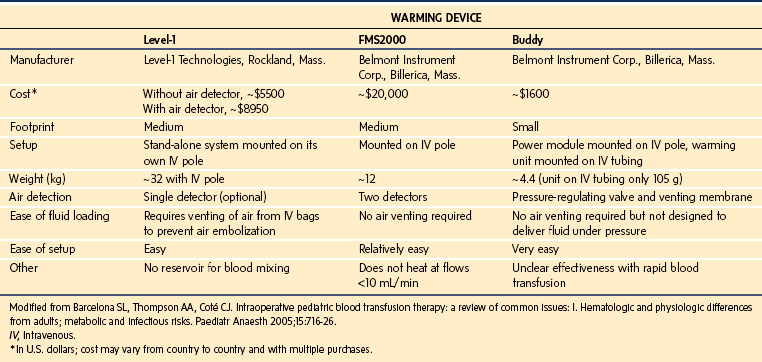
In general, the Level 1 Hotline (Smiths Medical) or the Belmont Buddy appear most suited for low-volume, relatively slow blood loss. The Level 1 system seems most appropriate for children weighing 40 kg or less with expected rapid blood loss and the Belmont FMS for larger children with expected massive and rapid blood loss. The advantages and disadvantages of these warming devices are compared in Table 51-1.30
Heated Humidifiers
Airstream warming and humidification devices are useful for maintaining body temperature.31–33 If a humidifier is used, the anesthesiologist should be familiar with the potential hazards: overheating, leaks, “rain out,” changes in compression volume, and obstruction if connected in reverse. Humidifiers with servomechanism-controlled temperature regulation may prevent overheating and overhydration of infants. Anesthesia breathing circuits with internal heating elements may further reduce the rainout problem and minimize the risk of overheating; these advantages must be weighed against the additional expense. However, with the advent of warm air mattresses and more common use of circle systems in children of all sizes, there appears to be less of a need for in-line humidification as a means of warming children. The main indication for in-line humidification is to prevent secretions from drying when nonrebreathing circuits are used.
Wrapping
Any form of wrapping can reduce radiant and convective heat losses; plastic wrap used for food is particularly effective and inexpensive. Covering an infant’s head is of greatest value because the head represents such a large proportion of the body surface area. As with any warming device, there are hazards; the plastic drapes reduce conductive losses but also eliminate sweating as a mechanism of thermal regulation, so that hyperthermia may result.34 “Space” blankets with reflective aluminized Mylar layers have been very effective in preventing heat loss in the past.35 With effective room temperature control and forced air heating mattresses, the importance of wrapping the child has diminished.
Intravenous Therapy
The volume of the infusion fluid container should not exceed the child’s estimated fluid deficit unless a volume-limiting device is interposed between the intravenous container and the child. Generally, an intravenous set with a microdrop outlet (60 drops/mL) is most practical for children weighing less than 50 kg. Infusion sets that include a one-way valve to prevent retrograde flow in the intravenous administration set are most useful. The infusion setup should include easily accessible injection ports; intravenous extension tubing may be required if the access is in the lower extremities. A three-way stopcock within the system is also helpful should an “adult” intravenous line be needed to give a rapid bolus of intravenous fluid. Recent practice has shifted from needle injection ports to needleless. Both the needleless ports and stopcocks are reservoirs for small volumes of air that may be injected into the intravenous tubing if the air is not cleared during the initial setup or aspirated into the medication syringe before delivering the syringe contents. It is best to purge infusion sets of all air bubbles, especially at each injection port, before use with infants and children.36 In children with known cardiac defects with shunts or arteriovenous malformations, and in infants with patent foramen ovale, the potential for paradoxical air emboli is always present (up to 27% of adults).37 In these circumstances, intravenous air traps may reduce this risk, but caution is advised because blood cannot pass through such devices.38 In the smallest patients, a T-connector placed at the hub of the intravenous catheter allows direct injection of drugs with minimal fluid dead space. It should also be noted that stopcocks and injection ports may act as a reservoir for administered drugs; it is thus important to always flush through any medications administered with these systems. With standard injection ports, long needles will bypass the injection port and deliver drug into the main body of the intravenous tubing (see later).
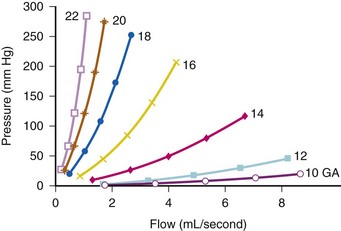
The flow of crystalloid is not a simple linear function of perfusion pressure; the relationship deviates substantially from linearity. Additionally, elements of the intravenous system have isolated effects on one factor or another. For example, a 5-µm filter increases resistance and reduces flow at all pressures. A check valve significantly increases resistance but reduces flow primarily at high flow rates.39 A similar nonlinear function describes the pressure and flow relationship of intravenous cannulas.40 Studies of crystalloid solutions demonstrate steep increases in resistance with increasing pressure in a nonlinear manner with smaller-gauge cannulas (E-Fig. 51-1). The effects of the tubing set add to the effects of the cannula; connecting large-diameter tubing to a smaller-diameter catheter limits flow according to the diameter of the catheter. In situations in which massive and rapid blood loss is anticipated, insertion of extra-large peripheral intravenous catheters such as 5, 6, 7 or 8F pulmonary artery flow catheter introducers or specially designed rapid infusion catheters will provide the most efficient method for rapid volume administration. Special placement kits are available that allow the dilation of small veins by the placement of a guidewire through either a small needle (Seldinger technique) or a small-gauge intravenous line followed by dilation of the vein and placement of a larger-diameter cannula (see Chapter 48).
Accelerating flow through an intravenous line is a concern in emergency situations. Rapid transfusion devices are the best method for increasing flow rates (see earlier).22,24,25 For bags of crystalloid, colloid, or blood components a pressure infusion cuff is a relatively fast, readily available, and the least expensive method for rapidly administering large volumes.41 It is important to remove air from these bags so as to avoid air embolization because an air detector is not used with this technique. Next in order of efficacy are in-line blood pumps or a stopcock and syringe arrangement; gravity is the slowest method of infusion. The syringe technique is often used in infants and children (primarily for accurate measurement in infants); it is similar in efficiency to the in-line blood pump. Intravenous tubing designed for administering blood has better flow characteristics than standard intravenous tubing.42 Large-bore “resuscitation” tubing provides the best flow characteristics; however, the final common pathway, the lumen of the intravenous catheter itself, is the major limitation of flow capacity.25,39–44 Larger-diameter, short catheters allow greater flow than long narrow-diameter catheters.44 This factor is particularly important when rapid volume administration is required but the only intravenous access is through a central venous catheter. This problem is greatly magnified when multilumen catheters are used.44–45 If rapid volume resuscitation is a significant potential problem, then one single-lumen, large-bore peripheral intravenous line is of much greater value than a multilumen central venous catheter. Peripherally inserted central catheters (PICC) lines are a potential problem as well because of their very large resistance they cannot be relied on for rapid volume administration (see further discussion of laminar and turbulent flow).
The choice of fluid and its temperature also have effects on the rate of infusion, primarily because of differences in viscosity. Crystalloid passes most readily, followed by colloid, whole blood, and packed red blood cells. Dilution of packed red blood cells with normal saline markedly improves the flow rate and decreases hemolysis during rapid infusion.46 Hemolysis and associated hyperkalemia of packed red blood cells transfused through short or long catheters under high pressure appears to not be clinically important when the rate of transfusion is moderate. However, rapid transfusion may be associated with hyperkalemia, particularly in infants.47–51
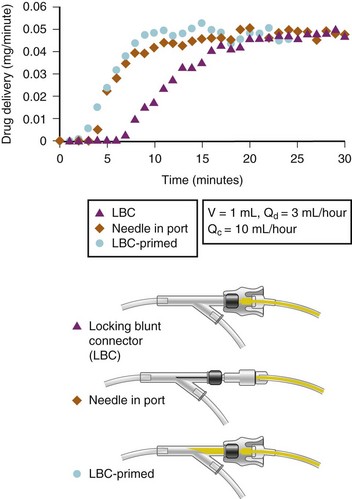
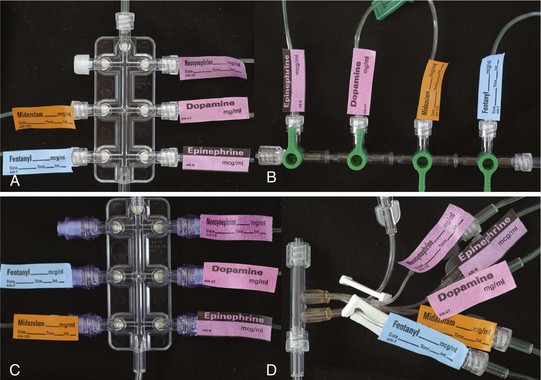
One further concern that has very important clinical implications is the interaction among intravenous tubing design, infusion pumps, and carrier flow rates regarding timing of actual drug delivery to the child. This is an even greater issue in infants and neonates in whom the volumes of drugs and the carrier infusion rates are small. Several studies of adult equipment have examined the interactions of dead space volumes and carrier rates on the time lag to initial drug delivery, time to steady-state drug delivery, and then offset once the infusion is stopped. These interactions also vary whenever a change in carrier flow rate occurs (e.g., when a slow carrier infusion is suddenly increased, this will result in a bolus of drug). Conversely, when a rapid carrier rate is suddenly reduced, this will result in a marked reduction in drug delivery. The importance of these interactions increases the further upstream in the system that a drug is administered; for example, the administration of a drug directly into the T-connector will be the least susceptible to these variables than a drug administered into a side port of an intravenous line 10 to 30 cm away from the intravenous catheter. Thus, when life-support medications are being administered, it is vital to avoid fluctuations or interruptions in carrier rate and to reduce as much as possible the dead space in the system between the child’s bloodstream and the point of drug entering the intravenous system. This is true for the OR, during transit to the intensive care unit (ICU), and at the time of transfer of care. This is particularly an issue when OR pumps and intravenous equipment are switched over to new tubing and pumps in the ICU. Figure 51-3 illustrates the time delay in drug delivery comparing the dead space of an injection port with and without a needle that bypasses the dead space of the injection port and how this time delay can be minimized by “priming” the dead space of the injection port.52,53 Figure 51-4 illustrates several multidrug administration manifolds that allow administration of many medications simultaneously, each with differing dead space characteristics that could cause considerable delay in drug delivery if the dead space within each is inadequately primed. The best way to avoid this problem is to flush each drug through the system from entry to exit point, shut off the infusion, flush the carrier through the system to remove any medications from the carrier portion of the manifold, then attach the system to the patient, so that all infusions will immediately be discharged into the carrier intravenous fluid when turned on, rather than having a delay while the dead space at the connection is filled.
Airway Apparatus
Masks
The importance of elimination of mechanical dead space increases with decreasing size of the child. Rendell-Baker/Soucek masks, developed from molds of the facial contours of Caucasian children, were designed to minimize mechanical dead space without the inflatable cuff or high dome of adult masks. Transparent disposable plastic models are preferable to the classic black conductive rubber because they allow observation of a child’s color and condensate from exhaled humidity with respirations. Plastic disposable masks with soft inflatable cuffs around the periphery of the mask are particularly well suited for children with anatomic or mechanical problems that interfere with normal mask application. An especially useful mask has a built-in port, which allows passage of an endotracheal tube over a fiberoptic scope, thus providing excellent fiberoptic intubation conditions while maintaining spontaneous respiration, oxygenation, and depth of anesthesia (see E-Fig. 12-13).54
Oral Airways
A complete selection of oral airways must be readily available. Infants have a relatively large tongue, which easily obstructs the airway once they lose consciousness. If too large an airway is inserted, damage to laryngeal structures (traumatic epiglottitis, uvular swelling) may result in postoperative airway obstruction (see Fig. 12-13).55 Improperly inserted airways, by obstructing venous and lymphatic drainage, also may result in airway obstruction secondary to swelling of the tongue.56,57 A tongue blade is useful to help insert the airway without kinking the tongue or catching the tongue or lips between the airway and the teeth.
Laryngeal Mask Airway
The laryngeal mask airway (LMA) has proved to be a great addition to the airway management of children. Minimal practice is required to develop the skills for insertion. In some circumstances the LMA can be lifesaving (see Chapter 12).58–64 This device generally provides a clear airway for maintaining spontaneous respirations; however, it cannot be relied on if controlled ventilation is required. Controlled ventilation has been described but holds the risk of gastric dilation.65 We think that this device should be used primarily for spontaneous ventilation and with great caution with controlled ventilation in infants and children. Modern anesthesia ventilators provide the alternative and likely safer pressure-assisted mode of ventilation.66 The LMA is particularly useful in children with anatomic airway abnormalities, because it provides a clear airway while other measures are planned for successful tracheal intubation or a tracheotomy.64,67 Fiberoptic intubation through the LMA is possible in children with midfacial hypoplasia syndromes and in those with redundant airway tissue such as mucopolysaccharidoses (see also E-Fig. 12-22, A through C). Fiberoptic intubation is easier through the LMA because the child can be well oxygenated while using the fiberoptic scope to find the laryngeal inlet. Emergency placement of this device can also be lifesaving in children who are difficult to ventilate with bag and mask with or without a nasal or oral airway.68
Variations to the classic LMA include disposable LMAs of all sizes, intubating LMAs in larger sizes, LMAs with reinforced tubing, and the ProSeal LMA (LMA, La Jolla, Calif.) with a distal opening that provides the ability to pass an orogastric tube (see Fig. 12-18, A and B). This variation to the classic LMA produces a better laryngeal seal and provides the ability to ventilate the lungs without a significant leak at greater peak inflation pressures.69 This variation would seem to offer the greatest advantage in the child with a known difficult airway or in the emergency management of the child with a difficult airway who also has a full stomach. Many other LMA variants are available, each with claimed advantages. Each practitioner should assess which device is most effective in the practice environment70 (see also Chapter 12 for a more in-depth discussion).
Tracheal Tubes
A variety of tracheal tubes must be available with appropriate sizes of stylets. There may be considerable variations from one manufacturer to another in wall thickness, external diameter, kink resistance, and direction and angle of bevel, as well as differences in tracheal tube cuff length and thickness of cuffed tracheal tubes.71 All tracheal tubes should be implantation tested and manufactured in accordance with the Z79 international agreement for safe products. Tracheal tubes are sized by the internal diameter (ID) in millimeters. Despite differences in endotracheal tube wall thickness among manufacturers, a first approximation for the correct tracheal tube size for an average child older than 2 years of age is72:
Preterm infants weighing less than 1500 g generally require a 2.5-mm ID tracheal tube, whereas preterm infants of more than 1500 g require a 3.0-mm ID tube, full-term neonates require a 3.0- to 3.5-mm ID tube, a 1-year-old requires a 4.0-mm ID, and a 2-year-old requires a 4.5- to 5.0-mm ID. Although it has been suggested that the correct diameter of the tracheal tube may be determined by examining the diameter of the distal phalanx of the child’s first or fifth digit, evidence suggests that this is unreliable.69 Tracheal tubes at least one half-size larger and smaller than estimated should be immediately available to accommodate airway size variability. The only true test for appropriate size selection is that the tube passes through the subglottis easily and has an appropriate leak (e.g., between 10 and 40 cm H2O peak inflation pressure). The leak may be easily assessed by closing the circuit pop-off valve and slowly increasing the pressure by gently squeezing the anesthesia bag while listening over the larynx with a stethoscope. This technique has been demonstrated to be a sensitive and accurate measure of fit between the tracheal lumen and the endotracheal tube.73 It should be borne in mind that a leak is dependent in part, upon the child’s head position and degree of paralysis.74 For example, if the neck is flexed or the head is turned from side to side, a leak may become worse or decrease. If the trachea was intubated without the use of muscle relaxants, the child must be well anesthetized to assess the leak properly. If a child is coughing, it is difficult to make this assessment. Conversely, if the child has laryngospasm around the tube, then it will appear to be too large because a leak may not be present. On the other hand, too large a leak may prevent adequate ventilation should positive-pressure ventilation be required, pollute the OR excessively, and dilute the inspired gases during spontaneous respirations. In this circumstance, the tracheal tube should be replaced with the next larger size and the leak reassessed. The relationship of age, tracheal tube sizes and inhalaton pressure leak is commonly used to estimate laryngeal size (see Fig. 31-13).
The past decade has seen a transition from the routine use of uncuffed tubes in children during general anesthesia to the routine use of cuffed tubes. Uncuffed tubes are available for use from 2.0-mm ID to 6-mm ID and have been used as a standard in children up to 8 years of age. Indeed, traditional teaching suggests that the use of uncuffed tubes is the most appropriate practice75; however, this is not evidence-based. The use of cuffed tracheal tubes has been demonstrated to reduce costs of inhalation agents use because of the ability to use lower fresh gas flows.76 With the introduction of the MICROCUFF (Kimberly-Clark Health Care, Roswell, Ga.) tube and publication of several clinical studies, practice has shifted from the routine use of uncuffed to cuffed tracheal tubes. The external diameter of cuffed tracheal tubes is approximately 0.5 mm larger than uncuffed because of the cuff, so one must use a smaller-ID tube for a given tracheal size. Cuffed tracheal tubes as small as 3.0 mm ID are available for use in elective and emergency surgery in infants and children. Some have proposed that the advantages of a cuffed tracheal tube for children include reduced number of reintubations, decreased leak around the tracheal tube leading to less contamination of the operating room, greater ease and consistency of ventilation, reduced cost if low-flow anesthesia is used, and a theoretical attenuated incidence of aspiration.76–82 If a cuffed endotracheal tube is used, the cuff is generally inflated just enough to provide a leak between 20 and 30 cm H2O peak inflation pressure or cuff pressure can be monitored using a device that continually monitors cuff pressure.83 It must recognized that the endotracheal tube cuff pressure will increase during a nitrous oxide (N2O)-supplemented anesthetic because of diffusion of N2O into the cuff,84 unless saline is used to fill the cuff.85 However, because the MICROCUFF tube seals the airway at a reduced pressure compared with polyurethane cuffed tubes, the time until the pressure within the cuff becomes excessive with the MICROCUFF tube is greater than with traditional tracheal tubes.86 Excessive cuff pressures may present a special additional hazard to children, in whom swelling of the pseudostratified columnar epithelium that lines the inside of the cricoid ring may critically reduce the diameter of the upper airway. To date, studies have not investigated long-term complications such as subglottic stenosis that might occur in the intensive care unit. Tracheal tubes used in the OR remain in the trachea for only a brief time; thus it is unlikely that such use could result in long-term complications. An important advantage of the MICROCUFF tube is that the cuff is located closer to the tip of the tube (thereby reducing the potential for cuff pressure on the vocal cords or the cricoid cartilage, but eliminating the Murphy eye), lower cuff sealing pressure (again potentially reducing injury to the mucosa of the trachea), and less leakage around the cuff (less microaspiration) (see Fig. 12-15).87–92 However, the marked nearly fivefold difference in cost will likely prohibit the routine use of this tracheal tube for short term intubation. Additionally, reports of easy kinking in the smaller size tubes resulted in a product recall.93 The authors’ practice has increased the use of the Mallinckrodt Lo-Pro Endotracheal Tubes (Nellcor, Pleasanton, Calif.), which is not a new product but anecdotally provides a low-pressure cuff that has a lower contour, aiding the view during tracheal intubation, and has a smaller effect on the size of the outer diameter of the tracheal tube. No specific studies have demonstrated any differences in complications when comparing different low-pressure endotracheal tubes. The original Khine formula (4 + age [years]/4)79 has been critically evaluated, and a new formula with greater tracheal tube size accuracy has been proposed (3.5 + age [years]/4).79,94 However, these studies did not adequately examine the infants in the lowest age range, nor did they examine these specific brands of tracheal tubes. The argument could be made that long-term follow-up data for complications has not been adequately investigated.95 Currently, we do not recommend the routine use of 3.0-mm ID cuffed tracheal tubes in neonates because the potential for laryngeal/tracheal damage far outweighs the advantage of avoiding an extra laryngoscopy and intubation.
Molded preformed tracheal tubes are especially useful for head and neck surgery because they remove the anesthesia circuit connections from the surgical field.96 When excessive external pressure may be applied to the tracheal tube or when extreme flexion of the neck is likely to kink a standard endotracheal tube, a tracheal tube with Tovell spiral wire reinforcement may be used. Its kink resistance is well-known, but the additional wall thickness limits its applicability in younger children. The spiral springlike construction has caused the tube to pop out of the airway; in addition, repeated improper sterilization of nondisposable models may lead to bubble formation within the rubber, leading to airway obstruction when used with N2O.97
The carbon dioxide (CO2) laser for treatment of laryngeal polyposis and other airway lesions has introduced the problem of ignition of the tracheal tube or other material in the airway. Avoiding high levels of O2 and N2O, which both support combustion, diminishes the risk of fire. Special stainless steel and metal implanted tracheal tubes are available but are quite expensive. Protecting the tube surface with aluminum or copper foil or wet sponges and red rubber tracheal tubes, which have a much reduced ignition potential, may offer the greatest protection (see Chapter 31).98–102 Electrocautery can ignite standard tracheal tubes, esophageal stethoscopes, oral airways, feeding tubes, tissue, and packs if the correct circumstances combine.103–105 Rubber tracheal tubes may soon no longer be manufactured because they are made of latex rubber but are still being advocated by some practitioners for laser surgery.
where NRe is the Reynolds number, D is the diameter of the airway or blood vessel, and V is the velocity, ρ is the density, and µ is the viscosity of the gas or fluid. An NRe less than 2100 denotes flow that is laminar, whereas an NRE of 3000 or greater denotes turbulent flow. Flows that have NRe between 2100 and 3000 may be either laminar or turbulent. Conceptually, laminar flow is organized flow in which each plate of fluid, air, or gas moves in parallel at the same speed, with zero velocity at the interface between the wall of the airway or blood vessel and the gas or fluid. Laminar flow occurs in blood beyond the aortic arch and in airways beyond the fifth bronchial division. In contrast, turbulent flow is chaotic, unpredictable flow in which the fluid, air, or gas does not flow in parallel plates but rather develop eddies and lateral as well as forward flow, thus dissipating large amounts of energy. Turbulent flow occurs in the aortic arch and in the large airways of the tracheobronchial tree down to the fifth bronchial division.
Laminar flow is characterized by the Hagen-Poiseuille equation:
Where ΔP is the pressure drop, Q is the flow rate, η is the viscosity of the fluid (air or gas), l is the length of the airway or blood vessel, and r is the radius of the airway or blood vessel. Thus pressure drop is proportional to the flow rate and viscosity of the fluid and inversely proportional to the radius to the fourth power.
Where ΔP is the pressure drop, Q is the flow rate, ρ is the density and r is the radius of the airway or blood vessel. Note that in contrast to laminar flow, pressure drop is proportional to the square of the flow rate and inversely related to the fifth power of the radius.
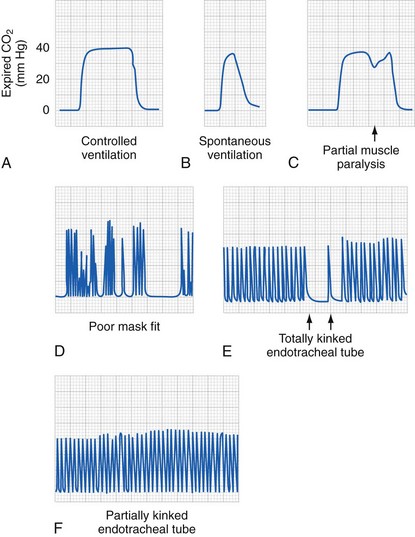
Therefore the resistance to gas or fluid flow is greater in relation to the longer and narrower tracheal tube (or intravenous catheter) used; anything that increases turbulence may proportionally increase the resistance to gas or fluid flow. In practical terms, this means that with a tracheal tube, when the respiratory rate is increased, turbulent flow results (meaning that resistance changes with radius to the fifth power, not the fourth power), decreasing the size of the delivered tidal volume.106 In addition, flow may vary from laminar to turbulent at different portions of the respiratory cycle. Studies with in vivo models have shown that this effect is less pronounced with progressively larger tracheal tubes; however, larger tubes may result in reductions in delivered tidal volume when the respiratory rate is increased in children who require a smaller than normal endotracheal tube; that is, an increase in respiratory rate from 10 to 20/min does not double the delivered minute ventilation.106–108 Data suggest that in children with poorly compliant lungs, lung compliance seems to be the most important factor, even with very small tracheal tubes.106 The implications of these observations are that accurate measurement of end-expired CO2 or arterial blood gases are the only way to be certain of the effectiveness of ventilation. In patients with severe pulmonary disease with ventilation-perfusion mismatch, only arterial blood gases or transcutaneous CO2 monitoring will provide the needed information.
Intubation Equipment
Routine Equipment
Intubation equipment must suit pediatric children of all sizes. Laryngoscopes are available with lightweight small handles and a full range of blades (see Chapter 12). An Oxyscope (Foregger, Inc. Langhorne, Pa.), which incorporates a small-bore O2 delivery tube, reduces the incidence of cyanosis and bradycardia in spontaneously breathing neonates should laryngoscopy be prolonged (see E-Fig. 12-12, A and B).109 Magill forceps are available in several sizes for children of different ages.
Special Airway Equipment
Difficult airways require special intubation equipment; flexible and rigid fiberoptic laryngoscopes and bronchoscopes are available for infants and children. Flexible fiberoptic laryngoscopes are available as small as 1.8 mm external diameter; these are suitable for passage of 2.5-mm ID endotracheal tube.110 The small scope is limited because it does not provide suction. Fiberoptic endoscopic skills should be developed during routine elective cases and in training exercises using airway or full manikin simulators to acquire these skills before encountering emergency cases or children with abnormal airway anatomy (see Chapter 12).110 Fiberoptic intubation through the LMA in infants and neonates with midfacial hypoplasia allows the maintenance of a clear airway, delivery of O2 with or without inhalation anesthetic and provides optimal conditions for successful placement of the tracheal tube.61,62,111–114
Lighted stylets provide an alternative technique for management of a difficult airway.115–118 Other airway devices such as the Bonfils fiberscope (Karl Storz, Tuttlingen, Germany), the Airtraq disposable optical laryngoscope (Prodol Meditec, Vizcaya, Spain), the GlideScope video laryngoscope (Verathon, Bothell Wash.), the Storz DCI video laryngoscope (Karl Storz, Tuttlingen, Germany), and the Trueview PCD Infant (Truphatek, Netanya, Israel) can optimize the management of difficult pediatric airways119–125 (see Chapter 12 for a more in-depth discussion).
Suction Apparatus
A functioning suction apparatus must be available before beginning any anesthetic administration. The device should be capable of regulated pressures; suctioning the oropharynx requires a greater vacuum level than suctioning the tracheal tube. Special circumstances (e.g., a full stomach and arterial bleeding in the oropharynx) may require a second suction device. A separate vacuum system is needed to scavenge waste anesthetic gases. Several sizes of suction catheters are helpful, depending on the indication—tracheal, gastric, or oropharyngeal suction. A 6 or 8F suction catheter with a thumb-controlled side port is useful for suctioning through small tracheal tubes, whereas 10, 12, and 14F catheters are useful for larger tracheal tubes. Routine suction of stomach contents after induction of anesthesia may reduce the potential for gastric fluid regurgitation and serious pulmonary aspiration of gastric contents. In larger patients, vented catheters (e.g., Salem Sump; Sherwood Medical, St. Louis) may be more efficacious than unvented catheters. Suctioning the stomach with the child in the supine position and in left and right lateral decubitus positions has been shown to be most efficient in evacuation of stomach contents.126
The Anesthesia Machine and Its Appendages
Anesthesia Machine
All children can be anesthetized with standard adult machinery as long as the anesthesiologist is aware of the pitfalls and limitations. No anesthesia machines are currently marketed solely for pediatric use; however, anesthesia machines that have the ability to be customized for pediatric use may be of value, but not necessary. If one wishes to use special circuits, a Mapleson D system may be permanently mounted, allowing the choice of either a circle or open system. Modifying the anesthesia machine has inherent danger, especially for inexperienced users; the circuit or other components could be assembled incorrectly and potentially be unsafe. In addition, air should be available from a central pipeline or a cylinder with an appropriate yoke and flow meter. This is particularly advantageous in circumstances in which N2O or a high inspired O2 concentration is contraindicated (avoiding hyperoxia in a premature infant, N2O in a patient with bowel distention, or laser airway surgery).127–129 Even though machines are designed not to allow the delivery of a hypoxic gas mixture, an in-line O2 analyzer and pulse oximetry are indicated to use an air–oxygen blend safely. The anesthesia machine should also be equipped with an alternative means of providing O2 and positive-pressure ventilation (self-inflating bag) in case of a machine failure or malfunction.
Scavenger
The scavenging of anesthetic waste gases to limit exposure of anesthetic gases to health care providers is important. However, during pediatric anesthesia, exposure to anesthetic gases occurs during inhalational inductions by mask where high fresh gas flows are used and a poor mask seal exists because of patient anxiety or movement. The use of uncuffed tracheal tubes with large leaks also increases exposure. Modern scavenging systems are designed to avoid applying either negative or positive pressure to a patient circuit by using an open system, in which a reservoir is used to collect the waste gases and active (suction) or passive pressures are used to remove waste gases from the OR.130 In a closed scavenging system, positive or negative relief values are used in the reservoir or waste gas exhaust hose to ensure that positive pressure does not build up in the system or if the system is blocked so negative pressure is not applied to the patient circuit.131,132 In addition to scavenging systems, exposure to anesthetic gases is also related to adequate ventilation of the OR.133
Circuits
A full spectrum of anesthetic circuits have been used in infants and children.134 Adolescents and older children may be anesthetized with a standard adult semiclosed circle absorber system, perhaps with the substitution of a smaller rebreathing bag. Younger children may be anesthetized with the circuit modified by replacing the hoses with small-diameter pediatric tubing and by substituting a smaller reservoir bag. In the past, most infants and neonates (10 kg or less) were anesthetized with nonrebreathing open systems, such as the Mapleson D or F variety. These circuits are used infrequently today and may soon be of historical interest only because the increasing pressure for cost containment and the expense of the newer anesthetic agents has forced most children’s hospitals to convert to circle systems. In our institutions, we use only circle systems; this has simplified teaching and markedly reduced anesthetic agent costs. However, because Mapleson D systems are still being used, the advantages and disadvantages of open and closed systems for infants and neonates are reviewed in the following discussion.
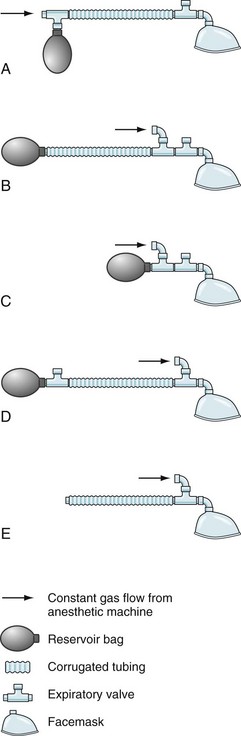
Mapleson D systems do not have directional valves or a CO2 absorber, thus eliminating the resistance intrinsic to the opening pressure of circuit valves and to turbulent flow through soda lime.135 This may be an advantage in spontaneously breathing neonates during induction before intubation. The main disadvantage of open systems is the need for relatively high fresh gas flows and waste of expensive anesthetic agents, as well as unnecessary pollution of the environment. The work of breathing may be important for spontaneously breathing infants, so most pediatric circuits and masks are designed to eliminate both dead space and resistance. The classic example of this type of system is the Ayre T-piece; this circuit includes no valves or reservoir bags (Fig. 51-5).136 Later modifications included changes to the T-piece and expiratory limb.137 The Jackson-Rees modification involves the addition of a reservoir bag to the expiratory limb (Mapleson F).138
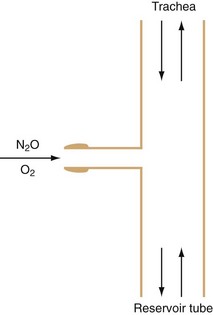
FIGURE 51-5 The T piece as described by Ayre.
(From Ayre P. The T-piece technique. Br J Anaesth 1956;28:520-3.)
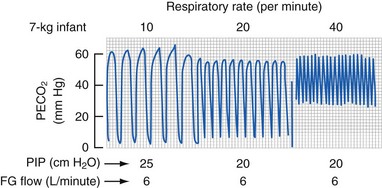
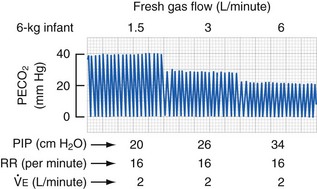
The Magill circuit and its various modifications, Mapleson A to E (Fig. 51-6) remain popular.139 These systems consist of a source of fresh gas flow into the circuit, a reservoir bag, a pressure-relief (pop-off ) valve, tubing of various lengths connecting all of these parts, and an adapter for the mask or tracheal tube. Each of these has advantages and disadvantages, which are well reviewed elsewhere.130,134,140,141 The Mapleson D variety is the most commonly used because of its safety and versatility with both controlled and spontaneous ventilation.141 The pop-off valve is at the end of the expiratory limb, just before the reservoir bag; thus, fresh gas washes alveolar gas out of the expiratory limb during expiration. The efficiency of this washout (to prevent rebreathing) depends on the volume of the expiratory limb, the fresh gas flow, and the size of the tidal volume.130 Larger children (heavier than 15 kg) require greater fresh gas flow rates to prevent rebreathing during spontaneous ventilation.141,142 Fresh gas flow rates as low as 1 times minute ventilation may be used with controlled ventilation without an increase in expired CO2 values.143 Rebreathing (an increase in inspired CO2) can occur in children with controlled ventilation when the respiratory rate increases above 20 breaths per minute; this apparent rebreathing can be diminished by using greater fresh gas flows (E-Fig. 51-2). The reason for this phenomenon may relate to inadequate washout of expired alveolar gas before the onset of the next breath. The advantages of minimal dead space and low resistance to flow with nonrebreathing circuits are counterbalanced by the disadvantages of heat and humidity lost to the anesthetic gases and significant waste of anesthetic agents because of the high flows required.134,141 A clinically important factor is that whenever a change in anesthetic gas concentration is made at the vaporizer, this change is immediately reflected at the airway. This allows a more rapid induction of anesthesia, which may be an advantage but also means a greater risk of producing an anesthetic overdose when compared with a circle system. The most common and efficacious use of the Mapleson D circuit is for the safe transport of a child to and from the OR and for intrahospital transport. In this situation the light weight of the system, the ability to easily adjust peak inflation pressure and provide positive end expiratory pressure, and the ability to use relatively low O2 flows make this system far superior to self-inflating (Ambu type) devices.
The circle system offers the advantage of using reduced fresh gas flows that decreases cost and decreases pollution of the atmosphere with waste anesthetic gases.144,145 The use of the circle system in neonates and small infants generally requires assisted or controlled ventilation. Newer anesthesia machines use valves that require reduced pressure to open and close and the CO2 absorbers are now wider and shorter, thus reducing the resistance. The main resistance to spontaneous ventilation in infants and neonates is the inner diameter and length of the tracheal tube. It is particularly important for practitioners using older systems with ventilators that do not compensate for circuit compliance or fresh gas flow to be aware of the different pressure and volume characteristics of circle systems compared with nonrebreathing systems (Fig. 51-7). Because most pediatric anesthesiologists recommend controlled ventilation in this age group, there no longer seems to be a great need for special circuits. The most important pitfall is that a tidal volume is selected based solely on weight rather than examining chest wall excursion, listening to breath sounds, observing the peak inflation pressure, and measuring end-tidal carbon dioxide (ETco2) tension. Our in vitro studies have found that the main determinants of delivered tidal volume are peak inflation pressure, inspired to expired ratio, and respiratory rate.106–108 As long as careful attention is paid to these important variables, even tiny infants can be successfully ventilated with adult circuits and adult bellows with pressure-limited ventilation (see further). No difference exists between adult circle systems and a Bain (Mapleson D) type system if pressure-limited ventilation is used and the same peak inflation pressure is generated.108 The most important factor is that tidal volume per kilogram will be very large with an adult circle system compared with nonrebreathing systems, especially in infants. Many of these issues of delivering accurate small tidal volumes relative to the compliance of the circuit are simplified with the newer generation ventilators that compensate for the compliance of the system and the fresh gas flow (see later). In the final analysis, however, the practitioner must be cognizant of the fact that the only physiologic measure of the adequacy of ventilation is the CO2 tension—ETco2 or arterial CO2.
Carbon Dioxide Absorption
When using a closed or semiclosed circle system, removal of CO2 from the circuit is required. This is accomplished by inserting a CO2 absorption canister into the anesthetic circuit. The canister is usually placed between the anesthesia bag or ventilator and the fresh gas inlet. In some anesthesia machines, the canister contains two levels of a CO2 absorbent in series. The replacement of the absorbent can create leaks in the circuit if the seal is not reapplied tightly,146 complete obstruction if the plastic packaging is not removed from the prepackaged absorbent before insertion, or intermittent obstruction if part of the packaging is left in place.147 The size of the absorbent is designed to maximize CO2 reabsorption while minimally increasing the resistance in the circuit. Many different commercially manufactured absorbents are available whose chemistry involves the use of materials such as calcium hydroxide, sodium hydroxide, potassium hydroxide, and barium hydroxide, so that CO2 is converted into calcium carbonate, water and heat. CO2 absorption, cost and chemistry are reviewed in detail elsewhere.148 The most common absorbents are soda lime and Baralyme (Allied Healthcare Products, Inc., St. Louis). Soda lime contains 95% calcium hydroxide, either sodium or potassium hydroxide and the balance as water. Baralyme has been voluntarily withdrawn from the market (see later). It contains 80% calcium hydroxide, 20% barium hydroxide, and the balance as water. Newer agents exist, such as Amsorb (Armstrong Medical Limited, Coleraine, Northern Ireland), which contains 70% calcium hydroxide, 0.7% calcium chloride, 0.7% calcium sulfate, 0.7% polyvinyl pyrrolidine, and the balance as water. Potassium hydroxide and sodium hydroxide have been incriminated in the degradation of halogenated anesthetic agents in CO2 absorbers; thus the absence of these compounds in Amsorb prevents the degradation of inhaled anesthetics to carbon monoxide (CO) and compound A (see later). Most absorbents contain a dye such as ethyl violet that turns color as the pH of the absorbent decreases because carbonic acid is formed as CO2 reacts with water. The change in the color in the absorbent indicates it is time to replace the absorbent.
Significant concern exists regarding the interaction of anesthetic agents with CO2 absorbents because this interaction is an exothermic reaction that produces potentially toxic degradation products and a large amount of heat. Sevoflurane is degraded in the presence of CO2 absorbent to produce five compounds, the most common of which is an olefin compound known as compound A.149 Although compound A has been shown to cause renal corticomedullary necrosis in rats, extensive studies in humans have not yielded any substantive consequences or similar renal damage.150–155 Factors that increase production of compound A include use of low-flow anesthesia, use of large sevoflurane concentrations, increased minute ventilation, anesthetics of prolonged duration, increased absorbent temperature, and fresh absorbent (Baralyme > soda lime  Amsorb).156–159 The safety of low-flow (less than 2 L/min fresh gas flow) sevoflurane is still a question because of the limited number of studies in children, although no anecdotal or animal evidence exists to suggest that children may be at greater risk with compound A.160 Other toxic degradation products, including CO, formaldehyde, and methanol are produced when inhaled anesthetic agents interact with desiccated carbon dioxide absorbents (see Chapter 6). Carbon monoxide poisoning has been reported in patients with carboxyhemoglobin levels reaching 30% or more.161–163 These case reports were often after several days of anesthesia machine inactivity, in particular, on a Monday morning (i.e., after a weekend with a large fresh gas left flowing through the machine all weekend while it was not in clinical use and usually without a reservoir bag attached).160,161,164 In some anesthetic machines in which the fresh gas enters at or near the CO2 canister rather than downstream of the inspiratory valve, fresh gas can dry the CO2 absorbent by flowing retrograde through the absorber and exiting where the reservoir bag would usually be connected, because of lower resistance to retrograde flow when a reservoir bag is not in the circle system. In vitro studies demonstrated that CO production from the currently used inhaled agents occurs only when the CO2 absorbent has been desiccated. The order of CO production from greatest to least is desflurane > isoflurane
Amsorb).156–159 The safety of low-flow (less than 2 L/min fresh gas flow) sevoflurane is still a question because of the limited number of studies in children, although no anecdotal or animal evidence exists to suggest that children may be at greater risk with compound A.160 Other toxic degradation products, including CO, formaldehyde, and methanol are produced when inhaled anesthetic agents interact with desiccated carbon dioxide absorbents (see Chapter 6). Carbon monoxide poisoning has been reported in patients with carboxyhemoglobin levels reaching 30% or more.161–163 These case reports were often after several days of anesthesia machine inactivity, in particular, on a Monday morning (i.e., after a weekend with a large fresh gas left flowing through the machine all weekend while it was not in clinical use and usually without a reservoir bag attached).160,161,164 In some anesthetic machines in which the fresh gas enters at or near the CO2 canister rather than downstream of the inspiratory valve, fresh gas can dry the CO2 absorbent by flowing retrograde through the absorber and exiting where the reservoir bag would usually be connected, because of lower resistance to retrograde flow when a reservoir bag is not in the circle system. In vitro studies demonstrated that CO production from the currently used inhaled agents occurs only when the CO2 absorbent has been desiccated. The order of CO production from greatest to least is desflurane > isoflurane  halothane and sevoflurane.165,166 Other factors that increase the production of CO include the degree of desiccation and type of CO2 absorbent (Baralyme > soda lime), increased temperature, increased anesthetic concentration, and low fresh gas flow rates.160,167–170 Newer CO2 absorbents with little or no potassium or sodium hydroxide produce significantly less or no CO and compound A when they become dehydrated.158,171,172 The Anesthesia Patient Safety Foundation developed a consensus statement in 2005 to address the problem of desiccation of CO2 absorbents: “The APSF recommends use of carbon dioxide absorbents whose composition is such that exposure to volatile anesthetics does not result in significant degradation of the volatile anesthetic.”173 The report discusses the difficulty and impracticality of monitoring CO2 absorbent for desiccation and makes specific recommendations (if absorbents are used that degrade anesthetic agents), including turning off all fresh gas flow when the machine is not in use, changing the CO2 absorbent frequently (e.g., every Monday morning), and changing the absorbent when there is concern about the state of hydration of the absorbent, such as when the machine is found with high fresh gas flows for an uncertain period.173 New multiwavelength pulse oximeters are now available (e.g., Masimo Rainbow SET Pulse CO-Oximetry; Masimo Corporation, Irvine, Calif.) that have the ability to measure carboxyhemoglobin and methemoglobin levels noninvasively (see later discussion of multiple wavelength pulse oximetry); however, the costs of this technology make its routine use prohibitive.
halothane and sevoflurane.165,166 Other factors that increase the production of CO include the degree of desiccation and type of CO2 absorbent (Baralyme > soda lime), increased temperature, increased anesthetic concentration, and low fresh gas flow rates.160,167–170 Newer CO2 absorbents with little or no potassium or sodium hydroxide produce significantly less or no CO and compound A when they become dehydrated.158,171,172 The Anesthesia Patient Safety Foundation developed a consensus statement in 2005 to address the problem of desiccation of CO2 absorbents: “The APSF recommends use of carbon dioxide absorbents whose composition is such that exposure to volatile anesthetics does not result in significant degradation of the volatile anesthetic.”173 The report discusses the difficulty and impracticality of monitoring CO2 absorbent for desiccation and makes specific recommendations (if absorbents are used that degrade anesthetic agents), including turning off all fresh gas flow when the machine is not in use, changing the CO2 absorbent frequently (e.g., every Monday morning), and changing the absorbent when there is concern about the state of hydration of the absorbent, such as when the machine is found with high fresh gas flows for an uncertain period.173 New multiwavelength pulse oximeters are now available (e.g., Masimo Rainbow SET Pulse CO-Oximetry; Masimo Corporation, Irvine, Calif.) that have the ability to measure carboxyhemoglobin and methemoglobin levels noninvasively (see later discussion of multiple wavelength pulse oximetry); however, the costs of this technology make its routine use prohibitive.
A very important interaction of the modern halogenated anesthetics and desiccated CO2 absorbent is a poorly defined exothermic reaction that has resulted in fires and explosions, specifically with sevoflurane and Baralyme. There have been several reports of extremely high heat in the CO2 canister and inspiratory circuit in conjunction with fires, explosions, and various detrimental effects to patients, including burns, acute respiratory distress syndrome, and carbon monoxide poisoning (Fig. 51-8).174–176 These cases often reported a very slow increase in sevoflurane concentration in the inspiratory circuit with a large gradient between the vaporizer setting and the measured inspiratory sevoflurane concentration along with a rapid color change of the CO2 absorbent presumably from rapid degradation of sevoflurane and possibly a rapid change in pH or other substances causing the indicator color change. In 2003, Abbott Laboratories (North Chicago, Ill.), in conjunction with the U.S. Food and Drug Administration (FDA), released a practice alert and revised the product information about this phenomenon. This highly exothermic reaction occurs with other inhalation agents and other absorbents such as soda lime, but the highest temperatures of 392° F (200° C) and fires were associated with sevoflurane and desiccated Baralyme; Baralyme was voluntarily withdrawn from the market in 2004.177–179
Humidifiers
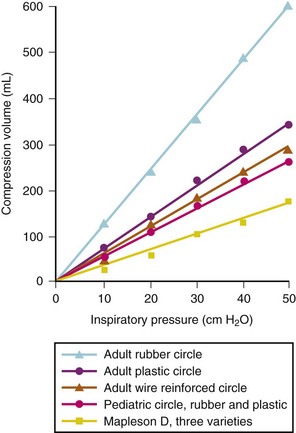
The benefits of heating and humidifying inspired gases include maintenance of body temperature, improved mucociliary function, and prevention of drying secretions, particularly with nonrebreathing circuits.180,181 A heated humidifier also helps maintain body temperature.31–33 When using a circle system partial heat and humidification occur as part of the exothermic reaction used in the CO2 absorber that adds heat and humidity to the circuit. Low fresh gas flows do increase the rebreathing of humidified and warmed gases, and when combined with passive humidification “artificial nose,” provides warm, humidified gases.15 However, small fresh gas flows add little heat and humidity in infants ventilated through a circle circuit.182 Humidification can be further increased by active humidification systems.15 Active humidification systems may create problems such as additional connections that may leak or become disconnected, “rain out” from humidified gas (resulting in water collection within the circuit or the tracheal tube and blockage of the capnograph), added resistance to gas flow, and bubbling noises that distract from the ability to auscultate heart tones and breath sounds. Inhalation of excessively heated inspiratory gases may result in “hot pot tracheitis.”183 Whenever a heated humidifier is added, the extra gas-containing volume of the tubing and humidifier increases the total volume of the anesthetic circuit,184 in turn increasing compression volume. The degree to which anesthetic circuit efficiency is affected depends on the gas-containing volume of the humidifier and the distensibility of the tubing used.184 Because of significant complexity and potential for complications, these devices are no longer commonly used and likely soon to be of historic interest only.
Ventilators
First-Generation Anesthesia Ventilators
The next generation of anesthesia machines, which have a switch at the CO2 canister to change from manual to mechanical ventilation, do not allow adjustment of peak inflation pressure at the pop-off valve because in the mechanical ventilation mode the pop-off valve is out of the circuit. When such a configuration is used, the pop-off valve of the ventilator must be used to limit the peak inflation pressure. The excursion of the ventilator bellows may also be adjusted to limit the size of each tidal volume. Additionally, limiting the inflow of gas to the bellows will limit the bellows inflation. Any of three techniques for adjusting the excursion of the adult bellows provides equal tidal volume as long as the anesthesiologist pays careful attention to peak inflation pressure.106–108 Using the adult ventilator in pressure pop-off mode allows safe use even in neonates with a very small tidal volume. The following is a suggested means for setting up an older adult ventilator for use with children in the pressure-controlled mode:
1. Set pop-off limit on ventilator to 20 cm H2O.
2. Set rate to appropriate age.
3. Set inspiratory to expiratory ratio to 1 : 2.
4. Set gas flow to the middle range.
5. Set tidal volume to a minimum of 10 mL/kg or minute ventilation (VE) to a minimum of 200 mL/kg/min.
6. Turn lever to ventilator mode (new systems) or attach ventilator hose to replace bag (old system).
7. Observe peak inflation pressure to be certain that it is 20 cm H2O or less. If the peak inflation pressure is less than 20 cm H2O, recheck pressure pop-off limit to make sure it is set at 20 cm H2O, and, if correctly set, slowly increase the size of the tidal volume until 20 cm H2O is achieved.
8. Auscultate both lungs and observe chest excursion and ETco2.
9. Make certain fresh gas flow is adequate to maintain bellows at full capacity.
10. Reassess the entire process based on Step 8.
11. For noncompliant lungs or with a small-diameter tracheal tube (e.g., 2.5 mm ID), greater peak inflation pressures may be required.106–108
It is our bias that manual bag ventilation provides immediate feedback about a child’s lung compliance or problems with the circuit (e.g., kinked tube, disconnections, or bronchospasm). However, the literature is unclear regarding this issue. Some experienced anesthesiologists have been able to detect an occluded endotracheal tube, whereas others have not.185,186 This may in part depend on fresh gas flow, the size of the child, and the configuration of the circuit. The use of a ventilator provides valuable assistance by freeing the anesthesiologist’s hands for other use when the immediate hand-bag feedback is given up for the convenience of a mechanical ventilator. A disconnect, apnea, or peak inflation pressure alarm system is helpful to aid vigilance for these types of adverse events, although these alarms are not perfect.187 A CO2 detector with the alarm enabled is optimal; the American Society of Anesthesiologists’ (ASA) Standards for Basic Anesthetic Monitoring require “continual monitoring for the presence of expired CO2” and the “alarm shall be audible to the anesthesiologist or the anesthesia care team personnel.”188
With many first-generation ventilators, fresh gas flow may have considerable impact on tidal volume during volume-controlled ventilation189; fresh gas flow into the circuit is added to the ventilator output during the inspiratory time. This augmentation effect may result in serious errors in calculating delivered VE during volume-controlled ventilation. The following examples illustrate this situation in volume-controlled ventilation:
1. An adult has a VE of 7 L/min, I : E ratio of 1 : 2, and fresh gas flow of 6 L/min. If there were no compliance or compression volume losses, the patient would receive 7 L/min + (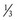 × 6 L/min) = 9 L/min. If the fresh gas flow were reduced to 3 L/min, the patient would receive 7 L/min + (
× 6 L/min) = 9 L/min. If the fresh gas flow were reduced to 3 L/min, the patient would receive 7 L/min + (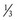 × 3 L/min) = 8 L/min. This is a change of about 11%.
× 3 L/min) = 8 L/min. This is a change of about 11%.
2. A child has a VE of 2 L/min, I : E ratio of 1 : 2, and fresh gas flow of 6 L/min. Again, assuming no compression or compliance volume losses, the child would receive 2 L/min + (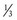 × 6 L/min) = 4 L/min. If the fresh gas flow were now reduced to 3 L/min, the child would receive 2 L/min + (
× 6 L/min) = 4 L/min. If the fresh gas flow were now reduced to 3 L/min, the child would receive 2 L/min + (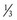 × 3 L/min) = 3 L/min. This is a 25% decrease in delivered minute ventilation (E-Fig. 51-3). Compared with the earlier example in adults, the impact on the minute ventilation in a child is magnified 2.5-fold.
× 3 L/min) = 3 L/min. This is a 25% decrease in delivered minute ventilation (E-Fig. 51-3). Compared with the earlier example in adults, the impact on the minute ventilation in a child is magnified 2.5-fold.
Thus when using first-generation anesthesia ventilators, increases and decreases in fresh gas flow during volume-controlled ventilation result in changes in delivered VE. The potential for clinically important effects on ventilation is greatest in infants (e.g., several study patients were found to have a 40% difference in VE when fresh gas flow was changed from 1.5 L/min to 6.0 L/min without any change in the ventilator settings.190 When using volume-controlled ventilation, if a change is made in fresh gas flow for any circuit used in children, the adequacy of chest expansion, breath sounds, peak inspiratory pressure, and ETco2 must be reevaluated, particularly when using older ventilators that do not automatically compensate for such changes (see later discussion of new ventilators).191 This effect does not occur during pressure-controlled ventilation because excess flow is simply diverted out of the pop-off valve. However, if fresh gas flows were reduced such that the tidal volume was less than the peak pressure setting, the tidal volume delivered to the child could be reduced.
Intensive Care Ventilators in the Operating Room
It has been suggested that ventilators used in the ICU may be of greater value compared with standard OR ventilators in children with very poor lung compliance.144 One study failed to demonstrate any clinically important differences among several commonly used ICU ventilators compared with standard operating room ventilators using pressure-controlled ventilation in an infant lung model with low compliance.192 However, other modes of ventilation such as volume-limited or pressure-assisted ventilation have not been systematically examined. It would appear that there may not be an advantage to using ICU ventilators in pressure-limited ventilation and there may even be a disadvantage because the anesthesiologist may not be as familiar with these ICU devices, thus leading to the potential for errors in ventilator adjustments. Additionally, this requires the use of total intravenous anesthesia because these devices do not interface with the anesthesia machine.
Current Anesthesia Ventilators
The newer modes of ventilation may offer advantages in certain patient populations under specific conditions, but further research is required to demonstrate whether they improve patient care or patient outcomes in the OR. Examples of potential uses of the alternative modes of ventilation include SIMV for children who have not received a neuromuscular blocking drug and situations in which the anesthesiologist is attempting to establish spontaneous ventilation; the child triggers a breath by creating negative pressure or positive-flow gas flow in the circuit. Pressure support ventilation may help overcome increased resistance and improve ventilation when patients are breathing spontaneously through an endotracheal tube or LMA193; pressure support ventilation can be used in combination with SIMV to ensure adequate ventilation if a child becomes apneic.
In limited comparison study of earlier versions of the modern anesthesia ventilation systems compared with ICU ventilation systems, the anesthesia systems performed reliably under a wide range of infant conditions, including high respiratory rates, poor compliance, and small tidal volumes.192
Equipment Cart
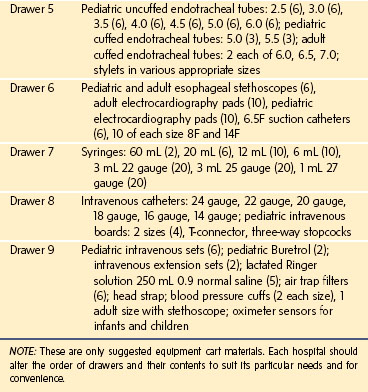
It is advantageous to use mobile multidrawer carts to stock the wide range and sizes of items necessary to care for the full spectrum of infants and children. The drawers should be organized for ease of use: airway equipment, drugs, intravenous supplies, monitoring equipment, circuits, and suction catheters, separated into appropriately labeled drawers. To facilitate efficient and safe delivery of anesthesia, it is prudent to design and stock each cart identically. Because pediatric anesthesia is often administered outside of the OR suite, these mobile carts simplify the safe practice of anesthesia for children and guarantee the availability of all necessary equipment in these remote locations (Table 51-2).
Defibrillator and External Pacemakers
Every operating room facility should be equipped with a direct-current defibrillator. It is not necessary to have a unit specifically for use with children, as long as the energy range may be adjusted to the appropriate levels (2 joules/kg) and pediatric paddles are kept with the device. Ideally, the design should incorporate all controls in the paddles, facilitating use without leaving the child’s side. A desirable feature is a sensing circuit, providing the capacity for synchronous defibrillation should it be needed. External defibrillators with disposable pads applied to the front and back or right chest and left lateral chest of the child have improved the rapidity of response in some circumstances. These can be placed on high-risk children (e.g., cardiac surgery, cardiomyopathy, conduction defects) before induction of anesthesia.194–197 Similar devices that allow for external cardiac pacing represent a major new advance for high-risk infants and children.198–200
Ultrasound for Regional Anesthesia and Vascular Cannulation
Ultrasound technology has advanced greatly in the last 10 years and now is routinely used in children for regional and neuraxial anesthesia and central venous cannulation (see Chapters 41, 42, and 48).201–209 The advent of portable, less expensive ultrasound machines has made this more practical for most pediatric institutions. Visualization of nerves and vascular structures in children requires small probes that can provide high-frequencies (10 to 14 MHz); high-frequency is necessary for visualization of shallow tissue structures.201 Most regional blocks described in children use a small high-frequency probe shaped like a hockey stick and use small needles (21 or 22 gauge) for single injection blocks or continuous infusion techniques and a Tuohy type of needle or an intravenous catheter (18 or 20 gauge) for threading an indwelling catheter.201,210–212 Ultrasound-guided techniques provide real-time visualization of nerves, blood vessels, and other structures that may improve the success rate of regional nerve blocks and central venous cannulation while decreasing the complication rate210 (see Chapter 41 for a more detailed description). This is particularly true in children, in whom the risk is greater than in adults because of the small size of the structures involved and the potential need to perform these procedures after induction of general anesthesia.201,202 An additional potential advantage is the greater ability to perform these procedures in awake-sedated children compared with the use of nerve stimulation or blind percutaneous cannulation, which can be painful. Other potential advantages are decreased adverse effects such as intravascular and intraneuronal injection, reduced dosage of local anesthetic, faster onset, longer duration, and improved quality of blocks.202,213,214 Studies of central venous and peripheral venous cannulation in infants and children demonstrate quicker times to cannulate the vessel, fewer attempts before success, and significantly fewer failures compared with landmark-based techniques.215–220 Quality randomized, controlled trials are difficult to perform owing to the difficulty in defining the level of training and experience of the care providers and the relatively low rates of complications.221,222 More studies are needed to better define the use of ultrasound techniques in infants and children; optimal outcomes require significant education, training, and hands-on experience.
Monitoring Equipment
The Anesthesia Record and Bar Code Drug Administration
Automated anesthesia records or automated anesthesia information management systems (AIMS) are increasingly used and will eventually replace hand-recorded records and likely improve the accuracy of data collection.223–226 These systems can provide timely reminders for administration of antibiotics, documentation of start and stop times, compliance with regulatory requirements, reminders of blood pressure measurement gaps, and automatically provide cumulative totals in infusions of drugs, such as propofol or remifentanil.227–230 Most importantly, the automated recording of physiologic data in real time provides improved accuracy, particularly at times when the anesthesiologist is multitasking or when an emergency occurs. In fact, such data may actually be protective from medical malpractice because the veracity of the data is far improved over retrospective data recording.231,232 Conversely some data recorded are spurious and may in fact be nonreflective of an actual event; neither system is perfect.233,234 Currently, the widespread introduction of automated record-keeping has been very slow for several reasons, including the large capital and contract expenses for implementation, difficulty integrating all hospital-wide digitized systems in real-time terms, and costs for 24-hour support staff.225
Medication errors are quite common, particularly in high acuity areas such as the OR and the ICU.235 The next area of technology improvement may be the adoption of bar code confirmation of drug administration in the operating room.236–242 Such systems are currently available to provide accurate labeling of syringes (date, time, concentration) and have had great success in reducing medication errors.235,243–251 Several studies have demonstrated reduced medication errors in the OR when bar coding is used.236,242,251 These systems will be incorporated into the automated anesthesia record in the future as well.
Precordial and Esophageal Stethoscope
In infants, neonates, and small children, an experienced ear can easily detect arrhythmias but also may be able to assess changes in cardiac output and blood pressure (soft heart tones compared with baseline).252 The continuous use of a stethoscope is of particular value in situations in which more advanced noninvasive blood pressure monitoring (NIBP) is unavailable. The precordial stethoscope may be stabilized with a double adhesive disk. For specialized needs, such as bronchography, angiography, or magnetic resonance imaging, a plastic stethoscope that is not radiopaque or ferromagnetic may be used. The optimal site where both heart tones and breath sounds can be heard is usually at the apex of the heart, but occasionally the suprasternal notch provides better listening conditions. The latter position may be more advantageous during induction and emergence, because information regarding airway patency is more readily obtained. The stethoscope can provide early indications of developing airway obstruction or laryngospasm, allowing the practitioner to take corrective action (PEEP) before a full-blown episode of laryngospasm develops. However, the perception is growing among clinicians that current monitors have superseded the need for the precordial stethoscope.253
Whenever the trachea has been intubated, the precordial stethoscope may be exchanged for an esophageal stethoscope. The pediatric size esophageal stethoscope may be introduced atraumatically, even in neonates. The optimal position for the stethoscope within the esophagus can be ascertained by inserting the scope while listening for the position that provides the loudest heart and breath sounds. The less expensive adult variety is often usable in children age 6 years or older. Some disposable esophageal stethoscopes incorporate a thermistor, allowing the introduction of two monitors at once. One possible relative contraindication to using or inserting an esophageal stethoscope is during a tracheostomy. Misidentification of an esophageal stethoscope as an endotracheal tube has resulted in the surgeon opening the esophagus.254 During a tracheostomy in a child, either the esophageal stethoscope should not be used or the surgeon should be informed that two stiff tubes pass through the neck structures, because this complication has occurred even in the hands of very experienced surgeons.255
Blood Pressure Devices
Blood pressure must be monitored in every child who requires our care. An appropriate-size blood pressure cuff should cover approximately two thirds of the length of the upper arm or thigh.256,257 The cuff bladder should rest over the artery; in older children a stethoscope may be secured over the artery to listen for Korotkoff sounds. In smaller children, the flicker of the needle in an aneroid sphygmomanometer dial may be used as an indicator of systolic pressure.258 If the flicker is not clearly visible, a distal pulse sensor may be used. Such a sensor can be either a photoelectric plethysmograph on a digit that detects the return of the signal as the cuff deflates or a Doppler flow detector that is positioned over a distal artery.259 Electronic oscillometer units (NIBP) are capable of repeated, frequent, and accurate measurements.260–263
If an automatic NIBP device is used, it is important that proper application, function, and adequate deflation time be ensured; venous stasis, petechiae, and nerve compression damage are possible, especially with early models.264 Most automated NIBP machines have specific cuff sizes for infants, neonates, and preterm infants, as well as a requirement that the monitor settings be matched with the size of the patient (neonate, child, adult). For neonates and infants, special tubing is required to connect the cuff with the monitor. If cuffs are improperly matched with the tubing and monitor settings, the built-in algorithms will produce factitious data.265 It appears that these devices offer reasonable accuracy for systolic blood pressure but may be less accurate for diastolic blood pressure.266,267 NIBP monitors provide very useful information during induction of anesthesia, particularly important during that period before establishing intravenous access. The results of one study showed greater consistency and accuracy with these devices than with standard auscultatory methods.263 One common practice is to place the cuff on the calf of infants as a substitute for upper extremity measurements. There is poor correlation between arm and calf blood pressures; because of this variability, one should not simply assume that one site is a substitute for the other.268–271 Another study determined that the loss of the pulse oximeter waveform correlated with systolic arterial pressure in children weighing less than 15 kg.272
Electrocardiograph
Although many arrhythmias may be diagnosed by careful attention to the heart sounds conducted through the precordial or esophageal stethoscope, the electrocardiogram (ECG) remains a mandatory monitor for all instances of sedation, regional anesthesia and general anesthesia. In a healthy child, lead placement may differ from that selected for adults because the principal need is for diagnosis and resolution of arrhythmias rather than detection of ischemia. One report emphasized that children undergoing cardiac surgery or children with severe anemia may develop ST depression consistent with ischemia, although it occurs extremely rarely.273 With the greater right heart predominance in the younger ages, a cross-chest lead generally provides the optimal combination of atrial and ventricular voltage signals. A QRS detector with a beeper is a useful accessory, particularly when vagal stresses occur. The ECG heart rate is used for comparison with the pulse oximeter heart rate to confirm appropriate application and function of the pulse oximeter. A built-in lead fault detector is also useful to check the cables and a patient’s leads when a poor signal is obtained. Cleaning, gently abrading, and defatting the skin with alcohol can improve the contact. Tincture of benzoin may also help maintain secure adhesion in areas near the application of surgical preparation solutions. Care must be taken not to allow the leads to become wet with preparation solutions and to isolate the leads from the electrocautery dispersive electrode to avoid electrical burns.274 Special ECG leads and monitors are required for use in the MRI scanner (see Chapter 45).
Oxygen Monitor
Assessment of the inspired O2 concentration is one aspect of the monitoring guidelines published by the ASA and Harvard Medical School.193,275 It is necessary in preterm infants and neonates to be alert to the possible relationship between high arterial O2 tension (Pao2) resulting from high inspired O2 concentrations and ocular toxicity, although recent evidence suggests that this relationship is not a quid pro quo.276 This monitor is also helpful in guiding inspired O2 concentrations in certain types of congenital heart disease in which low inspired O2 is key to optimizing pulmonary artery pressures and pulmonary blood flow. An O2 monitor allows easy blending of air and O2 to achieve the desired inspired O2 concentration. In all children, O2 monitoring can help prevent hypoxemia. The standard, so-called fail-safe device built into most anesthesia machines is generally keyed only to pressure in the O2 line; should the O2 flow be turned off, a child may receive a potentially hypoxic mixture (air plus a large concentration of inhalational anesthetic such as sevoflurane or desflurane) without warning from the fail-safe alarm. An O2 monitor on the inspiratory limb would indicate a decrease in the inspired fraction of O2 and provide an accurate alarm. With the more frequent use of closed-circuit anesthesia and air–O2 blends, O2 monitoring assumes even greater importance. Anesthesia machines generally provide a mechanical or electronic coupling system that delivers minimal O2 flow and prevents the delivery of hypoxic mixtures of N2O and O2. However, some newer machines do not have a minimum O2 flow and disable their electronic coupling system when air and O2 are combined; thus it is possible to intentionally or unintentionally turn on the air without O2 to deliver 21% O2. The delivery of low inspired O2 is sometimes desirable in certain conditions such as hypoplastic left heart syndrome or to decrease the risk of airway fire when laser or electrocautery are being used. When high concentrations of sevoflurane or desflurane are used with air it is possible to dilute the O2 and deliver a potentially hypoxic mixture. Careful monitoring of inspired O2 is vital to prevent this from occurring unintentionally. A high inspired O2 alarm device is also desirable; this may detect when an air tank is empty. The O2 analyzer should be calibrated and alarm limits set before anesthetic induction; a narrow band of alarm limits allows early detection of changes in gas flow ratio.
Temperature Monitors
Although there is medicolegal pressure to monitor temperature in every child because of the potential danger of malignant hyperthermia, in reality temperature monitoring is mandated for other reasons. Hypothermia is much more common in the OR than hyperthermia and may be associated with acidosis, myocardial irritability, coagulopathy, respiratory depression, prolonged neuromuscular blockade, greater absorption of inhalation agents, and delayed emergence from anesthesia.277–280
NonInvasive Oxygen Saturation Monitors
Pulse Oximetry
Nothing has changed anesthetic practice more in the past few decades than the introduction of mandatory pulse oximetry for all cases requiring sedation and general anesthesia. This monitor determines the O2 saturation of hemoglobin by spectrophotoelectric oximetric techniques. Measuring the amount of light transmitted through tissue between a two-wavelength light source (930 and 660 nm) and a detector allows continuous calculation of arterial O2 saturation.281 The use of two frequencies helps eliminate interfering absorption by other molecules, although intravenous dyes (indocyanine green, methylene blue) and colored nail polish may interfere with normal sensor function and cause the oximeter into reading a factitiously low O2 saturation value.275,282,283 Additionally, dyshemoglobinopathies such as carboxyhemoglobinemia and methemoglobinemia may result in significant artifact. Carboxyhemoglobinemia causes an overestimation of O2 saturation because the photodetector incorrectly interprets carboxyhemoglobin as oxyhemoglobin; this artifactual change is roughly proportional to the concentration of carboxyhemoglobin.284 Methemoglobinemia results in desaturation, but the saturation recorded tends to read greater than the actual saturation; at high levels of methemoglobin during episodes of desaturation, this disparity becomes greater.285 Fetal hemoglobin, hyperbilirubinemia, and sickle cell disease have minimal effect on pulse oximetry.286–290 This monitor is easy to use, noninvasive, and reasonably accurate, although not perfect.291,292 Of greatest importance to the clinician is appreciating that the current oximeter technology underestimates the actual hemoglobin saturation as the saturation is decreasing; the more rapidly the saturation decreases, the more the saturation reading underestimates the true saturation.
The clinical efficacy of this monitor was demonstrated in a prospective single-blind study of pediatric patients; 50% of the cases were conducted with the saturation data and alarms made available to the anesthesia team, whereas in the remainder, the data were blinded and the alarms silenced. Several observations were reported293,294:
1. Twice as many “major” desaturation events (defined as saturation 85% or less for 30 seconds or longer) occurred in the blinded group.
2. The oximeter detected a greater number of desaturation events before clinical recognition by the anesthesiologist.
3. Major desaturation events were not accompanied by changes in vital signs in most cases (i.e., in only 4 of 19 such events was any change in blood pressure, heart rate, or respiratory rate noted).
4. The incidence of these events in ASA physical status 3 and 4 patients was greater than in ASA 1 and 2 patients.
5. Desaturation events occurred in children who were managed by both experienced and inexperienced anesthesia personnel.
6. Correlation between the observation of cyanosis and true desaturation was poor.
7. Desaturation events were as likely in brief as in procedures of extended duration.293
In a subsequent study, the efficacy of combined pulse oximetry and capnography was examined to determine if patient safety was improved with the addition of capnography.294 In 400 children, 260 problems were observed (E-Fig. 51-4). The study confirmed that pulse oximetry was superior to the human eye in diagnosing hemoglobin desaturation. The incidence of major desaturation events when the oximeter data and alarms were blinded from the practitioners was threefold greater than when they were available. Capnography was not as helpful in diagnosing the majority of events leading to hemoglobin desaturation (E-Fig. 51-5). This is consistent with the Australia anesthesia incident study in which the authors reported that oximetry provides useful data in 85% of events.295 In the pediatric study, 15 problems fulfilled the criteria of a major capnograph event. These were problems that posed a threat to life (e.g., kinked tracheal tube, circuit disconnect, esophageal intubation, accidental extubation) and would have been detected immediately by a capnograph, but 8 were diagnosed initially by pulse oximetry (E-Fig. 51-5). Capnography may have helped initially to diagnose the seven remaining events; however, pulse oximetry would have diagnosed these events as desaturation developed, 30 to 60 seconds later. This observation is also consistent with the Australia incident study.296 This study also examined the incidence of “minor” capnograph events, defined as an abnormality of ventilation that was not a threat to a child’s life (e.g., hypercarbia or hypocarbia). A significantly greater incidence of both hypercarbia and hypocarbia occurred in children whose anesthesiologist was blinded from the capnograph data and alarms.
Infants 6 months of age or younger are at greatest risk for experiencing at least one major desaturation event (E-Fig. 51-6) or a major capnograph event. The number of children with multiple problems in the group in which neither pulse oximetry nor capnography data were available to the anesthesia team was twice as great as in the group in which both were available. An interesting observation was the relatively high incidence of clinically unrecognized endobronchial intubation that manifested as persistent, though minor, hemoglobin desaturation (93% to 95%).297
These studies confirm that the pulse oximeter provides an early warning of developing desaturation well before a clinician is able to detect it clinically. This is easily explained by the fact that approximately 5 g of desaturated hemoglobin is required to detect cyanosis; if a child’s hemoglobin level is 15 g/dL, the O2 saturation would have to decrease to 66% (i.e., less than the normal venous hemoglobin desaturation before cyanosis would be clearly evident). Pulse oximetry detects evolving hemoglobin desaturation in advance of this value being achieved. There is some variability among manufacturers, and accuracy declines as the saturation values decrease (70% or lower).284,298 The degree of inaccuracy of pulse oximeters is a function of many variables that should be carefully reviewed.286,299,300 Pulse oximetry is a reliable monitor for the majority of children who are free of cyanotic congenital heart disease.
Quite apart from the hemoglobin O2 saturation data, pulse oximetry provides additional information including data pertinent to the systemic blood pressure.272 Pulsus paradoxus may be observed in hypovolemic children. Loss of the peripheral pulse when using pulse oximetry may accompany hypovolemia, vasoconstriction resulting from hypothermia, or inadequate cardiac output as a result of hypovolemia, allergic reaction, or anesthetic overdose.301,302 Pulse oximetry is useful as a noninvasive trend monitor in individuals with anatomic or physiologic shunts, because the effects of anesthetic agents and positive-pressure ventilation are difficult to predict.303–305 Pulse oximetry also can be an effective monitor for preventing hyperoxia in preterm infants by guiding the inspired O2 concentration to values that correspond to hemoglobin saturations between 93% and 95%.306 Preductal and postductal oximeters indicate opening and closing of a patent ductus arteriosus (PDA).
Various common OR events can affect the accuracy and reliability of pulse oximetry including electrocautery, flickering operating room lights, movement, blood pressure cuff inflation, bright light, vasoconstriction, stereotactic positioning systems,307 and injected dyes (cardio green and methylene blue). Falsely high O2 saturation measurements may occur despite severe hemoglobin desaturation in the presence of interference with the oximeter sensor. Darkly pigmented skin color may also affect accuracy with up to a 10% error in low saturation ranges.308 Consequently, it is important to remember that this monitor is not foolproof.309 One very important artifact, the penumbra effect, is caused when an adult clip-on sensor is placed on an infant’s finger or toe.310 In this situation the oximeter senses the motion of the pulse but light is transmitted around the finger or toe, thus providing a falsely high hemoglobin saturation. Only appropriate-size oximeter probes should be used in infants and children. In critically ill children and those with thermal injuries, problems with finding a suitable site to apply the probe can be overcome by using a modified oximeter probe applied to the tongue (see E-Fig. 34-5, A to D).311,312 The tongue provides a rich blood supply unaffected by vasoconstriction, and electrocautery interference is less. If the tongue moves, the sensor may dislodge or record a false heart rate. Thermal injury secondary to the interface of incompatible sensor probes and cables (i.e., equipment from more than one manufacturer) has been reported, as has pressure necrosis resulting from too tight an application.313,314
Recent advances in pulse oximetry technology have attempted to make several major improvements, including (1) software that provides motion artifact compensation; (2) software that allows uninterrupted readings even in low flow states; and (3) software that provides more accurate assessment of oxygenation in cyanotic patients.315 Conventional pulse oximetry time-averages a signal over 5 to 10 seconds, depending on the default settings of the oximeter. This signal represents the ratio of transmitted red and infrared light, and the instrument uses this to determine the child’s hemoglobin saturation. This averaging smoothes motion-induced artifacts but may ultimately lessen the sensitivity and response time of a conventional oximeter in situations of hypoxemia or even delay the recognition of asystole or severe bradycardia by several seconds. More recent models are designed to recognize motion artifact and discard signal aberrations secondary to motion using proprietary signal-filtering techniques. Some devices combine two different approaches to signal processing to arbitrate the correct saturation based on dual signal filtration techniques using both pattern matching and adaptive comb filtering. This technique is designed to detect motion and reject analysis of data generated during motion. Other oximeter manufacturers take a different approach, using spectrophotometer principles to generate a saturation signal. That saturation signal may contain several component saturations corresponding to different absorption artifacts of blood and other tissues, including venous blood. The signal is then processed using a discrete saturation transform (DST) algorithm that then extracts the highest and presumably the arterial component saturation and reports this value as the hemoglobin O2 saturation.316–319 Conventional pulse oximeters can overestimate or underestimate the degree of desaturation and therefore may have less accuracy and greater signal dropout rate (i.e., no reading or poor quality signal associated with an alarm, especially in children with hypoxemia). These deviations may be improved with the newer generation pulse oximeters, but this has yet to be definitively demonstrated.320 The newer generation pulse oximeters perform better than conventional pulse oximetry in hypothermic low flow states during cardiopulmonary bypass.321 Movement artifact is the main cause of false alarms in sedated or awake children who require oximetry monitoring. This artifact is significantly reduced with the current generation of pulse oximeters in both adult and pediatric patient populations.322–326
Multiple Wavelength Pulse Oximetry
The latest advancement in pulse oximetry technology is multiple wavelength pulse oximetry, which was approved by the FDA in 2006 (Masimo Rainbow SET Pulse CO-Oximetry, Masimo Corporation, Irvine, Calif.). These systems use the principles of pulse oximetry and co-oximetry and at least seven wavelengths of light to calculate the standard measurements from new-generation pulse oximeters and continuous (1) hemoglobin, (2) carboxyhemoglobin, (3) methemoglobin, and (4) plethysmographic (Pleth) variability index. Several studies have examined the accuracy (bias and precision) of the measurements obtained via pulse co-oximetry, and data inconsistencies require further investigation. For the continuous measurement of hemoglobin it was estimated to be accurate within 1 g/dL in adult patients who had systematic hemodilution.327 More studies are needed to determine whether this level of accuracy is reproducible.328,329 The measurement of carboxyhemoglobin via pulse oximetry (SpCO) has been studied extensively because of its potential for screening large numbers of patients, especially in the emergency room setting. The accuracy data are not completely consistent, but in the authors’ opinions are likely acceptable for patient screening. However, with the known degree of false positive and false negative results, SpCO should be used in conjunction with clinical data and blood co-oximetry to verify results when indicated.315, 330–335 Initial studies of the accuracy of methemoglobin measurement by pulse co-oximetry (SpMet) demonstrated decreased accuracy with hypoxia315,336 but the more recent studies using newer technology appear to be even more accurate than the measurement of SpCO and possibly as good or better than SpO2 in the ranges measured in the studies—O2 saturation 74% to 100% and methemoglobin 0 to 14%.337–339
All the measurements from pulse co-oximetry likely have a significant degree of decreased accuracy in the presence of abnormal hemoglobins; more studies need to be done to examine these complex interactions and the effect on the accuracy of the obtained measurements with the coexistence of different forms of hemoglobin. Pleth variability index is a measure of the degree of hypovolemia and potentially might provide guidance for decisions regarding volume administration. The measurement comes from respiratory variations in the plethysmographic waveform amplitude and uses an algorithm to determine the likelihood of improved blood pressure and cardiac index with fluid or colloid administration.340 There are significant limitations in this technology and accurate assessment of volume status may require specific conditions, including general anesthesia, sinus rhythm, mechanical ventilation with a tidal volume of at least 8 mL/kg, PEEP less than 5 cm H2O, location of sensor, and an invasive arterial line depending on the algorithm or commercial product used.341,342 The use of invasive arterial blood pressure and plethysmographic variations in hemodynamic parameters during the respiratory cycle has potentially significant clinical utility but requires further investigation, including evaluation in the pediatric patient population.342,343
Reflectance Oximetry
Reflectance pulse oximetry has been available for many years, but with recent technologic improvements, several devices have received FDA approval and are currently marketed. Reflectance oximetry technology was first created to improve O2 saturation data in difficult clinical conditions, such as decreased perfusion, movement, and conditions in which optimal sites for standard transmission oximetry probes may not exist (e.g., burns). The technology behind reflectance oximetry involves the emission of multiple wavelengths of red and infrared light that are sensed in two or more photodetectors located a distance away from the diodes on the same probe sensor (Fig. 51-9). These devices measure reflected rather than transmitted light. The technology used to create these oximeter probes permit usage on the forehead, in the esophagus, and on the fetal scalp. The forehead devices have had the largest use among pediatric anesthesiologists. Studies of reflectance oximeters in general demonstrated that these devices are more susceptible to erratic measurements when the probe is placed directly over an artery344 and vein.345 Some potential advantages to using reflectance oximetry on the forehead include better signals during conditions of poor perfusion, lack of motion, and faster response time than conventional peripheral oximeter probes.346,347 However, possible disadvantages include a potentially high signal dropout rate (no measurable signal) and poor accuracy, often measuring lower than finger transmission oximetry, particularly in cases of venous congestion (e.g., Trendelenburg position or situations that impede venous return).348 Several studies, including more recent studies, report good correlation between concurrent measurements of O2 saturation comparing reflectance oximetry on the forehead,349–351 the esophagus,352 and around the chest of premature infants,353 compared with extremity pulse oximetry and arterial co-oximetry measurements; these studies also report a clear dropout rate.
Near-Infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is a noninvasive, optical technology that bears similarities to pulse oximetry in that it uses the relative absorption of near-infrared light, 700 to 900 nm, through biologic tissues to determine tissue oxygenation (E-Fig. 51-7).354 Oxyhemoglobin and deoxyhemoglobin absorb light at different frequencies, and the use of a probe that emits and detects different frequencies of near-infrared light can be used to estimate tissue oxygenation. This device has been most widely used to measure “regional” cerebral O2 saturation (rSO2),355,356 but measurement of oxygenation in other areas, such as kidney, bowel, spinal cord, free tissue flaps, and other areas, is possible.357–368 These recent developments provide an exciting new potential to detect ischemia to various tissues before it is evident clinically.369–376 Pulse oximetry requires the measurement of the pulsatile (arterial) component of the total light transmitted by the biologic tissue. Because NIRS does not require the subtraction of the pulsatile component, the signal is more than 100 times stronger and unaffected by poor perfusion compared with pulse oximetry. However, it is susceptible to motion artifact and ambient light noise but software algorithms have significantly decreased these sources of error. A probe placed on the child’s forehead measures the concentration of oxyhemoglobin and deoxyhemoglobin in the tissue underlying the probe. The device functionally measures the oxygenation of blood in the underlying tissues, including blood in the arterioles, capillaries, and venules. The majority (approximately 85%) of the signal originates from the venules; skin and bone and extracranial blood absorb only limited amounts of light, which is subtracted from the signal and does not have significant impact of the measurement in infants and children.357,358 NIRS thus measures the O2 saturation in venous and arterial blood and thus represents an average saturation across blood vessels and tissue. In cerebral oximetry, the O2 saturation of the blood in the tissue (i.e., between the sending and emitting probe) depends on factors that affect O2 transport, including cerebral blood flow, hemoglobin saturation, hemoglobin– O2 binding affinity, and oxygenation saturation in the arterial blood. Therapies aimed at improving O2 delivery or decreasing oxygenation consumption to the brain will potentially increase cerebral oxygenation (rSO2).359–361376
This technology is not new; however, only three devices are currently FDA approved for commercial use to measure cerebral oxygenation: the first, is the INVOS Cerebral Oximeter (Somanetics Corporation, Troy, Mich.). The absolute accuracy of the current Somanetics device is ±10% to 15%, making the measurement of absolute oxygenation unreliable. However, it is reasonably accurate for changes in oxygenation (±5%), making this device most useful for following trends in cerebral oxygenation.362–365 This device is currently FDA approved for trend monitoring. The Somanetics Cerebral Oximeter has demonstrated that normal regional cerebral oxygen saturation (rSO2) is 60% to 80%.366,367 In controlled hypoxic-ischemic states in animals, electroencephalogram (EEG) slowing and increased tissue lactic acid levels occur at rSO2 between 40% and 45%. The EEG becomes flat at rSO2 between 30% and 35%, and if the cerebral ischemia is protracted, it may be associated with tissue infarction.367
The most recent device to be approved by the FDA is the EQUANOX (Nonin Medical Inc., Minneapolis, Minn.), which uses light-emitting diode (LED) technology to transmit three wavelengths (EQUANOX Classic Sensor) and most recently four wavelengths (EQUANOX Advance Sensor, Model 8004CA).368,377 The EQUANOX sensor differs from the others by having dual emitter and sensor sites. The company claims this technology decreases surface tissue variability and can obtain quicker measurements. The first sensor did not have sufficient accuracy for absolute measurements and would be best used in trend monitoring. The newest sensor claims to be very accurate (4.1%), but further study needs to confirm the accuracy of these new systems.
Although some studies suggest improved outcomes when cerebral oximetry is used including pediatric congenital heart surgery, in which neurologic complications are high (2% to 25%),362,366,378,379 a fair degree of skepticism remains regarding the utility of cerebral oxygenation in clinical practice.380,381 There is hope, however, that these devices may at some time in the future provide real-time guidance of regional tissue oxygenation and the effectiveness of interventions to improve the oxygenation. Additional research, further improvements in the technology, and reduced per-patient costs are needed to clarify the role of reflectance oximetry in pediatric patient management.373
Carbon Dioxide Analyzers
The most commonly used gas monitor is the expired CO2 analyzer. This monitor is particularly useful in teaching about ventilation, CO2 elimination from alveoli, and airway management, provided the response time of the instrument is rapid enough to accurately reflect end-expired CO2 tension.382,383 Two configurations of infrared CO2 monitors are in clinical use. They differ only in the location of the CO2 analysis: (1) the side-stream analyzer, which involves the aspiration of expired gas from the anesthesia circuit and remote CO2 analysis, or (2) a mainstream analyzer in which an optical sensor within a cuvette is inserted into the circuit just proximal to the elbow and where analysis of the CO2 occurs based on a signal detected at the cuvette. Side-stream analyzers are less cumbersome than mainstream analyzers because the former requires no additional pieces to be inserted into the anesthetic circuit. Gas for side-stream analysis only requires a narrow-gauge flexible tube to transfer the expired gas unidirectionally from the elbow of the circuit to the analyzer. The gas sampling line and water trap can become obstructed with water or secretions, requiring replacement of the parts. Mainstream gas analyzers do not obstruct with secretions, but they are heavy, and if a tracheal tube of a small caliber is used, airway obstruction may occur as a result of kinking of the tracheal tube.383 Furthermore, mainstream systems require a large inventory of cuvettes, which must be sterilized between patients. Most OR monitors today use side-stream sampling. The current generation of these monitors have improved precision that reduces the gas sampling rate to just 50 mL/min and excellent accuracy even with small rapid tidal volumes.384–388
Capnography is the gold standard to ensure that the tracheal tube has been inserted within the trachea.188,389 If no expired CO2 is detected after a presumed tracheal intubation, one must assume that the endotracheal tube is in the esophagus. However, if the stomach has been distended by manual ventilation or crying before tracheal intubation or if carbonated soft drinks were present in the stomach, CO2 could be detected immediately after intubation, even after an esophageal intubation.390 In such a case, the expired CO2 will be very low or decrease rapidly in a stepwise manner after placing the tracheal tube. This artifact is very short lived. In addition, the presence of CO2 during cardiopulmonary resuscitation is a very important sign of pulmonary blood flow.391 Alternatively, its absence during resuscitation may indicate inadequate chest compressions and pulmonary blood flow (see Chapter 39).392–395
Measurement of expired CO2 tension is also helpful in detecting other clinical problems. Clinically important air embolism causes a transient but marked reduction in CO2 excretion because the lungs are ventilated but not perfused; hence dead space is suddenly increased (see Chapter 24). Quantitative measurement allows detection of change in circuit flows, disconnections, endotracheal tube kinking, or accidental extubations.296,396–398 Capnography also signals the earliest clinical sign of a malignant hyperthermic reaction and the effectiveness of treatment399 (see also Fig. 40-3). In critically ill neonates or children in a hypermetabolic state after thermal injuries, ventilatory requirements may be as much as 4 times normal and capnography may be an effective noninvasive means of assessing the adequacy of ventilation in these children as well (see Chapter 34).400
A capnograph with a recorder or a display is most valuable because the CO2 waveform may help in diagnosing other types of respiratory difficulties (Fig. 51-10). Bronchospasm and its response to treatment may be identified by changes in the CO2 waveform; bronchospasm causes an increasing slope of the plateau during expiration, and resolution of the bronchospasm restores the plateau to the horizontal. Data also suggest that the shape of the CO2 waveform changes with growth and development. The shape of the CO2 waveform achieves the normal adult configuration by adolescence.401 A slow-speed recorder also allows trending, which we have found particularly useful in diagnosing small circuit leaks, partially kinked endotracheal tubes, and rebreathing. In children free from pulmonary shunts, the arterial and alveolar CO2 values (Paco2 and PAco2 [as reflected by a true end-expired sample]) should be within 2 to 3 mm Hg. The severity of a shunt or the diagnosis of a shunt may be made if this difference is greater than 5 mm Hg and if the CO2 sensor is properly calibrated. Children with a variety of pulmonary problems may have significant differences between arterial and expired CO2 values. In such cases, expired CO2 monitoring may be used only for trending and as a disconnect alarm.402,403 This monitor is of great value in assessing the adequacy of ventilation in children whose trachea is intubated, but of possibly less value in children whose airway is managed by facemask when it is difficult to obtain an accurate end-expired sample.294,396 The accuracy of ETco2 monitoring is slightly better with an LMA than with a facemask.404
Transcutaneous Carbon Dioxide Measurement
Transcutaneous CO2 monitoring has been available for many years, but this technology has been limited because of difficulties preparing and fixing the sensor, preparing the skin, having to rotate the sensor to prevent burns, and calibration time, as well as questions of its reliability. The most common use for transcutaneous monitoring has been in critically ill neonates.405,406 Two monitors combine pulse oximetry with transcutaneous carbon dioxide (PtcCO2) measurement through a probe designed for application to the ear lobe (TOSCA, Linde Medical Sensors AG, Basel, Switzerland; V-Sign Sensor, SenTec Digital Monitoring System; SenTec AG, Steinhausen, Switzerland). These products use a variant of the Severinghaus-type CO2 electrodes in which the skin is warmed to 107.6° F (42° C) to increase blood flow to the capillary bed.407,408 CO2 is measured by change in pH of the electrode where pH is proportional to the logarithm of the change in CO2. The CO2 values are greater than the local arterial values because of the increased temperature and increased metabolic production locally. The system corrects for these changes, is relatively easy to use because of self-calibration, and requires infrequent need for repositioning and changes of the electrolyte layer of the probe. Both systems are reliable and accurately reflect the Paco2 in a variety of settings in both pediatric409,410 and adult patients.411–413 Proponents of this type of CO2 monitoring suggest that ETco2 monitoring is often inaccurate in some clinical conditions, including neonates with a large air leak around the tracheal tube, high respiratory rates, and sampling at the end of a tracheal tube such that a true end-tidal sample is not obtained, significant cardiopulmonary disease associated with increased dead space and/or right-to-left shunt, and conditions of frequent movement. A clear disadvantage is the lack of breath-to-breath monitoring capability for apnea and disordered breathing provided by end-tidal capnography.414 Transcutaneous CO2 monitoring may more closely approximate Paco2 in children who have large ventilation-perfusion mismatch.415–417 Transcutaneous CO2 monitoring may have a role in anesthetized children with severe pulmonary disease or other pathology that prevents accurate ETco2 measurement. More studies are needed to assess its role in the pediatric patient population in various settings such as the OR and ICU and for unintubated patients undergoing sedation.
Anesthetic Agent Analyzers
Most current anesthetic agent analyzers use ultraviolet or infrared monochromatic (only one agent detected) or polychromatic (more than one agent detected) absorption or mass spectroscopy and provide a rapid time response.418,419 Polychromatic infrared analyzers are most useful because they can detect erroneously selected anesthetic agents.420 Careful assessment of the end-expired vapor tensions may provide some indication of a child’s anesthetic depth, but each child must be assessed on an individual basis. Vaporizer malfunctions and miss-fills may also be diagnosed. Mass spectroscopy may be particularly valuable in providing an early warning of air embolism (after a child has been denitrogenated), because the sudden appearance of expired nitrogen is abnormal (entrainment of room air into the circuit and release of nitrogen from extremities with a tourniquet also may provide a source of nitrogen).421 The purported advantages of less waste of anesthetic gases and a more rapid awakening are effects that have not been proved. However, the ability to use low flows offers the potential for significant cost savings in the form of less waste of anesthetic agent and less pollution of the atmosphere.422 The report of accumulation of methane with closed circuit anesthesia and the inaccuracy of agent analysis (methane additive to the agent used) is worrisome but can be obviated with simple periodic flushing of the system.423,424
Blood Loss Monitors
Close observation of the surgical field is the best single monitor of blood loss. A small-volume trap on the suction line before the major evacuation trap is particularly useful for small children. Routinely weighing surgical sponges on a dietary scale, assuming that 1 g of weight is equivalent to 1 mL of blood, is also helpful. Greatest accuracy is obtained by immediate weighing as the sponges come off the surgical field, thus minimizing evaporative losses. Point-of-care testing devices such as the HemoCue (HemoCue AB, Ängelholm, Sweden) and the I-STAT (Abbott Point of Care Inc., Princeton, N.J.) provide a rapid means for assessment of hemoglobin values425–431 but each may underestimate the hematocrit at lower values.430,432–437 Noninvasive hemoglobin measurements using multiwavelength pulse oximetry in general may be useful for trend monitoring.328,438 However, overall it tends to underestimate hemoglobin values and may not be sufficiently accurate at this time to use these data for clinical transfusion decisions because discrepancy of up to 1 g of hemoglobin have been reported.327,329,436,437,439–442
Neuromuscular Transmission Monitors
Measuring the indirectly elicited twitch response is indicated when neuromuscular blocking agents are used. Residual or incomplete reversal of neuromuscular blockade has been shown in adults to be a common cause for delay in leaving the OR, prolonged postanesthesia care unit (PACU) recovery time, and respiratory complications in the early postoperative period.443–446 It has been clearly determined that even experienced anesthesiologists cannot determine decrement in the train-of-four (TOF) accurately. Clinical signs (e.g., sustained head lift) are often difficult to perform in older patients and can be done with a significant degree of residual blockade, thus leading to undetected residual neuromuscular blockade and presumably the potential for respiratory complications.444 In general, two types of neuromuscular monitoring devices are available, those using acceleromyography and those using mechanomyography. Mechanomyography models use constant current nerve stimulation. These devices self-calibrate and then deliver a constant stimulus (30 to 70 mA) to the skin regardless of other influences. Failure to deliver a supramaximal stimulus may result in overestimation of twitch suppression. The power should generally be left off between readings to avoid possible injury secondary to prolonged, repetitious exposure to electric current. Acceleromyography uses a piezoelectric sensor to quantitate the acceleration (proportional to force) of the thumb converting this to an electrical signal. There is considerable disagreement in the published literature as to which of these techniques is most accurate.447–449 Monitors based on acceleromyography are becoming more commonly available; however, these are difficult to use in infants because of the small arc of the displaced thumb. Some consider this monitor to be more accurate than the standard mechanomyography based train-of-four monitors, but they are difficult to use in infants and are not user friendly.445,446 Others think mechanomyography is more accurate because it is less influenced by external disturbances, that is, it does not go out of calibration.450 Therefore at present for clinical purposes in infants and children, mechanomyography still seems to be the simplest and most helpful clinical monitor during the use of nondepolarizing competitive blockers. The TOF does not require recording capability or baseline measurement because it works strictly by comparing the fourth twitch with its own internal standard, the first twitch.451,452 The unit may also be used with depolarizing relaxants, monitoring only the first twitch amplitude. A significant decrement (>50%) between the first and fourth twitch in this context suggests phase-two blockade (see E-Fig. 6-14).453 Needle electrodes are hazardous because they may cause bleeding, infection, burns, or nerve injury; current density may be very high, owing to the limited contact surface and the resistance of surrounding skin. Needle electrodes should not be used. Self-adhesive electrodes designed specifically for twitch monitors are available, but they are unnecessary and expensive and tend to be too large for infants and small children. A reasonable alternative is a pair of infant-sized ECG electrodes. The choice of monitoring location depends on the nature of the surgery. Any site where a motor nerve is close to the body surface and its associated muscle group is available for observation may be chosen. The most common stimulation site is the ulnar nerve in the forearm, observing the thumb (adductor pollicis brevis); this is the standard site for most research reports. It should be noted that the majority of studies have been standardized to the ulnar nerve, thus quantitating the response of other motor nerves is less reliable. Using the facial nerve may result in false-positive responses because the muscles may be easily stimulated directly, potentially causing an overdosing of nondepolarizing neuromuscular blocking agents.
Processed electroencephalogram Monitors
Monitoring the processed EEG has become a common means for monitoring patients who are anesthetized or sedated, but its role has yet to be fully defined in the pediatric patient population.454–457 Analysis of the EEG waveforms and EEG data from brain response to auditory input have been used to assess the depth of sedation and anesthesia and in turn to predict or avoid awareness under anesthesia in adults. Several excellent reviews are available regarding the technology and process of validation.458,459 Awareness under anesthesia has become a major source of litigation in the United States,460 and public concern prompted The Joint Commission to issue a Sentinel Event Alert.461 In response to this alert, the ASA instructed a taskforce to review the available literature and the existing monitors.462 Their review included studies from several processed EEG monitors (Bispectral Index [BIS], Aspect Medical Systems, Natick Mass.; Entropy, General Electric Healthcare Technologies, Waukesha, Wis.; and Narcotrend, MonitorTechnik, Bad Bramstedt, Germany) and data for auditory evoked potentials (AEP Monitor/2 (Danmeter Aps, Odense, Denmark). This task force reached several important conclusions:
A few studies have suggested that the use of these monitors may decrease awareness in adults463,464 but no definitive data exist in infants and children that these monitoring systems decrease the incidence of awareness under anesthesia.465 The vast majority of studies have examined the Bispectral Index (BIS); therefore this section will primarily focus on this device with the understanding that other devices are available but have not been adequately evaluated in children.
Bispectral analysis generates a value (0 to 100) derived from processed electroencephalographic data that provide a measure of the depth of anesthesia.458,466,467 There have been significant inconsistencies in data obtained from the BIS monitoring system in adults and even more inconsistencies in children. Some of the complexity and inconsistency of the BIS data will be reviewed to understand the possible uses and limitations of this and other EEG-based systems. Because of the nature of the data collection in the awake patient, artifacts may occur as a result of eye movement, blinking, and so forth at baseline if the device is applied before induction. Depth of anesthesia as measured by the bispectral analysis is more reliably assessed during propofol or inhalation-based anesthesia than during opioid-based anesthesia, although there is significant individual variation in the bispectral analysis reading.468–470 N2O has a minimal effect on the bispectral analysis, whereas ketamine paradoxically increases the bispectral analysis.458,471–473 Some well-controlled studies in adults examined the effects of a broad spectrum of medications on the bispectral analysis reading; this device monitored the effects of anesthetic drugs on awareness and patient responsiveness to painful and nonpainful stimuli.463,464,468,469,474–479 Studies also have demonstrated decreased anesthetic requirement and improved recovery parameters in adults who were monitored with bispectral analysis.480,481 However, although bispectral analysis technology appears to monitor the effects of hypnotics, it does not necessarily monitor the adequacy of anesthesia or analgesia.462
Bispectral analysis values in children anesthetized with sevoflurane were inversely proportional to end-tidal sevoflurane concentration in both infants and children, although the BIS values in anesthetized children had a much broader range than in adults.482,483 In one study, the BIS values decreased with increasing end-tidal concentration of sevoflurane until 3%, after which the bispectral analysis value paradoxically increased with higher inspired concentrations.482,483 At equi-minimal alveolar concentration (equi-MAC), bispectral analysis values are greater in children than in adults.483–485 Additionally, the bispectral analysis values at equi-MAC concentrations of volatile anesthetics has been inconsistent; several studies have shown that the bispectral analysis values during halothane anesthesia are greater than those during isoflurane, sevoflurane, or desflurane in children.486–488 These data indicate that BIS values may be substantially greater with halothane than with the other inhalational anesthetics.489 In addition, several studies have demonstrated conflicting results in children compared with infants, suggesting greater bispectral analysis values for halothane in children but not in infants at equi-MAC.482,485,488 All of these studies have suggested wide interindividual variability in the bispectral analysis values at comparable expired MAC concentrations of inhalational anesthetics. One disturbing study has found lower BIS values in intellectually impaired children compared with controls.490 Other studies have found differences between the right and left side of the brain.491 Limited data validate bispectral analysis readings in young children and infants. The algorithm was based on processed EEG readings from adults, and the EEG does change with age developmentally; therefore it seems reasonable that the algorithm will need to be adjusted for age if the bispectral analysis is to be used in infancy.
One study noted an incidence of awareness in children aged 5 to 12 years of approximately 0.8%.492 This is fourfold to eightfold greater than the incidence of awareness reported in adults, although the clinical implications are not clear at this time. Indeed, unlike in adults, none of the children who had awareness reported distress as a result.493,494 The use of N2O (when there is no contraindication) may be valuable in reducing the incidence of awareness without significantly adding to the duration of emergence.495 No randomized, controlled trials in children have demonstrated a decrease in awareness using bispectral analysis or other commercially available processed EEG devices. A recently introduced device is the SEDLine (Masimo Corp., Irvine, Calif.). This device uses four EEG electrodes and therefore simultaneously measures both sides of the brain. Preliminary experience at one institution has found that N2O interferes with SEDLine monitoring (personal communication, CJC). At present, no validating studies in children have been reported; until further data are developed, we do not recommend adopting this technology.
The BIS has the longest track record, but again its value in children is still quite unsubstantiated. One study in children 3 to 18 years of age demonstrated a more rapid recovery when bispectral analysis monitoring was used. However, this did not hold true for children younger than 3 years of age.496 Bispectral analysis monitoring is able to help predict voluntary patient movement during intraoperative wake-up tests during spinal fusions.497 Bispectral analysis also has been used to assess the depth of sedation in children and to correlate bispectral analysis values with other measures of recovery, exclusive of ketamine-induced sedation.498–500 Unfortunately, bispectral analysis values were unable to predictably separate moderate and deep sedation in children. In yet another study, a poor correlation was reported between the bispectral analysis values and a standardized Ramsay sedation score.501 Bispectral analysis values do not appear to correlate with arousal from sevoflurane anesthesia.502 Limitations on the usefulness of bispectral analysis monitoring in children include fear of probe placement while they are awake (thus in most cases the bispectral analysis monitor is applied after anesthetic induction). Because many procedures in children involve the head (e.g., strabismus, tonsillectomy, neurosurgical), the use of this monitor may be impractical in nearly half of the surgeries that involve children.
Intracranial Pressure Monitors
In children with increased intracranial pressure (ICP), direct monitoring of ICP may be helpful. Measurement may be accomplished by transduced pressures through either a ventricular cannula placed through a burr hole or by a subarachnoid bolt or equivalent. Measurement also can be performed noninvasively in infants with an open anterior fontanelle (see Chapter 24).
Apnea Monitors
Apnea monitors, whether based on transthoracic impedance, motion, or other patient parameters, are helpful in the perioperative and recovery room phases for former preterm infants younger than 60 weeks postconceptual age.503 Infants with a history of apnea spells and children with ongoing apnea spells are much more likely to develop apnea in the postoperative period. Even if a child has had a period of some months with a normal respiratory pattern at home, the anesthetic state may bring about a temporary return of apnea spells, and such a monitor should be used according to current guidelines (see Chapters 2, 4, 35, and 36).
Volume Meter and Spirometer
In some instances, monitoring a child’s respiratory gas flow may be desirable. A Wright turbine respirometer, Dräger positive displacement spirometer, and newer designs based on the pneumotachograph operating principle, vortex detectors, or thermistor flow meters are available. Some devices allow measurement of flow–volume loops, which can be very useful in both diagnosing and correcting ventilatory issues related to bronchospasm, airway compression, or single-lung ventilation.504–509 These monitors are not widely used in the clinical anesthesia care of children.
Inspiratory Pressure Gauge
In children who require ventilatory assistance, a pressure gauge applied at the airway allows the delivery of precise airway pressures and thus helps minimize barotrauma. When infants are treated with PEEP and continuous positive-airway pressure, airway pressures are best measured as close to the airway as possible. Such a device is useful in accurately determining leaks around an endotracheal tube when used to determine the size of the larynx (see Chapter 31).510
Invasive Blood Pressure Monitors
When clinically indicated, arterial, central venous, or very rarely pulmonary artery pressure should be monitored. Percutaneous arterial cannulation is performed with a 22- to 24-gauge catheter-over-needle device in infants and neonates, respectively, and a 20-gauge device in older children (see Chapter 48 and Fig. 48-10, A to D).511,512 Central venous and pulmonary artery cannulation are usually achieved using the Seldinger technique (see also Fig. 48-2, A to D).513,514 The proper reference location for all pressure transducers is usually the level of the child’s right atrium. If ICP monitoring is used or if the child is in the head-up or sitting position, a reference zero position at a reproducible cranial site (e.g., the external ear canal) is vitally important to accurately assess cerebral perfusion pressure. Placement of any of these invasive monitors provides additional benefits other than direct hemodynamic monitoring. Serial pH and arterial blood gas tension measurements from an arterial line or from determination of serial chemistries, ionized calcium, glucose concentrations, hematocrit, coagulation profiles, and osmolality allows continual assessment of a complex case. In operations performed in the sitting position, a right atrial catheter provides an approach to therapy for air embolism (see Chapter 24).
To withdraw a valid blood sample, an adequate volume of blood must be aspirated; 2 to 3 times the dead space (the point of aspiration to the tip of the catheter) usually accomplishes this.515 In infants, these systems must be flushed with small volumes at slow rates. In infant arterial lines, this amount is 0.5 mL/5 sec, to avoid dangerous fluctuations in blood pressure and retrograde flow with the risk of stroke.516
Echocardiography
Some reports have examined the usefulness of transesophageal echocardiography as a means of continuously monitoring cardiac output and the cardiac response to surgery and anesthetic agents. This equipment is very expensive and requires a great deal of skill and experience to take full advantage of its capacity. The practicality of this device in children appears to be primarily limited to those undergoing corrective cardiac surgery (see Chapters 14 to 16).517–524 However, this device is clearly of benefit in defining the source of an adverse cardiovascular event such as an air, clot, tumor embolism, or other source of right-sided outflow tract obstruction.525–529
Continuous Invasive and Noninvasive Cardiac Output Monitors
Traditionally, cardiac output has been estimated with thermodilution, Fick O2 consumption, or echocardiography. Continuous invasive techniques such as continuous analysis of central venous O2 saturation is feasible and accurate even in neonates but generally not applicable in the operating room.530,531 Arterial pulse contour analysis is another invasive method that requires arterial and central venous catheters; data regarding the accuracy and reliability for children are still preliminary.532–534 Other noninvasive techniques such as measurement of expired CO2 have been attempted but with variable success.535–542 The NICO device (Philips Respironics, Amsterdam, the Netherlands) estimates cardiac output as reflected by changes in expired CO2 using a Fick partial rebreathing calculation.541,542 This device requires that the child is intubated, and its accuracy in children is yet unproved.536,539,541,543 A new device, Cardiotronic EC (Cardiotronic, Inc., La Jolla, Calif.), has been approved by the FDA for use in children of all ages, including neonates.544–549 Electrical cardiometry continuously estimates stroke volume and left ventricular outflow to calculate cardiac output, cardiac index, and a variety of other parameters through quantitation of changes in impedance associated with changes in the orientation of red blood cells (Fig. 51-11). During diastole red cells are organized chaotically, but during systole they assume a position parallel to the direction of blood flow. Thus thoracic electrical bioimpedance relates to changes in thoracic aortic blood flow, and through the use of refined algorithms, noninvasive measurement of cardiac output is achieved.
The height (cm), gender, weight (kg), and age of the patient are entered into the handheld device. Four electrodes are applied to the neck and left chest at specified locations—in infants, one lead is placed on the cheek or forehead and the fourth lead placed on a thigh (see Fig. 51-11). The device records the heart rate and averages cardiac output every 10 to 60 heartbeats depending on how the device is configured. Studies in neonates, children, and adults suggest that this is a reasonable trend monitor to estimate cardiac output in a noninvasive manner. It certainly is not a monitor that will replace the gold standard of echocardiography or thermodilution cardiac output, but the r2 value of 0.86 and Bland-Altman plot suggest reasonable efficacy for monitoring and trending purposes in adults.550 Preliminary experience (CJC) with this device is favorable as an early warning device of a developing adverse cardiovascular event and as an indicator of successful intervention of such events.
Electrocautery
Although anesthesiologists are not directly responsible for electrocautery devices, they should have a basic knowledge of how they function and the necessary precautions to prevent burn injury or a fire. The most important means of preventing injury is to ensure that an appropriate-size grounding pad has been applied and that no blood, urine, or preparation solutions have been allowed to pool around or under the grounding pad. Additionally, the electrocautery can ignite any flammable implements, drapes, and an O2-rich environment. Severe injuries and deaths have occurred when high concentrations of O2 have been allowed to build up beneath drapes (supplemental O2 in awake or sedated patients) and have been ignited by the spark of the electrocautery on the other side of the surgical drape.551–556
Operating Table
The operating table is a vital piece of anesthesia equipment. The table should provide the full range of positioning, including Trendelenburg controls in case of either regurgitation or the need to increase venous return. It must have appropriate padding to prevent patient contact with metal structures. The ability to remove the head support and to lower the foot section provides a smaller table appropriate for infants. For extremely long procedures, an alternating-pressure air mattress or silicone gel padding placed between the patient and the operating table guards against decubitus injuries.557 Periodically changing the patient’s head position may prevent bald spots as a result of prolonged contact pressure in one location.558 The use of a silicone gel ring helps stabilize the head and reduces contact pressure at one point. The Jackson spinal table (Orthopedic Systems Inc., Union City, Calif.), along with other new tables with interchangeable modular components to maximize exposure of the surgical field and protect the patient from potentially harmful positions, have become popular for spine surgery in the past decade. This table may present unique challenges for the anesthesiologist, with access to the head of the table limited because of an immobile and obstructive block. If tracheal intubation must be performed in a smaller child who requires this table, provisions should be made to ensure that adequate access to the head can be obtained to facilitate laryngoscopy and intubation in advance of induction of anesthesia. An additional concern is to ensure that the operating table can handle the unfortunate increasing weight of the pediatric patient population; many centers are now performing bariatric surgery in teenagers.
Purchasing Anesthesia Equipment
The following are useful principles for any equipment purchase:
1. New purchases should interface with equipment that is already present. Considerable cost savings can occur if the same or compatible equipment is used in all the perioperative areas, including the OR, PACU, and ICU. Another issue to consider is how the equipment will interface with automated record-keeping systems and other hospital information systems if they are present. If an automated recording device is a possible plan for the future, the equipment should be evaluated for how well it will interface with systems that will possibly be purchased. Another compatibility issue to carefully review is the software that is required to use the equipment. Review the product information carefully to identify the need for software upgrades and compatibility, which can be important for safety and cost-effectiveness.
2. Recognize that the salesperson is inherently biased.
3. Always test proposed equipment in the environment in which it will be used, with the personnel who will be using it. What appears attractive in a display as presented by a salesperson may not function well in practice. It is also important to test the device in unusual circumstances to see how well it performs. The use of simulation is becoming an important modality to test devices under normal and unusual circumstances. Human factors must be considered, such as how easy it is to use the equipment clinically (i.e., the user interface), and equally important, the safety of the equipment or product. Evaluate systems issues that could lead to error or misuse. Software-driven products are harder to test for how they may potentially fail when connected to existing systems and products in your workstations; always test new equipment in the environment where it will be used.
4. Do not use equipment for any purpose other than that for which it has been designed.
5. Have your hospital biomedical staff be intimately involved with the safety issues of your systems, as described earlier, and periodically check for electrical leakage and other safety issues.
6. Decide explicitly how the product will be maintained; are the biomedical professionals going to perform the necessary calibration, testing, and repair of the equipment, or is a member of your department going to take on this role? Spend the necessary resources to properly maintain the product; this is typically on the order of 10% of the cost of the device per year. Consider a maintenance contract, if available. Ask that the company ensure the future availability of parts and the compatibility of subsequent modifications or design evolutions with your equipment.
7. Consider the cost and quality of disposable components of the equipment. This can also have an impact on the safety and cost of the product.
8. If two products are comparable but one has local service facilities, that one may be the better choice.
9. If areas of special needs have been recognized, be as detailed as possible with specifications. For example, if a monitor is to be used strictly in the OR, it may be reasonable to operate it from wall power. Conversely, if the monitor is to serve in the OR, and also for transport between the operating theater and the recovery room or ICU, it must have internal battery backup.