Pediatric Cardiothoracic Computed Tomographic Angiography
Remarkable advances have occurred in noninvasive imaging evaluation of pediatric cardiothoracic vascular disorders. One such technologic advancement is multidetector array computed tomography (MDCT). MDCT, using the computed tomography angiographic (CTA) technique, has become a primary imaging consideration for structural cardiovascular evaluation beginning as early as the newborn period.1–5 Attention to technique is fundamental for pediatric CTA.6–8 Without optimal or at least sufficient technical performance, diagnostic capabilities may be limited. This technical aspect (in addition to the diagnostic interpretation and communication of results) is the responsibility of the imaging expert. Therefore the objective of this chapter is to provide technical guidelines for performing pediatric thoracic CTA. The clinical examples provided illustrate these technical considerations.
Costs and Benefits
Pediatric CTA offers several advantages over other contemporary imaging modalities, including echocardiography, magnetic resonance imaging (MRI), and conventional cardiac catheterization and angiography. First, computed tomography (CT) provides the best global assessment of the lungs and airways, as well as other regional structures, in both congenital9 and acquired vascular disorders (e-Figs. 66-1 and 66-2).
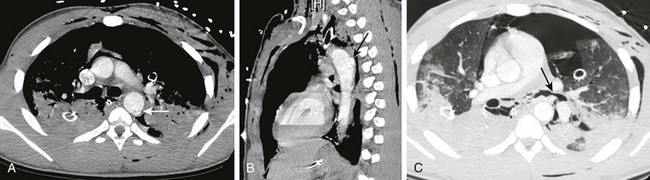
e-Figure 66-1 Posttraumatic aortic dissection in a 17-year-old male after a motor vehicle collision.
Axial computed tomographic angiography image (A) and sagittal reconstruction (B) demonstrate aortic luminal disruption (arrows). C, Note irregularity of the left bronchus (arrow) as a result of laceration with the adjacent pneumomediastinum and pneumothorax despite thoracostomy drainage. (From Frush DP. Thoracic cardiovascular CT: Technique and applications, Pediatr Radiol 39(3):464-70, 2009. Reprinted with permission.)
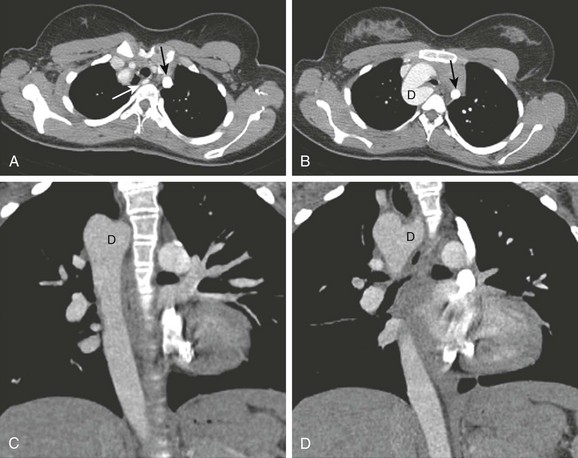
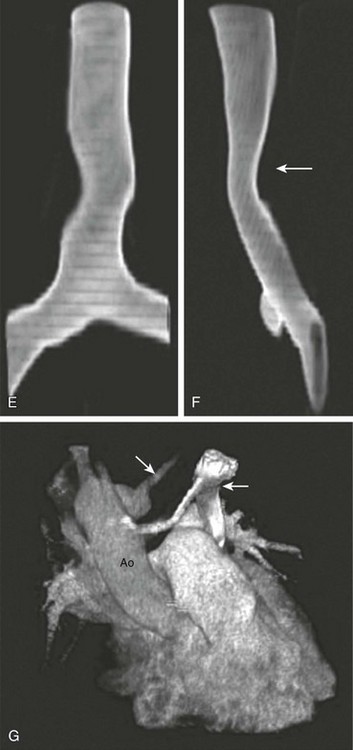
e-Figure 66-2 An 11-year-old girl with chronic cough on exertion due to a right aortic arch with an aberrant left subclavian artery.
A, Axial intravenous contrast-enhanced image from a computed tomography angiography scan at the level of the thoracic inlet shows the aberrant course of the left subclavian artery (white arrow). B, This is associated with an aortic diverticulum (D), resulting in airway narrowing. Note the left-sided superior vena cava (SVC) (black arrow in A and B). Coronal reformations (C, D) also show a right-sided arch and descending aorta, as well as a left-sided SVC seen adjacent to the left pulmonary artery in D. E) and lateral (F) projections shows mild displacement and effacement (arrow in F). G, Volume rendering shows the subclavian artery (large arrow) and left SVC (small arrow). Ao, Aorta. (From Frush DP. Technique of pediatric thoracic CT angiography, Radiol Clin North Am 43:419-433, 2005. Reprinted with permission.)
With newer technology, such as volume MDCT (e.g., 320 detector array single rotation acquisition)10 or dual-source MDCT (Fig. 66-3),11 a complete examination of the entire chest of an infant can be completed in less than 1 second. With echocardiography, MR vascular imaging, or angiography, imaging times typically exceed 20 minutes and may occur over hours. A reduced examination time means that CT is better tolerated by infants, children, and patients in the intensive care unit who may have a limited ability to hold still or require the examination to be performed quickly for other reasons. The technical quality, including display, also is more consistent with CT compared with echocardiography, which is a more operator-dependent examination, and compared with MR evaluation, where operators select parameters and sequences that may give a different study quality from one examination to the next. CT also allows better patient monitoring than does MRI. In addition, many of the contraindications of MRI (e.g., pacemakers, internal support apparatus, and some surgical devices) are not contraindications with CTA and produce less image artifact than with MR angiography. Unlike with conventional angiography, the multiplanar and three-dimensional capabilities of CTA provide for off-line review of information in virtually any plane. For conventional angiography, this feature is limited to the planes selected during a particular sequence, and for echocardiography, the examination planes or views recorded are the only information available for off-line evaluation. The cost of CT is comparable with that of Doppler echocardiography, in general is less than that of MR, and is much less than conventional angiography.
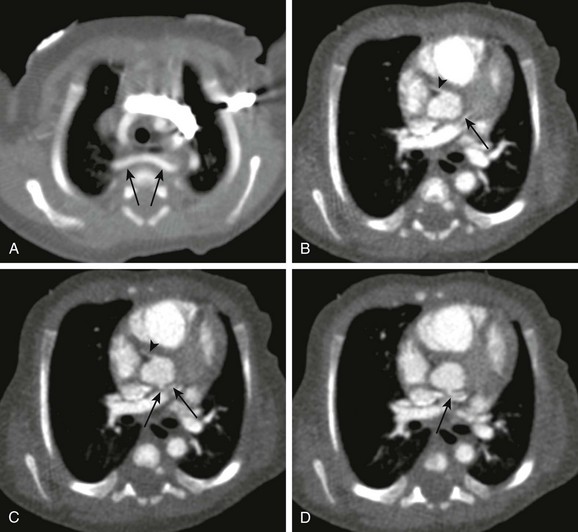
Figure 66-3 A newborn boy with labored stridor.
Aortic arch anatomy was incompletely assessed on echocardiography. Nongated dual-source cardiac computed tomographic angiography was performed using high-pitch 3.2, 80 kVp, 60 effective mA, and 6.0 mL of 300 mg/mL iodine yielding an estimated radiation dose of approximately 1.4 mSv. The scan was performed over about 0.6 seconds. A, An aberrant right subclavian artery (arrows) is seen to pass behind a normal caliber trachea. No cause for respiratory difficulty was identified. B to D, Note excellent depiction of small structures as a result of high-pitch scanning, demonstrating the normal origin of the right coronary artery (B and C) (arrowheads) and the relatively posterior origin and course of the left main coronary artery, probably still from the left coronary cusp in B to D (arrows).
CTA has some disadvantages. CTA involves the use of radiation, which is an issue with angiocardiography but not with MRI or echocardiography. CT radiation dosages depend on the technique used. In general, CTA can be performed with a dosage similar to or lower than that of a routine chest CT. CTA dose estimates in young children can be less than 1.0 mSv.12,13 With gated technology, employing prospective gating and newer volume scanning, doses also can be substantially lower than with older CT technology and retrospective gating.13–16 With rare exceptions, properly performed pediatric CTA results in a lower radiation dose than conventional diagnostic angiographic evaluation. Electrocardiographic-gated CTA usually involves a greater radiation dosage than limited diagnostic conventional angiography or a nongated CTA. Intravenous (IV) contrast material is required for CTA but also is required for conventional angiography and for MR angiography. The risk of major adverse effects (e.g., airway spasm and cardiovascular collapse) from iodinated IV contrast material is extremely small in children.17,18 Unlike with echocardiography, MRI, and conventional angiography, pediatric thoracic CTA typically is used for morphologic assessment, although cardiac function and other hemodynamic information can be obtained.19 CT for cardiovascular evaluation also is not portable.
Technique
Box 66-1 shows the steps taken in performing a pediatric CTA (see e-Fig. 66-2).6–8
Patient motion needs to be controlled (e-Fig. 66-4), which may require sedation of younger children. Breath holding is preferred during CTA but is not a requisite, especially with volume scanning and other fast (e.g., dual-source, high-pitch) scanning technology. If the child is intubated, an inspiratory hold during the CTA evaluation is recommended. Metal (e.g., pacemakers, intracardiac leads, sternal wires, stents, clips, and valves) and arms (Fig. 66-5) may cause streak artifact. Monitors and associated leads should be positioned outside the scan field if possible to minimize streak artifact across the chest. Knowing the location and caliber of angiocatheters used for IV contrast administration will help determine the rate of administration and the time it takes contrast material to reach the heart.20 Finally, because cardiac CT evaluation may involve patients with left-to-right shunts or admixture lesions, extreme care must be taken to avoid injection of air or a thrombus through the line when contrast is administered. This situation is rarely, if ever, a problem with adult cardiac evaluation.
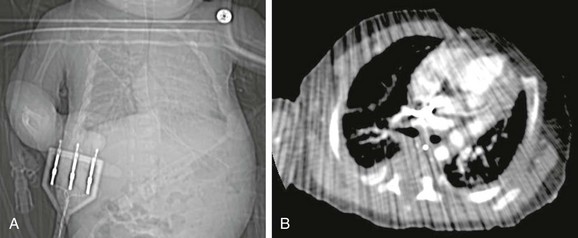
Figure 66-5 Artifact from leads.
A, A topogram demonstrates the leads that were in the scan range. B, Note substantial streak artifact. (From Frush DP: Thoracic cardiovascular CT: technique and applications, Pediatr Radiol. 2009;39(3):464-70. Reprinted with permission.)
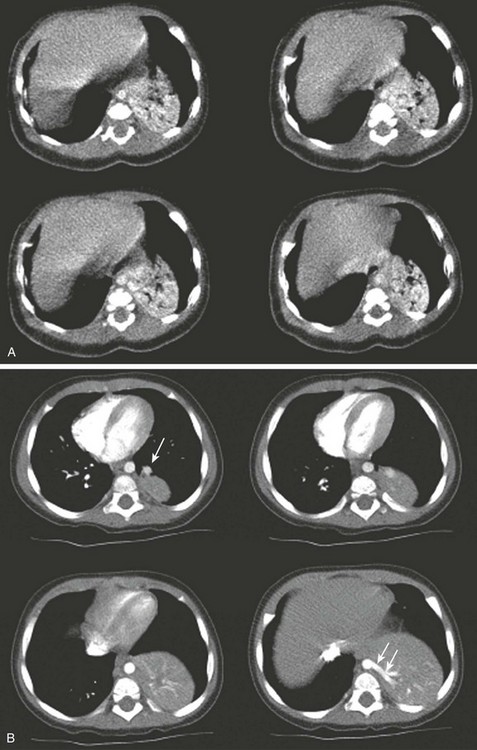
e-Figure 66-4 Infant with pulmonary sequestration.
A, Four basilar axial images from the initial computed tomography angiogram performed without sedation were inadequate because of movement and poor arterial bolus. B, A subsequent examination performed after adequate sedation and improved contrast administration shows pulmonary venous drainage (arrow in upper left image) and systemic arterial supply (arrows in lower right image).
Technical considerations regarding the IV contrast material include type and concentration, dosage, rate of administration, and timing of scan onset after administration.21 In general, low osmolar, nonionic contrast media are recommended for contrast-enhanced CT scanning in the pediatric population. The iodine concentration varies but usually is around 300 mg/mL. For newborns and small children, higher concentrations such as 370 mg/mL will afford better enhancement of their smaller vessels. A dosage of 1.5 mL/kg is usually adequate, with a maximum total volume of 125 mL. With gated cardiac evaluation, the contrast dose can be smaller (e.g., <100 mL). If a second CT scan must be performed while the patient remains on the scanner, then 1 to 1.5 mL/kg of additional contrast can be administered. In this case, the maximal dosage would be 3.0 mL/kg, which is still reasonable for a single examination.
The timing of contrast administration with respect to scanning initiation is a critical factor in CTA. In general, scanning is started either during administration of the contrast or immediately after administration is completed. Timing depends on the structures that need to be opacified. Later scan initiation is used for opacification of the thoracic aorta and major branches or systemic venous structures, whereas earlier scan initiation is appropriate for right-sided structures, especially pulmonary arteries. An empiric delay can be used, but because of the wide range of sizes that may be encountered in pediatric CTA (i.e., 1.0 to 100 kg), a single recommendation is impossible. A range of delays could be determined depending on the rate of administration. In general, most cardiac CTA, even in the smallest child, does not begin before 5 seconds after the onset of contrast administration. For large children, the empiric delay may be as long as 50 seconds. Bolus tracking technology, which obviates the need for this estimation, can be used to start the CTA. Alternatively, a test bolus can be administered and the arrival of contrast to the desired location (for cardiac CT, the right side versus the left side of the heart) can be used (Fig. 66-6).
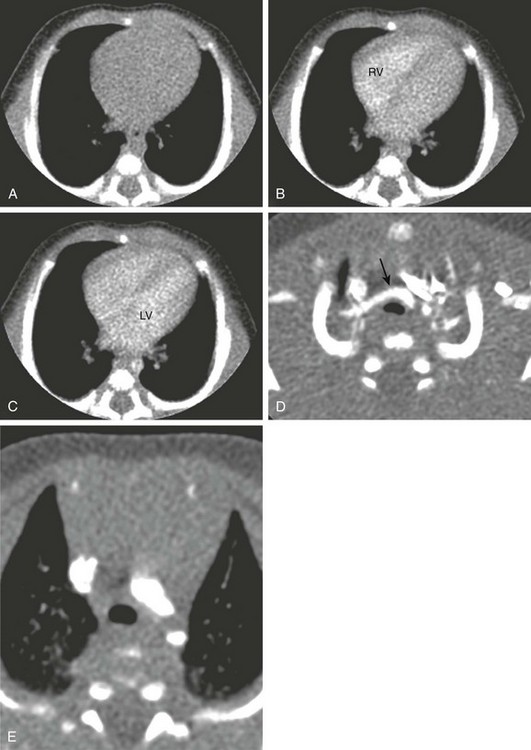
Figure 66-6 A 4-week-old infant boy with congenital stridor.
An echocardiogram was not able to fully assess for a suspected vascular ring. Axial serial isolevel monitoring images during bolus tracking at the midventricular level show precontrast appearance (A) and the arrival of a 0.6-mL test bolus in the right ventricle (RV) at 5 seconds (B) and the left ventricle (LV) and descending thoracic aorta at approximately 7 seconds (C). D, After the diagnostic computed tomography angiogram, the trachea is narrowed at the level of the thoracic inlet to about 50% of normal anteroposterior diameter brachiocephalic artery (arrow), but no distal tracheal or aortic branching abnormality was present (E). (From Frush DP. Technique of pediatric thoracic CT angiography, Radiol Clin North Am. 2005;43:419-433. Reprinted with permission.)
This test bolus technique works even in neonates. A test bolus of 10% of the total expected volume is used, which requires a tuberculin syringe and minimization of catheter and tubing dead space for a child receiving a test bolus of less than 1.0 mL. The arrival of the test bolus at the appropriate side of the heart is timed, and this time is used to set the delay for the start of scanning. In neonates, usually about 2 to 3 seconds is added to this arrival of the test bolus for optimal enhancement. Tracking without a test bolus is simple to perform, and a test bolus rarely is used for nongated CTA in children. Fontan circuits may require special contrast administration techniques.22
Scan techniques include adjustment of technical parameters such as the number of detector rows, the thickness of detectors, the gantry cycle time, tube current (milliamperes [mA]), and peak kilovoltage (kVp). Parameters such as scan thickness and interval are adjustable after the scan volume is obtained (these parameters usually are in protocols). Suggestions for a CTA technique are provided in Table 66-1.21
Table 66-1
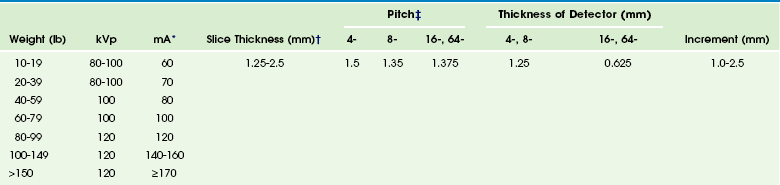
*Slightly higher than body CT protocols. Use fastest gantry rotation time.
†Displayed thickness. For coronal and sagittal reformats and three-dimensional reconstructions, reconstruct an axial data set at thickness of the detector (e.g., 0.625 for a 16-slice scanner) at 0.5- to 1.0-mm intervals, soft algorithm. Multiplanar thickness and interval should be similar to axial. For evaluation of larger structures (e.g., the aorta), especially in larger children, the larger detector configuration (2.5 for an 8-slice scanner and 1.25 for a 16-slice scanner) and a larger reconstructed thickness and interval can be used.
‡For larger children and larger vessels, the highest pitch can be used for multidetector array computed tomography.
Modified from Frush DP. Evidence-based principles and protocols for pediatric body multislice computed tomography. In Knollman F, Coakley FV, editors: Multislice CT: principles and protocols, Philadelphia: Elsevier; 2005.
The isotropic images obtained with 16-slice and higher MDCT scanners provide excellent multiplanar reformations and three-dimensional evaluation, which show complicated anatomy in a way to which clinical care providers (e.g., cardiac surgeons) are accustomed (e-Figs. 66-7 to 66-9). The thinnest detector option should be chosen for pediatric CTA, because thinner slices improve the quality of reformations. The fastest gantry cycle time is recommended to minimize motion artifact and to obtain the fastest scanning time possible, which is important in children who may be anxious or otherwise fidgety. Pitch, essentially representing table speed (mm/second) divided by effective collimation (mm), generally is in the range of 1 to 1.5 for nongated CTA. Tube current, including tube current modulation technology,23 varies depending on patient size; recommendations can be found in Table 66-1.
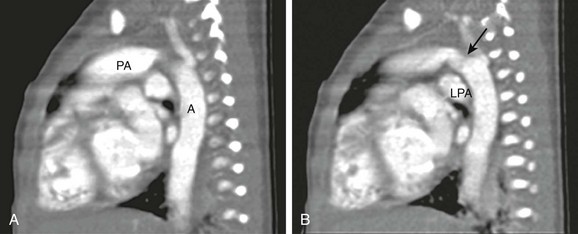
e-Figure 66-7 A 2-day-old infant boy with interrupted aortic arch identified by echocardiography.
Because the aortic branch anatomy was not well visualized and there was a suggestion of a possible systemic artery supplying a portion of the right lung, a computed tomography angiogram was performed. The examination was performed at 80 kVp. A and B, With sagittal reformation, the relationship between the large ductus arteriosus (arrow) connecting the main pulmonary artery (PA) and the aorta (A) is seen. LPA, Left pulmonary artery. (From Frush DP, Herlong RJ. Pediatric thoracic CT angiography, Pediatr Radiol 35:11-25, 2005. Reprinted with permission.)
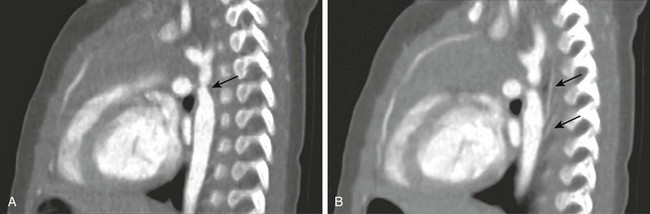
e-Figure 66-8 Sagittal reformations of aortic coarctation in a 4-month-old infant boy.
A, The location of the coarctation is well demonstrated (arrow). B, Collaterals (arrows) are seen originating distal to the coarctation. (From Frush DP. Technique of pediatric thoracic CT angiography, Radiol Clin North Am 43:419-433, 2005. Reprinted with permission.)
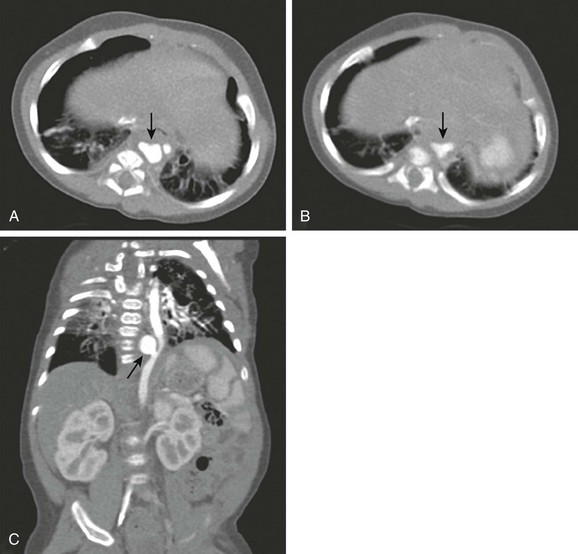
e-Figure 66-9 An infant with a descending thoracic aneurysm, probably from an infected umbilical arterial catheter in the neonatal period.
Axial images (A and B, slightly inferior to A) show an irregularity of the aorta (arrows). C, Coronal reconstruction shows an aneurysm (arrow), neck, and displacement of the adjacent aorta. The aneurysm was successfully occluded with a coil. (From Frush DP, Yoshizumi T. Conventional and CT angiography in children: dosimetry and dose comparisons, Pediatr Radiol 36:154-158, 2006. Reprinted with permission.)
Because contrast is relatively high in cardiothoracic CTA, consideration should be given to lowering the peak kilovoltage.8,24 This step will improve image contrast and, although some increased noise occurs, the increase in contrast is greater than the increase in noise in small children, which produces an improved contrast-to-noise ratio and better image quality. Therefore 80 kVp (Fig. 66-10) for newborn through young school-age children and 100 kVp for older but still young school-age children (up to about 10 years assuming normal body habitus) is acceptable. A value of 120 kVp can be used for older children.
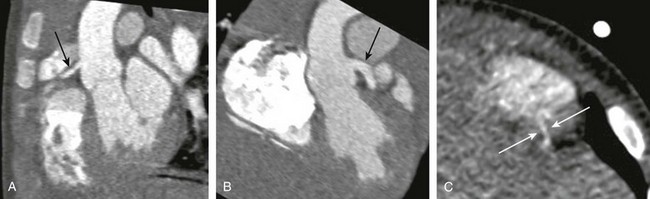
Figure 66-10 A coronary cameral fistula in a 10-month-old boy.
Oblique reformatted maximum intensity projection images through the origins of the left (A) (arrow) and right (B) (arrow) coronary arteries demonstrate a larger left coronary artery. C, A coned down axial image demonstrates the distal portion of the left sided coronary-cameral fistula (arrow) coursing through the myocardium to the right ventricular chamber. (From Lerner C, Frush DP, Boll DT. Evaluation of a coronary-cameral fistula: benefits of coronary dual source MDCT angiography in children, Pediatr Radiol. 2008;38:874-878. Reprinted with permission.)
Cardiac gated CTA became more practical after the availability of 16-detector row technology with improved spatial resolution. As previously noted, prospective gating reduces radiation dose. Technical considerations have been reviewed recently13; a detailed description of gated techniques is beyond the intent of this chapter. Even more than with the nongated angiographic technique, presence at the scanner by the imaging expert usually is necessary to determine contrast details, particularly with the timing bolus, and scan parameters (e.g., scan range, tube current, and peak kilovoltage), as well as performance of postacquisition processing. Applications include improved depiction of the great vessels (e.g., for aortic dissection). However, evaluation in children usually is focused on coronary artery anatomy. Recently, dual-source design (i.e., two x-ray tubes) has improved temporal resolution (obviating β-blockage of heart rates, even to heart rates around 120 beats/min) (see Figs. 66-10 and 66-11).25 Pediatric applications of coronary artery CTA include congenital abnormalities related to the number, site of origin, or course of the arteries, as well as postoperative effects on coronary arteries (e.g., after correction of transposition of the great vessels). In addition, the effect of systemic disorders, such as Kawasaki disease, on coronary arteries (and development of stenosis or aneurysm) increasingly is reported using cardiac gated techniques. As with adults, intracardiac anatomy is better displayed with use of gating, although dual-source high-pitch technology can provide improved intracardiac detail compared with non–high pitch and single-source scanners. Applying adult techniques to pediatric scanning can result in excessively high radiation dosages—as high as 30 millisieverts (mSv)—which is approximately 10 to 20 times the dosage of a normal chest CT examination in a child. Dosages and image quality for gated cardiac evaluation should reflect reduced-dosage techniques for other body applications in infants and children, with consideration of lower tube current and kilovoltage (see Fig. 66-6).
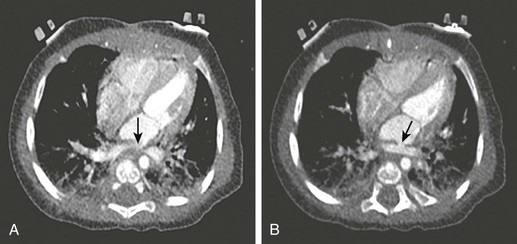
Figure 66-11 Postoperative evaluation of total anomalous pulmonary venous return in an infant.
Pulmonary vein stenosis was suspected after echocardiography was performed. A gated cardiac computed tomography examination, without β-blockade (heart rate, approximately 120 beats/min) was performed using 110 mA (approximately half of the adult tube current). Stenoses are present at insertion of the right upper pulmonary vein at the left atrium (arrow) (A) and the confluence of the remainder of the pulmonary veins (arrow) (B). A twisting of the confluence of all pulmonary veins was encountered in the operating room and corrected. (From Frush DP, Yoshizuma T. Conventional and CT angiography in children: dosimetry and dose comparisons, Pediatr Radiol. 2006;36:154-158.)
The use of bismuth breast shields to reduce breast dose has lowered radiation dose and maintained image quality for pediatric chest CT,26 although the benefit of shielding is debated given the availability of other techniques for dose reduction.27 The use of organ-based dose modulation, where the tube current is reduced over an arc to reduce surface dose such as for the breasts, has yet to be systematically studied in pediatric chest CT. In addition, iterative reconstruction technology28 likely will provide new opportunities to reduce dose or improve image quality for pediatric thoracic cardiovascular CTA.
Frush, DP. Thoracic cardiovascular CT: technique and applications. Pediatr Radiol. 2009;39(3):464–470.
Frush, DP. Evidence-based principles and protocols for pediatric body multislice computed tomography. In: Knollman F, Coakley FV, eds. Multislice CT: principles and protocols. Philadelphia: Elsevier, 2005.
Siegel, MJ. Computed tomography of pediatric cardiovascular disease. J Thorac Imaging. 2010;25:256–266.
Young, C, Taylor, A, Owens, C. Paediatric cardiac computed tomography: a review of imaging techniques and radiation dose consideration. Eur Radiol. 2011;21:518–529.
References
1. Shiraishi, I, Kajiyama, Y, Yamagishi, M, et al. The applications of non-ECG-gated MSCT angiography in children with congenital heart disease. Int J Cardiol. 2010;13108:1–6.
2. Dillman, J, Hernandez, R. Role of CT in the evaluation of congenital cardiovascular disease in children. AJR Am J Roentgenol. 2009;192:1219–1231.
3. Goo, H, Park, I, Ko, J, et al. CT of congenital heart disease: normal anatomy and typical pathologic conditions. Radiographics. 2003;23:S147–S165.
4. Haramati, L, Glickstein, J, Issenberg, H, et al. MR imaging and CT of vascular anomalies and connections in patients with congenital heart disease: significance in surgical planning. Radiographics. 2002;22:337–349.
5. Siegel, MJ. Computed tomography of pediatric cardiovascular disease. J Thorac Imaging. 2010;25:256–266.
6. Frush, DP. Technique of pediatric thoracic CT angiography. Radiol Clin North Am. 2005;43:419–433.
7. Frush, DP, Herlong, RJ. Pediatric thoracic CT angiography. Pediatr Radiol. 2005;35:11–25.
8. Frush, DP. Thoracic cardiovascular CT: technique and applications. Pediatr Radiol. 2009;39(3):464–470.
9. Lee, E, Boiselle, P, Shamberger, R. Multidetector computed tomography and 3-dimensional imaging: preoperative evaluation of thoracic vascular and tracheobronchial anomalies and abnormalities in pediatric patients. J Pediatr Surg. 2010;45(4):811–821.
10. Mousily, F, Shifrin, R, Fricker, F, et al. Use of 320-detector computed tomographic angiography for infants and young children with congenital heart disease. Pediatr Cardiol. 2011;32:426–432.
11. Lell, MM, May, M, Deak, P, et al. High-pitch spiral computed tomography: effect on image quality and radiation dose in pediatric chest computed tomography. Invest Radiol. 2011;46(2):116–123.
12. Hellinger, JC, Pena, A, Poon, M, et al. Pediatric computed tomography angiography: imaging the cardiovascular system gently. Radiol Clin North Am. 2010;48(2):439–467.
13. Young, C, Taylor, A, Owens, C. Paediatric cardiac computed tomography: a review of imaging techniques and radiation dose consideration. Eur Radiol. 2011;21:518–529.
14. Hollingsworth, CL, Frush, DP, Yoshizumi, TT, et al. Pediatric cardiac-gated CT angiography: assessment of radiation dose. AJR Am J Roentgenol. 2007;189:12–18.
15. Frush, DP, Yoshizumi, T. Conventional and CT angiography in children: dosimetry and dose comparisons. Pediatr Radiol. 2006;36:154–158.
16. Huang, B, Law, M, Mak, H, et al. Pediatric 64-MDCT coronary angiography with ECT-modulated tube current: radiation dose and cancer risk. AJR Am J Roentgenol. 2009;193:539–544.
17. Dillman, JR, Strouse, PJ, Ellis, JH, et al. Incidence and severity of acute allergic-like reactions to IV nonionic iodinated contrast material in children. AJR Am J Roentgenol. 2007;188:1643–1647.
18. Callahan, MJ, Poznauskis, L, Zurakowski, D, et al. Nonionic iodinated intravenous contrast material-related reactions: incidence in large urban children’s hospital—retrospective analysis of data in 12,494 patients. Radiology. 2009;250(3):674–681.
19. Goo, H. Haemodynamic findings on cardiac CT in children with congenital heart disease. Pediatr Radiol. 2011;41:250–261.
20. Yang, M, Mo, X, Jin, J, et al. Image quality and radiation exposure in pediatric cardiovascular CT angiography from different injection sites. AJR Am J Roentgenol. 2011;196:W117–W122.
21. Frush, DP. Evidence-based principles and protocols for pediatric body multislice computed tomography. In: Knollman F, Coakley FV, eds. Multislice CT: principles and protocols. Philadelphia: Elsevier, 2005.
22. Greenberg, S, Bhutta, S. A dual contrast injection technique for multidetector computed tomography angiography of Fontan procedures. Int J Cardiovasc Imaging. 2008;24:345–348.
23. Herzog, C, Mulvhill, D, Nguyen, S, et al. Pediatric cardiovascular CT angiography: radiation dose reduction using automatic anatomic tube current module. AJR Am J Roentgenol. 2008;190:1232–1240.
24. Yu, L, Bruesewitz, M, Thomas, K, et al. Optimal tube potential for radiation dose reduction in pediatric CT: principles, clinical implementations, and pitfalls. Radiographics. 2011;31:835–848.
25. Lerner, C, Frush, DP, Boll, DT. Evaluation of a coronary-cameral fistula: benefits of coronary dual source MDCT angiography in children. Pediatr Radiol. 2008;38:874–878.
26. Kim, S, Frush, D, Yoshizumi, T. Bismuth shielding in CT: support for use in children. Pediatr Radiol. 2010;40:1739–1743.
27. Geleijns, J, Wang, J, McCollough, C. The use of breast shielding for dose reduction in pediatric CT: arguments against the proposition. Pediatr Radiol. 2010;40(11):1744–1747.
28. Silva, AC, Lawder, HJ, Hara, A, et al. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol. 2010;194:191–199.




