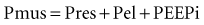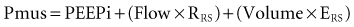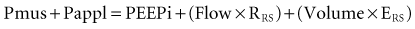49 Patient-Ventilator Interaction
The clinical management of patients with acute respiratory failure is based on the assumption that significant abnormalities in respiratory mechanics, respiratory muscle performance, and control of breathing are the underlying mechanisms responsible for acute respiratory failure.1 The effects of mechanical ventilation on gas exchange, respiratory muscle load, and dyspnea depend on the matching between the ventilator settings and the patient’s respiratory physiology. However, mechanical ventilation is rarely optimized, which would require that ventilator settings be based on accurate and reproducible measurements of lung and chest wall mechanics, respiratory muscle function, and respiratory drive.2–5
 Respiratory Physiology
Respiratory Physiology
The goal of the intrinsic ventilatory control system is to integrate the timing and intensity of the phrenic nerve signal, inputs from chemoreceptors and pulmonary stretch receptors, and variations in metabolic demands. Contraction of the respiratory muscles leads to the generation of flow and volume to provide adequate alveolar ventilation with minimal work of breathing.6 During spontaneous breathing,7 the respiratory muscles generate pressure (Pmus) to generate flow against the resistive properties (RRS) and volume against the elastic properties (ERS) of the respiratory system to eventually overcome intrinsic positive end-expiratory pressure (PEEPi). Under these circumstances, the act of spontaneous breathing can be described at any instant as follows:
where Pres represents the resistive pressure and is a function of flow (Pres = Flow × RRS), and Pel represents the elastic recoil pressure and is a function of volume (Pel = Volume × ERS). Assuming that RRS and ERS are linear, the equation becomes:
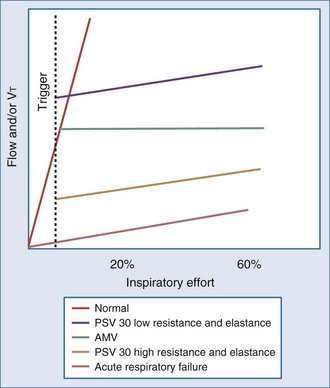
The complex interaction among all the variables in equation 3 can be summarized by the concept of neuroventilatory coupling (Figure 49-1).8 Under normal conditions, as well as at the onset of acute respiratory failure, the spontaneous contraction of the respiratory muscles suddenly generates flow and volume; the slope of the relationship between effort and ventilatory output is conditioned by the contractile properties of the respiratory muscles and the impedance of the respiratory system. When positive pressure is applied to assist the action of breathing in most common modes of mechanical ventilation (pressure-support or pressure-assist mandatory ventilation), the coupling between effort and output is compromised. During assist-control ventilation, flow and volume remain constant despite changes in muscle contraction; during pressure-support ventilation, despite coupling between inspiratory effort and ventilatory output, any increase in respiratory impedance decreases the amount of delivered flow and volume.8 During noninvasive ventilation (NIV), air leaks may further compromise the coupling between patient effort and ventilatory output.9
 Patient and Ventilator Variables
Patient and Ventilator Variables
Patient Variables
The patient interacts with the ventilator based on three physiologic variables2,10,11:
Ventilator Variables
Three phase variables define inspiration13,14:
Features of ventilators such as blowers and inspiratory, expiratory, and positive end-expiratory pressure (PEEP) valves are also important in determining the interaction between patient and ventilator.23–26
Total Ventilator-Controlled Mechanical Support
In this mode, the patient’s breathing pattern is totally controlled by the ventilator. The pressure generated by the respiratory muscles is abolished by paralysis or sedation. Flow, volume, and pressure are imposed by the ventilator, and the patient’s breathing pattern is totally replaced by that of the ventilator. The risk of patient-ventilator asynchrony is therefore abolished, but there are potential risks associated with sedation and paralysis,27 respiratory muscle atrophy,28 lung damage due to overdistention,29 patient discomfort,30 and difficulty weaning after prolonged controlled mechanical ventilation.1
Partial Patient-Controlled Mechanical Support
With this mode, spontaneous breathing activity is partially preserved.31 The need for sedation and paralysis may be reduced, disuse atrophy of the respiratory muscles may be minimized, and the weaning process may be accelerated, provided the patient’s ventilatory demand and ventilator settings are synchronized.32 The ability to restore gas exchange, unload respiratory muscles, and relieve patient dyspnea with partial patient-controlled mechanical support therefore depends on the absence of patient-ventilator asynchrony.33
Although there are no well-accepted definitions, patient-ventilator asynchrony is common; it is often unrecognized, underestimated, and inappropriately treated.3–5,22,33–35 The cause of patient-ventilator asynchrony can be described as occurring because of a mismatch between the three physiologic variables characterizing spontaneous breathing (ventilatory drive, ventilatory requirements, and duration and ratio of inspiratory time to total breath cycle duration) and the three technologic variables characterizing ventilator function (trigger function, gas delivery algorithm, and cycling criteria).
 Respiratory Drive–Ventilator Trigger Asynchrony
Respiratory Drive–Ventilator Trigger Asynchrony
During partial ventilatory assistance (assisted breath), the inspiratory synchronization system (inspiratory trigger) detects any patient inspiratory effort and activates a mechanical act. Therefore, inspiratory effort is tracked in order to couple the patient’s effort with the delivery of pressure, flow, or volume. The goal of a good inspiratory trigger is to reduce as much as possible the duration and intensity of the muscular effort that comes before mechanical support, while avoiding autotrigger effects.36 Auto-triggering can be defined as a mandatory breath not following a patient’s inspiratory effort.
It has been suggested that a trigger (independently of its algorithm) must have a response time less than 100 ms. However, the inspiratory effort necessary to trigger a breath may be a significant part of the total inspiratory effort, representing 17% and 12% of the total inspiratory effort during pressure and flow triggering, respectively.15–23,34,35 Aslanian and coworkers found that even though the time required for triggering was 43% shorter, and effort during the time of triggering was 62% less with flow triggering than with pressure triggering, effort during the post-triggering phase was equivalent for both pressure and flow triggering.37 The clinical benefit of flow triggering therefore appears to be much less relevant than commonly stated.3
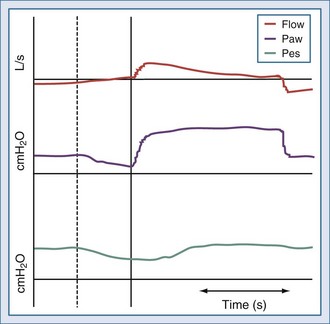
Inspiratory phase asynchrony may be due to problems with inspiratory triggering, and this can be correlated with respiratory drive. Phase lag quantifies the delay between the commencement of inspiratory muscle activity and the beginning of mechanical inflation (Figure 49-2).3,10,11 The presence of a threshold load, such as dynamic intrinsic PEEP, may further complicate patient-ventilator interaction during the triggering phase.15 Giuliani et al. suggested that effort during triggering determines patient effort during the remaining portion of inspiration.38 Leung and coworkers demonstrated that the higher the level of ventilator-applied pressure, the lower the respiratory drive, but the longer the time required to trigger the ventilator. As a result, respiratory muscles generate smaller inspiratory swings in intrathoracic pressure but over a longer inspiratory time.2 Another problem is related to the fact that pressure is mostly detected inside the ventilator; therefore, any resistive load (e.g., endotracheal tube or upper airways during noninvasive ventilation) reduces the responsiveness of the gas delivered by the ventilator in response to patient effort.22
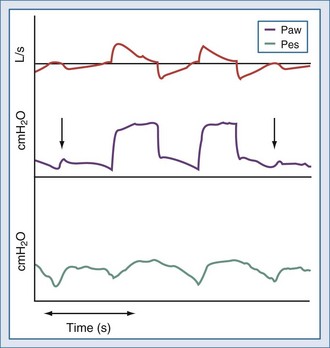
Ineffective triggering is due to the ventilator’s inability to detect the patient’s “request” for an assisted breath despite substantial inspiratory effort (Figure 49-3). This phenomenon usually occurs with high levels of ventilator assistance and short expiratory times. Mechanical characteristics that may induce ineffective triggering include low elastance, high resistance, and intrinsic PEEP; ineffective triggering is not correlated to an increase in the patient’s inspiratory effort.2 The application of external PEEP below the intrinsic PEEP level can reduce the inspiratory effort required to trigger the ventilator.39 Parthasarathy and coworkers demonstrated that prolonging mechanical inflation into neural expiration reduces the time available for unopposed exhalation, resulting in the need for a greater inspiratory effort to trigger the ventilator.40 Younes and colleagues found that ineffective triggering in ventilator-dependent patients exacerbates dynamic hyperinflation.41
New trigger algorithms aim at improving patient-ventilator interaction during sudden changes in flow or respiratory rate or in the presence of air leaks during NIV. This can be achieved with volume triggers, triggers linked to flow waveform algorithms, combining pressure and flow signals in the same trigger algorithm, or using both pressure and flow triggers. However, all inspiratory trigger drawbacks may be overcome by using a neural trigger obtained by means of a dedicated nasogastric tube with a multiple array of electrodes placed in the distal esophageal portion.8,42,43
 Ventilatory Requirement–Gas Delivery Asynchrony
Ventilatory Requirement–Gas Delivery Asynchrony
Gas delivery asynchrony occurs when ventilator-delivered flow, volume, and pressure are insufficient to meet the patient’s ventilatory demand. Ward and coworkers demonstrated that increasing the flow rate could be used as a means of reducing the patient’s respiratory drive and active respiratory muscle work,17 although doing so may exert an excitatory effect on respiratory rate and on the rate of rise of inspiratory muscle activity.3,20,21,44–50 Laghi and colleagues demonstrated that the imposed inspiratory time during mechanical ventilation determines respiratory frequency independent of inspiratory flow and tidal volume.20 Pressure-targeted breaths may better match the patient’s ventilatory requirements, because flow is the dependent variable during constant pressure delivery. In addition, rapid pressurization of the airways is coupled with high inspiratory flow only at the beginning of inspiration, thus reproducing the physiologic flow profile.51 However, during a pressure-targeted breath, the pressure-rise time setting—the time taken to reach the pressure set on the ventilator—may influence patient-ventilator interaction because its modification determines the dependent flow output.13,14
 Inspiratory Time–Ventilator Cycling Asynchrony
Inspiratory Time–Ventilator Cycling Asynchrony
A breath can be pressure-, time-, volume-, or flow-cycled.14 Ventilator-patient asynchrony occurs when the patient is trying to exhale, but the ventilator is still delivering gas.37,40,52 In patients ventilated with a time-cycled breath, expiratory phase asynchrony takes place when the patient’s neural inspiratory time is shorter or longer than the ventilator inflation time. For proper cycling off the ventilator and optimal patient-ventilator synchrony, the patient’s inspiratory flow and ratio of inspiratory time–to–total breath cycle duration must be tracked.
During flow-cycled breaths, inspiratory time is determined exclusively by the time taken for the exponentially declining flow to reach the flow threshold value (when cycling between inspiration and expiration occurs).34,53 As a consequence, a flow-cycled ventilation mode (e.g., pressure-support ventilation [PSV]) is cycled when inspiratory flow decay reaches a given threshold value. The inspiratory flow threshold value, also called expiratory trigger, thus controls the inspiration-to-expiration switch in these modalities.13,54 The aim is to detect the very end of patient inspiration through inspiratory flow measurement. The goal of these ventilatory modes is to optimize synchronization between spontaneous patient inspiratory time and ventilator inspiratory time. However, for proper cycling off and optimal patient-ventilator synchrony, the ventilator always has to track the patient’s inspiratory flow.40,55,56
 Patient-Ventilator Asynchrony During Pressure-Support Ventilation
Patient-Ventilator Asynchrony During Pressure-Support Ventilation
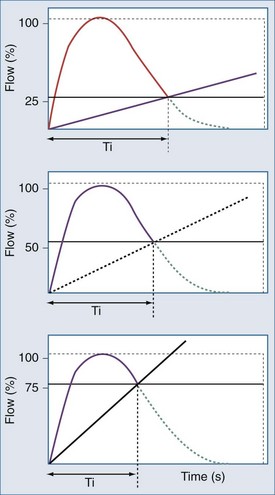
(1) The expiratory trigger sensitivity can be fixed (default at 25% of peak flow ) or can vary from 5% to 90% or from 5 to 25 L/min (Figure 49-4).57 It can also be linked to algorithms where there is a ranking logic of expiratory cycling criteria that links cycling to expiration. Setting the expiratory trigger at a higher percentage of peak inspiratory flow (i.e., 50% to 70% of decay of peak inspiratory flow) in patients with obstructive pulmonary disease improves patient-ventilator synchrony and reduces inspiratory muscle effort.58
In addition, the modification of cycling-off criteria may have a beneficial effect on reducing the dynamic hyperinflation and inspiratory effort in chronic obstructive pulmonary disease patients, especially at low levels of pressure support.59
The proper adjustment of expiratory trigger threshold may be also important in improving patient-ventilator synchrony and in decreasing the work of breathing during acute lung injury. Unlike in obstructive pulmonary disease, setting the lower threshold at 5% of the peak inspiratory flow might be the optimal value for patients with acute respiratory distress syndrome or acute lung injury.60
Chiumello et al. found in patients recovering from acute lung injury during PSV at 15 cm H2O, the lowest cycling-off criteria reduced the respiratory rate and increased the tidal volume without modifying the work of breathing.61
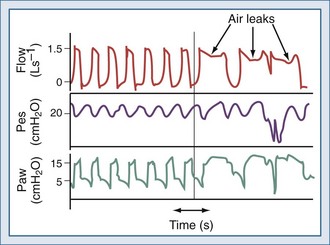
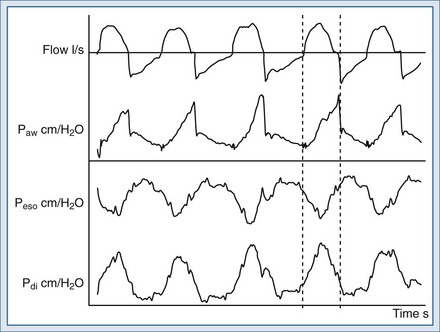
The expiratory sensitivity setting is crucial when ventilators are used to deliver NIV, because air leaks may cause an abnormal prolongation of the inspiratory time; during this time, the patient may make efforts to exhale against the machine or to inhale, without receiving any ventilatory support (inspiratory hang-up) (Figure 49-5).62–66 In addition, leak-compensating capabilities differ markedly between ventilators.56,67
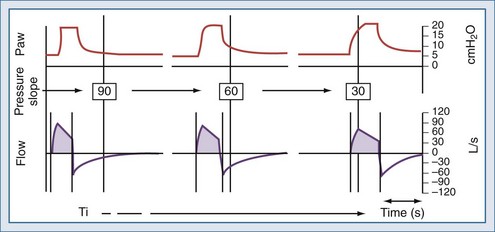
(2) The setting of the pressure-rise time (pressure slope) can also affect the expiratory threshold by modifying the dependent inspiratory flow.61,68–71 Although there is some evidence that rapid pressure-rise times might reduce a patient’s work of breathing,69 a fast pressure increase may lead to particularly high peak inspiratory flow, which may cause premature termination of inspiration when the fixed percentage criterion for expiratory cycling is reached (Figure 49-6).22,59,71
Prinianakis et al. assessed the effects of varying the rate of pressure change during noninvasive PSV on the breathing pattern of patients with COPD, as well as inspiratory effort, arterial blood gases, tolerance to ventilation, and amount of air leakage. No significant changes were observed in breathing pattern and arterial blood gases between the differing amounts of pressure change. The pressure-time product of the diaphragm, an estimate of its metabolic consumption, was significantly lower with all rates of pressure change than with spontaneous breathing, but it was significantly lower with the fastest rate. Interestingly, air leak—assessed by the ratio between expired and inspired tidal volumes—increased, and the patients’ tolerance of ventilation, measured using a standardized scale, was significantly poorer with the fastest rate of pressure change.72 In invasively ventilated patients recovering from acute lung injury, Chiumello et al. found that the shortest inspiratory rise time reduced the work of breathing.61
(3) The pressure-support level also determines the dependent flow output. During NIV, patient-ventilator asynchrony is a common occurrence, mainly owing to air leaks.9,63 Because air leaks may determine modification in flow output, reducing PSV level even by 1 or 2 cm H2O may reduce air leaks, thus improving patient-ventilator asynchrony.9
In conclusion, modifications of inspiratory rise time and cycling-off criteria must be carefully adjusted during PSV, as well as the level of PSV. Dyssynchrony at the termination of a PSV breath can be corrected by varying the cycling-off criteria (e.g., the expiratory trigger threshold) or modulating inspiratory flow (e.g., modifying the pressure slope or varying the set pressure level).9 Automated modes designed to achieve an optimal expiratory cycling during PSV may deserve further investigation.73–74
 Total Patient-Controlled Mechanical Support
Total Patient-Controlled Mechanical Support
Optimization of patient-ventilator interactions can be obtained only by continuous matching between the triggering, flow delivery, and cycling functions of the ventilator and the patient’s ventilatory drive, spontaneous inspiratory flow demand, and ratio of inspiratory time to total breath cycle. This implies continuous measurement of physiologic variables and continuous adaptation of the ventilator to the spontaneous variations in these variables. Future development in ventilator technology should be oriented toward systems with the capability to automatically interface between physiologic parameters and ventilator output. Such technology will be based on closed-loop algorithms able to achieve total patient-controlled mechanical support.4
 Proportional Assisted Ventilation (PAV), Proportional Pressure Support (PPS), and Proportional Assisted Ventilation Plus (PAV+)
Proportional Assisted Ventilation (PAV), Proportional Pressure Support (PPS), and Proportional Assisted Ventilation Plus (PAV+)
During proportional assisted ventilation (PAV) and proportional pressure support (PPS), the ventilator generates pressure in proportion to patient-generated flow and volume75,76; the ventilator amplifies patient effort without imposing any ventilatory or pressure targets. Ventilator-generated pressure rises as long as inspiratory muscle effort is produced by the patient. During PAV or PPS, the preset parameter is therefore not a pressure target. During these modes of mechanical support, the clinician adjusts the percentage of flow-assisted or volume-assisted ventilation after determining the patient’s resistance and elastance. In other words, the physician must determine how much to reduce the load imposed by the patient’s elastance77 and resistance.78,79
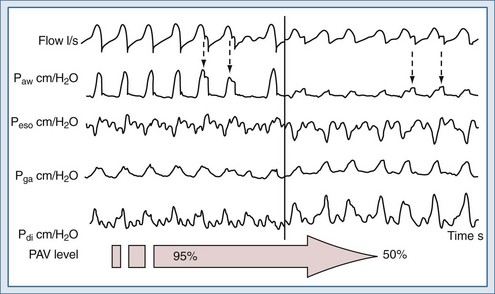
Despite the exciting potential of these techniques,79–82 applied either invasively or noninvasively,73–89 no large-scale studies have demonstrated an improvement in patient outcome compared with other modes of ventilation. PAV+ provides continuous measurement of the value of elastance and resistance of the patients according to the method described by Younes and coworkers.77–79,89,90 This option requires that the physician sets only a given percentage level of gain. During invasive ventilation, PAV+ seems to considerably reduce the incidence of patient-ventilator asynchronies compared to proportional ventilation with the manual adjustment of the percentage of flow-assisted or volume-assisted ventilation according to patient’s respiratory mechanics90 (Figure 49-7). As compared to PSV, PAV+ also was able to reduce the time for ventilatory settings and changes in sedative doses.91
 Neural-Adjusted Ventilatory Assistance
Neural-Adjusted Ventilatory Assistance
With neural-adjusted ventilatory assistance (NAVA), electrical activity of the diaphragm is measured by means of an electrode array inserted into a nasogastric tube and placed in the lower esophagus; this information is then used to control the ventilator to generate flow, volume, and pressure.8,42,43,92 Unlike with the proportional mode described earlier, estimates of respiratory mechanics are not needed; with NAVA, the patient’s respiratory center controls the assisted positive breaths in all phases of the ventilation cycle, from triggering to cycling off of inspiration. Any change in patient ventilatory output is matched breath by breath by the ventilator, even in the presence of variations in respiratory mechanics. A NAVA level equal to 1 corresponds to a support of 1 cm H2O for 1 µV of electrical diaphragm activity (EAdi). A representative tracing of NAVA is shown in Figure 49-8.
 Adaptive-Support Ventilation
Adaptive-Support Ventilation
Adaptive-support ventilation is an assist time–limited, pressure-targeted mode of ventilation (pressure-controlled ventilation) that relies on a negative closed-loop system of regulating ventilator settings in response to changes in both respiratory impedance (elastance and resistance) and the patient’s spontaneous efforts.93 The basic principle relies on the work of Otis and coworkers94 and Mead,6 demonstrating that for a given level of minute alveolar ventilation, there is a respiratory rate that is least costly in terms of respiratory work. With adaptive support ventilation, the operator enters the patient’s body weight and sets the desired percentage of minute ventilation. The expiratory time constant is determined by analysis of the expiratory flow-volume curve,95 adjusting inspiratory pressure, inspiratory-expiratory time ratio, and respiratory rate to obtain the prescribed minute ventilation. Adaptive support ventilation thus adjusts inspiratory pressure, inspiratory-expiratory time ratio, and mandatory respiratory rate to maintain the target minute ventilation and respiratory rate within a framework designed to avoid both rapid, shallow breathing and excessive inflation volumes. Spontaneous breathing triggers either a pressure-controlled or a spontaneous breath with inspiratory pressure support, the level of which is adjusted to meet the target respiratory rate–tidal volume combination.
Key Points
Appendini L, Purro A, Gudjonsdottir M, et al. Physiologic response of ventilator-dependent patients with chronic obstructive pulmonary disease to proportional assist ventilation and continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159(5):1510-1517.
Beck J, Sinderby C, Lindström L. Effects of lung volume on diaphragm EMG signal strength during voluntary contractions. J Appl Physiol. 1998;85(3):1123-1134.
Calderini E, Confalonieri M, Puccio PG, et al. Patient-ventilator asynchrony during noninvasive ventilation: the role of the expiratory trigger. Intensive Care Med. 1999;25(7):662-667.
Laghi F, Karamchandani K, Tobin MJ. Influence of ventilator settings in determining respiratory frequency during mechanical ventilation. Am J Respir Crit Care Med. 1999;160(5):1766-1770.
Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patients’ effort, and dyspnea. Am J Respir Crit Care Med. 1997;155(6):1940-1948.
Parthasarathy S, Jubran A, Tobin MJ. Cycling of inspiratory and expiratory muscle groups with the ventilator in airflow limitation. Am J Respir Crit Care Med. 1998;158(5):1471-1478.
Parthasarathy S, Tobin JM. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166(11):1423-1429.
Sinderby C, Navalesi P, Beck J, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5(12):1433-1436.
Tobert DG, Simon PM, Stroetz RW, Hubmayr RD. The determinants of respiratory rate during mechanical ventilation. Am J Respir Crit Care Med. 1997;155(2):485-492.
Younes M, Webster K, Kun J, et al. A method for measuring passive elastance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;164(1):50-60.
1 Jubran A, Tobin MJ. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med. 1997;155:906-915.
2 Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patients’ effort, and dyspnea. Am J Respir Crit Care Med. 1997;155:1940-1948.
3 Tobin MJ, Jubran A, Laghi L. Patient-ventilator interaction. Am J Respir Crit Care Med. 2001;163:1059-1063.
4 Ranieri VM. Optimization of patient-ventilator interactions: Closed-loop technology to turn the century. Intensive Care Med. 1997;23:936-939.
5 Cameron RD, Sassoon CSH. Patient-ventilator interactions. Clin Chest Med. 1996;17:423-438.
6 Mead J. Control of respiratory frequency. J Appl Physiol. 1960;15:325-336.
7 Mead J, Whittenberger JL. Physical properties of human lungs measured during spontaneous respiration. J Appl Physiol. 1953;5:770-796.
8 Beck J, Sinderby C, Lindström L. Effects of lung volume on diaphragm EMG signal strength during voluntary contractions. J Appl Physiol. 1998;85:1123-1134.
9 Vignaux L, Vargas F, Roeseler J, et al. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med. 2009;35:840-846.
10 Hubmayr RD, Abel MB, Rehder K. Physiologic approach to mechanical ventilation. Crit Care Med. 1990;18:103-111.
11 Iotti G, Braschi A, Locatelli A, Bellinzona G. Spontaneous respiration in artificial ventilation: Importance of valve resistance. Presse Med. 1985;14:165-168.
12 Whitelow WA, Derenne JP, Milic-Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol. 1975;23:181-199.
13 Kacmarek RM, Chipman D. Basic principles of ventilator machinery. In: Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. 2nd Edn. New York: McGraw-Hill; 2006:53-95.
14 Chatburn RL. Classification of mechanical ventilators. In: Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. 2nd Edn. New York: McGraw-Hill; 2006:37-52.
15 Ranieri VM, Mascia L, Petruzzelli V, et al. Inspiratory effort and measurement of dynamic intrinsic PEEP in COPD patients: Effects of ventilator triggering system. Intensive Care Med. 1995;21:896-903.
16 Alberti A, Gallo F, Fongaro A, et al. P01 is a useful parameter in setting the level of pressure support ventilation. Intensive Care Med. 1995;103:547-553.
17 Ward ME, Corbeil C, Gibbons W, et al. Optimization of respiratory muscle relaxation during mechanical ventilation. Anesthesiology. 1988;69:29-35.
18 Puddy A, Younes M. Effect of inspiratory flow rate on respiratory output in normal subjects. Am Rev Respir Dis. 1992;146:787-789.
19 Georgopulos G, Mitrousa I, Bshouty Z, et al. Effect of N-REM sleep on the response of respiratory output to varying inspiratory flow. Am J Respir Crit Care Med. 1996;153:1624-1630.
20 Laghi F, Karamchandani K, Tobin MJ. Influence of ventilator settings in determining respiratory frequency during mechanical ventilation. Am J Respir Crit Care Med. 1999;160:1766-1770.
21 Banner MJ, Paul B, Blanch PB, Gabrielli A. Tracheal pressure control provides automatic and variable inspiratory pressure assist to decrease the imposed resistive work of breathing. Crit Care Med. 2002;30:1106-1111.
22 MacIntyre NR. Improving patient/ventilator interactions. In: Vincent J-L, editor. Yearbook of Intensive Care and Emergency Medicine. Berlin: Springer-Verlag; 1999:235-243.
23 Sassoon CSH, Gruer SE. Characteristic of the ventilator pressure- and flow-trigger variables. Intensive Care Med. 1995;21:159-168.
24 Fabry B, Guttmann J, Eberhard L, et al. An analysis of desynchronization between the spontaneously breathing patients and ventilator during inspiratory pressure support. Chest. 1995;107:1387-1394.
25 Fabry B, Haberthur C, Zappe D, et al. Breathing pattern and additional work of breathing in spontaneously breathing patients with different ventilatory demand during inspiratory pressure support and automatic tube compensation. Intensive Care Med. 1997;23:545-552.
26 Fink JB. Device and equipment evaluations. Respir Care. 2004;49:1157-1164.
27 Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
28 Shanely RA, Zergeroglu MA, Lennon SL, et al. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med. 2002;166:1369-1374.
29 Imai Y, Parodo J, Kajikawa D, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104-2112.
30 Parthasarathy S, Tobin JM. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423-1429.
31 Marini JJ, Capps JS, Culver BH. The inspiratory work of breathing during assisted mechanical ventilation. Chest. 1985;87:612-618.
32 Evidence-based guidelines for weaning and discontinuing ventilatory support: A collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120:375-395S.
33 Younes M. Interactions between patients and ventilators. In: Roussos C, editor. Thorax. 2nd ed. New York: Marcel Dekker; 1995:2367-2420.
34 Ranieri VM, Giuliani R, Mascia L, et al. Patient-ventilator interaction during acute hypercapnia: Pressure support vs proportional assist ventilation. J Appl Physiol. 1996;81:426-437.
35 Nava S, Bruschi C, Fracchia C, et al. Patient-ventilator interaction and inspiratory effort during pressure support ventilation in patients with different pathologies. Eur Respir J. 1997;10:177-183.
36 Hill LL, Pearl RG. Flow triggering, pressure triggering, and autotriggering during mechanical ventilation. Crit Care Med. 2000;28:579-581.
37 Aslanian P, El Atrous S, Isabey D, et al. Effects of flow triggering on breathing effort during partial ventilatory support. Am J Respir Crit Care Med. 1998;157:135-143.
38 Giuliani R, Mascia L, Recchia F, et al. Patient-ventilator interaction during synchronized intermittent mandatory ventilation: Effects of flow triggering. Am J Respir Crit Care Med. 1995;151:159.
39 Gottfried SB. The role of PEEP or CPAP in the mechanically ventilated COPD patient. In: Roussos C, editor. Thorax. 2nd ed. New York: Marcel Dekker; 1995:2471-2500.
40 Parthasarathy S, Jubran A, Tobin MJ. Cycling of inspiratory and expiratory muscle groups with the ventilator in airflow limitation. Am J Respir Crit Care Med. 1998;158:1471-1478.
41 Younes M, Kun WebsterJK, Roberts D. Response of ventilator-dependent patients to delayed opening of exhalation valve. Am J Respir Crit Care Med. 2002;166:21-30.
42 Sinderby C, Navalesi P, Beck J, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5:1433-1436.
43 Navalesi P, Costa R. New modes of mechanical ventilation: Proportional assist ventilation, neurally adjusted ventilatory assist, and fractal ventilation. Curr Opin Crit Care. 2003;9:51-58.
44 Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol (Lond). 1972;222:267-295.
45 Younes M, Vaillancourt P, Milic-Emili J. Interaction between chemical factors and duration of apnea following lung inflation. J Appl Physiol. 1974;36:190-201.
46 Zuperku EJ, Hopp FA, Kampine JP. Central integration of pulmonary stretch receptor input in the control of expiration. J Appl Physiol. 1982;52:1296-1315.
47 Georgopoulos D, Mitrouska I, Bshouty Z, et al. Effects of breathing route, temperature and volume of inspired gas, and airway anesthesia on the response of respiratory output to varying inspiratory flow. Am J Respir Crit Care Med. 1996;153:168-175.
48 Corne S, Gillespie D, Roberts D, Younes M. Effect of inspiratory flow rate on respiratory rate in intubated ventilated patients. Am J Respir Crit Care Med. 1997;156:304-308.
49 Mitrouska I, Bshouty Z, Younes M, Georgopoulos D. Effects of pulmonary and intercostal denervation on the response of breathing frequency to varying inspiratory flow. Eur Respir J. 1998;11:895-900.
50 Tobert DG, Simon PM, Stroetz RW, Hubmayr RD. The determinants of respiratory rate during mechanical ventilation. Am J Respir Crit Care Med. 1997;155:485-492.
51 Boysen PG, McGough E. Pressure-control and pressure-support ventilation: Flow patterns, inspiratory time, and gas distribution. Respir Care. 1988;33:126-134.
52 Tobin MJ, Yang KL, Jubran A, Lodato RF. Interrelationship of breath components in neighboring breaths of normal eupneic subjects. Am J Respir Crit Care Med. 1995;152:1967-1976.
53 Brochard L. Pressure support ventilation. In: Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. New York: McGraw-Hill; 1994:239-257.
54 Hess DR. Ventilator waveforms and the physiology of pressure support ventilation. Respir Care. 2005;50:166-186.
55 Jubran A, Van de Graaff WB, Tobin MJ. Variability of patient-ventilator interaction with pressure-support ventilation in patients with COPD. Am J Respir Crit Care Med. 1995;152:129-136.
56 Vignaux L, Tassaux D, Jolliet P. Performance of noninvasive ventilation modes on ICU ventilators during pressure support: a bench model study. Intensive Care Med. 2007;33:1444-1451.
57 Tassaux D, Michotte JB, Gainnier M, et al. Expiratory trigger setting in pressure support ventilation: from mathematical model to bedside. Crit Care Med. 2004;9:1844-1850.
58 Tassaux D, Gainnier M, Battisti A, et al. Impact of expiratory trigger setting on delayed cycling and inspiratory muscle workload. Am J Respir Crit Care Med. 2005;172:1283-1289.
59 Chiumello D, Polli F, Tallarini F, et al. Effect of different cycling-off criteria and positive end-expiratory pressure during pressure support ventilation in patients with chronic obstructive pulmonary disease. Crit Care Med. 2007;35:2547-2552.
60 Tokioka H, Tanaka T, Ishizu T, et al. The effect of breath termination criterion on breathing patterns and the work of breathing during pressure support ventilation. Anesth Analg. 2001;92:161-165.
61 Chiumello D, Pelosi P, Taccone P, et al. Effect of different inspiratory rise time and cycling off criteria during pressure support ventilation in patients recovering from acute lung injury. Crit Care Med. 2003;31:2604-2610.
62 Stell IM, Paul G, Lee KC, et al. Noninvasive ventilator triggering in chronic obstructive pulmonary disease: A test lung comparison. Am J Respir Crit Care Med. 2001;164:2092-2097.
63 Calderini E, Confalonieri M, Puccio PG, et al. Patient-ventilator asynchrony during noninvasive ventilation: The role of the expiratory trigger. Intensive Care Med. 1999;25:662-667.
64 Hotchkiss JR, Adams AB, Dries DY, et al. Dynamic behavior during noninvasive ventilation chaotic support? Am J Respir Crit Care Med. 2001;163:374-378.
65 Nava S, Bruschi C, Rubini F, et al. Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med. 1995;21:871-879.
66 Yamada Y, Du HL. Analysis of the mechanisms of expiratory asynchrony in pressure support ventilation: A mathematical approach. J Appl Physiol. 2000;88:2143-2150.
67 Mehta S, McCool FD, Hill NS. Leak compensation in positive pressure ventilators: a lung model study. Eur Respir J. 2001;17:259-267.
68 Richard JC, Carlucci A, Breton L, et al. Bench testing of pressure support ventilation with three different generations of ventilators. Intensive Care Med. 2002;28:1049-1057.
69 Bonmarchand G, Chevron V, Menard JF, et al. Effects of pressure ramp slope values on the work of breathing during pressure support ventilation in restrictive patients. Crit Care Med. 1999;27:715-722.
70 Chatmongkolchart S, Williams P, Hess DR, Kacmarek RM. Evaluation of inspiratory rise time and inspiration termination criteria in new-generation mechanical ventilators: A lung model study. Respir Care. 2001;46:666-677.
71 Chiumello D, Pelosi P, Croci M, et al. The effects of pressurization rate on breathing pattern, work of breathing, gas exchange and patient comfort in pressure support ventilation. Eur Respir J. 2001;18:107-114.
72 Prinianakis G, Delmastro M, Carlucci A, et al. Effect of varying the pressurisation rate during noninvasive pressure support ventilation. Eur Respir J. 2004;23:314-320.
73 Dojat M, Harf A, Touchard D, et al. Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med. 2000;161:1161-1166.
74 Battisti A, Roeseler J, Tassaux D, et al. Automatic adjustment of pressure support by computer-driven knowledge-based system during noninvasive ventilation: a feasibility study. Intensive Care Med. 2006;32:1523-1528.
75 Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation: Results of an initial clinical trial. Am Rev Respir Dis. 1992;145:121-129.
76 Navalesi P, Hernandez P, Wongsa D, et al. Proportional assist ventilation in acute respiratory failure: Effects on breathing pattern and inspiratory effort. Am J Respir Crit Care Med. 1996;154:1330-1338.
77 Younes M, Webster K, Kun J, et al. A method for measuring passive elastance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;164:50-60.
78 Younes M, Kun J, Masiowski B, et al. A method for non-invasive determination of inspiratory resistance during proportional assist ventilation. Am J Respir Crit Care Med. 2001;163:829-839.
79 Farre R, Mancini M, Rotger M, et al. Oscillatory resistance measured during noninvasive proportional assist ventilation. Am J Respir Crit Care Med. 2001;164:790-794.
80 Ranieri VM, Grasso S, Mascia L, et al. Effects of proportional assist ventilation on inspiratory muscle effort in patients with chronic obstructive pulmonary disease and acute respiratory failure. Anesthesiology. 1997;86:79-91.
81 Bigatello LM, Nishimura M, Imanaka H, et al. Unloading the work of breathing by proportional assist ventilation in a lung model. Crit Care Med. 1997;25:267.
82 Appendini L, Purro A, Gudjonsdottir M, et al. Physiologic response of ventilator-dependent patients with chronic obstructive pulmonary disease to proportional assist ventilation and continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159:1510-1517.
83 Wrigge H, Golisch W, Zinserling J, et al. Proportional assist versus pressure support ventilation: Effects on breathing pattern and respiratory work of patients with chronic obstructive pulmonary disease. Intensive Care Med. 1999;25:790-798.
84 Giannouli E, Webster K, Roberts D, Younes M. Response of ventilator-dependent patients to different levels of pressure support and proportional assist. Am J Respir Crit Care Med. 1999;159:1716-1725.
85 Vitacca M, Clini E, Pagani M, et al. Physiologic effects of early administered mask proportional assist ventilation in patients with chronic obstructive pulmonary disease and acute respiratory failure. Crit Care Med. 2000;28:1791-1797.
86 Grasso S, Puntillo F, Mascia L, et al. Compensation for increase in respiratory workload during mechanical ventilation. Am J Respir Crit Care Med. 2000;161:819-826.
87 Gay PC, Hess DR, Hill NS. Non-invasive proportional assist ventilation for acute respiratory insufficiency. Am J Respir Crit Care Med. 2001;164:1606-1611.
88 Grasso S, Ranieri VM. Proportional assist ventilation. Respir Care Clin N Am. 2001;7:465-473.
89 Wysocki M, Richard JC, Meshaka P. Non-invasive proportional assist ventilation compared with non-invasive pressure support ventilation in hypercapnic acute respiratory failure. Crit Care Med. 2002;30:323-329.
90 Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med. 2008;34:2026-2034.
91 Xirouchaki N, Kondili E, Klimathianaki M, et al. Is proportional-assist ventilation with load-adjustable gain factors a user-friendly mode? Intensive Care Med. 2009;35:1599-1603.
92 Sinderby C, Beck J, Spahija J, et al. Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest. 2007;131:711-717.
93 Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient-ventilator interactions during partial ventilatory support: A preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med. 2002;30:801-807.
94 Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol. 1950;2:592-607.
95 Brunner JX, Laubscher TP, Banner MJ, et al. Simple method to measure total expiratory time constant based on the passive expiratory flow-volume curve. Crit Care Med. 1995;23:1117-1122.