CHAPTER 4 Pathophysiology of Telangiectasias
The term telangiectasia was first coined in 1807 by Von Graf to describe a superficial vessel of the skin visible to the human eye.1 These vessels measure 0.1 to 1 mm in diameter and represent an expanded venule, capillary or arteriole. Telangiectasias that originate from arterioles on the arterial side of a capillary loop tend to be small and bright red and do not protrude above the skin surface. Telangiectasias that originate from venules on the venous side of a capillary loop are blue, wider, and often protrude above the skin surface. Sometimes, telangiectasias, especially those arising at the capillary loop, are red at first but with time become blue, probably due to increasing hydrostatic pressure and backflow from deep veins.2,3
Classification
Redisch and Pelzer4 classified telangiectasias into four types based upon clinical appearance (Fig. 4.1):
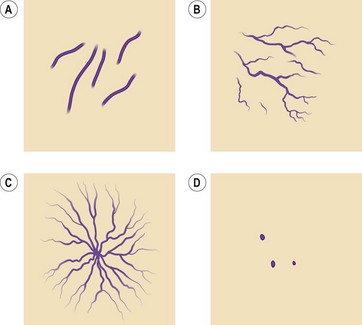
Figure 4.1 Four types of telangiectasias. A, Simple. B, Arborized. C, Spider. D, Papular.
(Adapted from Reddish W, Peltzer RH: Am Heart J 37:106, 1949.)
Raymond-Martimbeau and Dupuis3 have proposed another classification based on the relationship between telangiectasias and superficial as well as deep veins. Using duplex ultrasound, they evaluated 525 consecutive patients with 884 zones of telangiectasia without underlying saphenous or perforator vein incompetence. They found that 8.8% of the telangiectasias joined the deep venous system, 12.6% joined the superficial venous system, 71.2% were directly connected to reticular veins and 7.4% had no obvious connection. This is in contradistinction to the reports of very high incidence of arterial venous anastomoses for leg telangiectasias.5
The actual etiology of telangiectasias may be identical to that of varicose veins.6 However, the findings of Raymond-Martimbeau and Dupuis suggest that valvular damage occurs with subsequent venous hypertension that is transmitted to epidermal vessels, which then elongate and dilate. Our research has implicated a leukocyte–endothelial interaction that relates intercellular adhesion molecule-1 and monocytes to adherence and migration of cells.7 Valve and vein wall damage is produced by monocytes in the interstitial tissue.8 Thus, pharmacologic treatment of telangiectasias may be possible in the future.
Patterns
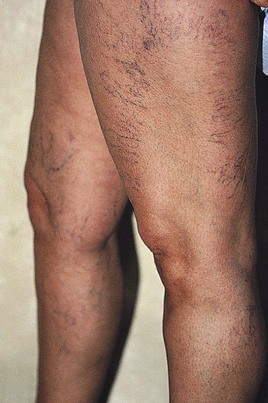
Two common patterns of telangiectasias on the legs of women, besides red or blue streaks, are the parallel linear pattern, usually found on the medial thigh (Fig. 4.2), and the arborizing or radiating cartwheel pattern, seen most often on the lateral thigh (Fig. 4.3).9 These two subsets of telangiectasias seem to run in families and may form anastomosing complexes as large as 15 cm in diameter. The arborizing type on the lateral thigh usually appears with ‘feeding’ reticular veins (see Fig. 1.11). These complexes have been termed venous stars, sunburst venous blemishes and spider leg veins by various authors.
Pathogenesis
The pathogenesis of each type of telangiectasia is somewhat different. Multiple factors may play a role in the development of new blood vessels or the dilation of existing blood vessels (see Chapters 2 and 8). Acquired telangiectasias probably result from the release or activation of vasoactive substances, such as hormones and other chemicals. Conditions associated with increased or activated vasoactive substances include anoxia, infection and certain physical factors that results in capillary or venular neogenesis.4,10,11 One common area for the development of telangiectasia is the medial thigh. This has been thought to be, in part, a result of pressure exerted by crossing the legs. A report on tissue atrophy in a woman with associated telangiectasia at the site of pressure where her legs crossed suggests that intermittent pressure results in subcutaneous tissue loss or atrophy.12 Unfortunately, to our knowledge, no formal studies on tissue pressure have been performed. Box 4.1, an extension of observations made by Shelley13 as well as Anderson and Smith,14 lists the major etiologies associated with telangiectasias arising on the lower extremities.
Incidence
The incidence of varicose and telangiectatic leg veins in the general population is presented in Chapter 2. The relationship between varicose veins and spider leg veins (telangiectasias) is profound. The anatomy and pathophysiology of telangiectasia is presented in Chapters 1 and 3. Telangiectasias increase in incidence with advancing age.15 Among neonates, the prevalence of telangiectasias is 3.8%, with 26% occurring on the legs.16
Two surveys have detailed the characteristics of patients seeking treatment of unwanted spider leg veins. Duffy,17 in a nonrandomized survey of his patients, reported a 90% family history of varicose or telangiectatic leg veins. Patients included three sets of identical twins with similar appearing leg telangiectasias. Sadick,18 in a nonrandomized survey of 100 patients seeking treatment, found a 43% family history of varicose or telangiectatic leg veins. Both surveys found that one third of the patients first noted the development of these veins during pregnancy. Among this subset of patients, veins became most severe after the third pregnancy.17 Between 20% and 30% of patients developed these veins before pregnancy, and 18% of women noted the onset of the veins while taking oral contraceptives. Both authors concluded that the development of leg telangiectasia is probably a partially sex-linked, autosomal dominant condition with incomplete penetrance and variable expressivity.
Pathophysiology
Genetic/congenital factors
Numerous genetic or congenital conditions (listed in Box 4.1) display cutaneous telangiectasia. The pathogenesis for the development of telangiectasia in these syndromes is unknown. Genetic syndromes associated with leg telangiectasias include nevus flammeus (alone or as a component of Klippel–Trénaunay syndrome (KTS)), nevus araneus, angioma serpiginosum, Bockenheimer’s syndrome (diffuse genuine phlebectasia), congenital neuroangiopathies (especially Maffucci’s syndrome), congenital poikiloderma, essential progressive or generalized telangiectasia, cutis marmorata telangiectatica congenita, and diffuse neonatal hemangiomatosis.
Nevus flammeus
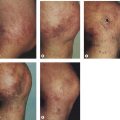
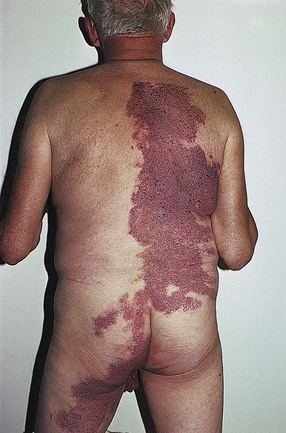
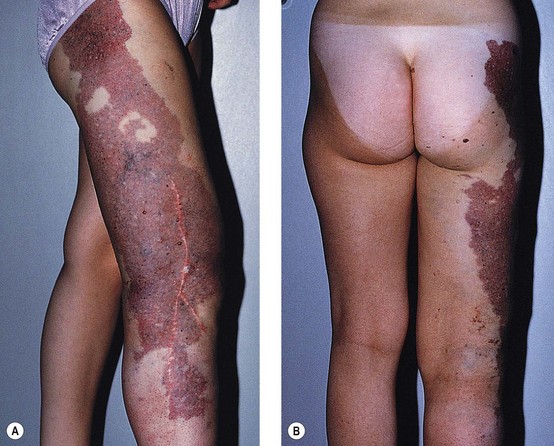
Nevi flammei (port-wine stains) affect 0.3% to 1% of the population,19,20 with women being twice as likely to be affected as men.21,22 Cases are usually sporadic, but a 10% familial incidence21 and an autosomal dominant inheritance have been described.23–26 Lesions occur in various shapes and sizes on any part of the body. They most commonly occur on the face but may cover large areas of the body, including an entire arm, leg, or trunk (Fig. 4.4). Lesions often overlay the distribution of peripheral nerves. Nevi flammei are usually macular and vary in color depending upon the extent and depth of vascular involvement. Lesions become progressively nodular and darker with time and may ulcerate and bleed from minor trauma.
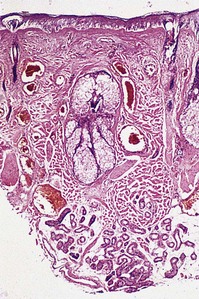
Histologic examination shows a collection of thin-walled capillary and cavernous vessels arranged loosely throughout the superficial and deep dermis (Fig. 4.5). These vessels represent dilations of postcapillary venules within the superficial dermis, with a mean depth of 0.46 mm.27 In infancy, histopathologic changes of cutaneous vasculature are minimal. With advancing age, however, these lesions usually undergo progressive ectasia and erythrocyte stasis.27 Although rare, cavernous hemangiomas arising from arteriovenous malformations may occur within the lesions.28 There may also be evidence of other vascular malformations or neovascularization.27 Further, some lesions on the legs may be associated with prominent telangiectasias and reticular varicose veins (Fig. 4.6).
Klippel–Trénaunay syndrome
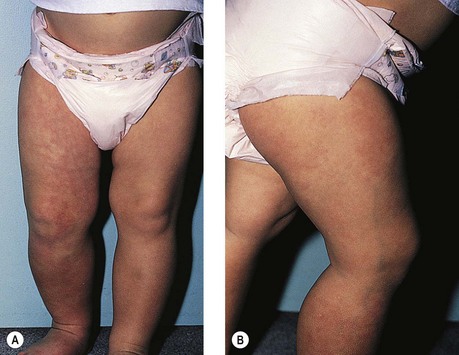
With KTS (Fig. 4.7), the cutaneous vascular abnormality is associated with underlying varicose and telangiectatic veins with or without significant abnormalities of the deep and superficial system or arteriovenous anastomoses. In addition, hypertrophy of soft tissue and bone may occur with overgrowth of the involved extremity (Figs 4.8 and 4.9).
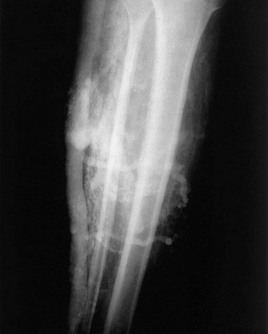
Figure 4.8 Venogram film of the right calf (anteroposterior projection) of the patient shown in Figure 4.7. There are multiple dilated collateral dermal venules and a grossly enlarged lateral accessory saphenous vein along the posterolateral aspect of the calf, which is avalvular. The deep venous system is absent.
(Courtesy Christopher Sebrechts MD)
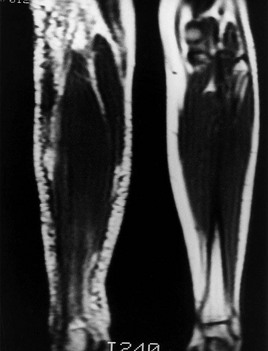
Figure 4.9 Magnetic resonance image, coronal T1-weighted, of the calves (TR 500, TE 20) of the patient in Figure 4.7. Multiple small collateral vessels in the subcutaneous fat of the right calf are shown as a spaghetti-like accumulation of dermal venulectases. Note the enlarged lateral accessory vein present along the posterolateral aspect of the right calf.
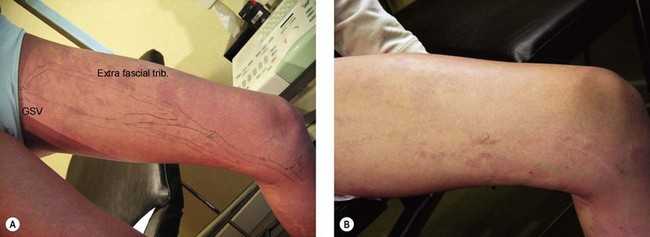
The cause of KTS is unknown. Its prevalence in newborns is estimated to be 1 : 25,000.29 There is no uniformly apparent hereditary factor.30 One study of 14 affected patients suggests an autosomal dominant inheritance,31 but some authors speculate that an atresia, agenesis or compression of the deep venous system by fibrotic tissue is the cause.32 Other authors suggest that a congenital weakness of the venous wall, in combination with vascular hypertension from an abnormal venous system, leads to the development of KTS.33 Baskerville et al34 studied 49 patients with KTS and found that 68% had a superficial, embryologic venous channel on the lateral aspect of the thigh (Fig. 4.10). Histologic and venous flow studies suggest that, because these veins are usually present at birth and avalvular, KTS is caused by a mesodermal abnormality during fetal development that leads to persistence of arteriovenous communications, causing the triad of a nevus flammeus, soft tissue hypertrophy and varicosities.35 This entire patient; population had clinical varicose veins, and 88% had pain and limb swelling. Twenty-two percent had severe hemorrhage from varicose vein rupture and 6% had a history of superficial thrombophlebitis. Baskerville et al34 recommended surgical excision-avulsion of symptomatic superficial varices, with Villavicencio,36 building upon his experience with 14 patients; also recommending surgical excision followed by sclerotherapy to treat patients with intractable symptoms.
Servelle37 presented his findings on 786 patients with KTS. He found venographic evidence for obstruction in most patients and postulated that changes seen in KTS are manifestations of this obstruction. In addition to cutaneous and soft tissue findings, Servelle noted a 36% incidence of varicose veins in his patient population. Concomitant malformations of the deep venous system (avalvulia, aneurysmata, aplasia, lateral marginal veins) have been found in up to 94% of patients.38 Servelle recommended surgical intervention to the deep venous system and cautioned against treating superficial varicosities, owing to the potential for increased outflow obstruction.
When associated with KTS, cutaneous telangiectasias, venulectasias and varicose veins occur in the distribution of the underlying vascular malformation of soft tissue and bone. Some patients have a persistent embryologic lateral limb bud vein. The large drainage capacity of this vein may limit venous hypertension. In this instance, microcirculatory change, rather than large vessel change, may account for limb hypertrophy.39 Thus, because KTS can be composed of a variable venous system, sclerotherapy treatment must be performed only after a thorough vascular evaluation.40 Extensive pure venous malformations without other sequelae have also been reported and are distinct from KTS.41 These lesions represent dilated venous tumors involving both skin and muscle. Coagulation studies are abnormal in 88% of patients.
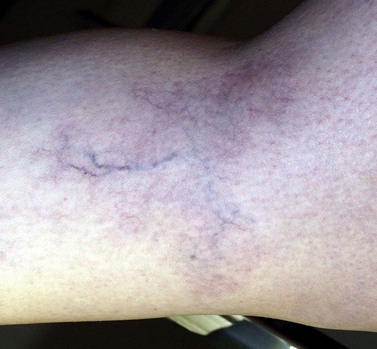
Most venous angiodysplasias demonstrate associated telangiectasias (Figs 4.11 and 4.12).
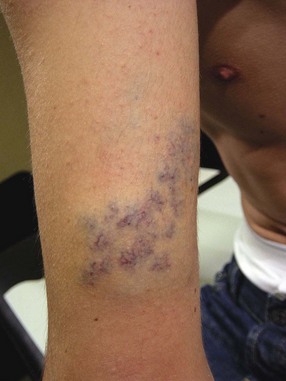
Figure 4.12 Telangiectasias associated with venous dysplasia of the upper limb in an 18-year-old male.
Proteus syndrome is a congenital hamartomatous condition that may have overlapping features with KTS.42 In 1983, Wiedemann et al43 described Proteus syndrome as consisting of partial gigantism of the hands or feet, hemihypertrophy, pigmented nevi, soft tissue tumors, macrocephaly and other hamartomatous changes. Patients with Proteus syndrome can also demonstrate prominent capillary hemangiomas, telangiectasia, and varicosities.44–47 Clinical findings are usually evident at or shortly after birth. This condition may represent a somatic mutation that influences the local regulation or production of tissue growth factors.48
Sclerotherapy to nontruncal varicose and telangiectatic veins can restore some degree of venous competency and relieve symptoms. In addition to experiencing a heavy, tired feeling of the affected limb, patients may have recurrent bleeding from cutaneous vascular blebs. These vessels are easily traumatized, with trauma occasionally leading to cutaneous and soft tissue infections. Sclerosing these vessels is helpful and has been practiced for more than 60 years.49
A complete discussion of the surgical management of KTS or the vascular component of Proteus syndrome is beyond the scope of this text. But, in short, if an incompetent feeding varicose vein is found alone with an intact deep venous system, the former can be avulsed safely. This procedure is often combined with sclerotherapy to distal varices. Foam sclerotherapy can be an important treatment modality, although dozens of treatment sessions may be required to minimize the volume of injected foam. Care must be taken to ensure adequate venous return from the remaining vessels. Treating perforating veins is usually quite difficult because there may be hundreds of connections between the superficial and deep venous systems.40
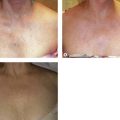
Laser coagulation or photocoagulation is reserved for cutaneous ectasia, which usually occurs within the nevus flammeus. These manifestations are not treated for cosmetic reasons but to prevent bleeding and infection (Fig. 4.13) (see Chapter 13).
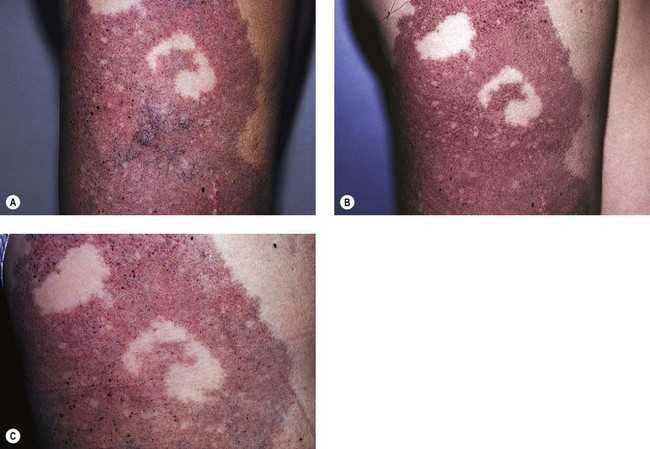
Figure 4.13 A, Before, and B after, treatment of a section of the nevus flammeus and associated superficial varicosity of the patient shown in Figure 4.6. The reticular veins were treated with polidocanol 0.75% (6 ml total) followed by multiple impacts with the Candela SPTL-I pulsed-dye laser at 8 J/cm2. C, Clinical appearance 5 years after last sclerotherapy/laser treatment. Note the recurrence of venules and vascular ectasia.
Nevus araneus
Nevi aranei (spider telangiectasias) may occur as a component of a number of congenital and acquired diseases. They are found in up to 15% of the normal population and increase in number during pregnancy, liver disease and multiple other conditions.50 Ninety-nine percent of nevi aranei occur superior to the umbilicus.50,51
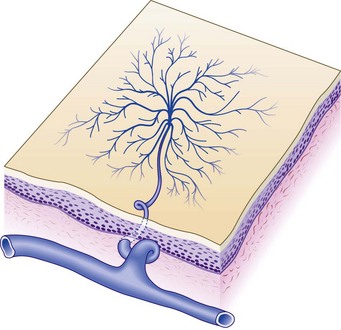
Lesions appear as bright red macules composed of a central red dot, with fine blood vessels radiating from the center (Fig. 4.14). The central vessel may pulsate, indicating its arteriolar origin. Point compression of the central dot blanches the radiating vessels. One genetic disease with this form of telangiectasia is ataxia telangiectasia, which may have lesions distributed within the popliteal fossae.52,53
Vascular spiders arise from the terminal arteriole (see Fig. 4.14).4 Within the spider telangiectasia, blood pressure is lower than systolic pressure but higher than venous pressure. One measurement demonstrated its pressure to be 85 mmHg when systolic pressure was 120 mmHg.54 The vessels arise within the deep dermis and push their way up into the superficial dermis as a space-occupying lesion. The central arteriole connects to dilated venous saccules with radiating venous legs in the papillary dermis.50 Detailed histologic studies have provided little understanding of the factors responsible for the initial growth of nevi aranei.55
Because spider telangiectasias are composed of a central arteriole, sclerotherapy as treatment usually produces ulcerations (see Chapter 8). Therefore, recommended treatment involves fibrosing the central feeding arteriole via the pulsed-dye laser (PDL) at 585 nm or 595 nm, continuous wave lasers at 511 nm to 577 nm, intense pulsed light (IPL), or electrodesiccation (see Chapter 12).
Angioma serpiginosum
Angioma serpiginosum is a rare nevoid disorder of the upper dermal vasculature. The disease usually occurs on the lower extremities in women and has its onset in childhood. Although most cases are sporadic, one family study suggests an autosomal dominant inheritance.56 Lesions appear as small erythematous puncta, which occur in groups. The lesions enlarge as new puncta form at the periphery, while those in the center fade. This results in a reticular or serpiginous pattern. A dilation of the subpapillary venous plexus may lead to telangiectasias. Histologic examination shows a number of ectatic capillaries in the superficial dermis. Endothelial cells are vacuolate, appear hyperplastic and have an increased number of interendothelial junctions. Capillary walls are thickened with prominant basal laminae and a ‘heavy’ precipitation of fine fibrillar material. The deeper dermis is unremarkable.57 Therefore, angioma serpiginosum may represent a type of capillary nevus.
Bockenheimer’s syndrome (diffuse genuine phlebectasia)
Diffuse genuine phlebectasia was first described by Bockenheimer58 in 1907. One review has documented 40 cases in the literature.59 This rare syndrome represents a deep venous malformation that is rarely present at birth, usually first manifesting in childhood. Multiple large venous sinusoids or cavernous hemangiomas develop, usually on an extremity. These frequently thrombose, hemorrhage, ulcerate and may ultimately progress to a gangrenous infection. Unilateral localization is common. Secondary cutaneous telangiectasia develops in response to venous hypertension. Late manifestations are soft tissue and/or bone hypotrophy or hypertrophy.
Compression therapy is generally beneficial to prevent manifestations of both venous hypertension as well as thromboses. Surgical excision and phlebectomy have produced varying results, generally with recurrence.60,61 Sclerotherapy has been successful in one of two cases.62,63
Maffucci’s syndrome
The syndrome affects all races, affects men and women equally, and has no evidence of a familial tendency.64 Twenty-five percent of patients have symptoms manifest within the first year of life; 78% manifest symptoms before puberty.64 From 25% to 30% of patients develop malignancies, including chondrosarcoma, angiosarcoma, lymphangiosarcoma, glioma, fibrosarcoma, pancreatic carcinoma and ovarian teratomas.64–67 Surgical excision or sclerotherapy treatment to symptomatic vascular lesions is helpful.36
Congenital poikiloderma
Congenital poikiloderma (Rothmund–Thomson syndrome) is a rare neurocutaneous syndrome that has its onset in the first year of life. There appears to be a female predominance. Although an autosomal dominant inheritance pattern has been demonstrated, 70% of cases show a familial recessive inheritance.68 A fine telangiectatic network first appears on the cheeks and progresses within a year to involve the head, arms, buttocks and legs. There may be associated scaling of the skin and lichenoid papules. Affected patients often have sparse hair, as well as soft and translucent skin. Dwarfism, cataracts, dental abnormalities, mental retardation, hypogenitalism and diabetes mellitus may also occur.68
Essential progressive telangiectasia
Essential progressive telangiectasia (EPT) is a rare entity that has been reported in association with bronchogenic carcinoma,69 angiokeratomas,70 chronic sinusitis71 and autoimmune disorders.72 However, EPT is most commonly an isolated entity. EPT has been reported to be associated with small varicose veins that occur many years after disease onset.73
Histochemical examination establishes the vessels in EPT to be venular in origin.74 Lesions appear as blue to bright red telangiectasias 0.1 to 0.4 mm in diameter. There may be associated peritelangiectatic atrophy of the subcutaneous tissue. Lesions most commonly appear on the feet and distal leg but rarely involve the entire leg.
The treatment of these lesions is often fraught with complications because many of the telangiectasias are intimately associated with arterioles. This leads to frequent recurrence of lesions after treatment. However, cautious treatment with sclerotherapy, PDL, or, best, IPL is usually successful (see Chapter 13).73
Cutis marmorata telangiectatica congenita
Cutis marmorata telangiectatica congenita is a rare congenital cutaneous vascular anomaly consisting of a sharply demarcated, reticulated vascular network of blue–violet venules associated with telangiectasias, with or without varicose veins. There have been about 300 cases reported to date in the world literature. The typical clinical findings consist of persistent cutis marmorata, telangiectasia, phlebectasia, occasional ulceration and atrophy; an associated nevus flammeus may be present. The cutis marmorata component is neither transient nor related to temperature. Telangiectasias may not appear in the first 2 years.75–77 Lesions are most prominent on the lower extremities but may involve any cutaneous surface. Involvement may be unilateral or bilateral. Atrophy and ulcerations of the overlying skin may occur over time.78 Lesions have been reported to improve spontaneously in up to three-quarters of patients within the first 2 years.77
Most cases of cutis marmorata telangiectatica congenita occur sporadically, and two-thirds of the approximately 300 reported cases occurred in women.75–7779 The pathogenesis is most likely multifactorial; genetic (autosomal dominant inheritance)80,81 and teratogenic factors are most commonly cited.
Histologic examination demonstrates an abnormal dilation of capillaries and veins.82,83 Associated congenital abnormalities have been reported in up to 50% of patients; these include structural defects of the musculoskeletal system, ocular and dental malformations and also arteriovenous malformations.84 Nevus telangiectaticus and hypertrophy or atrophy of the affected limbs are also reported.85
Diffuse neonatal hemangiomatosis
Diffuse neonatal hemangiomatosis is a rare congenital vascular disorder, presenting in infancy with multiple visceral hemangiomas. The combination of hepatic and cutaneous hemangiomas occurs twice as often in girls as in boys.86 Hemangiomas are usually present on the skin and may be associated with large areas of telangiectasias and venulectasias (Fig. 4.15).
Infants with diffuse neonatal hemangiomatosis often die of high-output cardiac failure (HOCM), resulting from arteriovenous shunting of blood flow through the hemangiomas. Recognition of the cutaneous component, which may be minimal in some infants, will help to prevent confusion of HOCM with a congenital heart disease.87 Therapy consists of systemic steroids, selective embolization, or surgical excision.64 Selective sclerotherapy of the telangiectasias may be performed for cosmesis.
Acquired disease with a secondary cutaneous component
In some conditions (e.g. mastocytosis), a component of the disease itself, such as the release of mast cell vasoactive factors including histamine or heparin, may lead to the development of telangiectasias.88,89 Histologic studies in a patient with unilateral facial telangiectasia macularis eruptiva perstans demonstrated an accumulation of mast cells.90
Component of a primary cutaneous disease
Varicose veins
Varicose veins lead to the development of telangiectasias, most likely through associated venous hypertension with resulting angiogenesis, vascular dilation50 or an increased distensibility of the telangiectatic vein wall (see Chapter 3). Although telangiectasias associated with varicose veins may first appear as erythematous streaks, they turn blue with time. Blue telangiectasias have an average oxygen concentration of 68.7%, versus 75.86% for red telangiectasias.91 Thus, the decrease in oxygen content of blue telangiectasias probably represents their association with the venous portion of the capillary loop. The relatively low oxygen concentration of red telangiectasia is possibly related to the backflow of venous blood into the capillary loop. Often telangiectasias are directly associated with underlying varicose veins, so the distinction between telangiectasias and varicose veins becomes blurred.50
Bihari et al92 have demonstrated that some leg telangiectasias have a high flow consistent with arteriovenous anastomoses (AVAs). They found a high flow in 5 of 22 limbs studied with Doppler flow analysis. They speculate that telangiectasias associated with AVAs account for the fast refilling of the former, as well as for their bright red color, the arterial pulsation seen in some, and the occurrence of punctate ulceration with injection of dilute solutions (see Chapter 9).
Corona phlebectatica (Fig. 4.16) describes the appearance of telangiectasia on the medial malleolar area. An evaluation of 411 limbs with great saphenous vein incompetence demonstrated corona phlebectasia in 204 (49.6%) patients.93 The frequency of corona phlebectasia was significantly greater in limbs with skin changes such as pigmentation and dermatitis than in limbs with normal skin (77.2% versus 40.6%). Blue telangiectasias were also more frequently observed in limbs with skin changes. Therefore the degree of venous hypertension is directly related to the development of both the telangiectasias comprising the corona phlebectasia as well as their blue appearance. One epidemiologic study evaluated 3072 members of a general population to determine whether corona phlebectatica could be used as a marker to predict more severe stages of chronic venous disease (CVD).94 Those with corona phlebectatica were classified as having either ‘mild’ involvement (up to one half of the foot edge involved) or ‘severe’ (entire foot edge involved). Approximately 50% of the population with C1–C6 stages of disease (CEAP classification, see Chapter 2) demonstrated mild corona. However, severe corona was associated with more advanced stages of CVD (C4 and C5). Thus, the authors conclude that while mild corona phlebectatica is not a good marker to differentiate between different stages of CVD, severe corona does frequently signify more advanced stages of CVD.
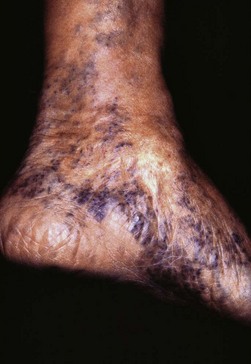
Figure 4.16 Severe corona phlebectatica around the scar of a healed ulcer. Medial malleolus of a 70-year-old female with chronic venous insufficiency and postthrombotic syndrome (C5s Es Asdp Pr; See Table 5.1 for further information on the CEAP classification for chronic venous disease).
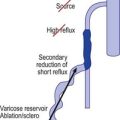
Telangiectasias have been shown by multiple investigators with various techniques to be associated with underlying reticular veins. Tretbar95 studied 100 patients with telangiectasias (0.1 mm diameter) with venous Doppler and found that the telangiectasias were connected with associated ‘feeding’ reticular veins. These veins were separate from any truncal varicosities that may have been present. All blue reticular veins demonstrated reflux that did not appear to originate from the great or small saphenous veins. In patients with blue reticular veins, 50% demonstrated reflux from incompetent calf perforating veins. Weiss and Weiss96 confirmed these findings with Doppler examination of 700 patients. They noted audible reflux in 88% of patients whose telangiectasias were associated with a ‘feeding’ reticular vein. In addition, they confirmed that enhanced therapeutic efficacy with decreased postsclerotherapy hyperpigmentation occurred when the reticular veins were treated before distal telangiectasias were treated.97 Duplex scanning has also demonstrated telangiectasias to be associated with ‘feeding’ reticular veins.98,99 Somjen et al99 found that 89% of telangiectasias had closely situated incompetent reticular veins. Furthermore, Raymond-Martimbeau and Dupuis3 found that 71.2% of telangiectasias had direct connections to reticular veins. Finally, direct radiographic imaging and duplex examination have shown connections between telangiectasia and both the deep as well as superficial venous systems.3,100 Importantly, 2 of 15 telangiectasias examined were found to connect directly to the deep venous system without any obvious cutaneous sign. Mariani et al101 evaluated 200 telangiectatic areas with a 10-MHz probe duplex ultrasound and transillumination. They found that 100% of telangiectasias in 106 female patients were connected to reticular veins (1–3 mm in diameter). In 73.5% of the telangiectatic areas, one or more incompetent perforator veins were present.
Only one author has reported a lack of importance of reticular veins with telangiectasias.102 In this singular ‘treatise’, successful eradication of telangiectasias was not dependent upon simultaneous eradication of reticular veins. However, the author failed to recognize that placing a Doppler probe over a vessel such as a reticular vein will extinguish the sound of reflux. Even ‘light’ pressure on a Doppler probe can generate hundreds of pounds per square inch and accounts for the fact that even the largest axial veins can be closed with light finger pressure.
The relationship between telangiectasias and reticular veins emphasizes the importance of postsclerotherapy compression when treating telangiectasias (see Chapters 6, 8, and 12). It is clear that treatment of the feeding varicosity results in treatment of the distal telangiectasia (see Chapters 9 and 12). Perhaps a more appropriate term instead of ‘feeding veins’ would be ‘back pressure recipient veins’. When no apparent connection exists between a telangiectasia and deep collecting or reticular vessels, the telangiectasia may arise from a terminal arteriole or arteriovenous anastomosis.103
Telangiectasias may be associated with underlying venous disease even when there are no other clinical abnormalities. Thibault et al104 evaluated 83 patients with spider leg veins using duplex and Doppler examinations. They found that 23% of these patients without clinically apparent varicose veins had incompetence of the superficial venous system. In addition, 1.2% had incompetence of a perforator vein. Nineteen patients, each with one clinically abnormal leg and one clinically normal leg, were also evaluated. Interestingly, 37% of clinically normal legs demonstrated incompetence of the superficial system. The abnormal legs in this group had a 74% incidence of incompetence of the superficial system, and 21% had saphenofemoral incompetence. This study demonstrates the need for both a clinical and noninvasive diagnostic workup in patients who have spider veins and reinforces the view that spider veins arise from underlying varicose veins through venous hypertension.
Engelhorn et al105 similarly advocated ultrasound mapping of the great saphenous vein (GSV) in women with telangiectasias, even in asymptomatic patients. This study evaluated 269 limbs of women with telangiectasias (CEAP class C1); exclusion criteria included the presence of concomitant varicosities, overlying skin abnormalities including ulcerations, and limb edema. Venous reflux was present in 46% of the 269 extremities, with 44% of patients having reflux of the GSV. Seven percent of the extremities had reflux of the small saphenous vein (SSV), 5% had reflux of both the GSV and SSV, and only 2% had reflux isolated solely to the SSV. Seventy-eight percent of these telangiectatic limbs were symptomatic. Interestingly, reflux prevalence was similar in symptomatic (47%) and asymptomatic (44%) extremities. Since treatment of telangiectasias with sclerotherapy is rarely effective if one fails to also detect and treat any underlying incompetent superficial veins, the authors advocate pretreatment saphenous vein ultrasound mapping, even in asymptomatic patients.
A proposed mechanism for the development of leg telangiectasia associated with underlying venous disease is that pre-existing vascular anastomotic channels open in response to venous stasis.106 Venous stasis with resulting venous hypertension leads to a reversal of flow from venules back to capillaries. The resulting capillary hypertension leads to opening and dilation of normally closed vessels. Consequently, relative anoxia, as a result of reversed venous flow, leads to angiogenesis. Merlen103 stated that capillaries and venules have an enhanced neogenic potential and a remarkable tendency toward neogenesis in a hypoxic atmosphere.
In addition, Braverman107 distinguished between the arterial and venous sides of the microcirculation based on the ultrastructural characteristics of the vascular basement membrane, which appears homogeneous in arterial vessels and multilaminated in venous vessels. With this finding, he demonstrated changes from arterial to venous vessels in various cutaneous disorders, such as psoriasis, within 48 to 72 hours. It is postulated that this change occurs as a result of changes in circulation pressure. Thus, the development of venulectasias and telangiectasias in venous hypertension may represent the conversion of pre-existing capillaries into venules.
Keratosis lichenoides chronica
Prominent telangiectasias on the feet and legs have been described in patients with keratosis lichenoides chronica,108 a condition not usually associated with telangiectasias.109 Severe pruritus was also present in these patients, and, although the authors ascribed the development of the telangiectasias and lichenoid papules to the same process, it appears to be equally likely that both physical manifestations could be caused by chronic rubbing and scratching. Thus it is difficult to separate the development of telangiectasias into primary versus secondary processes in this disease.
Other acquired primary cutaneous diseases
Progressive pigmented purpuric dermatitis (Schamberg’s disease), pigmented purpuric lichenoid dermatitis (Gougerot–Blum capillaritis), lichen aureus, and purpura annularis telangiectodes (Majocchi’s disease) share the common pathogenic denominator of dilatation of the superficial papillary dermal capillaries with occasional endothelial proliferation. These conditions are manifested by punctate purpuric lesions and telangiectasias, predominately on the lower legs. Most patients do not have manifestations of venous hypertension. Capillary microscopy in 12 patients with pigmented purpura disclosed ectatic dilated venules in the subpapillary plexus.110 Iwatsuki et al111 found fibrinoid degeneration and occlusive damage with swollen endothelia in three of eight patients with this condition. On direct immunofluorescence examination, each of the eight patients had evidence of C3, C1q, and fibrin within papillary vessels. These findings suggest an immunologic etiology.
Treatment of telangiectasias caused by an acquired or primary cutaneous disease is usually best accomplished by treatment of the acquired disease itself. The telangiectatic component of these lesions is usually asymptomatic and requires no treatment except for cosmesis. In that case, electrodesiccation or the tunable continuous dye laser, PDL, or IPL may be the treatment of choice (see Chapter 13).
Hormonal factors
Pregnancy and estrogen therapy
The hormonal influence on the development of telangiectasias is well known but a survey of 61 phlebologists from the International Union of Phlebology demonstrated many different opinions as to the role of hormones and telangiectasia.112 Seventy-two percent of responders thought that hormones have a causal or worsening effect on telangiectasia and 38% thought that hormones may have an effect on sclerotherapy treatment – but only 25% recommended that patients stop hormone therapy before treatment.
Pregnancy is perhaps the most common physiologic condition that leads to the development of telangiectasias. Corbett54 first suggested this in 1914. Bean50 has estimated that almost 70% of women develop telangiectasias during pregnancy, the majority of which disappear between 3 and 6 weeks postpartum (Fig. 4.17). Pregnant women often develop telangiectasias in the legs within a few weeks of conception, even before the uterus has enlarged to compress venous return to the pelvis.113,114 Also, pregnant women and those taking birth control pills have been shown to have an increase in the distensibility of vein walls.115 This increase in distensibility has also been noted to fluctuate with the normal menstrual cycle.116 It was found that leg volume was greatest just before ovulation and during menses. The increased distensibility did not fully correlate with one specific hormone but was related to both estrogen and progesterone levels or ratios.
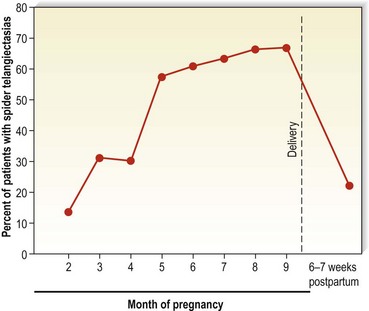
Figure 4.17 Incidence of spider telangiectasias in white pregnant women as a function of the duration of pregnancy.
(From Bean WB: Vascular spiders and related lesions of the skin, Springfield, Ill, 1958, Charles C Thomas.)
Hormonal stimulation may lead to the development of telangiectasias, independent of the effect on venous distensibility. Paradoxically, estrogen has been found to be beneficial in controlling symptoms of venous distensibility during pregnancy (see Chapter 3).117 Thus, some hormonal influence is involved in the development of varicose veins and their associated telangiectasias in women (see Chapter 2), but the exact hormonal mechanism for telangiectasia development is unknown.
Davis and Duffy118 reported an apparent association of estrogen excess states with the development of telangiectatic matting after sclerotherapy of spider leg veins. In their selected patient population of 160, 29% of women who developed ‘new’ telangiectasias were taking systemic estrogens or became pregnant, compared with 19% of patients on hormones who did not develop telangiectatic matting. One additional patient noted the disappearance of both spider veins and telangiectatic matting after taking the antiestrogen tamoxifen citrate (Nolvadex). Biopsies of these patients were not performed.
Estrogen acts by entering its target cell and associating with an extranuclear receptor protein. This complex then enters the nucleus and modulates ribonucleic acid (RNA) synthesis.119 Because endothelial cells have been found to possess estrogen receptors, they may be potential target cells.120 Sadick and Neidt121 assayed 20 patients with leg telangiectasias for estrogen receptors. Interestingly, they failed to find any evidence of such receptors in their patient population. Sadick and Urmacher122 also assayed patients with telangiectatic matting for estrogen receptors and again found no evidence of their presence. They postulated that estrogen receptors are not present in increased numbers in leg telangiectasias. The measurement technique was not sensitive enough to ascertain their presence, or that estrogen acts on endothelium through an indirect pathway. This pathway may be a stimulation of angiogenic factors (see Chapter 8) or an increase in vascular distensibility.
The hormonal influence on telangiectasia neogenesis has been noted in 17 of 46 reported cases of unilateral nevoid telangiectasia syndrome.123,124 Another study noted this in 10 of 15 reported cases. In these female patients, the telangiectasias did not occur until triggered by pregnancy or puberty and correlated with high serum estrogen levels. Of the remaining five cases, four were associated with alcoholic cirrhosis and one was congenital. There was no association with the development of varicose veins. Progesterone receptors have also been reported in two patients with unilateral nevoid telangiectasia in one of the patients’ receptors developed following metastases from a carcinoid tumor of the stomach.125,126
Malignancy
Although exceptionally rare, cutaneous patches of telangiectasias can represent metastases of an underlying malignancy.127–129 When this phenomenon does occur, the most common associated malignancy is breast cancer. However, there have also been at least two reported cases of cutaneous metastases from prostate carcinoma presenting as telangiectatic patches.130,131 Reddy et al130 reported a man who presented with an asymptomatic nontender, nonblanching telangiectatic patch on his chest. The patch had been enlarging since its onset 3 months previously. A biopsy from the patch showed ectatic papillary dermal blood vessels within which were aggregates of PSA-positive adenocarcinoma cells. Similar histologic findings were seen in lesional tissue from the other reported case of telangiectatic cutaneous metastases of prostate cancer.131 Interestingly, the former patient had been diagnosed with prostate cancer 12 years prior to the appearance of the telangiectatic patch; he subsequently died from metastatic prostate cancer 6 months after presenting with the telangiectasias. The authors speculate that the telangiectatic cutaneous metastases resulted from hematogenous dissemination of the neoplastic cells; the intravascular PSA-positive tumor cells found within the lesional skin biopsy support this theory. Regardless of the mechanism, cutaneous metastases of an underlying systemic malignancy usually herald advanced disease and a grave prognosis.
Topical corticosteroid preparations
A common form of iatrogenic telangiectasias may occur from the use of high-potency topical steroid preparations. Leyden132 first reported the development of rosacea associated with telangiectasias in 10 patients who regularly used topical fluorinated steroids on the face. Katz and Prawer133 demonstrated clinical cutaneous vascular dilation and network development within 2 weeks of treatment with superpotent topical steroids (betamethasone dipropionate in an optimized vehicle and clobetasol ointment). This type of steroid-induced telangiectasia probably reflects the loss of perivascular ground substance, allowing distention of existing vessels, or the capillary elongation and distortion generated to meet the requirement of the hyperplastic epidermis.134 Accordingly, covert vascular visualization has been shown to represent the early changes of cutaneous atrophy that occur as a result of steroid use.135 Interestingly, facial telangiectasias have been reported to develop in association with long-term application of a topical corticosteroid to the scalp.136 In this report, the long-term use of betamethasone valerate lotion allowed percutaneous absorption of a sufficient amount to spread locally from the scalp to the face through the dermal vasculature. Therefore, topical application of corticosteroid preparations can induce the development or appearance of telangiectasias. There has been a rise in incidence with the use of bootleg topical bleaching preparations which are hydroquinone adulterated with high potency topical steroids such as clobetasol.
Physical factors
Actinic neovascularization and vascular dilation
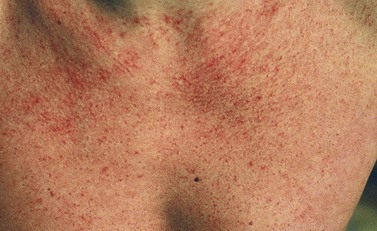
Physical factors are commonly responsible for acquired telangiectasia. Telangiectasias are noted to appear after many types of physical trauma. The most common form of physical damage to the skin is that caused from sun exposure.137,138 Telangiectasia on the face is probably a manifestation of persistent active arteriolar vasodilation caused by weakness in the vessel wall, resulting from degenerative elastic changes or weakness in the surrounding connective tissue caused by chronic sun exposure. Alternatively, ultraviolet B (UVB) exposure has been shown to elevate epidermal tumor necrosis factor (TNF),139 which promotes angiogenesis by various mechanisms that may also induce dilation of existing vessels.140 Vascular endothelial growth factor (VEGF) has also been shown to be elevated in the epidermis following UVB exposure, with an increase in the number of cutaneous blood vessels.141 Finally, the aging process itself may lead to vessel dilation. Perivascular veil and adventitial cells are believed to be responsible for the synthesis and maintenance of the peripheral portion of vascular walls in the dermis.107 These cells decrease in number with aging, correlating with a histologic thinning of vascular walls.142 Thus, both direct and indirect ultraviolet light, as well as the aging process, contribute to the development of telangiectasia.
Ultraviolet-induced telangiectasias most often arise from arterioles and are seen frequently in individuals with fair complexions, often on the nose (especially on the ala and nasolabial crease). A similar mechanism for the pathogenesis of type I telangiectasias may also apply to their occurrence on the legs. An examination of more than 20,000 Americans143 demonstrated the presence of fine telangiectasias in 17.3% of men and 11.6% of women with low sun exposure, compared with 30.1% of men and 26.2% of women who reported high sun exposure. More significantly, 15.5% of men and 40.9% of women with actinic skin damage were shown to have spider leg veins, compared with 6% of men and 28.9% of women without actinic skin damage. Solar-induced damage to cutaneous and subcutaneous tissue is a significant etiologic factor in the appearance of telangiectasias (Fig. 4.18).
Trauma
Contusion
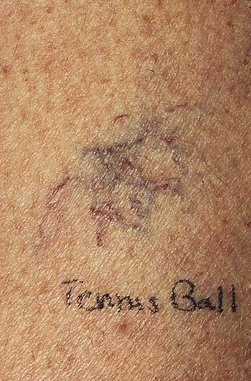
Various forms of physical trauma may lead to the growth of new blood vessels. Contusion injuries are a common mechanism for the development of a localized growth of telangiectasias (Fig 4.19). In these cases, neovascularization probably results from epidermal and endothelial damage, which induces the release of angiogenic factors, including fibrin.144 Trauma also causes a rapid change in the permeability of cutaneous blood vessels through the release of various mediators.89 The increased permeability of endothelium may lead to angiogenesis through multiple mechanisms.145
A solitary giant spider angioma with an overlying pyogenic granuloma was described in a patient with alcoholic cirrhosis.146 The authors postulated that local mechanical irritation led to a reactive proliferation of endothelial cells. When the central pyogenic granuloma was removed, the surrounding spider telangiectasia disappeared.
Surgical Incisions or Lacerations
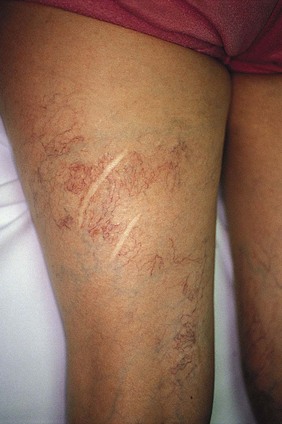
Surgical incisions or cutaneous lacerations are common events that require physiologic neovascularization. Here, angiogenesis is a prerequisite for the progression of wound healing.144 Fibrin deposition appears to play a prominent role in wound healing by stimulating new blood vessel growth.144,147 The resulting formation of blood vessels has been demonstrated to represent a dilation and extension of existing blood vessels.89 Unfortunately, some postsurgical patients develop an exaggerated angiogenic response manifested by cutaneous telangiectasias. Although this may occur at sites of ligation for vein stripping (Fig. 4.20), the most common surgical event that leads to telangiectasia development is the skin flap procedure. When a skin flap is under excessive tension, telangiectasias may develop at the edge of the flap. This excessive blood vessel growth may be induced by mechanical forces on the wound. Histologic examination demonstrates the orientation of vascular fiber networks along lines of tension. This orientation may be related to the activation of cell growth through stretching.148 Thus, the vascularization of skin flaps and grafts supports the concept of an epidermal stimulus for vasculogenesis.149,150
Infection
Generalized Essential Telangiectasia
Various infections have been associated with the development of the form of telangiectasia just described. Bacteria may stimulate endothelial proliferation in vitro.151 In 1926, Becker152 reviewed a series of patients with generalized telangiectasia and associated this phenomenon with syphilitic infection. Of these patients, 16 had evidence of systemic syphilis and 4% had evidence of a focal infection. Ayres et al153 later described a patient with generalized telangiectasia and a sinus infection. Resolution of the sinusitis with antibiotic treatment resulted in the disappearance of the telangiectasia.
Progressive Ascending Telangiectasia
Shelley,154 and Shelley and Fierer,155 described two cases of essential progressive telangiectasia that resolved after empirical treatment with tetracycline and ketoconazole, respectively. Electron microscopy studies in one patient demonstrated focal fibrin clots in some of the dilated vessels, which disappeared within a month of ketoconazole therapy.155 The authors postulated a microbial-induced focal intravascular coagulation as the causal factor in the pathogenesis of this rare form of acquired telangiectasia.
Human Immunodeficiency Virus
Immunosuppressed populations are frequently infected with a wide variety of bacterial, fungal and yeast species. The first report of neovascularization in immunosuppressed patients was in an HIV-positive hemophiliac with numerous telangiectasias on the shins; the telangiectasias resolved after treatment with tetracycline.156 Unfortunately, the specific infection involved is usually difficult to identify because a myriad of infections occur in immunosuppressed patients. Also, a survey of homosexual men with and without lymphadenopathy demonstrated that 47% had focal telangiectasias in a broad distribution across the anterior chest.157 The presence of telangiectasias was significantly, although not exclusively, associated with HIV seropositivity. Telangiectasias in this group did not appear to be related to underlying bacterial or fungal infection or to sun exposure. The authors postulated that this clinical finding might have been a direct manifestation of HIV infectivity.
Although not telangiectatic, bacillary angiomatosis (also known as epithelioid angiomatosis) is a disease most often characterized by multiple reddish cutaneous papules. Tissue sections of these lesions demonstrate weakly reactive gram-negative bacilli. The cutaneous lesions resolve with antibiotic therapy. Thus, this new entity confirms an infectious etiology for the stimulation of vascular proliferation.158
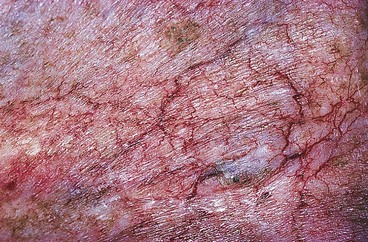
Radiodermatitis
Therapeutic radiation therapy may lead to the development of telangiectasias (Fig. 4.21). Chronic radiodermatitis has noticeably decreased with the advent of mega-voltage equipment and better technique. Mega-voltage radiation beams are more penetrating than the older, lower-energy beams; this minimizes the radiation dose to the skin. However, at least 5% of patients receiving therapeutic radiation therapy develop cutaneous telangiectasias.159 The fundamental pathology of chronic radiodermatitis is fibrosis of the vessels with occlusion and varying degrees of homogenization of the connective tissue. Residual superficial blood vessels are usually dilated.160
Erythema ab igne
Infrared radiation or heat exposure can lead to the appearance of telangiectasia. Erythema ab igne is a localized dermatosis that occurs as reticular pigmentation and telangiectasia produced by repeated exposures to heat. It is commonly seen on the legs of women who sit close to heating units in countries without central heating; recently there have been reports of erythema ab igne appearing on the anterior thighs in patients exposing these sites to heat generated by laptops.161–164 Histologic findings include epidermal atrophy, vasodilation, a dermal mixed cellular infiltrate, and an increase in melanophages as well as free-lying melanin granules.165
1 Merlen JF. Red telangiectasias, blue telangiectasias. Soc Franc Phlebol. 1970;22:167.
2 Goldman MP, Bennett RG. Treatment of telangiectasia: a review. J Am Acad Dermatol. 1987;17:167.
3 Raymond-Martimbeau P, Dupuis JL. Telangiectasias: incidence, classification, and relationship with the superficial and deep venous systems: a double-blind study. In: Negus D, editor]. Phlebology ’95. 1995;1:169.
4 Redisch W, Pelzer RH. Localized vascular dilatations of the human skin: capillary microscopy and related studies. Am Heart J. 1949;37:106.
5 De Faria JL, Moraes IN. Histopathology of the telangiectasias associated with varicose veins. Dermatologica. 1963;127:321.
6 Bergan JJ. Development of primary varicose veins. Phlebolymphology. 1997;18:3.
7 Ono T, Bergan JJ, Schmid-Schönbein GW, Takase S. Monocyte infiltration into venous valves. J Vasc Surg. 1998;27:158.
8 Takase S, Delano F, Lerond L, et al. Inflammation in chronic venous insufficiency: is the problem insurmountable? J Vasc Res. 1999;38(Suppl):3.
9 Kaplan I, Peled I. The carbon dioxide laser in the treatment of superficial telangiectasias. Br J Plast Surg. 1975;28:214.
10 Ayres SJr, Burrows LA, Anderson NP. Generalized telangiectasias and sinus infection: report of a case with cure by treatment of chronic sinusitis. Arch Dermatol Syph. 1931;26:56.
11 Noe JM, Finley J, Rosen S, Arndt KA. Postrhinoplasty ‘red nose’: differential diagnosis and treatment by laser. Plast Reconstr Surg. 1981;67:661.
12 Gormley DE. Tissue atrophy caused by intermittent mechanical pressure on the legs (subcutaneous sartorial tissue atrophy). Dermatol Surg. 1995;21:354.
13 Shelley WB. Essential progressive telangiectasia. JAMA. 1971;210:1343.
14 Anderson RL, Smith JGJr. Unilateral nevoid telangiectasia with gastric involvement. Arch Dermatol. 1975;111:617.
15 Widmer LK. Peripheral venous disorders: prevalence and socio-medical importance: observations in 4529 apparently healthy persons, Basle study III, Berne. Switzerland: Hans Huber; 1978.
16 Rivers JK, Frederiksen PC, Dibdin C. A prevalence survey of dermatoses in the Australian neonate. J Am Acad Dermatol. 1990;23:77.
17 Duffy DM. Small vessel sclerotherapy: an overview. Adv Dermatol. 1988;3:221.
18 Sadick N. Treatment of varicose and telangiectatic leg veins with hypertonic saline: a comparative study of heparin and saline. J Dermatol Surg Oncol. 1990;16:24.
19 Pratt AG. Birthmarks in infants. Arch Dermatol Syph. 1953;67:302.
20 Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1970;58:218.
21 Cosman B. Clinical experience in the laser therapy of port-wine stains. Lasers Surg Med. 1980;1:133.
22 Hobby LW. Treatment of port-wine stains and other cutaneous lesions. Contemp Surg. 1981;18:21.
23 Shelley WB, Livingood CS. Familial multiple nevi flammei. Arch Dermatol Syph. 1949;59:343.
24 Kaplan P, Hollenberg RD, Fraser FC. A spinal arteriovenous malformation in hereditary cutaneous hemangiomas. Am J Dis Child. 1976;130:1329.
25 Nova HR. Familial communicating hydrocephalus, posterior cerebellar agenesis, mega cisterna magna, and port-wine nevi. J Neurosurg. 1979;51:862.
26 Zaremba J, Stepien M, Jelowicka M, Ostrowska D. Hereditary neurocutaneous angioma: a new genetic entity? J Med Genet. 1979;6:443.
27 Barsky SH, Rosen S, Geer DE, Noe JM. The nature and evolution of port wine stains: a computer-assisted study. J Invest Dermatol. 1980;74:154.
28 Finley JL, Noe JM, Arndt KA, et al. Port-wine stains: morphologic variations and developmental lesions. Arch Dermatol. 1984;120:1453.
29 Higurashi M, Oda M, Iijima K, et al. Livebirth prevalence and follow-up of malformation syndromes in 27,472 newborns. Brain Dev. 1990;12:770.
30 Gloviczki P, Hollier LH, Telander RL, et al. Surgical implications of Klippel-Trénaunay syndrome. Ann Surg. 1983;19:353.
31 Lorda-Sanchez I, Prieto L, Rodriguez-Pinilla E, et al. Increased parental age and number of pregnancies in Klippel-Trenaunay-Weber syndrome. Ann Hum Genet. 1998;62:235.
32 Servelle M, Bastin R, Loygue J, et al. Hematuria and rectal bleeding in the child with Klippel and Trénaunay syndrome. Ann Surg. 1976;183:418.
33 Vollmar JF, Paes E, Irion B, et al. Aneurysmatische transformation des Venensystems bei venosen Angiodysplasien der Gliedmassen. Vasa. 1989;18:96.
34 Baskerville PA, Ackroyd JS, Lea Thomas M, Browse NL. The Klippel-Trénaunay syndrome: clinical, radiological and haemodynamic features and management. Br J Surg. 1985;72:232.
35 Baskerville PA, Ackroyd JS, Browse NL. The etiology of the Klippel-Trénaunay syndrome. Ann Surg. 1985;202:624.
36 Villavicencio L. Treatment of varicose veins associated with congenital vascular malformations. In: Bergan JB, Goldman MP, editors. Varicose and telangiectatic leg veins: diagnosis and treatment. St Louis: Quality Medical Publishers, 1993.
37 Servelle M. Klippel and Trénaunay’s syndrome. Ann Surg. 1985;197:365.
38 Paes E, Vollmar J. Diagnosis and surgical aspects of congenital venous angiodysplasia in lower extremities, Sixth annual meeting of the American Venous Forum, Maui, February 23–5, 1994.
39 Colver GB. The clinical relevance of abnormal cutaneous vascular patterns and related pathologies. In: Ryan J, Cherry GW, editors. Vascular birthmarks: pathogenesis and management. New York: Oxford University Press, 1987.
40 Myers TT, Janes JM. Comprehensive surgical management of cavernous hemangioma of the lower extremity with special reference to stripping. Surgery. 1955;37:184.
41 Enjolras O, Ciabrini D, Mazoyer E, et al. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36:219.
42 Hulsmans RFHJ, Schrander-Stumpel DTRM, Koopman RJJ, et al. ‘Chimp’s’ feet and ‘Tarzan’s’ chest constitute clinical hallmarks in many pediatric patients with Proteus syndrome. Scr Phlebologica. 1994;2:7.
43 Wiedemann HR, Burgio GR, Aldenhoff P, et al. The Proteus syndrome: partial gigantism of the hands and/or feet, nevi, hemihypertrophy, subcutaneous tumors, macrocephaly or other skull anomalies and possible accelerated growth and other visceral afflictions. Eur J Pediatr. 1983;140:5.
44 Mayatepek E, Kurczynski TW, Ruppert ES, et al. Expanding the phenotype of the Proteus syndrome: a severely affected patient with new findings. Am J Med Genet. 1989;32:402.
45 Cohen MMJr, Hayden PW. A newly recognized hamartomatous syndrome. In: Bergsma D, editor. Penetrance and variability in malformation syndromes. New York: Alan R Liss, 1979.
46 Fay JT, Schow SR. A possible cause of Maffucci’s syndrome: report of a case. J Oral Surg. 1968;26:739.
47 Kontras SB. Case report 19. Synd Indent. 1974;2:1.
48 Clark RD, Donnai D, Rogers J, et al. Proteus syndrome: an expanded phenotype. Am J Med Genet. 1987;27:99.
49 De Takats G. Vascular anomalies of the extremities. Surg Gynecol Obstet. 1932;55:227.
50 Bean WB. Vascular spiders and related lesions of the skin. Springfield, Ill: Charles C Thomas; 1958.
51 Pasyk KA. Classification and clinical and histopathological features of haemangiomas and other vascular malformations. In: Ryan J, Cherry GW, editors. Vascular birthmarks: pathogenesis and management. New York: Oxford University Press, 1987.
52 Reed WB, Epstein WL, Boder E, Sedgwick R. Cutaneous manifestations of ataxia-telangiectasia. JAMA. 1966;195:746.
53 Patek AJ, Post J, Victor JC. The vascular ‘spider’ associated with cirrhosis of the liver. Am J Med Sci. 1946;200:341.
54 Corbett D. Discussion. Br J Dermatol. 1914;26:200.
55 Martini GA, Staubesand J. Zur morphologie der gefäcbspinnen (‘vascular spider’) in der hatu leverknankev. Virchows Archiv. 1953;324:147.
56 Marriott P, Munro D, Ryan TJ. Angioma serpiginosum – familial incidence. Br J Dermatol. 1975;93:701.
57 Kumakiri M. Angioma serpiginosum. J Cutan Pathol. 1980;7:410.
58 Bockenheimer PH. Uber die genuine diffuse Phlebektasie deroberen Extremitat. Leipzig. Festschrift fur Georg Eduard von rindfleisch, 1907.
59 Hulsmans RF, Woltil HA, Groeneweg DA. Genuine diffuse phlebectasia (Bockenheimer’s syndrome): report of 5 cases and a review of the literature. Scr Phlebologica. 1993;1:4.
60 Sonntag E. Das Rankenagion, sowie die genuine diffuse Phlebarteriektasie und Phlebektasie. Ergebn Chir Orthop. 1919;11:99.
61 Malan E, Puglionisi A. Congenital angiodysplasias of the extremities. J Cardiovasc Surg. 1964;5:87.
62 Thompson AW, Shafer JC. Congenital vascular anomalies. JAMA. 1951;145:869.
63 Kluken N, Heller W. Die genuine diffuse Phlebektasie. Folia Angiol. 1972;20:66.
64 Young AE, Ackroyd AE, Baskerville P. Combined vascular malformations. In: Mulliken JB, Young AE, editors. Vascular birthmarks. Philadelphia: WB Saunders, 1988.
65 Lewis RJ, Ketcham AS. Maffucci’s syndrome: functional and neoplastic significance. J Bone Joint Surg. 1973;55:1465.
66 Cremer H, Gullotta F, Wolf L. Maffucci-Kast syndrome. J Cancer Res Clin Oncol. 1981;101:231.
67 Cheng FC. Maffucci’s syndrome with fibroadenomas of the breasts. J R Coll Surg Edinb. 1981;26:181.
68 Silver HK. Rothmund-Thomson syndrome: an oculocutaneous disorder. Am J Dis Child. 1966;111:182.
69 Ochshorn M, Ilie B, Blum I. Multiple telangiectasias preceding the appearance of undifferentiated bronchiogenic carcinoma. Dermatologica. 1982;165:620.
70 Draelos ZK, Hansen RC, Hays SB. Symmetric progressive telangiectatic nevi with hemorrhagic angiokeratomas. Pediatr Dermatol. 1986;3:212.
71 Ayers SJr, Burrows LA, Anderson NP. Generalized telangiectasia and sinus infection: report of a case with cure by treatment of chronic sinusitis. Arch Dermatol Syph. 1932;26:56.
72 Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21:1094.
73 Girbig P, Voigtlander V. Besenreiservarizen in rahmen progressiver essentieller Telangiektasien. Phlebol Proctol. 1988;17:116.
74 McGrae JD, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histologic study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909.
75 Paller AS. Vascular disorders. Dermatol Clin. 1987;5:239.
76 Way BH, Herrmann J, Gilbert EF, et al. Cutis marmorata telangiectatic congenita. J Cutan Pathol. 1974;1:10.
77 Powell ST, Su WPD. Cutis marmorata telangiectatica congenita: report of nine cases and review of the literature. Cutis. 1984;34:305.
78 Picascia DD, Esterly NB. Cutis marmorata telangiectatica congenita: report of 22 cases. J Am Acad Dermatol. 1989;20:1098.
79 South DA, Jacobs AH. Cutis marmorata telangiectatica congenita (congenital generalized phlebectasia). J Pediatr. 1978;93:944.
80 Andreev VC, Pramatarov K. Cutis marmorata telangiectatic congenita in two sisters. Br J Dermatol. 1979;101:345.
81 Kurczynski TW. Hereditary cutis marmorata telangiectatic congenita. Pediatrics. 1982;70:52.
82 Van Lohuizen CHJ. Uber eine seltene angeborene hautanomalie (cutis marmorata telangiectatica congenita). Acta Derm Venerol (Stockh). 1922;3:202.
83 Fujita M, Darmstadt GL, Dinulos JG. Cutis marmorata telangiectatica congenita with hemangiomatous histopathologic features. J Am Acad Dermatol. 2003;48:950.
84 Cohen PR, Zalar GL. Cutis marmorata telangiectatica congenita: clinicopathologic characteristics and differential diagnosis. Cutis. 1988;42:518.
85 Kennedy C, Oranje A, Keizer K. Cutis marmorata telangiectatica congenita. Int J Dermatol. 1992;31:349.
86 De Lorimier AA. Hepatic tumors of infancy and childhood. Surg Clin North Am. 1977;57:443.
87 Hurwitz S. Clinical pediatric dermatology: a textbook of skin disorders of childhood and adolescence. Philadelphia: Saunders; 1981.
88 Azizkhan G, Azizkhan JC, Zetter BR, Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980;152:931.
89 Ryan TJ, Kurban AK. New vessel growth in the adult skin. Br J Dermatol. 1970;82(Suppl):92.
90 Fried SZ, Lynfield L. Unilateral facial telangiectasia macularis eruptiva perstans. J Am Acad Dermatol. 1987;16:250.
91 Hirai M. Clinical significance of corona phlebectatica associated with varicose veins. Phlebology. 1999;14:77.
92 Bihari I, Muranyi A, Bihari P. Laser-Doppler examination shows high flow in some common telangiectasia in the lower limb. Dermatol Surg. 2005;31:388.
93 Sommer A, Van Mierlo PL, Neumann HAM, et al. Red and blue telangiectasias: Differences in oxygenation? Dermatol Surg. 1997;23:55.
94 Rabe E, Pannier F. Does corona phlebectatica indicate chronic venous insufficiency? Phlebology. 2008;23:238.
95 Tretbar LL. The origin of reflux in incompetent blue reticular/telangiectasia veins. In: Davy A, Stemmer R, editors. Phlébologie ’89. Montrouge, France: John Libbey Eurotext, 1989.
96 Weiss RA, Weiss MA. Doppler ultrasound findings in reticular veins of the thigh subdermic lateral venous system and implications for sclerotherapy. J Dermatol Surg Oncol. 1993;19:947.
97 Goldman MP. Commentary: rational sclerotherapy techniques for leg telangiectasia. J Dermatol Surg Oncol. 1993;19:933.
98 Salles-Cunha SX. Telangiectasias: classification of feeder vessels with color flow. Radiology. 1993;186:615.
99 Somjen GM, Ziegenbein R, Johnston AH, Royle JP. Anatomical examination of leg telangiectases with duplex scanning. J Dermatol Surg Oncol. 1993;19:940.
100 Bohler-Sommeregger K, Karnel F, Schuller-Petrovic S, Santler R. Do telangiectases communicate with the deep venous system? J Dermatol Surg Oncol. 1992;18:403.
101 Mariani F, Bianchi V, Mancini S, Mancini S. Telangiectases in venous insufficiency: point of reflux and treatment strategy. Phlebology. 2000;15:38.
102 Green D. Reticular veins, incompetent reticular veins, and their relationship to telangiectases. Dermatol Surg. 1998;24:1129.
103 Merlen JF. Telangiectasies rouges, telangiectasies bleues. Phlebologie. 1970;23:167.
104 Thibault P, Bray A, Wlodarczyk J, Lewis W. Cosmetic leg veins: evaluation using duplex venous imaging. J Dermatol Surg Oncol. 1990;16:612.
105 Engelhorn CA, Engelhorn AL, Cassou MF, et al. Patterns of saphenous venous reflux in women presenting with lower extremity telangiectasias. Dermatol Surg. 2007;33:282.
106 Curri GB, Lo Brutto ME. Influenza dei mucopolisaccaridi e dei loro costituenti sulla neoformazione vascolare. Riv Pat Clin Sper. 1967;8:379.
107 Braverman IM. Microcirculation in psoriasis. In: Roenigk HHJr, Maibach HA, editors. Psoriasis. New York: Marcel Dekker, 1985.
108 David M, Filhaber A, Rotem A, et al. Keratosis lichenoides chronica with prominent telangiectasia: response to etretinate. J Am Acad Dermatol. 1989;21:1112.
109 Margolis MH, Cooper GA, Johnson SAM. Keratosis lichenoides chronica. Arch Dermatol. 1972;105:739.
110 Davis MJ, Lawler JC. Capillary alterations in pigmented purpuric disease of the skin. Arch Dermatol. 1958;78:723.
111 Iwatsuki K, Aoshima T, Tagami H, et al. Immunofluorescence study in purpura pigmentosa chronica. Acta Derm Venereol. 1980;60:341.
112 Ricci S. Results of an electronic mail survey on hormones and telangiectasias. Dermatol Surg. 2005;31:246.
113 Miller SS. Investigation and management of varicose veins. Ann R Coll Surg Engl. 1974;55:245.
114 Mullane DJ. Varicose veins of pregnancy. Am J Obstet Gynecol. 1952;63:620.
115 Goodrich SM, Wood JE. Peripheral venous distensibility and velocity of venous blood flow during pregnancy or during oral contraceptive therapy. Am J Obstet Gynecol. 1964;90:740.
116 Keates JS, Fitzgerald DE. An interim report on lower limb volume and blood flow changes during a normal menstrual cycle. Bibl Anat. 1969;10:189.
117 McPheeters HO. The value of estrogen therapy in the treatment of varicose veins complicating pregnancy. Lancet. 1949;69:2.
118 Davis LT, Duffy DM. Determination of incidence and risk factors for post-sclerotherapy telangiectatic matting of the lower extremity: a retrospective analysis. J Dermatol Surg Oncol. 1990;16:327.
119 Jensen EV, Greene GL, Closs LE, et al. Receptors reconsidered: a 20-year perspective. Recent Prog Horm Res. 1982;38:1.
120 Colburn P, Buonassisi V. Estrogen binding sites in endothelial cell cultures. Science. 1978;201:817.
121 Sadick NS, Niedt G. A study of estrogen receptors in spider telangiectasia of the lower extremities. J Dermatol Surg Oncol. 1990;16:620.
122 Sadick NS, Urmacher C. Estrogen and progesterone receptors: their role in postsclerotherapy angiogenesis telangiectatic matting. Dermatol Surg. 1999;25:539.
123 Wilkin JK, Smith JG, Cullison DA, et al. Unilateral dermatomal superficial telangiectasia: nine new cases and a review of unilateral dermatomal superficial telangiectasia. J Am Acad Dermatol. 1983;8:468.
124 Wilkin JK. Unilateral nevoid telangiectasias. Arch Dermatol. 1977;113:486.
125 Beacham BE, Kurgansky D. Unilateral naevoid telangiectasia syndrome associated with metastatic carcinoid tumour. Br J Dermatol. 1991;124:86.
126 Uhlin SR, McCarty KS. Unilateral nevoid telangiectatic syndrome: the role of estrogen and progesterone receptors. Arch Dermatol. 1983;119:226.
127 Dobson CM, Tagore V, Myint AS, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635.
128 Lin JH, Lee JY, Chao SC, et al. Telangiectatic metastatic breast carcinoma preceded by en cuirasse metastatic breast carcinoma. Br J Dermatol. 2004;150:523.
129 Zanca A, Ferracini U, Bertazzoni MG. Telangiectatic metastasis from ductal carcinoma of the parotid gland. J Am Acad Dermatol. 1993;28:113.
130 Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:575.
131 Boswell JS, Davis MDP. Violaceous plaque on the forehead clinically resembling angiosarcoma: cutaneous metastasis in a patient with prostatic adenocarcinoma. J Am Acad Dermatol. 2005;53:744.
132 Leyden JJ, Thew M, Kligman AM. Steroid rosacea. Arch Dermatol. 1974;110:619.
133 Katz HI, Prawer SE. Morphologic diagnosis of pre-atrophy of the skin. Scientific exhibit presented at the American Academy of Dermatology, New Orleans, December 6–11, 1986.
134 Zheng P, Lavker RM, Lehmann P, Kligman AM. Morphologic investigations on the rebound phenomenon after corticosteroid-induced atrophy in human skin. J Invest Dermatol. 1984;82:345.
135 Katz HI, Prawer SE, Mooney JJ, Samson CR. Preatrophy: covert sign of thinned skin. J Am Acad Dermatol. 1989;20:731.
136 Hogan DJ, Rooney ME. Facial telangiectasia associated with long-term application of a topical corticosteroid to the scalp. J Am Acad Dermatol. 1989;20:1129.
137 O’Brian JP. Solar and radiant damage to elastic tissue as a cause of internal vascular disease. Aust J Dermatol. 1980;21:1.
138 Kumakari M, Hashimoto K, Willis I. Biologic changes due to long wave ultraviolet irradiation on human skin. J Invest Dermatol. 1977;69:392.
139 Oxholm A, Oxholm P, Staberg B, Bendtzen K. Immunohistochemical detection of interleukin I-like molecules and tumour necrosis factor in human epidermis before and after UVB-irradiation in vivo. Br J Dermatol. 1988;118:369.
140 Braverman IM, Fonferko E. Studies in cutaneous aging. II. The microvasculature. J Invest Dermatol. 1982;78:444.
141 Blaudschun R, Sunderkotter C, Brenneisen P, et al. Vascular endothelial growth factor causally contributes to the angiogenic response upon ultraviolet B irradiation in vivo. Br J Dermatol. 2002;146:581.
142 Piquet PF, Grau GE, Vassali P. Subcutaneous perfusion of tumor necrosis factor induces local proliferation of fibroblasts, capillaries, and epidermal cells, or massive tissue necrosis. Am J Pathol. 1990;136:103.
143 Engel A, Johnson ML, Haynes SG. Health effects of sunlight exposure in the United States: results from the first National Health and Nutrition Examination Survey, 1971–1974. Arch Dermatol. 1988;124:72.
144 Dvorak HF. Tumors: wounds that do not heal: similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;26:1650.
145 Ryan TJ. Factors influencing the growth of vascular endothelium in the skin. Br J Dermatol. 1970;82(Suppl 5):99.
146 Okada N. Solitary giant spider angioma with an overlying pyogenic granuloma. J Am Acad Dermatol. 1987;5:1053.
147 Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442.
148 Ryan TJ, Barnhill RL. Physical factors and angiogenesis. In: Nugent J, O’Connor M, editors. Development of the vascular system, Ciba Foundation Symposium. London: Pitman, 1983.
149 Smahel J. The healing of skin grafts. Clin Plast Surg. 1977;4:409.
150 Myers MB. Attempts to augment survival in skin flaps – mechanism of the delay phenomenon. In: Grabb WC, Myers MB, editors. Skin flaps. Boston: Little, Brown, 1975.
151 Garcia FN, Ojta T, Hoover RL. Stimulation of human umbilical vein endothelial cells (HUVEC) by Bartonella bacilliformis (BB). FASEB J. 1988:A1189.
152 Becker SW. Generalized telangiectasia. Arch Dermatol Syph. 1926;14:387.
153 Ayres SJr, Burrows A, Anderson NP. Generalized telangiectasia and sinus infection: report of a case with cure by treatment of chronic sinusitis. Arch Dermatol Syph. 1932;26:56.
154 Shelley WB. Essential progressive telangiectasia: successful treatment with tetracycline. JAMA. 1971;216:1343.
155 Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10:876.
156 Jimenez-Acosta F, Fonseca E, Magallon M. Response to tetracycline of telangiectasias in a male hemophiliac with human immunodeficiency virus infection. J Am Acad Dermatol. 1988;19:369.
157 Fallon TJr, Abell E, Kingsley L, et al. Telangiectases of the anterior chest in homosexual men. Ann Intern Med. 1986;105:679.
158 Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501.
159 Clarke D, Martinez A, Cox RS. Analysis of cosmetic results and complications in patients with stage I and II breast cancer treated by biopsy and irradiation. Int J Radiat Oncol Biol Phys. 1983;9:1807.
160 Goldschmidt H, Sherwin WK. Reactions to ionizing radiation. J Am Acad Dermatol. 1980;3:551.
161 Bilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973.
162 Jagtman BA. Erythema ab igne due to a laptop computer. Contact Dermatitis. 2004;50:105.
163 Mohr MR, Scott KA, Rariser RM. Laptop computer-induced erythema ab igne: a case report. Cutis. 2007;79:59.
164 Bachmeyer C, Bensaid P, Begon E. Laptop computer as a modern cause of erythema ab igne. J Eur Acad Dermatol Venereol. 2009;23:736.
165 Dover JS, Phillips TJ, Arndt KA. Cutaneous effects and therapeutic uses of heat with emphasis on infrared radiation. J Am Acad Dermatol. 1989;20:278.

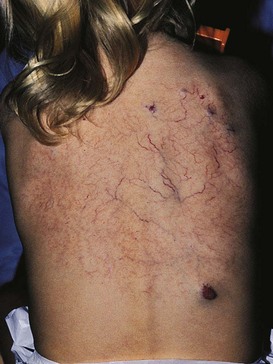
 -year-old girl. The telangiectatic component was present at birth. Within the first few months, cutaneous hemangiomas began to appear. A large mediastinal hemangioma was also noted surrounding the esophagus. Systemic treatment with corticosteroids did not result in any notable decrease in the size of the internal or cutaneous hemangiomas. The patient was still well at age 13 years.
-year-old girl. The telangiectatic component was present at birth. Within the first few months, cutaneous hemangiomas began to appear. A large mediastinal hemangioma was also noted surrounding the esophagus. Systemic treatment with corticosteroids did not result in any notable decrease in the size of the internal or cutaneous hemangiomas. The patient was still well at age 13 years.