Chapter 34 Pathophysiology, Clinical Evaluation, and Medical Management of Aortic Dissection
Acute aortic dissection is an uncommon but life-threatening emergency that requires prompt diagnosis, rapid triage, and immediate medical, endovascular, or surgical treatment. A unified effort across several international centers over the past 15 years has led to the establishment of a detailed registry that describes major aspects of presentation, management, and outcomes of patients with acute aortic dissection.1 This longitudinal experience has given new clinical insights into an old disease and spawned additional multicenter efforts to explore its genetics and pathobiology. Although gains have been made in the delivery of life-saving care to patients with acute aortic dissection, hospital mortality rates remain distressingly high. Enhanced awareness of risk factors for aortic dissection, presentation features, diagnostic pathways, and medical, endovascular, and surgical treatment strategies is a critical first step toward improving outcomes.
Epidemiology
Published figures on the incidence of aortic dissection likely underestimate the actual occurrence rate, since misdiagnosis of this condition is common and the percentage of acute aortic dissection patients who expire before hospital presentation cannot be accurately estimated.2 Nevertheless, acute aortic dissection is a rare event. Analysis of the Swedish National Cause of Death Register between 1987 and 2002 estimated the incidence of thoracic aortic aneurysm or dissection to be 16.3 per 100,000 men and 9.1 per 100,000 women; others have reported acute aortic dissection to affect 30 in 1 million individuals per year.3 By comparison, acute myocardial infarction (AMI) is 140-fold more common.1 Data from the International Registry of Aortic Dissection (IRAD) indicate that the mean age of patients at presentation with aortic dissection is 63 years, with men accounting for 63% of cases.1
Classification
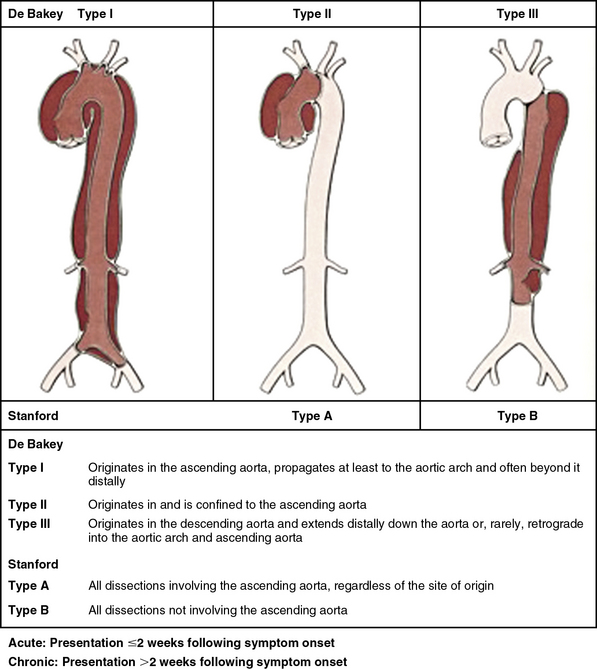
Classifying aortic dissection according to anatomical location and time from onset of symptoms helps stratify risk and guide selection of initial treatment strategy (Fig. 34-1). The Stanford classification system designates dissections that involve the aorta proximal to the brachiocephalic artery (i.e., root and ascending aorta) as type A, and those that do not as type B.4 This distinction is clinically important because dissection involving the ascending aorta is a key determinate of early death and major morbidity. Location of the intimal tear does not influence Stanford dissection type. In the older DeBakey classification scheme, a type I dissection originates within the ascending aorta and extends for a variable distance beyond the take-off the innominate artery. A DeBakey type II dissection is confined to the ascending aorta, and a type III dissection originates in the descending thoracic aorta beyond the origin of the left subclavian artery and terminates above (type IIIA) or extends below (type IIIB) the level of the diaphragm.5 Although there is no single universally accepted classification system, the Stanford classification scheme is most often used in practice today and will be used throughout this chapter. The terms communicating and noncommunicating refer to the presence or absence, respectively, of blood flow between the true and false lumens of the aorta.
Pathogenesis
Forces that weaken the medial layer of the aorta increase the probability of dilation, aneurysm formation, and dissection (Box 34-1). Acquired and genetic diseases that mediate this process are discussed later. In classic acute aortic dissection, the initiating event is an intimal tear through which blood rapidly surges distally into the media under systolic pressure, splitting the layers of the aortic wall and creating an intimal flap that separates the true from the false lumen.
Intimal Tear
Contemporary imaging modalities or autopsy findings identify the primary entry tear in approximately 90% of cases. It is most frequently located a few centimeters above the level of the aortic valve along the greater curvature of the aorta in cases of type A dissection and accounts for nearly 60% of all cases. Compared with other locations in the ascending aorta, the proximal few centimeters of the greater curvature are exposed to relatively greater hemodynamic, shear, and torsional force. A pivot region located in the descending thoracic aorta just beyond the insertion of the ligamentum arteriosum where the relatively mobile arch meets the fixed descending thoracic aorta is the second most common entry site for intimal tears, which will then propagate as a type B dissection (30% of cases). Arch entry occurs in 7% of cases. The abdominal aorta is the least common site for entry (3% of cases), despite the high prevalence of intima media ulcers in patients with atherosclerotic disease in this segment.6–8
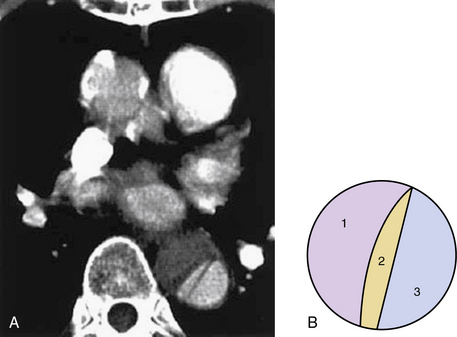
The dissecting hematoma usually propagates in an anterograde direction, although retrograde extension can occur. By this mechanism, as many as 20% of dissections that originate in the distal arch or descending thoracic aorta may involve the ascending aorta.9 In rare cases, a second tear may occur, resulting in a three-channel dissection10 (Fig. 34-2).
Aortic Rupture and End-Organ Malperfusion
Aortic rupture, defined as tearing in the vessel wall that results in extravascular hemorrhage, most commonly occurs with trauma (e.g., motor vehicle collision) but may occur as a complication of the primary dissection.11 Rupture into the pericardial space resulting in cardiac tamponade occurs in type A dissection, whereas rupture into the left pleural space is usually encountered with type B dissection. Dissection-mediated end-organ ischemia or infarction occurs from (1) mechanical compression of aortic branch vessels by false lumen hematoma, (2) extension of the dissection plane across the ostium of the branch vessel, or (3) dynamic vessel inlet obstruction caused by an oscillating intimal flap. Compromise of coronary, brachiocephalic, mesenteric, renal, spinal, and iliac circulations can occur and result in a myriad of clinical presentations. Occlusion of the left ventricular (LV) outflow tract by an intimal flap has been reported (Fig. 34-3).
False Lumen Thrombosis
Thrombosis of blood within the false lumen may seal the entry tear, thus eliminating communication with the true lumen and interruption of false lumen expansion. Partial thrombosis of the false lumen, however, has been identified as a risk factor for long-term death in patients with type B dissection.12,13 Elevation of pressure with partial thrombosis within the false lumen may lead to further extrinsic compression of the true lumen and impairment of blood flow to critical organs. Alternatively, it has been proposed that partial thrombosis of the false lumen is associated with worse clinical outcomes by promoting vascular inflammation, hypoxia, and/or neovascularization with weakening of adjacent vascular structures and an increased risk for aortic rupture.13–17
Persistent patency of the false lumen is also associated with a higher risk of long-term complications such as late rupture or false aneurysm formation requiring operative intervention.12,15–17 Although native aortic aneurysm disease is often a risk factor for dissection, a dissection need not result in aneurysm formation. The term “dissecting aneurysm” is an inaccurate anachronism, and these diseases are not synonymous, a distinction that is particularly important when considering their natural histories and treatments.
Predisposing Genetic Factors
As is true for aneurysms, any process that leads to destruction or degeneration of the major supporting elements of the aortic media (elastin, collagen, smooth muscle cells [SMCs]) can predispose to development of dissection. The histopathological term medial degeneration refers to noninflammatory destruction or fragmentation of elastic lamellar units, dropout of SMCs, and accumulation of mucopolysaccharide ground substances (not always in distinct cystic spaces), which characterize the final common pathway for a variety of processes that affect the integrity of the aortic media (Fig. 34-4).
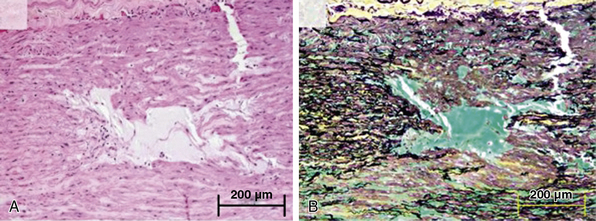
Figure 34-4 Cystic medial degeneration.
(From Maleszewski JJ, Miller DV, Lu J, et al: Histopathologic findings in ascending aortas from individuals with Loeys-Dietz syndrome [LDS]. Am J Surg Pathol 33:194–201, 2009.)
Marfan’s syndrome (MFS) is the most common inherited connective tissue disorder, with an estimated prevalence of 1 case per 3000 to 5000 individuals irrespective of racial, ethnic, or geographic considerations.18–21 If untreated, aortic disease in MFS is progressive and incompatible with normal longevity. Half of patients in whom aortic dissection occurs at younger than 40 years of age have a history of MFS.22 Mutations in the gene encoding fibrillin-1 (FBN1), a critical component of the microfibrils that help form cellular adhesions in the extracellular matrix (ECM), are largely responsible for disease expression.20 Medial degeneration is not pathognomic of MFS and may be present in numerous other conditions.23
Marfan’s syndrome disease expression is heterogenous, but the presence of an aortic root aneurysm (aortic diameter z score ≥2) and ectopia lentis are sufficient to make the diagnosis even in the absence of a family history24 (Box 34-2A). Conversely, in the absence of either of these two clinical features, the revised Ghent nosology for MFS outlines a scoring system based on the presence of FBN1 mutation and/or other key systemic features of MFS to make the diagnosis (Box 34-2B). Systemic features suggestive of MFS include positive wrist sign (entire distal phalanx of adducted thumb extends beyond ulnar border of the palm), positive thumb sign (tip of thumb covers entire fingernail of fifth finger when wrapped around contralateral wrist), pectus excavatum, pneumothorax, dural ectasia, hindfoot deformity, and protrusio acetabuli. Other clinical features less strongly associated with MFS include mitral valve prolapse, various abnormal facial features, and thoracolumbar kyphosis.
![]() Box 34-2A Revised Ghent Criteria for Diagnosis of Marfan’s Syndrome and Related Conditions*
Box 34-2A Revised Ghent Criteria for Diagnosis of Marfan’s Syndrome and Related Conditions*
In the Absence of Family History
In the Presence of Family History
(5) EL and FH of MFS (as defined above) = MFS
(6) Syst (≥7 pts) and FH of MFS (as defined above) = MFS†
(7) Ao (z ≥2 above 20 years old, ≥3 below 20 years old) + FH of MFS (as defined above) = MFS†
Ao, aortic diameter at the sinuses of Valsalva above indicated z-score or aortic root dissection; EL, ectopia lentis; ELS, ectopia lentis syndrome; FBN1, fibrillin-1 mutation; FBN1 not known with Ao, FBN1 mutation that has not previously been associated with aortic root aneurysm/dissection; FBN1 with known Ao, FBN1 mutation that has been identified in an individual with aortic aneurysm; MASS, myopia, mitral valve prolapse, borderline (z <2) aortic root dilation, striae, skeletal findings phenotype; MFS, Marfan’s syndrome; MVPS, mitral valve prolapse syndrome; Syst, systemic score (see Box 34-2B); z, z-score.
* In general, MFS is diagnosed in the presence of aortic root dilation/dissection and ectopia lentis; aortic root dilation/dissection plus FBN1 mutation; aortic root dilation plus sufficient systemic findings (Box 34-2B, ≥7 points); ectopia lentis plus FBN1 mutation previously associated with aortic disease; or in an individual with a positive family history of MFS, the diagnosis is made in the presence of ectopia lentis, or a systemic score ≥7 points, or aortic root dilation.
† Caveat: without discriminating features of Shprintzen-Goldberg’s syndrome, Loeys-Dietz’s syndrome, or vascular Ehlers-Danlos’ syndrome and after TGFBA1/2, collagen biochemistry, COL3A1 testing if indicated. Other conditions/genes will emerge with time.
![]() Box 34-2B Scoring of Systemic Features
Box 34-2B Scoring of Systemic Features
Wrist and thumb sign: 3 (wrist or thumb sign: 1)
Pectus carinatum deformity: 2 (pectus excavatum or chest asymmetry: 1)
Hindfoot deformity: 2 (plain pes planus: 1)
Reduced US/LS and increased arm/height and no severe scoliosis: 1
Scoliosis or thoracolumbar kyphosis: 1
Facial features (3/5): 1 (dolichocephaly, enophthalmos, downslanting palpebral fissures, malar hypoplasia, retrognathia)
mAXIMUM tOTAL: 20 points; score ≥7 indicates systemic involvement.
US/LS, upper segment/lower segment ratio.
From Loeys BL, Dietz HC, Braverman AC, et al: The revised Ghent nosology for the Marfan’s syndrome. J Med Genet 47:476–485, 2010.24
Using an in vivo murine model of MFS, Dietz et al. demonstrated that fibrillin-1 is a key target for binding of transforming growth factor (TGF)-β, a molecule involved in activation of inflammatory, fibrotic, and metalloproteinase cell signaling pathways.25 To investigate the possibility that angiotensin I (AT1)-mediated TGF-β activation could represent a pharmacological target to modify development of aortic dilation in MFS, Habashi et al. studied the effects of losartan, an AT1 receptor antagonist, on aortic aneurysm formation in transgenic mice encoding a cysteine-to-glutamine substitution at position 1039 in the fibrillin-1 gene (Fbn1C1039G/−). Mice with this fibrillin-1 mutation, the most common class of mutation associated with MFS, demonstrate significant and progressive aortic root dilation compared with wild-type mice.26 Treatment with losartan attenuated TGF-β signaling in the aortic wall and resulted in full normalization of aortic wall thickness and a marked improvement in aortic wall architecture (i.e., a decrease in elastin fiber disruption) compared with placebo or β-adrenergic receptor antagonist therapy with propranolol.
These landmark observations have advanced understanding of the molecular basis of MFS and provided new targets for therapy. In one small clinical trial in young MFS patients, initiation of losartan resulted in a significant decrease in rate of growth of the aortic root (mean change 0.46 ± 0.62 mm/yr vs. 3.5 ± 2.8 mm/yr prior to treatment; P < 0.001).27 These findings were supplemented by Ahimastos et al., who conducted a randomized placebo-controlled clinical trial testing the effect of the angiotensin-converting enzyme inhibitor (ACEI) perindopril on aortic root diameter and aortic stiffness in 17 MFS patients.29 At 24 weeks, circulating TGF-β levels and central pulse wave velocities were lower and aortic root diameters were smaller in perindopril-treated patients. Several larger randomized clinical trials directed at modulating TGF-β signaling are ongoing (clinicaltrials.gov; NCT00683124, NCT00782327 NCT00429364, NCT00723801).
Limited forms (i.e., forme frustes) of MFS that feature cardiovascular manifestations include mitral valve prolapse syndrome (MVPS) (mitral valve prolapse, pectus excavatum, scoliosis, and mild arachnodactyly) and the MASS phenotype (myopia, mitral valve prolapse, borderline and nonprogressive aortic root dilation, skeletal findings and striae). There is increasing awareness of familial thoracic aortic aneurysm disease (FTAAD), though candidate genes affecting both matrix and SMC components have been identified in only 20% of such patients. Vascular-type Ehlers-Danlos’ syndrome (EDS) is associated with arterial rupture and dissection, including the aorta.24,28
Ehlers-Danlos’ syndrome (1:5000 births) comprises a heterogeneous group of disorders characterized clinically by hypermobile joints, hyperextensible skin, tissue fragility, and a predisposition to spontaneous vascular rupture. Aortic involvement occurs in EDS type IV, an autosomal dominant disorder attributed to structural defects in the pro-α1 (III) chain of type III collagen, encoded by the COL3A1 gene on chromosome 2q31.30
FTAAD has been mapped to other genetic loci, including 16p13.11 (MYH11 gene), 5q13-14, and 11q23.2-q24, which are not associated with abnormalities of fibrillin or collagen.31,32 More than five mutations in the fibrillin-1 gene have been identified in patients with familial or spontaneous thoracic aortic aneurysm and dissection, with histopathological changes characteristic of medial degeneration, yet with no demonstrable abnormalities of collagen or fibrillin in fibroblast culture.33,34 Polymorphisms encoding the gene vitamin K epoxide reductase complex subunit 1 (VKORC1), which results in under-carboxylation of specific matrix proteins, are associated with calcification of the arterial wall. In patients carrying the C allele (CT or CC), a relative two-fold increase in the probability of developing aortic dissection has been observed.35 A missense mutation in the ACTA2 gene that encodes for actin filaments in SMCs is linked to 14% of patients with FTAAD.36 As a consequence of this mutation, intracellular actin filament assembly is disrupted, promoting focal areas of SMC disarray, decreased SMC contraction, and medial degeneration of the aorta. This phenotype is felt to be associated with aortic wall weakening and increased predisposition to dissection.
Bicuspid aortic valve (BAV) disease is the most common congenital cardiac anomaly in adults (4:1000 live births) and is often accompanied by an aortopathy that is histologically similar but less severe than that observed in patients with MFS. Similar pathological changes have been described in patients with aortic coarctation (many of whom have BAV disease), Noonan’s syndrome, Turner’s syndrome, and polycystic kidney disease. Dilation of the root and (more commonly) the ascending aorta is present in up to 40% of patients with BAV disease and is a risk factor for dissection or rupture (Fig. 34-5).
Acquired Disorders
Systemic hypertension is the most common treatable risk factor for aortic dissection and is present in approximately 75% of patients.2 Hypertension accelerates the normal aging process and leads to intimal thickening, SMC apoptosis, fibrosis, loss of elasticity, and compromise of nutritive blood supply. Decreased aortic compliance and vulnerability to pulsatile forces predispose to injury and create a substrate for dissection.
Iatrogenic Dissection
In the IRAD registry, iatrogenic aortic dissection after cardiac surgery or catheterization accounted for 5% of the total reported.37 Older age, hypertension, and severe peripheral vascular disease are risk factors associated with procedure-related dissection.38 Pain may be absent in iatrogenic dissection. Retrograde dissections created at the time of catheterization usually seal spontaneously on withdrawal of the catheter. Aortic atherosclerotic plaques may prevent longitudinal propagation of a dissection. Dissections arising from sites where the aorta has been incised or cross-clamped may occur intraoperatively or at any time following surgery. Deceleration injury from high-speed accidents results in aortic transection with false aneurysm formation and rupture, most commonly in the region of the aortic isthmus just beyond the origin of the left subclavian artery. Transection results in a transmural tear that is different both pathologically and etiologically from aortic dissection.
Dissection in Pregnancy
Aortic dissection as a complication of pregnancy is rare, although by some estimates 50% of all dissections in women younger than 40 occur during labor, delivery, or early after childbirth.39 Histopathological changes affecting the aortic media of pregnant women have been described, including alterations in elastic fibers and SMCs.40 Both estrogen and relaxin are associated with alterations in matrix metalloproteinase (MMP) homeostasis and contribute to vascular remodeling and a susceptibility to injury independent of the hemodynamic stress of labor and delivery. In many cases, pregnancy unmasks primary conditions that predispose to aortic dissection (e.g., MFS). In those patients with preexisting MFS or BAV disease, aortic root size greater than 4.0 cm is a contraindication to pregnancy, owing to increased risk for spontaneous rupture or dissection.2
Drug Use and Other Acquired Conditions
Recent cocaine use, particularly among young men who smoke tobacco, is an additional risk factor for aortic dissection.41 Among 38 patients with acute aortic dissection occurring over a 20-year period in an urban center, 37% reported cocaine (in particular, crack cocaine) use within the preceding 24 hours (mean 12 hours). Chronic amphetamine use and/or dependence appear to increase the probability of developing a thoracoabdominal aortic dissection in those aged 18 to 49 years.42 Presumed mechanisms for aortic injury from cocaine and amphetamine use involve oxidant stress–mediated endothelial dysfunction and extreme catecholamine-induced shear forces, with abrupt hypertension and tachycardia that collectively lead to weakening of the aortic media and predisposition to tearing.
Inflammatory diseases of the aorta can lead to destruction of ECM proteins and SMCs, with subsequent aneurysm formation and/or dissection. Aortic dissection has been reported in patients with Takayasu’s disease, giant cell aortitis, Behçet’s disease, relapsing polychondritis, systemic lupus erythematosus (SLE), and the aortitis associated with inflammatory bowel disease.2 Syphilitic aortitis, on the other hand, does not predispose to dissection, perhaps because of intense reactive medial scarring and fibrosis that occurs in response to the spirochetal infection. Pheochromocytoma and weight lifting (believed due to intense or repetitious Valsalva maneuvers) also predispose to aortic dissection.
Clinical Presentation
The most important element of any diagnostic algorithm for suspected acute aortic syndrome is a high clinical index of suspicion, based foremost on the presenting history and physical examination (Box 34-3). Absent an appreciation for the cardinal features of dissection, the diagnosis can be missed in a substantial number of patients. Simple clinical prediction rules have been developed to estimate probability of acute aortic dissection.43 The IRAD investigators have recently confirmed the sensitivity of 12 clinical risk markers proposed in the 2010 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) thoracic aortic disease guidelines2 (Table 34-1). These markers, assessed at bedside, were divided into three distinct categories: predisposing factors, characteristics of the pain at time of presentation, and key physical examination findings.44 The presence of risk factor(s) from at least one category identified 95.7% of acute aortic dissection patients in the IRAD database.
![]() Box 34-3 Acute Aortic Syndromes
Box 34-3 Acute Aortic Syndromes
Adapted from Hiratzka LF, Bakris GL, Beckman JA, et al: 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol 55:e27, 2010.2
Table 34-1 Percentage of Patients in the International Registry of Acute Aortic Dissection (1996–2009) with Each of 12 High-Risk Clinical Markers Observed at Time of Presentation with Acute Aortic Dissection*
| Aortic Dissection Detection Risk Category | Clinical Characteristics | % of Patients (N = 2538) |
|---|---|---|
| 1 | MFS | 4.3 |
| 1 | Family history of aortic disease | 1.9 |
| 1 | Known aortic valve disease | 11.9 |
| 1 | Recent aortic manipulation | 2.8 |
| 1 | Known thoracic aortic aneurysm | 14.7 |
| 2 | Abrupt onset of pain Severe pain intensity |
79.3 72.7 |
| 2 | Ripping or tearing pain | 21.7 |
| 3 | Pulse deficit or systolic blood pressure differential | 20.3 |
| 3 | Focal neurological deficit (in conjunction with pain) | 10.8 |
| 3 | Murmur of aortic insufficiency (new or in conjunction with pain) | 23.6 |
| 3 | Hypotension/shock | 16.0 |
MFS, Marfan’s syndrome.
* The aortic dissection detection (ADD) score aims to enhance early diagnosis of acute aortic dissection. The ADD score is calculated by determining the number of categories in which any of 12 high-risk clinical features are present in patients with symptoms suggestive of acute aortic dissection. For example, in a patient with a family history of aortic disease (category 1) and known thoracic aneurysm (also category 1), the ADD score would be 1. Likewise, the ADD score is 2 in a patient with Marfan’s syndrome (category 1) and a blood pressure differential (category 3). A retrospective analysis of the International Registry of Acute Aortic Dissection determined that among 2538 patients with acute aortic dissection, 95.7% had an ADD score ≥1. The ADD score may therefore provide the clinician with a simple and effective bedside method to inform further diagnostic testing and/or treatment in patients with suspected aortic dissection. Importantly, the negative predictive value for acute aortic dissection in patients with an ADD score of 0 has not yet been established.
From Rogers AM, Hermann LK, Booher AM, et al: Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection on initial presentation. Circulation 123:2213–2218, 2011.44
History
Diagnosis of aortic dissection may be missed on initial clinical evaluation in about a third of cases, and an equal number are detected only at autopsy.1,44 Chest pain is the dominant feature of the clinical presentation and occurs in over 90% of patients.2 It is qualitatively severe and may in many cases be distinguished from coronary ischemia by abrupt onset and maximal intensity at inception. More than 84% of aortic dissection patients described chest pain as “worst ever” in the IRAD registry.2,45 The pain is characterized as sharp more often than tearing or ripping in nature, and may radiate or be sensed anteriorly (suggestive of type A dissection) or in the interscapular, lower back, or abdominal area (suggestive of type B dissection). Visceral discomfort or limb pain may be indicative of aortic branch vessel ischemia from malperfusion.
Syncope is a particularly ominous presenting symptom and may reflect cardiac tamponade from intrapericardial aortic rupture, cerebral malperfusion, and/or neurally mediated hypotension in response to the intense pain of the dissection. In the IRAD registry, patients with syncope were more likely to die in the hospital or suffer a stroke. Neurological complications are noted in up to 20% of aortic dissection patients. For example, paraplegia may develop when critical impairment of flow to the anterior spinal artery, thoracic intercostals, or the artery of Adamkiewicz occurs. Abdominal pain is an underrecognized symptom of acute aortic dissection; when present, it is associated with elevated in-hospital mortality and increased frequency of malperfusion syndromes.2,43,44
Physical Examination
Patients with acute aortic dissection appear ill, uncomfortable, and apprehensive. Hypertension is present in more than two thirds of type B dissection patients and in approximately one third of type A patients.1,2 A murmur of aortic regurgitation can be heard in approximately 40% of patients with type A dissection.37 Due to rapid equilibration of aortic and LV diastolic pressure from acute aortic valve regurgitation, the murmur is usually of shorter duration, lower in pitch, and of lesser intensity than the diastolic murmur of chronic severe aortic regurgitation. Additional auscultatory findings include a soft first heart sound and a grade 1 or 2 midsystolic murmur at the base or along the left sternal border.
An inverse correlation between the presence of pulse deficits and mortality is observed in acute aortic dissection.46 Furthermore, pulse deficits may obscure accurate blood pressure assessment, as in pseudohypotension, which arises from an inability to measure central aortic pressure when bilateral subclavian and/or femoral artery compromise is present. Thus, invasive intraarterial monitoring may be necessary in aortic dissection patients.
Laboratory Testing
Biomarkers
Plasma smooth muscle myosin heavy chain protein, D-dimer, and high-sensitivity C-reactive protein (CRP) have been proposed as potentially useful biomarkers to assist with point-of-care diagnosis of aortic dissection. In one study of 95 patients with acute aortic dissection, elevated levels of circulating smooth muscle myosin heavy chain protein (>2.5 μg/L) had a sensitivity of 90% and a specificity of 98% compared with healthy controls when measured within 3 hours of presentation.47 In this analysis, smooth muscle myosin heavy chain protein levels were elevated in all patients presenting with a proximal or type A dissection.
Soluble elastin fragment (sELAF) levels have also been proposed to be a useful biomarker for the early detection of acute aortic dissection. Despite a natural rise with age in the concentration of sELAF levels detected in plasma, a level more than 3 standard deviations above normal for age is associated with a 64% positivity rate in acute aortic dissection, compared with 2% for patients with AMI. Interestingly, patients with complete false lumen thrombosis appear to have no detectable sELAF.48
Suzuki et al. conducted a multicenter study of 220 patients with suspected acute aortic dissection.49 A D-dimer level of less than 500 ng/mL when drawn within 24 hours of symptom onset was associated with a negative likelihood ratio (LR) for aortic dissection of 0.07. Consistent with these data, findings from one large meta-analysis of 734 patients demonstrated that an elevated D-dimer level had a 97% sensitivity and 96% negative predictive value for identifying acute aortic dissection. Conversely, an elevated D-dimer is less effective at “ruling-in” aortic dissection, with a specificity of 56% and positive predictive value of 60%.50 An early rapid increase in CRP levels following acute aortic dissection has been observed, with levels falling rapidly 24 hours following symptom onset.51 Although less well studied in aortic dissection, calponin, a counterpart protein to troponin in vascular SMCs, may provide enhanced specificity for early detection of type A aortic dissection, but requires comprehensive testing in advance of clinical application.52
Other Point-of-Care Tests
The chest x-ray is abnormal in 80% to 90% of patients with aortic dissection, but is an insufficient tool to rule out this condition, particularly when pathology is confined to the ascending aorta.53 Findings suggestive of aortic dissection include mediastinal widening, disparity in the caliber of the ascending and descending thoracic aortic segments, a localized bulge or angulation along the normally smooth border of the aorta, displacement of intimal calcium (especially in the region of the aortic knob), and a double density appearance. Associated findings may include cardiomegaly (pericardial effusion) and pleural effusion (left > right). Effusions that occupy more than 50% of the chest cavity may be indicative of rupture with hemothorax.
Nonspecific electrocardiographic (ECG) repolarization abnormalities are present in approximately 40% of dissection patients.37 Changes indicative of active ischemia may be found in 15% of patients, and findings suggestive of AMI (new Q waves, ST-segment elevation) are present in a small minority (3%) of cases.37 A thorough assessment is critical to avoid initiation of acute reperfusion therapy in this setting.
Diagnostic Imaging
Retrograde aortography, the original diagnostic gold standard for aortic dissection, has been almost completely replaced by transesophageal echocardiography (TEE) and computed tomographic angiography (CTA). Magnetic resonance imaging/angiography (MRI/MRA) is much less frequently performed in the acute setting. Sensitivity and specificity of these three noninvasive techniques are essentially equivalent and exceed 90% in most series.2 Choice of imaging technique depends chiefly on availability, speed, safety, and local expertise in performance and interpretation. A second test is frequently needed for clarification when the first study is abnormal but nondiagnostic. Regardless of the diagnostic sequence employed, an institutional commitment to rapid imaging of critically ill patients is critical. Essential features to be defined for both treatment and prognosis include presence or absence of ascending aortic involvement, entry and reentry sites, pericardial and aortic valve involvement, extent of the dissection, major branch vessel compromise, and the anatomical substrate for potential malperfusion syndrome(s).
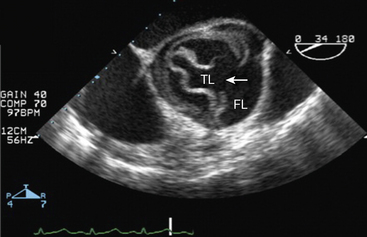
Transesophageal Echocardiography
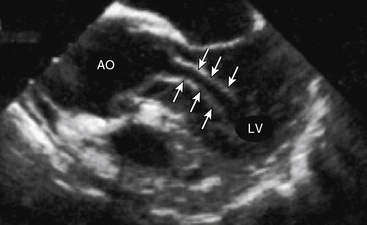
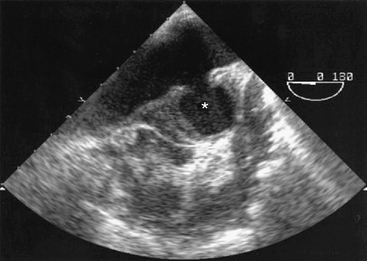
A surface transthoracic echocardiogram (TTE) alone is not sufficient for diagnosis and characterization of aortic dissection in most cases.54 However, when combined with TEE, the sensitivity and specificity of these tests reaches 99% and 89%, respectively54 (Fig. 34-6). Transthoracic echocardiogram should not delay performance of TEE, which can be accomplished at the bedside in the emergency department or in the operating room within 15 to 20 minutes. Oropharyngeal anesthesia and conscious sedation are required, with simultaneous monitoring of heart rate and rhythm, blood pressure, and oxygen saturation. Orthogonal and longitudinal scan planes combined with M-mode, two-dimensional (2D), and Doppler profile interrogation provide information regarding: (1) entry and reentry sites, (2) longitudinal extent and oscillation of the intimal flap, (3) flow velocity and direction within the true and false lumens, (4) spontaneous contrast or thrombus within the false lumen, (5) aortic valve competence and mechanism of regurgitation, (6) ostial coronary artery involvement, (7) pericardial effusion, and (8) global and regional LV function. In most cases, the true lumen is differentiated from the false lumen by observing systolic expansion and diastolic collapse, absence or minimal spontaneous echo contrast, and/or an antegrade Doppler signal. However, vessel diameter alone is not sufficient for making this determination. In ambiguous cases (e.g., with a large false lumen), a pressure gradient between true and false lumen between 10 and 25 mmHg may be observed by continuous wave Doppler interrogation.55,56
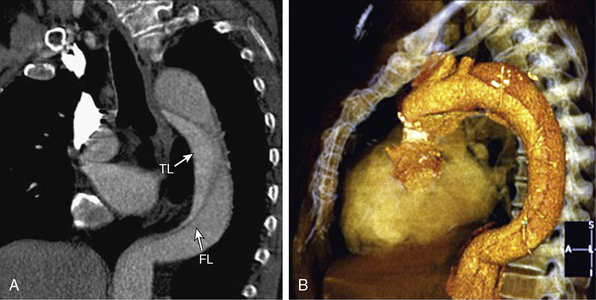
Computed Tomographic Angiography
Multislice CTA using rapid acquisition protocols and postprocessing of the volumetric data (multiplanar reformatting, maximum intensity projection (MIP), shaded surface display, volumetric rendering) provides highly detailed and visually familiar anatomical images (Fig. 34-7; also see Chapter 14). The diagnostic accuracy of 64-slice CTA approaches 100% for aortic dissection.57 The intimal flap appears as a thin, low attenuation, linear or spiral structure that separates the true and false lumens. Additional findings include displacement of intimal calcium, delayed contrast enhancement of the false lumen, and aortic widening. Branch vessel involvement anywhere along the course of the aorta to the level of the iliac arteries can be precisely displayed. In addition, CTA can visualize the proximal third of the coronary arteries. Limitations to CTA include exposure to intravenous contrast and ionizing radiation. In addition, CTA is an anatomical study; neither aortic valve nor LV function can be rapidly assessed. Motion artifact, mural thrombi, and image artifacts may negatively affect study accuracy.57 Dedicated emergency department scanners are now widespread, and studies can be obtained, reconstructed, and interpreted within 15 to 20 minutes. Computed tomographic angiography has several advantages relative to MRA, including wider availability, quicker throughput, higher spatial resolution, absence of arterial flow-related artifacts, and the capability to visualize calcification and metallic implants.
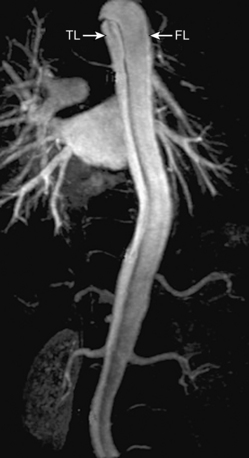
Magnetic Resonance Imaging/Angiography
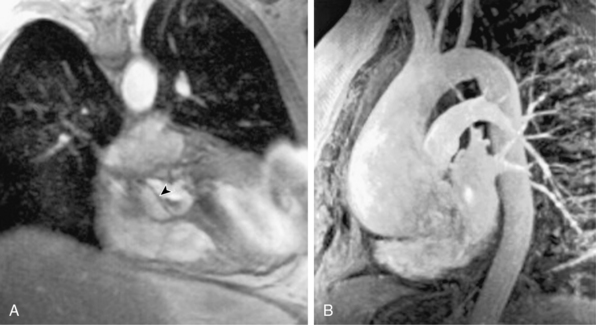
Contemporary MRI technology affords rapid scanning with the ability to cover a wide field of view and a comprehensive analysis of dissection anatomy and extraaortic involvement57,58 (Fig. 34-8; also see Chapter 13). Magnetic resonance imaging allows for assessment of pericardial involvement, aortic regurgitation, proximal coronary artery involvement, and LV function. The 0.5-tesla (T) magnet and modern gating software allow for expedited scanning across multiple levels during a single breath hold. Despite these advances, MRI is infrequently used as the initial imaging study in patients with suspected acute aortic syndromes. Reasons for its limited use in the acute setting include lack of widespread availability, difficulties with patient transport to and monitoring within MRI scanners, and presence of implanted cardiac devices or metallic clips. Nevertheless, MRI can provide excellent imaging of false lumen thrombus, intramural hematoma, and penetrating atherosclerotic ulcers.57,58
Invasive Aortography
The risk for catheter-related injury, length of time required to assemble necessary personnel in an emergency situation, use of contrast and ionizing radiation, low sensitivity (77%), and availability of highly accurate noninvasive imaging techniques have significantly decreased use of invasive aortography as an initial diagnostic test for acute aortic dissection.59 Aortography is particularly limited in diagnosing noncommunicating aortic dissections, intramural hematomas, and penetrating ulcers.2 Inadvertent injection into the false lumen or equal and rapid opacification of true and false lumens without obvious aortic dilation may make correct diagnosis of aortic dissection difficult. Invasive angiography is a feature of any catheter-based intervention.
Intravascular Ultrasound
Low-frequency (<20 MHz) intravascular ultrasound (IVUS) affords maximal signal penetration of the aortic wall and nearly 100% diagnostic accuracy for aortic dissection in a procedure that can be completed in less than 10 minutes.58,60 This method provides clear delineation of several key findings, including entry points, longitudinal and circumferential extent, luminal dimensions and contour, and thrombus if present. Intravascular ultrasound is infrequently used as a second imaging technique for diagnosis in patients for whom false-negative results on invasive aortography are suspected, and femoral access has been obtained. Intravascular ultrasound may also have a role during performance of endovascular procedures.
Coronary Angiography
Selective coronary angiography is neither indicated nor advisable in anticipation of emergency surgery for type A dissection.61 Operative mortality is generally not related to myocardial ischemia but rather to aortic rupture, so performance of angiography consumes valuable time before life-saving surgery. Systematic preoperative coronary angiography for hemodynamically stable chronic type A dissection patients is a subject of debate.54,62 Preoperative coronary angiography is reasonable in type A dissection patients who have a history of previous coronary artery bypass graft surgery, or in type B patients with unstable angina prior to planned aortic and/or coronary intervention. Identification of high-grade atherosclerotic disease of native coronary arteries and/or coronary artery bypass graft(s) affords determination of the optimal operation for patients requiring ascending aortic surgery. However, in these instances, the potential for incorporating additional surgical procedures beyond repairing the dissection should be evaluated on a case-by-case basis.
Differential Diagnosis
Other Acute Aortic Syndromes
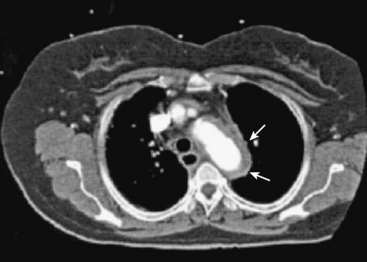
Aortic Intramural Hematoma
Intramural hematoma (IMH) is defined as a contained collection of blood within the wall of the aorta, without evidence of an intimal flap, entry tear, or double lumen (Fig. 34-9). Mechanisms to account for IMH include primary rupture of the nutrient vasa vasorum or a limited intimal tear that cannot be detected with imaging.62–64 Intramural hematoma is observed clinically in about 20% of cases of suspected acute aortic dissection and is discovered at autopsy in 5% to 13% of acute aortic syndrome cases.2,64 Approximately 10% of aortic IMHs undergo spontaneous resorption. Predicting evolution to dissection, rupture, aneurysm formation, or false aneurysm development is difficult. Type A IMH thickness greater than 11 mm is an independent risk factor for death, surgery, or progression to dissection.64,65 Likewise, an ascending aortic diameter greater than 4.8 cm is a high-risk feature.2,62–65 Aortic IMH is managed according to the same principles that pertain to aortic dissection, including surgery for type A disease, surveillance imaging, and intervention for downstream complications.
Penetrating Atherosclerotic Aortic Ulcer
An inflamed atherosclerotic plaque that disrupts normal aortic wall architecture may result in erosion of the internal elastic membrane, allowing luminal blood to burrow into the media of the aorta and beyond. Penetrating atherosclerotic aortic ulcers (PAUs) are most commonly seen in the mid- to distal descending thoracic aorta in older persons with a heavy burden of atherosclerotic disease. They appear as irregular craters or outpouchings of contrast (Fig. 34-10) and may result in IMH formation or frank dissection. Ganaha et al. observed in a retrospective analysis of 65 symptomatic IMH patients with PAU that ulcer depth (>1.0 cm) and diameter (>2.0 cm) positively correlated with disease progression (i.e., IMH expansion, aortic rupture, propagation of dissection).66 Others suggest that PAU location in the proximal segment of the descending thoracic aorta, and refractory symptoms rather than presence of an ulcer per se is most worrisome.67 Medical management with vigilant clinical and radiological follow-up is advised for the initially uncomplicated descending thoracic PAU. Surgery or endovascular stent grafting when feasible can be undertaken for failed medical therapy, pseudoaneurysm, or rupture.
Initial Medical Treatment
Patients with acute aortic syndromes should be treated with intravenous medications to lower the arterial blood pressure as expeditiously as possible (Fig. 34-11). Since aortic wall strain is a function of LV contraction velocity (expressed mathematically as change in pressure divided by change in time [dP/dT]), β-adrenergic receptor antagonists, given to attenuate LV systolic contractile force and decrease heart rate, are first-line therapeutic agents (Table 34-2). In patients with a contraindication or intolerance to β-adrenergic receptor antagonists, a heart rate–slowing nondihydropyridine calcium channel blocker, such as diltiazem or verapamil, may be an effective substitute.
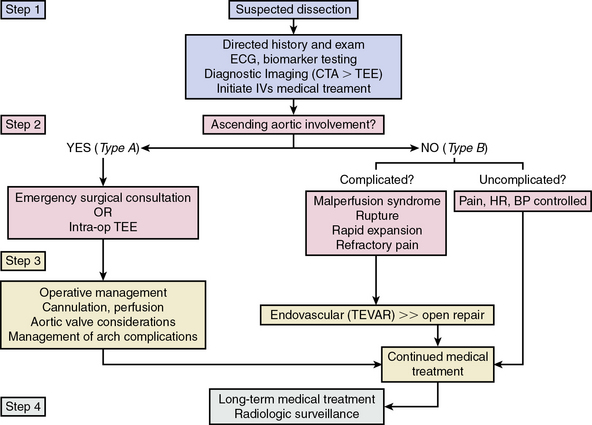
Figure 34-11 One proposed management pathway for acute aortic dissection.
(Adapted from Hiratzka LF, Bakris GL, Beckman JA, et al: 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol 55:e27–e129, 2010.)2
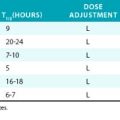
Table 34-2 Intravenous β-Adrenergic Receptor Antagonists for Management of Acute Aortic Dissection
| Therapy | Dose | Receptor Selectivity (Half-Life) |
|---|---|---|
| Metoprolol | 5 mg bolus every 5 min for 3 doses; additional doses of 5-10 mg every 4-6 h as needed | β1 > β2 (3-6 h) |
| Labetalol | 10-20 mg bolus, repeat 20-40 mg bolus every 10-15 min as needed Maintenance infusion 1-2 mg/min; maximum total dose of 300 mg |
α1-, β1-, and β2 (≈︀ 5.5 h) |
| Esmolol | 0.5 mg/kg bolus, then 50 μg/kg/min infusion | β1 (9 min) |
| Propranolol | 0.05-0.15 mg/kg every 4-6 h as needed | β1 ≈︀ β2 (5-7 h) |
Target systolic blood pressure and heart rate are 110 mmHg and 60 beats/min or less, respectively, but medications may require titration according to clinical evidence of impaired end-organ perfusion. β-Adrenergic receptor antagonists alone are often insufficient for achieving blood pressure control, so administration of a direct vasodilator may be necessary. Sodium nitroprusside is the agent of first choice in aortic dissection patients with hypertension refractory to initial β-blocker therapy, but should not be initiated without adequate heart rate control because reflex tachycardia unfavorably influences the dP/dT profile. The starting dose is 25 μg/min by continuous infusion, and adjustments are usually made in increments of 10 to 25 μg. At infusion rates above 2 μg/kg/min, the circulating concentration of the metabolite cyanide (CN−) exceeds the rate of excretion by the kidneys. After a total nitroprusside load of 500 μg/kg, endogenous molecular CN− buffers are depleted, increasing the probability of complications from drug toxicity, including death. In clinical practice, measuring levels of thiocyanate, a byproduct of CN− metabolism, is critical to prevent drug-induced CN− toxicity, particularly in patients with renal insufficiency. Alternative intravenous vasodilators available for use in the acute setting include enalaprilat, hydralazine, and nicardipine.68 Concomitant analgesia for pain control is essential and may favorably influence blood pressure and heart rate.
Indications for Surgery
Anatomical location of disease, patient comorbidities, initial complications from the dissection, and acuity of presentation (i.e., acute vs. chronic) are key factors that influence surgical indications for treatment of aortic dissection (Box 34-4). There is evolving evidence to support a relationship between clinical outcome and operator experience in repair of aortic disease. Increasing hospital volume for open abdominal aortic aneurysm repair is associated with improved survival, particularly at centers that perform over 50 abdominal aortic aneurysm repairs annually.69,70 Less well established are clinically useful parameters for evidence-based referral of patients with thoracic aortic disease. Ongoing public health initiatives have proposed examining the following variables to define centers of excellence for surgical repair of thoracic aortic disease: procedural volumes (operator and facility), outcome, time to diagnosis and intervention, and logistical measures including distance to nearest referral center and services available.2,71
![]() Box 34-4 Indications for Surgery
Box 34-4 Indications for Surgery
Adapted from Hiratzka LF, Bakris GL, Beckman JA, et al: 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol 55:e27, 2010.2
Type A Aortic Dissection
Recommendations pertaining to patient selection for surgical, endovascular, or medical treatment of acute aortic dissection are derived from consensus expert opinion because randomized trials are lacking.2 Emergency surgery is indicated for all acute type A dissections, regardless of the site of entry.2,72 Surgery is performed to prevent rupture with exsanguination or tamponade and to relieve aortic regurgitation when present. The extent and complexity of surgery (resection/grafting of the ascending aorta, valve resuspension or replacement, coronary artery reimplantation) is determined on a case-by-case basis. Incorporation of the aortic arch in the primary repair is indicated when the tear traverses this segment of the aorta or when it has become acutely aneurysmal.73 Indications for and timing of surgical repair for the unusual case of chronic stable type A aortic dissection are unresolved. In this situation, surgeon preference and patient comorbidities weigh heavily in decision making, as does any information related to aortic enlargement over time. Outcomes with conservative management may not be inferior to surgical repair in the chronic phase, as suggested by limited single center experiences and retrospective data.12,75 Endovascular stent grafts are not approved by the U.S. Food and Drug Administration (FDA) for use in the ascending aorta or arch.
Type B Aortic Dissection
Uncomplicated type B dissection is treated medically, with emphasis on tight heart rate and blood pressure control. Serial imaging is performed to monitor disease evolution. Lifestyle modifications, including the possibility of career change, may be necessary to avoid strenuous lifting, pushing, or straining that requires intense or repetitive Valsalva maneuvers.2
Surgery for acute type B dissection is generally reserved for those patients who have failed initial conservative therapy and have a complicated course, as indicated by refractory or recurrent pain, continued extension, early aneurysmal expansion, rupture, malperfusion syndrome, dissection location within a previously known aneurysmal aortic segment, and for patients with MFS. The importance of refractory pain in otherwise uncomplicated type B dissection is increasingly appreciated. In one recently published prospective analysis of 365 type B dissection patients without conventional high-risk features, the presence of pain or persistent hypertension despite medical therapy was associated with a 35-fold increase in mortality, compared with the absence of these clinical features.74
Presently there are insufficient data to provide comprehensive guidelines for appropriateness of endovascular stent grafting, percutaneous fenestration, and branch vessel stenting as alternatives to surgery for type B aortic dissection (Table 34-3; also see Chapter 36). Several nonrandomized small prospective trials and registries have shown that endovascular stent grafting for acute, subacute, or chronic type B dissection can be an effective lower-risk alternative to surgery.75–77 Recent years have seen increasing adoption of endovascular stent graft treatment for complicated type B dissection, although randomized trial data are lacking. Most high-volume centers have moved in this direction, and it is unlikely that a pivotal trial versus surgery will be conducted in patients with traditional indications for surgery in type B dissection.
Table 34-3 Society of Thoracic Surgeons Class I and II Recommendations for Thoracic Stent Graft Insertion
| Patient Subgroup | Classification | Level of Evidence |
|---|---|---|
| Acute traumatic dissection | I | C |
| Acute type B dissection with ischemia | I | A |
| Symptomatic PUA/AIH | IIa | C |
| Chronic dissection from trauma | IIa | B |
| Acute type B dissection without ischemia | IIb | C |
| Subacute dissection | IIb | B |
| Chronic dissection | IIb | B |
| Degenerative descending aortic dissection >5.5 cm | IIa with comorbidities IIb without comorbidities |
B/C |
| Aortic arch dissection with morbidity prohibitive for surgery | IIb | C |
AIH, aortic intramural hematoma; PUA, penetrating atherosclerotic aortic ulcer.
Adapted from Svensson LG, et al: Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 82:S1, 2008.
The Investigation of Stent Grafts in Aortic Dissection (INSTEAD) trial randomized 140 stable type B patients 2 weeks following dissection to optimal medical therapy or optimal medical therapy plus endovascular stent grafting.78 Although this trial was underpowered for the primary endpoint of aorta-related death at 2 years following randomization, a substantially greater number of patients who underwent stent grafting demonstrated recovery of true lumen size and contour and false lumen thrombosis (91%) compared with those who received optimal medical therapy alone (19%; P <0.001). These data are concordant with others suggesting positive aortic remodeling in type B dissection patients following endovascular stent graft placement. It is unclear whether positive aortic remodeling will impact clinical outcomes longer term. Endoleak, stroke, and other device complications including migration and thrombosis have been reported.76
Indications for percutaneous balloon fenestration include false lumen compression of the true lumen with end-organ hypoperfusion. In this procedure, a balloon catheter is used to create a transverse tear across the dissection flap to attenuate compressive forces on the true lumen and improve flow to compromised organs. Placement of a bare metal stent (BMS) into side branch vessels to restore blood flow may be performed to enhance regional perfusion.7
Surgery for chronic type A aortic dissection is indicated for treatment of symptomatic aortic regurgitation with LV dysfunction or for management of aneurysmal disease according to conventional size criteria (≥5.5 cm for ascending aortic aneurysm, ≥5.5-6.0 cm for descending thoracic aneurysm, ≥5.5 cm for thoracoabdominal aortic aneurysm, or ≥1.0 cm/yr increase in maximal dimension).2 Of note, in high-risk patients, such as those with MFS, elective aneurysm repair may be recommended at smaller aortic diameters. Aneurysmal enlargement and recurrent dissection are more likely with long-term patency or partial thrombosis of the false lumen.2 It has been proposed that partial thrombosis of the false lumen confers a worse outcome on patients with type B aortic dissection due to associated increases in pressure within the false lumen that may compromise true lumen-mediated blood flow to critical organs.15–17
Prognosis
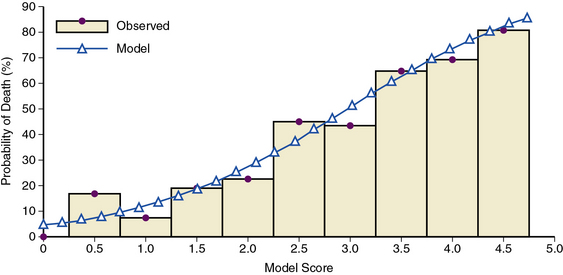
The European Cooperative study group reported 1- and 2-year mortality rates for patients with type A dissection of 60% and 50%, respectively.54 Approximately one third of aortic dissection survivors will experience rupture, extension, or require surgery for aneurysm formation within 5 years of recovery from the initial event.7 Outcome in acute type A dissection is heavily influenced by treatment strategy; in-hospital mortality rates following presentation are 65% and 6% with medical therapy and surgical repair, respectively.79 Nevertheless, surgical outcomes are poor in patients demonstrating signs of ischemia in renal, mesenteric, or peripheral arterial circulatory beds prior to dissection repair.7 A bedside risk prediction tool for in-hospital mortality incorporating these variables offers clinicians, patients, and families a useful method by which to understand the complexities and hazards of the acute dissection process72 (Fig. 34-12).
For type B dissection, overall in-hospital mortality rates approach 15%.37 For patients with uncomplicated type B dissection managed medically, 1-month survival is 90%, whereas for patients who require surgical intervention for the indications listed previously, 1-month survival is only 75%. Independent predictors of early mortality include advanced age, rupture, and malperfusion syndromes. The excess mortality risk imposed by early complications necessitating surgical treatment, and thus operation on acutely sicker patients, has prompted investigation of endovascular stent grafting for selected patients. Nearly 2 decades of experience with thoracic endovascular aortic repair have yielded encouraging results regarding short- and long-term efficacy rates for this treatment strategy. One retrospective analysis of 87 patients undergoing endovascular stent placement to treat acute type B dissection demonstrated a 30-day survival rate of 81%, despite the presence of hemodynamic instability or shock in 62% of the study population.80 In a type B dissection patient cohort for whom the prevalence of hemodynamic collapse was only 16%, endovascular graft placement was associated with short- and long-term survival rates of 90% and 87%, respectively.81
The most feared complications of type B aortic dissection are rupture, redissection, or development of malperfusion syndromes. Complete or partial false lumen patency or maximal descending thoracic aortic diameter of 4.0 cm or greater are risk factors for development of subsequent descending thoracic aortic aneurysms.78
Long-Term Surveillance
Because of the lifelong risk of subsequent aortic and cardiovascular complications, vigilant clinical and radiographic follow-up is mandatory for all hospital survivors. Medical management remains targeted to strict blood pressure (≤130/80 mmHg) and heart rate (≤60 beats/min) goals.2 Statin therapy is indicated for treatment of atherosclerosis. Strenuous exercise is discouraged, and patients need be educated regarding the chronic nature of this disease, self-awareness of dissection-associated symptoms, and the importance of medication adherence. Imaging of the entire aorta is recommended pre-discharge and at 1, 3, 6, and 12 months, then annually thereafter.82
1 Hagan P.G., Nienaber C.A., Isselbacher E.M., et al. The International Registry of Acute Aortic Dissection (IRAD). JAMA. 2000;283:897.
2 Hiratzka L.F., Bakris G.L., Beckman J.A., et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol. 2010;55:e27.
3 Olson C., Thelin S., Ståhle E., et al. Thoracic aortic aneurysm and dissection. Increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611.
4 Karthikesalingam A., Holt P.J., Hinchliffe R.J., et al. The diagnosis and management of aortic dissection. Vasc Endovascular Surg. 2010;44:165.
5 DeBakey M.E., Beall A.C.Jr, Cooley D.A., et al. Dissecting aneurysms of the aorta. Surg Clin North Am. 1966;46:1045.
6 Eggebrecht H., Baumgart D., Schmermund A., et al. Penetrating atherosclerotic ulcer of the aorta: treatment by endovascular stent-graft placement. Curr Opin Cardiol. 2003;18:431.
7 Nienaber C., Eagle K.A. Aortic dissection: new frontiers in diagnosis and management: part I: from etiology to diagnostic strategies. Circulation. 2003;108:628.
8 Hirst A.E.Jr, Johns V.J.Jr, Kime S.W.Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore). 1958;37:217–279.
9 Lansman S.L., McCullough J.N., Nguyen K.H., et al. Subtypes of acute aortic dissection. Ann Thorac Surg. 1999;67:1975.
10 Ando M., Okita Y., Tangusari O., et al. Surgery in three-channeled aortic dissection. A 31-patient review. Jpn J Thorac Cardiovasc Surg. 2000;48:339.
11 Richen D., Kotidis K., Neale M., et al. Rupture of the aorta following road traffic accidents in the United Kingdom 1992-199. The results of the co-operative crash injury study. Eur J Cardiothorac Surg. 2003;23:143.
12 Trimarchi S., Nienaber C.A., Rampoldi V., et al. Results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2006;114:I357.
13 Tsai T.T., Evangelista A., Nienaber C.A., et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357:349.
14 Li Z.Y., U-King-Im J., Tang T.Y., et al. Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm. J Vasc Surg. 2008;23:47.
15 Fillinger M.F., Racusin J., Baker R.K., et al. Anatomic characteristics of ruptured abdominal aortic aneurysm of conventional CT scans: implications for rupture risk. J Vasc Surg. 2004;39:1243.
16 Kazi M., Thyberg J., Religa P., et al. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283.
17 Vorp D.A., Lee P.C., Wang D.H., et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34:291.
18 Nataatmadja M., West M., West J., et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(Suppl 1):II329.
19 LeMaire S.A., Russell. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103.
20 Judge D.P., Dietz H.C. Marfan’s syndrome. Lancet. 2005;366:1965.
21 Keane M.G., Pyeritz R.E. Medical management of Marfan syndrome. Circulation. 2008;117:2802.
22 Januzzi J.L., Isselbacher E.M., Fattori R., et al. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol. 2004;43:665.
23 Gary T., Seinost G., Hafner F., et al. Cystic medial necrosis Erdheim Gsell as a rare reason for spontaneous rupture of the ascending aorta. Vasa. 2011;40:147.
24 Loeys B.L., Dietz H.C., Braverman A.C., et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476.
25 Dietz H.C., Cutting G.R., Pyeritz R.E., et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337.
26 Habashi J.P., Judge D.P., Holm T.M., et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117.
27 Brooke B.S., Habashi J.P., Judge D.P., et al. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med. 2008;358:2787.
28 Malfait F., Wenstrup R.J., De Paepe A. Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. 2010;12:597.
29 Ahimastos A.A., Dart A.M., Kingwell B.A. Angiotensin II blockade in Marfan’s syndrome. N Engl J Med. 2008;359:1732.
30 Smith L.B., Hadoke P.W., Dyer E., et al. Haploinsufficiency of the murine Col3a1 locus causes aortic dissection: a novel model of the vascular type of Ehlers-Danlos syndrome. Cardiovasc Res. 2011;90:182.
31 Guo D., Hasham S., Kuang S.Q., et al. Familial thoracic aortic aneurysms and dissections: genetic heterogeneity with a major locus mapping to 5q13-14. Circulation. 2001;103:2461.
32 Vaughan C.J., Casey M., He J., et al. Identification of a chromosome 11q23.2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation. 2001;103:2469.
33 Robinson P.N., Godfrey M. The molecular genetics of Marfan syndrome and related microfibrillopathies. J Med Genet. 2000;37:9.
34 Pereira L., Lee S.Y., Gayraud B., et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A. 1999;96:3819.
35 Wang Y., Zhang W., Zhang Y., et al. VKORC1 haplotypes are associated with arterial vascular diseases (stroke, coronary heart disease, and aortic disease). Circulation. 2006;113:1615.
36 Guo D-C, Pannu H., Tran-Fadulu V., et al. Mutations in smooth muscle α-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488.
37 Januzzi J.L., Sabatine M.S., Eagle K.A., et al. Iatrogenic aortic dissection. Am J Cardiol. 2002;89:623.
38 Ketenci B., Enc Y., Ozay B., et al. Perioperative type I aortic dissection during conventional coronary artery bypass surgery: risk factors and management. Heart Surg Forum. 2008;11:E231.
39 Braverman A.C. Acute aortic dissection. Circulation. 2010;122:184.
40 Manalo-Estrella P., Barker A.E. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol. 1967;83:336.
41 Hsue P.Y., Salinas C.L., Bolger A.F., et al. Acute aortic dissection related to crack cocaine. Circulation. 2002;105:1592.
42 Westover A.N., Nakonezny P.A. Aortic dissection in young adults who abuse amphetamines. Am Heart J. 2010;160:315.
43 von Kodolitsch Y., Schwartz A.G., Nienaber C.A. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160:2977.
44 Rogers A.M., Hermann L.K., Booher A.M., et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection on initial presentation. Circulation. 2011;123:2213–2218.
45 Tsai T.T., Trimarchi S., Nienaber C.A. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg. 2009;37:149.
46 Bossone E., Rampoldi V., Nienaber C.A., et al. Usefulness of pulse deficit to predict in-hospital complications and mortality in patients with acute type A aortic dissection. Am J Cardiol. 2002;89:851.
47 Suzuki T., Katoh H., Tsuchio Y., et al. Diagnostic implications of elevated levels of smooth-muscle myosin heavy-chain protein in acute aortic dissection. The smooth muscle myosin heavy chain study. Ann Intern Med. 2000;133:537.
48 Shinohara T., Suzuki K., Okada M., et al. Soluble elastin fragments in serum are elevated in acute aortic dissection. Atherioscler Thromb Vasc Biol. 2003;23:1839.
49 Suzuki T., Distante A., Zizza A., et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on biomarkers (IRAD-Bio) experience. Circulation. 2009;119:2702.
50 Shimony A., Fillon K.B., Mottillo S., et al. Meta-analysis of usefulness of D-dimer to diagnose acute aortic dissection. Am J Cardiol. 2011;107:1227–1234.
51 Schillinger M., Domanovits H., Bayegan K., et al. C-reactive protein and mortality in patients with acute aortic disease. Intensive Care Med. 2002;28:740.
52 Ranasinghe A.M., Bonser R.S. Biomarkers in acute aortic dissection and other aortic syndromes. J Am Coll Cardiol. 2010;56:1535.
53 von Kodolitsch Y., Nienaber C.A., Dieckmann C., et al. Chest radiography for the diagnosis of acute aortic syndrome. Am J Med. 2004;116:73.
54 Erbel R., Alfonso F., Boileau C., et al. Diagnosis and management of aortic dissection: recommendations of the task force on aortic dissection, European Society of Cardiology. Eur Heart J. 2001;22:1642.
55 Bossone E., Evangelista A., Isselbacher E., et al. Prognostic role of transesophageal echocardiography in acute type A aortic dissection. Am Heart J. 2007;253:1013.
56 Flachskampf F.A. Assessment of aortic dissection and hematoma. Semin Cardiothorac Vasc Anesth. 2006;10:83.
57 Macura K.J., Szarf G., Fishman E.K., et al. Role of computed tomography and magnetic resonance imaging in assessment of acute aortic syndromes. Semin Ultrasound CT MR. 2003;24:232.
58 Clough R.E., Schaeffter T., Taylor P.R. Magnetic resonance imaging for aortic dissection. Eur J Endovasc Surg. 2010;39:514.
59 Shiga T., Wajima Z., Apfel C.C., et al. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166:1350.
60 Hayashi H., Matsuoka Y., Sakamoto I., et al. Penetrating atherosclerotic ulcer of the aorta: imaging features and disease concept. Radiographics. 2000;20:995.
61 Motallebzadeh R., Batas D., Valencia O., et al. The role of coronary angiography in acute type A dissection. Eur J Cardiothorac Surg. 2004;25:231.
62 Motoyoshi N., Moizumi Y., Komatsu T., et al. Intramural hematoma and dissection involving ascending aorta: the clinical features and prognosis. Eur J Cardiothorac Surg. 2003;24:237.
63 Nienaber C.A., von Kodolitsch Y., Petersen B., et al. Intramural hemorrhage of the thoracic aorta. Diagnostic and therapeutic implications. Circulation. 1995;92:1465.
64 Song J.K., Kim H.S., Kang D.H., et al. Different clinical features of aortic intramural hematoma versus dissection involving the ascending aorta. J Am Coll Cardiol. 2001;47:1604.
65 Song J.K., Yim J.H., Ahn J.M., et al. Outcomes of patients with acute type a aortic intramural hematoma. Circulation. 2009;120:2046.
66 Ganaha F., Miller C., Sugimoto K., et al. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer. Circulation. 2002;106:342.
67 Singhai P., Lin Z. Penetrating atheromatous ulcer of ascending aorta: a case report and review of the literature. Heart Lung Circ. 2008;17:380.
68 Kim K.H., Moon I.S., Park J.S., et al. Nicardipine hydrochloride injectable phase IV open-label clinical trial: study on the anti-hypertensive effect and safety of nicardipine for acute aortic dissection. J Int Med Res. 2002;30:337.
69 The Leap Frog Group. Factsheet: evidence-based hospital referral. Available at: http://www.leapfroggroup.org/media/file/FactSheet_EBHR.pdf Accessed. April 23, 2009
70 Landon B.E., O’Malley J.A., Giles K., et al. Volume-outcome relationships and abdominal aortic aneurysm repair. Circulation. 2010;122:1290.
71 Luft H.S., Bunker J.P., Enthoven A.C. Should operations be regionalized? The empirical relation between surgical volume and mortality. Clin Orthop Relat Res. 2007;457:3.
72 Mehta R.H., Suzuki T., Hagan P.G., et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200.
73 Sun L., Qi R., Zhu J., et al. Total arch replacement combined with stented elephant trunk implantation: a new “standard” therapy for type A dissection involving repair of the aortic arch. Circulation. 2011;123:971.
74 Trimarchi S., Eagle K.A., Nienaber C.A., et al. Importance of refractory pain and hypertension in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2010;122:1283.
75 Nienaber C.A., Fattori R., Lud G., et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539.
76 Cambria R.P., Crawford R.S., Cho J.S., et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50:1255.
77 Dake M., Kato N., Mitchell R.S. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1524.
78 Nienaber C.A., Rousseau H., Eggebrecht H., et al. Randomized comparison of strategies for type B aortic dissection. The INSTEAD trial. Circulation. 2009;120:2519.
79 Yanagisawa S., Yuasa T., Suzuki N., et al. Comparison of medically versus surgically treated acute type A aortic dissection in patients <80 years old versus >80 years old. Am J Cardiol. 2011;108:453.
80 Jonker F.H., Verhagen H.J., Lin P.H., et al. Outcomes of endovascular repair of ruptured descending thoracic aortic aneurysms. Circulation. 2010;121:2718.
81 Steuer J., Eriksson M.O., Nyman R., et al. Early and long-term outcome after thoracic endovascular aortic repair (TEVAR) for acute complicated type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;41:318.
82 Yeh C.H., Chen M.C., Wu Y.C., et al. Risk factors for descending aortic aneurysm formation in medium-term follow-up of patients with type A aortic dissection. Chest. 2003;124:989.