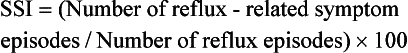Pathophysiology and investigation of gastro-oesophageal reflux disease
Introduction
Gastro-oesophageal reflux disease (GORD) describes symptoms or mucosal damage caused by reflux of gastric contents into the oesophagus.1 Symptoms are variable and along with damage are associated with many permutations of motility, endoscopic and physiological abnormalities. GORD is the major factor leading to the increasing incidence of oesophageal adenocarcinoma,2 and is one of the commonest health problems in the developed world.
Epidemiology
Gastro-oesophageal reflux is universal, and as such can be viewed as a normal physiological process. Such physiological episodes are asymptomatic and rapidly cleared. They occur mainly after meals, in the upright position and when awake.3,4 By contrast, pathological reflux results in chronic symptoms or mucosal damage. However, the subjectivity of symptoms is such that the boundary between the two is blurred, with many people viewing occasional reflux symptoms as normal, without seeking medical attention. For example, the reflux of air (belching) due to gastric distension is a universal experience, yet depending upon frequency and patient perception may be either normal or or a symptom of GORD. Unsurprisingly, the epidemiology of GORD is difficult to determine. A 2011 population-based cohort study of 45 000 people from Norway5 found the prevalence of any symptoms to be 41%, weekly symptoms to be 17% and severe symptoms to be 6.7% The figures had increased by 30%, 24% and 47%, respectively, over the previous decade. Incidence of any symptoms was 3%, with that of severe symptoms 0.2%, with spontaneous resolution of these symptoms occurring in 2% and 1%, respectively. A systematic review of 15 studies estimated a similar prevalence of weekly symptoms in 10–20% in the Western world (5% in Asia).6
These findings were similar to a smaller Finnish study of 1562 consecutive patients referred for endoscopy. With the caveat of this selection bias, the overall incidence was estimated as 3%, of which 2% had endoscopic mucosal damage.7 Two additional studies have reached similar conclusions, with 32–38% of those with symptoms having normal endoscopies.8,9 Importantly, the converse may be true, with up to 20% of those with oesophagitis or Barrett’s being asymptomatic.10
Traditionally, GORD has been viewed as a spectrum of a single disease, with endoscopy-negative symptoms at the mild end, increasing grades of oesophagitis representing progressively severe disease, culminating in Barrett’s metaplasia.1 However, this approach is undermined by a number of clinical, endoscopic and physiological findings, suggesting that the three may in fact be distinct groups and disease processes in themselves (Box 12.1).11,12 Most importantly, endoscopic progression from one end of the spectrum to the other is rare.12
Symptoms
Symptoms typically vary between the three GORD groups discussed above. Those with symptomatic endoscopy-negative disease (or non-erosive reflux) tend to have severe, often atypical symptoms with variable response to acid suppression (presumably representing oesophageal hypersensitivity to acid and non-acid reflux).12 By contrast, those with erosive reflux and oesophagitis tend towards more typical symptoms responding well to acid suppression.12 Finally, those with Barrett’s often have minimal symptoms, possibly due to the relative insensitivity of metaplastic epithelium to acid.13
Normal oesophageal physiology
On swallowing the upper and lower oesophageal sphincters relax, and the food or liquid bolus is propelled distally by peristalsis to the stomach. The responsible mechanisms involve complicated coordination between central and local neuromuscular mechanisms. Peristalsis is the sequential contraction of the oesophageal body. Animal models demonstrate progression aborally in both longitudinal and circular layers, without torque.14 Primary peristalsis is instigated centrally in the swallowing centre by swallowing, with the wave arising in the pharynx. A persistent bolus distends the oesophagus, triggering local neural mechanisms and a secondary peristaltic wave.15 Tertiary contractions are aberrant, synchronous contractions of oesophageal segments, which play no role in peristalsis. During peristalsis, the oesophageal body contracts segmentally, with the greatest pressure at the mid-point of the contracted segment, a few centimetres behind the bolus16 (Fig. 12.1).
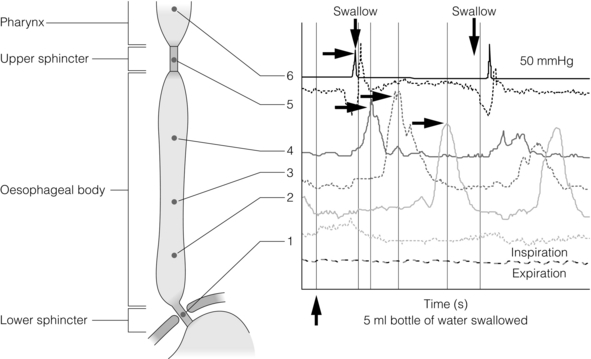
Figure 12.1 A standard six-sensor manometry trace of normal oesophageal peristalsis. Adapted from Anggiansah A, Marshal R. Use of the oesophageal laboratory, 1st edn. Oxford: Isis Medical Media, 2000. With permission from Isis Medical Media.
Whether the longitudinal and circular layers contract synchronously or not is disputed. A number of studies have suggested that the circular layer initially hyperpolarises, to contract two seconds after the longitudinal layer.17–19 However, this has been disputed, and it has been suggested that any delay is a function of the manometric techniques used.20 In any case, the involvement of both layers confers mechanical advantages, with longitudinal contractions shortening the circular layer, allowing greater contractile force. In addition, contraction of both reduces wall tension at the site of contraction.21
Primary peristalsis is initiated centrally (via the vagus) and modified peripherally (via local myogenic and neuronal mechanisms). Vagal efferents from the nucleus ambiguous initiate skeletal muscle contraction, and those from the dorsomotor nucleus innervate smooth muscle. These do so via intrinsic neurons of the myenteric plexus (between the muscle layers). Modification within the oesophageal body occurs in response to volume, temperature and acid receptors, with central feedback via vagal afferents. Warm boluses initiate an exaggerated peristaltic wave, whereas cold boluses (e.g. ice cream) may not provoke distal peristalsis.22 This is true for wet and dry swallows, respectively. In addition, oesophageal acid receptors are believed to allow protective clearance of refluxate. Crucial to successful peristalsis is the oesophageal latency period. Following initial stimulation of the circular muscle layers, a variable period of membrane hyperpolarisation occurs, primarily mediated by intrinsic nitric oxide inhibition.23 This period increases progressively, moving distally along the oesophageal body, with greater inhibitory inhibition.24 Initial (or deglutitive) inhibition describes a refractory period of the oesophageal body due to myogenic and inhibitory properties. Swallowing for a second time within seconds causes the first peristaltic wave to be aborted.25 This allows rapid sequences of swallows (used primarily for drinking).
Antireflux mechanisms
A positive pressure gradient of approximately 10 mmHg exists between the stomach and oesophagus. The stomach and abdominal oesophagus lie within 5 mmHg of positive intra-abdominal pressure, with the thoracic oesophagus exposed to 5 mmHg of negative pressure. That gastro-oesophageal reflux is the exception rather than the rule is due to several factors: the lower oesophageal sphincter (LOS), the diaphragmatic sphincter or ‘pinch-cock’, distal oesophageal compression, and other mechanical barriers such as the cardio-oesophageal angle (of His) and the mucosal rosette (Box 12.2).
Lower oesophageal sphincter
The LOS is the primary antireflux mechanism. Although not an anatomically discrete sphincter, it is a resting high-pressure zone in the distal oesophagus, which relaxes appropriately to allow swallowing, belching and vomiting.26 It is composed of specialised smooth muscle, arranged in either clasp or sling formation, running in the distal 1–4 cm of the oesophagus and blending with the cardia.27 The basal tone of the LOS is both intrinisic (inherent muscular properties) and extrinsic (excitatory cholinergic vagal input). Clasp and sling fibres vary in resting tone and responsiveness to stimulation; sling fibres have lower tone but greater responsiveness, probably due to differing proportions of contractile proteins. These fibres are also asymmetrically arranged, potentially accounting for the longitudinal and radial asymmetry of basal pressure (being greatest posteriorly and on the right).28 LOS muscle cells are tonic, and as such distinct from the phasic cells of the oesophageal body (which have no intrinsic tone).
Neurological control of the LOS is complicated. Central control is mediated by vagal efferents originating in the dorsal motor nucleus (with excitatory cell bodies cephalad and inhibitory cells caudally).29 Sensory feedback from the LOS is relayed via the tractus solitarius. Central neurotransmitters include glutamate, adrenaline, dopamine, acetylcholine and nitric oxide. Peripherally, vagal fibres synapse in the myenteric plexus via acetylcholine.30 Both atropine and vagotomy reduce resting LOS pressure significantly.31 Nitric oxide is the primary inhibitory neurotransmitter of the LOS: in murine models nitric oxide knockout increases resting LOS pressure and prevents the transient LOS relaxations (TLOSRs) required for swallowing32 (Fig. 12.2). This inhibition may be further modified by the interstitial cells of Cajal.33
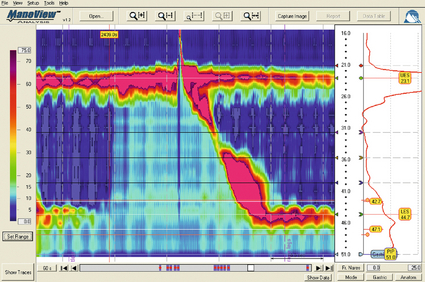
Figure 12.2 High-resolution manometry demonstrating transient lower oesophageal sphincter relaxation (TLOSR). The upper oesophageal sphincter is demonstrated by the upper red and yellow zone, and the LOS by the lower red and yellow zone. On relaxation of the lower oesophageal sphincter a common cavity is created between the stomach and oesophagus – demonstrated by the light blue zone on the spatiotemporal plot (centre). These events are also observed on the axial pressure plot (right). The event is terminated and oesophagus cleared by primary peristalsis with intra-oesophageal pressure returning to baseline levels. Images acquired by 36-channel SSI Manoscan 360. Reproduced from Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut 2008; 57(3):405–23. With permission from BMJ Publishing Group Ltd.
LOS resting pressure varies physiologically, in particular reducing with meals.34 It also reduces in response to pharyngeal tactile stimulation (in the absence of swallowing or peristalsis).35 In control subjects, transient increases in intra-abdominal pressure (e.g. induced by straight leg raising, coughing and straining) are met with reflex increase in LOS pressure that is both greater and faster than the increase in gastric pressure.36 This response is due in part to neuromuscular reflexes (vagally mediated) and transmitted pressure from the crus of the diaphragm. Similarly, protective increases in pressure occur in response to drops in intrathoracic pressure.37
TLOSRs are appropriate short-lived (usually less than 10 seconds) relaxations instigated by primary or secondary peristalsis, gastric distension and vomiting.38,39 In asymptomatic controls, TLOSRs account for 94% of reflux episodes,40 primarily occurring after meals and when upright rather than supine34,41 (correlating with the distribution of asymptomatic physiological reflux episodes discussed above). Fifty per cent of TLOSRs result in reflux episodes.40,42 Their genesis is incompletely understood, but seems to be a vagally mediated response to proximal gastric distension sensed by mechanoreceptors.41,43 Fundoplication reduces the frequency of TLOSRs, potentially via reducing cardiac distensibility.44,45 Manometrically, TLOSRs are similar to the belch reflex, and many reflux episodes follow belching,46,47 suggesting that the former are aberrations of the latter. Pathological and physiological (swallow-induced) TLOSRs appear to be distinct, with pathological lasting longer (up to 45 seconds).48
In a small controlled study of patients with oesophagitis, pathological reflux was found to be due to three primary mechanisms: TLOSRs (65%), spontaneous reflux due to low resting LOS pressure (18%) and transient increased intra-abdominal pressure (17%).40 However, the commonest mechanisms vary between individuals.
Diaphragmatic sphincter
The slings of the right crus constitute a ‘pinch-cock’ mechanism. In the absence of a hiatus hernia, it is difficult to separate the relative contributions of the diaphragmatic sphincter and LOS. In those with a surgically resected LOS, however, a basal high-pressure zone is detectable (oscillating in line with ventilation), suggesting that the diaphragmatic sphincter has a tonic component.50 Such increases in high-pressure zone pressure with inspiration are abolished with curare in feline models, so as with the LOS the diaphragmatic sphincter seems to have both basal and reactive tonicity.51 These increases in pressure are related to depth of inspiration49 and are maximal during sleep (when the gastro-oesophageal pressure gradient is greatest).52 Functionally, in the absence of a hiatus hernia, both LOS and diaphragmatic sphincter contribute to the high-pressure zone.53 However, the mechanism of diaphragmatic sphincter relaxation differs neurochemically and functionally. Oesophageal distension (due to swallowing) triggers incomplete diaphragmatic sphincter relaxation, whereas TLOSRs cause complete relaxation.54 As with the LOS, this relaxation is both central and local. Central control is mediated via vagal afferents modifying control of the diaphragm,55 although this may be distal to the medulla in site.56 Local control may be due to stretching of diaphragmatic sphincter fibres by contraction of longitudinal oesophageal fibres.57 Variation in the distensibility of the diaphragmatic sphincter affects reflux; greater distensibility predisposes to more reflux episodes.58
Distal oesophageal compression
The abdominal oesophagus is exposed to positive pressure within the abdomen. This has a compressive effect on the distal oesophagus/LOS, with a shorter length correlating with more frequent reflux.59 This is particularly important with increasing age and a recumbent position. The phreno-oesophageal ligament is a prolongation of abdominal fascia originating from the abdominal surface of the diaphragm, which anchors the oesophagus. As it approaches the oesophagus, it decussates into upper and lower leaves. The former inserts just above the squamocolumnar junction, with the latter inserting a few centimetres below, but is less well defined and often absent. The upper leaf inserts into the submucosa and intramuscular septae. This fibromuscular anchor is not absolute, allowing the oesophagus to slide 2 cm cranially when swallowing and, crucially, up to 4 cm during TLOSRs.60,61 Whether the oesophagus herniates into the thorax under physiological conditions is unlikely, however, as the diaphragmatic hiatus moves almost as far cranially. This arrangement serves to keep the distal oesophagus within this positive pressure environment, ensuring that any increases in intra-abdominal pressure are transmitted to the LOS.62 Anatomical variations in strength and height of insertion of the phreno-oesophageal ligament presumably influence the length of oesophagus to which this applies. Disruption of the ligament predisposes to sliding hiatus hernias, although the hernia sac will still envelop the LOS within the physiological (but not anatomical) abdomen.
Other mechanical barriers
The acute angle of the gastro-oesophageal junction may represent a partial barrier to reflux, functioning as a ‘flap valve’ (the cardio-oesophageal angle of His). This angle disappears after death, suggesting that the angle is maintained by tonic contraction of the oblique sling fibres of the cardia.63 This ‘valve’ lies below the physiological high-pressure zone determined with radiological contrast, and so its contribution is therefore limited. The mucosal rosette of the gastro-oesophageal junction is also reported to act as a partial barrier.
Oesophageal mucosal acid defence mechanisms
The main defence mechanisms comprise oesophageal clearance and tissue resistance. Reflex peristalsis is induced by oesophageal acid receptors and aided by gravity, both of which are impaired, particularly during sleep.64
The oesophagus has a number of further protective mechanisms, both intrinsic and extrinsic, termed tissue resistance. These are such that continuous in vivo exposure of oesophageal epithelium to hydrochloric acid and pepsin does not cause damage for 1 hour.65 Mechanisms are classed as pre-epithelial, epithelial and post-epithelial. Pre-epithelial resistance is conferred by the oesophageal buffer layer, augmented by saliva. In contrast to the gastroduodenal buffer layer, the (non-Barrett’s) oesophageal epithelium lacks a mucous gel barrier and the ability to secrete bicarbonate. However, the buffer needs only to raise pH above 3 to prevent pepsin-induced damage.66
This buffer is augmented by the neutralising properties of saliva. Swallowing saliva contributes to approximately 5% of acid clearance by neutralising acid remaining after peristaltic clearance.67 It has a pH of 7.02,68 but the solubility of its mucins means that it cannot contribute permanently to the oesophageal buffer.69 Consequently, when salivary production and primary oesophageal peristalsis cease at night, the oesophagus is particularly vulnerable.70
The epithelium itself possesses a combination of properties: a protective transmural electrochemical gradient, tight cell junctions, pH-dependent cation channels and intracellular buffers. Post-epithelial characteristics include adaptive perfusion and epithelial repair. For refluxed acid to cause epithelial cell death, hydrogen ions must enter the epithelial cytosol in sufficient quantities and for long enough to disrupt cytosol homeostasis and induce apoptosis/necrosis. One mechanism is transit of acid into the intercellular space, disrupting cell junctions and increasing permeability into the space, resulting in greater levels of acid. However, acid levels below that required to cause cellular damage may still be symptomatic. Nociceptors underlying heartburn can be found within the intercellular space within three cell layers of the oesophageal lumen,71 thus helping to explain the existence of heartburn without frank oesophagitis. Quite why pain and oesophagitis may be independent is not clear. Potentially this represents visceral hypersensitivity,72 or a reduction in acid sensitivity due to chronic reflux acid.73
Risk factors for reflux
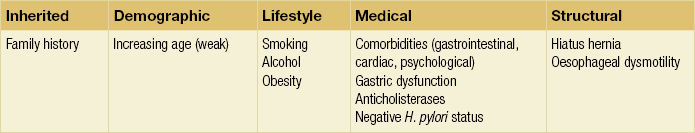
The complexity of the body’s control of swallowing and reflux means that there are numerous opportunities for disruption (and therefore pharmacological and surgical intervention). Pathological reflux is caused by excessive frequency of TLOSRs, inadequate high-pressure zone resting pressure, and inability of the LOS and diaphragmatic sphincter to compensate for increases in abdominal pressure. A number of factors have been implicated in these mechanisms (Table 12.1).
Inherited factors
A small number of studies have demonstrated the role of a positive family history. A parental history of GORD conferred an odds ratio (OR) of 1.5 in one study,74 with an OR of 2.6 when symptoms are extended to that of an immediate relative.75 In the latter study, no association was found with GORD prevalence between spouses, implying genetic effects to be greater than shared environmental effects (although this has been disputed).76 Greater concordance has also been demonstrated between monozygotic than dizygotic twins.74
Demographic factors
No association between sex and GORD has been demonstrated. Findings assessing age have been equivocal; both European and US studies assessing symptomatology have found contradictory results. Overall, a marginally increased risk with increasing age seems likely (OR 1.1)77 up to the age of 55, and possibly beyond.78
Lifestyle factors
Longitudinal studies show that smoking increases the risk of GORD (OR 1.1–2.6)77,78 due to a chronic reduction in LOS pressure, combined with acute provocation of reflux (via coughing/deep inspiration). Particular foods and drinks are often felt to predispose to reflux episodes, although a Swedish case–control study of over 1000 subjects found no association between an extensive range of foodstuffs (including chocolate, coffee, onions and acidic fruits) and chronic symptoms of reflux (although the authors admit this may be due to avoidance in sufferers).79 The study also found no correlation between portion size and eating late in the evening (albeit with the same caveat). Coffee is consistently cited as a factor, although one not demonstrated by additional studies.74 Any underlying mechanism is believed to be due to caffeine-mediated inhibition of phosphodiesterase, inducing LOS relaxation.80 Alcohol is generally agreed to lead to reflux – the US study above78 found an OR of 1.8 between alcohol consumption and GORD. Again, this is thought to be via a reduction in LOS pressure, combined with direct irritation.80
Obesity has been repeatedy shown to correlate with GORD, with ORs between 1.378 and 2.8.77 A meta-analysis of nine studies found an OR of 1.4 for a body mass index (BMI) of 25–30, and 1.9 for a BMI greater than 30.81 Multiple mechanisms have been proposed: impaired LOS pressure, increased intra-abdominal pressure and delayed gastric emptying.81 A combination of GORD and obesity is a potent risk factor for oesophageal adenocarcinoma.82 The effect of weight loss on symptoms varies between studies, having been shown to improve symptoms83 or have no effect.84 Pregnancy induces progesterone-mediated LOS relaxation, in addition to increasing abdominal pressure.
Medical factors
GORD has been associated with a number of gastrointestinal and extragastrointestinal conditions, including irritable bowel syndrome, peptic ulceration, angina,78 psychosomatic symptoms, anxiety and depression.75,85 Anticholinergic medications increase risk (OR 1.5),74 but non-steroidal anti- inflammatory drugs do not.
Gastric function can influence GORD via a number of mechanisms. Overall, Helicobacter pylori seems to protect against GORD and its complications, via atrophic gastritis.86 A Japanese study found H. pylori to be present in 71% of controls, 30% of those with oesophagitis and almost 0% of those with Barrett’s.87 However, this relationship is not simple, and is dependent upon site of infection, extent and strain type. Indeed, antral infection induces acid hypersecretion and therefore may increase GORD. Helicobacter pylori eradication might therefore worsen GORD rates – however, associations with gastric carcinoma and ulceration are strong, and in practice eradication does not hamper treatment of oesophagitis with proton-pump inhibitors.88 The hypersecretion of acid seen in Zollinger–Ellison syndrome is unsurprisingly associated with higher rates of GORD and oesophagitis;89 however, supraphysiological levels of acid secretion in those with ‘normal’ GORD has not been demonstrated.90 Delayed gastric emptying seems a plausible factor in GORD, and a recent study reported a prevalence of 26% in those with GORD.91 The consequent distension hypothetically may induce more TLOSRs and acid secretion, although this is unproven.
Hiatus hernia
For the reasons outlined above, sliding hiatus hernia is a strong risk factor for GORD. A multicentric study found the endoscopic prevalence of hiatus hernia overall to be 5.8%, rising to 32% in those with oesophagitis. One radiological study of those with oesophagitis found a much higher rate of 90%.92 Two-thirds of those with a hiatus hernia have GORD, and oesophagitis is more common.93 The underlying mechanisms involve both an increase in duration and frequency of reflux episodes94 (Fig. 12.3).
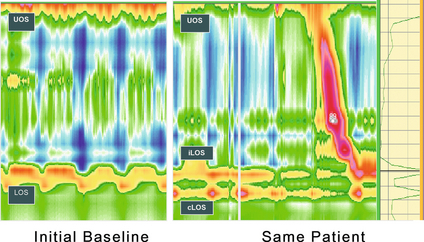
Figure 12.3 High-resolution manometry showing a single high-pressure zone in the lower oesophagus crossing the diaphragm on the left (LOS). In this spatiotemporal plot higher pressures are presented in the yellow–red spectrum and lower pressures in the green–blue spectrum. On the right there is separation of the lower oesophageal high-pressure zone (iLOS) and the high-pressure zone created by the diaphragmatic crura (cLOS) suggestive of a transient hiatus hernia. The longitudinal red and yellow zone on the right demonstrates the propagation of a peristaltic wave down the oesophagus. LOS, lower oesophageal sphincter; cLOS, crural LOS; iLOS, intrinsic LOS; UOS, upper oesophageal sphincter. Reproduced from Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut 2008; 57(3):405–23. With permission from BMJ Publishing Group Ltd.
Oesophageal dysmotility and GORD: cause or effect?
As discussed above, peristaltic acid clearance is an integral component of the body’s antireflux mechanism. Surrogate radiological and manometric simulation using barium fluoroscopy in one study demonstrated complete clearance with a single effective peristaltic wave greater than 20 mmHg.94 In 1968, Booth et al.95 described their standard acid clearance test, having demonstrated that those with GORD required more swallows to clear acid than controls. However, whilst subsequent studies have confirmed this, the utility of this test is very limited by both poor sensitivity and specificity96 for assessing GORD. The potential role of reduced or delayed acid clearance in GORD as a function of dysmotility is plausible and supported by a number of studies. Pathological reflux episodes last longer than physiological episodes, and this has been suggested to be due to impaired acid clearance. Corroborating evidence by DeMeester et al.97 expanded upon the different patterns of reflux seen between cases and controls. The former experienced longer nocturnal supine episodes, compared with the more physiological transient upright episodes in the latter. These nocturnal episodes are due to reduced peristaltic acid clearance,97 both in terms of reduced frequency and probably quality of peristaltic waves,98,99 compounded by a lack of gravitational clearance.100 Elevating the head of the bed compensates partially, and improves both acid clearance and microscopic oesophagitis.101
In one of the first such studies, Olsen and Schlegel102 assessed motility in 50 subjects with oesophagitis, finding normal activity in 28%, incoordinated peristalsis in 32%, hypotensive peristalsis in 37% and complete motor failure in 8%. Progressively worse motility was seen with worsening degrees of oesophagitis. In a separate study, 48% of those with severe oesophagitis displayed peristaltic dysfunction.103 A later study supported this, finding non-specific dysmotility or aperistalsis in 64% of those with benign peptic stricutres, compared to 32% of those with non-stricturing GORD.104
It is highly relevant, of course, that oesophageal sensitivity to acid is often reduced,1 and this combination of motor and sensory dysfunction may lead to particularly severe acid exposure, with resultant significant mucosal damage.105 Abnormal motility has been found in 46% of those with Barrett’s oesophagus, with a longer segment correlating with worse motility.106 These findings have recently been corroborated in a 1000-patient (with proven GORD) study by Diener et al.107 Peristalsis was normal in 56%, ineffective (hypotensive or incoordinated) in 21% and non-specifically abnormal in 23%. Those with ineffective peristalsis had worse symptoms, slower acid clearance and greater mucosal injury.
Whether this dysmotility with consequent delayed acid clearance is a primary phenomenon or occurs secondary to reflux-induced damage is unclear. Eriksen et al.,108 using the solid bolus oesophageal egg transit test, compared the transit times of those with GORD and controls. Whilst delayed transit times correlated with the frequency of prolonged reflux episodes, no correlation was demonstrated between symptoms and oesophagitis and motility. The study authors argued that this dysmotility may be a primary phenomenon. Contradictory evidence exists in the form of improvements in oesophageal motility seen after antireflux surgery.109 Other studies have found no such improvements, despite objective improvements in reflux, suggesting that either reflux-induced dysmotility may be permanent or it is a primary phenomenon.110,111 Perhaps both may be true, with reflux-induced dysmotility perpetuating a vicious cycle of impaired peristalsis and LOS function.
Certainly, molecular mechanisms exist for this. Inflammation has been shown to impair both oesophageal motility and LOS function. Proinflammatory cytokines such as interleukin (IL)-1B, IL-6 and IL-8 have been shown to inhibit in vitro motility.112 IL-1B induces prostaglandin E2 (PGE2)-mediated relaxation of the LOS,113 and IL-6 induces PGE2- and platelet activating factor-mediated relaxation of the LOS.114 Inflammation also increases nitric oxide and consequent inhibition of motility.115
Role of duodenogastric reflux
Whilst gastric acid (potentiated by pepsin) is the predominant source of inflammation and symptoms, the role of non-acidic duodenogastric reflux is not as clear cut. Observationally, oesophagitis is more common in those with both acid and duodenal reflux, compared with acid alone,116 and duodenal reflux is more frequently seen in those with peptic strictures and Barrett’s.117 Bile acids contribute to the sequence of oesophagitis, metaplasia and dysplasia, and with progressively severe disease (from endoscopy-negative reflux, to simple oesophagitis, to peptic stricturing, and on to Barrett’s metaplasia and finally dysplasia) so the relative proportion of bile acids in the refluxate increases.118 Despite this, abolition of acid seems adequate to prevent progression.119 Bile acids cause pain independently of acid,120 but the two are probably synergistic, with biliary reflux the lesser culprit.121,122 Duodenogastric reflux is difficult to assess, but can be detected using bilirubin as a surrogate (via spectrophotometry).123 This topic is being further assessed by the use of impedance techniques.
Investigation and diagnosis
Symptomatic diagnosis
Symptoms alone have limited utility. Whilst heartburn and regurgitation are typical symptoms and highly suggestive of GORD, their utility is limited by subjectivity and lack of evidence comparing them with investigations. One systematic review concluded that reflux symptoms had limited sensitivity (30–76%) and specificity (62–96%) in diagnosing oesophagitis.119 One meta-analysis reported response of chest pain to proton-pump inhibitors to be suggestive of GORD, but significantly less specific (74%) and sensitive (80%) than 24-hour pH studies.120 A number of standardised symptom questionnaires have been developed to aid diagnosis, but these are limited in terms of their diagnostic validity.121 They may have a greater role to play in assessing and standardising functional end-points of treatment (particularly in clinical trials), such as the Reflux Questionnaire (ReQuest).122
Endoscopy
Flexible endoscopy is typically first line for investigation of oesophageal symptoms. The current UK National Institute for Health and Clinical Excellence (NICE) guidelines endorsed by the British Society of Gastroenterology suggest urgent endoscopy for patients of any age with dyspeptic symptoms plus any of: chronic gastrointestinal bleeding, progressive unintentional weight loss, progressive dysphagia, persistent vomiting, iron deficiency anaemia, epigastric mass or suspicious contrast swallow radiology. In those aged 55 years or more, unexplained and persistent dyspepsia alone mandates endoscopy.121 In reality, endoscopy is used much more liberally. It allows diagnosis via inspection of the mucosa, and biopsy for histology, as well as various therapeutic interventions. Clues towards underlying dysmotility may be apparent, such as a dilated oesophagus, the presence of food residue and diverticula. Oesophagitis and Barrett’s mucosa indicate reflux, but a subset of those with GORD will have normal endoscopies. Consequently, whilst the sensitivity and specificity of diagnosing mucosal lesions is high (and assessment of oesophagitis is reliable between observers),122 those for diagnosing GORD are low relative to 24-hour pH monitoring. The roles of newer techniques (such as high-resolution, narrow-band and chromendoscopy) have yet to be determined.
Contrast radiology
Contrast radiology, whilst lacking the ability to biopsy and perform therapuetic procedures, has an important complementary role to endoscopy. A double-contrast barium swallow is highly sensitive at diagnosing significant complications of GORD (mucosal abnormalities such as strictures or malignancy).123 It will also demonstrate associated anatomical abnormalities such as hiatus hernias and diverticula, and may give useful information as to motility via the presence and quality of peristalsis. In particular it may demonstrate the classical appearances of achalasia or diffuse oesophageal spasm (‘corkscrew oesophagus’). However, the diagnostic ability of contrast radiology for GORD is limited; radiological features of finely nodular or granular mucosa may be visualised, or frank reflux may occur during the study. One review found radiological evidence of reflux in just 35% of symptomatic patients.124 One study (n = 112) compared barium swallow to 24-hour pH monitoring, concluding that just 30% of those with pH monitoring-diagnosed GORD had radiological abnormalities.125 This poor sensitivity may be somewhat improved by provocative manoeuvres (such as the water siphon test).
pH studies
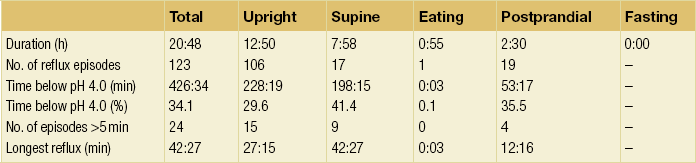
Since the original description in 1969 by Spencer, advances in nasogastric pH catheters, portable digital recorders and computer software have allowed 24-hour ambulatory pH studies to become widely available. Of great importance is the ability of the patient to mark symptoms by pressing a button, allowing correlation of symptoms with reflux episodes (Fig. 12.4). Computer analysis of the resultant data generates multiple variables, which are then compared to those of controls (Table 12.1). Indications for 24-hour pH monitoring include refractory typical symptoms, assessment of atypical symptoms and suspected motility disorders (as pH studies are usually combined with manometry), and as such are a prerequesite when considering anti-reflux surgery.124
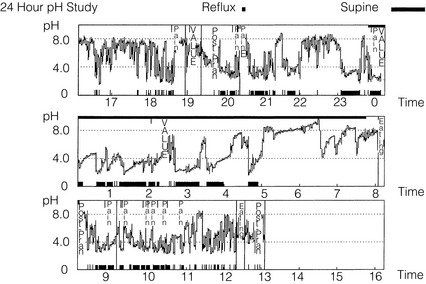
Figure 12.4 A 24-hour pH study showing excessive acid reflux in a patient with Barrett’s oesophagus. Note the correlation of the patient’s pain with acid in the oesophagus.
Twenty-four-hour studies are considered the gold standard, although there is some evidence that shorter studies may be representative and better tolerated.126 Antireflux medications are usually stopped to increase diagnostic yield,127 although performing the test whilst on treatment may be useful in assessing refractory symptoms. Patients should take a normal diet, but complete a food and drink diary to allow elimination of acidic foodstuffs from the analysis.
Parameters generated include the number of reflux episodes, those that are prolonged beyond 5 minutes and the total reflux time (expressed as a percentage of the study time). This should be less than 5% of a 24-hour period.128 These parameters can be subdivided into periods, such as when supine or upright. These may then be used to generate composite scores with reference to controls. The most commonly used is the DeMeester score. This combines the parameters in Tables 12.2 and 12.3 (number of episodes, number over 5 minutes and longest episode, with total reflux, upright and supine times (weighted towards supine)).129 A normal value is < 14.72.
Table 12.3
Scores according to Johnson and DeMeester for pH < 4 for findings in Table 12.1 (study of patient illustrated in Fig. 12.4)
Symptom Index (SI) is the proportion of symptoms relating to reflux:
An SI of 50% or more is usually considered to be positive, although with the caveats that it does not take account of the frequency or severity of symptoms. Consequently, in those with frequent reflux episodes or infrequent symptoms these may coincide with symptoms purely by chance.130 Despite this, a positive SI has been demonstrated to correlate with greatest improvement with proton-pump inhibitor acid suppression.131
The Symptom Sensitivity Index (SSI) takes into account the number of reflux episodes:
An SSI ≥ 10% is considered positive, and strengthening evidence of GORD.
The Symptom Association Probability (SAP) was introduced by Weuston and colleagues, and represents a more complex probability calculation. This aims to calculate the probability that the association of reflux and symptoms is not due to chance. This is performed by generating a 2 × 2 contingency table analysing each 2-minute segment of the study period. An SAP > 95% represents a P value < 0.05 and so is considered positive. Whilst the caveat of probability should not be forgotten, it has been shown to have (limited) success in predicting successful response to both proton-pump inhibitors132 and antireflux surgery.133
The Ghillebert Probability Estimate (GPE) was similarly developed to assess the probability of symptoms occurring by chance. It uses a binomial formula, inputting the number of symptom episodes, the number occurring within 2 minutes of demonstrated reflux, and the individual probability of an episode of pain coinciding with reflux.134
The concordance of SI, SAP and GPE was assessed in a recent study of 772 subjects. SAP and GPE were extremely concordant, with major discordance in 2.8%. GPE underestimated symptom association, whereas SAP overestimated. This discordance increased with greater frequency of symptoms and total reflux time. Similarly, SI became discordant with SAP when symptoms were less frequent than two or greater than 18. The authors concluded that SAP and GPE were interchangable.130 Taghavi et al.,132 using 50% reduction in heartburn with proton-pump inhibitor treatment as an end-point, calculated positive predictive vaules of SI (73%), SSI (81%) and SAP (79%). The imperfect diagnostic and predictive characteristics of these indices reinforce the need to interpret pH studies within the context of symptoms, endoscopic and radiological findings, manometry and any response to acid suppression. This is especially so when considering surgical intervention.
Wireless pH monitoring
A major drawback to catheter-based pH studies is the catheter; this may preclude testing in the 5–10% who are intolerant,73 or inhibit the ability to eat or function normally in the remainder (due to discomfort or social embarrassment), possibly causing a false-negative test. Wireless capsule-based systems have been designed to avoid this. The second major drawback is reproducibility; significant day-to-day variation occurs (up to the order of 3.2),136 with overall reproducibility of 70–80%.137
The Bravo® pH system (Medtronic, Minneapolis, MN, USA) is a wireless capsule system that may avoid both disadvantages (Fig. 12.5). It consists of a 26 mm × 6 mm capsule with an antimony electrode and radiofrequency transmitter, which is placed endoscopically 6 cm above the z-line (the high pressure zone being 1–1.5 cm above the squamocolumnar junction). Data are transmitted to a recorder attached to the patient’s belt. It is better tolerated than conventional catheter-based studies138 and allows prolonged measurement (up to 96 hours), which may be particularly useful in those with intermittent symptoms.139 One study found the Bravo® system to under-record acid exposure, although it concluded that this did not affect sensitivity provided it was allowed for.140 It has a particularly useful role in those intolerant of a catheter (and as such is recommended by NICE for this subgroup;141 the largest study to date successfully used the Bravo® system in 129 of 134 (96%) patients who failed a catheter system.142 The authors also reported less oropharyngeal discomfort, dysphagia and chest pain.
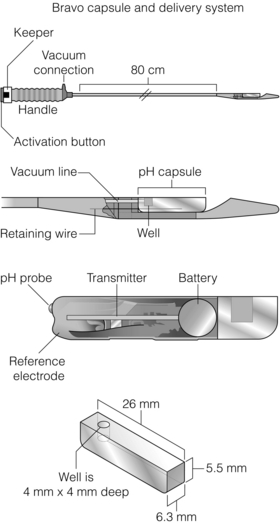
Figure 12.5 Bravo® delivery system and capsule. The delivery system is inserted in the same way as a nasogastric tube, although it is usually delivered orally. Markings on the side depict the distance from the incisors. The capsule is deployed 6 cm above the anatomical z-line (or 5 cm above the proximal LOS high-pressure zone). The delivery system is then retracted and the receiver is synchronised. It remains attached to the patient (via belt clip or shoulder pouch) for 48 hours at least. Capsules all fall off inevitably within 10 days (usually 5–7). Complications requiring early removal are almost unheard of. Reprinted by permission from Macmillan Publishers Ltd: Pandolfino JE, Richter JE, Ours T et al. Ambulatory oesophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98 (4):740–9, copyright 2003.
There are a number of disadvantages, however. Foremost is that of cost. A very small minority develop pain on implantation of the capsule, requiring removal. Furthermore, one small study reported that 25% of patients experienced significant hypercontractillity on placement of the capsule, leading to chest pain in six of 40.143
Oesophageal impedance monitoring
Multichannel intraluminal impedance (MII) was described by Silny in 1991144 and represents a novel direction in monitoring of refluxate. Impedance is the opposite of current flow, measured by detecting resistance to alternating current. This allows detection and differentiation of gas and liquid reflux (irrespective of pH). Paired electrodes are placed in the oesophageal lumen, separated by a non-conducting catheter (Fig. 12.6). The circuit is completed by ions within the oesophageal mucosa and lumen, the availability of which varies with luminal content. When empty, ionic conduction is relatively stable. The presence of liquid (either swallowed or refluxing), with its greater ionic concentration, improves conduction and therefore impedance drops. Conversely, the presence of air reduces conduction and increases impedance. Impedance for both returns to baseline when the refluxate has been cleared. Multiple electrodes along the catheter determine the direction of flow. Sensitivity is high, and boluses of just 1 mL can be detected.145
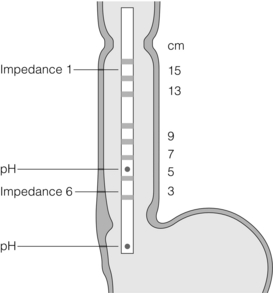
Figure 12.6 Example of impedance catheter. This one has six impedance sensors and two pH sensors. Reprinted by permission from Macmillan Publishers Ltd: Sifrim D, Blondeau K. Technology insight: the role of impedance testing for esophageal disorders. Nat Clin Pract Gastroenterol Hepatol 2006; 3(4):210–9, copyright 2006.
MII can therefore determine the following:
• whether the bolus is swallowed or refluxed;
• how long it takes to be swallowed or cleared;
• whether the bolus is liquid or gas;
• how proximally reflux extends;
• whether superimposed episodes of reflux occur (re-reflux).
Importantly, MII cannot determine the volume of refluxate.
This more comprehensive assessment allows a more sensitive approach to reflux, correlating symptoms beyond the somewhat arbitrary threshold of a pH of 4 for reflux.146 This is important for both gastroduodenal reflux and postprandial reflux (which may be non-acidic yet still symptomatic). It also allows consideration of the role of disturbances of bolus transport in oesophageal symptoms. It is reproducible147 and, consequently, MII–pH represents the most sensitive investigation for GORD, potentially increasing the detection of pathological reflux by a factor of four148,149 and improving symptom correlation by 10–20%.150 It has a particularly useful role in investigating the troublesome subgroup increasingly referred to surgeons: those with symptoms despite acid suppression. This group are often considered not to have GORD as determined by conventional pH studies, yet may be shown to have symptoms correlating with non-acidic or weakly acidic reflux.151 Consequently, a negative MII–pH study is a more discriminatory tool in the selection of patients who will benefit from antireflux surgery.148
Manometry
Standard static manometry
This measures a number of parameters. Multiple pressure sensors can be used to map and characterise peristalsis, and determine the resting and functional pressures of the oesophageal sphincters. Consequently, circumferential contraction, peristaltic wave duration and velocity can be determined. Normal peristaltic sequences (as previously discussed) consist of upper and lower oesophageal sphincter relaxation, followed by segmental contraction of adjacent oesophageal body segments, coordinated to give a smooth peristaltic wave. Contractions typically last for up to 6 seconds (in individual segments) and can reach pressures of 180 mmHg distally, with an overall velocity of 5 cm/s.151
Clearly, this limited assessment has a number of caveats. Firstly, measuring just 10 of the approximately 1000–2000 peristaltic swallows occurring per day means that intermittent or subtle dysmotility may be missed. Secondly, the experimental conditions are different to those of normal swallows; small-volume water swallows may not trigger symptoms occurring during normal activity. This is particularly true of GORD (with often postprandial symptoms triggered by physical activity or position). Thirdly, anatomical resolution is limited: focal anatomical or physiological abnormalities may lie between measurement ports and be missed. In addition, the presence of a hiatus hernia may distort the position of the LOS and affect its measurement. However, the availability and cost-effectiveness of standard manometry is such that it is the commonest investigation used in assessing motility.152 Although diagnosis may be limited to characteristic disorders such as achalasia and diffuse oesophageal spasm (with the rest characterised as inneffective or non-specific dysmotility), this may be adequate for the purposes of GORD assessment.
High-resolution manometry (HRM)
To circumvent some of these limitations, Clouse and Staiano developed HRM.153,154 A larger number of more closely spaced ports has become possible with the development of both micromanometric solid-state and perfused transducers. Up to 36 pressure sensors can be used to generate a three-dimensional representation, combining time, position and amplitude with greatly increased spatial resolution. This can be represented as a two-dimensional plot, with pressure represented with a colour spectrum (Fig. 12.7 and 12.8), and allows better characterisation of peristalsis.152 This has three specific advantages within the assessment of GORD: firstly, associated or causative dysmotility can be better defined; secondly, acid clearance can be better predicted; and thirdly the LOS can be better assessed (in both function and anatomy; Table 12.4).152 A sophisticated development is that of the ‘e-sleeve’. This is a software emulation of the sleeve device of standard pull-through manometry. This allows better characterisation of the LOS and also more rapid positioning of the catheter array. Furthermore, HRM can be used more easily to assess the dynamics of more frequent liquid swallows, and also solids, which may improve the sensitivity of GORD assessment (by being more physiologically representative and challenging).153–155 Manometry can be combined with MII to provide a valuable functional perspective.156
Table 12.4
Comparison between standard and high-resolution manometry
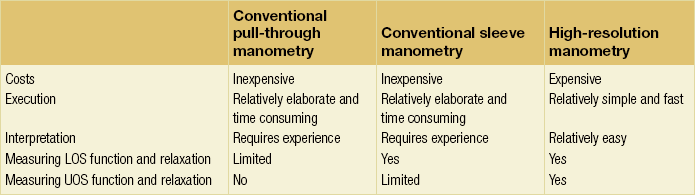
LOS, lower oesophageal sphincter; UOS, upper oesophageal sphincter.
Reproduced from Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut 2008; 57(3):405–23. With permission from BMJ Publishing Group Ltd.
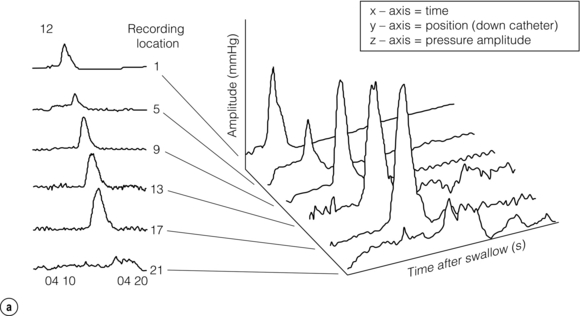
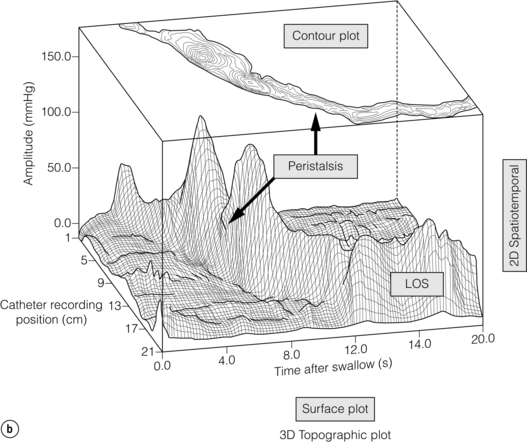
Figure 12.7 (a) Early 1990s (Clouse and Staiano), foundations of HRM laid: time, catheter position and average pressure were reconstructed into pseudo-3-D ‘topographic plots’ that demonstrated the functional anatomy of the oesophagus. (b) Topographic display of normal oesophageal pressure data. The pseudo-3-D surface plot displays the characteristic peaks and troughs of the peristaltic pressure wave proceeding from the proximal oesophagus (background), until it merges with the LOS after contraction (foreground). The contour plot of the same swallow superimposed at the top of the figure demonstrates how 3-D data are represented using concentric rings at 10-mmHg intervals to indicate increasing amplitudes. Reproduced from Clouse RE, Staiano A, Bickston SJ et al. Characteristics of the propagating pressure wave in the oesophagus. Dig Dis Sci 1996; 41(12):2369–76. Used with permission from the American Physiological Society.
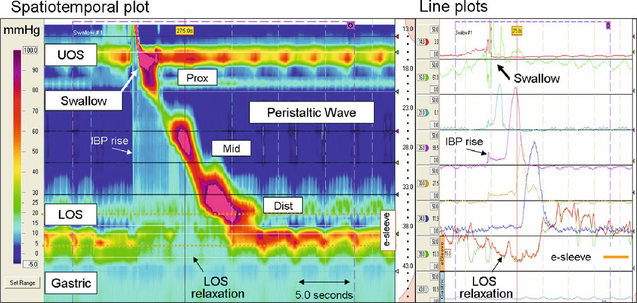
Figure 12.8 High-resolution manometry depicts oesophageal pressure activity from the pharynx to the stomach via pressure sensors spaced at < 2 cm intervals apart. Recordings can be analysed and presented either as line plots (similar to conventional manometry) or spatiotemporal plots. The spatiotemporal plot presents the same information as the line plots. Time is on the x-axis, distance from the nares is on the y-axis and pressure amplitude on the z-axis (each pressure is assigned a colour (legend left)). The segmental functional anatomy of the oesophagus is clearly demonstrated. The synchronous relaxation of the upper oesophageal sphincter (UOS) and LOS (deglutitive inhibition) is obvious, as is the increasing pressure and duration of the peristaltic wave as it passes distally. The virtual ‘e-sleeve’ application provides a summary measurement of LOS pressure and relaxation (bold brown line plot). Similar to a conventional sleeve sensor, the maximum pressure over a 6-cm distance is displayed. Images acquired by 36-channel SSI Manoscan 360. Reproduced from Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut 2008; 57(3):405–23. With permission from BMJ Publishing Group Ltd.
References
1. Fox, M., Forgacs, I., Gastro-oesophageal reflux disease. Br Med J. 2006;332(7533):88–93. 16410582
2. Lagergren, J., Bergstrom, R., Lindgren, A., et al, Symptomatic gastroesophageal reflux as a risk factor for oesophageal adenocarcinoma. N Engl J Med. 1999;340(11):825–831. 10080844
3. Johnson, L.F., DeMeester, T.R., Twenty-four-hour pH monitoring of the distal oesophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62(4):325–332. 4432845
4. Dent, J., Dodds, W.J., Friedman, R.H., et al, Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65(2):256–267. 7356677
5. Ness-Jensen, E., Lindam, A., Lagergren, J., et al, Changes in prevalence, incidence and spontaneous loss of gastro-oesophageal reflux symptoms: a prospective population-based cohort study, the HUNT study. Gut. 2012;61(10):1390–1397. 22190483
6. Dent, J., El-Serag, H.B., Wallander, M.A., et al, Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–717. 15831922
7. Voutilainen, M., Sipponen, P., Mecklin, J.P., et al, Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion. 2000;61(1):6–13. 10671769
8. Johansson, K.E., Ask, P., Boeryd, B., et al, Oesophagitis, signs of reflux, and gastric acid secretion in patients with symptoms of gastro-oesophageal reflux disease. Scand J Gastroenterol. 1986;21(7):837–847. 3775250
9. Fuchs, K.H., DeMeester, T.R., Albertucci, M., Specificity and sensitivity of objective diagnosis of gastroesophageal reflux disease. Surgery. 1987;102(4):575–580. 3660234
10. Sloan, S., Rademaker, A.W., Kahrilas, P.J., Determinants of gastroesophageal junction incompetence: hiatal hernia, lower oesophageal sphincter, or both. Ann Intern Med. 1992;117(12):977–982. 1443984
11. Fitzgerald, R.C., Onwuegbusi, B.A., Bajaj-Elliott, M., et al, Diversity in the oesophageal phenotypic response to gastro-oesophageal relux: immunological determinants. Gut. 2002;50(4):451–459. 11889061
12. Vela, M.F., Camacho-Lobato, L., Srinivasan, R., et al, Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120(7):1599–1606. 11375942
13. Rex, D.K., Cummings, O.W., Shaw, M., et al, Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125(6):1670–1677. 14724819
14. Dodds, W.J., Stewart, E.T., Hodges, D., et al, Movement of the feline esophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest. 1973;52(1):1–13. 4682383
15. Castell, D. Anatomy and physiology of the oesophagus and its sphincters. In: Castell D.O., Richter J.E., Dalton C.B., eds. Esophageal motility testing. New York: Elsevier Science; 1987:13–27.
16. Clouse, R.E., Staiano, A., Bickston, S.J., et al, Characteristics of the propagating pressure wave in the esophagus. Dig Dis Sci. 1996;41(12):2369–2376. 9011445
17. Sugarbaker, D.J., Rattan, S., Goyal, R.K., Mechanical and electrical activity of oesophageal smooth muscle during peristalsis. Am J Physiol. 1984;246(2, Pt 1):G145–G150. 6696111
18. Sugarbaker, D.J., Rattan, S., Goyal, R.K., Swallowing induces sequential activation of esophageal longitudinal smooth muscle. Am J Physiol. 1984;247(5, Pt 1):G515–G519. 6496741
19. Pouderoux, P., Lin, S., Kahrilas, P.J., Timing, propogation, coordination, and effect of esophageal shortening during peristalsis. Gastroenterology. 1997;112(4):1147–1154. 9097997
20. Bhall, V., Liu, J., Puckett, J.L., et al, Symptom hypersensitivity to acid infusion is associated with hypersensitivity of esophageal contracility. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G65–G71. 14977636
21. Pal, A., Brasseur, J.G., The mechanical advantage of local longitudinal shortening on peristaltic transport. J Biomech Eng. 2002;124(1):94–100. 11871611
22. Winship, D.H., Viegas de Andrade, S.R., Zboralske, F.F., Influence of bolus temperature on human oesophageal motor function. J Clin Invest. 1970;49(2):243–250. 5411782
23. Gidda, J.S., Goyal, R.K., Regional gradient of local inhibition and refractoriness in esophageal smooth muscle. Gastroenterology. 1985;89(4):843–851. 4029565
24. Crist, J., Gidda, J.S., Goyal, R.K., Intramural mechanism of esophageal peristalsis: roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci U S A. 1984;81(11):3595–3599. 6587375
25. Meyer, G.W., Gerhardt, D.C., Castell, D.O., Human esophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol 1981; 241:G129–G136. 7270690
26. Higgs, B., Shorter, R.G., Ellis, H. A study of the anatomy of the human esophagus with special reference to the gatroesophageal sphincter. J Surg Res. 1965; 5(11):503–507.
27. Liebermann-Meffert, D., What anatomic structures are undoubtedly responsible for gastroesophageal competence. Gastroenterology 1979; 76:31–39. 81791
28. Bemelman, W., Van Der Hulst, V., Dijkhuis, T., et al, The lower oesophageal sphincter shown by a computerized representation. Scand J Gastroenterol 1990; 25:601–608. 2359991
29. Rossiter, C.D., Norman, W.P., Jain, M., et al, Control of the lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol. 1990;259(6, Pt 1):G899–G906. 1701973
30. Gonella, J., Niel, J.P., Roman, C., Vagal control of lower oesophageal sphincter motility in the cat. J Physiol. 1977;273(3):647–664. 604452
31. Rattan, S., Goyal, R.K., Neural control of the lower oesophageal sphincter: influence of the vagus nerves. J Clin Invest. 1974;54(4):899–906. 4430720
32. Mashimo, H., He, X.D., Huang, P.L., et al, Neuronal constructive nitric oxide synthetase is involved in murine enteric inhibitory neurotransmission. J Clin Invest. 1996;98(1):3–13. 8690799
33. Ward, S.M., Morris, G., Reese, L., et al, Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115(2):314–329. 9679037
34. Dent, J., Dodds, W.J., Friedman, R.H., et al, Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65(2):256–267. 7356677
35. Dodds, W.J., Dent, J., Hogan, W.J., et al, Effect of atropine on esophageal motor function in humans. Am J Physiol. 1981;240(4):G290–G296. 6784581
36. Mittal, R.K., Fisher, M., McCallum, R.W., et al, Human lower esophageal sphincter pressure response to increased intra-abdominal pressure. Am J Physiol. 1990;258(4, Pt 1):G624–G630. 2333975
37. Mittal, R.K., Rochester, D.F., McCallum, R.W., Electrical and mechanical activity in the human lower esophageal sphincter during diaphragmatic contraction. J Clin Invest. 1988;81(4):1182–1189. 3350968
38. Dent, J., A new technique for continuous sphincter pressure measurement. Gastroenterology. 1976;71(2):263–267. 939387
39. Mittal, R.K., McCallum, R.W., Characteristics of transient lower oesophageal sphincter relaxation in humans. Am J Physiol. 1987;252(5, Pt 1):G636–G641. 3578522
40. Dodds, W.J., Dent, J., Hogan, W.J., et al, Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307(25):1547–1552. 7144836
41. Wyman, J.B., Dent, J., Heddle, R., et al, Control of belching by the lower oesophageal sphincter. Gut. 1990;31(6):639–646. 2379867
42. Dent, J., Holloway, R.H., Toouli, J., et al, Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastrooesophageal reflux. Gut. 1988;29(8):1020–1028. 3410327
43. Penagini, R., Carmagnola, S., Cantu, P., et al, Mechanoreceptors of the proximal stomach: role in triggering transient lower oesophageal sphincter relaxation. Gastroenterology. 2004;126(1):49–56. 14699486
44. Ireland, A.C., Holloway, R.H., Toouli, J., et al, Mechanisms underlying the antireflux action of fundoplication. Gut. 1993;34(3):303–308. 8472975
45. Scheffer, R.C., Akkermans, L.M., Bais, J.E., et al, Elicitation of transient lower oesophageal sphincter relaxations in response to gastric distension and meal ingestion. Neurogastroenterol Motil. 2002;14(6):647–655. 12464087
46. Barham, C.P., Gotley, D.C., Mills, A., et al, Precipitating causes of acid reflux episodes in ambulant patients with gastro-oesophageal reflux disease. Gut. 1995;36(4):505–510. 7737554
47. Barham, C.P., Gotley, D.C., Miller, R., et al, Pressure events surrounding oesophageal acid reflux episodes and acid clearance in ambulant healthy volunteers. Gut. 1993;34(4):444–449. 8491388
48. Holloway, R.H., Penagini, R., Ireland, A.C., Criteria for objective definition of transient lower esophageal sphincter relaxation. Am J Physiol. 1995;268(1, Pt 1):G128–G133. 7840195
49. Welch, R.W., Gray, J.E., Influence of respiration on recordings of lower oesophageal sphincter pressure in humans. Gastroenterology. 1982;83(3):590–594. 7095363
50. Klein, W.A., Parkman, H.P., Dempsey, D.T., et al, Sphincterlike thoracoabdominal high pressure zone after esophagogastrectomy. Gastroenterology. 1993;105(5):1362–1369. 8224640
51. Boyle, J.T., Altschuler, S.M., Nixon, T.E., et al, Role of the diaphragm in the genesis of lower esophageal sphincter pressure in the cat. Gastroenterology. 1985;88(3):723–730. 3967808
52. Mittal, R.K., Rochester, D.F., McCallum, R.W., Effect of the diaphragmatic contraction on lower oesophageal sphincter pressure in man. Gut. 1987;28(12):1564–1568. 3428682
53. Kaye, M., Showater, J., Manometric configuration of the lower oesophageal sphincter in normal human subjects. Gastroenterology 1971; 61:213–223. 5563404
54. Mittal, R.K., Holloway, R.H., Penagini, R., et al, Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109(2):601–610. 7615211
55. Altschuler, S.M., Boyle, J.T., Nixon, T.E., et al, Simultaneous reflex inhibition of lower esophageal sphincter and crural diaphragm in cats. Am J Physiol. 1985;249(5, Pt 1):G586–G591. 4061647
56. Oyer, L.M., Knuth, S.L., Ward, D.K., et al, Patterns of neural and muscular electrical activity in the costal and crural portions of the diaphragm. J Appl Physiol 1989; 66:2092–2100. 2745278
57. Liu, J., Puckett, J.L., Takeda, T., et al, Crural diaphragm inhibition during esophageal distension correlates with contraction of the esophageal longitudinal muscle in cats. Am J Physiol 2005; 288:G927–G932. 15626730
58. Pandolfino, J.E., Shi, G., Trueworthy, B., et al, Esophagogastric junction opening during relaxation distinguishes nonhernia reflux patients, hernia patients, and normal subjects. Gastroenterology. 2003;125(4):1018–1024. 14517784
59. Joelsson, B.E., DeMeester, T.R., Skinner, D.B., et al, The role of the oesophageal body in the antireflux mechanism. Surgery. 1982;92(2):417–424. 7101132
60. Edmundowicz, S.A., Clouse, R.E., Shortening of the esophagus in response to swallowing. Am J Physiol. 1991;260(3, Pt 1):G512–G516. 2003613
61. Shi, G., Pandolfino, J.E., Joehl, R.J., et al, Distinct patterns of oesophageal shortening during primary peristalsis, secondary peristalsis and transient lower oesophageal sphincter relaxation. Neurogastroenterol Motil. 2002;14(5):505–512. 12358678
62. De Caestecker, J., Heading, R. The pathophysiology of reflux. In: Hennessy T.P.J., Cuschieri A., Bennett J.R., eds. Reflux oesophagitis. London: Butterworth; 1989:1–36.
63. Atkinson, M., Summerling, M., The competence of the cardia after cardiomyotomy. Gastroenterologia 1959; 92:123–134. 13794972
64. Madsen, T., Wallin, L., Boesby, S., et al, Oesophageal peristalsis in normal subjects. Influence of pH and volume during imitated gastro-oesophageal reflux. Scand J Gastroenterol. 1983;18(4):513–518. 6669927
65. Salo, J.A., Lehto, V.P., Kivilaakso, E., Morphological alterations in experimental esophagitis. Light microscopic and scanning and transmission electron microscopic study. Dig Dis Sci. 1983;28(5):440–448. 6404615
66. Tobey, N.A., Hosseini, S.S., Caymaz-Bor, C., et al, The role of pepsin in acid injury to esophageal epithelium. Am J Gastroenterol. 2001;96(11):3062–3070. 11721751
67. Helm, J.F., Dodds, W.J., Pelc, L.R., et al, Effect of oesophageal emptying and saliva on clearance of acid from the oesophagus. N Engl J Med. 1984;310(5):284–288. 6690951
68. Helm, J.F., Dodds, W.J., Hogan, W.J., et al, Acid neutralizing capacity of human saliva. Gastroenterology. 1982;83(1, Pt 1):69–74. 7075945
69. Arul, G.S., Moorghen, M., Myerscough, N., et al, Mucin gene expression in Barrett’s oesophagus: an in situ hybridisation and immunohistochemical study. Gut. 2000;47(6):753–761. 11076872
70. Lichter, I., Muir, R.C., The pattern of swallowing during sleep. Electroenceph Clin Neurophysiol. 1975;38(4):427–432. 46823
71. Rodrigo, J., Hernández, C.J., Vidal, M.A., et al, Vegetative innervation of the esophagus. III. Intraepithelial endings. Acta Anat (Basel). 1975;92(2):242–258. 1155013
72. Mehta, A.J., De Caestecker, J.S., Camm, A.J., et al, Sensitization to painful distention and abnormal sensory perception in the esophagus. Gastroenterology. 1995;108(2):311–319. 7835571
73. Lee, J., Anggiansah, A., Anggiansah, R., et al, Effects of age on the gastroesophageal junction, oesophageal motility, and reflux disease. Clin Gastroenterol Hepatol. 2007;5(12):1392–1398. 17936081
74. Mohammed, I., Cherkas, L.F., Riley, S.A., et al, Genetic influences in gastro-oesophageal reflux disease: a twin study. Gut. 2003;52(8):1085–1089. 12865263
75. Locke, G.R., 3rd., Talley, N.J., Fett, S.L., et al, Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106(6):642–649. 10378622
76. Diaz-Rubio, M., Moreno-Elola-Olaso, C., Rey, E., et al, Symptoms of gastro-oesophageal reflux: prevalence, severity, duration and associated factors in a Spanish population. Aliment Pharmacol Ther. 2004;19(1):95–105. 14687171
77. Kotzan, J., Wade, W., Yu, H.H., et al, Assessing NSAID prescription use as a predisposing factor for gastroesophageal reflux disease in a Medicaid population. Pharm Res. 2001;18(9):1367–1372. 11683254
78. Ruigómez, A., García Rodríguez, L.A., Wallander, M.A., et al, Natural history of gastro-oesophageal reflux disease diagnosed in general practice. Aliment Pharmacol Ther. 2004;20(7):751–760. 15379835
79. Kahrilas, P.J., Gupta, R.R., Mechanisms of acid reflux associated with cigarette smoking. Gut. 1990;31(1):4–10. 2318431
80. Castell, D.O., Diet and the lower esophageal sphincter. Am J Clin Nutr 1975; 28:1296–1298. 1190108
81. Hampel, H., Abraham, N.S., El-Serag, H.B., Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143(3):199–211. 16061918
82. Whiteman, D.C., Sadeghi, S., Pandeya, N., et al, Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57(2):173–180. 17932103
83. Fraser-Moodie, C.A., Norton, B., Gornall, C., et al, Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol. 1999;34(4):337–340. 10365891
84. Kjellin, A., Ramel, S., Rossner, S., et al, Gastroesophageal reflux in obese patients is not reduced by weight reduction. Scand J Gastroenterol 1996; 31:1047–1051. 8938895
85. Hu, W.H., Wong, W.M., Lam, C.L., et al, Anxiety but not depression determines health care-seeking behaviour in Chinese patients with dyspepsia and irritable bowel syndrome: a population-based study. Aliment Pharmacol Ther. 2002;16(12):2081–2088. 12452941
86. Vicari, J.J., Peek, R.M., Falk, G.W., et al, The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115(1):50–57. 9649458
87. Abe, Y., Ohara, S., Koike, T., et al, The prevalence of Helicobacter pylori infection and the status of gastric acid secretion in patients with Barrett’s esophagus in Japan. Am J Gastroenterol. 2004;99(7):1213–1221. 15233656
88. Muramatsu, A., Azuma, T., Okajima, T., et al, Evaluation of treatment for gastro-oesophageal reflux disease with a proton pump inhibitor, and relationship between gastro-oesophageal reflux disease and Helicobacter pylori infection in Japan. Aliment Pharmacol Ther. 2004;20(Suppl. 1):102–106. 15298614
89. Miller, L.S., Vinayek, R., Frucht, H., et al, Reflux esophagitis in patients with Zollinger–Ellison syndrome. Gastroenterology. 1990;98(2):341–346. 1967239
90. Hirschowitz, B.I., A critical analysis, with appropriate controls, of gastric acid and pepsin secretion in clinical esophagitis. Gastroenterology. 1991;101(5):1149–1158. 1936784
91. Buckles, D.C., Sarosiek, I., McMillin, C., et al, Delayed gastric emptying in gastroesophageal reflux disease: reassessment with new methods and symptomatic correlations. Am J Med Sci 2004; 327:1–4. 14722388
92. Baldi, F., Ferrarini, F., Labate, A., et al. Prevalence of esophagitis in patients undergoing routine upper endoscopy: a multicenter survey in Italy. In: DeMeester T.R., Skinner D.B., eds. Esophageal disorders: pathophysiology and therapy. New York: Raven Press; 1985:213–219.
93. Savas, N., Dagli, U., Sahin, B., The effect of hiatal hernia on gastroesophageal reflux disease and influence on proximal and distal esophageal reflux. Dig Dis Sci. 2008;53(9):2380–2386. 18205046
94. Kahrilas, P.J., Dodds, W.J., Hogan, W.J., Effect of peristaltic dysfunction on oesophageal volume clearance. Gastroenterology. 1988;94(1):73–80. 3335301
95. Booth, D.J., Kemmerer, W.T., Skinner, D.B., Acid clearing from the distal oesophagus. Arch Surg. 1968;96(5):731–734. 5653000
96. Barham, C.P., Gotley, D.C., Mills, A., et al, Oesophageal acid clearance in patients with severe reflux oesophagitis. Br J Surg. 1995;82(3):333–337. 7796001
97. DeMeester, T.R., Johnson, L.F., Joseph, G.J., et al, Patterns of gastroesophageal reflux in health and disease. Ann Surg. 1976;184(4):459–470. 13747
98. Lichter, I., Muir, R.C., The pattern of swallowing during sleep. Electroenceph Clin Neurophysiol. 1975;38(4):427–432. 46823
99. Orr, W.C., Robinson, M.G., Johnson, L.F., Acid clearance during sleep in the pathogenesis of reflux esophagitis. Dig Dis Sci. 1981;26(5):423–427. 7249882
100. Kjellen, G., Tibbling, L., Influence of body position, dry and water swallows, smoking, and alcohol on oesophageal acid clearing. Scand J Gastroenterol. 1978;13(3):283–288. 39328
101. Johnson, L.F., DeMeester, T.R., Evaluation of elevation of the head of the bed, bethanechol, and antacid form tablets on gastroesophageal reflux. Dig Dis Sci. 1981;26(8):673–680. 7261830
102. Olsen, A.M., Schlegel, J.F., Motility disturbances caused by esophagitis. J Thorac Cardiovasc Surg. 1965;50(5):607–612. 5843967
103. Kahrilas, P.J., Dodds, W.J., Hogan, W.J., et al, Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology. 1986;91(4):897–904. 3743966
104. Ahtaridis, G., Snape, W.J., Jr., Cohen, S., Clinical and manometric findings in benign peptic strictures of the oesophagus. Dig Dis Sci. 1979;24(11):858–861. 520106
105. Jones, M.P., Sloan, S.S., Jovanovic, B., et al, Impaired egress rather than increased access: an important independent predictor of erosive oesophagitis. Neurogastroenterol Motil. 2002;14(6):625–631. 12464084
106. Shah, A.K., Wolfsen, H.C., Hemminger, L.L., et al, Changes in oesophageal motility after porfimer sodium photodynamic therapy for Barrett’s dysplasia and mucosal carcinoma. Dis Esophagus. 2006;19(5):335–339. 16984528
107. Diener, U., Patti, M.G., Molena, D., et al, Esophageal dysmotility and gastroesophageal reflux disease. J Gastrointest Surg. 2001;5(3):260–265. 11360049
108. Eriksen, C.A., Sadek, S.A., Cranford, C., et al, Reflux oesophagitis and oesophageal transit: evidence for a primary oesophageal motor disorder. Gut. 1988;29(4):448–452. 3371713
109. Escandell, A.O., De Haro, L.F.M., Paricio, P.P., et al, Surgery improves defective oesophageal peristalsis in patients with gastro-oesophageal reflux. Br J Surg 1991; 78:1095–1097. 1933194
110. Eckardt, V.F., Does healing of esophagitis improve oesophageal motor function. Dig Dis Sci. 1988;33(2):161–165. 3338364
111. Baldi, F., Ferrarini, F., Longanesi, A., et al, Oesophageal function before, during, and after healing of erosive oesophagitis. Gut. 1988;29(2):157–160. 3345925
112. Reider, F., Cheng, L., Harnett, K.M., et al, Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology 2007; 132:154–165. 17241868
113. Cheng, L., Cao, W., Behar, J., et al, Inflammation induced changes in arachidonic acid metabolism in cat LES circular muscle. Am J Physiol Gastrointest Liver Physiol 2005; 288:G787–G797. 15550558
114. Eastwood, G.L., Beck, B.D., Castell, D.O., et al, Beneficial effect of indomethacin on acid-induced esophagitis in cats. Dig Dis Sci. 1981;26(7):601–608. 7249895
115. Tomita, R., Tanjoh, K., Fujisaki, S., et al, Physiological studies on nitric oxide in the lower esophageal sphincter of patients with reflux esophagitis. Hepatogastroenterology. 2003;50(49):110–114. 12630004
116. Vaezi, M.F., Richter, J.E., Contribution of acid and duodenogastro-oesophageal reflux to oesophageal mucosal injury and symptoms in partial gastrectomy patients. Gut. 1997;41(3):297–302. 9378381
117. Stein, H.J., Barlow, A.P., DeMeester, T.R., et al, Complications of gastroesophageal reflux disease: role of the lower esophageal sphincter, esophageal acid/alkaline exposure and duodenogastric reflux. Ann Surg 1992; 216:35–43. 1632700
118. Nehra, D., Howell, P., Williams, C.P., et al, Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44(5):598–602. 10205192
119. Moayyedi, P., Talley, N.J., Fennerty, M.B., et al, Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295(13):1566–1576. 16595759
120. Cremonini, F., Wise, J., Moayyedi, P., et al, Diagnostic and therapeutic use of proton pump inhibitors in non-cardiac chest pain: a metaanalysis. Am J Gastroenterol. 2005;100(6):1226–1232. 15929749
121. NICEDyspepsia: Management of dyspepsia in adults in primary care. London, UK: National Institute for Health and Clinical Excellence, 2004.
122. Lundell, L.R., Dent, J., Bennett, J.R., et al, Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–180. 10403727
123. Levine, M.S., Chu, P., Furth, E.E., et al, Carcinoma of the esophagus and esophagogastric junction: sensitivity of radiographic diagnosis. Am J Roentgenol. 1997;168(6):1423–1426. 9168701
124. Ott, D.J., Gastroesophageal reflux: what is the role of barium studies? Am J Roentgenol. 1994;162(3):627–629. 8109510
125. Chen, M.Y., Ott, D.J., Sinclair, J.W., et al, Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic findings. Radiology. 1992;185(2):483–486. 1410359
126. Dobhan, R., Castell, D.O., Prolonged intraesophageal pH monitoring with 16-hr overnight recording. Comparison with “24-hr” analysis. Dig Dis Sci. 1992;37(6):857–864. 1587190
127. Kushnir, V.M., Sayuk, G.S., Gyawali, C.P., The effect of antisecretory therapy and study duration on ambulatory esophageal pH monitoring. Dig Dis Sci. 2011;56(5):1412–1419. 21046245
128. Bodger, K., Trudgill, N.Guidelines for oesophageal manometry and pH monitoring. BSG Guidelines in Gastroenterology, 2006. [p. 1–11].
129. Johnson, L.F., DeMeester, T.R., Twenty-four-hour pH monitoring of the distal oesophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62(4):325–332. 4432845
130. Kushnir, V.M., Sathyamurthy, A., Drapekin, J., et al, Assessment of concordance of symptom reflux association tests in ambulatory pH monitoring. Aliment Pharmacol Ther 2012; Mar 20. Epub ahead of print. 22428660
131. Watson, R.G., Tham, T.C., Johnston, B.T., et al, Double blind cross-over placebo controlled study of omeprazole in the treatment of patients with reflux symptoms and physiological levels of acid reflux – the “sensitive oesophagus”. Gut. 1997;40(5):587–590. 9203934
132. Taghavi, S.A., Ghasedi, M., Saberi-Firoozi, M., et al, Symptom association probability and symptom sensitivity index: preferable but still suboptimal predictors of response to high dose omeprazole. Gut. 2005;54(8):1067–1071. 15845561
133. Diaz, S., Aymerich, R., Clouse, R., et al. The symptom association probability (SAP) is superior to the symptom index (SI) for attributing symptoms to gastroesophageal reflux: validation using outcome from laparoscopic antireflux surgery (LARS). Gastroenterology. 2002; 122:A75.
134. Ghillebert, G., Janssens, J., Vantrappen, G., et al, Ambulatory 24 hour intraoesophageal pH and pressure recordings v provocation tests in the diagnosis of chest pain of oesophageal origin. Gut. 1990;31(7):738–744. 2370009
135. Gotley, D., Cooper, M. The investigation of gastrooesophageal reflux. Surg Res Commun. 1987; 2:1–17.
136. Wiener, G.J., Morgan, T.M., Copper, J.B., et al, Ambulatory 24-hour oesophageal pH monitoring. Reproducibility and variability of pH parameters. Dig Dis Sci. 1988;33(9):1127–1133. 3044715
137. Bredenoord, A.J., Weusten, B.L., Smout, A.J., Symptom association analysis in ambulatory gastrooesophageal reflux monitoring. Gut. 2005;54(12):1810–1817. 16284291
138. Pandolfino, J.E., Richter, J.E., Ours, T., et al, Ambulatory oesophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98(4):740–749. 12738450
139. Scarpulla, G., Camilleri, S., Galante, P., et al, The impact of prolonged pH measurements on the diagnosis of gastroesophageal reflux disease: 4-day wireless pH studies. Am J Gastroenterol. 2007;102(12):2642–2647. 17850412
140. des Varannes, S.B., Mion, F., Ducrotté, P., et al, Simultaneous recordings of oesophageal acid exposure with conventional pH monitoring and a wireless system (Bravo). Gut. 2005;54(12):1682–1686. 15843417
141. NICECatheterless Oesophageal pH Monitoring. National Institute for Health and Clinical Excellence, July 2006.
142. Sweis, R., Fox, M., Anggiansah, R., et al, Patient acceptance and clinical impact of Bravo monitoring in patients with previous failed catheter-based studies. Aliment Pharmacol Ther. 2009;29(6):669–676. 19183144
143. Tharavej, C., Hagen, J.A., Portale, G., et al, Bravo capsule induction of esophageal hypercontractility and chest pain. Surg Endosc. 2006;20(5):783–786. 16544080
144. Silny, J. Intraluminal multiple electric impedance procedure for measurement of gastrointestinal motility. J Gastrointest Motil. 1991; 3:151–162.
145. Srinivasan, R., Vela, M.F., Katz, P.O., et al, Esophageal function testing using multichannel intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G457–G462. 11171628
146. Sifrim, D., Castell, D., Dent, J., et al, Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53(7):1024–1031. 15194656
147. Bredenoord, A.J., Impedance–pH monitoring: new standard for measuring gastro-oesophageal reflux. Neurogastroenterol Motil. 2008;20(5):434–439. 18416700
148. Mainie, I., Tutuian, R., Agrawal, A., et al, Combined multichannel intraluminal impedance–pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg. 2006;93(12):1483–1487. 17051602
149. Shay, S., Richter, J., Direct comparison of impedance, manometry, and pH probe in detecting reflux before and after a meal. Dig Dis Sci. 2005;50(9):1584–1590. 16133955
150. Bredenoord, A.J., Weusten, B.L., Curvers, W.L., et al, Determinants of perception of heartburn and regurgitation. Gut. 2006;55(3):313–318. 16120760
151. Anggiansah, A., Marshal, R. Use of the oesophageal laboratory, 1st ed. Oxford: Isis Medical Media; 2000.
152. Fox, M., Hebbard, G., Janiak, P., et al, High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16(5):533–542. 15500509
153. Fox, M.R., Bredenoord, A.J., Oesophageal high- resolution manometry: moving from research into clinical practice. Gut. 2008;57(3):405–423. 17895358
154. Fox, M., Multiple rapid swallowing in idiopathic achalasia: from conventional to high resolution manometry. Neurogastroenterol Motil. 2007;19(9):780–781 author reply 782. 17727398
155. Fox, M., Young, A., Anggiansah, R., et al, A 22 year old man with persistent regurgitation and vomiting: case outcome. Br Med J. 2006;333(7559):133–137. 16840471
156. Tutuian, R., Vela, M.F., Balaji, N.S., et al, Esophageal function testing with combined multichannel intraluminal impedance and manometry: multicenter study in healthy volunteers. Clin Gastroenterol Hepatol. 2003;1(3):174–182. 15017488