Pathogenesis of Soft Tissue and Bone Repair
Boris A. Zelle and Freddie H. Fu
Musculoskeletal injuries usually result from supraphysiologic stresses that overwhelm the intrinsic stability of the musculoskeletal apparatus. The consequence is injury to the bone, tendon, muscle, ligaments, or a combination of these structures. The physiologic healing response varies among these tissues and is influenced by various intrinsic and extrinsic factors. Among these are the degree and anatomic location of the injury, the patient’s physiology, and the mode of treatment rendered. The aim of this chapter is to review the concept of soft tissue and bone healing and to describe the factors that influence the healing response.
Incision And Wound Healing
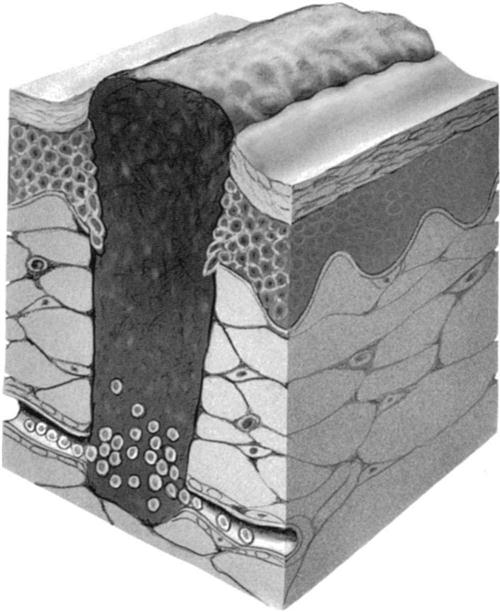
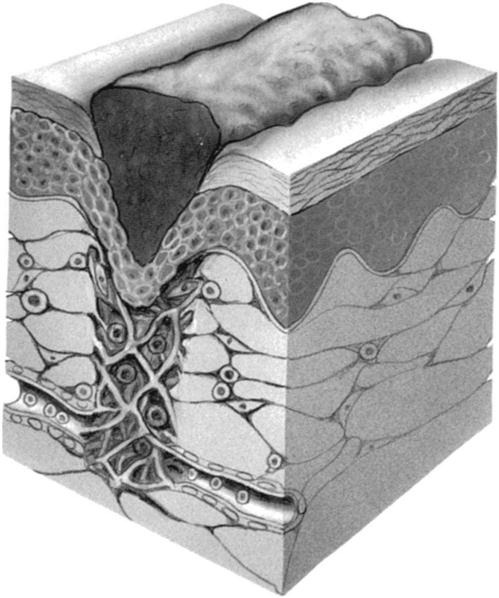
![]() With regard to epithelial tissue, the surgical incision is considered to be a “controlled trauma.” Incision and wound healing begins immediately after surgery and progresses through four distinct phases: (1) the coagulation phase (Fig. 1-1), (2) the inflammatory phase, (3) the granulation phase (Fig. 1-2), and (4) the scar formation and maturation phase. Table 1-1 gives an approximate time frame for each of these phases with hallmarks of what each phase accomplishes. Wound healing requires a clean environment, good circulation, appropriate approximation of wound edges, and a balance of the cellular mechanisms that ensure a proper immune response in the wound environment. Wound healing occurs through scar formation. Many intrinsic factors (e.g., age, metabolic and circulatory disorders, patient physiology, and comorbidities) and extrinsic factors (e.g., nutrition, hydration, smoking, wound exposure, and wound management) will influence the healing response and formation of the scar.
With regard to epithelial tissue, the surgical incision is considered to be a “controlled trauma.” Incision and wound healing begins immediately after surgery and progresses through four distinct phases: (1) the coagulation phase (Fig. 1-1), (2) the inflammatory phase, (3) the granulation phase (Fig. 1-2), and (4) the scar formation and maturation phase. Table 1-1 gives an approximate time frame for each of these phases with hallmarks of what each phase accomplishes. Wound healing requires a clean environment, good circulation, appropriate approximation of wound edges, and a balance of the cellular mechanisms that ensure a proper immune response in the wound environment. Wound healing occurs through scar formation. Many intrinsic factors (e.g., age, metabolic and circulatory disorders, patient physiology, and comorbidities) and extrinsic factors (e.g., nutrition, hydration, smoking, wound exposure, and wound management) will influence the healing response and formation of the scar.
TABLE 1-1
| Coagulation Phase (see Fig. 1-1) | Vasoconstriction, platelet aggregation, clot formation | Begins immediately and lasts minutes |
| Inflammatory Phase | Vasodilation, polymorphonuclear (PMN) leukocytes, phagocytes | At the edges of wounds, epidermis immediately begins thickening; within the first 48 hours entire wound is epithelialized; lasts hours |
| Granulation Phase | Fibroplasia, epithelialization, wound contraction | Fibroblasts appear in 2-3 days and are dominant cell by day 10 |
| Scar Formation/Maturation Phase (see Fig. 1-2) | Collagen synthesis; rarely regain full elasticity and strength | Lasts weeks to months and even up to 1 year |
Adapted from Browner BD, et al: Skeletal trauma—basic science, management, and reconstruction, ed 3, Philadelphia, 2003, Saunders.
Ligament Injuries And Healing
Ligament Anatomy and Function
Ligaments are anatomic structures of dense, fibrous connective tissue. They can be divided into two major subgroups: (1) ligaments connecting the elements of the bony skeleton (skeletal subgroup) and (2) ligaments connecting other organs, such as suspensory ligaments in the abdomen (visceral subgroup). The skeletal ligaments are the focus of this chapter. The nomenclature of the ligaments usually relates to their anatomic location and bony attachments (i.e., medial collateral, posterior talofibular), as well as their shape and function (i.e., triangular, cruciate, or deltoid ligament).
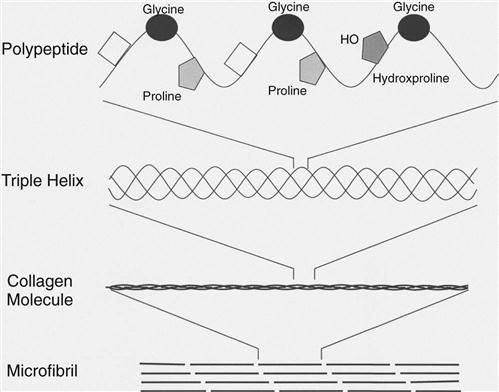
Structurally, ligaments contain rows of fibroblasts within parallel bundles of collagen fibers. Approximately two thirds of the wet weight of a ligament is water, whereas collagen fibers account for approximately 70% of the dry weight. More than 90% of the collagen in ligaments is type I collagen. Trace amounts of other collagens exist, such as type III, V, X, XII, and XIV.1 The primary structure of the type I collagen consists of a polypeptide chain with high concentrations of glycine, proline, and hydroxyproline. Almost two thirds of the primary structure of type I collagen consists of these three amino acids. Intermolecular forces cause three polypeptide chains to combine into a triple helical collagen molecule. This ropelike configuration imparts great tensile strength properties (Fig. 1-3). Within the ligament, the collagen fibrils are usually organized in a longitudinal pattern and are held in place by the extracellular matrix (see Fig. 1-1).2 Collagen fibers in the extracellular matrix are surrounded by water-soluble molecules, such as proteoglycans, glycosaminoglycans, and structural glycoproteins. Although these molecules represent only approximately 1% of the dry weight of ligaments, they are important for proper ligament formation and organization of the ligament meshwork. Their hydrophilic properties are crucial for the viscoelastic capacity of ligament tissue and ensure adequate tissue lubrication and proper gliding of the fibers. Moreover, proteoglycans couple adjacent collagen fibrils together and support the mechanical integrity of the ligaments.3
Ligament Injury
From the clinical standpoint, ligamentous injuries are classified into three grades.4 Grade I injuries include mild sprains. The structural integrity of the ligament is intact, although edema, swelling, and punctate ligament bleeding may be present. In grade II injuries, individual fibrils are torn, but the overall continuity of the ligament is maintained. Significant edema and bleeding is usually noted, and ligament stability is reduced. Grade III injuries are characterized by complete disruption of the ligament substance. Most ligamentous injuries can be diagnosed through a clinical examination and joint stability tests. Magnetic resonance imaging (MRI) represents the most commonly performed imaging study for diagnosing ligamentous injuries.
Multiple healing studies involving the medial collateral ligament (MCL) of the knee have been performed and have contributed to our knowledge of ligament healing. The healing phases of ligaments are traditionally divided by their morphologic appearance into an inflammatory phase (first days postinjury), a proliferative phase (1 to 6 weeks postinjury), and a remodeling phase (beginning at 7 weeks postinjury) (Table 1-2).5 It is important to appreciate that these three phases represent a continuum rather than distinct phases. The predominant cell types in the inflammatory phase are inflammatory cells and erythrocytes. As the ligament ruptures, its torn ends retract and have a ragged, “mop-end” appearance. The gap between these torn ends is filled with hematoma from ruptured capillaries. Histologically, the inflammatory reaction is characterized by increased vasodilation, capillary permeability, and migration of leukocytes. During the inflammatory phase, water and glycosaminoglycans are increased in the injured tissue. During the proliferative phase, a highly cellular scar develops, with fibroblasts as the dominating cell type. New collagen fibrils can be identified as early as 4 days after the injury. After approximately 2 weeks, the newly formed collagen fibrils bridge the gap between the torn ligament ends. However, the water content of the scar remains elevated, the collagen density remains low, and the collagen fibrils still appear less organized than in normal ligament tissue. During the remodeling phase, cellularity and vascularity decrease while collagen density increases. Moreover, the collagen arrangement becomes more organized along the axis of the ligament.
TABLE 1-2
| Inflammatory Phase | Vasodilation, fibrin clot formation, increased capillary permeability, and migration of leukocytes | Begins immediately and lasts minutes to hours |
| Proliferative Phase | Fibroblasts are the dominate cell type, collagen fibrils (as early as 4 days postinjury) | 1-6 wk postinjury |
| Remodeling Phase | Collagen synthesis and increased density; rarely regain full elasticity and strength | 7 wk up to 1 yr |
MCL healing studies in rabbits demonstrated that the remodeling phase is a long, ongoing process.6 At 10 months after ligament midsubstance injuries, the scar could be identified macroscopically and a significantly increased cross-sectional area of the scar was noticed. This scar tissue demonstrated an increased cellularity and highly organized scar tissue was not achieved, even at 10 months postinjury. Although the water concentration returned to normal value at 10 months, the glycosaminoglycan concentration of the scar tissue remained elevated and the collagen concentration remained lower. Despite a gradual increase throughout the healing phase, the collagen concentration plateaued at 70% of uninjured ligament tissue. In addition, the collagen types in the ligament scar varied from the normal tissue, with type III collagen being increased in the scar tissue.6
The healing response varies among the different ligaments. While MCL injuries have the potential to heal spontaneously, other ligament injuries, such as anterior cruciate ligament (ACL) injuries, rarely show a spontaneous healing response. Recent experimental studies in rabbits have demonstrated an increased expression of myofibroblasts and growth factor receptors in the injured MCL as compared with the injured ACL.7 Various reasons may account for the superior healing response of the MCL as compared with the ACL. It must be assumed that the high stress carried by the ACL prevents the ruptured ligament ends from having sufficient contact. In addition, the ACL is not embedded in a strong soft tissue envelope. Moreover, the ACL is an intraarticular structure; when it ruptures, the blood is diluted by the synovial fluid, preventing hematoma formation and hence initiation of the healing mechanism. Finally, it has been suggested that the synovial fluid is a hostile environment for soft tissue healing. Thus in ACL-deficient knees, the levels of proinflammatory cytokines are elevated, leading to a potentially unfavorable intraarticular microenvironment.8
Effect of Mobilization and Immobilization on Ligament Healing
An important aspect of the rehabilitation of patients with ligament injuries represents the timing of postinjury mobilization. Although aggressive mobilization obviously results in disruption of the scar tissue, prolonged immobilization may decrease the morphologic and biomechanical properties of the newly formed scar. It remains unclear what degree of immobilization is appropriate for healing ligaments.
The role of mobilization versus immobilization on ligament healing has been investigated in numerous animal studies.9–11 In an MCL healing study in rats, Vailas and associates11 compared the healing properties of the transected MCL across the following four groups: (1) surgical repair with 2 weeks of immobilization and 6 weeks of normal cage activity; (2) surgical repair with 2 weeks of immobilization and 6 weeks of treadmill exercise; (3) surgical repair with 8 weeks of immobilization; and (4) no surgical repair and no exercise. All animals were sacrificed at 8 weeks. The authors reported that the wet ligament weight, dry ligament weight, total collagen content of the ligament, and the ultimate load at failure of the ligament substance was lowest in the completely immobilized group and highest in the exercised group.11 In an MCL transsection model in the rabbit, Gomez and associates9 investigated the effect of continuous tension, as achieved by the implantation of a steel pin applying continuous stress on the healing MCL. At 12 weeks after MCL transection, the additional implantation of a tension pin resulted in a significantly decreased varus and valgus laxity, decreased cellularity of the scar tissue, and a more longitudinal alignment of the collagen fibers. These authors concluded that the application of controlled stress helped to augment the biochemical, morphologic, and biomechanical properties of the healing MCL.9 In a more recent study, Provenzano and associates10 investigated the effect of hind limb immobilization on the healing response of transected MCLs in a rat model. The authors reported significantly superior biomechanical ligament properties in the mobilized group. Microscopic analysis revealed abnormal scar formation and cell distribution in the immobilized group, as suggested by disoriented fiber bundles and discontinuities in the extracellular ligament matrix.10
These experimental data clearly emphasize the importance of stress and motion for the functional recovery of healing ligaments. However, the ideal amount of mobilization and immobilization during ligament healing is difficult to determine by animal studies, because animal studies are limited by the differing physiology and joint kinematics of animals. In addition, the amount of mobilization is difficult to control, and an exact titration of the stress cannot be performed with current in vivo models. Future clinical trials are necessary to determine the optimal amount of applied stress for the various ligament injuries.
Tendon Injuries And Healing
Tendon Anatomy and Function
Tendons are bands of dense, fibrous connective tissue interposed between muscles and bones. They transmit the forces created in the muscles to the bone, making joint motion possible. Some tendons may also connect two muscle bellies (e.g., digastrics, omohyoid). The gross tendon structure varies considerably from tendon to tendon, ranging from cylindrical rounded cords to flattened bands, called aponeuroses. The cross-sectional area of the more rounded tendons usually correlates with the isometric strength of the muscle from which they arise. The bony insertion site of the tendon is often accompanied by a small synovial bursa (e.g., subacromial bursa, pes anserinus, retrocalcaneal bursa). The tendon bursae are usually located in those anatomic sites where a bony prominence would otherwise compress the gliding tendon.
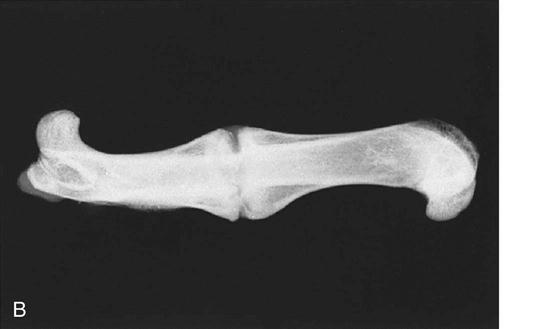
Microscopically, tendons and ligaments are similar. The tendon tissue is a complex composite of parallel collagen fibrils embedded in a matrix; cells are relatively rare, and fibroblasts represent the predominant cell type within the tendon; and the fibroblasts are arranged in parallel rows between the collagen fibrils (Fig. 1-4, A and B). The biochemical composition of ligaments and tendons are also very similar. Water is the major constituent of the wet tendon weight, whereas type I collagen accounts for approximately 70% to 80% and elastin for approximately 1% to 2% of the dry tendon substance. As in ligaments, other collagen types exist only in small amounts. The proteoglycans and glycosaminoglycans in the extracellular matrix play an important role for the viscoelastic properties and the tensile strength of the tendon. Their hydrophilic capacity provides the tendon with lubrication and facilitates gliding of the fibrils during tensile stress.
According to their envelope, tendons can be divided into tendons within a synovial sheath (i.e., sheathed tendons) and paratenon-covered tendons. In particular, tendons in the hand and foot are often enclosed in a synovial tendon sheath. The tendon sheath directs the path of the tendon and produces a synovial fluid, which allows tendon gliding and contributes to tendon nutrition. True tendon sheaths are only found in areas with an increased friction or sharp bending of the tendons (e.g., flexor tendons of the hand). A simple membranous thickening of the surrounding soft tissue, called the paratenon, usually surrounds tendons without a true synovial tendon sheath, such as the Achilles tendon. The paratenon is composed of loose fibrillar tissue. It also functions as an elastic sleeve and permits free movement of the tendon against the surrounding tissue, although it is not as efficient as a true tendon sheath.
Just like ligaments, tendons have a limited blood supply. The vascular supply of tendons has been described by injection studies, which demonstrated that tendons are usually surrounded by a network of blood vessels.12 Arteries supplying the tendon might come from the attached muscle, the bony insertion site, the paratenon, or the tendon sheath along the length of the tendon. However, there seems to be a difference between the nutrition of the sheathed tendons and the paratenon-covered tendons. The paratenon-covered tendons receive the majority of their blood supply from vessels in the paratenon. In sheathed tendons, the synovial sheath minimizes the vascular supply to the tendon substance, and avascular regions have been identified within the midsubstance of these tendons.12–14 Hence the diffusion of nutrients through the synovial fluid of sheathed tendons is critical for their homeostasis. Indeed, in sheathed tendons this process may be even more important than vascular perfusion. The digital flexor tendons, for example, receive up to 90% of their nutrition by diffusion.15 For this reason, sheathed tendons have also been referred to as avascular tendons, whereas the paratenon-covered tendons have been referred to as vascular tendons.
Tendon Injury
Tendon injuries may occur as a result of direct or indirect trauma (Fig. 1-5, A and B). Direct trauma includes contusions and lacerations, such as lacerations of the flexor tendons of the hand. Indirect tendon injuries are usually a consequence of tensile overload. Because most tendons can withstand higher tensile forces than their associated muscles or osseous insertion sites, avulsion fractures and ruptures at the myotendinous junctions are more likely than midsubstance ruptures. Midsubstance ruptures of the tendon after indirect trauma are usually associated with preexisting tendon degeneration. This has been supported by histologic investigations of ruptured Achilles tendons, which demonstrated increased tenocyte necrosis, loss of fiber structure, increased vascularity, decreased collagen content, and increased glycosaminoglycan content in previously ruptured tendons.16–18
Tendon Healing
The repair process in paratenon-covered tendons is also initiated by the influx of extrinsic inflammatory cells. As in ligaments, the healing of the ruptured tendon proceeds through an inflammatory phase, a proliferative phase, and a remodeling phase.19–21 During the inflammatory phase, healing is initiated by the formation of a blood clot bridging the gap between the ruptured ends. During the first few days after the injury, the proliferative phase begins; disorganized fibroblasts are the dominating cell types, and collagen synthesis can be detected. The collagen fibers orient themselves along the axis of the tendon during the remodeling phase. The remodeling phase continues for many months. It is characterized by increased organization of the collagen fibers, an increase in the number of intermolecular bonds between the collagen fibers, subsequent reduction of scar tissue, and increased tensile strength (Table 1-3).
TABLE 1-3
| Inflammatory Phase | Vasodilation, hematoma formation to bridge the defect (or gap), increased capillary permeability, and migration of leukocytes; influx of extrinsic/intrinsic inflammatory cells | Begins immediately and lasts minutes to hours |
| Proliferative Phase | Fibroblasts are the dominant cell type, collagen synthesis | 1-6 wk postinjury |
| Remodeling Phase | Collagen synthesis and increased density | Can last for several months |
Although it seems well accepted that the healing response in paratenon-covered tendons is initiated by the influx of inflammatory cells, the initiation of the healing response of sheathed tendons remains controversial. Both an intrinsic mechanism and an extrinsic mechanism have been proposed. The extrinsic concept suggests that similar to paratenon-covered tendons, the healing of sheathed tendons occurs by granulation from the tendon sheath and the surrounding tissue, although the tenocytes themselves play no important role in this repair. According to the intrinsic theory, cells from within the tendon proliferate at the wound site, leading to production of collagen and extracellular matrix.15,20 With regard to the initiation of the healing response, it appears probable that both intrinsic and extrinsic healing exist.
Effect of Mobilization and Immobilization on Tendon Healing
The ideal mobilization regimen for the various tendon injuries is beyond the scope of this discussion. Clearly, overaggressive early mobilization may result in rerupture of the tendon. ![]() Scientific evidence suggests that motion and stress increase collagen production, accelerate remodeling, and improve the biomechanical properties of healing tendons.22–24 However, the optimal level of stress and motion of the healing tendon must be established based on clinical evidence.
Scientific evidence suggests that motion and stress increase collagen production, accelerate remodeling, and improve the biomechanical properties of healing tendons.22–24 However, the optimal level of stress and motion of the healing tendon must be established based on clinical evidence.
Skeletal Muscle Injuries And Healing
Anatomy and Function of Skeletal Muscle
Skeletal muscle represents the largest tissue mass in the body and accounts for almost 50% of the total body weight. Skeletal muscle originates from bone and inserts into bone via tendon. The primary function of skeletal muscle is to provide mobility to the bony skeleton. This is accomplished by muscle contraction (i.e., shortening) and force transmission through the muscle-tendon-bone complex.
The basic structural element of the skeletal muscle is the muscle fiber. The muscle fiber is a syncytium of many cells fused together with multiple nuclei. The muscle fibers consist of contractile elements, the myofibrils, which give skeletal muscle a striated appearance by light microscopy. The myofibrils consist of myofilaments (actin and myosin filaments). Individual muscle fibers are organized into muscle by surrounding connective tissue (i.e., endomysium, perimysium, epimysium) that provide integrated motion among the muscle fibers. The endomysium surrounds the individual muscle fibers. Groups of muscle fibers are arranged together to fascicles surrounded by the perimysium. The fascicles are grouped together by the epimysium to form the whole muscle belly.
Skeletal muscle contraction is accomplished by sliding of its filaments.25,26 The basic contractile unit is the sarcomere. The active contractile units within the sarcomere are the actin and the myosin filaments. These myofilaments are arranged in a parallel fashion in which the larger myosin filaments interdigitate between the small actin filaments. The actin filaments actively slide along the surface of the myosin filaments through cross-bridges originating from the myosin. The coordinated sliding of actin and myosin filaments throughout the muscle translates into contraction of the entire unit, which generates force and motion.
Muscle Injuries and Healing
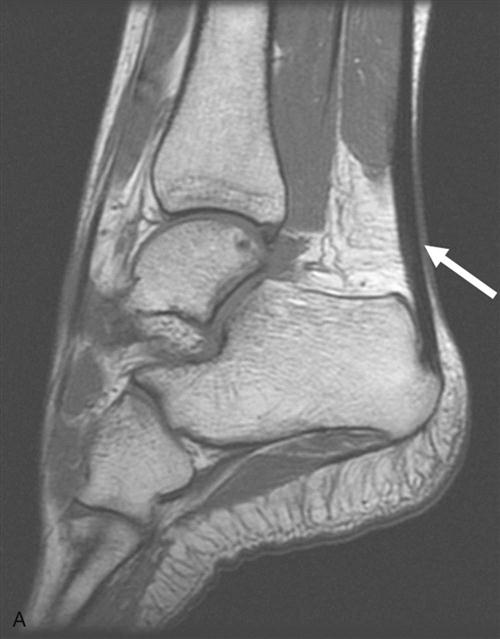
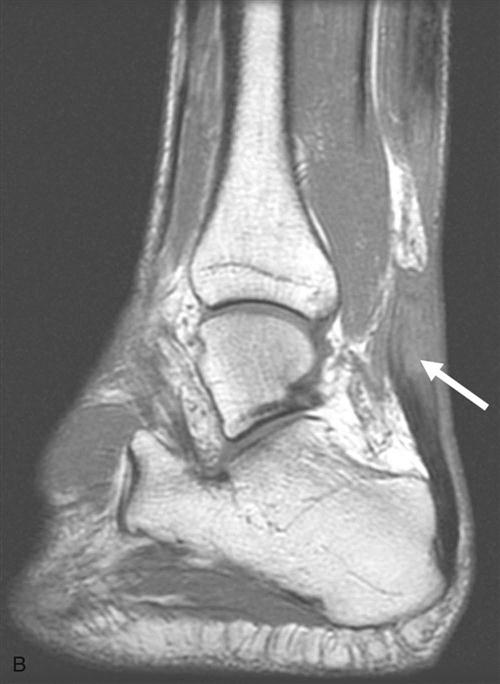
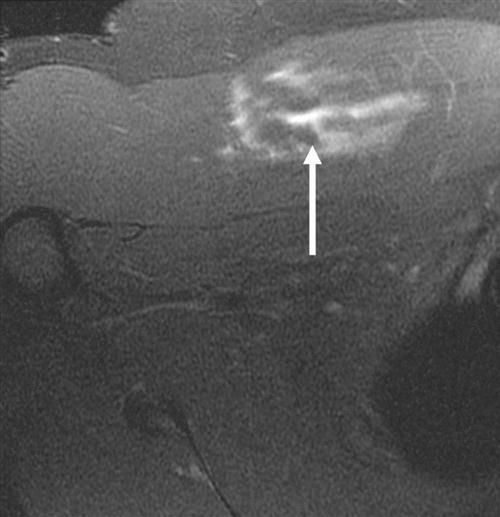
Skeletal muscle injuries constitute the majority of sports-related injuries.27,28 Skeletal muscle injuries can be classified as indirect and direct injuries. Indirect injuries result from an overload that overwhelms the muscle’s ability to respond normally, such as muscle strains and delayed-onset muscle soreness. Direct injuries are usually a result of external forces, such as muscle contusions and muscle lacerations. Most injuries are diagnosed clinically. MRI has been found to be highly sensitive to muscle edema and hemorrhage and is the primary imaging modality for determining the type of injury and the degree of muscle involvement (Fig. 1-6).18,29 Large muscle injuries have a limited healing capacity, and the repair process usually results in the formation of scar tissue. Severe muscle injuries may result in the inability to train or compete for several weeks, and they have a high tendency to recur.30,31
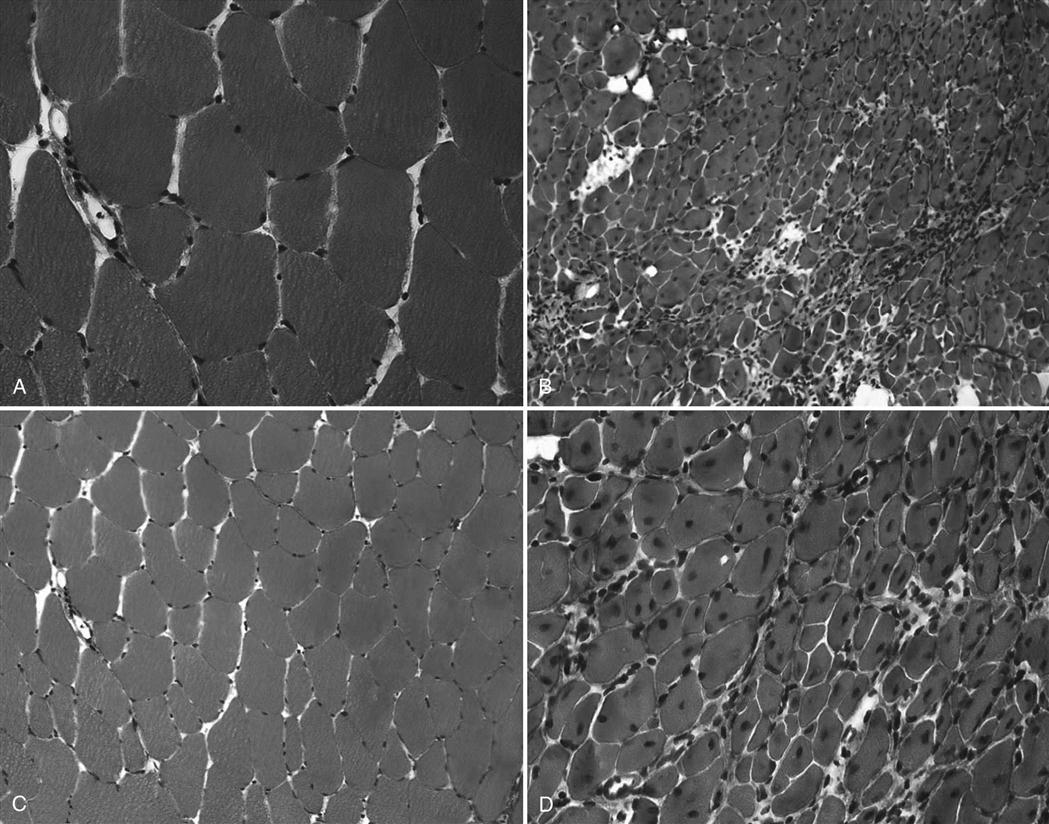
Similar to ligaments and tendons, injured skeletal muscle undergoes phases of disruption and degeneration, inflammation, proliferation, and fibrosis (Table 1-4). After trauma to the muscle, the disrupted muscle ends retract and the gap is filled by a local hematoma. Disruption of the muscle fibers leads to increased extracellular calcium levels, activation of the complement cascade, and myofiber necrosis. Inflammation is an early response to muscle tissue injury. Neutrophils rapidly invade the injury site and release inflammatory cytokines followed by an increase in macrophages that phagocytose cell debris. Structural damage of the muscle fibers usually heals with formation of scar tissue (Fig. 1-7, A to D).29
TABLE 1-4
Muscle Healing (Involving Disruption of Muscle Cell Structure)
| Inflammatory Phase (Disruption and Degeneration) | Vasodilation, hematoma formation, increased capillary permeability, increased extracellular calcium, and migration of leukocytes | Begins immediately and lasts minutes to hours |
| Proliferative Phase | Neutrophil and macrophage migration | 1-6 wk postinjury |
| Remodeling/Fibrosis Phase | Collagen synthesis and increased density; scar formation | Can last for several months |
The most common muscle injuries include delayed-onset muscle soreness (DOMS), muscular contusion, muscular strain, and muscular laceration. Among these types of injuries, the mechanism of injury, pathologic changes, treatment, and outcome vary greatly. Therefore these issues will be discussed in detail for each of these muscle injuries.
Delayed Onset Muscle Soreness
DOMS is a consequence of extensive exercise and usually occurs approximately 12 to 48 hours after exercise. The symptoms of DOMS occur when the amount of stress applied to the muscle exceeds its ability to elongate without disrupting the structural integrity. The symptoms of DOMS are particularly intense after eccentric muscle contraction exercises, whereas repetitive submaximal muscle contractions cause less severe symptoms.32,33 DOMS is characterized by alterations of the structural integrity, an inflammatory response, and the loss of functional capacity.34,35 The inflammatory component is most likely a response to the damage of the structural muscle integrity and usually lasts for a few days. To reduce the inflammatory response, the treatment during the first 2 to 3 days consists of rest, ice, compression, and elevation (RICE). Stretching exercises are recommended thereafter to allow superior scar tissue remodeling and fiber alignment of the repair tissue. However, most competitive athletes resume their normal activities quickly after DOMS onset. Permanent impairment after DOMS does not occur.32
Muscular Contusion
Muscular contusions are caused by direct blunt trauma to the muscle resulting in damage and partial disruption of the muscle fibers. Frequently, muscle contusions are associated with capillary rupture and local hematoma formation. This is associated with an inflammatory reaction, including increased neutrophil and phagocytic activity, release of inflammatory cytokines, prostaglandin production, and local edema. Clinical signs and symptoms may include ecchymosis, superficial and deep soft tissue swelling, pain, local tenderness, and decreased or abnormal range of motion (ROM). Jackson and Feagin36 classified the muscular contusions into three degrees, according to the clinical symptoms. A mild contusion is characterized by localized tenderness, near normal ROM, and near normal gait pattern. A moderate contusion usually includes a swollen tender muscle mass, a 50% decrease in ROM, and an antalgic gait. A severe contusion is characterized by marked tenderness and swelling, a 75% decrease in ROM, and a severe limp.36
The initial treatment consists of RICE to prevent further hemorrhage. This is followed by active and passive ROM exercises and eventually the use of heat, a whirlpool, and ultrasound. Functional rehabilitation includes strengthening exercises. Muscular contusions heal by formation of dense connective scar tissue with variable amounts of muscle regeneration. Early stretching exercises of the injured muscle play an important role in the functional scar tissue remodeling process and normal alignment of the newly formed collagen fibers. In contrast, it seems that prolonged immobilization is associated with an inferior recovery of muscle function.36
Muscular Strain
Muscular strains are tears in the muscle, which may occur as a result of excessive stress (i.e., acute strain) or constant overuse (i.e., chronic strain).33 In particular, muscles that cross two joints, such as the hamstring muscles and the gastrocnemius, seem to be particularly susceptible to muscular strains. Chronic muscle strains usually occur as a result of repetitive overuse, causing fatigue of the muscle. Acute strains, on the other hand, are the result of an excessive force applied to the muscle. The injury usually occurs at the weakest part of the muscle, the myotendinous junction. Histologically, muscle strains are characterized by hemorrhage and an inflammatory response. However, the extent of muscle strain may vary. Mild strains occur when no appreciable structural damage exists to the muscle tissue and pathologic changes are confined to an inflammatory response with swelling and edema, causing discomfort with exercise. With moderate damage, an appreciable muscular defect occurs and the inflammatory response, edema, and discomfort are increased as compared with mild strains. Severe strains are characterized by complete rupture of the muscle belly or the myotendinous junction.
The treatment of muscular strains is completely dependent on the grade of the injury. Although mild strains are usually treated symptomatically with RICE, severe strains may require surgical reconstruction. Muscular strains usually heal with the formation of fibrous scar tissue that can be visualized by MRI.29
Muscle Laceration
Muscle lacerations may be caused by penetrating trauma to the muscle and the surrounding soft tissue. Recovery of the muscle function depends on the orientation of the laceration. Lacerations perpendicular to the muscle fibers may create a denervated segment, which is associated with a poor recovery.37,38 Suture repair of these lesions usually results in scar formation across the laceration. Thus muscle regeneration does not occur across the laceration site, and the functional continuity is usually not restored after muscle laceration.37,38 In addition, the distal segment is often denervated, and even surgical repair of the muscle belly may not restore the innervation of this part of the muscle. Therefore the functional recovery is usually limited after muscle laceration.
Myositis Ossificans
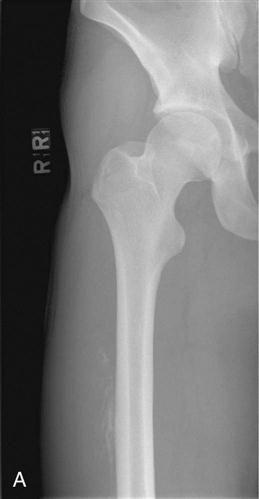
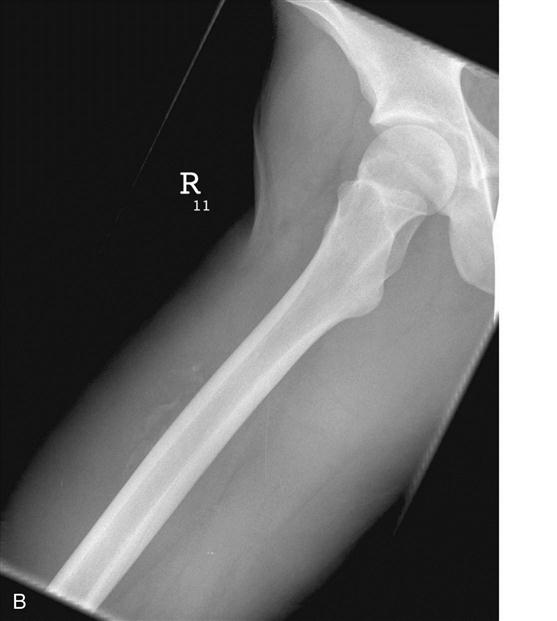
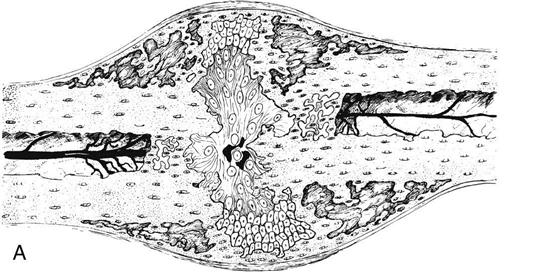
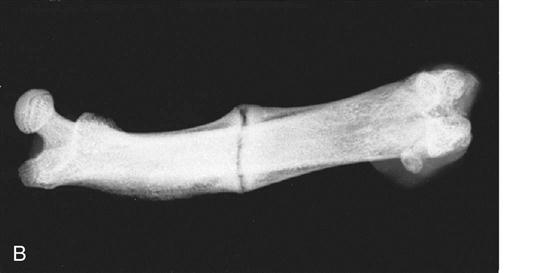
The term myositis ossificans is used to describe ectopic bone formation within a muscle. Myositis ossificans represents a common complication after muscle injuries. Although common locations of myositis ossificans are the anterior thigh and the upper arm, it may occur in any muscle of the body. The clinical symptoms suggesting myositis ossificans include localized tenderness, swelling, and muscle weakness. Myositis ossificans can usually be detected on plain radiographs (Fig. 1-8, A and B). MRI studies may provide additional information with regard to location within the muscle and extent of the lesion. In addition, nuclear bone scans may play a role in the early detection of the lesion and may help judging the maturity and activity of the process. The pathogenesis of myositis ossificans is not completely understood. Myositis ossificans commonly occurs adjacent to the bone shaft, suggesting that bone-forming cells from the periosteum migrate into the muscle tissue and produce bone within the injured muscle or the muscle hematoma.39,40 In some cases, however, the ectopic calcifications occur within the muscle and are not in the close proximity of the bone. For these lesions, the pathogenesis remains unclear. One theory is that after the muscle injury, the circulation within the traumatized muscle decreases and leads to ossification.41 Other authors theorize that after the muscle injury, rapidly proliferating nondifferentiated connective tissue forms calcifications within the muscle.42
In the early stages of myositis ossificans, RICE is the preferred treatment. Ectopic calcification in the soft tissue surrounding the hip joint after total hip replacement or treatment of acetabular fractures represent a major clinical challenge. A meta-analysis of the literature demonstrated that treatment with moderate to high doses of nonsteroidal antiinflammatory drugs (NSAIDs), such as indomethacin, at the time of surgery decreases the risk of ectopic bone formation.43 The method by which NSAIDs prevent ectopic bone formation has not been completely understood, but early inhibition of prostaglandin-dependent osteogenic cells appears to be the likely mechanism. Early surgery is contraindicated in myositis ossificans because reossification commonly occurs. Surgical exploration and excision of heterotopic bone formations is only indicated in symptomatic patients and when bone scans and consecutive radiographs suggest low activity and mature bone formation.40
Bone Injury And Healing
Bone Morphology
Bone is a composite of material consisting of minerals, proteins, water, cells, and other macromolecules (i.e., lipids, sugar). The composition of the bone tissue varies and depends on age, diet, and general health status. In general, minerals account for 60% to 70% of the bone tissue, water accounts for 5% to 10% of the bone tissue, and the organic bone matrix makes up the remainder. The mineral component is mainly composed of calcium hydroxyapatite (Ca10(PO4)6(OH)2. Approximately 90% of the organic bone matrix is type I collagen; the remainder consists of minor collagen types, noncollagenous proteins, and other macromolecules.
The major cell types of bone tissue are osteoblasts, osteocytes, and osteoclasts. The osteoblasts represent the bone-forming cells. The osteoblasts line the surface of the bone matrix and the osteocytes are encased within the mineralized bone matrix. Both cell types are derived from the same osteoprogenitor cell type. A layer of unmineralized bone matrix (osteoid) lies between the osteoblast and the mineralized bone matrix. Once an osteoblast becomes surrounded by mineralized bone matrix, it is referred to as an osteocyte. Osteocytes are characterized by a higher nucleus-to-cytoplasm ratio and contain fewer cell organelles than osteoblasts. Osteoclasts are the major resorptive cells of bone and are characterized by their large size and multiple nuclei. Osteoclasts derive from pluripotent cells of the bone marrow, which are hematopoietic precursors that also give rise to monocytes and macrophages. They lie in regions of bone resorption in pits called Howship lacunae.
Bone Injuries and Healing
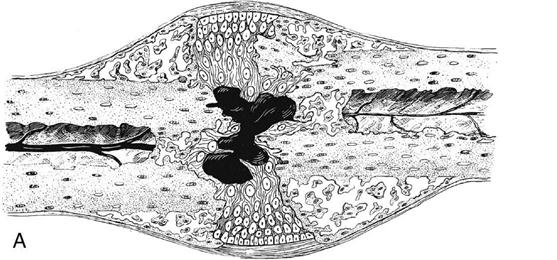
A fracture of a bone is a complete or partial break in the continuity of the bone. Fractures usually occur as a result of trauma and may arise from low energy forces that are cyclically repeated over a long time period (i.e., stress fractures) or from forces having sufficient magnitude to cause structural failure after a single impact. Most fractures can be identified on plain radiographs. In some cases, computed tomography (CT) scans or MRI may provide additional information on the fracture pattern. Fracture repair is unique in that healing occurs without scar formation, and only mature bone remains in the fracture site at the end of the repair process. This repair process consists of four stages, including inflammation, soft callus, hard callus, and remodeling (Table 1-5).
TABLE 1-5
Bone Healing for a Stable Fracture
| Inflammatory Phase | Hemorrhage, necrotic cells; hematoma and fibrin clot formed to bridge the gap | Begins immediately |
| Soft Callus Phase | Fibrous and cartilaginous tissue forms between the fracture ends, increase in vascularity and ingrowth of capillaries into the fracture callus; increase in cellular proliferation, osteoclasts remove dead bone fragments | 1-6 wk postinjury |
| Hard Callus Phase | Woven bone develops when the callus converts from fibrocartilaginous; osteoclasts continue removing dead bone; osteoblast activity abundant | 4-6 wk postinjury |
| Remodeling Phase | Woven bone slowly changes to lamellar bone; medullary canal is then reconstituted; fracture diameter decreases to the original width | 6 wk and up to several months or years (depends on a number of anatomic and physiologic factors) |
The inflammation period begins immediately after the fracture is sustained and is characterized by the presence of hemorrhage, necrotic cells, hematoma, and fibrin clots. The predominant cell types are platelets, polymorphonuclear neutrophils, monocytes, and macrophages. Shortly thereafter, fibroblasts and osteoprogenitor cells appear and blood vessels start growing into the defect. This neoangiogenesis is initiated and maintained by a tissue oxygen gradient and is enhanced by angiogenic factors.
The stage of soft callus is characterized by fibrous or cartilaginous tissue within the fracture gap and a great increase in vascularity (Fig. 1-9, A and B). The bony ends are no longer freely moveable. Clinically, subsiding pain and swelling characterize this stage.
During the stage of hard callus, the fibrous callus is replaced by immature woven bone. (Fig. 1-10, A and B). The transition of soft callus to hard callus is somewhat arbitrary, and overlap exists between these two stages because different regions may progress at different rates. During the remodeling process, the woven bone slowly converts to lamellar bone and the trabecular structure responds to the loading conditions according to Wolff’s law.44 The remodeling process may continue for years after the fracture.
The vast majority of fractures (90% to 95%) are treated successfully.45 However, a variety of local and systemic factors may affect fracture healing. Local factors that may impede fracture healing include extensive injury to the surrounding soft tissue envelope, decreased local blood supply, inadequate reduction, inadequate mobilization, local infection, or malignant tissue at the fracture site. Systemic factors may include endocrinologic factors (e.g., diabetes mellitus, menopause), general bone loss (e.g., osteopenia, osteoporosis), patient nutrition (smoking, insufficient vitamin or calcium uptake), and peripheral circulation (vascular disease). In many fractures that do not heal, multiple risk factors may exist. Impaired bone healing may present as delayed osseous union or osseous nonunion. Delayed union is usually defined as the failure of the fractured bone to heal within the expected time course, while maintaining the potential to heal. Nonunion is defined as a state in which all healing processes have ceased before fracture healing has occurred.
![]() Nonunions can be classified as hypertrophic and atrophic. Nonunions lack the potential to heal and require further interventions. Hypertrophic nonunions demonstrate excessive vascularity and callus formation. They are typically the result of biomechanical instability and have a good biologic healing potential. Treatment of hypertrophic nonunions requires biomechanical stabilization of the fracture site. Atrophic nonunions have a limited healing potential, decreased vascularity, and show decreased callus formation. Treatment of atrophic nonunions is challenging, and the treatment may include débridement, stabilization, and use of bone grafts or other bone stimulating agents.
Nonunions can be classified as hypertrophic and atrophic. Nonunions lack the potential to heal and require further interventions. Hypertrophic nonunions demonstrate excessive vascularity and callus formation. They are typically the result of biomechanical instability and have a good biologic healing potential. Treatment of hypertrophic nonunions requires biomechanical stabilization of the fracture site. Atrophic nonunions have a limited healing potential, decreased vascularity, and show decreased callus formation. Treatment of atrophic nonunions is challenging, and the treatment may include débridement, stabilization, and use of bone grafts or other bone stimulating agents.
Biologic Treatment Approaches
The successful treatment of musculoskeletal injuries remains challenging. Both ligaments and tendons have a poor vascular supply and a low cell turnover. Recent experimental investigations have attempted to establish novel biologic treatment methods, such as growth factor stimulation. Although most of these novel techniques have not been established in the clinical practice, we provide a brief review of the current research and discuss future perspectives in this area.
Growth Factors and Gene Therapy
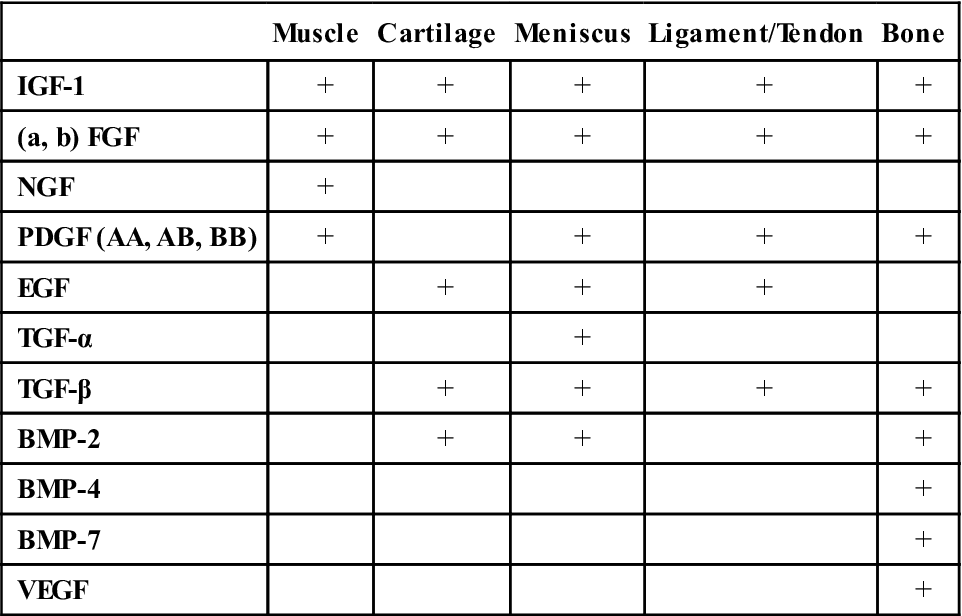
Growth factors are proteins that can be synthesized by both the resident cells (e.g., fibroblasts) and by immigrating cells (e.g., macrophages). Growth factors have the ability to stimulate cell proliferation, cell migration, and cell differentiation.46 Several authors have investigated the role of growth factors in stimulating the musculoskeletal healing response. Stimulating effects of various growth factors have been demonstrated in a variety of tissue (Table 1-6).47
TABLE 1-6
Effects of Growth Factors on Musculoskeletal Tissues
< ?comst?>
| Muscle | Cartilage | Meniscus | Ligament/Tendon | Bone | |
| IGF-1 | + | + | + | + | + |
| (a, b) FGF | + | + | + | + | + |
| NGF | + | ||||
| PDGF (AA, AB, BB) | + | + | + | + | |
| EGF | + | + | + | ||
| TGF-α | + | ||||
| TGF-β | + | + | + | + | |
| BMP-2 | + | + | + | ||
| BMP-4 | + | ||||
| BMP-7 | + | ||||
| VEGF | + |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
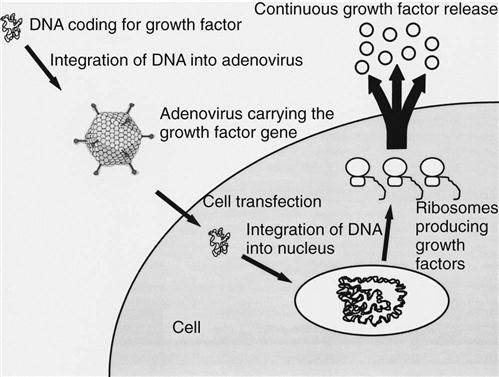
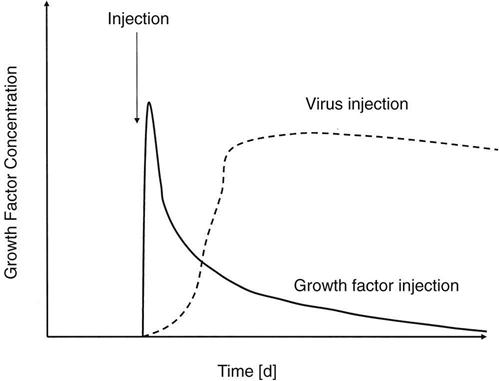
The use of most of these growth factors is limited by their short biologic half-lives, requiring repeated applications.48,49 To overcome this problem, gene transfer techniques have been tested in experimental studies. Gene therapy is based on the modification of cellular genetic information (Fig. 1-11). Thus the genes encoding for growth factors are transferred into local cells at the injury site to modify their genetic codes so that growth factors are continuously produced. This continuous excretion of growth factors will result in an uninterrupted stimulation of the injured musculoskeletal tissue. To achieve gene expression, the DNA encoding for the growth factors must be transferred into the nucleus of the host cells. After gene transfer, the treated cells generously express the intended factor (Fig. 1-12). Usually, viral vectors are used for the gene transfer, with adenoviruses and retroviruses representing the most commonly used vectors. Two strategies are used for the transfection of the host cells: (1) the in vivo approach and (2) the ex vivo approach.48,49 The in vivo approach includes the injection of a virus (usually an adenovirus) encoding the growth factor gene at the injury site. The ex vivo approach includes harvesting cells from the host, genetic modification in vitro (usually by a retrovirus), and reinjection of the modified cells to the injury site. Although the in vivo approach appears to be technically simpler, the ex vivo approach appears to be safer because the transfection of the cells occurs under controlled conditions in vitro.
Although experimental data have demonstrated the great potential of gene therapy techniques, gene therapy has not been established as a standard treatment in patients with musculoskeletal injuries. The major concern surrounding gene therapy is the safety of this technique. Potential risk factors include uncontrolled overstimulation and overgrowth of the repair tissue, mutation of the viral vectors, development of malignancies, and immunologic reactions. Future research is required to investigate and optimize the safety of gene therapy to translate this treatment approach into clinical practice.
Clinical Case Review
1 What can patients do to improve wound healing?
Wound healing occurs through scar formation. Extrinsic factors (e.g., nutrition, hydration, smoking, wound exposure, and wound management) will influence the healing response and the scar formation.
2 Why can some ligaments heal, whereas others need to be repaired?
The healing response varies among the different ligaments. For this answer, let us consider the knee as an example. While MCL injuries have the potential to heal spontaneously, other ligament injuries, such as ACL injuries, rarely show a spontaneous healing response due to: