CHAPTER 19 Partial-Thickness Rotator Cuff Tears
Our knowledge and understanding of partial-thickness rotator cuff tears (PTRCTs) have grown with the advent of shoulder arthroscopy and refinements in magnetic resonance imaging (MRI) techniques. Despite improvements in detection, the magnitude of pain and disability attributable to partial cuff tears remains uncertain and the natural history of these lesions is largely unknown. PTRCTs have shown a limited capacity for spontaneous healing and tend to progress over time.1 Considerations in the management of partial cuff tears include the patient’s age, activity preference, shoulder demands, response to nonsurgical treatment, location and extent of tearing, associated pathology, and surgeon’s level of skill and experience.
ANATOMY, PATHOANATOMY, AND BIOMECHANICS
A detailed understanding of rotator cuff anatomy will enable the clinician to appreciate and effectively manage the observed pathology. The tendons of the supraspinatus and infraspinatus blend together 15 mm medial to their insertion onto the greater tuberosity whereas the infraspinatus and teres minor coalesce closer to their musculotendinous junctions.2 As the individual rotator cuff tendons splay out and interdigitate, they form a common insertion onto the greater tuberosity. A superficial anterior extension of the supraspinatus contributes to a sheath that becomes the roof of the bicipital groove and a deep superior extension of the subscapularis helps form the floor.3 This latter relationship contributes to the anterior sling of the biceps and may help to explain, in part, the frequent coincident findings of a partial tear of the superior boarder of the subscapularis with anterior subluxation of the long head of the biceps. The coracohumeral ligament consists of a condensation of capsular tissue without a true ligamentous structure.4 This thick band originates on the base of the coracoid, reinforces the biceps sheath, and then blends with the capsule deep to the supraspinatus and infraspinatus.
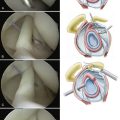
On histologic evaluation, the rotator cuff was found to consist of five distinct lamina.2 Layer 1 extends from the base of the coracoid to the greater tuberosity and is composed of superficial fibers that overlie the cuff tendons. Layers 2 and 3 contain the supraspinatus and infraspinatus tendons. Layer 2 is composed of relatively large collagen fibers, which are parallel to the axis of the tendon. The fibers of layer 3 are smaller, more random, and form a meshlike network. Layer 4 constitutes the deep extension of the coracohumeral ligament, coursing from anterior to posterior, and layer 5 is comprised of the true capsule, which extends from the glenoid rim and labrum to the humeral neck. Differential strain in the decussating fibers of different tendon layers contributes to intratendinous delamination. The bursal layers have approximately double the ultimate tensile strength and are more elastic than those near the articular surface.5 The primary vascular supply for the rotator cuff derives from an anastomotic network between layers 2 and 3, with contributions from the suprascapular and subscapular arteries.6 Additional blood supply is via retinacular vessels from the humeral circumflex arteries that enter the tuberosities. The deeper articular side of the cuff contains smaller arterioles and is relatively hypovascular, particularly in the supraspinatus region.7
The region on the greater tuberosity where the rotator cuff inserts has been termed the footprint. This attachment site varies in breadth, averaging 12.7 mm for the supraspinatus, 13.4 mm for the infraspinatus, and 17.9 mm for the subscapularis.8 The distance of the tendinous insertion to the articular surface increases from approximately 1 mm near the anterior supraspinatus to 13.9 mm at the inferior aspect of the teres minor. The mean anteroposterior distance of the supraspinatus is 1.63 cm, the infraspinatus 1.64 cm, and the subscapularis 2.43 cm.9 Gross cadaveric dissections have helped identify a thick band of tissue termed the rotator cable, which courses from anterior to the biceps to the posterior aspect of the infraspinatus. Within this arc lies the rotator crescent, consisting of the distal portions of the supraspinatus and infraspinatus insertions.10 The cable is approximately 2.5 times thicker than the crescent that it surrounds. The cable appears functionally similar to a suspension bridge and stress-shields the crescent to some degree. The prevalence of this distinct cable-crescent anatomic pattern is uncertain, but appears to increase with age.
Biomechanical research has improved our understanding of rotator cuff dysfunction. It has been shown that the articular surface of the rotator cuff has an ultimate stress to failure approximately half that of the bursal surface,11 and that tear propagation often progresses from the articular to the bursal surface of the cuff. With extension of the tear, small defects have the capacity to develop into full-thickness tears. A cadaveric study was conducted to create three partial-thickness tear patterns—articular surface, bursal surface, and midsubstance.12 Finite element analysis demonstrated that in all three types of tears, as tension was placed on the cuff muscles, a high stress concentration appeared at the articular surface and at the site of the tear. In a separate cadaveric investigation, tears of 25%, 50%, and 75% thickness were created and strain was measured at 45, 60, and 90 degrees of abduction.13 Articular-sided tendon strain increased consistently across the supraspinatus tendon with tear depths of 50% and 75%, but not 25%. Repair returned the supraspinatus strain almost to the intact state.
These findings lend biomechanical support for the clinical practice of repairing partial tears equal to or greater than 50%. Joint position and its relation to articular-sided tears has also been studied in a cadaveric model. An MRI-based technique to was used quantify cuff strain in shoulder positions of 15, 30, 45, and 60 degrees glenohumeral abduction in the scapular plane.14 Intratendinous strain was seen to increase significantly across all tendon regions with increasing abduction. These findings are particularly relevant to overhead athletes and workers with repetitive overhead demands.
CAUSES, INCIDENCE, AND CLASSIFICATION
Although the final common pathology for a partial-thickness rotator cuff tear is tendon fiber failure, the causes are mulifactorial.15 Both intrinsic and extrinsic causes have been hypothesized. Intrinsic causes first proposed by Codman and more recently advocated by Nirschl include age-related metabolic and vascular changes, including decreased cellularity, thinning of collagen fascicles, accumulation of granulation tissue, and dystrophic calcification. Those alterations may lead to degenerative tearing whereas shear stresses may contribute to the development of intratendinous lesions, including delamination. As degenerative changes occur, the ability of the rotator cuff to depress the humeral head during elevation may be significantly compromised. Consequently, superior migration and secondary wear and fraying on the bursal surface of the involved tendons may occur because of abutment on the inferior surface of the acromion.
The prevalence of rotator cuff disease is strongly associated with age, with the peak incidence of PTRCTs appearing to occur in the fifth and sixth decades of life. Many tears are minimally or asymptomatic and patients are not motivated to seek medical evaluation to detect them. Even a partial tear identified at diagnostic arthroscopy may be an incidental finding and not necessarily related to the patient’s primary symptoms. Of the partial-thickness tears that are symptomatic and create dysfunction, the supraspinatus is the most commonly involved. In a cadaveric study of 306 shoulders, 32% were found to have partial-thickness cuff tears and 19% full-thickness tears of the supraspinatus.16 Clinically, articular-sided tears are identified up to three times more frequently than those originating on the bursal surface. Intratendinous tears can be particularly difficult to detect. In one study of 249 cadavers in which the specimens were noted to be older, 13% had PTRCTs (18% bursal, 27% articular, 55% intratendinous).17 In a separate evaluation of 249 cadaveric specimens, intratendinous tears were the most common type of partial-thickness tears (7.2%), followed by articular-sided tears (3.6%) and bursal-sided tears (2.4%).18 Partial tears of the subscapularis tendon also occur. In a study of 46 cadaveric shoulders, 17 were found to have partial articular-sided tears of the subscapularis near the superior boarder.19 Of those tears, 30.4% also had a lesion of the long head of the biceps, suggesting a significant association.
Useful classifications of partial-thickness rotator cuff tears were lacking prior to the publication of Ellman’s scheme which included location (articular, bursal, intratendinous), depth (grade 1 < 3 mm deep; grade 2, 3 to 6 mm deep; and grade 3 > 6 mm deep), and area (in mm2).20 Snyder offered a somewhat more geographic and qualitative classification, including location (articular, bursal, complete), and tear severity (0 = normal cuff; 1 = synovial irritation < 1cm; 2 = superficial fraying or failure of cuff fibers < 2 cm; 3 = substantial tearing of cuff fibers < 3 cm; 4 = severe partial cuff tearing or flap > 3 cm).21 Intratendinous tears have been difficult to accurately classify, in part because of the difficulty in defining the extent arthroscopically. To date, most estimates of the tear depth have relied on the extent of the footprint exposed after débridement of the damaged tissue from the greater tuberosity.9 An instrument of known dimension, (e.g., a 5.5-mm shaver) is used to measure the breadth of the region on the tuberosity devoid of normal fiber attachment. Calibrated probes have also been developed in an attempt to improve the accuracy in measuring tear thickness. A study designed to assess the preoperative interobserver grading in the classification of PTRCTs has shown poor agreement in predicting the depth of a partial-thickness tear.22 Our current methods to grade partial cuff tears likely provide only a rough estimate.
DIAGNOSTIC IMAGING
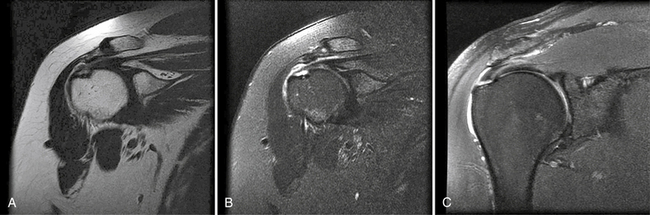
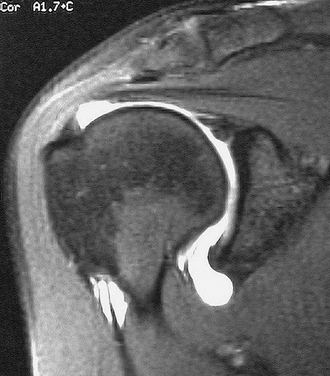
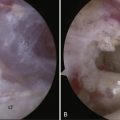
In the past, MRI demonstrated only moderate success in identifying partial-thickness tears of the rotator cuff. Enhancements to MRI evaluation including sequence manipulation (fat suppression), contrast arthrography, and differential arm positioning, have improved the accuracy of evaluating partial-thickness cuff tears. Of added value is the ability of MRI to identify associated pathology, including biceps and labral disorders. The presence of a PTRCT is suggested by an increased signal in the rotator cuff on T1-weighted images (without evidence for loss of continuity; Fig. 19-1A) combined with a focal defect on T2-weighted images that is intratendinous or limited to the articular or bursal surface (see Fig. 19-1B and C).23,24 It may be difficult to differentiate a high-grade partial-thickness tear from a small full-thickness tear. The accuracy in identifying articular surface and delaminating tears is improved with the addition of intra-articular contrast (Fig. 19-2). In a review of 76 patients who underwent MR arthrography (MRA) and subsequent arthroscopy, 26 of 28 patients with a partial articular-sided cuff tear were accurately identified preoperatively.25 Sensitivity was 84%, with a positive predictive value of 93%. A specificity of 96% and overall accuracy of 91% (69 of 76) were reported. In another evaluation of 50 patients in which MRA and arthroscopy were again correlated, the sensitivity and specificity of MRA were 91% and 85%, respectively, with a positive predictive value of 84% and a false-negative rate of 9%.26 MRA with the patient’s shoulder placed in the abduction, external rotation (ABER) position improves the sensitivity for depicting PTRCT,27 particularly those in whom a horizontal or delaminating component is present.28 In that position, tension within the posterior cuff is decreased and the contrast can more readily infiltrate an articular-sided cuff defect. For most patients with suspected partial-thickness cuff tears, especially young, overhead throwing athletes, MRA is the imaging modality of choice.
TREATMENT OPTIONS
Arthroscopic Treatment
Diagnostic Arthroscopy and Bursoscopy
The timing for surgical intervention is not well-defined. When a thorough nonoperative treatment program has failed to result in acceptable pain relief and functional return, arthroscopy is considered. A careful examination under anesthesia should be performed prior to final patient positioning on the operating table, taking particular note of ROM and translational laxity in all planes. Surgeon choice dictates the setup position, either lateral decubitus or beach-chair. Hypotensive anesthesia (systolic blood pressure [BP] approximately 100 mm Hg) will minimize bleeding and improve visualization but requires careful monitoring, particularly with the patient in the beach chair position. In the sitting orientation, the blood pressure at the level of the cranium may be significantly less than at the height of the arm cuff, resulting in hypoperfusion of the brain. Furthermore, normal physiologic compensatory mechanisms that protect blood flow to the brain may be compromised under general anesthesia. Once the patient is appropriately prepped and draped, a routine posterior portal is established. An anterior portal is created midway between the anterolateral acromion and tip of the coracoid. The arthroscope must alternately be placed into anterior and posterior portals to afford a comprehensive evaluation. A systematic diagnostic glenohumeral evaluation is essential because partial-thickness rotator cuff tears are often accompanied by additional pathology, including labral tears, biceps pathology, chondral defects, and loose bodies. These associated lesions should be addressed arthroscopically in the manner deemed appropriate. Partial articular surface tears of the supraspinatus are readily evaluated from the posterior portal and those of the infraspinatus from the anterior portal. The subscapularis, particularly the insertion of the superior border, may require the use of the 70- degree arthroscope from the posterior portal. A dynamic assessment of the potential for internal impingement is accomplished by removing traction and placing the arm in abduction and external rotation while viewing with the arthroscope in the posterior portal. Support for the diagnosis of internal impingement is present if a partial articular surface tear of the posterior supraspinatus abuts the posterosuperior glenoid and adjacent labrum with the arm in the overhead throwing position.
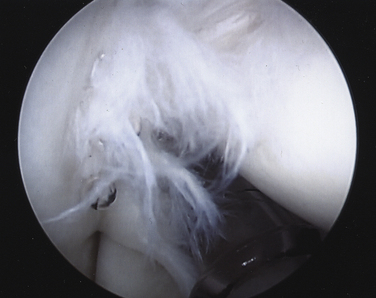
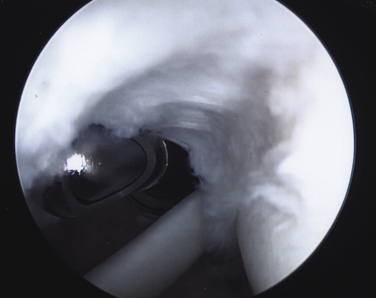
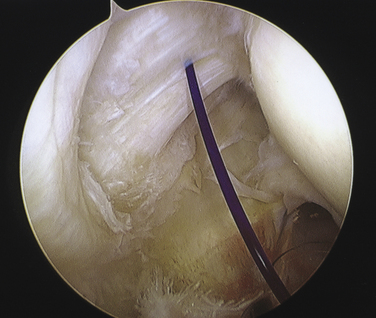
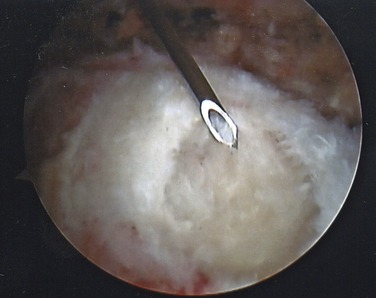
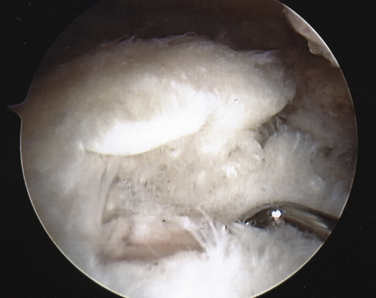
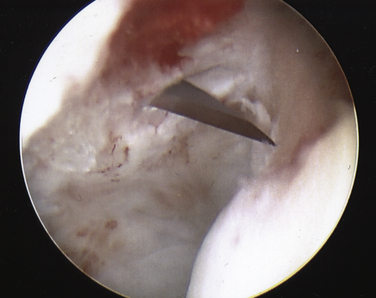
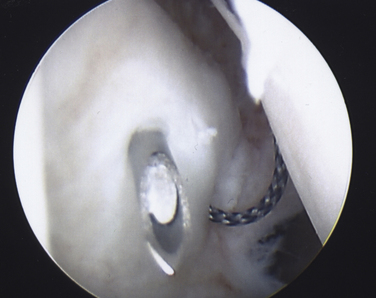
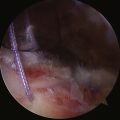
On completing the glenohumeral evaluation, if a partial-thickness articular surface tear is identified (Fig. 19-3),1 débridement is performed to create healthy tissue margins (see later; Fig. 19-4). An 18-gauge spinal needle is then introduced through the skin over the anterolateral shoulder and through the cuff defect under direct arthroscopic visualization. A monofilament suture is introduced through the spinal needle, which is then withdrawn (Fig. 19-5). The suture serves as a marker in the subacromial space for the corresponding location of the articular surface defect. The arthroscope is withdrawn and reestablished in the subacromial space. A lateral subacromial portal is established 2 cm lateral and just posterior to the anterolateral acromial corner. While viewing from the posterior portal, a shaver or radiofrequency device introduced through the lateral subacromial portal is used to remove thickened, inflamed, or hemorrhagic bursal tissue. It is essential to then place the arthroscope in the lateral portal, and complete resection of the posterior bursal curtain from a posterior portal. This preparation affords an accurate examination of the entire bursal surface of the rotator cuff, inferior surface of the acromion, coracoacromial ligament, and acromioclavicular joint. The previously placed marker suture may be hidden by a lateral bursal shelf, which must be carefully separated to avoid inadvertent transection of the suture. Alternating internal and external rotation of the shoulder affords a relatively complete view of the bursal surface of the cuff (Figs. 19-6 and 19-7).
Débridement
Following débridement, an instrument of known size is used to estimate the tear depth based on the size of the exposed footprint. Regardless of the specific classification scheme used, both the breadth and anteroposterior dimension of the tear should be documented. Despite having a good impression of the thickness of the remaining cuff, it is much more difficult to know the integrity and durability of the remaining intact portion with certainty. For those tears with a defect less than or equal to 3 mm (A1), débridement alone is sufficient. Tears of similar magnitude on the bursal surface are managed in the same manner, with the addition of an acromioplasty. Intratendinous tears can be difficult to identify because of relatively normal-appearing articular and bursal surfaces. Occasionally, firm palpation of these tears from the bursal surface with a probe will suggest subsurface irregularity, and unroofing the tear with a shaver may be necessary for more accurate identification. Alternatively, saline injection into the suspected defect with a syringe and 22-gauge needle may demonstrate an atypical bulbous distention of the cuff.
Little evidence exists to indicate a significant spontaneous healing potential for partial cuff tears. In one study of 40 articular-sided tears followed up arthrographically at 2 years, 80% progressed in size and only 10% gave the impression that the tear decreased in size.29 The influence of tear depth and area in relation to patient outcomes is not well understood. Although a defect of more than 50% on the articular side has been commonly referred to as an indicator of when débridement alone is likely to be insufficient, no data exists to establish that figure firmly as a guide. For sedentary individuals, débridement alone of tears between 30% and 50% thickness is often sufficient. In higher demand patients such as an overhead athlete or manual laborer, a tear defect larger than 30% of the tendon thickness (>4 mm), may require repair because of the limited ability of the tear to heal and the propensity to enlarge. Bursal surface tears may be less amenable to débridement alone. In one study of 162 patients with a diagnosis of primary impingement, all shoulders underwent débridement of the cuff defect and an anterior acromioplasty. A 5% failure rate was noted for 44 patients with an A2 tear in contrast to a 38% failure rate for 8 patients with a B2 tear. The patient’s activity level also plays a role in determining treatment.
Acromioplasty
Various methods have been described to resect the anterolateral acromion and decompress the subacromial space accurately and adequately. In the past, the expressed intent was to attempt to create a type I or flat acromion in most cases, which often led to an excessive and unnecessary amount of bone removal and substantial detachment of the anterior deltoid. The removal of a 6-mm thickness of anterior acromion can result in the loss of 74% of the deltoid origin at that site.30 Only the prominent anterior rim of bone and any associated osteophytes need be resected. Furthermore, it is unnecessary to intentionally detach or resect the coracoacromial ligament.
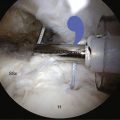
A safe approach is to use a cutting bar technique. While viewing from the lateral subacromial portal, a barrel-shaped burr is introduced from the posterior subacromial portal immediately inferior to the posterior border of the acromion, similar to the cutting block technique. The burr tip, however, begins to resect bone first from the most inferior extent of the anterior acromion and works progressively superior rather than beginning near the apex of the acromial arch. It is rarely necessary to resect more than a 5- to 6-mm thickness of the bone anteriorly. Once the appropriate level of resection has been established in the saggital plane, the arthroscope is then switched to the posterior viewing portal. With the burr in the lateral subacromial portal, the anterolateral aspect of the acromion is planed to make it flush with the initial plane of resection. Slight feathering at the posterior extent of the resection completes the acromioplasty while substantially preserving the integrity of the coracoacromial arch. Alternatively, if the acromion has a flatter saggital profile, the burr can be introduced through the lateral portal initially and used simply to smooth the inferior surface, with no attempt to alter the shape. Somewhat greater anterolateral resection is required if there is a significant lateral downslope to the acromion.
Transtendon Repair of Articular-Sided Tears
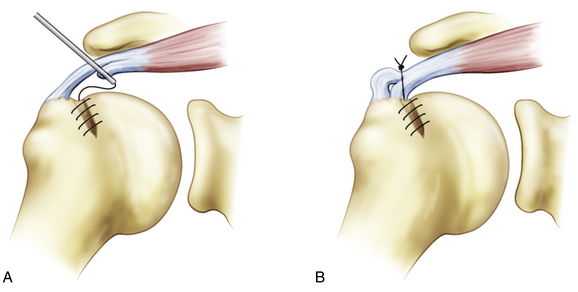
No proven guidelines exist for the optimal management of type A2 or mild A3 partial cuff tears (approximately 4 to 8 mm of exposed greater tuberosity footprint following débridement). For articular-sided tears, the options include a transtendon repair or completing the tear followed by repair. In a cadaveric model, 50% thickness articular surface tears were created. Transtendon repair outperformed tear completion and dual-row repair of the full-thickness defect in these paired specimens, with significantly less gap formation and greater ultimate load to failure.31 The intact bursal layer of these created tears was likely relatively normal and underscores the importance of carefully evaluating the integrity of the remaining intact bursal layer if a transtendon repair is contemplated.
Once an acceptable entry site is chosen, a no. 11 blade scalpel is introduced through the skin and intact bursal cuff surface (Fig. 19-8). The 4- to 5-mm slit that is created near the tuberosity should parallel the tendon fibers. If the need for two anchors is anticipated, separate approaches through the cuff are necessary. Anchors double-loaded with high-strength suture are used. Although metal anchors may be inserted directly through the cuff slit, nonmetallic anchors generally require that the hole be prepared with an awl and tap. Once implanted, the anchor is tested for security by placing firm traction on the sutures that exit the skin. Sequential suture passage through the cuff progresses from anterior to posterior. A disposable obturator cannula is established at the anterior portal site. An 18-gauge spinal needle is introduced through the skin anterior to the sutures and through the anterior tear margin from a lateral approach (Fig. 19-9). If the tear margin is retracted medially, graspers are used to reduce the tear prior to penetration with the spinal needle.
Failure to reduce the tear tends to promote a relatively perpendicular penetration of the spinal needle through the intact bursal surface of the cuff too far medially (Fig. 19-10A). Consequently, the suture passes through the bursal surface further medial than the articular surface. As the sutures are tied, a nonanatomic buckling of the bursal surface is created (see Fig. 19-10B).
After proper insertion of the spinal needle through the cuff, a 0 monofilament suture is introduced through the needle and retrieved out the anterior cannula with a loop grasper. The most anterior limb of anchor suture is then also retrieved out the anterior cannula. A simple overhand throw is created with the monofilament suture around the anterior limb of anchor suture. By placing traction on the lateral limb of monofilament suture that exits the skin, the anchor limb will be shuttled through the cuff margin and out the skin (Fig. 19-11). Using the same technique, the remaining anchor sutures are sequentially delivered and evenly spaced along the articular tear margin (Fig. 19-12). If two anchors are used, it is preferable to place the posterior anchor and pass those repair sutures first, followed by the anterior anchor to avoid crowding and difficult suture management.
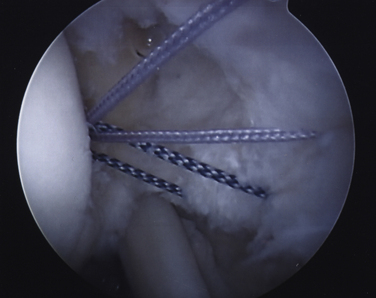
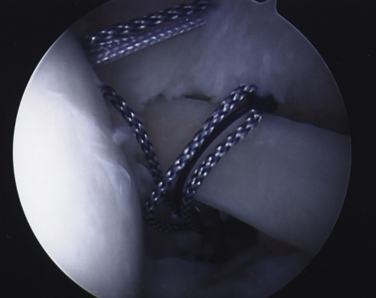
FIGURE 19-12 All transtendon sutures in place through the articular margin prior to tying in the subacromial space.
Once all repair sutures have been passed, the arthroscope is reestablished in the subacromial space through the posterior portal. Abduction of the arm to 45 degrees will reduce tension on the articular cuff margin as the sutures are tied. A lateral subacromial cannula is established and the most anterior suture retrieved. By placing enough tension on the suture to cause it to be withdrawn slowly, its mate can easily be identified and retrieved. The surgeon’s preferred knot-tying technique is used with corresponding pairs to complete the transtendon repair. Replacement of the arthroscope into the glenohumeral joint will enable probing and confirmation that the repair is secure.
Repair of Partial Bursal-Sided Tears
Analogous to preservation of the bursal surface during a transtendon repair of an articular-sided tear, the articular surface can be left intact with a bursal-sided partial repair. The intact articular side maintains the appropriate length for the cuff and serves as the medial row fixation. Once the subtotal bursectomy is completed, a posterolateral viewing portal is established. While viewing from a posterior subacromial portal, an 18-gauge spinal needle is introduced 3 to 4 cm posterior to the lateral subacromial portal and is directed toward the center of the cuff tear. With a 30-degree arthroscope, the view from this portal provides a “50-yard line” vantage of the cuff tear while maintaining availability of all the other portals for working instruments. The bursal surface of the cuff is débrided with a shaver back to acceptably healthy tissue. The length of the remaining bursal layer will determine the appropriate location for the lateral anchors. If sufficient length is present, the anchors can be placed 5 to 7 mm over the edge of the greater tuberosity laterally in a tension band configuration. Alternatively, if there is significant loss of bursal layer length, the anchors may need to be placed on the exposed tuberosity. For suture passage, cannulated suture hooks from an anterior or posterior portal or a lasso device from a Neviaser supraclavicular portal can be used to introduce a monofilament suture through the bursal layer margin. One limb of each pair of anchor sutures can be shuttled through the cuff with a previously placed monofilament suture. Suture pairs are then tied in simple fashion. Alternatively, the anchor suture limbs can both be shuttled to create a horizontal mattress configuration. A knotless anchor is then used to secure the suture limbs to the lateral aspect of the greater tuberosity. Using either method, as the sutures are secured, the bursal layer is reapproximated to the prepared greater tuberosity.
Tear Completion and Repair
Many instruments are available for suture passage and numerous strategies exist for suture repair patterns. Generally, by antegrade or retrograde methods, the medial row sutures are placed in a horizontal mattress configuration. If a suture bridge technique is preferred, the medial row pairs are tied, left uncut, and retrieved out an anterior or posterior subacromial portal. Through the lateral subacromial portal, appropriate preparation for the particular lateral row anchor chosen is made 5 to 7 mm over the lateral edge of the tuberosity. One limb of each of the medial suture pairs is retrieved out the lateral cannula. Those medial repair sutures are inserted in a manner appropriate for the particular lateral anchor, which is then introduced into the prepared hole. The sutures are tensioned and the anchor deployed. In a similar manner, the remaining sutures are brought out to a second, appropriately spaced anchor and secured. If a single-row construct is planned, a triple-loaded anchor is optimal. All the sutures can be placed in simple fashion, or one of the various locking configurations can be used. It is best to pass all the sutures through the cuff before beginning to tie or fix them to knotless anchors.
PEARLS& PITFALLS
PEARLS
PITFALLS
OUTCOMES
Most studies evaluating nonoperative and operative treatment of partial-thickness rotator cuff tears are poorly controlled, use different outcome measures, contain small numbers of patients, and lack randomization. Although available studies report satisfactory outcomes in 75% to 85% of patients following débridement of tears less than 50% thickness, with or without acromioplasty, those results tend to deteriorate with time. Débridement with acromioplasty has not been shown to prevent the progression of rotator cuff disease.32–34 In one study, patients with a B2 PTRCT had a statistically significantly higher failure rate (38%) with débridement and acromioplasty alone, suggesting that this group may have experienced a better outcome with a cuff repair. Few reports evaluating transtendon repair are available. In one study, 22 patients underwent an arthroscopic transtendon suture anchor repair of defects ranging from 30% to 70%.35 After an average follow-up of 16 months, 86% of patients had a good or excellent result and 91% were satisfied. In a separate clinical evaluation of patients who underwent arthroscopic transtendon repair for A3 partial cuff tears, good or excellent outcomes were documented in 16 of 17 patients at an average of 39 months using the UCLA rating scale.36 An evaluation of 54 patients who underwent a transtendon repair for articular-sided tears averaging 5.2 mm was conducted, with a minimum 2-year follow-up. UCLA, Constant, and simple shoulder test assessments revealed that 98% had a good or better outcome. Better results were documented in patients with less tendon retraction, a larger footprint exposure, younger age, and a clinical history of trauma. In a nonrandomized group of patients who had more than 50% PTRCTs (88% articular, 12% bursal tears), 32 who underwent débridement and acromioplasty were compared with 33 who had an acromioplasty and miniopen repair.33 Although the débridement group had less postoperative pain and a faster recovery, three progressed to a full-thickness tear. There were no reruptures and no reoperations in the repair group. A prospective study evaluated 41 patients with tears greater than 50% who underwent repair.37 The tear was converted to a full-thickness defect and repaired with suture anchors and simple sutures. Isometric testing showed strength equal to the uninvolved shoulder for all patients, 98% of whom were satisfied with their result. Intra-articular suture anchor repair of partial articular surface tears of the superior subscapularis resulted in a good or excellent outcome in 28 of 29 patients at an average 27-month follow-up,38 and 90% of the patients resumed their normal activities. A treatment recommendation summary is provided in Table 19-1.
| Defect Thickness | Treatment |
|---|---|
| Articular Surface Tears | |
POSTOPERATIVE REHABILITATION PROTOCOL
When a cuff repair has been performed, a protective padded sling is worn for 4 weeks following surgery. Gentle pendulum rotations are again initiated POD 1. Formal physical therapy begins at approximately POD 14 for gentle, progressive, passive ROM exercises, with a goal of full passive motion at 10 to 12 weeks following surgery. Periscapular isometrics are initiated 2 weeks following surgery, and light rotator cuff strengthening begins at 6 to 8 weeks, depending on the size of the repair. Light sports-specific activities (e.g., chipping, putting) are undertaken at 3 to 4 months. More detailed descriptions of rotator cuff repair protocols are present in subsequent chapters on repair of full-thickness cuff defects.
1. DePalma AF. Surgery of the Shoulder. Philadelphia: JB Lippincott; 1950.
2. Clark JM, Harryman DT Tendons, ligament and capsule of the rotator cuff: gross and microscopic anatomy. J Bone Joint Surg Am, 74; 1992:713-725.
3. Flatow EL, Kelkar R, Raimondo RA, et al Active and passive restraints against superior humeral translation: the contributions of the rotator cuff, the biceps tendon and the coracoacromial arch. J Shoulder Elbow Surg, 5; 1996:S111.
4. Cooper DE, O’Brien SJ, Arnoczky SP, et al The structure and function of the CHL: an anatomic and microscopic study. J Shoulder Elbow Surg, 2; 1993:70-77.
5. Rathbun JB, MacNab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg Br. 1970;52:540-553.
6. Moseley HF. Goldie I. The arterial pattern of the rotator cuff of the shoulder. J Bone Joint Surg Br. 1963;45:780-789.
7. Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res. 1990;254:35-38.
8. Dugas JR, Campbell DA, Warren RF, et al. Anatomy and dimensions of the rotator cuff insertions. J Shoulder Elbow Surg. 2002;11:498-503.
9. Ruotolo CV, Fow JE, Nottage WM The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy, 20; 2004:246-249.
10. Burkhart SS, Esch JC, Jolson RS The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge”. Arthroscopy, 9; 1993:611-616.
11. Nakajima T, Rokuuma N, Hamada K, et al Histologic and biomechanical characteristics of the supraspinatus tendon: reference to rotator cuff tearing. J Shoulder Elbow Surg, 3; 1994:79-87.
12. Sano H, Wakabayashi I, Itoi E Stress distribution in the supraspinatus tendon with partial-thickness tears: an analysis using two-dimensional finite element model. J Shoulder Elbow Surg, 15; 2006:100-105.
13. Mazzocca AD, Rincon LM, O’Connor RW, et al Intra-articular partial-thickness rotator cuff tears: analysis of injured and repaired strain behavior. Am J Sports Med, 36; 2008:110-116.
14. Bey MJ, Song HK, Wehrli FW, Soslowsky LJ Intratendinous strain fields of the intact supraspinatus tendon: the effect of glenohumeral joint position and tendon region. J Orthop Res, 20; 2002:869-874.
15. Wolff AB, Sethi P, Sutton KM, et al. Patrtial-thickness rotator cuff tears. J Am Acad Orthop Surg. 2006;14:715-725.
16. Lohr JF, Uhthoff HK. The pathogenesis of degenerative rotator cuff tears. Orthop Trans. 1987;11:237.
17. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;223:51-58.
18. Yamanaka K, Fukuda H. Pathologic studies of the supraspinatus tendon with reference to incomplete partial thickness tears. In: Takagishi N, editor. The Shoulder. Tokyo: Professional Postraduate Services; 1987:220-224.
19. Sakurai G, Osaki J, Tomita Y, et al Incomplete tears of the subscapularis tendon associated with tears of the supraspinatus tendon: cadaveric and clinical studies. J Shoulder Elbow Surg, 7; 1998:510-515.
20. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;254:64-74.
21. Snyder SJ, Pachelli A, Del Pizzo W et al. Partial thickness rotator cuff tears: results of arthroscopic treatment. Arthroscopy, 7; 1991:1-7.
22. Spencer EEJr, Dunn WR, Wright RW, et al. Shoulder Multicenter Orthopaedic Outcomes Network. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging. Am J Sports Med. 2008;36:99-103.
23. Rafii M, Firooznia H, Sherman O, et al Rotator cuff lesions: signal patterns at MR imaging. Radiology, 177; 1990:817-823.
24. McConville OR, Iannotti JP Partial-thickness tears of the rotator cuff: evaluation and management. J Am Acad Orthop Surg, 7; 1999:32-43.
25. Meister K, Thesing J, Montgomery WJ, et al MR arthrography of partial thickness tears of the undersurface of the rotator cuff: an arthroscopic correlation. Skeletal Radiol, 33; 2004:136-141.
26. Stetson WB, Phillips T, Deutsch A. The use of magnetic resonance arthrography to detect partial-thickness rotator cuff tears. J Bone Joint Surg Am. 2005;87(suppl 2):81-88.
27. Herold T, Bachthaler M, Hamer OW, et al Indirect MR arthrography of the shoulder: use of abduction and external rotator to detect full and partial-thickness tears of the supraspinatus tendon. Radiology, 240; 2006:152-160.
28. Lee SY, Lee JK Horizontal component of partial-thickness tears of the rotator cuff: imaging characteristics and comparison of ABER view with oblique coronal view at MR arthrography—initial results. Radiology, 224; 2002:470-476.
29. Yamanaka K, Masumoto T The joint side tear of the rotator cuff: a follow-up study by arthrography. Clin Orthop Relat Res, 304; 1994:68-73.
30. Torpey B, Ikeda K, Weng M, et al The deltoid muscle origin: histologic characteristics and effect of subacromial decompression. Am J Sports Med, 26; 1998:379-383.
31. Gonzalez-Lomas G, Kippe MA, Brown GD, et al. In situ transtendon repair outperforms tear completion and repair for partial articular-sided supraspinatus tendon tears. J Shoulder Elbow Surg. 2008;17:722-728.
32. Hyvonen P, Lohi S, Jalovaara P Open acromioplasty does not prevent the progression of an impingement syndrome to a tear: Nine-year follow-up of 96 cases. J Bone Joint Surg Br, 80; 1998:813-816.
33. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15:126-131.
34. Kartus J, Kartus C, Rostgard-Christensen L, et al. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22:44-49.
35. Waibl B, Buess E Partial-thickness articular surface supraspinatus tears: a new transtendon suture technique. Arthroscopy, 21; 2005:376-381.
36. Ide J, Maeda S, Takagi K Arthroscopic transtendon repair of partial-thickness articular-side tears of the rotator cuff: anatomical and clinical study. Am J Sports Med, 33; 2005:1672-1679.
37. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16:193-201.
38. Kim SH, Oh I, Park JS, et al. Intra-articular repair of an isolated partial articular-surface tear of the subscapularis tendon. Am J Sports Med. 2005;33:1825-1830.