45 Parkinson’s disease
Salient features
History
• Tremor: usually unilateral at onset; usually starts in upper limbs. Also seen in the legs and jaws
• Rigidity: ask about history of falls, poor balance, pain and muscle stiffness
• Poverty of movement: ask about drooling of saliva, difficulty in writing (micrographia), difficulty in turning in bed and change in voice (softness of voice)
• Family history of disease (susceptibility genes include α-synuclein, leucine rich repeat kinase 2 and glucocerebrosidase)
• History of smoking (never smokers are twice as likely to develop disease) and caffeine intake (those who take no or very low quantities of daily caffeine, are at increased risk, ~25%) (Lancet 2009;373;2055–66)
• History of exposure to managanese dust, carbon disulfide or carbon monoxide
• Use of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) for recreational purposes
• Elicit a drug history, particularly regarding neuroleptics (reserpine, metoclopramide)
• History of herbal medications, particularly Pacific sedative kava kava and Indian snake root Rauwolfia serpentina
• History of severe head injury, encephalitis, hypertension or cerebrovascular disease.
Examination
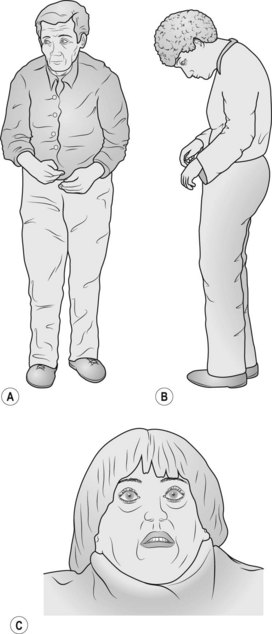
• Comment on the expressionless face, pill-rolling movement and drooling of saliva so that the examiner knows that you have observed these abnormalities. Elicit bradykinesia by asking the patient to touch her thumb with each finger in turn.
• Examine the tone, in particular at the wrist for cog-wheel rigidity.
• Proceed to do the glabellar tap (tap the forehead just above the bridge of the nose repeatedly (about twice per second): in normal subjects, the blinking will stop whereas the patient with Parkinson’s disease continues to blink: referred to as Myerson’s sign. (It must be remembered that this sign is unreliable.)
• Ask the patient to walk and comment on the paucity of movement including the absence of arm swing and festinating gait (the patient walks with a stooped posture as if trying to catch up with her centre of gravity). The feet may scrape the floor in taking steps so the patient trips easily (be prepared to prevent the patient from falling when examining the gait).
Questions
What comprises Parkinson’s disease?
• Upper body dyskinesia must be present; it is a symptom complex containing many of the following features:
• Rigidity is usually but not always present:
• Postural instability is usually a late feature
Advanced-level questions
What are the pathological changes in Parkinson’s disease?
The most typical pathological hallmarks of Parkinson’s disease are:
• neuronal loss with depigmentation of the substantia nigra
• Lewy bodies, which are eosinophilic cytoplasmic inclusions in neurons consisting of α-synuclein.
The following associations have been made with clinical features and pathological changes:
| Clinical deficit | Pathology |
|---|---|
| Motor symptoms | Degeneration of dopaminergic nigrostriatal pathway |
| Cognitive defects | Degeneration of dopaminergic mesocortical and mesolimbic pathways |
| Autonomic dysfunction | Dopamine depletion in the hypothalamus |
| ‘Freezing phenomenon’ | Degeneration of the noradrenergic locus ceruleus |
| Dementia | Degeneration of the cholinergic nucleus |
What is the mental status of patients with Parkinson’s disease?
• In the initial stages, intellect and senses are usually preserved. Many patients have some intellectual deterioration—a slowness of thought and of memory retrieval (bradyphrenia), and subtle personality changes.
• Global dementia may develop in one-fifth of patients.
What are the causes of Parkinson’s disease?
True parkinsonism
• Idiopathic (caused by degeneration of the substantia nigra); also known as Parkinson’s disease
• Drug induced (chlorpromazine, metaclopramide, prochlorperazine)
• Anoxic brain damage such as cardiac arrest, exposure to manganese and carbon monoxide
• Post-encephalitic, as a result of encephalitis lethargica or von Economo disease
• MPTP toxicity, seen in drug abusers
• Progressive supranuclear atrophy
• Familial: mutation of the gene for α-synuclein or linkage to a region on chromosome 2.
What differences are seen in rigidity, spasticity and gegenhalten?
• Rigidity indicates increased tone affecting opposing muscle groups equally and is present throughout the range of passive movement. When smooth it is called lead-pipe rigidity and when intermittent is termed cog-wheel rigidity. It is common in extrapyramidal syndromes: Wilson’s disease and Creutzfeldt–Jakob disease.
• Spasticity of the clasp-knife type is characterized by increased tone, which is maximal at the beginning of movement and suddenly decreases as passive movement is continued. It occurs chiefly in the flexors of the upper limb and extensors of the lower limb (antigravity muscles).
• Gegenhalten, or paratonia, is where the increased muscle tone varies, and becomes worse the more the patient tries to relax.
What is the role of protein diets in patients who have episodes of sudden and substantial loss of mobility?
How is the severity of Parkinson’s disease graded?
Hoehn–Yahr staging grades Parkinson’s disease into five stages:
How would you manage a patient with Parkinson’s disease?
Step I: replacing dopamine neurotransmitter that is lost as the dopamine neurons degenerate is the mainstay treatment. All drugs are started at low doses and doses are increased slowly to reduce adverse effects (start low, go slow). Withdrawal of therapy also should be done slowly to avoid worsening of parkinsonism or precipitating neuroleptic malignant syndrome:
Step II: transplanting fetal nerve tissue to replace dopamine neurons that have been lost.
Step III: halting neuronal loss altogether with trophic factors; this is in the early stages of clinical testing. At present, there are no neuroprotective therapies, although clinical trials with monoamine oxidase B inhibitors, dopamine agoists and coenzyme Q0 may slow progression. Glial cell line-derived neurotrophic factor (GDNF) is under investigation. Data are still needed to clarify neuroprotective therapies.
What is the role of thalamotomy in treatment of Parkinson’s disease?
• Thalamotomy is used for intractable tremor and radiofrequency ablation of an area of the venterointermediate nucleus of the thalamus. It is unsuitable for bilateral tremor as bilateral thalamotomy tends to cause impairment of speech.
• Thalamic stimulation is achieved with placement of a fine electrode in the venterointermediate nucleus and insertion of a pacemaker under the skin on the chest. This relieves tremor and can be performed bilaterally. Neither procedure improves akinesia, the most disabling aspect of Parkinson’s disease.
• Deep-brain stimulation of the globus pallidus and subthalamic nucleus may result in striking improvements in parkinsonism and dyskinesia, resulting in large reductions of levodopa dose and thus improvements in levodopa-induced dyskinesias (Lancet 1995;345:91–5).
• Unilateral posteroventral medial pallidotomy ameliorates contralateral parkinsonian symptoms and medication-related dyskinesia and the effect is sustained for up to 5.5 years. Improvements in ipsilateral and axial symptoms are not sustained and many patients undergo, a second contralateral procedure (N Engl J Med 2000;342:1708–14).
Mention some heredo-degenerative parkinsonian disorders
• Hallervorden–Spatz disease: autosomal recessive; patients also have dementia, dystonia, choreoathetosis, retinitis pigmentosa. There is increased iron deposition and increased cysteine in the globus pallidus.
• Fahr’s disease or familial basal ganglia calcification: patients also have chorea, dementia and palilalia.
• Olivopontocerebellar and spinocerebellar degenerations: autosomal dominant; associated cerebellar ataxia and retinitis pigmentosa.
How would you manage autonomic and psychological symptoms?
• Insomnia: adjust Parkinson’s disease drugs; use sleep hygiene techniques or clonazepam
• Depression: serotonin and noradrenergic reuptake inhibitors or amitryptiline
• Rapid eye movement behaviour disorders: adjust Parkinson’s disease drugs or give clonazepam
• Fatigue: amantidine or selegiline
• Day time sleepiness: modafinil
• Psychosis and hallucinations: adjust Parkinson’s disease drugs or use an antipsychotic (clozapine, quetiapine or aripiprazole). Quetapine typically does not worsen motor function and is often used as first-line therapy
• Constipation: osmotic laxatives (macrogol)
• Urinary urgency: check drugs; use anticholinergic bladder stabilizers, and desmopressin for nocturia
• Impotence: sildenafil, tadalafil and vardenafil
• Pain: adjust Parkinson’s disease drugs and give muscle relaxants
• Restless legs: dopamine agonists
• Orthostatic hypotension: adjust Parkinson’s disease drugs; increase water and salt intake; give fludrocortisone, ephedrine or midodrine
• Drooling: 0.5% atropine eye drops sublingually, scopoderm patch or botulinum toxin injections into salivary glands
• Excessive sweating: adjust Parkinson’s disease drugs; give propantheline, propranolol or topical aluminium creams.