Chapter 31 Parkinson’s disease
With contribution from Dr Lily Tomas
Introduction
Parkinson’s disease (PD) is a degenerative neurological disorder that becomes more prevalent with age and is of unknown aetiology. It is characterised by a progressive degeneration of the nigrostriatal dopaminergic pathway subsequently leading to progressive tremor, bradykinesis, rigidity and postural instability. As such, the primary features of PD relate to a deficiency of dopamine, with the development of traditional pharmaceutical therapies being based around the replacement of this particular neurotransmitter. However, effective as these are, they do not address all features of the disease that may not be secondary to low dopamine.1 This appears to be 1 of the prime reasons that complementary and alternative medicine (CAM) therapies are widely used by patients afflicted with this disease. Surveys throughout the world demonstrate that 40–76% patients with PD have used at least 1 CAM therapy since their original diagnosis.2–5
Incidence/prevalence in Australia
Patients using CAM are seeking to improve their motor symptoms (57.6%), for fatigue (19.6%), for pain relief (4.3%), for constipation (5.4%) or for no specific reason (13%).2 The most common therapies used by Westerners include vitamins, herbs, massage and acupuncture. Users of CAM tend to be younger and have a younger age of onset of PD, and have a higher income and education level than non-users.5 Patients with more severe motor dysfunction symptoms at onset are also more likely to use CAM.4
In 1998, the Parkinson’s Disease Society (PDS) set up a working group to look at complementary therapies and PD. Their first initiative was to survey PDS members about their experiences of complementary therapies. Over 2000 people replied and the results are presented in Table 31.1.6, 7
Table 31.1 Complementary therapies utilised and reported effective, together with the percent benefits
| CAM modality | Benefit described by those who reported a positive effect |
|---|---|
| Acupuncture/acupressure |
(Source: adapted and modified from Paccetti C, Mancini F, Agliera R et. al. Active music therapy in PD: an integrative method for motor and emotional rehabilitation. Psychosom Med 2000:62(3):386–93)
Lifestyle
It has been suggested that there are several environmental, lifestyle, and physical attributes that appear to be precursors of PD.8 In summary these include:
Factors that showed an inverse association with PD included:
Mind–body medicine
Cognitive behavioural therapy (CBT)
There have been several studies demonstrating a benefit of CBT for those individuals suffering with PD. There is a strong comorbidity between PD and depression and for these patients there is a faster progression of physical symptoms, greater cognitive decline and poorer quality of life. It is important to note that depressed PD patients often differ from depressed non-PD patients and therefore CBT may need to be adapted to their individual needs.10, 11
One such study of individually tailored CBT demonstrated that PD patients with depression experienced a significant reduction in depressive symptoms and negative cognitions with a greater perception of social support over the course of treatment. These gains were maintained at 1 month follow-up. Larger randomised control trials (RCTs) are needed to further evaluate the efficacy of this intervention.12 CBT has also been shown to be efficacious for carers of those with PD.13
Alexander technique (AT)
Alexander technique is a process of psycho–physical re-education. A systematic review of controlled clinical trials has shown that AT is effective in reducing disability of patients suffering with PD.14 Earlier studies have also shown that AT may be effective for reducing depression for PD patients on drug therapy.15, 16
Imagery
One recent study has shown that the combination of motor imagery and real exercise practice was effective in the treatment of PD, particularly for reducing bradykinesia.7, 17
Music therapy
Available data shows that music therapy is effective on motor, affective and behavioural functioning in PD and ideally should be included in rehabilitation programmes.18
Repetitive transcranial magnetic stimulation (rTMS)
rTMS is a non-invasive brain stimulation technique that can produce lasting changes in excitability and activity in cortical regions underneath the stimulation coil (designated as a local effect), but also within functionally connected cortical or sub-cortical regions (designated as remote effects).19 Moreover, although the results from a small efficacy study support the beneficial effects of rTMS on Parkinsonian symptoms, long-term studies with large numbers of participants should be conducted to assess the efficacy of the rTMS on PD.20 A recent study has demonstrated that rTMS is significantly safe for use in PD patients.21 However, a comprehensive screening schedule should include electroencephalogram (EEG) before higher-frequency rTMS is applied.
Physical activity/exercise
A systematic review has stated that regardless of the strength of the evidence, the published literature that was reviewed reported that exercise resulted in improvements in postural stability and balance task performance in patients with PD.22 It was also recognised that despite these improvements, the number and quality of the studies and the outcomes used were limited. The authors concluded that there is a need for longer term follow-up so as to establish the trajectory of change in PD patients and to determine if any gains are retained long term. The optimal delivery and content of physical activity interventions (i.e. dosing, component exercises) at different stages of the disease are not clear and require further study.
A recent RCT investigating changes in walking activity and endurance following rehabilitation for people with PD demonstrated that an interdisciplinary rehabilitation program can improve walking activity and endurance depending on baseline walking levels in people diagnosed with PD.23
Despite treatment with drugs or neurosurgery, people with PD are faced with progressively increasing mobility problems.24 Recent meta-analyses have confirmed evidence to support exercise being beneficial with regard to physical functioning, health-related quality of life, strength, balance and gait speed for people with PD.25, 26, 27 Tango dancing, in fact, has been shown to ameliorate some functional mobility deficits in those that are frail and elderly when compared to a general exercise programme.28 A systematic review has also indicated that the effect of a physical treatment declines after the treatment has ended, suggesting the need for permanent treatment for patients with PD.29
A further study has demonstrated that the effects of aerobic exercise are more beneficial than qigong for people with advanced PD.30
There is currently insufficient evidence to support or refute the value of exercise in reducing falls or depression.25 Those with PD are twice as likely to fall compared with the non-PD elderly. An Australian RCT is currently under way to investigate whether exercises focusing on balance, leg muscle strength and gait will benefit those with PD.31
Tai chi
The objective of a critical review was to assess the effectiveness of tai chi as a treatment option for PD.32 The review concluded that the evidence was insufficient to suggest tai chi was an effective intervention for PD. Further research is hence required to investigate whether there are specific benefits of tai chi for people with PD, such as its potential effect on balance and on the frequency of falls.
Recently a pilot study has examined the effects of tai chi on balance, gait and mobility in people with PD.33 This study reported that all tai chi participants reported satisfaction with the program and improvements in wellbeing. Furthermore, the tai chi program appeared to be an appropriate, a safe and effective form of exercise for some individuals with mild–moderately severe PD.33
Environment
Toxins
Health and disease are shaped for all individuals by interactions between their genes and environment, such is the phenomenon now known as epigenetics.34, 35 In recent years, an increased emphasis has been placed on an integrating role of medicine in the prevention, early diagnosis and treatment of diseases that have been linked to environmental factors.36
A lack of heritability in idiopathic PD has implicated adulthood environmental factors in the aetiology of the disease. However, there is increasing evidence that exposure in the womb to a variety of environmental factors (such as exposure to pesticides, heavy metals and an iron-enriched diet) can either directly cause a reduction in the number of dopaminergic neurons, or cause an increased susceptibility to degeneration of these neurons with subsequent environmental insults or with aging alone.37
It should be noted that previous work in an electronics plant, use of chlorpyrifos products and exposure to fluorides are also associated with a significantly increased risk of developing PD. The association exists but is not as strong for previous work in a paper/lumber mill and exposure to cadmium.38
Repeated traumatic loss of consciousness is associated with increased risk of PD. Hypnotic, anxiolytic or anti-depressant drug use for more than 1 year and a family history of PD show significantly increased odds ratios for developing PD. Tobacco use is protective against developing PD.39
Recent evidence has demonstrated that cigarette smoking, alcohol use, fish intake and carbon monoxide intoxication are associated with reduced risk of PD.36, 38 Consuming well-water and living/working on a farm are not associated with PD.40
Heavy metals
There have been a multitude of studies regarding the neurotoxic effects of certain heavy metals and their relationship to PD.28 Several of these reports concern a village in Italy, Valcamonica, that was previously exposed to heavy metals, especially manganese. A high prevalence of PD was observed within the close vicinity (407/100 000) and it was determined that such Parkinsonian patients who had exposure to heavy metals developed a more severe neuropsychological phenotype, without detectable contribution from genetic factors.41
It is important to note, however, that manganese exposure can cause neuro-behavioural and neurological symptoms at concentrations much less than is currently considered to be the minimum acceptable level. The relationship appears to be dose-dependent, with adults presenting primarily with motor symptoms and children with dysfunctions in cognition and behaviour.42 Preliminary studies show that manganese may be transported into the brain by either transferrin or non-transferrin-dependent mechanisms which may include calcium channels.43
Chronic occupational exposure to manganese or copper, individually, or dual combinations of lead, iron and copper have also been associated with PD.44, 45
Recently, PD has been characterised by elevated tissue iron (not currently connected with hemochromatosis) and mis-compartmentalisation of copper and zinc.46 Zinc levels appear to be greater in PD independently from metal exposure whereas the perturbation of copper metabolism seem to be associated with exposure to environmental toxins or metals and could, indeed, be involved in the progression of the disease itself.47
Although brain uptake mechanisms for some metals have been identified, the efflux of metals from the brain has received little attention, preventing the integration of all processes that contribute to brain metal concentrations.43
As such new information comes to light, however, there has been steadily growing interest in how particular metal ions (especially zinc, copper and iron) are involved in neurobiological processes such as the regulation of synaptic transmission. Increasingly sophisticated medicinal chemistry approaches (that are correcting these metal imbalances without resulting in systemic disturbances of these essential minerals) are being investigated. Furthermore, this process shows great promise of being disease-modifying.46
Pesticides
There has been considerable research in the last decade as to the association between the neurotoxic effects of pesticides and the development of PD. Epidemiological evidence certainly suggests that exposure to pesticides may play a significant role in the aetiology of idiopathic PD.35, 38, 40, 48 Indeed, most studies reveal that moderate pesticide exposure is linked to neurological symptoms and altered neuro-behavioural performance, reflecting cognitive and psychomotor dysfunction. There is less evidence that exposure is related to sensory/motor deficits or peripheral nerve conduction, but fewer studies have considered these outcomes. It is also possible that general malaise and mild cognitive dysfunction may be the most sensitive manifestation of symptoms of pesticide neurotoxicity.49
Many pesticides (organophosphates, carbamates, pyrethroids, organochlorines) target the nervous system of insect pests and, because of the similarity in brain biochemistry, such chemicals may also be neurotoxic to humans. Indeed, adverse effects on brain development can be severe and irreversible.50 This may be of more concern in the case of developing brains that are particularly vulnerable to the adverse effects of neurotoxins.50, 51
There is strong human epidemiological evidence for persistent nervous system damage following acute intoxication with several pesticide groups such as organophosphates and certain fumigants.52 Investigations are now concentrating on whether chronic low-level exposure to pesticides in adults, children or in-utero may also result in persistent nervous system damage.51 There is evidence that exposure to the common agricultural pesticides such as maneb, paraquat and rotenone increases the risk of PD, particularly when exposure occurs at a younger age.35, 53 Such an association suggests a causative role.39 However, despite definitive evidence from animal and cell models that pesticides cause a neurodegenerative process leading to PD, human data are insufficient to support this claim for any specific pesticide primarily because of ethical challenges in exposure assessment.53
It is recommended that the residues of pesticides in food and other types of human exposures should be prevented with regards to those pesticide groups that are known to be neurotoxic. ‘Whilst awaiting more definitive evidence, existing uncertainties should be considered in light of the need for precautionary action to protect brain development.’50
Nutritional influences
Diets
There has been much research attempting to elucidate the most beneficial diet in order to prevent and/or treat PD. Caloric restriction has been proposed to counteract neuronal loss and be associated with extended lifespan. Although there are mixed results, recent animal models with PD have not confirmed this association.54, 55
The dietary patterns of approximately 50 000 men and 80 000 women were followed for 16 years. During this time, 508 new cases of PD were diagnosed. It was found that a high intake of vegetables, fruit, legumes, whole grains, nuts, fish and poultry with a low intake of saturated fat and a moderate intake of alcohol was protective against PD. In comparison, a typical Western diet high in saturated/animal fats was directly associated with PD risk. Indeed, the benefits of a whole-food, plant-based diet with fish warrants further investigation.56, 57
Previous epidemiological studies have shown that consumption of diets rich in antioxidant and anti-inflammatory agents, such as those found in fruits and vegetables, may lower the risk of PD and other age-related neurodegenerative diseases. Further research suggests that the polyphenolic compounds found in fruits such as blueberries exert their positive effects through signal conduction and neuronal communication.58
Uric acid is a natural antioxidant and recent studies demonstrate that it may play a neuroprotective role in the pathogenesis of PD. Dietary manipulation of uric acid through increased consumption of purines (meat/crustaceans) may slow progression of the disease.59 This finding was supported by several earlier studies which show that uric acid levels are lower in individuals with PD than controls. It should also be noted that plasma uric acid correlated strongly with serum ferritin in both patients and control groups.60, 61
As can be easily seen, correct levels of protein intake are currently debatable, with mixed results of studies showing positive benefits for both ketogenic and low-protein dietary regimes.55, 62, 63 A further recent investigation demonstrated that low saturated fat/cholesterol, especially in the presence of high iron intake, may be associated with an increased risk of PD.64
The dietary patterns of approximately 58 000 men and 73 000 women from the American Cancer Society’s Cancer Prevention Study II Nutrition Cohort were also followed for 9 years. It was found that dairy product consumption was positively associated with risk of PD, particularly in men. This is once again controversial and more studies are required to confirm these findings and explore possible underlying mechanisms.65
There have been multiple studies demonstrating the association between caffeine intake and PD. Results are mixed, with some studies showing a protective effect of caffeine and others with no effect.66, 67, 68 A recent study showed that consumption of green tea was unrelated to risk of PD, however, black tea, a caffeine-containing beverage, showed an inverse association with risk of PD. Therefore, it was concluded that the ingredients of black tea other than caffeine appear to be responsible for this inverse association.69
Previous dietary exposure to food contaminants such as polychlorinated biphenyls (PCBs) and methyl mercury (MeHg) have also been positively associated with PD.70
Nutritional supplements
Vitamins and minerals
The nutraceutical treatment for PD is still in its infancy. For example, it has been postulated that imbalances in body metal levels could be a significant risk factor as the homeostasis of trace metals in the brain is important for brain function and also for the prevention of brain diseases.71 Hair mineral analyses indicate significantly lower levels of iron in the hair of patients with PD compared with controls. Calcium and magnesium were slightly lower while zinc was higher in PD, but these differences were not significant.72
Atomic absorption spectrophotometer studies have correspondingly revealed a significant zinc deficiency combined with elevated iron and selenium in the CSF of patients with PD on L-dopa. This provided clear evidence of increased iron and selenium in the brain which could be correlated with decreased dopamine levels and increased oxidative stress in PD patients.73
B group vitamins
Vitamin B6
Adequate dietary vitamin B6 has also been shown to decrease the risk of PD, probably through its antioxidant effects rather than mechanisms related to homocysteine metabolism.75
Folate and vitamin B12
L-dopa treated PD patients have significantly lower serum folate and vitamin B12 than controls.76 In fact, L-dopa treatment may represent an acquired cause of hyperhomocysteinaemia, as evidenced by studies in animals as well as PD patients.77 PD patients with depression have significantly lower folate levels compared with non-depressed PD, and cognitively impaired PD patients have significantly lower vitamin B12 levels compared with non-cognitively impaired PD. Supplementation with cobalamin and folate is effective in reducing homocysteine, and this may have important implications in the management of PD patients who are at risk for vascular diseases, cognitive impairment or dementia. The effects of vitamin supplementation certainly warrant further attention and investigation given that elevated concentrations of total homocysteine in plasma (>12 micromol/l) is epidemiologically associated as a risk factor for several diseases of the central nervous system (CNS).76
A recent review has concluded that treatment with folate, B12, and B6 can improve cerebral function. Hence, preventive vitamin B supplementation and sufficient intake seem very important for secondary and primary prevention of neuropsychiatric disorders, especially in those individuals with a low intake or status of these vitamins.78
Vitamin D
Based upon several lines of evidence, vitamin D deficiency may also be a significant risk factor in the pathogenesis of PD.79 Significantly more patients with PD have been found to have insufficient vitamin D compared with controls.80 Furthermore, animal studies show that 1,25(OH)(2)D(3) may help to prevent dopaminergic neuron damage.81
More studies are warranted to assess the role of vitamin D deficiency in PD.
Zinc
Zinc has many critical functions in brain development and maintenance.82 The CNS concentrates almost 10% total body zinc, a large proportion of which serves as zinc metalloproteins in neurons and glial cells. Ionic zinc exists in the synaptic vesicles, serving as an endogenous neuromodulator in synaptic neurotransmission. Dietary zinc deficiency significantly affects zinc homeostasis in the brain, causing deficiencies of neurotransmitters such as serotonin, gamma-butyric acid (GABA) and melatonin and brain dysfunctions such as cognitive impairment, olfactory dysfunction and depression.83, 84 It is interesting to note that patients with PD often complain of these symptoms.85
PD is characterised by the loss of dopamine-producing cells which is believed to be caused by defects in mitochondrial oxidative phosphorylation and enhanced oxidative stress.86–90 Zinc is required for the synthesis of superoxide dismutase (SOD), a powerful antioxidant. SOD is normally found in high concentrations in the substantia nigra where it protects neurons by scavenging free radicals.91 PD patients show a significantly decreased zinc status compared with controls and zinc supplementation has been shown to significantly increase SOD in vitro.85
Zinc is also necessary for the synthesis of metallothionein, which has been shown to be neuroprotective against the nitric oxide synthase activation and peroxynitrite ion overproduction that may be involved in the aetiopathogenesis of PD.86 Additional studies on the role of zinc in the development and treatment of PD are therefore warranted.85
Copper
Copper is a trace element whose role in the pathogenesis of PD has been widely discussed.90, 92 Low copper may result in incomplete CNS development whereas excess copper may be injurious. It may be involved in free radical production that results in mitochondrial damage, DNA breakage and neuronal injury.93 Free copper is increased in the CSF and thus appears to be a good biomarker for PD.91 Furthermore, a person who gradually accumulates copper will tend to experience a gradual reduction in zinc, with a subsequent increase in oxidative stress, which may eventually lead to PD.82
Iron
Iron accumulation in human and animal PD brains is beyond that observed in non-PD brains of a similar age.71, 94, 95 For animal models, pre-treatment with iron chelators is neuro–protective.96 Several case-control studies have described the association of high dietary iron and PD, but prospective data are lacking. In 1 study, total iron intake was not associated with an increased risk of PD but the risk was significantly increased among individuals with high nonheme iron and low Vitamin C.97 A significantly decreased ferroxidase activity has also been found in patients with PD, confirming previous findings of iron deposition in this disorder.91 At this time, there are only hypotheses as to the possible role of iron in the pathogenic processes of PD, including potential interactions between iron and other factors associated with PD.94
Magnesium
Magnesium deficiency has recently been shown to be involved in the pathogenesis of substantia nigra neuronal degeneration, hence contributing to the risk of PD.36, 98, 99 Results from animal studies show that magnesium might protect dopaminergic neurons in the substantia nigra from degeneration. Thus, magnesium exerts both preventative and ameliorating effects with regards to neurite and neuron pathology in a PD model.100, 101
Other supplements
Coenzyme Q10 (CoQ10)
The concentration of CoQ10 has been reported recently to be in deficit in a number of brain regions (substantia nigra, cerebellum, cortex and striatum) from individuals with PD as compared to controls.102 CoQ10 may therefore also be involved in the pathophysiology of PD.103 A study that employed doses as high as 1200mg/day of CoQ10 in early PD showed that it was safe and well tolerated. Moreover, a recent nano-particular CoQ10 at a dosage of 300mg/day was safe and well tolerated and led to plasma levels similar to 1200mg/day of standard formulations. However, it did not improve primary or secondary outcome measures.104 A large phase III clinical trial is currently under way to examine if high-dose CoQ10 will slow disease progression.105
Further, a new anti-parkinsonian agent has been tested in combination with CoQ10; namely, GPI 1485 (a neuroimmunophilin ligand that binds FK-506-binding proteins) and the RCT study concluded that the combination may warrant further study in PD, even though the data are inconsistent.106
Omega-3 fatty acids
A recent nutrition review related to neurodegenerative diseases suggested that adequate omega-3 fatty acids in the diet will probably prevent most psychotic episodes and prove that neurodegenerative disorders with dementia are also to a large extent not only preventable but avoidable.107 In addition, an RCT study has demonstrated that PD patients, with or without anti-depressants, show improvement in depressive symptoms when supplemented with omega-3 fish oils supplements.108
Levodopa (L-dopa)
L-dopa-induced dyskinesia (LID) is 1 of the major complications that occur in PD patients after prolonged treatment with L-dopa. Animal studies have demonstrated that combining L-dopa (a naturally occurring amino acid found in food and made from l–tyrosine in the human body) with a diet high in creatine (found in, for example, salmon, tuna, sashimi, sushi, lean red meat) could attenuate LID, possibly representing a novel way to control the motor complications associated with L-dopa therapy.109
Alpha-lipoic acid/acetyl L-carnitine
In a review of the in vitro and animal data, Beal has suggested that the considerable evidence that is available suggests that mitochondrial dysfunction may play a role in the pathogenesis of PD and that supplements such as alpha-lipoic acid and acetyl L-carntine may have a role in ameliorating symptoms by modulating cellular energy metabolism.102 Moreover, anecdotal evidence has suggested that alpha-lipoic acid at a dose of 80mg/day and acetyl L-carnitine at 400mg/day in combination with a number of other antioxidant substances (e.g. CoQ10 + vitamin C + vitamin E) could be useful. However there are no clinical data to substantiate this notion.111
Herbal medicines
Curcumin and naringenin
The nutritional supplements, curcumin and naringenin, are phenolic antioxidant compounds that have also recently been shown to prevent significant loss of tyrosine-hydroxylase-positive cells, thereby increasing dopamine levels, in the substantia nigra of animals with induced PD.112
Gingko biloba
Gingko biloba is 1 of the most extensively studied herbal supplements for neurodegenerative conditions that has been reported to directly improve brain metabolism and increase brain blood flow. In a double-blind RCT Gingko biloba was reported to stabilise Alzheimer’s disease and many of the participants demonstrated an improvement as noted in various standardised psychological tests.113 A recent review suggests that Gingko biloba EGb 761 extracts exerts a combination of antioxidative, antiamyloidogenic and anti-apoptotic effects that are in tune with cognitive deficits, however, it may also have a metabolic role to play in PD.114 Clinical trials are hence warranted.
Ayurvedic medicine
Ayurveda is a comprehensive natural health care system that originated in India more than 5000 years ago. Still being used in India as a primary health care system, it is now creating more interest worldwide.115 PD or paralysis agitans and its pharmacological treatment was in fact described in this ancient medical system under the name Kampavata.116
Mucuna pruriens
Mucuna pruriens (MP) contains L-dopa and is the traditional herb used in Ayurveda to treat PD. It is interesting that PD existed before current environmental toxins, suggesting again that PD is of multi-factorial origin.117, 118, 119
The pharmaceutical preparation, HP-200, which contains MP has been shown to be effective in the treatment of PD. Indeed, it has been demonstrated, in animal models, to be more effective than conventional L-dopa.120 MP possesses significantly higher anti-Parkinson activity compared with levadopa in the 6-hydroxydopamine lesioned rat model of PD.121 Unlike synthetic levadopa treatment, MP cotyledon powder significantly restored the endogenous L-dopa, dopamine, noradrenaline and serotonin content in the substantia nigra. It should be noted that nicotine adenine dinucleotide and CoQ10 were also included in this powder.121
MP has been shown to possess anti-Parkinson and neuroprotective effects in animal models of PD. Its antioxidant activity was demonstrated by its ability to scavenge DPPH radicals, ABTS radicals and reactive oxygen species. Furthermore, MP inhibited the oxidation of lipids and deoxyribose sugar and exhibited divalent iron chelating activity. The results suggest that the neuroprotective and neurorestorative effects of MP may be related to its antioxidant activity, independent of the symptomatic effect. In addition, MP appears to be safe in the treatment of patients with PD.122
Traditional Chinese medicine (TCM)
The description and treatment of PD can also be found in the ancient Chinese medical system. When herbs-interposed moxibustion at Shenque acu-point CV8 was added to the routine Western medical therapy, the total effective rate was significantly better than in the control group.123 Recent reviews of trials involving TCM, however, have concluded that larger, more well-designed RCTs need to be performed before any conclusion can be drawn regarding the efficacy of TCM for PD.124, 125
Physical therapies
Allied health care
Allied health care (e.g. occupational therapy, speech therapy, physiotherapy) is used by many patients with PD. Referral rates have been estimated to be 63% for physical therapy, 9% for occupational therapy and 14% for speech therapy. It is advisable to refer patients with PD to an allied health care professional with PD-specific expertise.126
Scientific evidence is beginning to emerge with specific interventions being increasingly integrated in rehabilitation programmes.127 Recent Cochrane reviews, however, have revealed that there is insufficient evidence to support or refute the efficacy of OT, physiotherapy and speech therapy in PD. Larger placebo-controlled RCTs are needed to determine a consensus for ‘best practice’ regimens in these areas.128, 129, 130
Acupuncture
There is a plethora of studies, including meta-analyses, suggesting beneficial effects of acupuncture in the treatment of PD.131–136 Acupuncture is safe and well tolerated in patients with PD.134 Similar results have been obtained with electro-scalp acupuncture (ESA).137 Tremor, rigidity and bradykinesia, in particular, have shown to be improved.138 It has been found that ESA may possibly reduce the loss of dopamine transporters in the basal ganglia, delaying the progression of PD and alleviating clinical symptoms.139 Larger, placebo-controlled RCTs with rigorous methods of randomisation are warranted.132
There have also been several studies combining traditional pharmaceutical therapies with acupuncture. It has been demonstrated that the combination can be synergistic and, furthermore, that the adverse effects of drugs may be reduced.140, 141 The dose of medication required has also been shown to be reduced.142
Conclusion
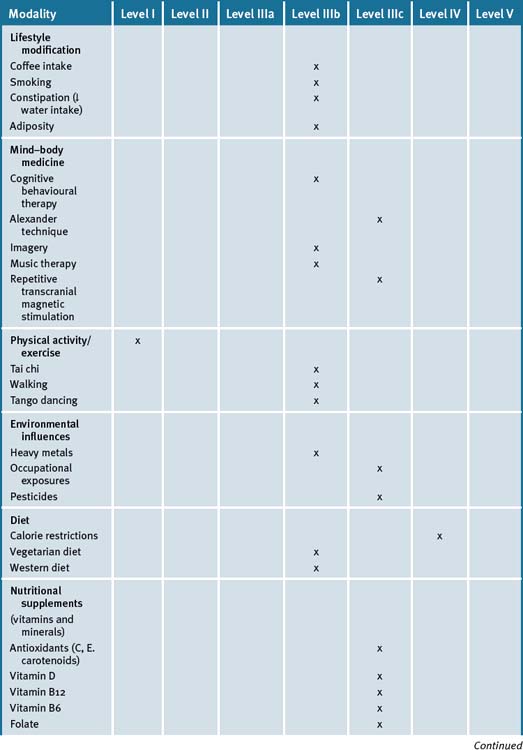
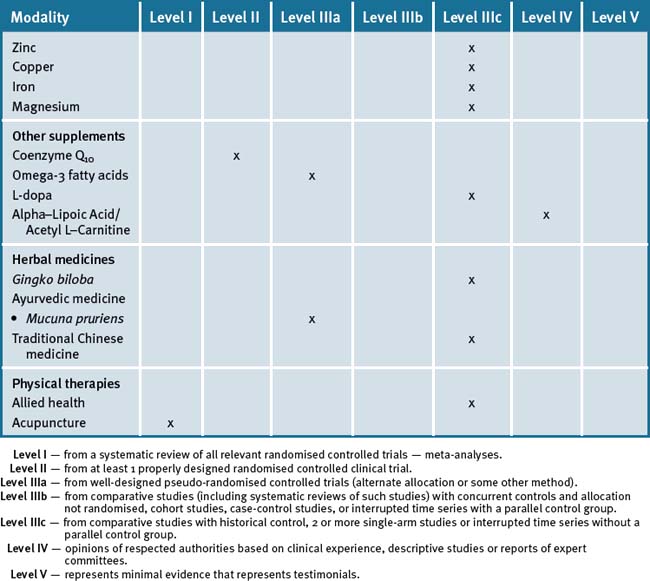
Hence, an integrative approach to the management of PD can include a number of CAM modalities to improve quality of life. Furthermore these can also be integrated with other nutritional and lifestyle modification modalities (see Table 31.2).
Clinical tips handout for patients — Parkinson’s disease
1 Lifestyle advice
3 Mind–body medicine
4 Environment
5 Dietary modification
7 Supplementation
Supplements to reduce the risk of Parkinson’s disease
Vitamin B6
Vitamin D3 (cholecalciferol)
Supplements to treat PD
Vitamin B12
Folic acid
Tyrosine
1 Stoessl J. Potential therapeutic targets for PD. Expert Opin Ther Targets. 2008;12(4):425-436.
2 Kim S.R., Lee T.Y., Kim M.S., et al. Use of CAM by Korean patients with PD. Clin Neurol Neurosurg. 2009;111(2):156-160.
3 Murphy S.M., Rogers A., Hutchinson M., et al. Counting the cost of CAM therapies in an Irish neurological clinic. Eur J Neurol. 2008;15(12):1380-1383.
4 Tan L.C., Lau P.N., Jamora R.D., et al. Use of complementary therapies in patients with PD in Singapore. Mov Disord. 2006;21(1):86-89.
5 Rajendran P.R., Thompson R.E., Reich S.G. The use of alternative therapies by patients with PD. Neurology. 2001;57(5):790-794.
6 Parkinson’s Disease Society. UK. Online. Available: www.parkinsons.org.uk/pdf/comptherapiesOct05.pdf. (accessed May 2009).
7 Paccetti C., Mancini F., Agliera R., et al. Active music therapy in PD: an integrative method for motor and emotional rehabilitation. Psychosom Med. 2000;62(3):386-393.
8 Abbott R.D., Ross G.W., White L.R., et al. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: recent findings from the Honolulu-Asia Aging Study. J Neurol. 2003;250(Suppl 3):III30-III39.
9 Ueki A., Otsuka M. Life style risks of Parkinson’s disease: association between decreased water intake and constipation. J Neurol. 2004;251(Suppl 7):vII18-vII23.
10 Dobkin R.D., Menza M., Bienfait K.L. CBT for the treatment of depression in PD: a promising nonpharmacological approach. Expert Rev Neurother. 2008;8(1):27-35.
11 Cole K., Vaughan F.L. The feasibility of using CBT for depression associated with PD: a literature review. Parkinsonism Relat Disord. 2005;11(5):269-276.
12 Dobkin R.D., Allen L.A., Menza M. CBT for depression in PD: a pilot study. Mov Disord. 2007;22(7):946-952.
13 Secker D.L., Brown R.G. CBT for carers of patients with PD: a preliminary RCT. J Neurol Neurosurg Psychiatry. 2005;76(4):491-497.
14 Ernst E., Canter P.H. The AT: a systematic review of controlled clinical trials. Forsch Komplementarmed Klass Nuturheilkd. 2003;10(6):325-329.
15 Stallibrass C. An evaluation of the AT for the management of disability in PD- a preliminary study. Clin Rehabil. 1997;11(1):8-12.
16 Stallibrass C., Hampson M. The AT: its application in midwifery and the results of preliminary research into Parkinsons. Complement Ther Nurs Midwifery. 2001;7(1):13-18.
17 Tamir R., Dickstein R., Huberman M. Integration of motor imagery and physical practice in group treatment applied to subjects with PD. Neurorehabil Neural Repair. 2007;21(1):68-75.
18 Paccetti C., Aglieri R., Mancini F., et al. Active music therapy and PD: methods. Funct Neurol. 1998;13(1):57-67.
19 Helmich R.C., Siebner H.R., Bakker M., et al. Repetitive transcranial magnetic stimulation to improve mood and motor function in Parkinson’s Disease. J Neurol Sci. 2006;248(1–2):84-96.
20 Derejko M., Niewiadomska M., Rakowicz M. The diagnostic and therapeutic application of transcranial magnetic stimulation. Neurol Neurochir Pol. 2005;39(5):389-396.
21 Benninger D.H., Lomarev M., Wassermann E.M. Safety study of 50 Hz repetitive transcranial magnetic stimulation in patients with Parkinson’s disease. Clin Neurophysiol. 2009;120(4):809-815.
22 Dibble L.E., Addison O., Papa E. The effects of exercise on balance in persons with Parkinson’s disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33(1):14-26.
23 White D.K., Wagenaar R.C., Ellis T.D., et al. Changes in walking activity and endurance following rehabilitation for people with Parkinson disease. Arch Phys Med Rehabil. 2009;90(1):43-50.
24 Keus S.H., Munneke M., Nijkrake M.J., et al. Physical therapy in PD: evolution and future challenges. Mov Disord. 2009;24(1):1-14.
25 Goodwin V.A., Richards S.H., Taylor R.S., et al. The effectiveness of exercise interventions for people with PD: SR and meta-analysis. Mov Disord. 2008;23(5):631-640.
26 Keus S.H., Bloem B.R., Hendriks E.J., et al. Evidence-based analysis of physical therapy in PD with recommendations for practice and research. Mov Disord. 2007;22(4):451-460.
27 Crizzle A.M., Newhouse I.J. Is physical exercise beneficial for persons with PD? Clin J Sport Med. 2006;16(5):422-425.
28 Hackney M.E., Kantorovich S., Levin R., et al. Effects of tango on functional mobility in PD: a preliminary study. J Neurol Phys Ther. 2007;31(4):173-179.
29 Kwakkel G., de Goede C.J., van Wegen E.E.. Impact of physical therapy for PD: a critical review of the literature. Parkinsonism Relat Disord. 2007;13 Suppl. 3:S478-S487..
30 Burini D., Farabollini B., Iacucci S., et al. A RC cross-over trial of aerobic training vs Qigong in advanced PD. Eura Medicophys. 2006;42(3):231-238.
31 Canning C.G., Sherrington C., Lord S.R., et al. Exercise therapy for prevention of falls in people with PD: a protocol for a RCT and economic evaluation. BMC Neurology. 2009;9:4.
32 Lee M.S., Lam P., Ernst E. Effectiveness of tai chi for Parkinson’s disease: a critical review. Parkinsonism Relat Disord. 2008;14(8):589-594.
33 Hackney M.E., Earhart G.M. Tai Chi improves balance and mobility in people with Parkinson disease. Gait Posture. 2008;28(3):456-460.
34 Edwards T.M., Myers J.P. Environmental exposures and gene regulation in disease aetiology. Cien Saude Colet. 2008;13(1):269-281.
35 Brown T.P., Rumsby P.C., Capleton A.C., et al. Pesticides and PD-is there a link? Environ Health Perspect. 2006;114(2):156-164.
36 Guzeva V.I., Chukhlovina M.L., Chuklovin B.A. Environmental factors and Parkinsonian syndrome. Gig Sanit. 2008;2:60-62.
37 Barlow BK, Cory-Slechta DA, Richfield EK, et al. The gestational environment and Parkinson’s disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol 07;23(3):457-70
38 Dhillon A.S., Tarbutton G.L., Levin J.L., et al. Pesticide/environmental exposures and PD in EastTtexas. J Agromedicine. 2008;13(1):37-48.
39 Dick F.D., De Palma G., Ahmadi A., et al. Environmental risk factors for PD and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64(10):666-672.
40 Hancock D.B., Martin E.R., Mayhew G.M., et al. Pesticide exposure and risk of PD: a family-based case-control study. BMC Neurol. 2008;8:6.
41 Luccini R., Albini E., Benedetti L., et al. Neurological and neuropsychological features in PD patients exposed to neurotoxic metals. G Ital Med Lav Ergon. 2007;29(3 Suppl):280-281.
42 Zoni S., Albini E., Luccini R. Neuropsychological testing for the assessment of manganese neurotoxicity: a review and a proposal. Am J Ind Med. 2007;50(11):812-830.
43 RA Yokel. Blood-brain barrier flux of aluminium, manganese, iron and other metals suspected to contribute to metal-induced neurodegenration. J Alzheimers Dis. 2006;10(2–3):223-253.
44 Gorell J.M., Johnson C.C., Rybicki B.A., et al. Occupational exposure to Mn, Cu, Pb, Fe, Hg and Zn and the risk of PD. Neurotoxicology. 1999;20(2–3):239-247.
45 Coon S., Stark A., Peterson E., et al. Whole-body lifetime occupational lead exposure and risk of PD. Environ Health Perpect. 2006;114(12):1872-1876.
46 Barnham K.J., Bush A.I. Metals in Alzheimer’s and PD. Curr Opin Chem Biol. 2008;12(2):222-228.
47 Squitti R., Gorgone G., Binetti G., et al. Metals and oxidative stress in PD from industrial areas with exposition to environmental toxins or metal pollution. G Ital Med Lav Ergon. 2007;29(3 Suppl):294-296.
48 Li A.A., Mink P.J., McIntosh L.J., et al. Evaluation of epidemiologic and animal data associating pesticides with PD. J Occup Environ Med. 2005;47(10):1059-1087.
49 Kamel F., Hoppin J.A. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112(9):950-958.
50 Bjorling-Poulson M., Anderson H.R., Grandjean P. Potential developmental neurotoxicity of pesticides in Europe. Environ Health. 2008;7:50.
51 Neurotoxicity of pesticides: a review. Costa LG, Giordano G, Guizzetti M, et al. Front Biosci 2008;13:1240–9.
52 Keifer M.C., Firestone J. Neurotoxicity of pesticides. 2007;12(1):17-25.
53 Costello S., Cockburn M., Bronstein J., et al. PD and residential exposure to maneb and paraquat from agricultural applications in the central valley of Caifornia. Am J Epidemiol. 2009;169(8):919-926.
54 Armentero M.T., Levandis G., Bramanti P., et al. Dietary restriction does not prevent nigrostriatal degeneration in the 6-hydroxydopamine model of PD. Exp Neurol. 2008;212(2):548-551.
55 Barichella M., savardi C., Mauri A., et al. Diet with LPP for renal patients increases daily energy expenditure and improves motor function in parkinsonian patients with motor fluctuations. Nutr Neurosci. 2007;10(3–4):129-135.
56 Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of PD. Am J Clin Nutr 07;86(5):1486-94
57 de Luis Roman D., Aller R., castano O. Vegetarian diets: effects on health. Rev Clin Esp. 2007;207(3):141-143.
58 Lau F.C., Shukitt-Hale B., Joseph J.A. Nutritional intervention in brain aging: reducing the effects of inflammation and oxidative stress. subcell Biochem. 2007;42:299-318.
59 Schlesinger I., Schlesinger N. Uric acid in PD. Mov Discord. 2008;23(12):1653-1657.
60 Gao X., Chen H., Choi H.K., et al. Diet, urate and PD. Am J Epidemiol. 2008;167(7):831-838.
61 Annanmaki T., Muuronen A., Murros K. Low plasma uric acid level in PD. Mov Disord. 2007;22(8):1133-1137.
62 Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifing effects of the ketogenic diet. Behav Pharmacol 06;17(5–6):431–9
63 Ma L., Zhang L., Gao X.H., et al. Dietary factors and smoking as risk factors for PD in a rural population in china: a nested case-control study. Acta Neurol Scand. 2006;113(4):278-281.
64 Powers K.M., Smith-weller T., Franklin G.M., et al. Dietary fats, cholesterol and iron as risk factors for PD. Parkinsonian Relat Disord. 2009;15(1):47-52.
65 Chen H, O’Reilly E, McCullough ML, et al. Consumption of dairy products and risk of PD. Am J Epidemiol 07;16599:998–1006
66 Nakaso K., Ito S., Nakashima K. Caffeine activates the P13K/Akt pathway and prevents apoptotic cell death in a PD model of SH-SY5Y cells. Neuro Sci Lett. 2008;432(2):146-150.
67 Facheris M.F., Schneider N.K., Lesnick T.G., et al. Coffee, caffeine-related genes and PD: a case-control study. Mov Disord. 2008;23(14):2033-2040.
68 Tan E.K., Chua E., Fook-Chong S.M., et al. Association between caffeine intake and risk of PD among fast and slow metabolisers. Pharmacogenet Genomics. 2007;17(11):1001-1005.
69 Tan L.C., Koh W.P., Yuan J.M., et al. Differential effects of black vs green tea on risk of PD in the Singapore Chinese Health Study. Am J Epidemiol. 2008;167(5):553-560.
70 Petersen M.S., Halling J., Bech S., et al. Impact of dietary exposure to food contaminants on the risk of PD. Neurotoxicology. 2008;2994:584-590.
71 Takeda A. Essential trace metals and brain function. Yakugaku Zasshi. 2004;124(9):577-585.
72 Forte G., Alimonte A., Violante N., et al. Ca, Cu, Fe, mg, Si and Zn content of hair in PD. J Trace Elem Med Biol. 2005;19(2–3):195-201.
73 Qureshi G.A., Qureshi A.A., Memon S.A., et al. Impact of Se, Cu and Zn in on/off PD patients on L-dopa therapy. J Neural Transm Suppl. 2006;71:229-236.
74 Etminan M., Gill S.S., Samii A. Intake of Vit E, C and carotenoids and the risk of PD: a meta-analysis. Lancet Neurol. 2005;4(6):362-365.
75 de Lau L.M., Koudstaal P.J., Witteman J.C., et al. Dietary folate, Vitamin B12 and Vitamin B6 and the risk of PD. Neurology. 2006;67(2):315-318.
76 Triantafyllou N.I., Nikolau C., Boufidou F., et al. Folate and Vit B12 levels in L-dopa treated PD patients: their relationship to clinical maifestations, mood and cognition. Parkinsonism Relat Disord. 2008;14(4):321-325.
77 Lamberti P., Zoccolella S., Armenise E., et al. Hyperhomocysteinaemia in L-dopa treated PD patients: effect of cobalamin and folate administration. Eur J Neurol. 2005;12(5):365-368.
78 Herrmann W., Lorenzl S., Obeid R. Review of the role of hyperhomocysteinemia and vitamin B deficiency in neurological and psychiatric disorders–current evidence and preliminary recommendations. Fortschr Neurol Psychiatr. 2007;75(9):515-527.
79 Newmark H.L., Newmark J. Vit D and PD- a hypothesis. Mov Disord. 2007;22(4):461-468.
80 Evatt M.L., Delong M.R., Khazai N., et al. Prevalence of Vit D deficiency in patients with PD and Alzheimers. Arch Neurol. 2008;65(10):1348-1352.
81 Sanchez B., Relova J.L., Gallego R., et al. 1,25 Dihydroxyvitamin D3 administration to 6-hydroxydopamine-lesioned rats increases glial cell line-derived neurotrophic factor and partially restores tyrosine hydroxylase expression in SN and striatum. J Neurosci Res. 2009;87(3):723-732.
82 Johnson S. Micronutrient accumulation and depletion in schizophrenia, epilepsy, autism and PD? Med Hypothese. 2001;56(5):641-645.
83 Takeda A. Zinc homeostasis and functions of zinc in the brain. Biometals. 2001;14(3–4):343-351.
84 Ciubotariu D., Nechifor M. Zinc involvements in the brain. Rev Med Chir Soc Med Nat Iasi. 2007;111(4):981-985.
85 Forsleff L., Schauss A.G., Bier I.D., et al. Evidence of functional zinc deficiency in PD. J Altern Comp Med. 1999;5(1):57-64.
86 Ebadi M., Sharma S.K., Ghafourifar P., et al. Peroxynitrite in the pathogenesis of PD and the neuroprotective role of metallothioneins. Methods Enzymol. 2005;396:276-298.
87 Henchcliffe C., Beal M.F. Mitochondrial biology and ox stress in PD pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600-609.
88 Chen C.M., Liu J.L., Wu Y.R., et al. Increased ox damage in peripheral blood correlates with severity of PD. Neurobiol Dis. 2009;33(3):429-435.
89 Mandel S, Packer L, Youdim, et al Proceedings from the ‘3rd International Conference on Mechanism of Action of Nutraceuticals.’. J Nutr Biochem 2005;16(9):513–20.
90 Chwiej J., Adamek D., Szczerbowska-Boruchowska M., et al. Study of Cu chemical state inside single neurons from PD and control substantia nigra using the micro-XANES technigue. J Trace Elem Med Biol. 2008;22(3):183-188.
91 Boll M.C., Alcaraz-Zubeldia M., Montes S., et al. A different marker profile in 4 neurodegenerative diseases. Free Cu, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. Neurochem Res. 2008;33(9):1717-1723.
92 Gaggelli E., Kozlowski H., Valensin D., et al. Cu homeostasis and neurodegerative disorders (Alzheimers, prion, PD and ALS). Chem Rev. 2006;106(6):1995-2044.
93 Desai V., Kaler S.G. Role of copper in human neurological disorders. Am J Clin Nutr. 2008;88(3):855S-858S.
94 Rhodes S.L., Ritz B. Genetics of iron regulation and the possible role of iron in PD. Neurobiol Dis. 2008;32(2):183-195.
95 Youdim M.B. Brain iron deficiency and excess: cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotox Res. 2008;14(1):45-56.
96 Youdim M.B., Grunblatt E., Mandel S. The copper chelator, D-peniccamine, does not attenuate MPTP induced dopamine depletion in mice. J Neural Transm. 2007;114(2):205-209.
97 Logroscino g, Gao X., Chen H., et al. Dietary iron intake and risk of PD. Am J Epidem. 2008;168(12):1381-1388.
98 Oyanagi K., Kawakami E., Kikuchi-Horie K., et al. mg deficiency over generations in rats with special references to the pathogenesis of the P-dementia complex and ALS of Guam. Neuropathology. 2006;2692:115-128.
99 Oyanagi K.. The nature of the P-dementia complex and ALS of Guam and Mg deficiency. Parkinsonism Relat Disord. 2005;11 suppl. 1:S17-S23..
100 Hashimoto T., Nishi K., Nagasao J., et al. Mg exerts both preventative and ameliorating effects in an in vivo rat PD model involving MPP+ toxicity in dopaminergic neurons. Brain Res. 2008;1197:143-151.
101 Tariq M., Khan H.A., al Moutaery K., et al. Effect of chronic administration ofmgSO4 on MPTP induced neurotoxicity in mice. Pharmacol Toxicol. 1998;82(5):218-222.
102 Hargreaves I.P., Lane A., Sleiman P.M. The coenzyme Q10 status of the brain regions of Parkinson’s disease patients. Neurosci Lett. 2008;447(1):17-19.
103 Hargreaves I.P., Lane A., Sleiman P.M. The CoQ10 status of the brain regions of PD patients. Neurosci Lett. 2008;447(1):17-19.
104 Storch A., Jost W.H., Vieregge P., et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. German Coenzyme Q(10) Study Group. Arch Neurol. 2007;64(7):938-944.
105 Henchcliffe C., Beal M.F. Mitochondrial biology and ox stress in PD pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600-609.
106 A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Investigators., The NINDS NET-PD. Neurology. 2007;68(1):20-28.
107 Are neurodegenerative disorder and psychotic manifestations avoidable brain dysfunctions with adequate dietary omega-3? LF, Saugstad. Nutr Health 2006;18(3):203–15.
108 Depression in PD. a double-blind, placebo-controled pilot study of omega-3 fatty acid supplementation. J Affect Disord. 2008;111(2–3):351-359.
109 da Silva T.M., Munhoz R.P., Alvarez C., et al. Oral creatine supplementation attenuates L-dopa induced dyskinesia in 6-hydroxydopamine lesioned rats. Valastro B, Dekundy A, Danysz W, et al. Behav Brain Res. 2009;197(1):90-96.
110 Beal M.F.. Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann Neurol. 2003;53 Suppl. 3:S39-S47..
111 Kidd P.M. Parkinson’s disease as multifactorial oxidative neurodegeneration: implications for integrative management. Altern Med Rev. 2000;5(6):502-529.
112 Zbarsky V., datla K.P., Parkar S., et al. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of PD. Free Radic Res. 2005;39(10):1119-1125.
113 Le Bars P., Katz M.M., Berman N., et al. Placebo Controlled, Double-blind Randomized Trial of an Extract of Gingko Biloba for Dementia. JAMA. 1997;278(16):1327-1332.
114 Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545(1):51-64.
115 Sharma H., Chandola H.M., Sing G., et al. Utilization of Ayurveda in helath care: an approach for prevention, health promotion and treatment of disease. Part 2-Ayurveda in primary health care. J Altern Complement Med. 2007;13(10):1135-1150.
116 Manyam B.V. Paralysis agitans and levo-dpa in ‘Ayurveda’-ancient Indian medical treatise. Mov Disord. 1990;5(1):47-48.
117 Gourie-Devi M., Ramumg, Venkatarum B.S. Treatment of PD in Ayurveda(ancient system of medicine): discussion peper. J R Soc Med. 1991;84(8):491-492.
118 Manyam B.V., Sanchez-Ramos J.R. Traditional and complementary therapies in PD. Adv Neurol. 1999;80:565-574.
119 Nader T., Rothenberg S., Averbach R., et al. Improvements in chronic diseases with a comprehensive natural medine approach:a review and case series. Behav Med. 2000;26(1):14-46.
120 Manyam B.V., Dhanasekaran M., Hare T.A. Effectv of antiparkinson drug HP-200 (Mucuna pruirns) on the central monoaminergic neurotransmitters. Phytother Res. 2004;18(2):97-101.
121 Manyam B.V., Dhanasekaran M., Hare T.A. Neuroprotective effects of the anti-parkinson drug Mucuna pruriens. Phytother Res. 2004;18(9):706-712.
122 Dhanasekaran M., Tharakan B., Manyam B.V. Antiparkinson drug-Mucuna pruriens shows antioxidant and metal chelating activity. Phytother Res. 2008;22(1):6-11.
123 Zhang J.F., Sun G.S., Zhao G.H. Observation on therapeutic effect of herbs-partitioned moxibustion on PD of 54 cases. Zhongguo Zhen Jiu. 2005;25(9):610-612.
124 Yang Z.M., Tang X.J., Lao Y.R. Systematic evaluation on clinical literature related with treatment of PD with TCM. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25(7):612-615.
125 Li Q., Zhao D., Bezard E. TCM for PD: a review of Chinese literature. Behav Pharmacol. 2006;17(5–6):403-410.
126 Nijkrake M.J., Keus S.H., Oostendorp R.A., et al. Allied health care in PD: referral, consulation and professional expertise. Mov Disord. 2009;24(2):282-286.
127 Nijrake M.J., Keus S.H., Kalf J.G., et al. Allied health acre interventions and complementary therapies in PD. Parkinsonism Relat Disord. 2007;13 Suppl. 3:S488-S494..
128 Dixon L., Duncan D., Johnson P., et al. OT for patients with PD. Cochrane database Syst Rev. (3):2007. CD002813
129 Deane K.H., Jones D., Playford Ed, et al. Physio for patients with PD: a comparison of techniques. Cochrane Database Syst Rev. (3):2001. CD002817
130 Deane K.H., Whurr R., Playford E.D., et al. A comparison of speech and language therapy techniques for dysarthria in PD. Cochrane Database Syst Rev. (2):2001. CD002814
131 Lee M.S., Shin B.C., Kong J.C., et al. Effectiveness of acupuncture for PD: a systematic review. Mov Disord. 2008;23(11):1505-1515.
132 Lam Y.C., Kum W.F., Durairajan S.S., et al. Efficacy and safety of acupuncture for isiopathic PD: a systematic review. J Altern Complement Med. 2008;14(6):663-671.
133 Cheng X.R., Cheng K. Survey of studies on the mechanism of acupuncture and moxibustion treating diseases abroad. Zhongguo Zhen Jiu. 2008;28(6):463-467.
134 Eng M.L., Lyons K.E., Greene M.S., et al. Open-label trial regarding the use of acupuncture and yin tui na in PD outpatients: a pilot study on efficacy, tolerabolity and QOL. J Altern Comp Med. 2006;12(4):395-399.
135 Cristan A., Katz M., Cutrone E., et al. Evaluation of acupuncture in the treatment of PD: a double-blind pilot study. Mov Disord. 2005;20(9):1185-1188.
136 Shulman L.M., Wen X., weiner W.J., et al. Acupuncture therapy for the symptoms of PD. Mov Disord. 2002;17(4):799-802.
137 Wang X., Liang X.B., Li F.Q., et al. Therapeutic strategies for PD: the ancient meets the future-TCM, electroacupuncture, gene therapy and stem cells. Neurochem Res. 2008;33(10):1956-1963.
138 Jiang X.M., Huang Y., Zhuo Y., et al. Therapeutic effect of ESA on PD. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26(1):114-116.
139 Huang Y., Jiang X.M., Li D.J. Effects of ESA on cerebral dopamine transporter in patients with PD. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26(4):303-307.
140 Chen X.H., Li Y., Kui Y. Clinical observation on abdominal acupuncture plus Madopa for treatment of PD. Zhongguo Zhen Jiu. 2007;27(8):562-564.
141 Chang X.H., Zhang L.Z., Li Y.J. Observation on therapeutic effect of acupuncture combined with medicine on PD. Zhongguo Zhen Jiu. 2008;28(9):645-647.
142 Ren X.M. 50 cases of PD treated by acupuncture combined with Madopar. J Tradit Chin Med. 2008;28(4):255-257.