32 Parkinson’s disease
Aetiology
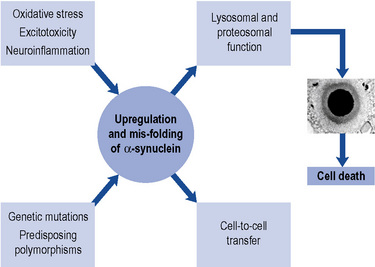
In a small number of patients, genetic factors are dominant. The discovery of a mutation in the gene coding for a synaptic protein called α-synuclein has provided tremendous impetus for further research. Such mutations have been described in fewer than 10 families worldwide. Nevertheless, because α-synuclein is a major component of the pathological hallmark of Parkinson’s disease, the Lewy body (see below), the challenge is to discover how a mutation in this protein in a tiny minority can relate to the formation of Lewy bodies in the vast majority. In recent years, eight genetic loci and a further four genes (parkin, DJ-1, PINK1 and LRRK-2) have been identified (Healy et al., 2004). The intriguing thing is that the protein products of these genes are involved in a cellular system called the ubiquitin-proteasome system, which plays a crucial role in removing and recycling abnormal or damaged proteins. Current thinking is that abnormalities in the way in which the cell handles mutated or abnormal proteins may ultimately lead to its death, through increased oxidative stress and/or reduced mitochondrial energy production. The Lewy body may actually represent a defence mechanism by the cell to ‘parcel up’ potentially damaging proteinaceous material (Olanow et al., 2004).
More recently, cell-to-cell transfer of α-synuclein has been demonstrated in vitro and also in engrafted stem cell tissue. This suggests that the pathology of Parkinson’s disease may be propagated between neurones and could have major implications for the spread of Lewy body pathology within the brain, as well as its treatment (Olanow and Prusiner, 2009) (Fig. 32.1).
Pathophysiology
The characteristic pathological features of Parkinson’s disease are neuronal loss in pigmented brainstem nuclei, together with the presence of eosinophilic inclusion bodies, called Lewy bodies, in surviving cells. The pars compacta of the substantia nigra in the midbrain is particularly affected. Dopaminergic neurones within this nucleus project to the striatum, which is, therefore, deprived of the neurotransmitter dopamine. In Parkinson’s disease, there is a loss of over 80% of nigral neurones before symptoms appear. The ‘Braak hypothesis’ has been proposed to account for spread of pathology within the Parkinsonian brain and suggests that α-synuclein may first accumulate in the lower brainstem and then gradually ascend rostrally to affect critical brain regions including the substantia nigra and ultimately the cerebral cortex (Braak et al., 2003).
Differential diagnosis
It is important to remember that, while Parkinson’s disease is a common form of Parkinsonism, there are numerous other degenerative and symptomatic causes. Further, ‘all that shakes is not Parkinson’s disease’. Table 32.1 gives a differential diagnosis for causes of Parkinsonism. These are separated into degenerative and symptomatic categories. The list is not exhaustive and excludes, for instance, rare Parkinsonian manifestations in uncommon diseases. A detailed description of these different causes of Parkinsonism is beyond the scope of this chapter, but a few points should be highlighted. Essential tremor is not included in Table 32.1, as this common condition does not cause bradykinesia. Nevertheless, it may be very difficult to differentiate from tremor-dominant Parkinson’s disease. A positive family history and good response to alcohol may provide vital clues towards the diagnosis of essential tremor, although in practice these are not always reliable.
| Degenerative causes | Symptomatic causes |
|---|---|
| Parkinson’s disease | Dopamine receptor blocking agents |
Several clinical and clinicopathological series have confirmed our fallibility in not making a correct diagnosis of Parkinson’s disease. If clinical criteria, such as those produced by the UK Parkinson’s Disease Brain Bank, are not applied, then the error rate (false-negative diagnosis) may be as high as 25–30%. These criteria are listed in Box 32.1. Degenerative conditions commonly masquerading as Parkinson’s disease include progressive supranuclear palsy, multiple system atrophy and Alzheimer’s disease.
Box 32.1 Clinical criteria for diagnosis of Parkinson’s disease
Step 1 Diagnosis of Parkinsonian syndrome
The patient has bradykinesia, plus one or more of the following:
Drug-Induced Parkinsonism
Perhaps the most important differential diagnosis to consider when a patient presents with Parkinsonism is whether their symptoms and signs may be drug induced. This is because drug-induced Parkinsonism is potentially reversible upon cessation of the offending agent. Reports linking drug-induced Parkinsonism with the neuroleptic chlorpromazine were first published in the 1950s. Since then, numerous other agents have been associated with drug-induced Parkinsonism. Many of these are widely recognised, although others are not (Box 32.2). Compound antidepressants were a problem in the past because they contained neuroleptic drugs. For example, fluphenazine was found with nortriptyline in Motival® (discontinued in the UK in 2006) and often overlooked as a potential culprit. Repeat prescription of vestibular sedatives and anti-emetics such as prochlorperazine and cinnarizine are other commonly encountered causes of drug-induced Parkinsonism. The pathogenesis of drug-induced Parkinsonism is unlikely to be only due to dopamine receptor blockade. If this were the case, the incidence and severity should correlate with the drug dosage and length of exposure, and this is not clearly observed. Sodium valproate is also now recognised to cause an encephalopathy dominated by Parkinsonism and cognitive impairment which is reversible upon drug cessation. Again, there is considerable idiosyncrasy in who develops this encephalopathy when exposed to valproate.
Box 32.2 Examples of non-neuroleptic drugs associated with drug-induced Parkinsonism
a Lithium causes postural tremor. Reports of Parkinsonism occurring with lithium have usually been in the context of prior exposure to neuroleptics.
b Only single case reports of drug-induced Parkinsonism with these drugs.
c One case report of drug-induced Parkinsonism in a child after bone marrow transplantation and a second in association with cytosine arabinoside therapy.
Investigations
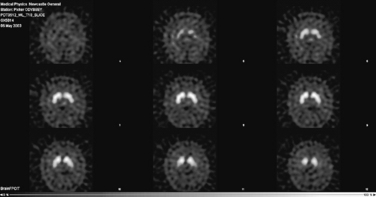
The diagnosis of Parkinson’s disease is a clinical one and should be based, preferably, upon validated criteria. In young-onset or clinically atypical Parkinson’s disease, a number of investigations may be appropriate. These include copper studies and DNA testing to exclude Wilson’s disease and Huntington’s disease, respectively. Brain imaging by computed tomography (CT) or magnetic resonance imaging (MRI) may be necessary to exclude hydrocephalus, cerebrovascular disease or basal ganglia abnormalities suggestive of an underlying metabolic cause. When there is difficulty in distinguishing Parkinson’s disease from essential tremor, a form of functional imaging called FP-CIT SPECT (also known as DaTSCAN) may be useful, as this technique can sensitively identify loss of nigrostriatal dopaminergic terminals in the striatum (Fig. 32.2). Thus, in essential tremor, the SPECT scan is normal, whereas in Parkinson’s disease, reduced tracer uptake is seen (Jennings et al., 2004).
Treatment
General approach
When treatment becomes necessary, it is impossible to generalise about which drug should be commenced. All currently available drugs for Parkinson’s disease are symptomatic, as no agent has yet been shown, beyond reasonable doubt, to have disease-modifying or neuroprotective properties. There is no accepted algorithm for the treatment of Parkinson’s disease, although a clinical management guideline has been produced (NICE, 2006).
A number of factors, including patient preference, age, severity and type of disease (tremor-dominant versus bradykinesia-dominant) and co-morbidity, need to be taken into account. The efficacy and tolerability of levodopa in Parkinson’s disease was first described 1967, when the drug was started in low doses and gradually increased thereafter (Cotzias et al., 1967).
Drug treatment
Levodopa preparations
Catechol-O-methyl transferase inhibitors
Inhibitors of the enzyme catechol-O-methyl transferase (COMT) represent a novel addition to the range of therapies available for Parkinson’s disease (Schrag, 2005). Use of the first agent in this class, tolcapone, was originally suspended in Europe because of fears over hepatotoxicity, although the drug became available again in 2005, accompanied by strict prescribing and monitoring guidelines. Entacapone is also available and studies have not shown derangement of liver function with this drug.
The optimal way to use COMT inhibition is unknown. A patient experiencing end-of-dose deterioration, or generally underdosed, would seem to be the ideal candidate. However, there are few comparative studies of COMT inhibitors versus dopamine agonists available to provide guidance as to which class of drug is best to use, and when. The STRIDE-PD study (Stocchi et al., 2010) assessed the potential benefit of combined treatment with levodopa and entacapone in de novo Parkinson’s disease patients to address whether this combined treatment was associated with a lower incidence of dyskinesias. Unfortunately, the opposite was actually found, with a higher incidence of dyskinesias in patients randomised to levodopa and entacapone compared with levodopa alone.
Monoamine oxidase type B inhibitors
The propargylamines selegiline and rasagiline are inhibitors of monoamine oxidase type B. Inhibition of this enzyme slows the breakdown of dopamine in the striatum. These agents effectively have a ‘levodopa-sparing’ effect and may delay the onset of, or reduce existing, motor complications. Both drugs may also have an antiapoptotic effect (apoptosis is a form of programmed cell death thought to be important in several neurodegenerative conditions, including Parkinson’s disease). Whether or not the drugs have a neuroprotective effect by this or some other means remains controversial. A recent study suggested that 1 mg of rasagiline may have a disease-modifying benefit in early Parkinson’s disease, although this study was difficult to interpret since the same effect was not seen with the 2 mg dose (Olanow et al., 2009). Further, the magnitude of effect was very modest and of uncertain clinical relevance. The findings, therefore, need to be interpreted with caution but do offer some cause for optimism.
Following publication of a study (Lees, 1995) which showed excess mortality in a group of patients taking selegiline, it was suggested that the drug was best avoided in patients with falls, confusion and postural hypotension. A subsequent meta-analysis, including nine trials of selegiline, did not, however, identify any excess mortality in patients taking selegiline (Ives et al., 2004). Selegiline can cause hallucinations and confusion, particularly in moderate-to-advanced disease. The withdrawal of selegiline may then be associated with significant deterioration in motor function. Unlike selegiline, rasagiline is not metabolised to amfetamine-like products, so neuropsychiatric side effects are less frequent. Selegiline should not be co-prescribed with selective serotonin reuptake inhibitors, as a serotonin syndrome, including hypertension and neuropsychiatric features, has been reported in a small minority of cases. Caution is also required for rasagiline when co-prescribing with a selective serotonin reuptake inhibitor.
Amantadine
Amantadine was introduced as an anti-Parkinsonian treatment in the late 1960s. It has a number of possible modes of action, including facilitation of presynaptic dopamine release, blocking dopamine reuptake, an anticholinergic effect, and also as a N-methyl-d-aspartate (NMDA) receptor antagonist. Initially employed in the early stages of treatment, where its effects are mild and relatively short-lived, interest has focused more recently upon the use of amantadine as an antidyskinetic agent in advanced disease (Blanchet et al., 2003).
Anticholinergic drugs
The use of anticholinergic agents has declined because of troublesome side effects, including constipation, urinary retention, cognitive impairment and toxic confusional states. In selected younger patients, an anticholinergic drug may still be helpful but close monitoring is advised. Postmortem studies have suggested that long-term anticholinergic use may have adverse disease-modifying effects in Parkinson’s disease, by increasing cortical levels of Alzheimer-type pathology (Perry et al., 2003).
Surgical treatment
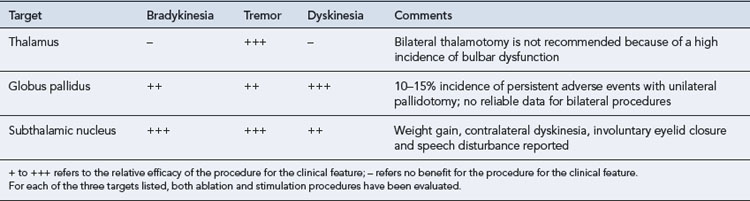
There has been renewed interest in the use of neurosurgical techniques for the treatment of Parkinson’s disease (Walter and Vitek, 2004). This has resulted not only from recognition of the shortcomings of medical treatment currently available but also from an improved understanding of basal ganglia circuitry and better neuroimaging methods. Table 32.2. summarises techniques currently being employed and evaluated. The functional effects of lesioning (-otomy) and the use of deep brain stimulation are similar, in that the high frequency used in stimulation is believed to act by blocking, or ‘jamming’ neurones. Deep brain stimulation has the advantage of being reversible but is costly, and programming the stimulator may be very time consuming.
Patient care
Common therapeutic solutions to problems encountered in the management of people with Parkinson’s disease are presented in Table 32.3. After diagnosis, the provision of an explanation of the condition, education and support are essential. The Parkinson’s Disease Society (www.parkinsons.org.uk) produces an excellent range of literature to help the newly diagnosed patient come to terms with the condition. In accordance with advice given by the Society itself, patients who drive are advised to inform their insurance company and also the Driver and Vehicle Licensing Agency.
Table 32.3 Common therapeutic solutions in the management of Parkinson’s disease
| Problem | Cause | Possible Solution |
|---|---|---|
| Early-onset dyskinesias in young Parkinson’s disease patient | Exposure to levodopa? Biological factors? |
Delay introduction of levodopa, or use lowest possible dose, or use alternative agent (e.g. agonist, MAOB inhibitor) |
| One dose of levodopa does not last until the next (wearing off) | Advanced disease (pre- and post-synaptic changes) | More frequent, smaller doses of levodopa, COMT inhibitor, dopamine agonist or MAOB-inhibitor |
| Pain and immobility during the night | Evening dose of levodopa not lasting long enough | Use slow release levodopa preparation or dopamine agonist |
| Freezing episodes and/or unpredictable motor fluctuations | Advanced disease (pre- and post-synaptic changes) | Apomorphine, duodopa or surgeryPhysiotherapy helpful for freezing |
| Mismatch between patient’s symptoms and signs | Underlying depression? | Consider antidepressant |
| Confusion and hallucinations with preserved cognition | Toxic (drug-related) psychosis | Exclude intercurrent infection or other medical problem |
| Review and reduce anti-Parkinsonian therapy Consider atypical anti-psychotic agent | ||
| Confusion and hallucinations with impaired cognition | Underlying brain pathology, and cholinergic deficit | Reduce anti-Parkinsonian therapy |
| Cholinesterase inhibitor |
Psychosis and dementia
Cholinesterase inhibitors have shown promise in treating the neuropsychiatric features of Parkinson’s disease and may also have modest cognitive-enhancing benefits. Visual hallucinations, delusions, apathy and depression seem to be particularly responsive to these agents. These effects have been demonstrated for rivastigmine in dementia associated with Parkinson’s disease in a large, multicentre, double-blind, placebo-controlled study (Emre et al., 2004). A randomised controlled trial of memantine in dementia associated with Parkinson’s disease (and also patients with the closely related dementia with Lewy bodies) showed a modest benefit for memantine in the primary end-point, the Clinician’s Global Impression of Change (Aarsland et al., 2009).
Autonomic problems
Answers
Answers
Answers
Answers
Aarsland D., Ballard C., Walker Z., et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8:613-618.
Blanchet P.J., Verhagen-Metman L., Chase T.N. Renaissance of amantadine in the treatment of Parkinson’s disease. Adv. Neurol.. 2003;91:251-257.
Braak H., Del Tredici K., Rüb U., et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197-211.
Cotzias G.C., Van Woert M.H., Schiffer L.M. Aromatic amino acids and modification of parkinsonism. N. Engl. J. Med.. 1967;276:374-379.
Emre M., Aarsland D., Albanese A., et al. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med.. 2004;351:2509-2518.
Healy D.G., Abou-Sleiman P.M., Wood N.W. PINK, PANK or PARK. A clinician’s guide to familial parkinsonism. Lancet Neurology. 2004;3:652-662.
Ives N., Stowe R.L., Marro J., et al. Monoamine oxidase type B inhibitors in early Parkinson’s disease: meta-analysis of 17 randomised trials involving 3525 patients. Br. Med. J. 2004;329:593-596.
Jennings D.L., Seibyl J.P., Oakes D., et al. (123I)beta-CIT and single-photon emission computed tomographic imaging vs. clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch. Neurol. 2004;61:1224-1229.
Lees A.J., on behalf of the Parkinson’s Disease Research Group of the United Kingdom. Comparison of therapeutic effects and mortality data of levodopa and levodopa combined with selegiline in patients with early, mild Parkinson’s disease. Br. Med. J.. 1995;311:1602-1607.
National Institute for Health and Clinical Excellence. Parkinson’s disease: diagnosis and management in primary and secondary care. Clinical Guideline 35. London: NICE, 2006. Available at: http://www.nice.org.uk/page.aspx?o=CG035
Olanow C.W., Perl D.P., DeMartino G.N., et al. Lewy-body formation is an aggresome-related disorder: a hypothesis. Lancet Neurol. 2004;3:496-503.
Olanow C., Rascol O., Hauser R., et althe ADAGIO Study Investigators. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N. Engl. J. Med.. 2009;361:1268-1278.
Olanow C.W., Prusiner S.B. Is Parkinson’s disease a prion disorder? Proc. Natl. Acad. Sci.. 2009;106:12571-12572.
Perry E.K., Kilford L., Lees A.J., et al. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann. Neurol.. 2003;54:235-238.
Schrag A. Entacapone in the treatment of Parkinson’s disease. Lancet Neurol. 2005;4:366-370.
Stocchi F., Rascol O., Kieburtz K., et al. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann. Neurol. 2010;68:18-27.
Walter B.L., Vitek J.L. Surgical treatment for Parkinson’s disease. Lancet Neurol.. 2004;3:719-728.
Chaudhuri K.R., Healy D.G., Schapira A.V.H. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235-245.
Clarke C.E. Parkinson’s disease. Br. Med. J. 2007;335:441-445.
Hauser R.A. Levodopa: past, present and future. Eur. J. Neurol. 2009;62:1-8.
Lees A.J., Hardy J., Revesz T. Parkinson’s disease. Lancet. 2009;373:2055-2066.
Olanow C.W., Stern M.B., Sethi K. The scientific and clinical basis for the treatment of Parkinson’s disease. Neurology. 2009;72(21 Suppl 4):S1-136.