Parathyroid disease
Part 1
Parathyroid disease, syndromes and pathophysiology
Introduction
Hyperparathyroidism is a disease characterised by elevated serum calcium and inappropriately elevated parathyroid hormone (PTH) levels, which occurs with a prevalence of 3 per 1000 in the general population.1 The modern era of treating parathyroid disease began in 1925, when Mandl performed the first parathyroidectomy in a patient with severe bone disease. Early in the history of hyperparathyroidism, patients presented with advanced clinical disease, including fractures, skeletal deformities, kidney stones and kidney failure. The discovery of the peptide PTH in the early 1970s, coupled with the development of a chemical analyser to measure calcium, permitted the biochemical diagnosis of hyperparathyroidism much earlier in the disease course.2
Embryology and anatomy
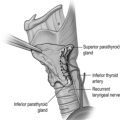
In order to successfully diagnose and treat disorders of the parathyroid glands, a keen understanding of parathyroid embryology and anatomy is essential. The parathyroid glands are small, brownish-tan glands located in the space around the thyroid gland. During the fifth week of foetal development, the inferior parathyroid glands arise from the dorsal aspect of the third pharyngeal pouch.5 Following development of the thymus from the ventral aspect of the third pharyngeal pouch, the inferior parathyroid glands and thymus descend in a caudal and medial direction to rest in the inferior neck and thorax respectively. The superior parathyroid glands arise from the dorsal wing of the fourth pharyngeal pouch and descend in a caudal direction with the thyroid gland.5
In an autopsy series of 503 human subjects, Akerstrom et al. showed that four parathyroid glands were present in 84% of cases, whereas 3% of patients had only three glands and 13% had supernumerary glands.6 The presence of missed hyperfunctioning supernumerary glands is an important but infrequent cause of persistent hyperparathyroidism and should be considered in all cases of persistent disease. In 80% of cases, the location of the inferior and superior glands is symmetrical when compared with the glands on the contralateral side of the neck.6 The superior parathyroid glands are most commonly found immediately superior to the junction of the recurrent laryngeal nerve and the inferior thyroid artery and can be located inside the thyroid gland in 0.2% of cases.6
Calcium and parathyroid hormone (PTH) regulation
The parathyroid glands play a central role in regulating serum levels of calcium through a complex feedback loop involving PTH, serum ionised calcium levels and vitamin D. The key organ systems involved in this process include the parathyroid glands, gastrointestinal tract, kidneys and skin. Although multiple factors influence parathyroid function, it is now clear that calcium is the single most potent stimulator of PTH release. Calcium-sensing receptors (CSRs), which are located on the surface of the parathyroid chief cells and are coupled with a G-protein receptor, are able to detect minuscule changes in serum levels of extracellular ionised calcium.7,8 When serum levels of calcium decrease the CSRs are activated, thereby stimulating the synthesis and release of PTH.9 In primary hyperparathyroidism (PHP), the set point of the CSRs is adjusted upwards, probably through a mutation of unknown aetiology, causing the parathyroid chief cell to ‘believe’ that serum calcium levels are low when in fact they are not. As a result of this alteration in the CSR set point, the parathyroid chief cell increases production of PTH, ultimately leading to hypercalcaemia. Calcium-sensing receptors are also present in other tissues such as the kidneys and gastrointestinal tract, where calcium homeostasis is influenced.8,10,11 In the kidney, the CSRs regulate renal calcium excretion and influence the transepithelial movement of water and other electrolytes.8 In the gastrointestinal tract, CSRs are present in the gastrin-secreting G cells and acid-secreting parietal cells, thereby providing a molecular link between hypercalcaemia and acid hypersecretion.10 These facts also underscore the complexity of calcium homeostasis in influencing cellular function throughout the body.
PTH is an intact 84-amino-acid peptide with amino and carboxy terminals.12 Production of PTH begins in the endoplasmic reticulum of the parathyroid chief cells as a 115-amino-acid molecule, which undergoes a series of cleavages before being released from the cytoplasm as the biologically active (1–84)PTH molecule. The circulating (1–84)PTH molecule, which has a half-life of 3–5 minutes in patients with normal renal function, is initially cleaved in the liver, yielding an inactive C-terminal fragment, which is ultimately cleared by the kidneys.12,13 The N-terminal fragment is the part of the peptide that is responsible for the biological activity of PTH in peripheral tissues.
PTH acts directly on the kidneys, bone and gastrointestinal tract to activate several intracellular second messengers, including cyclic AMP and calcium.14,15 In the kidneys, PTH increases serum calcium levels by acting on the renal tubule to increase resorption of calcium and to increase the hydroxylation of 25-hydroxyvitamin D to the biologically active 1,25-dihydroxyvitamin D.15 PTH also stimulates the renal tubular secretion of phosphate and bicarbonate. In the bone, PTH acts on osteoblasts and osteoclasts to increase bone turnover, thereby providing a large source of calcium for the extracellular space.16
Vitamin D is a fat-soluble vitamin that is prevalent in dairy products. After being absorbed by the gastrointestinal tract, it is hydroxylated in the liver to become 25-hydroxyvitamin D, which in turn is hydroxylated in the kidneys to become 1,25-dihydroxyvitamin D. The latter plays an important role in calcium homeostasis by increasing the resorption of phosphorus in the kidneys and increasing the absorption of calcium from the gastrointestinal tract. Calcitonin, which is synthesised by the parafollicular C cells of the thyroid gland, acts as the physiological antagonist to PTH. Calcitonin decreases serum levels of calcium by decreasing bone turnover and in fact can be used to treat patients in hypercalcaemic crisis.17
Primary hyperparathyroidism
PHP occurs more frequently in women than men, but the overall incidence increases with age in both sexes. In North America, the incidence of PHP in the general population is 4.3 per 1000, whereas in Europe the incidence is 3 per 1000.1,18 In women aged between 55 and 75 years, the incidence of PHP is 21 per 1000.1 Possible explanations for the increased incidence with age include the lower rate of biochemical screening in patients less than 50 years of age and the increased use of bone density measurements in postmenopausal women as a routine part of healthcare screening. The detection of osteopenia and/or osteoporosis that is out of proportion to age-matched controls often leads the clinician to measure serum calcium and PTH levels, thereby identifying hyperparathyroidism as the cause of increased bone loss. Vitamin D deficiency also influences the true detected incidence of PHP as this condition may cause serum calcium levels to be normal in patients with hyperparathyroidism. For example, the incidence of vitamin D deficiency in southern Europe is high, leading to an underestimation of the true incidence of hyperparathyroidism in this region of the world.1
Clinical manifestations
The clinical presentation of patients with PHP is highly variable, ranging from none to profound symptoms of hypercalcaemia, such as excessive thirst, dehydration, kidney stones, muscle weakness and pathological fracture. Generally, the clinical manifestations of PHP can be broadly classified by organ system (Box 1.1). Since many of these symptoms overlap with other clinical conditions, particularly in the elderly, the diagnosis of hyperparathyroidism is often delayed until hypercalcaemia is recognised on biochemical screening. Often the presence of a classic symptom, such as nephrolithiasis, will lead the astute clinician to assess the patient for PHP. By far, fatigue is one of the most common symptoms of hyperparathyroidism, being present in > 80% of patients.18 Numerous studies have shown that a high percentage of patients that are thought to be asymptomatic actually have occult symptoms attributable to PHP.18
Diagnosis
• Elevated serum calcium. While a useful screening tool, many conditions can lead to inaccuracies in the measured total serum calcium levels. For example, hypoalbuminaemia and acidosis can create ‘normal’ serum calcium levels. Given these variables, many groups favour measuring the ionised serum calcium level instead. Monchik found in a number of series that an elevated serum ionised calcium correlated better with the presence of PHP as confirmed by surgery.19
• Elevated serum PTH. Current antibody-driven assays for serum intact parathyroid hormone (iPTH) levels are highly accurate.
• Chloride:phosphate ratio. A recent retrospective study suggests that a chloride:phosphate ratio ≥ 33 is indicative of PHP in both hypercalcaemic and normocalcaemic patients.20
• Hypercalciuria. The presence of hypercalciuria rules out benign familial hypercalcaemic hypocalciuria, which can mimic PHP.
• Hypophosphataemia. Due to the decreased resorption of phosphate by the renal tubule, phosphate levels decrease in approximately 50% of patients with PHP.
Normocalcaemic hyperparathyroidism
There is a small subset of patients with PHP who present with normal or only intermittently elevated calcium levels. Mather first described normocalcaemic hyperparathyroidism in 1953 in a woman who presented with osteitis fibrosa cystica. Since that time, this variation of PHP has been an infrequent but recognised entity. While still uncommon when compared with hypercalcaemic PHP, recent population studies have shown that this variant of the disease may be more prevalent than previously believed and that improved screening may help identify mildly symptomatic or asymptomatic patients.21
The exact biochemical mechanisms of normocalcaemic PHP remain elusive. Some investigators postulate that the normocalcaemic variant of PHP represents an early or preclinical phase that progresses to typical hypercalcaemic PHP.22,23 Others have found distinct differences in the biological response to PTH in patients with normocalcaemic vs. hypercalcaemic hyperparathyroidism. For example, Maruani et al. found that patients with normocalcaemic hyperparathyroidism displayed a resistance to the renal and bony effects of PTH as measured by a lower fasting urine calcium excretion and renal tubular calcium resorption, as well as lower values of markers of bone turnover.24
The majority of patients with normocalcaemic PHP present with renal calculi and hypercalciuria. However, the most common cause of renal calculi and hypercalciuria is idiopathic hypercalciuria (IH). To further confound the matter, some variants of IH have elevated PTH levels. It is vitally important to distinguish between these two entities since surgical parathyroidectomy effectively cures normocalcaemic PHP, whereas postsurgical IH patients continue to form stones.25
• Thiazide administration. Administration of thiazide diuretics leads to a decrease in urinary calcium excretion. Patients with normocalcaemic PHP will have persistently elevated PTH levels, whereas those with IH will have a normalisation of PTH.27
• Phosphate deprivation. After restricting phosphate to 350 mg/day and administering 650 mg of aluminium hydroxide four times a day (while on a normal calorie and normal calcium diet), serum calcium and phosphorus levels are checked every day for 4 days. Patients with subsequent hypercalcaemia or persistent hypercalciuria usually have normocalcaemic PHP. This test is no longer used routinely.
• Calcium loading test. After administration of either 350 or 1000 mg of oral calcium, serum calcium and urine calcium are measured. Patients with normocalcaemic PHP have a significant increase in serum calcium (due to increased intestinal absorption) and an increase in urine calcium excretion, whereas intestinal absorption of calcium varies widely in patients with IH.28 In a recent study, after administration of 1 g of oral calcium, the combined parameters of (i) circulating PTH nadir (pg/mL) × peak calcium concentration (mg/dL) and (ii) relative PTH decline/relative calcium increment diagnosed normocalcaemic PHP with 100% sensitivity and 87% specificity.29 Furthermore, calcium loading suppressed urinary cyclic AMP28 but did not suppress PTH levels below 70% of baseline.19
• Serum ionised calcium. An elevated ionised calcium, in conjunction with an elevated PTH, is increasingly gaining acceptance as an excellent means of distinguishing normocalcaemic PHP from IH.19
Hypercalcaemic crisis
Hypercalcaemia is seen in approximately 0.5% of the general population and up to 5% of the hospital population.30,31 The majority of cases of hypercalcaemia are classified as mild to moderate (< 12 or 12–14 mg/dL respectively) and the patient is asymptomatic. This group responds to dietary measures and treatment of the underlying aetiology. However, a subset of patients will present in hypercalcaemic crisis, with serum calcium > 14 mg/dL, and are severely symptomatic. These patients require hospitalisation and aggressive reduction of serum calcium. Fortunately, except in cases of malignancy, treatment for hypercalcaemia is typically successful.
Since the calcium ion plays a crucial role in membrane potentials throughout the body, the symptoms of hypercalcaemia are varied and potentially life-threatening. The classic presentation of severe hypercalcaemia includes acute confusion, abdominal pain, vomiting, dehydration and anuria. In addition, patients may develop lethal arrhythmias due to decreased conduction velocities and shortened refractory periods, which manifest on an electrocardiogram as a prolonged P–R interval, a shortened Q–T interval, and arrhythmia. Hypercalcaemic crisis is the most extreme form of hypercalcaemia and is defined as severe hypercalcaemia in association with profound dehydration and obtundation.32 At serum calcium levels of 15–18 mg/dL, coma and cardiac arrest may occur.
The most common aetiology of hypercalcaemia in non-hospitalised patients is PHP, while malignancy accounts for almost two-thirds of the hypercalcaemic inpatient population. It is crucial to identify the underlying cause of hypercalcaemia in order to effectively and definitively address the acute event. Box 1.2 lists the differential diagnoses for hypercalcaemia. The treatment of severe hypercalcaemia revolves around aggressive rehydration, increasing renal excretion of calcium, blunting of calcium release from skeletal stores, and treating the underlying cause of the hypercalcaemia.33
The primary goal of treatment is to achieve adequate volume resuscitation, which in turn increases calcium excretion in the kidneys.33,34 Patients are invariably dehydrated due to poor oral intake and vomiting. The resultant decrement in glomerular filtration rate leads to a decrease in renal excretion of calcium. Typically, 200–500 mL/h of normal saline are given to maintain urine output above 100 mL/h, with the caveat that comorbidities may limit the rate of resuscitation. Using normal saline lends substrate for the resultant natriuresis. Once the intravascular volume is restored, loop diuretics such as furosemide may be given to enhance calciuresis by inhibiting calcium resorption in the thick ascending limb of the loop of Henle. During the resuscitative phase, the patient must be monitored closely for signs of fluid overload, hypokalaemia and hypomagnesaemia. Serum calcium levels can be reduced by 1.6–2.5 mg/dL within 24 hours by volume repletion and loop diuretic administration alone.32 However, when serum calcium exceeds 12 mg/dL or hypercalcaemia is caused by malignancy, intravenous fluids and diuretics alone are usually insufficient to normalise calcium levels.
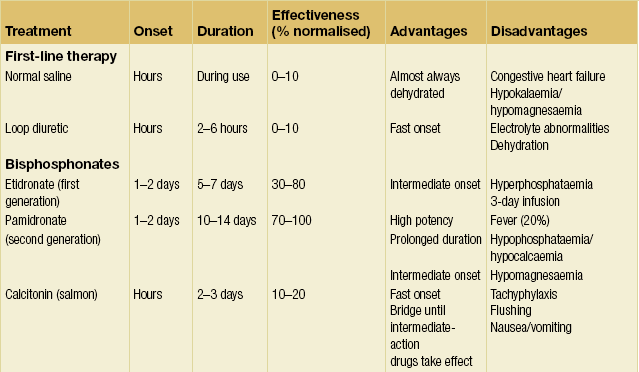
Numerous agents are available to blunt the release of calcium from bone resorption and treat the underlying disease.32–34 Table 1.1 provides an overview of agents available to combat hypercalcaemia and their relative strengths and weaknesses.
• Bisphosphonates: pamidronate 60–90 mg i.v. Bisphosphonates are pyrophosphate analogues that are concentrated in areas of high bone turnover and inhibit osteoclast activity. Endogenous phosphatases cannot hydrolyse the central carbon–phosphorus–carbon bond, making this drug stable in vivo. Bisphosphonates should be given intravenously due to their poor absorption by the gastrointestinal tract. In the USA, only etidronate (first generation) and pamidronate (second generation) are approved for use in treating hypercalcaemia. Pamidronate has widely supplanted etidronate as the bisphosphonate of choice due to its faster onset, increased duration of action, increased efficacy and minimal adverse effect on mineralisation. One dose of intravenous pamidronate normalises serum calcium for 10–14 days in 80–100% of patients with hypercalcaemia of malignancy. Newer, more potent generations of bisphosphonates may replace pamidronate as the standard as more clinical data become available.35
• Calcitonin: salmon calcitonin 4–8 U/kg s.c./i.v. Calcitonin diminishes osteoclast activity and increases calciuresis within minutes of administration. However, the duration of action is limited to only a few days. Calcitonin therapy only rarely results in normocalcaemia. Tachyphylaxis limits the long-term use of calcitonin. Currently, calcitonin is used primarily as an immediate hypocalcaemic agent that temporises until the more sustained effects of other agents begin.
• Gallium nitrate: 200 mg/m2 i.v. q.d. for 5 days. Gallium nitrate inhibits bone resorption by reducing the solubility of hydroxyapatite crystals. This drug induces normocalcaemia within 2–3 days that lasts for 5–6 days in approximately 75% of patients. The use of gallium nitrate has been limited by its nephrotoxicity, the need for continuous infusion and lack of clinical data.
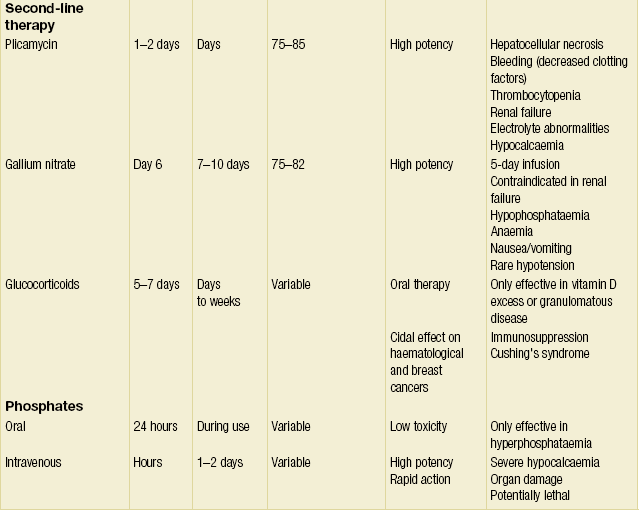
• Plicamycin: 25 μg/kg. Plicamycin is an osteoclast cytotoxin originally used in chemotherapy. Due to its serious side-effects (hepatic, renal and bone marrow toxicity), plicamycin is reserved for patients who fail bisphosphonate therapy. Since toxicities are related to the frequency and total dosage, administration is limited to one dose, with additional dosing only if hypercalcaemia recurs.
• Glucocorticoids: prednisone 40–100 mg p.o. q.d. or hydrocortisone 200–300 mg i.v. for 3–5 days. Glucocorticoids are used primarily to augment the effect of calcitonin or in diseases associated with vitamin D excess (i.e. granulomatous diseases, vitamin D toxicity and multiple myeloma). Glucocorticoids increase calciuresis, decrease intestinal absorption of calcium and have a direct tumoricidal effect on certain haematological malignancies as well as breast cancer.
• Oral inorganic phosphate: phosphate 1–1.5 g p.o. q.d. Oral inorganic phosphate has a limited effect in normalising serum calcium in patients who are hypophosphataemic by increasing calcium uptake by bone and intestinal absorption of calcium. Intravenous phosphate is one of the swiftest means to reduce serum calcium levels. However, it can cause fatal hypocalcaemia and severe organ failure by calcium phosphate precipitation. As such, intravenous phosphate is reserved for life-threatening hypercalcaemia, and even then must be used with extreme caution.
• Dialysis. This is the treatment of choice for patients with hypercalcaemia and renal or heart failure. Dialysis may also be considered in hypercalcaemic patients who fail standard therapies. Haemodialysis and peritoneal dialysis can remove up to 250 mg of calcium/hour. Care must be taken to avoid the hypophosphataemia that often accompanies dialysis.
The underlying cause of hypercalcaemic crisis must always be addressed as part of the definitive management.33 In patients with an elevated PTH level and clinical factors suggestive of PHP, parathyroidectomy is the fastest way to decrease PTH levels and consequently serum calcium levels. Therefore, expedient operative intervention should always be considered in this subgroup of patients.36
Surgical indications
The treatment of PHP is primarily surgical as medical interventions do not address the underlying pathology. Medical treatment is generally temporary and is reserved for acute hypercalcaemic crises or for patients who have mild disease with low risk of long-term sequelae or are poor operative candidates based on age or comorbidities. Definitive therapy is focused on removal of the offending gland or glands. Box 1.3 lists the current indications for surgery in PHP, which include (1) symptoms, (2) age less than 50, (3) significant hypercalcaemia, (4) osteoporosis and (5) decreased renal filtration. Recommendations for parathyroidectomy in asymptomatic patients were updated in 2008, as surgical intervention decreases the long-term risks of hypercalcaemia on bone health and nephrolithiasis in broad patient populations.37
Imaging and localisation
In the hands of experienced surgeons, bilateral neck exploration for PHP cures 95% of cases.38,39 Furthermore, prior to recent advances in imaging technology, the sensitivity of localisation studies was approximately 60–70%.
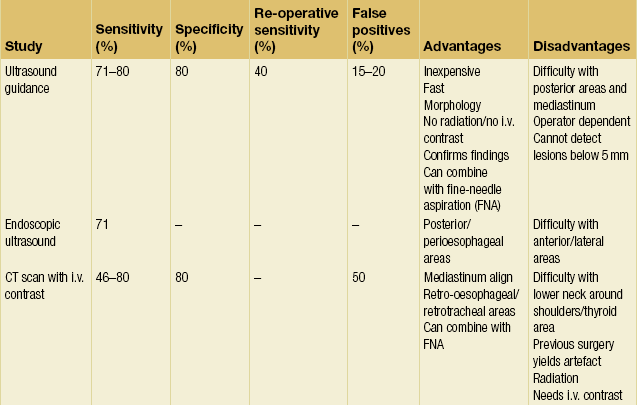
These statistics are consistent across the literature.42 The fact that the overwhelming majority of patients have unilateral disease or bilateral disease that can be identified by unilateral exploration raises the issue of whether bilateral exploration is mandated in every case. Is it reasonable to expose the patient to the increased morbidity of bilateral exploration to identify the less than 3% of people who will have a second adenoma on the contralateral side? These issues have led many endocrine surgeons to investigate the feasibility of preoperative localisation and directed unilateral exploration. This trend, along with the need to localise pathology in re-operative situations, has spurred the refinement of imaging techniques for parathyroid disease. Table 1.2 provides a summary of the current imaging modalities.
Ultrasound (US)
Ultrasound was one of the first localisation techniques to be widely used. Typically this test is performed with the 7.5- or 10-MHz probes to optimise penetration and resolution. It is fast, non-invasive, non-irradiating and inexpensive. Furthermore, it allows visualisation of the thyroid, carotid, jugular and cervical areas. However, ultrasound is dependent on operator experience and size of pathology (limit is approximately 5 mm). This technique also has difficulty locating abnormalities in the retro-oesophageal, retrosternal, retrotracheal and deep cervical areas. False-positive results (15–20%) are due to muscles, vessels, thyroid nodules, lymphadenopathy and oesophageal pathology.43,44 Image quality may be limited by patient motion or metallic clips from previous operations. The reported sensitivity of ultrasound is between 71% and 80%, but falls to 40% for re-operative localisation.45
Endoscopic US has also been used to evaluate posterior, deep cervical and perioesophageal glands. Endoscopic US correctly identified 12 of 23 adenomas (the remaining 11 were in either the anterior or lateral neck) in one series and had a sensitivity of 71% in another.46,47 Endoscopic US appears to have a role in localising certain parathyroid lesions for recurrent or persistent hyperparathyroidism.
Given these limitations, US is perhaps most useful when used in conjunction with other modalities. US combined with thyroid scintigraphy has the specific benefits of identifying intrathyroidal adenomas and distinguishing adenomas from thyroid nodules.48–50 Performing US-guided fine-needle aspiration (FNA) increases the sensitivity of US localisation by confirming the presence of PTH in the mass. Cytological studies of the aspirate are not useful and often cannot even distinguish between thyroid and parathyroid tissue. In one small series, PTH analysis of the aspirate made the diagnosis in 100% of cases.51 Finally, US provides a useful means to define the depth and singularity of adenomas found by scintigraphy.
Computed tomography (CT)
With the new-generation CT scanners and alterations in technique, the accuracy of CT has improved greatly over the last 5 years. In the past, the limitations of CT were based primarily on the size of the adenoma in that smaller parathyroid adenomas were more difficult to visualise. CT scan had difficulty in localising adenomas in the lower neck (at the level of the shoulders) and close to or within the thyroid. Furthermore, CT scan was inaccurate in differentiating between upper and lower pole glands.52,53 CT scans with intravenous contrast had sensitivities in the 80% range, but prior operations in the neck can produce artefacts, such as the ‘sparkler effect’ (seen with surgical clips), which reduce this number.54 The false-positive rate, at 50%, is higher than in other imaging modalities.55,56
The accuracy of CT scanning is largely dependent on the technique utilised, as well as the experience and dedication of the radiologist interpreting the study. Whereas in the past most reports of CT scanning utilised 5-mm cross-sectional cuts, accurate parathyroid CT localisation mandates the use of 2.5-mm cuts as well as a dedicated radiologist committed to conducting the time-consuming review of parathyroid CT scans. Comparing pre- and post-intravenous contrast scans permits identification of parathyroid adenomas due to the increased vascularity of hyperfunctioning parathyroid tissue. Thin-cut parathyroid CT scanning provides precise anatamomical information regarding gland location (anterior, posterior, superior, inferior or mediastinum) as well as information regarding parathyroid gland relationship to the thyroid gland. Thyroid nodules can be differentiated from parathyroid adenomas due to the difference in shape and vascularity. Moreover, parathyroid gland weight can be estimated by determining the volume of the visualised parathyroid gland. As with US, CT may be used in conjunction with FNA to increase diagnostic yield.57 In a retrospective review from Columbia and Cornell Universities, Harari et al. demonstrated that in patients with negative sestamibi localisation, thin-cut CT scanning permitted a focused parathyroidectomy in 66% of patients.58 Four-dimensional reconstruction is now feasible and permits a remarkable appreciation and increased accuracy of parathyroid gland location and relationship to surrounding structures.
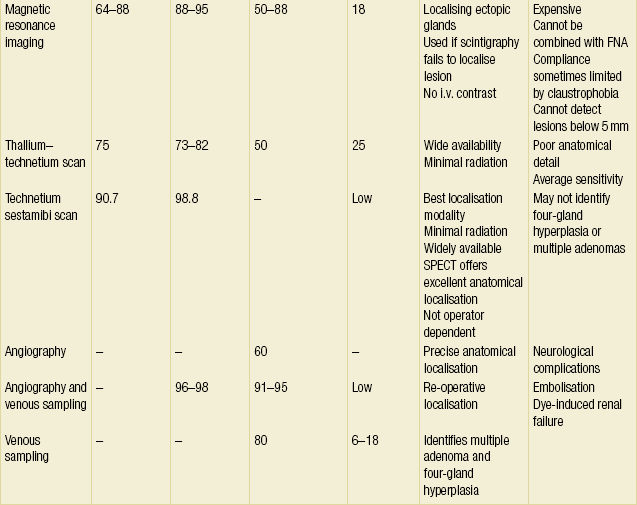
Magnetic resonance imaging (MRI)
MRI is superior to CT scanning in that it does not require intravenous contrast nor is it subject to the ‘sparkler effect’ or shoulder artefact. On T2-weighted imaging, enlarged parathyroid glands have significantly increased intensity. T2-weighted MRI is an excellent means of localising ectopic glands in patients undergoing re-operation for PHP, although it was less useful for identifying lesions in normal positions. Aufferman et al. found that MRI located 79% of ectopic adenomas while localising only 59% of those in the normal anatomical position.59 Overall sensitivities are in the 50–88% range for re-operative localisation.60 Despite better sensitivities (64–88%) than CT scanning, MRI has significant drawbacks.55,56,61,62 This modality cannot image normal glands or adenomas less than 5 mm in size. Furthermore, it has difficulty localising superior parathyroid glands since they lie posterior to the thyroid. False positives can result from thyroid nodules and lymphadenopathy.63 Finally, MRI is expensive, cannot be combined with FNA, and patient compliance is sometimes limited by claustrophobia. Given all these factors, MRI is best reserved for localisation in re-operation for PHP or when parathyroid scintigraphy is negative or equivocal.64,65
Thallium-201–technetium-99 m pertechnetate scan (Tl–99mTc scan)
Tl–Tc scanning is an image subtraction technique that is rapidly being replaced by sestamibi scanning (see below). Tl–Tc scanning relies on the fact that the thyroid and parathyroid tissues (especially hyperfunctioning glands) take up thallium while the thyroid alone takes up technetium. By subtracting the two images, one can localise the parathyroid tumour. The sensitivity of Tl–Tc scanning is 75% for first-time operations and only 50% for re-operations.66 The false-positive rate is approximately 25% and occurs with metastatic nodal disease and thyroid pathology.66 Given the average sensitivity and poor anatomical detail of Tl–Tc scanning, this mode has been relegated to second-line imaging status.
Technetium-99 m sestamibi scan (sestamibi scan)
Ever since Coakley et al. fortuitously discovered that technetium-99 m sestamibi concentrated in abnormal parathyroid glands, sestamibi scanning has revolutionised the practice of parathyroid surgery, making directed unilateral exploration a reasonable alternative to routine bilateral exploration.67 Sestamibi is a derivative of technetium that avidly incorporates itself into mitochondria. The large amount of mitochondria in hyperactive parathyroid glands allows more intense labelling of parathyroid tumours relative to the thyroid and surrounding tissue.68 The radiotracer also washes out much more slowly from the parathyroid than the thyroid. This differential uptake can be accentuated by pretest medical thyroid suppression. Sestamibi exploits these differences in uptake and retention to localise parathyroid adenomas. This radioisotope has a short half-life and produces high-energy photon emission that allows for low doses of radiation and high-definition imaging. Also, sestamibi scanning images both in the anteroposterior and lateral views, which allows for more precise localisation of the pathology.
There are three basic protocols for sestamibi scanning in current use:
• Single-isotope dual-phase scan. After intravenous administration of 15–25 mCi of sestamibi, images are taken at 10, 15, 120 and 180 minutes post-injection. A positive scan demonstrates increased uptake of tracer in the thyroid gland and parathyroid adenoma in early phases with washout of tracer from the thyroid gland but not the parathyroid adenoma in the late-phase images. This is the simplest and most widely used protocol. However, two potential pitfalls of this technique are: (i) sestamibi can accumulate and remain in thyroid nodules; and (ii) rapid washout of sestamibi can lead to false-negative results. To counter the first problem, many investigators are experimenting with dual-isotope subtraction scanning. Figure 1.1 illustrates a typical parathyroid adenoma in the early-phase scan.
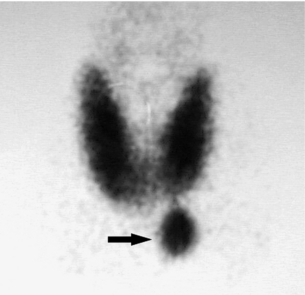
Figure 1.1 Single-isotope dual-phase sestamibi scan. Sestamibi tracer can be seen concentrating in both the thyroid gland and a left lower pole parathyroid adenoma (arrow) in this early-phase image. Delayed-phase images would demonstrate washout of tracer from the thyroid gland but not the parathyroid adenoma.
• Dual-isotope subtraction scanning. Sestamibi and another radioisotope that amasses in the thyroid (such as 123I or thallium chloride) are administered and the two views are subtracted to reveal the parathyroid pathology. Images are taken in both early and late phases. Late-phase imaging helps to exclude false-positive results by allowing more time for thyroid nodules to wash out. Numerous protocols and isotopes are currently being investigated but none has yet proven superior to the rest. Figure 1.2 demonstrates a dual-isotope subtraction scan. Panel (a) demonstrates 123I tracer uptake in only the thyroid gland. Panel (b) demonstrates an early-phase image with uptake of sestamibi tracer in the right lower parathyroid adenoma (arrow) and parts of the thyroid gland. Panel (c) demonstrates persistent tracer in the parathyroid adenoma (arrow) and washout of tracer from the thyroid gland.
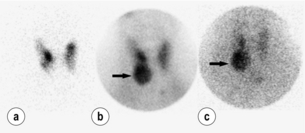
Figure 1.2 Dual-isotope dual-phase sestamibi scan using both sestamibi and iodine-123. (a) The 123I-only scan that delineates the thyroid gland only. (b) A right lower lobe parathyroid adenoma (arrow) in the early-phase image with concomitant thyroid uptake of 123I. (c) The same right lower lobe parathyroid adenoma (arrow) with thyroid ‘washout’ seen in this late-phase image.
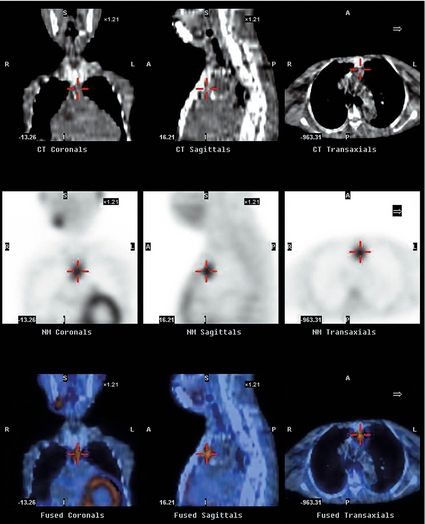
• SPECT (single-photon emission computed tomography) analysis. This protocol allows for three-dimensional images to be created, which allows for better anatomical localisation, especially within the mediastinum, without any significant increase in sensitivity.69,70 While this enhanced anatomical delineation may be useful in re-operative PHP, the significantly increased cost of this modality does not justify its routine use in preoperative localisation. Figure 1.3 demonstrates a CT-enhanced SPECT scan (CT-SPECT). The top two rows of images mark the parathyroid adenoma on a CT scan (see Fig. 1.3). The bottom row of images marks the parathyroid adenoma on SPECT scan (see Fig. 1.3).
Irrespective of the protocol, depending on the series quoted, sestamibi scanning localises parathyroid adenomas in 80–100% of cases and has a specificity of around 90%.41,72–75
The false-negative rate is low and is usually related to small-sized glands or failure to recognise hyperplasia. In a meta-analysis of the English-language literature over 10 years, comprising 6331 patients, Denham and Norman71 found that 87% of patients had a single adenoma that sestamibi scan localised with an average sensitivity and specificity of 90.7% and 98.8%, respectively.41 Sestamibi-guided unilateral exploration led to an average cost saving of US $650 per operation. This study demonstrated that preoperative localisation with sestamibi scan was specific enough to make unilateral exploration both safe and cost-effective. If a single focus of uptake is noted, then unilateral exploration is likely to be successful. If no uptake or multiple areas of uptake are seen then bilateral exploration should be planned. Other radioisotopes, such as [99mTc]tetrofosmin and 2[18F]-fluoro-2-deoxyglucose, are currently being evaluated. For the time being, sestamibi scanning remains the standard for non-invasive localisation modalities.
Parathyroid angiography and venous sampling for PTH
Parathyroid angiography involves examination of both thyrocervical trunks, both internal mammary arteries and both carotids, with occasional selective superior thyroid artery catheterisation. The highly vascular parathyroid adenomas appear as a persistent oval or round ‘stain’ on angiography. Glands 4 mm in size or greater may be readily visualised. False positives are typically due to thyroid nodules or inflamed lymph nodes. Due to potentially serious complications like dye-induced renal failure, embolisation and neurological damage, angiography is usually reserved for re-operative localisation. The sensitivity of parathyroid angiography in this situation approaches 60%.45,76,77
Selective venous sampling for PTH allows for precise localisation of adenomas in the hands of an experienced interventionalist. The venous drainage of the lesion is established when there is a twofold drop in PTH between the sampled blood and the serum PTH. Figure 1.4 demonstrates venous sampling data. The technique has a sensitivity of 80% and is equally effective in localising mediastinal and cervical adenomas.44,45,78,79 Venous sampling also allows for the identification of pathology in multiple glands. Venous sampling without concomitant angiography has a false-positive rate of 6–18%.79
Pathology
Adenoma
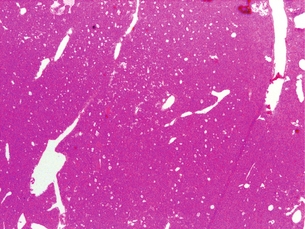
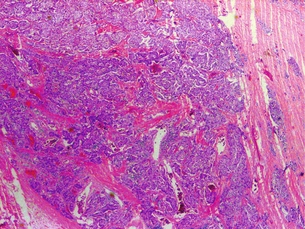
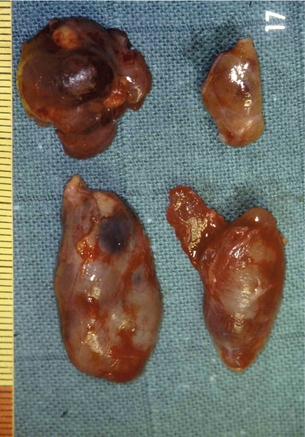
The gross appearance of an adenoma is typically large and tan or beefy red. Some authors have described the classic adenoma as a ‘little kidney in the neck or mediastinum’.80 The other glands appear atrophic or normal in size. While normal parathyroids contain predominantly chief cells with scattered oxyphil cells, adenomas contain solid sheets of chief cells, oxyphil cells or a combination of both surrounded by a fibrous capsule. Classically, there is a rim of compressed normal parathyroid surrounding the adenoma, which can be found in 20–30% of patients. Figure 1.5 demonstrates the characteristic hypercellularity, loss of fat, loss of lobulation and oxyphilic change of adenomatous degeneration. Pleomorphism and multinucleation may be present, but mitotic figures are rare and more strongly associated with carcinoma. There is less stromal fat in adenomas compared with normal parathyroids. Research demonstrates that parathyroid adenomas are typically monoclonal and may have very specific mutations in certain genes, such as the MEN1 tumour suppressor gene and the PRAD1 oncogene.81
Hyperplasia
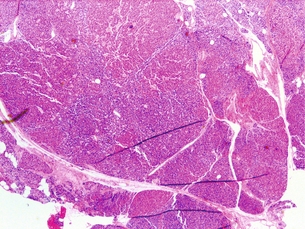
A polyclonal expansion of parathyroid cells is called hyperplasia. This is more typical of familial hyperparathyroidism but may be found in sporadic cases. Grossly, the hyperplasia is typically not uniform. One gland may appear much larger than the rest, giving the false impression of adenomatous disease, but on histological examination each gland is hyperplastic. Microscopically, the chief cells are mainly affected. More so than with adenomas, the absence of parathyroid fat supports the diagnosis of hyperplasia. Figure 1.6 demonstrates the characteristic hypercellularity, loss of fat, and retained lobulation of parathyroid hyperplasia. Diffuse hyperplasia warrants four-gland exploration with three-and-a-half-gland parathyroidectomy or four-gland excision with autotransplantation.
Carcinoma
A rare finding, parathyroid carcinoma is a difficult diagnosis to make preoperatively and often is a retrospective diagnosis made only after metastatic disease develops. Patients tend to be younger (fifties cf. sixties) than in benign disease and there is an equal distribution among men and women. On preoperative evaluation, carcinoma produces a palpable mass in 30–75% of patients (far more frequently than in benign disease) and serum calcium tends to be higher than for adenomatous disease. Furthermore, recurrent laryngeal nerve involvement is suggestive of malignancy. Classic operative findings for parathyroid carcinoma include adherence or invasion into surrounding structures and dense scarring. Typical histological findings include bizarre nuclear atypia, mitotic figures, and capsular or vascular invasion. Figure 1.7 demonstrates the characteristic thickened fibrous septa, nuclear atypia and capsular invasion. The only definitive criteria for malignancy are metastatic disease (lung, lymph node, liver) and local invasion. There is a recurrence rate of 66%. The 5-year survival is approximately 69%, with death caused by metabolic sequelae of hypercalcaemia.
Secondary hyperparathyroidism (SHP)
Pathogenesis
Bony resistance to PTH
Typically, PTH induces bone resorption with a subsequent rise in serum calcium levels by activating osteoclasts. However, experiments have shown that excessive PTH blunts the mobilisation of calcium from osseous stores.82
Changes in PTH set point
The PTH set point is defined as the serum calcium level that decreases PTH levels by 50%.83 As the set point rises, inhibition of PTH secretion is lost and SHP results. Research suggests that changes in set point may be due to alterations in the expression or sensitivity of the calcium-sensing receptor, but no genetic links have yet been found.81
Presentation
Pruritus
Pruritus is found in 85% of patients on haemodialysis and may become so severe as to be disabling. Significant symptomatic relief is achieved after parathyroidectomy.84
Treatment
The initial therapy of SHP is primarily medical and revolves around bringing the serum calcium and phosphate to physiological levels. Normalising these removes the major impetus for PTH overproduction. Non-operative therapy includes calcium supplementation (1500 mg/day), phosphate-poor diets, phosphate binders (< 1000 mg/day) and vitamin D supplementation. Other therapies include aluminium-binding agents (desferrioxamine) and haemodialysis with calcium-enriched dialysates. However, hypercalcaemia often complicates these treatment regimens. Newly developed calcimimetics bind to the calcium-sensing receptor and lower parathyroid hormone levels without increasing calcium and phosphate levels. Agents like cinacalcet have been shown to effectively reduce PTH levels in contrast to placebo in randomised double-blind studies.85 The definitive therapy for SHP is renal transplant, although some patients will develop tertiary hyperparathyroidism postoperatively. Operative parathyroidectomy (four-gland with autotransplantation or three-and-a-half-gland) is indicated in the 5–10% of patients who fail medical management. Other indications include: (1) intractable bone pain; (2) intractable pruritus; (3) fractures; and (4) symptomatic ectopic calcifications.
References
1. Adami, S., Marcocci, C., Gatti, D., Epidemiology of primary hyperparathyroidism in Europe. J Bone Miner Res. 2002;17(Suppl. 2):N18–N23. 12412773
2. Heath, H., 3rd., Hodgson, S.F., Kennedy, M.A., Primary hyperparathyroidism. Incidence, morbidity, and potential economic impact in a community. N Engl J Med. 1980;302(4):189–193. 7350459
3. Russell, C.F., Edis, A.J., Surgery for primary hyperparathyroidism: experience with 500 consecutive cases and evaluation of the role of surgery in the asymptomatic patient. Br J Surg. 1982;69(5):244–247. 7074331
4. Thompson, N.W., Eckhauser, F.E., Harness, J.K., The anatomy of primary hyperparathyroidism. Surgery. 1982;92(5):814–821. 7135202 An excellent overall review.
5. Moore, M.A., Owen, J.J., Experimental studies on the development of the thymus. J Exp Med. 1967;126(4):715–726. 4861748
6. Akerstrom, G., Malmaeus, J., Bergstrom, R., Surgical anatomy of human parathyroid glands. Surgery. 1984;95(1):14–21. 6691181
7. Brown, E.M., The pathophysiology of primary hyperparathyroidism. J Bone Miner Res. 2002;17(Suppl. 2):N24–N29. 12412774
8. Goodman, W.G., Calcium-sensing receptors. Semin Nephrol. 2004;24(1):17–24. 14730506
9. Tfelt-Hansen, J., Schwarz, P., Brown, E.M., et al, The calcium-sensing receptor in human disease. Front Biosci 2003; 8:s377–s390. 12700051
10. Conigrave, A.D., Franks, A.H., Brown, E.M., et al, l-Amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr. 2002;56(11):1072–1080. 12428172
11. Hofer, A.M., Brown, E.M., Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4(7):530–538. 12838336
12. Hoare, S.R., Usdin, T.B., Molecular mechanisms of ligand recognition by parathyroid hormone 1 (PTH1) and PTH2 receptors. Curr Pharm Des. 2001;7(8):689–713. 11375776
13. Libutti, S.K., Alexander, H.R., Bartlett, D.L., et al, Kinetic analysis of the rapid intraoperative parathyroid hormone assay in patients during operation for hyperparathyroidism. Surgery. 1999;126(6):1145–1151. 10598200
14. Fujita, T., Meguro, T., Fukuyama, R., et al, New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells. Checkpoint of modulation by cyclic AMP. J Biol Chem. 2002;277(25):22191–22200. 11956184
15. Brown, E.M., Extracellular Ca2 + sensing, regulation of parathyroid cell function, and role of Ca2 + and other ions as extracellular (first) messengers. Physiol Rev. 1991;71(2):371–411. 2006218
16. Carmeliet, G., Van Cromphaut, S., Daci, E., et al, Disorders of calcium homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):529–546. 14687587
17. Austin, L.A., Heath, H., 3rd., Calcitonin: physiology and pathophysiology. N Engl J Med. 1981;304(5):269–278. 7003392
18. Melton, J.L., The epidemiology of primary hyperparathyroidism in North America. J Bone Miner Res. 2002;17(Suppl. 2):N12–N17. 12412772
19. Monchik, J.M., Normocalcemic hyperparathyroidism. Surgery. 1995;118(6):917–923. 7491534
20. Boughey, J.C., Ewart, C.J., Yost, M.J., et al, Chloride/phosphate ratio in primary hyperparathyroidism. Am Surg. 2004;70(1):25–28. 14964541
21. Lundgren, E., Rastad, J., Thrufjell, E., et al, Population based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997;121(3):287–294. 9092129
22. Silverberg, S.J., Bilezikian, J.P., “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88(11):5348–5352. 14602772
23. Carnaille, B.M., Pattou, F.N., Oudar, C., et al, Parathyroid incidentalomas in normocalcemic patients during thyroid surgery. World J Surg. 1996;20(7):830–834. 8678958 Overview of normocalcaemic hyperparathyroidism.
24. Maruani, G., Hertig, A., Paillard, M., et al, Normocalcemic primary hyperparathyroidism: evidence for a generalized target-tissue resistance to parathyroid hormone. J Clin Endocrinol Metab. 2003;88(10):4641–4648. 14557434
25. Siperstein, A.E., Shen, W., Chan, A.K., et al, Normocalcemic hyperparathyroidism. Biochemical and symptom profiles before and after surgery. Arch Surg. 1992;127(10):1157–1163. 1417479
26. Wu, P.H., Wang, C.J., Normocalcemic primary hyperparathyroidism with fractures. J Arthroplasty. 2002;17(6):805–809. 12216040
27. Parks, J., Coe, F., Favus, M., Hyperparathyroidism in nephrolithiasis. Arch Intern Med 1980; 140:1479–1481. 7436644
28. Broadus, A.E., Dominguez, M., Bartter, F.C., Pathophysiological studies in idiopathic hypercalciuria: use of an oral calcium tolerance test to characterize distinctive hypercalciuric subgroups. J Clin Endocrinol Metab. 1978;47(4):751–760. 233682
29. Hagag, P., Revet-Zak, I., Hod, N., et al, Diagnosis of normocalcemic hyperparathyroidism by oral calcium loading test. J Endocrinol Invest. 2003;26(4):327–332. 12841540
30. Greenfield, M.W. Parathyroid glands. In: Lazar J., et al, eds. Surgery: scientific principles and practice. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001:1290.
31. Carroll, M.F., Schade, D.S., A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959–1966. 12751658
32. Bardin, C.W. Hypercalcemia. In Current therapy in endocrinology and metabolism, 6th ed., New York: Mosby; 1997:552.
33. Bilezikian, J.P., Management of acute hypercalcemia. N Engl J Med 1992; 326:1196–1203. 1532633
34. Edelson, G.W., Kleerekoper, M., Hypercalcemic crisis. Med Clin North Am 1995; 79:79–92. 7808096
35. Oura, S., Malignancy-associated hypercalcemia [in Japanese]. Nippon Rinsho. 2003;61(6):1006–1009. 12806951
36. Ziegler, R., Hypercalcemic crisis. J Am Soc Nephrol. 2001;12(Suppl. 17):S3–S9. 11251025
37. Bilezikian, J.P., Khan, A.A., Potts, J.T., Jr., Third International Workshop on the Management of Asymptomatic Primary Hyperthyroidism. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94(2):335–339. 19193908
38. Van Heerden, J., Lessons learned. Surgery. 1997;122(6):978–988. 9426410 Overview of surgical approach to primary hyperparathyroidism.
39. Weber, C., Burke, G.J., McGarity, W.C., Persistent and recurrent sporadic primary hyperparathyroidism: histopathology, complications, and results of reoperation. Surgery 1994; 116:991. 7985107
40. Consensus Development Conference Panel, Diagnosis and management of asymptomatic primary hyperparathyroidism: Consensus Development Conference statement. Ann Intern Med 1991; 114:593–597. 1900404
41. Ruda, J., Hollenbeak, C.S., Stack, B.C., A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132(3):359–372. 15746845 This study is a large review that details the pathology of primary hyperparathyroidism in patients undergoing parathyroidectomy.
42. Attie, J.N., Bock, G., August, L.J., Multiple parathyroid adenomas: report of thirty three cases. Surgery 1990; 108:1014. 2247825
43. Grant, C., Van Heerden, J.A., Charboneau, E.M., Clinical management of persistent and/or recurrent primary hyperparathyroidism. World J Surg 1986; 10:555. 3529648
44. Rodriquez, J.M., Tezelman, S., Siperstein, A.E., et al, Localization procedures in patients with persistent or recurrent hyperparathyroidism. Arch Surg. 1994;129(8):870–875. 8048861
45. Miller, D., Doppman, M.D., Shawker, M.D., et al, Localization of parathyroid adenomas who have undergone surgery. Radiology 1987; 162:133–137. 3538145
46. Henry, J.F., Audiffret, J., Denizot, A., et al, Endosonography in the localization of parathyroid tumors: a preliminary study. Surgery. 1990;108(6):1021–1025. 2247826
47. Catargi, B., Raymond, J.M., Lafarge-Gense, V., et al, Localization of parathyroid tumors using endoscopic ultrasonography in primary hyperparathyroidism. J Endocrinol Invest. 1999;22(9):688–692. 10595832
48. Casara, D., Rubello, D., Pelizzo, M.R., et al, Clinical role of 99mTcO4/MIBI scan, ultrasound, and intra-operative gamma probe in the performance of unilateral and minimally invasive surgery in hyperparathyroidism. Eur J Nucl Med 2001; 28:1351–1359. 11585294
49. Uden, P., Aspelin, P., Berglund, J., et al, Preoperative localization in unilateral parathyroid surgery. A cost-benefit study on ultrasound, computed tomography and scintigraphy. Acta Chir Scand. 1990;156(1):29–35. 2108524
50. De Feo, M.L., Colagrande, S., Biagini, C., et al, Parathyroid glands: combination of (99m)Tc MIBI scintigraphy and US for demonstration of parathyroid glands and nodules [see comment]. Radiology. 2000;214(2):393–402. 10671586
51. Tikkakoski, T., Stenfors, L.E., Typpo, T., et al, Parathyroid adenomas: pre-operative localization with ultrasound combined with fine-needle biopsy. J Laryngol Otol. 1993;107(6):543–545. 8345304
52. Dijkstra, B., Healy, C., Kelly, L.M., et al, Parathyroid localisation – current practice. J R Coll Surg Edinb. 2002;47(4):599–607. 12365423
53. Giuliano, M., Gulec, S.A., Rubello, D., et al, Preoperative localization and radioguided parathyroid surgery. J Nucl Med 2003; 44:1443–1458. 12960191
54. Weber, A.L., Randolph, G., Aksoy, F., The thyroid and parathyroid glands: CT and MR imaging and correlation with pathological and clinical findings. Radiol Clin North Am 2000; 38:1105–1128. 11054972
55. Erdman, W.A., Breslau, N.A., Weinreb, J.C., et al, Noninvasive localization of parathyroid adenomas: a comparison of X-ray computerized tomography, ultrasound, scintigraphy and MRI. Magn Reson Imaging. 1989;7(2):187–194. 2541298
56. Levin, K.E., Gooding, G.A., Okerlund, M., et al, Localizing studies in patients with persistent or recurrent hyperparathyroidism. Surgery. 1987;102(6):917–925. 3317961
57. Doppman, J., Krudy, A.G., Marx, S.J., Aspiration of enlarged parathyroid glands for parathyroid hormone assay. Radiology 1983; 148:31–35. 6856859
58. Harari, A., Zarnegar, R., Lee, J., Kazam, E., Inabnet, W.B., 3rd., Fahey, T.J., 3rd., Computed tomography can guide focused exploration with primary hyperparathyroidism and negative sestamibi scanning. Surgery. 2008;144(6):970–976. 19041005
59. Aufferman, W., Gooding, G., Okerlund, M., Diagnosis of recurrent hyperparathyroidism: comparison of MR imaging and the other techniques. Am J Roentgenol 1988; 150:1027–1033. 3282400
60. Stark, D., Clark, O.H., Moss, A., Magnetic resonance imaging of the thyroid, thymus, and parathyroid glands. Surgery. 1984;96(6):1083–1090. 6390767
61. Kang, Y., Rosen, K., Clark, O.H., et al, Localization of abnormal parathyroid glands of the mediastinum with MR imaging. Radiology 1993; 189:137–141. 8372183
62. Kurbskack, A., Wilson, S.D., Lawson, T., Prospective comparison of radionuclide, computed tomography, sonographic, and magnetic resonance localization of parathyroid tumors. Surgery 1989; 106:639. 2678555
63. Higgins, C.B., Role of magnetic resonance imaging in hyperparathyroidism. Radiol Clin North Am. 1993;31(5):1017–1028. 8362052
64. Fayet, P., Hoeffel, C., Fulla, Y., Technetium-99m sestamibi, magnetic resonance imaging, and venous blood sampling in persistent and recurrent hyperparathyroidism. Br J Radiol 1997; 70:459–464. 9227226 Overview of current state of localisation studies for primary hyperparathyroidism.
65. Gotway, M., Reddy, G., Webb, W., et al, Comparison between MR imaging and 99mTc-MIBI scintigraphy in the evaluation of recurrent or persistent hyperparathyroidism: results and factors affecting parathyroid detection. Am J Roentgenol 2001; 218:783–790. 11230657
66. Hewin, D.F., Brammar, T.J., Kabala, J., et al, Role of preoperative localization in the management of primary hyperparathyroidism. Br J Surg. 1997;84(10):1377–1380. 9361592
67. Coakley, A.J., Kettle, A.G., Wells, C.P., et al, 99Tcm sestamibi – a new agent for parathyroid imaging. Nucl Med Commun. 1989;10(11):791–794. 2532313
68. Sandrock, D., Merino, M.J., Norton, J.A. Light and electronmicroscopic analyses of parathyroid tumors explain results of Tl201Tc99 m parathyroid scintigraphy. Eur J Med. 1989; 15:410.
69. Pattou, F., Huglo, D., Proye, C., Radionuclide scanning in parathyroid diseases. Br J Surg. 1998;85(12):1605–1616. 9876061
70. McHenry, C., Lee, K., Saddey, J., et al, Parathyroid localisation with technetium-99 m-MIBI scintigraphy to identify anatomy in secondary hyperparathyroidism. J Nucl Med 1996; 37:565–569. 8691240
71. Denham, D.W., Norman, J., Cost-effectiveness of preoperative sestamibi scan for primary hyperparathyroidism is dependent solely upon the surgeon’s choice of operative procedure. J Am Coll Surg. 1998;186(3):293–305. 9510260
72. O’Doherty, M.J., Kettle, A.G., Parathyroid imaging: preoperative localization. Nucl Med Commun. 2003;24(2):125–131. 12548036
73. Thule, P., Thakore, K., Vansant, J., et al, Preoperative localization of parathyroid tissue with technetium-99m sestamibi 123I subtraction scanning. J Clin Endocrinol Metab. 1994;78(1):77–82. 8288719
74. Casas, A.T., Burke, G.J., Mansberger, A.R., Jr., et al, Impact of technetium-99m-sestamibi localization on operative time and success of operations for primary hyperparathyroidism. Am Surg. 1994;60(1):12–17. 8273968
75. Caixas, A., Berna, L., Hernandez, A., et al, Efficacy of preoperative diagnostic imaging localization of technetium 99m-sestamibi scintigraphy in hyperparathyroidism. Surgery. 1997;121(5):535–541. 9142152
76. Miller, D.L., Preoperative localization and interventional treatment of parathyroid tumors: when and how? World J Surg 1991; 15:706. 1767536
77. Miller, D.L., Chang, R., Doppman, J., et al, Superselective DSA versus superselective conventional angiography. Radiology 1989; 170:1003. 2644666
78. Sugg, S.L., Fraker, D.L., Alexander, R., et al, Prospective evaluation of selective venous sampling for parathyroid hormone concentration in patients undergoing reoperations for primary hyperparathyroidism. Surgery. 1993;114(6):1004–1010. 8256203
79. Granberg, P.O., Hamberger, B., Johansson, G., et al, Selective venous sampling for localization of hyperfunctioning parathyroid glands. Br J Surg. 1986;73(2):118–120. 3947900
80. Van Heerden, J., Farley, D. Parathyroid. In Schwartz S., ed.: Principles of surgery, 7th ed., New York: McGraw-Hill, 1999.
81. Miedlich, S., Krohn, K., Paschke, R., Update on genetic and clinical aspects of primary hyperparathyroidism. Clin Endocrinol 2003; 59:539–554. 14616876
82. Rodriguez, M., Martin-Malo, A., Martinez, M., Calcemic response to parathyroid hormone in renal failure: role of phosphorus and its effect on calcitriol. Kidney Int 1991; 40:1055. 1762306
83. Felsenfeld, A., Rodriguez, M., Dunlay, R., et al, A comparison of parathyroid gland function in haemodialysis patients with different forms of renal osteodystrophy. Nephrol Dial Transpl 1991; 6:244–251. 1881578
84. Demeure, M., McGee, D., Wilkes, W., et al, Results of surgical treatment for primary hyperparathyroidism associated with renal disease. Am J Surg 1990; 160:337. 2221230 An overview of the treatment of secondary hyperparathyroidism.
85. Block, G., Martin, K., de Francisco, A., et al, Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–1525. 15071126
Part 2
Operative strategy for the management of parathyroid disease
Primary hyperparathyroidism
For many years bilateral cervical exploration has been the preferred surgical approach for primary hyperparathyroidism (PHP). The success rate is reported to be 95–98%, the morbidity is minimal, the mortality is close to zero and cosmetic results are excellent.1
1. The improvement of imaging techniques such as high-resolution ultrasonography, sestamibi and computed tomography (CT) scanning.
2. The introduction of the intraoperative parathyroid hormone assay (ioPTH).
3. The advancement and refinement of instrumentation (gamma probe, tissue sealing devices, endoscopic instruments, miniaturised cameras).
Conventional open parathyroidectomy
Management of surgical procedure
The operation is usually performed under general anaesthesia but regional anaesthesia2 and hypnosedation3 can also be used.
The search for superior parathyroid (P IV)
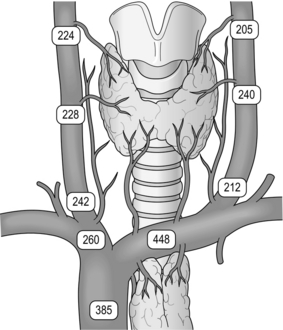
In 85% of cases this simple exposure allows identification of the normal P IV in its orthotopic site. It ‘floats’ in a loose fatty setting immediately adjacent to the inferior cornu of the thyroid cartilage, very close to the recurrent nerve and the most cranial branch of the inferior thyroid artery. These structures constitute three basic landmarks in the search for P IV (Fig. 1.8).
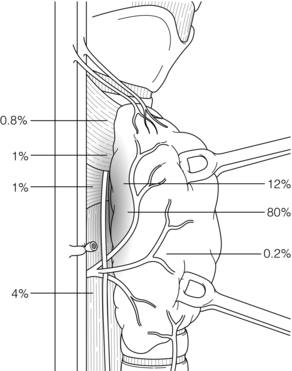
Figure 1.8 Location of superior parathyroid glands (P IV). The numbers represent the percentages of glands found at different locations in an autopsy study of 503 cases. Adapted from Akerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of human parathyroid glands. Surgery 1984; 95:14–21. With permission from Elsevier.
When a P IV is abnormal, it tends to migrate posteriorly and downwards (Fig. 1.9). Therefore, if it is not found in immediate contact with the thyroid capsule, it should be sought beside or behind the oesophagus. Its migration may drag it down very low, well below the inferior thyroid artery, behind whose trunk it crosses during its descent. The lower a P IV, the more posterior it becomes. These adenomas are revealed by their vascular pedicles, whose origin is found at the middle or upper third of the thyroid lobe. They emerge with simple traction on their pedicle. They are closely related to the recurrent nerve, which may be adherent to their capsule, so that their mobilisation calls for prior identification of the nerve and possibly its dissection. If no gland, normal or abnormal, is discovered, the search should be transferred to the perithyroid visceral sheath, carefully exploring the posterior aspect of the lobe from the trunk of the inferior thyroid artery to the superior thyroid pedicle. Particular attention must be devoted to the posterior aspect of the upper pole of the thyroid lobe, where some very flattened adenomas, closely adherent to the surface of the thyroid capsule, may easily pass unnoticed.
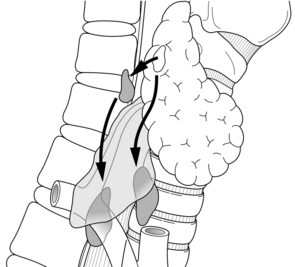
Figure 1.9 Acquired migration paths of enlarged superior parathyroids (P IV). The enlarged glands tend to migrate posteriorly and downwards. Adapted from Randolph GW, Urken ML. Surgical management of primary hyperparathyroidism. In: Randolph GW (ed.) Surgery of the thyroid and parathyroid glands. Philadelphia: WB Saunders, 2003; pp. 507–28. With permission from Elsevier.
The search for inferior parathyroid (P III)
The usual range of position of P III is more extensive than that of P IV (Fig. 1.10). The search must be made from the inferior thyroid artery to the inferior thyroid pole, and along the thyrothymic ligament. The P IIIs are rarely posterior and become more anterior the lower they are.
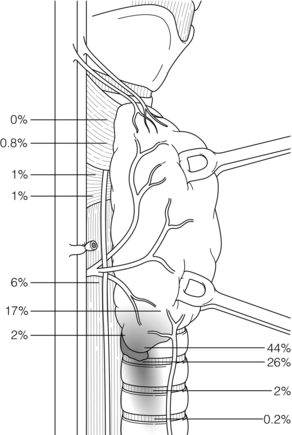
Figure 1.10 Location of inferior parathyroid glands (P III). The numbers represent the percentages of glands found at different locations in an autopsy study of 503 cases. Adapted from Akerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of human parathyroid glands. Surgery 1984; 95:14–21. With permission from Elsevier.
The search should first be made at the posterior aspect of the thyroid lobe from the inferior thyroid artery to the lower pole of the lobe. At this site, a normal P III is always situated in front of the recurrent nerve. When it is adenomatous its posterior surface may adhere to the nerve. The exploration must be carried all around the inferior pole of the thyroid lobe, checking its lateral, anterior and inferior aspects in turn. During this dissection one must safeguard the thyroid attachments, i.e. the thyrothymic ligament and the inferior thyroid veins. Then the dissection must be carried as low as possible along the thyrothymic ligaments and the thymus. Nearly 25% of P IIIs are situated along the thyrothymic ligaments or at the upper poles of the thymus.4,5 Very often they are discovered only after incision of the thymic sheath.
Evaluation of the initial bilateral exploration
The exploration can be abandoned in two circumstances:
1. The four glands have been discovered and one or more are abnormal. A continued search for a supernumerary gland is justified only in cases of familial hyperparathyroidism.
2. One gland is pathological, the other gland(s) identified are normal, but fewer than four glands have been discovered. Except in cases of familial hyperparathyroidism the low risk of multiglandular disease that might pass unnoticed does not justify obstinate pursuit of an exploration that risks proving more dangerous than beneficial for the patient. The diagnosis of a solitary adenoma becomes more likely as the number of normal glands found approaches three.
The exploration should be pursued in three circumstances:
1. No gland or fewer than four glands have been discovered and none is pathological. A probable adenoma remains to be discovered.
2. Fewer than four glands have been discovered and at least two of these are enlarged. The surgeon is dealing with a multiglandular disease (MGD). The missing gland(s) must be identified.
3. All four glands have been discovered but all are normal. The surgeon must remain convinced of his or her diagnosis and consider the possibility of a probable ectopic supernumerary adenoma.
Continuation of the exploration
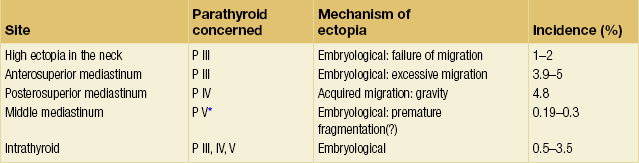
The surgeon must keep in mind that: (i) congenital ectopias, in the neck or in the anterior mediastinum, respectively caused by defective or excessive embryological migration, are related to P III (Fig. 1.11); and (ii) acquired ectopias in the posterior mediastinum caused by migration affected by gravity, secondary to adenomatous pathology, are essentially related to P IV (Fig. 1.8, Table 1.3).6 Therefore, it is essential to know whether the missing gland is a P IV or a P III.
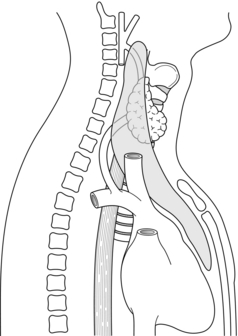
Figure 1.11 The embryonic migration of the inferior parathyroid gland (P III)–thymus complex results in an extensive area of dispersal of the normal P III from the angle of the mandible to the pericardium.
1. Re-explore the juxta-oesophageal regions, as far down as possible in the posterior mediastinum.
2. Remember the defective migration of P IV and explore the superior thyroid pedicle region.
3. Ligate the superior thyroid pedicle and mobilise the upper pole of the lobe for scrupulous dissection of its posteromedial aspect.
4. Carefully palpate the thyroid lobe to seek an intraparenchymatous parathyroid adenoma.
1. Remember that migration of P III may have been excessive and extend the dissection downwards by performing a thymectomy by the cervical route. The exploration must be made, not by endeavouring to progress downwards, since the space between the manubrium and the trachea is very narrow, but by bringing the thymic lobe upwards by gentle progressive traction, which requires securing several veins. The thymus may thus be exteriorised over 8–10 cm. The elevated thymic lobe must then be dissected since some adenomas are deeply embedded in its substance.
2. Remember the defective migration of P III (undescended gland) and explore the carotid sheath up to the angle of the mandible.
1. The diagnosis should be confirmed.
2. The adenoma should be precisely localised.
3. Left thoracoscopy7 or anterior mediastinotomy via an incision over and removal of the second costal cartilage8 can be less invasive alternative approaches.
The parathyroidectomy
Sporadic multiglandular disease
When all four glands are enlarged (Fig. 1.13), in addition to excision of three glands, the fourth, if possible the smallest, should be reduced so as to leave in place a fragment of a weight estimated as identical to that of a normal gland, i.e. around 40–60 mg.
Familial hyperparathyroidism
Primary hyperparathyroidism in MEN1
The basic principles of parathyroid surgery in patients with MEN1 include:
1. Obtaining and maintaining normocalcaemia for the longest time possible, avoiding persistent/recurrent hypercalcaemia.
• subtotal parathyroidectomy, leaving a remnant of no more than 60 mg in the neck;
• total parathyroidectomy with immediate autotransplantation of 10–20 1-mm3 pieces of parathyroid tissue;
Selective surgery for hyperparathyroidism in MEN1 is effectively a palliative procedure for the majority of patients. The underlying disease process predisposes patients to persistent or recurrent disease. Total parathyroidectomy has been reported to have a higher initial ‘cure’ rate than subtotal resection. Total parathyroidectomy and autotransplantation does carry an increased risk of hypoparathyroidism of up to 47%.9,10 Cryopreservation of some resected parathyroid tissue should therefore be considered after total parathyroidectomy. Delayed autotransplantation using cryopreserved parathyroid can be useful in the case of persistent hypoparathyroidism.
A large series of re-operations for persistent and recurrent hyperparathyroidism in MEN1 patients has been reported.11 Neck re-exploration resulted in normocalcaemia in 91% of patients, with a rate of 2.1% of permanent injury to the recurrent laryngeal nerve (RLN). Autograft removal was more problematic and resulted in normocalcaemia in 58% of patients. The use of parathyroid autografts does not always simplify subsequent treatment.12
Primary hyperparathyroidism in MEN2A
Before treating hyperparathyroidism in patients with MEN2A one must rule out a possible coexistent phaeochromocytoma. Hyperparathyroidism in MEN2A patients is less aggressive than in MEN1 patients. The main risk of parathyroid surgery in these patients is hypoparathyroidism. Although MEN2A patients should be considered to have multiglandular disease, most often not all glands are enlarged and aggressive resections are not recommended. Identification of four glands and excision of only macroscopically enlarged glands is associated with a low rate of persistent or recurrent hyperparathyroidism and avoids postoperative hypoparathyroidism. If they look normal, superior glands should be preserved in preference to inferior. Normal inferior glands (which are at higher risk of necrosis during thyroidectomy for medullary carcinoma, lymph node resection and thymectomy) may be preferably autotransplanted. Some authors recommend total parathyroidectomy with autotransplantation in the forearm.13 The surgeon must bear in mind that permanent hypoparathyroidism can be a worse disease than mild hyperparathyroidism.
Parathyroid carcinoma
1. The diagnosis has been established or seriously considered at the first operation. Severe hypercalcaemia with very high parathyroid hormone (PTH) levels in a patient with a palpable neck tumour are the classic ‘at-risk’ signs to suggest malignancy. Carcinoma is often suspected by the surgeon, as frozen section often cannot conclusively confirm a diagnosis. At operation the tumour appears as a grey enlarged parathyroid, often of hard consistency, with a thick capsule with adherence to the surrounding structures. The surgeon must proceed to an en bloc excision of the parathyroid tumour, the thyroid lobe, the other ipsilateral parathyroid, and the recurrent, jugulocarotid and pretracheal lymph nodes. The diagnosis by frozen section may be indeterminate but is facilitated by this monobloc resection, which provides some idea of the extent of local invasion. Some surgeons remove the lymph nodes only if they are clinically invaded or seen to be so on frozen section. The recurrent nerve should be sacrificed only when it is obviously invaded. The contralateral parathyroids are routinely explored.
2. The diagnosis is only made postoperatively, from the definitive paraffin section histology. In equivocal cases, parafibromin immunochemistry may be used to distinguish parathyroid carcinoma from atypical adenoma.14 The initial operation will usually have been a simple removal of the tumour. It is advisable to re-operate and to resect the structures adjacent to the tumour.15,16
Parathyroid carcinomas are relatively slow growing and should be followed up for life, essentially by clinical evaluation and blood calcium levels. Local recurrences develop in up to 50% of patients. Distant metastases can be expected in 30% of patients.17 Most authors advocate, wherever possible, an aggressive surgical policy towards recurrences and metastases.15–17 Residual tumoral tissue in the neck must be removed en bloc, if necessary together with invaded neighbouring organs such as the trachea or muscular wall of the oesophagus. Distant metastases are most often found in the lungs and bones, and may or may not be associated with local recurrence. Any subsequent operations are rarely curative. The 1999 National Cancer Data Base Report of 286 patients with parathyroid carcinomas in the USA reported a 5-year survival rate of 86% and a 10-year survival rate of 49% for all patients.18 The threat to life is related to the degree of hypercalcaemia, so that long-term survival is possible in the presence of metastases if biochemical control is adequate.15,17
Parathyroidectomy associated with thyroid excisions
Explorations combining thyroidectomy and parathyroidectomy are frequent. Primary parathyroid exploration is recommended first. Indeed, excision of the thyroid lobe first would lead to section of all the landmarks and moorings used by the surgeon to direct the search for parathyroid tissue. It may also be responsible for an accidental parathyroidectomy, which may pass unnoticed. In cases of a benign thyroid lesion, there should be no hesitation in preserving a layer of thyroid parenchyma so as not to compromise the vascularisation of a normal parathyroid. Excluding MEN2A patients, definitive hypoparathyroidism is observed in 4.3% of patients who undergo concomitant thyroidectomy and parathyroidectomy.6
Overall results of conventional open parathyroidectomy
The immediate operative outcome is usually very straightforward. The plasma calcium returns to normal in 24–48 hours. Nowadays so few patients have bone involvement to a severe degree that significant postoperative hypocalcaemia is relatively uncommon. Preventive treatment for hypocalcaemia is not justified. Apart from hypocalcaemia, the morbidity of parathyroidectomy is mainly represented by laryngeal nerve palsy and haematomas, but this is now reported in only 1% or less of cases.1 The mortality of parathyroidectomy is very low, close to zero.
PTH levels decrease and are almost undetectable 4 hours after surgery, then begin to return within the normal range on day 1. One month after surgery elevated serum PTH levels are observed in up to 30% of patients despite normalisation of serum calcium levels. In some cases elevated PTH levels are an adaptive reaction to renal dysfunction or vitamin D deficiency. It has also been demonstrated recently that patients operated on for primary hyperparathyroidism (PHP) show decreased peripheral sensitivity to PTH.19
When conventional open parathyroidectomy is done by an expert surgeon, 95–98% of patients become normocalcaemic.1 With MGD the results are less satisfactory than with solitary adenomas. A multicentre study showed that 20% of MEN1 patients were still hypercalcaemic immediately after surgery.20 Therefore, patients with familial PHP must be managed in specialised centres.
Minimally invasive parathyroidectomy (MIP)
1. They all have a limited incision when compared with classic open transverse cervical incision.
2. The surgery is targeted on one specific parathyroid gland. In most cases the exploration of other glands is not performed or is limited.
Unilateral neck exploration
Initially, the concept of unilateral exploration was based on finding an enlarged gland and an ipsilateral normal gland.21 Since the introduction of the quick parathyroid hormone (QPTH) assay, attempts to identify the ipsilateral gland are no longer made, and in most cases unilateral exploration is focused on one gland alone.
Open minimally invasive parathyroidectomy (OMIP)
This procedure is suitable for day-case surgery.22 Accurate preoperative localisation is a prerequisite condition for OMIP. The procedure is carried out through a 2- to 4-cm incision, which may be placed in the standard location or adjusted to a location that targets the site of pathology. For upper adenomas, the incision is made on the anterior border of the sternocleidomastoid muscle (SCM) and a posterolateral, or ‘back-door’, approach is used to reach the retrothyroid space. For anterior lower adenomas the incision is made at the suprasternal notch level. This technique, when compared with bilateral neck exploration, has shown fewer overall complications (1.2% vs. 3.0%), a 50% reduction in operating time and a substantial reduction in postoperative stay.22
Minimally invasive radio-guided parathyroidectomy (MIRP)
MIRP is characterised by the use of an intraoperative gamma-probe to direct the dissection according to the level of radioactivity.23 The operation must be carried out within 3.5 h of radiopharmaceutical injection of 99mTc-sestamibi. The incision (2–3 cm) is placed according to the expected location of the adenoma as determined by both sestamibi scanning and measurement of gamma emissions on the skin. There is no need to use QPTH measurements. The operation is complete if the excised adenoma has more than 20% of background activity. Gratifying results have been obtained with this technique.23
Endoscopic parathyroidectomy
Endoscopic techniques are particularly suitable for parathyroid surgery for several reasons:
1. They are ablative procedures that do not require any elaborate surgical reconstruction.
2. Most parathyroid tumours are small and benign.
3. Reduction in the length of the scar to about 10–15 mm is appealing to many patients.
The first endoscopic removal of enlarged parathyroid glands was from the mediastinum. Thoracoscopy has successfully allowed excision of mediastinal parathyroid adenomas located deep in the anterior mediastinum or in the middle mediastinum.7
The three endoscopic neck procedures in most widespread use are:
1. The pure endoscopic parathyroidectomy.24 This technique includes constant gas insufflation and four trocars. A large subplatysmal space is created by blunt dissection. Then the midline is opened and the strap muscles retracted in order to expose the thyroid lobes. A bilateral parathyroid exploration is possible.
2. Minimally invasive video-assisted parathyroidectomy (MIVAP).25 A 15-mm skin incision is made at the suprasternal notch. The cervical midline is opened and complete dissection of the thyroid lobe is obtained by blunt dissection under endoscopic vision. Small conventional retractors maintain the operative space. This gasless procedure is carried out only through the midline incision and also permits a bilateral exploration.
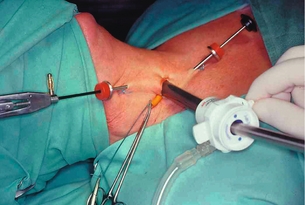
3. Endoscopic parathyroidectomy by lateral approach.26 A 15-mm transverse skin incision is made on the anterior border of the SCM and a back-door approach is used to reach the retrothyroid space. Three trocars (one 10 mm and two 2–3 mm) are inserted on the line of the anterior border of the SCM (Fig. 1.14). The working space is maintained with low-pressure CO2 at 8 mmHg. During this unilateral exploration, one can identify both the adenoma and the ipsilateral parathyroid gland. The lateral approach is applicable in all cases where the parathyroid lesions are located posteriorly.
Other endoscopic techniques, avoiding scars in the neck area, have been proposed but are less commonly used: axillary approach27 and anterior chest approach.28
Depending on the type of access employed, conversion to conventional parathyroidectomy is necessary in 8–15% of cases. The main causes for conversion include difficulties of dissection, capsular ruptures of large adenomas, false-positive results of imaging studies and MGD not detected by preoperative imaging but correctly predicted by QPTH assay results. In experienced hands endoscopic parathyroid techniques are as safe as the standard open procedure. There is virtually no associated mortality. The incidence of recurrent nerve palsy is less than 1%. Insufflation is harmless as long as the procedure is performed under low pressure. Endoscopic operations can be completed in less than 1 hour and the operating time improves dramatically after the first few procedures. Nevertheless, these operations are technically more challenging than standard cervical exploration. Endoscopic techniques have the main advantage of offering a magnified view that permits a precise and careful dissection with minimal risks (Fig. 1.15). By direct vision through mini-incisions it is probably more difficult to get an adequate view of structures, and optimal conditions for exploration are not met even if surgeons use frontal lamps and surgical loops.
MIP in the broader context
After MIP 95–100% of patients are normocalcaemic.21–23,32,33
In contrast to open surgery, the MIP surgeon depends on multiple technologies:
1. The adenoma should be clearly localised before the operation. If the lesion is singular and confirmed by imaging studies, MIP can be advocated. One can choose a lateral or central approach depending on whether the lesion has a posterior or anterior location.
2. The availability of the ioPTH assay is of utmost importance. The overall accuracy of intraoperative ioPTH monitoring is reported to be 97%.36 This test may be especially useful when localisation studies are less certain.
3. MIP, and particularly endoscopic techniques, require dedicated instruments.
Demonstrating the advantages of minimally invasive techniques for parathyroid surgery is not easy. Whether MIP is actually less costly than conventional parathyroidectomy is difficult to quantify. Randomised trials have shown that MIP reduces operating time and early symptomatic hypocalcaemia.34,35 The true advantages of MIP to the patient in terms of comfort and cosmetic results are especially impressive on the first postoperative days.
Intraoperative parathyroid hormone assay (ioPTH)
The increased use of the ioPTH assay has been utilised to limit the extent of operation and to guide parathyroidectomy in single versus multigland disease. Since its efficacy was demonstrated in the late 1990s,36 it has become an increasingly prevalent adjunct to parathyroid surgery. The short half-life (3–5 min) of parathyroid hormone makes it an excellent determinant of whether the pathological gland(s) have been removed. Parathyroid hormone levels are drawn at baseline and at selected time intervals after gland removal, and decrease greater than 50% of the baseline at 10 minutes indicates successful surgery in 97% of patients.37 The ideal protocol for measuring ioPTH is debated and some authors suggest that varying the timing of measurements can further improve outcomes.38,39 Regardless of criteria, ioPTH has become a useful component of the parathyroid surgery arsenal in appropriate cases.
Re-operation for persistent or recurrent primary hyperparathyroidism (PHP)
Analysis of causes of failure
Recurrent PHP constitutes a more complex and controversial problem. The significance of a normocalcaemic interval of at least 6 months between the first operation and the reappearance of hypercalcaemia is debatable. The question is whether this is a true cure of the PHP or a persistent PHP masked by transient return to normal calcaemia. Recurrences may develop after normocalcaemic intervals of several years. In most cases they are seen in patients with familial history of PHP and initially operated on for MGD. The development of a second adenoma in a normal gland, which has been checked at the first operation, is less common and is seen most commonly in patients with history of neck radiation.40 Persistent PHP is much more commonly observed than recurrent PHP: 80–90% vs. 10–20%.
Management
Confirmation of the diagnosis
The diagnosis of PHP can only be raised again after elimination of other causes of hypercalcaemia and fresh confirmation of the biochemical syndrome. Among the many causes of persistent hypercalcaemia after unsuccessful parathyroidectomy, thought should be given to the syndrome of familial hypocalciuric hypercalcaemia (FHH).41
Case history
The sporadic or familial nature of the PHP should be determined by searching for a family history or another associated endocrinopathy that may fit into the picture of MEN1 or MEN2A. Study of the operation notes is vital to gain details of the operative and histology reports from previous operations. This will supply the surgeon with information that is helpful in planning operative tactics (Table 1.4). Preoperative evaluation of the vocal cords similarly plays an important role in operative management.
Table 1.4
Re-operation for persistent-recurrent primary hyperparathyroidism: information supplied from study of case records and surgical implications
| Information | Procedure indicated |
| Familial hyperparathyroidism | Complete exploration of all residual parathyroid tissue |
| MEN1 or MEN2A | Adapt resection to type of familial hyperparathyroidism |
| Multiglandular lesions | Complete exploration of all residual parathyroid tissue |
| Three normal glands | Adenoma not found. Re-operation guided by preoperative localisation studies |
| P III identified (thymus) | Search for homolateral P IV |
| P IV identified | Search for homolateral P III |
| Four normal glands in neck; experienced surgeon | Adenoma in major ectopic site: mediastinal site very probable |
| Cancer suspected or atypical adenoma | Suspect local recurrence and look for visceral or bony metastases |
| Several normal glands removed | Arrange for cryopreservation |
Preoperative evaluation
While preoperative localisation studies may not seem essential, or even desirable, in the case of a primary bilateral exploration most authors consider that ultrasonography and sestamibi scan should be performed routinely in the work-up for any persistent or recurrent PHP. CT scan and magnetic resonance imaging should be reserved for patients in whom the former imaging techniques have failed or when a mediastinal location is strongly suspected. Invasive procedures, including selective venous sampling of PTH or selective angiography, should be performed only if non-invasive procedures are inconclusive. Sometimes, image-guided fine-needle aspiration (FNA) may help distinguish a parathyroid tumour from other structures. The topographic diagnosis should ideally be established by convergence of the results of at least two different investigations. With concordant co-localisation, imaging techniques correctly identify abnormal glands in nearly 95% of cases.42–46
Methods of re-operation
According to the case history and the results of localisation studies, the surgeon must clearly establish if there is or is not a suspicion of MGD (Fig. 1.17). If the lesion sought is a solitary adenoma, an open focused approach can be proposed. Conversely, if there is confirmation or strong suspicion of MGD, revision of the transverse cervicotomy is recommended.
Mediastinal approaches
Most mediastinal adenomas located in the posterior mediastinum or in the anterior mediastinum above the aortic arch can be excised through the neck.42–46 Only adenomas located deep in the anterior mediastinum or in the middle mediastinum require a thoracic approach. The appropriate approach will be dependent upon careful consideration of localising studies and the depth of the lesion in the mediastinum. Precise localisation can allow a less invasive approach than sternal split: anterior mediastinotomy8 or left thoracoscopy7 may be preferable to partial or total sternotomy.
Other focused approaches
These approaches concern glands located in a major ectopic cervical area and not accessible via the previous cervicotomy. The skin incision is placed according to the expected location of the adenoma as determined by imaging studies: undescended glands, or within the carotid sheath in association with the vagus nerve.47
Additional procedures
Graft recurrences must be proven before re-operation on the graft site. Hyperfunctioning grafts are sometimes palpable, or may be located by ultrasound or sestamibi scan. In every case, the possibility of recurrence in residual cervical or mediastinal tissue must be eliminated before re-operating on the arm, and assessing PTH levels after induced ischemia in the graft-bearing arm (Casanova test) may be very helpful.48
Results
With experienced parathyroid surgeons, the success rate of re-operations can be as high as 95%.42–46,49 The overall perioperative morbidity, on average 20%, is much higher than in cases of primary neck exploration. There is a dramatically increased risk of permanent recurrent laryngeal nerve paralysis (up to 10%) compared with initial parathyroid surgery. In up to 20% of cases permanent hypoparathyroidism may result. Transplantation of hyperfunctional tissue can result in recurrent disease in 7–17% of patients. Grafts may fail to function in 6–50% of transplanted tissue, with failure occurring more frequently when using cryopreserved tissue.
Secondary hyperparathyroidism (SHP)
Hyperparathyroidism secondary to compensatory stimulation of parathyroid hormone
Surgical strategies
• obtain and maintain correction of hyperparathyroidism for the longest time possible, avoiding persistent disease;
Localisation diagnosis is often considered unnecessary because patients will systematically undergo bilateral neck exploration. However, imaging studies may be performed to reduce the operation time and to detect supernumerary and ectopic glands, of which the incidence (6.5–25%) is increased due to the ongoing stimulation of renal failure.50,51
The surgical treatment of SHP is performed by using one of three main techniques:
1. Subtotal parathyroidectomy (SPTX).52 This involves identifying four glands and removing at least three but leaving a parathyroid remnant in the neck (approximately 50 mg). The most diffusely hyperplastic gland should be selected. The main disadvantage of this approach is that a second cervical exploration would be needed if persistent or recurrent SHP occurs.
2. Total parathyroidectomy with autotransplantation (TPTX + AT).53 This involves the resection of at least four glands combined with the transplantation of 10–20 1-mm3 pieces of parathyroid tissue into individual pockets created in soft tissues. The brachioradialis muscle of the forearm is used most commonly, principally to aid future surgery under local anaesthetic for recurrent disease. Autotransplantation may also be performed in the musculature in the neck or in subcutaneous pockets in the forearm or anterior chest wall.
3. Total parathyroidectomy without autotransplantation (TPT − AT). This involves the removal of at least four glands without transplantation of a parathyroid remnant into a muscular pocket.
The glands may be markedly enlarged. They are often pale and hard, with fibrosis and calcifications, and may be difficult to distinguish from thyroid tissue or lymph nodes (Fig. 1.16). To exclude supernumerary glands resection of fatty tissue from the central neck compartment and bilateral thymectomy can also be performed in all techniques. Theoretically, there should be no difference in outcome in terms of persistent or recurrent hypercalcaemia between SPTX and TPTX + AT, as both involve the controlled excision of all parathyroid tissue, but for a remnant in the neck or as grafts in the arm. However, rates of recurrence/persistence are decreased in the TPTX − AT group.
The intraoperative selection of the tissue to be left in the neck or the forearm is of particular importance. Neck-remnant or graft-dependent recurrences are observed most often in patients with nodular tissue at initial surgery.54–56 In practice, this intraoperative tissue selection is easier to perform ex vivo prior to autotransplantation rather than during the parathyroidectomy. Because parathyroid remnants or grafts can undergo ischaemic necrosis and result in permanent hypoparathyroidism, cryopreservation of ‘spare’ parathyroid tissue should be performed whenever feasible.
Each technique has advantages and disadvantages (Table 1.5), and no definitive answer can be given to the question of which method is superior as no large randomised controlled trails comparing one surgical approach to another exist.57–59 Total parathyroidectomy is recommended for patients with four markedly enlarged glands and for patients who are not transplant candidates.55,58 Subtotal parathyroidectomy is favoured for children, for patients scheduled to receive a renal transplant and for patients with any normal-sized parathyroid detected at surgery. Total parathyroidectomy alone, without autotransplantation, may also be used in selected patients. Advocates of TPT − AT quote lower rates of recurrence,60 but these patients will require permanent therapy with oral calcium and vitamin D postoperatively.
Table 1.5
Advantages and disadvantages of subtotal parathyroidectomy (SPTX) and total parathyroidectomy with (TPTX + AT) or without (TPTX − AT) autotransplantation in patients with secondary hyperparathyroidism
| Surgical procedure | Advantages | Disadvantages |
| SPTX | Short or no period of hypocalcaemia postoperatively | Tissue selection not always possible Morbidity of cervical re-operation |
| TPTX + AT | Tissue selection possible Low morbidity of re-operations on the autografts |
Longer period of hypocalcaemia postoperatively Problems in localising the hyperactive tissue: autografts or supernumerary gland? Identification and resection of autografts not always easy (seeding in the muscle) |
| TPTX − AT | Recurrence/persistence rate decreased | Permanent postoperative calcium requirement |
| All procedures | Do not avoid persistent/recurrent disease due to ectopic supernumerary gland |
Perioperative care
Patients may receive oral calcitriol before operation to decrease the severity and the duration of postoperative hypocalcaemia. They should undergo dialysis within 1 day of operation and then 48 hours postoperatively or as needed. The risk of bleeding is increased as heparin is used during haemodialysis. Hypocalcaemia is found after subtotal and total parathyroidectomy with autotransplantation in 6.3% and 1.4% respectively.55 Hypocalcaemia may be severe in patients who have marked bone disease, and this may require intravenous calcium. Prolonged hypocalcaemia should be treated with calcitriol (1–4 μg/day) and oral calcium. Calcium infusion during dialysis may decrease the oral calcium supplementation.
Delayed autotransplantation of cryopreserved tissue may be helpful in correcting hypoparathyroidism but should not be performed within 6 months following surgery. However, the functional results are less good than after immediate autotransplantation of fresh parathyroid tissue.61,62
Persistent and recurrent SHP
It is known that up to 15% of haemodialysis patients have a supernumerary parathyroid gland in the neck or mediastinum.4,63–65 After removal of four parathyroids, these supernumerary ‘missed’ glands are capable of causing persistent or recurrent SHP and represent a third cause of surgical failure. During the initial operation, they are usually small and often appear to be embryological rests of parathyroid cells. Most of these glands are associated with the thymus, either in the mediastinum or the neck. In healthy individuals these have little physiological importance, but they can develop functional significance following chronic stimulation over many years in patients with renal failure.An additional cause of recurrent SHP is parathormatosis, in which capsular rupture of the pathological gland causes spillage and inadvertent autotransplantation of cells in the operative site. This tissue can grow and lead to recurrent disease.
In persistent/recurrent SHP, all patients who require re-operation should undergo localisation studies. After TPTX + AT it must be kept in mind that recurrences can occur not only on the grafts but also on supernumerary glands in the neck or the mediastinum. The Casanova test45 can be used to evaluate whether the origin of recurrence is a residual gland or grafted tissue. The incidence of persistent/recurrent SHP is similar after SPTX and TPTX + AT, namely 5.8% and 6.6%, respectively,58 while the rate after TPTX − AT is 5.4%.66
Cervical re-operations in this setting are more invasive, require greater surgical expertise and are associated with a higher morbidity than the excision of an autograft in the forearm. Nevertheless, re-operations on autografted parathyroid fragments are not always technically simple. Not all the grafts grow in the same way. Some become hypertrophic whereas others atrophy. Attempts to locate them precisely are difficult because they are embedded in the muscle at varying depths. The volume of the tissue that has to be removed or left is difficult to evaluate. This is particularly true when there is exuberant pseudo-invasive overgrowth that sometimes requires repeated graft resections. In these cases some surgeons prefer to remove all the transplanted tissue as completely as possible. In some cases the problem of persistent or recurrent SHP is no easier to solve after TPTX + AT than after SPTX.67 Cryopreservation of parathyroid tissue is strongly recommended in re-operations.62
Lithium-induced hyperparathyroidism
Approximately 10–15% of lithium-treated patients become hypercalcaemic. This condition is often reversible if lithium is withdrawn. Lithium-induced hyperparathyroidism was first reported in 1973.68 Hypercalcaemia is generally mild with slight elevation of PTH. It has been suggested that lithium stimulates the entire parathyroid tissue, resulting in hyperplasia,69,70 but several cases of patients presenting a single adenoma as the cause of hyperparathyroidism have also been reported.71 Alternatively, it has been suggested that lithium may unmask underlying PHP.71 For patients who require ongoing treatment with lithium, surgery is indicated.69–72 The incidence of MGD in this setting contraindicates minimally invasive surgery. Excision should be limited to evidently enlarged glands.
Tertiary hyperparathyroidism
Tertiary hyperparathyroidism is a persistent autonomous hypercalcaemic hyperparathyroidism despite reversal of the underlying cause and often occurs after kidney transplantation. After renal transplantation the hypercalcaemia resolves in 50% of patients in the first month, in 85% in the first 6 months and in 95% after 6 months. However, elevated PTH and abnormal bone biopsy persist in up to 70% of patients with long-term kidney grafts.73
• impaired renal graft function;
• non-suppressible PTH secretion;
Only 0.2–0.3% of all patients with kidney transplants are reported to require parathyroid surgery.77 Indications for parathyroidectomy are subacute severe hypercalcaemia (> 3 mmol/L) and symptomatic persistent (> 2 years) hypercalcaemia. Because transient hypoparathyroidism may provoke reduced graft perfusion, which may be a cause of kidney graft deterioration associated with TPTX, one should consider SPTX instead of TPTX + AT78 or TPTX − AT. Transplant patients rarely develop recurrent hyperparathyroidism.
References
1. Van Heerden, J.A., Grant, C.S., Surgical treatment of primary hyperparathyroidism: an institutional perspective. World J Surg 1991; 15:688–692. 1767534
2. Lo Gerfo, P., Kim, L.J. Technique for regional anesthesia: thyroidectomy and parathyroidectomy. In: Van Heerden J.A., Farley D.R., eds. Operative technique in general surgery. Surgical exploration for hyperparathyroidism. Philadelphia: WB Saunders; 1999:95–102.
3. Meurisse, M., Hamoir, E., Defechereux, T., Bilateral neck exploration under hypnosedation. A new standard of care in primary hyperparathyroidism. Ann Surg 1999; 229:401–408. 10077053
4. Akerstrom, G., Malmaeus, J., Bergstrom, R., Surgical anatomy of human parathyroid glands. Surgery 1984; 95:14–21. 6691181
5. Thompson, N.W. The techniques of initial parathyroid exploration and reoperative parathyroidectomy. In: Thompson N.W., Vinik A.I., eds. Endocrine surgery update. New York: Grune & Stratton; 1983:365–383.
6. Henry, J.F., Denizot, A. Anatomic and embryologic aspects of primary hyperparathyroidism. In: Barbier J., Henry J.F., eds. Primary hyperparathyroidism. Paris: Springer-Verlag; 1992:5–18.
7. Prinz, R.A., Lonchina, V., Carnaille, B., et al, Thoracoscopic excision of enlarged mediastinal parathyroid glands. Surgery 1994; 116:999–1005. 7985108
8. Schinkert, R.T., Whitaker, M.D., Argueta, R., Resection of select mediastinal parathyroid adenomas through an anterior mediastinotomy. Mayo Clin Proc 1991; 66:1110–1113. 1943241
9. O’Riordain, D.S., O’Brien, T., Grant, C.S., et al, Surgical management of primary hyperparathyroidism in multiple endocrine neoplasia type 1 and 2. Surgery 1993; 114:1031–1039. 7903001
10. Hellman, P., Skogseid, B., Oberg, K., et al, Primary and reoperative operations in parathyroid operations in hyperparathyroidism of multiple endocrine neoplasia type 1. Surgery 1998; 124:993–999. 9854574
11. Kivlen, M.H., Bartlett, D.L., Libutti, S.K., et al, Reoperation for hyperparathyroidism in multiple endocrine neoplasia type 1 (MEN 1). Surgery 2001; 130:991–998. 11742328
12. Hubbard, J.G.H., Sebag, F., Maweja, S., et al, Primary hyperparathyroidism in MEN I – how radical should surgery be? Langenbecks Arch Surg 2002; 368:553–557. 11914930
13. Wells, S.A., Farndon, J.R., Dale, J.K., et al, Long term evaluation of patients with primary parathyroid hyperplasia managed by total parathyroidectomy and heterotopic autotransplantation. Ann Surg 1980; 192:451–458. 7425691
14. Gill, A.J., Clarkson, A., Gimm, O., et al, Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism–jaw tumors (HPT-JT) syndrome related adenomas from sporadic parathyroid adenomas and hyperplasias. Am J Surg Pathol 2006; 30:1140–1149. 16931959
15. Rodgers, S.E., Perrier, N.D., Parathyroid carcinoma. Curr Opin Oncol 2006; 18:16–22. 16357559
16. Busaidy, N., Jimenez, C., Habra, M., et al, Parathyroid carcinoma: a 22-year experience. Head Neck 2004; 26:716–726. 15287039
17. Sandelin, K., Tullgren, O., Farnebo, L.O., Clinical course of metastatic parathyroid cancer. World J Surg 1994; 18:594–598. 7725750
18. Hundahl, S.A., Flemming, I.D., Fremgen, A.M., et al, Two hundred eighty-six cases of parathyroid carcinoma treated in the US between 1985–1995: a national cancer data base report. The American College of Surgeon Commission on Cancer and the American Cancer Society. Cancer 1999; 86:538–544. 10430265
19. Nordenstrom, E., Westerdahl, J., Isaksson, A., Patients with elevated serum parathyroid hormone levels after parathyroidectomy showing signs of decreased peripheral parathyroid hormone sensitivity. World J Surg 2003; 27:212–215. 12616439
20. Goudet, P., Cougard, P., Vergès, B., et al, Hyperparathyroidism in multiple endocrine neoplasia type 1: surgical trends and results of a 256-patient series from Group d’Etude des Néoplasies Endocriniennes Multiples study group. World J Surg 2001; 25:886–890. 11572029
21. Tibblin, S.A., Bondeson, A.G., Ljunberg, O., Unilateral parathyroidectomy in hyperparathyroidism due to single adenoma. Ann Surg 1982; 195:245–252. 7059236
22. Udelsman, R., Donovan, P.I., Sokoll, L.J., One hundred consecutive minimally invasive parathyroid explorations. Ann Surg 2000; 232:331–339. 10973383
23. Norman, J., Murphy, C. Minimally invasive radioguided parathyroidectomy. In: Van Heerden J.A., Farley D.R., eds. Operative technique in general surgery. Surgical exploration for hyperparathyroidism. Philadelphia: WB Saunders; 1999:28–33.
24. Gagner, M., Rubino, F. Endoscopic parathyroidectomy. In: Schwartz A.E., Pertsemlidis D., Gagner M., eds. Endocrine surgery. New York: Marcel Dekker; 2004:289–296.
25. Miccoli, P., Bendinelli, C., Conte, M., Endoscopic parathyroidectomy by a gasless approach. J Laparoendosc Adv Surg Tech A 1998; 8:189–194. 9755909
26. Henry, J.F. Endoscopic exploration. In: Van Heerden J.A., Farley D.R., eds. Operative technique in general surgery. Surgical exploration for hyperparathyroidism. Philadelphia: WB Saunders; 1999:49–61.
27. Ikeda, Y., Takami, H., Sasaki, Y., et al, Endoscopic neck surgery by the axillary approach. J Am Coll Surg 2000; 191:336–340. 10989910
28. Okido, M., Shimizu, S., Kuroki, S., et al, Video-assisted parathyroidectomy for primary hyperparathyroidism: an approach involving a skin-lifting method. Surg Endosc 2001; 15:1120–1123. 11727083
29. Miccoli, P., Bendinelli, C., Berti, P., et al, Video-assisted versus conventional parathyroidectomy in primary hyperparathyroidism: a prospective randomized study. Surgery 1999; 126:1117–1122. 10598196
30. Henry, J.F., Raffaelli, M., Iacobone, M., et al, Video-assisted parathyroidectomy via lateral approach versus conventional surgery in the treatment of sporadic primary hyperparathyroidism. Results of a case–control study. Surg Endosc 2001; 15:1116–1119. 11727082
31. Barczynski, M., Cichon, S., Konturek, A., et al, Minimally invasive video-assisted parathyroidectimy versus open minimally invasive parathyroidectomy for solitary parathyroid adenoma: a prospective, randomized, blinded trial. World J Surg 2006; 30:721–731. 16547619
32. Miccoli, P., Berti, P., Materazzi, G., et al, Results of video-assisted parathyroidectoy: single institution’s six years experience. World J Surg 2004; 28:1216–1218. 15517483
33. Henry, J.F., Sebag, F., Tamagnini, P., et al, Endoscopic parathyroid surgery: results of 365 consecutive procedures. World J Surg 2004; 28:1219–1223. 15517493
34. Russell, C.F., Dolan, S.J., Laird, J.D., Randomized clinical trial comparing scan-directed unilateral versus bilateral cervical exploration for primary hyperparathyroidism due to solitary adenoma. Br J Surg 2006; 93:418–421. 16392100
35. Westerdalh, J., Bergenfelz, A., Unilateral versus bilateral neck exploration for primary hyperparathyroidism: five-year follow-up of a randomized controlled trial. Ann Surg 2007; 246:976–980. 18043099
36. Irvin, G.L., Carneiro, D.M. Rapid parathyroid hormone assay guided exploration. In: Van Heerden J.A., Farley D.R., eds. Operative technique in general surgery. Surgical exploration for hyperparathyroidism. Philadelphia: WB Saunders; 1999:18–27.
37. Carneiro, D.M., Solorzano, C.C., Nader, M.C., et al, Comparison of intraoperative iPTH assay (QPTH) criteria in guiding parathyroidectomy: which criterion is the most accurate? Surgery. 2003;134(6):973–981. 14668730
38. Richards, M.L., Thompson, G.B., Farley, D.R., et al, An optimal algorithm for intraoperative parathyroid hormone monitoring. Arch Surg. 2011;146(3):280–285. 21422358
39. Heller, K.S., Blumberg, S.N., Relation of final intraoperative parathyroid hormone level and outcome following parathyroidectomy. Arch Otolaryngol Head Neck Surg. 2009;135(11):1103–1107. 19917922
40. Ippolito, G., Palazzo, F., Sebag, F., et al, Long-term follow-up after parathyroidectomy for radiationinduced hyperparathyroidism. Surgery 2007; 142:819–822. 18063062
41. Heath, H., III., Familial benign hypercalcemia – from clinical description to molecular genetics. West J Med 1994; 160:554–561. 8053177
42. Shen, W., Duren, M., Morita, E., et al, Reoperation for persistent or recurrent hyperparathyroidism. Arch Surg 1996; 131:861–869. 8712911
43. Jaskowiak, N., Norton, J.A., Alexander, H.T., et al, A prospective trial evaluating a standard approach to reoperation for missed parathyroid adenoma. Ann Surg 1996; 224:308–322. 8813259
44. Thompson, G.B., Grant, C.S., Perrier, N.D., et al, Reoperative parathyroid surgery in the era of sestamibi scanning and intraoperative parathyroid hormone monitoring. Arch Surg 1999; 134:699–704. 10401818
45. Wadstrom, C., Zedenius, J., Guinea, A., et al, Reoperative surgery for recurrent or persistent primary hyperparathyroidism. Aust N Z J Surg 1998; 68:103–107. 9493999
46. Mariette, C., Pellissier, L., Combemale, F., et al, Reoperation for persistent or recurrent primary hyperparathyroidism. Langenbecks Arch Surg 1998; 383:174–179. 9641894
47. Chan, T.J., Libutti, S.K., McCart, J.A., et al, Persistent primary hyperparathyroidism caused by adenomas identified in pharyngeal or adjacent structures. World J Surg 2003; 27:675–679. 12734681
48. Casanova, D., Sarfati, E., De Francisco, A., et al, Secondary hyperparathyroidism: diagnosis of site or recurrence. World J Surg 1991; 15:546–554. 1891942
49. Lo, C.Y., Van Heerden, J.A. Parathyroid reoperations. In: Clark O.H., Duh Q.Y., eds. Textbook of endocrine surgery. Philadelphia: WB Saunders; 1997:411–417.
50. Perie, S., Fessi, H., Tassart, M., et al, Usefulness of combination of high-resolution ultrasonography and dual-phase dual-isotope iodine 123/technetium Tc99m sestamibi scintigraphy for the preoperative localization of hyperplastic parathyroid glands in renal hyperparathyroidism. Am J Kidney Dis 2005; 45:344–352. 15685513
51. De La Rosa, A., Jimeno, J., Membrilla, E., et al, Usefulness of preoperative Tc-mibi parathyroid scintigraphy in secondary hyperparathyroidism. Langenbecks Arch Surg 2008; 393:21–24. 17294211
52. Stanbury, S.W., Lumb, G.A., Nicholson, W.F., Elective subtotal parathyroidectomy for autonomous hyperparathyroidism. Lancet 1960; 1:793–798. 13833730
53. Wells, S.A., Gunnels, J.C., Shelbourne, J.D., et al, Transplantation of the parathyroid glands in man: clinical indications and results. Surgery 1975; 78:34–44. 1138398
54. Tanaka, Y., Seo, H., Tominaga, Y., et al, Factors related to the recurrent hyperfunction of autografts after total parathyroidectomy in patients with severe secondary hyperparathyroidism. Surg Today 1993; 23:220–227. 8467173
55. Ohta, K., Manabe, T., Katagiri, M., et al, Expression of proliferating cell nuclear antigens in parathyroid glands of renal hyperparathyroidism. World J Surg 1994; 18:625–629. 7725755
56. Tominaga, Y., Tanaka, Y., Sato, K., et al. DNA studies in graft-dependent hyperparathyroidism. Acta Chir Austriaca. 1996; 28(Suppl. 124):65–68.
57. Takagi, H., Tominaga, Y., Uchida, K., et al, Subtotal versus total parathyroidectomy with forearm autograft for secondary hyperparathyroidism in chronic renal failure. Ann Surg 1984; 200:18–23. 6732323
58. Rothmund, M., Wagner, P.K., Schark, C., Subtotal parathyroidectomy versus total parathyroidectomy and autotransplantation in secondary hyperparathyroidism: a randomized trial. World J Surg 1991; 15:745–750. 1767541
59. Gagne, E.R., Urena, P., Leite-Silva, S., et al, Short- and long-term efficacy of total parathyroidectomy with immediate autografting compared with subtotal parathyroidectomy in hemodialysis patients. J Am Soc Nephrol 1992; 3:1008–1017. 1450363
60. Madorin, C., Owen, R.P., Fraser, W.D., et al, The surgical management of renal hyperparathyroidism. Eur Arch Otorhinolaryngol. 2012;269(6):1565–1576. 22101574
61. Tanaka, Y., Funahashi, H., Imai, T., et al, Functional and morphometric study of cryopreserved human parathyroid tissue transplanted into nude mice. World J Surg 1996; 20:692–699. 8662154
62. Niederle, B. The technique of parathyroid cryopreservation and the results of delayed autotransplantation. A review. Acta Chir Austriaca. 1996; 28(Suppl. 24):68–71.
63. Edis, A.J., Levitt, M.D., Supernumerary parathyroid glands: implications for the surgical treatment of secondary hyperparathyroidism. World J Surg 1987; 11:398–401. 3604250
64. Numano, M., Tominaga, Y., Uchida, K., et al, Surgical significance of supernumerary parathyroid glands in renal hyperparathyroidism. World J Surg 1998; 22:1098–1103. 9747174
65. Hibi, Y., Tominoga, Y., Sato, T., et al, Reoperation for renal hyperparathyroidism. World J Surg 2002; 26:1301–1307. 12205559
66. Coulston, J.E., Egan, R., Willis, E., et al, Total parathyroidectomy without autotransplantation for renalhyperparathyroidism. Br J Surg 2010; 97:1674–1679. 20641052
67. Henry, J.F., Denizot, A., Audiffret, J., et al, Results of reoperations for persistent or recurrent secondary hyperparathyroidism in hemodialysis patients. World J Surg 1990; 14:303–307. 2368433
68. Garfinkel, P.E., Ezrin, C., Stancer, H.C., Hypothyroidism and hyperparathyroidism associated with lithium. Lancet 1973; 2:331–332. 4124823
69. McHenry, C.R., Racke, F., Meister, M., et al, Lithium effects on dispersed bovine parathyroid cells grown in tissue culture. Surgery 1991; 110:1061–1066. 1745976
70. Hundley, J.C., Woodrum, D.T., Saunders, B.D., et al, Revisiting lithium-associated hyperparathyroidism in the era of intraoperative hormone monitoring. Surgery 2005; 138:1027–1032. 16360387
71. Awad, S.S., Miskulin, J., Thompson, N.W., Parathyroid adenomas versus four-gland hyperplasia as the cause of primary hyperparathyroidism in patients with prolonged lithium therapy. World J Surg 2003; 27:486–488. 12658498
72. Nordenstrom, J., Strigard, K., Perkeck, L., et al, Hyperparathyroidism associated with treatment of manic-depressive disorders by lithium. Eur J Surg 1992; 158:207–211. 1352133
73. Sitges-Serra, A., Esteller, E., Ricart, M.J., et al, Indications and late results of subtotal parathyroidectomy for hyperparathyroidism after renal transplantation. World J Surg 1984; 8:534–539. 6385493
74. Saha, H.H., Salmela, K.T., Ahonen, P.J., et al, Sequential changes in vitamin D and calcium metabolism after successful renal transplantation. Scand J Urol Nephrol 1994; 28:21–25. 8009188
75. Straffen, A.M., Carmichael, D.J., Fainety, A., et al, Calcium metabolism following renal transplantation. Ann Clin Biochem 1994; 31:125–129. 8060089
76. Sancho, J.J., Stiges-Serra, A. Metabolic complications for patients with secondary hyperparathyroidism. In: Clark O.H., Duh Q.Y., eds. Textbook of endocrine surgery. Philadelphia: WB Saunders; 1997:394–401.
77. Fassbinder, W., Brunner, F.P., Brynger, H., et al, Combined report on regular dialysis and transplantation in Europe. Nephrol Dial Transpl. 1991;6(Suppl.):5–35. 2038432
78. Schlosser, K., Endres, N., Celik, I., et al, Surgical treatment of tertiary hyperparathyroidism: the choice of procedure matters!. World J Surg 2007; 31:1947–1953. 17665243