Chapter 33 Parasitic infestations
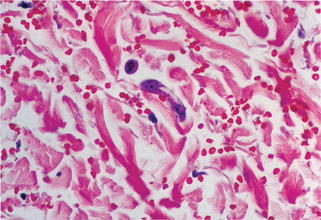
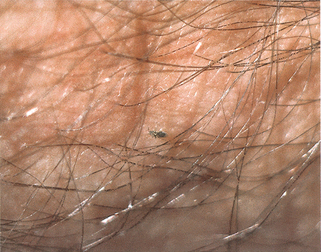
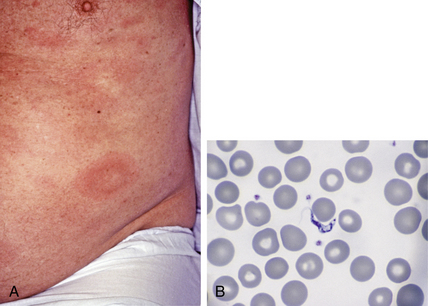
Figure 33-2. Larva currens. Biopsy demonstrates migrating larva of Strongyloides stercoralis in the dermis.
(Courtesy of the Fitzsimons Army Medical Center teaching files.)
Table 33-1. Parasitic Infestations of the Skin
| PARASITIC INFESTATION | VECTOR OR MODE OF TRANSMISSION |
|---|---|
| Filariasis | Mosquito |
| Onchocerciasis | Black fly |
| Creeping eruption | Soil contact and larval penetration |
| African trypanosomiasis | Tsetse fly |
| American trypanosomiasis | Kissing bug |
| Leishmaniasis | Sand fly |
| Schistosomiasis | Water contact and cercarial penetration |
| Dracunculiasis, sparganosis | Ingestion of larva |
| Echinococcosis, cysticercosis | Ingestion of cysts |
| Amebiasis | Direct contact or ingestion of cysts |
| Loiasis | Horse and deer flies |
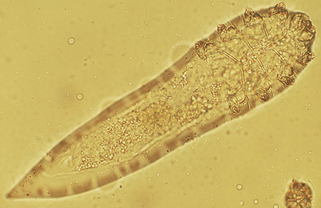
| Demodex | Person-to-person contact in childhood |
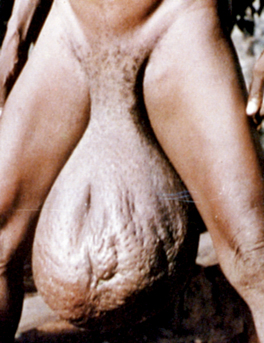
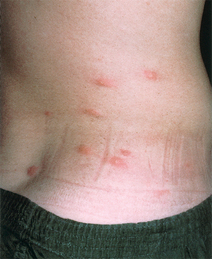
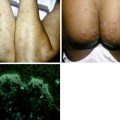
Figure 33-4. Marked scrotal enlargement in elephantiasis.
(From Zaiman H, Jong EC: Parasitic diseases of the skin and soft tissue. In Stevens DL, editor: Atlas of infectious diseases, vol II, New York, 1995, Churchill Livingstone.)
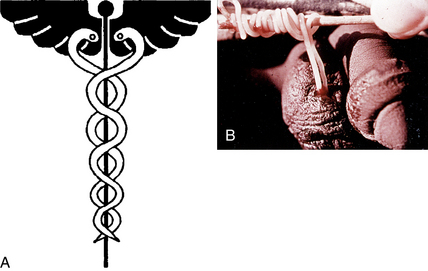
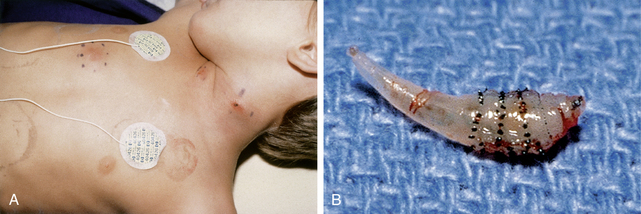
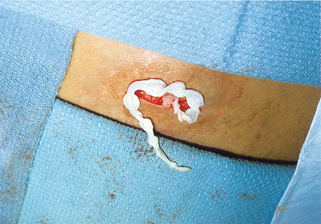
Figure 33-5. A, Caduceus. B, The classic matchstick recovery technique used in extracting the adult female worm.
(From Zaiman H, Jong EC: Parasitic diseases of the skin and soft tissue. In Stevens DL, editor: Atlas of infectious diseases, vol. II, New York, 1995, Churchill Livingstone.)
| SPECIES | DISEASE | DISTRIBUTION |
|---|---|---|
| L. donovani group | Visceral leishmaniasis, kala-azar | India, Asia, Middle East, Africa |
| L. tropica group | Old World cutaneous leishmaniasis, oriental sore, newly discovered viscerotropic disease | India, Middle East |
| L. viannia group (L. braziliensis group) | New World mucocutaneous leishmaniasis, espundia | Latin America |
| L. mexicana group | American cutaneous leishmaniasis | Mexico, Central America, Texas, South America |
Key Points: Parasitic Infestations
Centers for Disease Control (CDC): Unexplained dermopathy: http://www.cdc.gov/unexplaineddermopathy/. Accessed July 25, 2010.