Pancreaticoduodenectomy
Introduction
Resection of tumors of the periampullary region has its origins in the writings of Kausch (1912) and Whipple (1935). Pancreaticoduodenectomy, or pancreatoduodenectomy, previously was accompanied by a mortality rate of 20% to 25%. Currently, however, most experienced pancreatic surgery centers report a mortality rate of 3% or less. Complication rates remain 20% to 50%, with the most troublesome complication being leakage at the pancreatic anastomosis.
Principles of Pancreatic Cancer Treatment
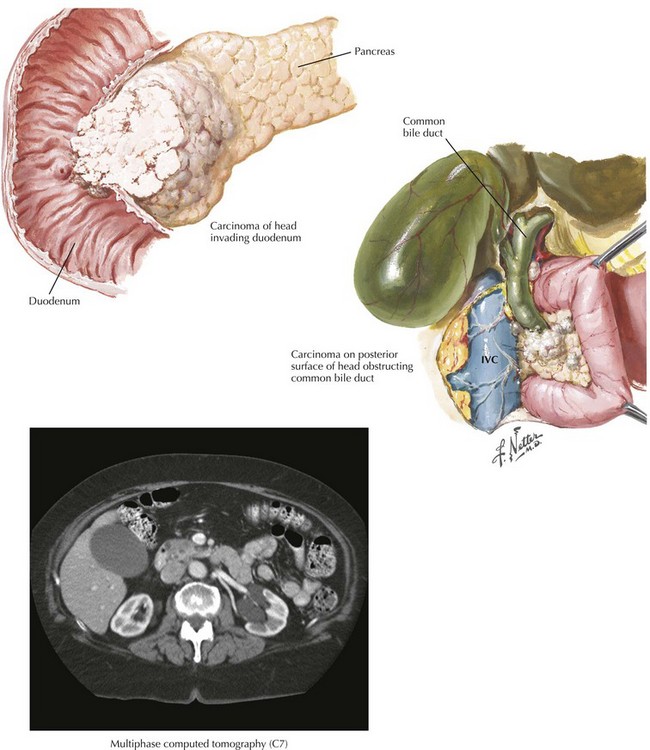
The treatment of pancreatic cancer begins with accurate staging, including a complete history and physical examination. The most important component of staging is a multiphase computed tomography (CT) scan of the abdomen using a multidetector scanner (Fig. 16-1). With CT of the chest, this allows patients to be staged clinically as resectable (15% to 25%), borderline resectable or locally advanced/unresectable (30% to 40%), or metastatic (40% to 50%). Endoscopic ultrasound is rarely needed for staging purposes, and laparoscopy is favored by some authors. Debate continues about the utility of preoperative biliary decompression in jaundiced patients. Recently, laparoscopic approaches to pancreaticoduodenectomy have been described, but these remain nascent.
Surgical Approach
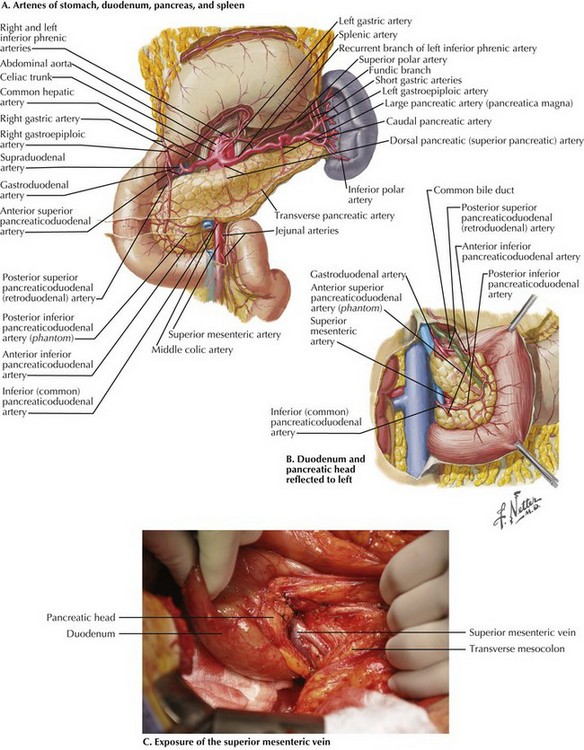
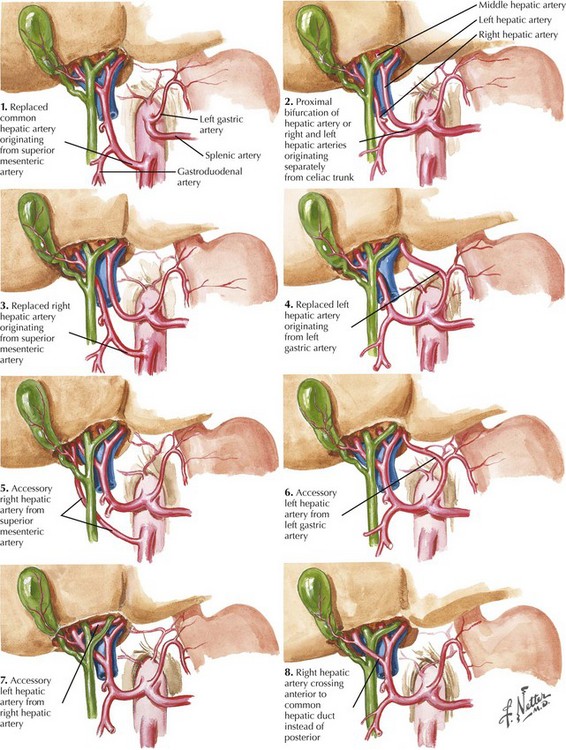
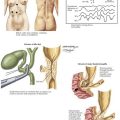
The dissection begins with a generous Kocher maneuver to lyse the lateral retroperitoneal attachments of the duodenum (Fig. 16-2, A and B). This elevates the duodenum and head of the pancreas out of the retroperitoneum. By taking the Kocher maneuver to its fullest extent, the surgeon identifies the superior mesenteric vein (SMV) in the groove between the head of the pancreas and the transverse mesocolon (Fig. 16-2, C). Further, the relationship of the tumor in the head of the pancreas to the SMV and superior mesenteric artery (SMA) is assessed. At this point, the surgeon should feel for evidence of a replaced or accessory right hepatic artery coming off the SMA (Fig. 16-3).
With the SMV identified, dissection is carried along this vessel cranially toward the inferior border of the pancreatic neck. The right gastroepiploic vein and its branches are identified and ligated, allowing the development of the plane posterior to the neck of the pancreas and anterior to the SMV (Fig 16-4).
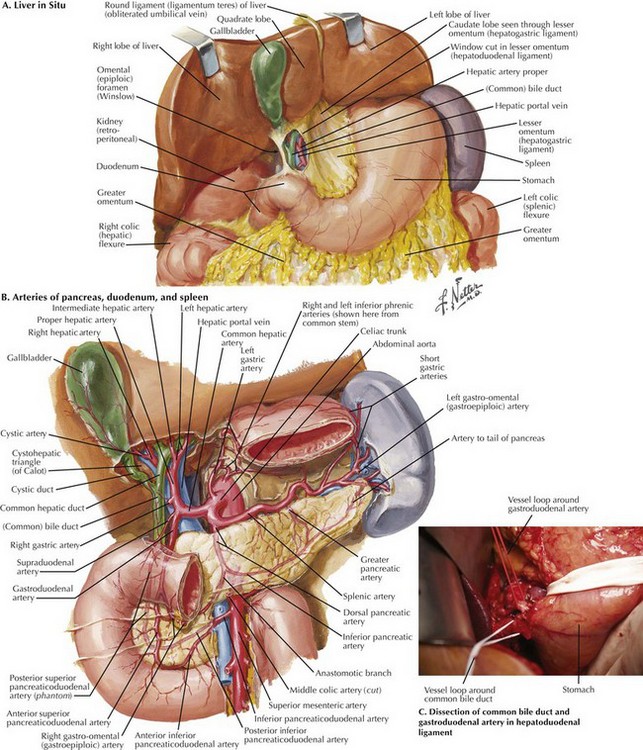
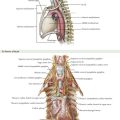
When the plane has been developed as far as possible from the inferior aspect, attention is turned to the hepatoduodenal ligament (Fig. 16-5, A and B). Care is taken to identify and preserve a replaced or accessory right hepatic artery. The common bile duct (CBD) is identified and encircled with a vessel loop. Some surgeons prefer to divide the CBD at this point. The gastroduodenal artery (GDA) is identified and encircled with a vessel loop as well (Fig. 16-5, C). Before ligating the GDA, the surgeon must be sure that when it is occluded, there is still a pulse in the hepatic artery going to the liver. Loss of that pulse may indicate an arcuate ligament syndrome, celiac stenosis, or variant arterial anatomy (see Fig. 16-3).
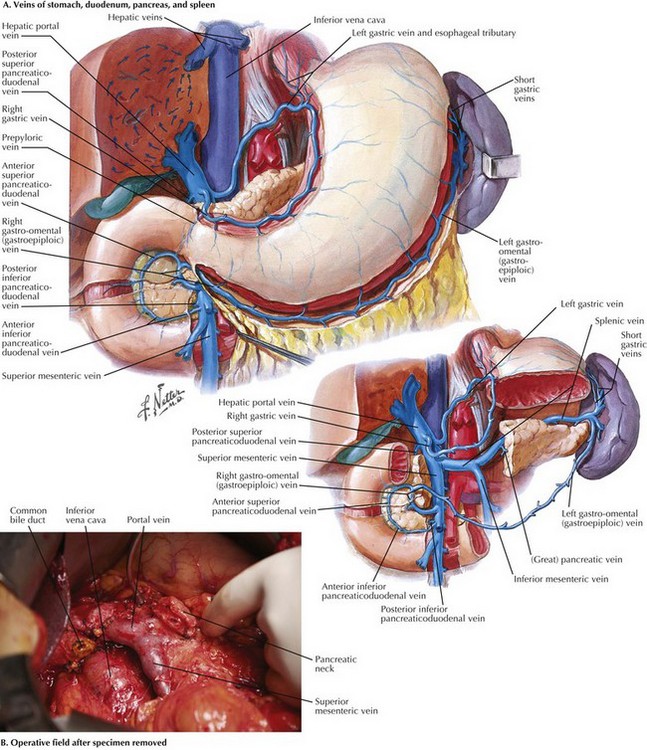
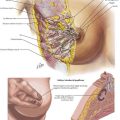
The duodenum and head of the pancreas are dissected away from the portal vein and SMV. There are few, if any, anterolateral small venous branches. The posterior superior pancreaticoduodenal vein is reliably identified and ligated (Fig. 16-6, A). The uncinate process is then dissected away from the SMA, taking care to ligate the pancreaticoduodenal arteries (see Fig. 16-2). Usually, one half to two thirds of the uncinate dissection is completed at this time.
Attention is then turned to the proximal jejunum, which is transected about 10 to 15 cm distal to the ligament of Treitz with a GIA stapler. The distal duodenal and proximal jejunal mesenteries are ligated, and then the bowel is fed underneath the SMA and SMV to the right upper quadrant. The uncinate dissection is completed, again flush with the SMA, and the specimen is marked and sent to pathology. While the surgeon is completing the uncinate dissection, the first jejunal branch off the SMV generally enters the vein on the right posterolateral surface and runs underneath the SMV to the patient’s left. This branch can be problematic if not identified, and it usually is ligated. With the specimen removed, the operative field appears as shown in Figure 16-6, B.
Cameron, JL. Rapid exposure of the portal and superior mesenteric veins. Surg Gynecol Obstet. 1993;176:395.
Farnell, MB, Pearson, RK, Sarr, MG, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618.
Kausch, W. Das carinom de papilla duodeni und seine radikale entfernung. Beltrage sur Klinischen Cirurgie. 1912;78:439.
Lillemoe, KD, Cameron, JL, Hardacre, JM, et al. Is Prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg. 1999;230:322.
Lillemoe, KD, Cameron, JL, Kaufman, HS, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer: a prospective randomized trial. Ann Surg. 1993;217:447.
Neoptolemos, JP, Stocken, DD, Bassi, C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection. JAMA. 2010;304:1073.
Oettle, H, Post, S, Neuhaus, P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer. JAMA. 2007;297:267.
Regine, WF, Winter, KA, Abrams, RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma. JAMA. 2008;299:1019.
Whipple, AO, Parsons, WB, Mullins, CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935;102:763.
Yeo, CJ, Cameron, JL, Lillemoe, KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma. Part 2. Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355.