CHAPTER 50 PANCREATIC INJURIES
The pancreas is relatively protected deep within the confines of the retroperitoneum. As such, injuries to the pancreas are uncommon, but not rare, and can present a diagnostic dilemma. In fact, despite advances in modern trauma care, there remains significant morbidity and mortality, with mortality rates ranging from 9%–34%.1 Frequent complications are also common following pancreatic injuries, occurring in 30%–60% of patients. The high complication rate associated with these injuries is primarily secondary to diagnostic delays and missed injuries. When identified early, the treatment of most pancreatic injuries is straightforward. It is the delayed recognition and/or treatment of these injuries that can result in devastating outcomes.
There are few well-documented historical accounts about the management of pancreatic injuries. The first documented case of pancreatic trauma was an autopsy report from St. Thomas Hospital in London in 1827 in which a patient struck by the wheel of a stagecoach suffered a complete pancreatic body transection.2 Over the next several decades, reports of pancreatic injuries were scattered. In 1903, after extensive review of the literature, only 45 cases of pancreatic trauma, 21 resulting from penetrating injuries and 24 from blunt trauma could be identified.3 The occurrence of complications following pancreatic injury was also noted early. In 1905, Korte4 reported a case of an isolated pancreatic transection with resultant pancreatic fistula. The fistula closed spontaneously and the patient survived.
ANATOMY
A complete understanding of pancreatic relational anatomy is essential for providing appropriate treatment and understanding the potential for associated injuries. The pancreas is about 15–20 cm in length, 3.1 cm wide, and 1–1.5 cm thick. The average mass is 90 g (ranging from 40 to 180 g).5 The inferior vena cava, aorta, left kidney, both renal veins, and right renal artery lie posterior to the pancreas. The head of the pancreas is nestled in the duodenal sweep, with the body crossing the spine and the tail resting within the hilum of the spleen. The splenic artery and vein can be found along the superior border of the pancreas. The superior mesenteric artery and vein reside just behind the neck of the pancreas and are enclosed posteriorly by the uncinate process. This process can be absent or can almost completely encircle the superior mesenteric artery and vein.
PHYSIOLOGY
The pancreas is a compound tubuloalveolar gland with both endocrine (insulin, glucagon, somatostatin) and exocrine (digestive enzyme precursors, bicarbonate) function. The endocrine cells are separated histologically into nests of cells known as the islets of Langerhans. There are three predominant subtypes of islet cells: alpha cells (which produce glucagon), beta cells (which produce insulin), and delta cells (which produce somatostatin). Although these cells are distributed throughout the substance of the pancreas, most reside primarily within the tail. Consequently, it would seem that a distal pancreatectomy would be poorly tolerated in terms of endocrine function. However, it is well known that resection of more than 90% of the pancreas must occur before endocrine insufficiency develops, provided the remainder of the gland is normal. In fact, partial resection induces hypertrophy and increased activity of the residual islet cells. In animal studies, Dragstedt6 was the first to show that removal of 80% of the pancreas did not significantly alter carbohydrate or fat metabolism or the digestion and/or absorption of food, provided that the remaining gland is normal and that pancreatic secretions still have access to the upper digestive tract via the ductal system.
DIAGNOSIS
It is important to remember that whenever there is trauma to the pancreas, particular attention must be given to the possibility of a major ductal injury for this is the single most important determinant of outcome after pancreatic injury. In fact, this concept was first recognized as early as 1962.7 Subsequent investigators have confirmed and reemphasized the necessity of determining the status of the pancreatic duct. In fact, Heitsch et al.8 found that distal resection of ductal injuries significantly lowered postoperative morbidity and mortality when compared with drainage alone. This finding was confirmed over a decade later when investigators documented a drop in mortality rate from 19%–3% after pancreatic resection proximal to the site of ductal injury.9
Elevated serum amylase is not a reliable indicator of pancreatic trauma. In fact, the use of amylase as a screening tool in blunt trauma carries a negative predictive value of 95%.10 Measurement of the pancreatic isoamylase fraction has failed to substantially improve both the sensitivity and specificity of this value as a marker of pancreatic injury.
Asymptomatic patients with elevated serum pancreatic isoamylase require observation and repeat amylase determination. Persistently elevated serum amylase or the development of abdominal symptomatology warrants further investigation and may include computed tomography (CT) scan, endoscopic retrograde cholangiopancreatography (ERCP), or operative exploration. Abdominal CT scans have a reported sensitivity and specificity as high as 80% in diagnosing pancreatic injury.11 Patton and colleagues12 reported that in 26 patients that sustained blunt pancreatic trauma, early CT scan was suspicious for injury in 15. CT failed to demonstrate injury in four patients (21%), resulting in a delay in operative intervention (mean, 3.8 days). The remaining patients had other indications for exploration.
Endoscopic retrograde cholangiopancreatography can be useful in the diagnosis of pancreatic duct rupture. In addition, it can aid in the diagnosis of and occasionally the management of the complications of missed pancreatic injuries. A report from the University of Louisville documents ERCP as a useful diagnostic tool in the evaluation of the pancreatic duct in the early postinjury period in hemodynamically stable patients with elevated amylase levels, persistent abdominal pain, and abnormal or questionable abdominal CT findings.13 ERCP is also extremely helpful in the evaluation of patients in whom the diagnosis of pancreatic injury was missed during the initial evaluation. It is in these patients that ERCP can aid in diagnosing the injury, planning the surgical approach if necessary, determining internal transpancreatic stent placement, and transductal drainage of a pancreatic abscess. However, ERCP may not always be available and should not delay operation in patients with progressive clinical deterioration.
Magnetic resonance (MR) imaging, specifically MRCP (magnetic resonance cholangiopancreatography), has emerged as an alternative technique for evaluating the pancreatic duct. Although primarily used in elective circumstances, MRCP has been reported as a viable option for evaluating the status of the duct in those patients with pancreatic injuries.14 However, it frequently is not practical for use in trauma patients.
Once again, it must be stressed that, if possible, it is important to determine the status of the duct at the time of exploration. Most of these injuries can be diagnosed by local exploration. Injuries to the duct occur in approximately 15% of pancreatic trauma and are generally the result of penetrating injury.15 Blunt injury can also result in transection of the major duct with or without complete transection of the gland. Minor contusions and/or lacerations of the pancreatic parenchyma usually do not require further evaluation of the duct. However, an intact pancreatic capsule does not eliminate the possibility of complete transection of the pancreatic duct.9
Nevertheless, pancreatography can be performed either by directly cannulating the ampulla of Vater through a duodenotomy or the main pancreatic duct through the amputated tail of the pancreas. A 5F pediatric feeding tube is used along with 2–5 ml of contrast. Cannulating the ampulla of Vater entails creating a duodenotomy unless there is an associated duodenal injury. It should be stressed that identifying the ampulla can be difficult and that resection of the tail does not always ensure visualization of the pancreatic duct.
CLASSIFICATION OF PANCREATIC INJURIES
Although a number of classification systems have been devised to categorize pancreatic injuries, the American Association for the Surgery of Trauma (AAST) Committee on Organ Injury Scaling addresses the key issues of treatment of parenchymal disruption and major pancreatic ductal injury by focusing on the anatomic location of the injury (Table 1). Proximal duct injuries require different management than do distal duct and parenchymal injuries. The difficulty arises in those patients with parenchymal disruption and major duct injury. This classification scheme provides a useful management guide by focusing on the anatomic location of the duct and parenchymal injury (proximal vs. distal).
Table 1 Pancreatic Organ Injury Scale: American Association for the Surgery of Trauma
| Grade | Injury Description | |
|---|---|---|
| I | Hematoma | Minor contusion without duct injury |
| Laceration | Superficial laceration without duct injury | |
| II | Hematoma | Major contusion without duct injury or tissue loss |
| Laceration | Major laceration without duct injury or tissue loss | |
| III | Laceration | Distal transection or parenchymal injury with duct injury |
| IV | Laceration | Proximal (right of superior mesenteric vein) transection or parenchymal injury |
| V | Laceration | Massive disruption of pancreatic head |
SURGICAL MANAGEMENT OF PANCREATIC INJURIES
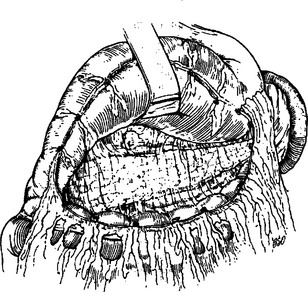
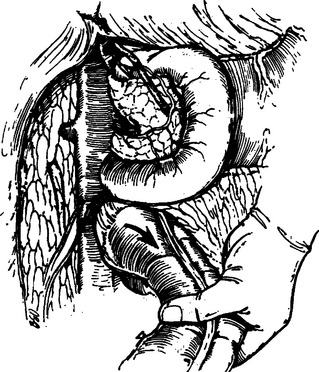
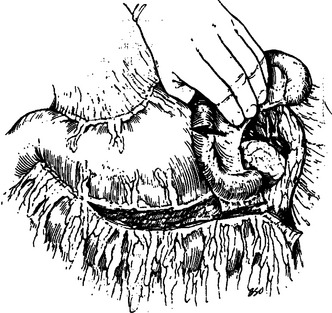
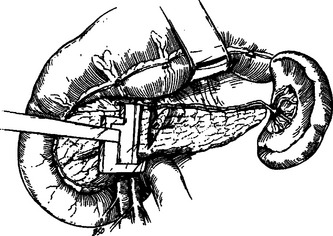
Proper evaluation of the pancreas requires complete exposure of the gland. Access to the pancreas is best accomplished by opening the lesser sac. That is, by dividing the gastrocolic omentum inferior to the gastroepiploic vessels, the anterior surface and the superior and inferior borders of the body and tail of the pancreas can be visualized. The transverse colon is retracted inferiorly and the stomach superiorly (Figure 1). The nasogastric tube may be advanced along the greater curvature of the stomach and can be used as a handle for retraction of the stomach. An adequate Kocher maneuver will allow complete visualization of the pancreatic head and uncinate process. This is accomplished by incising the lateral peritoneal attachments of the duodenum and sweeping the second and third portions medially with a combination of both blunt and sharp dissection (Figure 2). If a large retroperitoneal hematoma is encountered, the nasogastric tube should be advanced through the pylorus and used as a palpable guide to avoid iatrogenic injury to the duodenal wall. The Kocher maneuver should be extensive enough that the left renal vein is easily identified. Occasionally, mobilization of the hepatic flexure is necessary to adequately evaluate the pancreatic head. If the tail of the pancreas is involved, exposure of the splenic hilum is necessary. Division of the peritoneal attachments lateral to the spleen and colon facilitate mobilization. A plane is then created between the spleen, colon and pancreas anteriorly and the kidney posteriorly. This maneuver allows for inspection of the posterior surface of the pancreas (Figure 3).
Approximately 60% of all pancreatic injuries consist of minor contusions, hematomas, and capsular lacerations (Figure 4). Lacerations of the pancreatic parenchyma without major ductal disruption or tissue loss account for an additional 20% of pancreatic injuries (Figure 5). These injuries require only hemostasis and adequate external drainage.12 The temptation to repair capsular lacerations should be resisted, as this tends to lead to pseudocyst formation, whereas a controlled pancreatic fistula is usually self-limited. Closed-suction drains should be used for drainage of any pancreatic injury. These drains are better tolerated by the patient in terms of decreased intra-abdominal abscess formation, more reliable collection of the effluent and less skin excoriation.16 Typically, these drains are left in place for a minimum of 10 days, because if a fistula is going to develop, it should be evident by that time.
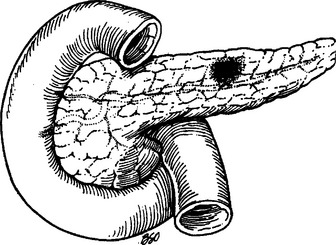
Figure 4 Pancreatic contusion or minor hematoma does not violate the capsule.
(From Asensio JA, Demetriades D, Berne TV: Atlas and Textbook of Techniques in Complex Trauma Surgery. Philadelphia, Saunders, 2005.)
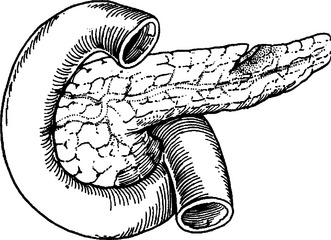
Figure 5 Pancreatic parenchymal injury without ductal injury.
(From Asensio JA, Demetriades D, Berne TV: Atlas and Textbook of Techniques in Complex Trauma Surgery. Philadelphia, Saunders, 2005.)
Nutritional support can be provided via either the oral or gastric route almost immediately. However, with more severe injuries, prolonged gastric ileus and potential pancreatic complications may preclude standard feeding. In addition, the majority of tube feed formulations increase pancreatic stimulation and, in turn, pancreatic effluent and amylase concentration. Elemental diets (low fat, higher pH) are less stimulating to the pancreas, and may be useful in these situations.17 Intraoperative placement of a feeding jejunostomy at the time of initial exploration should be considered for all patients with grade III–V injuries. These allow for early postoperative enteral feeding and avert the need for total parenteral nutrition in those patients unable to tolerate either oral or gastric feedings.
Distal parenchymal transection, especially with disruption of the main pancreatic duct (Figure 6), is best treated with distal pancreatectomy. In general, the anatomic distinction between proximal and distal pancreas is defined by the superior mesenteric vessels passing behind the pancreas at the junction of the head and body. Provided that the proximal duct is normal, the transected duct should be closed with either a “U” stitch or a “figure of eight” with direct suture ligation.18 Although normal endocrine and exocrine function has been reported after 90% pancreatectomy, efforts should be made to leave at least 20% residual pancreatic tissue to minimize postoperative complications.
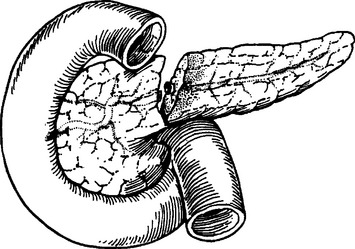
Figure 6 Pancreatic parenchymal disruption with ductal injury.
(From Asensio JA, Demetriades D, Berne TV: Atlas and Textbook of Techniques in Complex Trauma Surgery. Philadelphia, Saunders, 2005.)
The technique of pancreatic transection depends on individual preference. Interlocking “U” stitches with nonabsorbable sutures placed through the full thickness of the gland from anterior to posterior capsule help minimize potential leak from the transected parenchyma. Others prefer to use stapling devices for closure of the pancreatic parenchyma19 (Figure 7). Whatever technique is used for resection and closure of the pancreatic parenchyma, the duct itself (if visible) should be identified and individually ligated. If available, a small omental patch can be placed over the area of resection to buttress stump closure. A closed-suction drain should be left near the transection line, similar to injuries undergoing external drainage.
Distal pancreatectomy can be performed with or without splenectomy. The technical challenge in pancreatectomy without splenectomy involves isolating splenic branch vessels and avoiding injury to the splenic hilum (Figure 8). These, in turn, lead to increased operative time and potential blood loss. Nevertheless, the decision to proceed with splenic salvage requires a completely hemodynamically stable normothermic patient. The risk for postsplenectomy sepsis must also be considered. Generous mobilization of the entire pancreatic gland and spleen must be accomplished before even attempting splenic salvage. Transection of the gland just proximal to the point of injury is followed by elevation of the distal body with meticulous attention to individual ligation of the numerous arterial and venous tributaries found along the superior border of the gland.
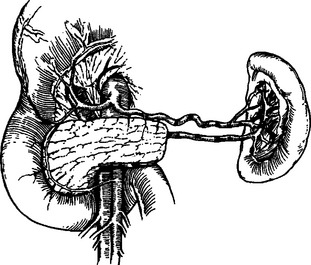
Figure 8 Distal pancreas resection with preservation of the spleen.
(From Asensio JA, Demetriades D, Berne TV: Atlas and Textbook of Techniques in Complex Trauma Surgery. Philadelphia, Saunders, 2005.)
The most challenging management problems arise with injuries to the pancreatic head. Although important with all pancreatic injuries, it is essential to define ductal anatomy for all proximal pancreatic injuries. Intraoperative pancreatography is an important technique for these situations. If local inspection and exploration of the defect fails to exclude ductal injury and intraoperative pancreatography is not an option, wide external drainage with postoperative ERCP is a viable alternative. In fact, because of the high morbidity associated with proximal pancreatic duct injury, closed-suction drainage should be used in virtually all cases of proximal pancreatic injury.12
Adequate external drainage is effective for injuries to the pancreatic head and neck in the absence of major ductal injury. Similarly, if the patient is hemodynamically unstable and the status of the proximal duct is uncertain, wide external drainage with postoperative ERCP is recommended. Patton and colleagues report the effectiveness of drainage alone for proximal pancreatic injuries.12 Of the 37 patients with proximal pancreatic injuries managed with closed-suction drainage, only 13.5% developed either a fistula or abscess.
In the case of incomplete pancreatic parenchymal transection, some surgeons have described an end jejunum to side pancreas anastomosis. This technique is mentioned for historical interest only and is not recommended because of the difficulty in ensuring the integrity of the anastomosis and potential for a high output pancreatic fistula from the posterior aspect of the injury. Stone and coworkers20 have illustrated the high complication rate associated with this dated technique. Of the 7 patients out of 238 in whom this technique was used, 5 (71%) developed a fistula and 3 (43%) died.
Fortunately, severe combined pancreatic head and duodenal injuries are rare. These injuries are most commonly caused by penetrating wounds and occur in association with multiple intra-abdominal injuries. Because of the large number of possible injury patterns, no single therapeutic intervention is right for all patients. The best treatment option is determined by the integrity of the distal common bile duct and ampulla, coupled with the severity of the duodenal injury. For that reason, any patient with a combined injury to the pancreas and duodenum must, at a minimum, have an intraoperative cholangiogram performed before an adequate treatment decision can be made.
During a 6-year period, 10 of 117 patients at Harborview Medical Center in Seattle underwent Whipple resection for nonreconstructible injury to the ampulla or severe combined pancreaticoduodenal injuries. Postoperative complications included four intra-abdominal abscesses, two cases of pancreatitis, and one pancreatic fistula. More importantly, all patients survived.21
Morbidity and Complications Management
Although most complications related to pancreatic injury are self-limiting and/or treatable, the possibility of sepsis and multiple organ failure leading to death is real and results in nearly 30% of the deaths after pancreatic trauma.22
A fistula is the most common complication after pancreatic injury, with an incidence of 7%–20%.11 For the most part, these are minor (<200 ml/day) and resolve spontaneously with adequate external drainage.23 However, those with output greater than 700 ml/day (high output) generally require longer periods of external drainage and may require operative intervention. During this period, nutritional support is paramount. Low-fat, higher pH elemental formulas result in less pancreatic stimulation and should be tried before total parental nutrition.17 Placement of a feeding jejunostomy at the time of initial or subsequent exploration is extremely helpful in those patients with prolonged fistula output in order to provide enteral nutrition.
The use of the long-acting somatostatin analog, octreotide acetate, has been reported in the management of postoperative complications after elective pancreatic resections.24 The use of this synthetic analog has been extended to the treatment of posttraumatic pancreatic fistulas, but there are few data in the literature documenting its efficacy. In fact, the reports that do exist are contradictory.25,26
Abscess formation after pancreatic trauma depends on the number and type of associated injuries and ranges from 10%–25%. The intra-abdominal abscess is often subfascial or peripancreatic. Although a true pancreatic abscess is rare, it is usually the result of inadequate debridement of dead tissue and/or initial drainage and often requires open debridement and drainage. In any case, the mortality rate in this group of patients remains about 25%27 underscoring the need for prompt drainage (either percutaneous or open).
Another common complication after operative management of pancreatic trauma is pancreatitis, occurring in 8%–18% of patients.20 This type of pancreatitis, characterized by transient abdominal pain and a rise in serum amylase, is amenable to bowel rest, with or without nasogastric decompression and nutritional support. In these cases, the course is usually self-limited and resolves spontaneously. A less common complication is hemorrhagic pancreatitis, occurring in less than 2% of postoperative patients.28
Secondary hemorrhage after operative management of pancreatic trauma may occur in 5%–10% of patients.29 This is particularly common with inadequate external drainage after pancreatic debridement or in the face of a postoperative intra-abdominal abscess. These patients often require re-exploration for hemorrhage but angioembolization remains a viable option.
Neither exocrine nor endocrine insufficiency after pancreatic injury is commonly observed. In fact, in both animal and human studies, it has been shown that only 10%–20% of normal pancreatic tissue is needed for normal pancreatic function.6 Thus, distal resection should be well tolerated with little if any physiologic sequelae. This conclusion was confirmed by a multicenter study in which there was only one case of endocrine insufficiency and no exocrine abnormalities were identified.23
1 Wilson R, Moorehead R. Current management of trauma to the pancreas. Br J Surg. 1991;78:1196-1202.
2 Travers B. Rupture of the pancreas. Lancet. 1827;12:384.
3 Mickulicz-Radecki JV. Surgery of the pancreas. Ann Surg. 1903;38:1-27.
4 Korte W. The Pancreas: Its Surgery and Pathology. Philadelphia: Saunders, 1907. Quoted by Robson AVM, Cambridge PJ
5 Innes J, Carey L. Normal pancreatic dimensions in the adult human. Am J Surg. 1994;167:261-264.
6 Dragstedt LR. Some physiologic problems in surgery of the pancreas. Ann Surg. 1943;118:576-593.
7 Baker R, Dippel W, Freeark R. The surgical significance of trauma to the pancreas. Trans West Drug Assoc. 1962;70:361-367.
8 Heitsch RC, Knutson CO, Fulton RL, Jones CE. Delineation of critical factors in the treatment of pancreatic trauma. Surgery. 1976;80:523-529.
9 Smego DR, Richardson JD, Flint LM. Determinants of outcome in pancreatic trauma. J Trauma. 1985;25:771-776.
10 Olsen W. The serum amylase in blunt abdominal trauma. J Trauma. 1973;13:200.
11 Peitzman A, et al. Prospective study of computed tomography in initial management of blunt abdominal trauma. J Trauma. 1986;26:585-592.
12 Patton JHJr, et al. Pancreatic trauma: a simplified management guideline. J Trauma. 1997;43:234-239.
13 Harrell D, Vitale G, Larson G. Selective role for endoscopic retrograde cholangiopancreatography in abdominal trauma. Surg Endosc. 1998;12:400-404.
14 Soto JA, et al. Traumatic disruption of the pancreatic duct: diagnosis with MR pancreatography. Am J Roentgenol. 2001;176:175-178.
15 Graham J, Mattox K, Jordan G. Traumatic injuries of the pancreas. Am J Surg. 1978;136:744-748.
16 Fabian TC, et al. Superiority of closed suction drainage for pancreatic trauma. A randomized, prospective study. Ann Surg. 1990;211:724-728.
17 Kellum J, Holland G, McNeill P. Traumatic pancreatic cutaneous fistula: comparison of enteral and parenteral feedings. J Trauma. 1988;28:700-704.
18 Fitzgibbons T, et al. Management of the transected pancreas following distal pancreatectomy. Surg Gynecol Obstet. 1982;154:225-231.
19 Anderson D, et al. Management of penetrating pancreatic injuries: subtotal pancreatectomy using the auto suture stapler. J Trauma. 1980;20:347-349.
20 Stone H. Experiences in the management of pancreatic trauma. J Trauma. 1981;21:771-776.
21 Oreskovich M, Carrico C. Pancreaticoduodenectomy for trauma: a viable option? Am J Surg. 1984;147:618-623.
22 Leppaniemi A, et al. Pancreatic trauma: acute and late manifestations. Br J Surg. 1988;75:165-167.
23 Cogbill TH, et al. Distal pancreatectomy for trauma: a multicenter experience. J Trauma. 1991;31:1600-1606.
24 Buchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992;163:126-131.
25 Amirata E, Livingston DH, Elcavage J. Octreotide acetate decreases pancreatic complications after pancreatic trauma. Am J Surg. 1994;168:345-347.
26 Nwariaku FE, et al. Is octreotide beneficial following pancreatic injury? Am J Surg. 1995;170:582-585.
27 Feliciano D, et al. Management of combined pancreatoduodenal injuries. Ann Surg. 1987;205:673-679.
28 Jones R. Management of pancreatic trauma. Am J Surg. 1985;150:698-704.
29 Campbell R, Kennedy T. The management of pancreatic and pancreaticoduodenal injuries. Br J Surg. 1980;67:845-850.