Chapter 11 Pancreatic Ductal Adenocarcinoma
Epidemiology and Risk Factors
Worldwide, approximately 230,000 people are afflicted yearly with pancreatic cancer. It is the fourth leading cause of cancer-related deaths in the United States, as reported by the American Cancer Society, for 2009. It is estimated that 33,000 to 34,000 patients will die of pancreatic cancer in the United States, representing approximately 6% of total U.S. cancer deaths.1
Pancreatic cancer is typically a cancer of the elderly. Only 13% of cases occur in patients younger than 55 years, whereas 69% of cases occur in those older than 65.2 There is a slight predilection for men over women in most countries.3 It has been speculated that, although there may be a hormonal element accounting for this, it is more likely secondary to the higher rate of smoking for males.4
Tobacco smoke exposure plays a significant part in the development of pancreatic adenocarcinoma in as many as 20% to 30% of pancreatic cancers.5 A study of 194 patients showed that the risk of pancreatic cancer doubled in those with a history of smoking.6 In contrast, although excessive alcohol consumption is a common risk factor for chronic pancreatitis, it has not been shown to result in an increased risk of pancreatic cancer.4
The relationship of diabetes to pancreatic cancer is complex. Pancreatic cancer itself can induce diabetes secondary to its destructive effects on the pancreatic parenchyma. Nevertheless, a meta-analysis of 20 studies showed a doubling of the pooled relative risk of pancreatic cancer in those patients with diabetes for 5 years.7 The findings suggest that mechanisms for this increased risk included insulin resistance, elevated insulin levels, and decreased glucose tolerance. This may also explain the finding that moderate physical activity, such as walking as little as 1.5 hours every week, was associated with reducing the risk of pancreatic cancer by half.8
Findings have been mixed regarding the impact of diet on the incidence of pancreatic cancer.9,10 Whereas some studies suggest excessive intake of saturated fat may have a detrimental effect,9 others have failed to show such a pattern.10
The activation of oncogenes and the inactivation of tumor suppressor genes play a significant role in the manifestation of pancreatic cancer.11 Mutations such as DPC4, p53, and p16 are each present in over half of all cases. Overall, it has been estimated that between 5% and 20% of pancreatic cancers are hereditary, depending on the population that is being studied.2,12 The degree of risk increases greatly with the number of affected family members.13
A variety of syndromes are also associated with an increased risk of pancreatic cancer. Individuals who are carriers of the germline BRCA2 mutation have up to a 10-fold greater risk of developing pancreatic cancer over the general population. Other syndromes, and their associated genetic alteration, include hereditary pancreatitis (PRSSI), hereditary nonpolyposis colorectal cancer—the Lynch II variant (hMSH2, hMLH1), familial atypical multiple mole melanoma (FAMMM) syndrome (p16), Peutz-Jeghers syndrome (STK11/LKB1), and ataxia telangiectasia (ATM).2
Anatomy and Pathology
The pancreas, measuring between 12 and 15 cm in length, is located deep within the retroperitoneum and lacks a capsule, which allows surrounding fat to extend into clefts between glandular components.14
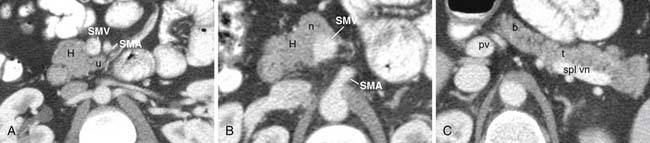
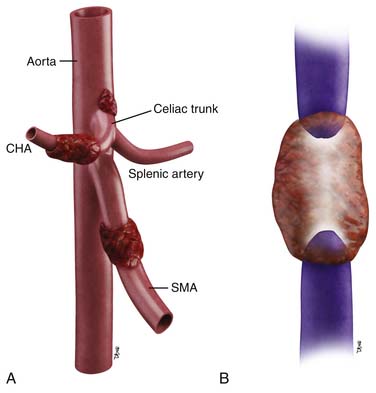
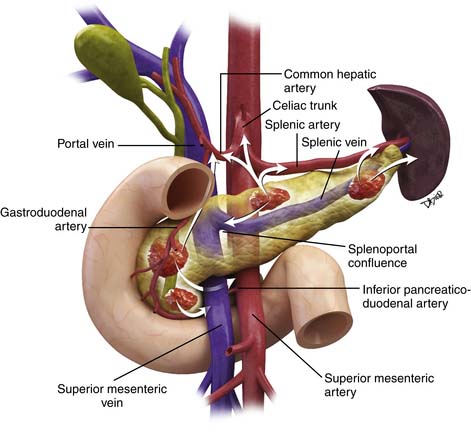
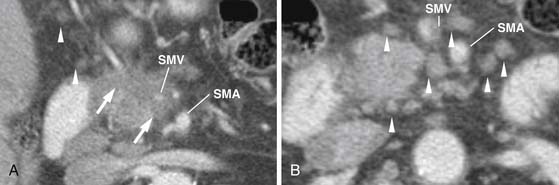
The pancreas can be divided into five components: the head, uncinate process, neck, body, and tail (Figure 11-1). It is bounded on the right by the descending duodenum, inferiorly by the horizontal duodenum, and anteriorly by the stomach. The pancreatic head is defined as that portion to the right of the left border of the superior mesenteric vein (SMV). Extending to the left of the pancreatic head is a narrow projection called the uncinate process posterior to the SMV. In contrast, the neck is a narrowed segment that joins the pancreatic head to the body. The body is defined as that portion located between the left border of the SMV and the left border of the aorta. The tail extends from the left border of the body laterally into the region of the splenic hilum.14 The pancreatic head is bounded on the right by the descending portion of the duodenum and inferiorly by the horizontal portion of the duodenum. The pancreatic body is located posterior to the stomach. Vascular structures also provide important boundaries of the pancreas that can be utilized to localize disease either inside or outside of the pancreas. The gastroduodenal artery, originating from the common hepatic artery (CHA), forms the right lateral and anterior boundary of the pancreatic head. The inferior pancreaticoduodenal artery courses along the posterior border of the pancreatic head and the SMV, as described previously, is an important landmark.
The arterial supply of the pancreas is as follows: the gastroduodenal artery from above and the inferior pancreaticoduodenal artery from below supply the anterior and posterior pancreaticoduodenal arcades to supply the pancreatic head and the uncinate process. Branches of the dorsal pancreatic artery, which typically arises from the celiac trunk, also provide supply to the pancreatic head and anastomose with the pancreaticoduodenal arcade and small vascular branches from the gastroduodenal artery and CHA. The pancreatic body is supplied by branches of the dorsal pancreatic artery and the great pancreatic artery, which in turn, arises from the splenic artery. Multiple splenic artery branches provide supply to the pancreatic tail.15 Knowledge of the vascular supply is important because it also provides a pathway for spread of tumor.
The venous drainage of the pancreas is via branches that drain back to the SMV and splenic veins, which in turn, fuse to form the portal vein (PV) at the splenoportal confluence posterior to the pancreatic neck.16 The splenic vein runs along the posterior pancreatic body, which is the etiology for frequent occlusion of the structure secondary to pancreatic cancer or pancreatitis.
Both sympathetic and parasympathetic innervations of the pancreas are present. The sympathetic supply controls pancreatic blood flow, and the parasympathetic innervations, originating from the posterior vagus nerve and the celiac plexus, promotes pancreatic secretions.17 Both the parasympathetic and the sympathetic tracts contain nerve fibers that transmit pain. These nerve fibers provide a means for tumor migration; their involvement is also a source of debilitating pain.
Histologically, the pancreas is composed of both exocrine and endocrine cell types. The exocrine component consists of acinar and ductal cells responsible for a variety of digestive enzymes and bicarbonate that are ultimately emptied, at the ampulla of Vater, into the duodenum to facilitate digestion.17 The endocrine cellular types include alpha cells (that produce glucagon), beta cells (which produce insulin), delta cells (which produce somatostatin), and PP cells (that produce pancreatic polypeptides).17
Ductal adenocarcinoma is the most common solid malignant neoplasm of the pancreas. This tumor type is an invasive epithelial form that, at least focally, shows sites of ductal or glandular differentiation.18 Typically, it induces an intense desmoplastic reaction made up of a variety of cellular types including myofibroblasts, inflammatory cells, such as lymphocytes and plasma cells, in addition to dense collagen.19 The tumor cells in the well-differentiated type form well-defined glands with mild pleomorphism.18 Moderately differentiated forms exhibit poorly defined glands, whereas poorly differentiated forms do not show well-defined glands. The poorly defined forms grow as individual cells or in sheets and with extensive nuclear pleomorphism.20 Growth tends to be haphazard and most forms show vascular invasion and lymphatic as well as perineural invasion.20
On gross examination, these tumors form firm, but poorly defined, masses that are white to yellow in color. They are typically of variable size, and vary from poorly seen small forms to those that are large, with central regions that can be necrotic, show cystic change, or manifest mucinous features.20
Clinical Presentation
Because the pancreas is located deep within the retroperitoneum, clinical symptoms typically do not manifest until tumor has involved local vessels, caused perineural infiltration, or in pancreatic head tumors, biliary obstruction. Therefore, patients usually present with jaundice, weight loss, and/or abdominal pain.21 In addition, symptomatology will depend on a tumor’s location within the pancreas and extent of metastatic disease. Tumors of the pancreatic head, neck, and sometimes, the body often obstruct the common bile duct, causing jaundice. Tumors in the pancreatic tail often cause left-sided pain, whereas those in the pancreatic body often manifest with midepigastric pain.22 Other symptoms include fatigue, new-onset diabetes, and steatorrhea related to pancreatic insufficiency.17,22 There should be a high suspicion for pancreatic cancer when patients without discernible risk factors present with pancreatitis.23
Unfortunately, symptoms are typically nonspecific, resulting in a median time of 6 months between onset of symptoms and presentation.17 Uncommon symptoms include nausea and vomiting secondary to gastric outlet obstruction and increasing abdominal distention secondary to accumulation of ascites.2 Unexplained progressive weight loss is a common symptom and can be related to jaundice, nausea, or catabolic syndrome and is noted in patients with resectable as well as advanced pancreas cancers. Patients with pancreatic cancer are prone to hypercoagulable states including deep venous thrombosis, pulmonary embolism and less often aseptic/marantic endocarditis—sometimes these are part of the initial presentation.
Physical signs may be very limited. Cachexia with temporal wasting is noted in patients with advanced disease and significant weight loss. Only a third will have a palpable gallbladder (Courvoisier’s sign), and an even smaller percentage will have other palpable masses or ascites.24 Virchow’s (left supraclavicular) or Sister Mary Joseph (periumbilical) adenopathy may be present.2
Biliary obstruction typically causes elevated serum bilirubin levels and transaminitis. Liver function tests can be significantly abnormal with extensive liver metastases as well. Serum amylase may be normal, but when greater than 300, advanced disease is typically present. Often, serum glucose levels are mildly to moderately elevated.2,25
A variety of tumor markers have been considered for screening for pancreatic cancer such as carcinoembryonic antigen (CEA), CA50, and cell adhesion molecule 17-1 (CAM 17-1), but the most useful so far has been CA19-9.2,26 Unfortunately, CA19-9 is not specific for pancreatic cancer but does serve as a prognostic indicator in some patients. It is elevated in a variety of gastrointestinal tumors including stomach, colon, and biliary tree and is often elevated in the setting of biliary obstruction alone.2 In addition the 10% of patients who are Lewis antigen a and b negative have undetectable or markedly suppressed levels of CA19-9.27
Staging Classification
Accurate staging is vital to stratify patients correctly to the most appropriate care, given the severe prognosis of this cancer. It is important to balance the need to give potentially curative surgery to the small percentage of patients who may benefit, at the cost of remaining months of fair to good quality of life with postoperative morbidity in those patients who undergo surgery without potential benefit because of locally advanced or metastatic disease. A review of cases from the United States from 1996 to 2002 shows that the vast majority had metastatic or regionally advanced disease with only a small fraction being resectable.28 When patients presenting with all stages are pooled together, it is found that 5-year survival overall is only 5%.29 Importantly, surgery provides no survival benefit if microscopic or gross positive margins are present.30,31
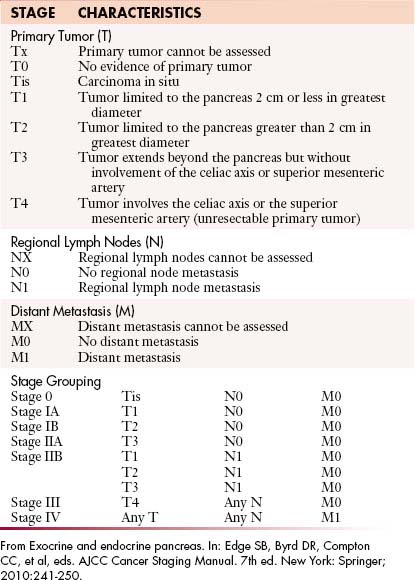
The staging system most extensively utilized is that from the American Joint Committee on Cancer and the International Union Against Cancer (Table 11-1). This system evaluates the primary tumor (T), the presence or absence of nodal disease (N), and the presence or absence of metastatic disease (M), to come up with TNM grades that are, in turn, utilized to determine staging.
Also important is pathologic staging that, by definition, is limited to patients who have undergone surgery. This assessment alone provides truly accurate information regarding nodal disease. Full assessment includes examination of the surgical specimen with no less than 10 lymph nodes resected from the region including those along such major vasculature structures as the CHA and celiac trunk as well as the regions near the distal stomach/pylorus and near the splenic hilum.26
Primary Tumor (T)
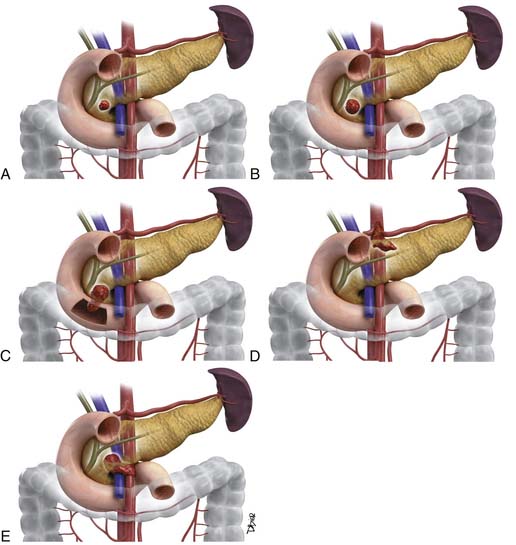
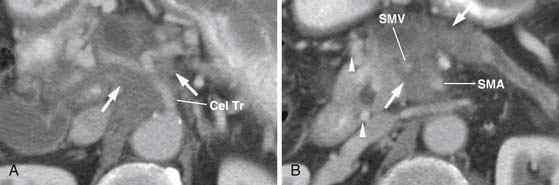
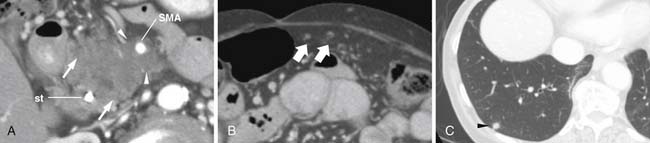
As shown in Table 11-1 and Figure 11-2, T staging is based on tumor size, whether it is limited to the pancreas, and whether tumor involves major local arterial structures (the SMA and celiac artery) (Figure 11-3). Tumors that are 2 cm or less in size but confined to the pancreas are identified as T1, but called T2 if more than 2 cm in diameter (while still confined to the pancreas). Once there is extension beyond the confines of the pancreas, tumor is graded at T3. When that extension is such that it involves the celiac axis or the SMA, it is identified as T4, which is unresectable disease in essentially all medical institutions. T3 disease is considered to be in the realm of resectable disease, but this will vary with the institution. It is notable that originally venous involvement was considered to be unresectable disease; surgical advances such as the venous bypass graft have allowed for en bloc resection.32 Nevertheless, such surgical techniques are not practiced at all institutions. Therefore, it is imperative to understand the local criteria for accepting patients for potential surgical resection. For example, some institutions may identify venous occlusion as an absolute contraindication. Others, such as our institution, have developed a criterion of “borderline” resectable pancreatic disease. These are patients in whom the primary tumor meets certain criteria, and in our case, in the setting of preoperative therapy, are considered to be possibly resectable after a prolonged course of preoperative therapy.
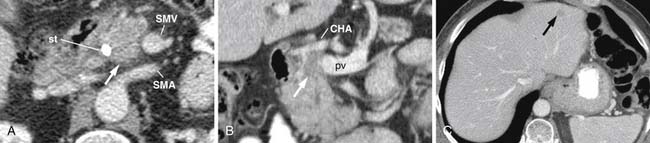
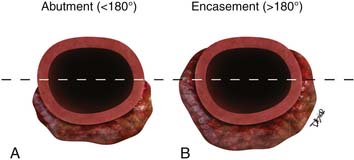
The M. D. Anderson criteria for borderline resectable disease are based largely on the appearance of the tumor on cross-sectional imaging, particularly computed tomography (CT; Figure 11-4). Borderline resectable disease includes abutment of up to 180 degrees of the SMA, short segment encasement/abutment of the CHA (but > 1 cm uninvolved CHA at the level of the origin from the CHA), or venous involvement up to short segment occlusion with suitable conditions above and below this region to allow for placement of a venous bypass graft (Figures 11-5 and 11-6).31 As can be seen from the prior description, the borderline resectable classification supplements or extends the T portion of TNM staging.
Nodal Disease (N)
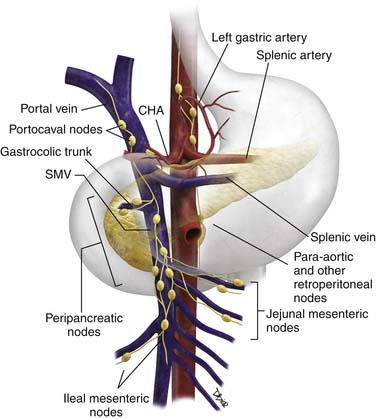
The N descriptor in TNM staging refers to regional nodal disease (Figure 11-7). This criterion is very difficult to assess preoperatively and is described further under “Imaging.” It commonly indicates pathologic staging and, therefore, requires an adequate lymphadenectomy; typically, the histologic evaluation should include no less than 10 regional nodes located in the celiac, CHA, gastric (pyloric), and splenic regions. Recent large studies that performed extended lymphadenectomy including such stations as celiac, greater and lesser omental, portal, SMA, and periaortic nodes have shown no improvement in survival.33,34
Nodal involvement and the more recently described variable of lymph node ratio (number of positive nodes divided by total number examined) have both been described as prognostic factors after resection of pancreatic cancer.35,36 A recent study at our institution of 326 patients showed median survival with nodal status of N0 was 31.9 months and 21.6 months in patients with N1 disease.37
Patterns of Tumor Spread
The most important factor in the spread of a patient’s pancreatic tumor is the location of the tumor within the pancreas (Figure 11-8). This is because of the scaffolding of vessels and neural structures that provide a pathway for tumor to spread outward from its site of origin.38,39
Tumors located within the anterior portion of the pancreatic head extend along the local vasculature, the anterior pancreaticoduodenal arcades superiorly, until reaching their closest supplying vessel, the gastroduodenal artery. Tumor then tends to extend further superiorly along the gastroduodenal artery to its origin from the proper hepatic artery. For this reason, close attention should be paid to this region on cross-sectional imaging for tumors originating in the anterior pancreatic head.
Tumors that are instead located in the much more medially and posteriorly located uncinate process commonly extend along the inferior pancreaticoduodenal arcade to the inferior pancreaticoduodenal artery. Tumor then uses this vessel to infiltrate toward the SMA from which this vessel originates, via a common trunk with the first jejunal artery. Uncinate tumors, therefore, typically involve the SMA before involvement of the celiac trunk or its tributaries. Tumor can also spread via the first jejunal artery into the jejunal mesentery. The SMV is frequently involved and is at risk for occlusion; there also is risk for involvement of the first jejunal venous branch and the ileal branch of the SMV. Tumor can also extend into the ileocolic vasculature.
Typically, the pattern of nodal involvement is that of adjacent regional nodes (see Figure 11-7). Pancreatic head and body tumors are of concern for left gastric, common hepatic, and portacaval nodes, and subsequently, celiac axis nodes. Pancreatic head and uncinate lesions are also of concern for peripancreatic nodes and jejunal mesenteric nodes. Pancreatic body tumors are of concern for left gastric distribution nodes in addition to common hepatic nodes. In contrast, pancreatic tail lesions may be associated with peripancreatic/splenic nodes, although with medial extension, as with body, neck, and head tumors, spread may occur to celiac trunk nodes. Inferior pancreatic head tumors and uncinate tumors can also spread to nodes along the proximal ileocolic vessels where they originate from the ileal vein. With sufficiently advanced disease, tumor can extend to retroperitoneal nodes, and in our anecdotal experience, nodes can occasionally be identified in the periesophageal region.
Metastatic disease typically goes initially to the liver and/or peritoneum. Lesions may initially be very small, requiring careful evaluation by the reading radiologist and close comparison with any available prior studies. Pulmonary metastases typically occur later and, in our experience, can have a variety of forms including small nodules, irregular lesions, small cavitary lesions, and infrequently as lymphangitic spread. Osseous lesions are less frequent, as low as 2.2% in one recent study,40 and in our experience are typically later in appearance than other forms of metastatic disease.
Imaging
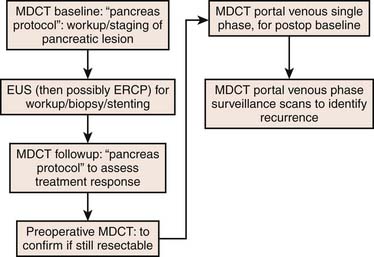
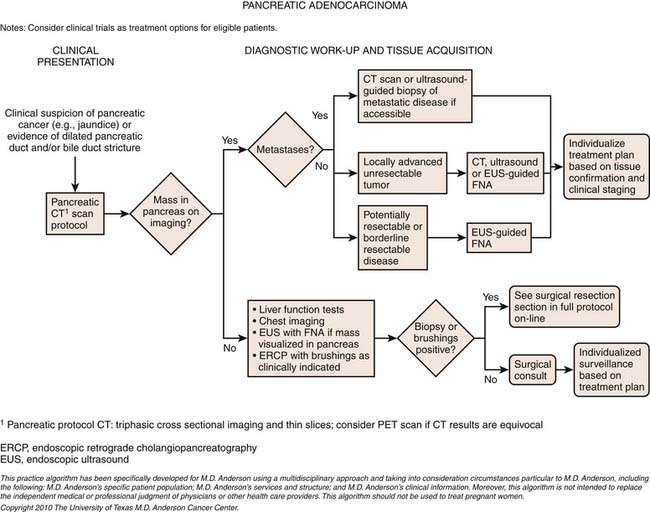
A combination of modalities is crucial for the accurate diagnosis and staging of pancreatic cancer. The following reviews the utility of integrating multiple modalities; see the algorithm in Figure 11-9.
Primary Tumor
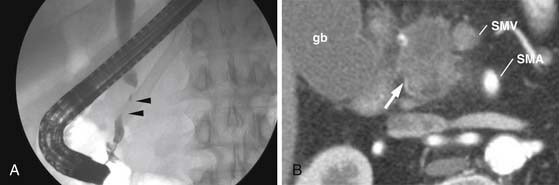
Often endoscopic retrograde cholangiopancreatography (ERCP) is subsequently performed for common bile duct obstruction identified on ultrasound. ERCP is able to diagnose and treat biliary obstruction. Tumor caused strictures are typically irregular with abrupt cutoff of the duct (Figure 11-10). In contrast, benign strictures are usually smooth and tapering. However, there is significant overlap. Brushings of the stricture region can provide a tissue diagnosis. In addition, ERCP can guide biliary stent placement. Unfortunately, ERCP-induced pancreatitis degrades subsequent cross-sectional imaging. For this reason, cross-sectional imaging should be performed first in patients who present with suspicion for pancreatic or biliary cancer.
Multidetector row computed tomography (MDCT) has become a workhorse for workup of suspected pancreatic cancer. Close attention must be paid to technique. Typically, contrast is injected rapidly, approximately 4 to 5 mL/sec, with an injection duration of 30 seconds to improve tumor conspicuity.41–43 Imaging also has to be obtained during both peak parenchymal and hepatic enhancement. Peak pancreatic parenchymal enhancement, during a 30-second injection duration, is approximately 35 to 50 seconds after the start of contrast enhancement and maximizes contrast between tumor and normal pancreatic parechyma (Figure 11-11).44 In contrast, liver metastases are best seen during the portal venous phase of enhancement.44 Thin-section imaging (1-3 mm) is important to evaluate the interface between tumor and vasculature. We typically obtain images of both phases at 2.5-mm slice thickness. We also obtain reconstructed images as thin as 0.625 mm in both phases for problem-solving and for multiplanar coronal and sagittal reconstructions useful for identifying whether disease is intrapancreatic or extrapancreatic and involvement of vasculature.
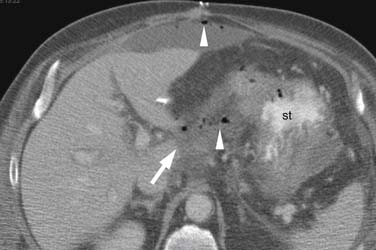
On MDCT, pancreatic cancer usually has the appearance of an ill-defined, slightly hypodense (to normal pancreatic parenchyma) solid mass (see Figure 11-3), 60% originating in the pancreatic head, 15% occurring in the body, and only 5% in the tail with reportedly as much as 20% manifesting as diffuse pancreatic involvement.45 Secondary signs include pancreatic atrophy upstream of the duct obstructing primary tumor, focal pancreatic enlargement, extrapancreatic extension of tumor, and cutoff of the pancreatic duct and/or common bile duct. Overall, MDCT has a sensitivity of 86% to 97% when considering tumors of all size, but for tumors under 2 cm, sensitivity drops to 77%.44,46–49
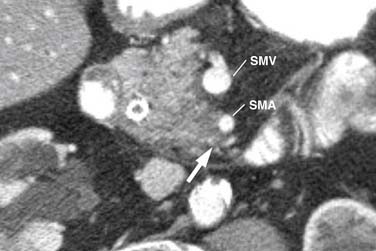
Identifying the local extent of disease with respect to neighboring vasculature is critical for local staging. The determination of vascular involvement is commonly made by identifying the circumferential extent of vascular involvement (Figure 11-12; see also Figures 11-3, 11-5, and 11-10). Using as a criterion for unresectable disease greater than 180 degrees of vascular involvement (Figure 11-13) resulted in a sensitivity for unresectable disease of 84% and a specificity of 98%.50 With the development of venous interposition grafts, the focus for identifying unresectable vascular involvement has shifted to that of the SMA, celiac artery, and CHA. In our experience, it still remains important to identify the extent of venous occlusion because extension of tumor to ileocolic vessels precludes the placement of a bypass graft and renders patients unresectable.
Recent developments in MRI include parallel imaging and high-field-strength platforms. MRI has inherently greater soft tissue contrast than CT before the administration of intravenous contrast, is a suitable substitute when patients are allergic to iodinated contrast, and provides useful information in the setting of renal failure with unenhanced imaging. A combination of typically useful imaging includes T2-weighted fat-suppressed, T1-weighted, and T1-weighted fat-suppressed (pre- and postcontrast, and dynamically obtained) images. Dynamically obtained images are typically acquired beginning 20, 60, and 120 seconds after the start of contrast injection, providing pancreatic parenchymal, portal venous, and delayed phase imaging.51,52 A combination of gradient recalled echo (GRE) and three-dimensional acquisitions with parallel imaging facilitates rapid, single-breathhold, thin-section overlapping dynamic imaging of the entire abdomen in a single breathhold for each phase.53,54 A variety of techniques including some form of either respiratory triggered/compensated or breathhold such as fast-recovery fast spin echo (FRFSE; GE Medical Systems, Milwaukee, WI) allows for either more detailed and/or faster T2-weighted acquisitions.53 In our experience, new sequences such as fast imaging employing steady-state acquisition (FIESTA; GE Medical Systems, Milwaukee, WI) provide new opportunities to accentuate contrast between vessels, fat, pancreas, and tumor.
Primary tumor typically has the appearance of a hypointense mass on the pancreatic parenchymal phase of dynamic imaging (Figures 11-14 and 11-15) but, as with MDCT, can appear isointense on later phases of dynamic imaging.55 MRCP sequences, in which long echo times suppress signal from soft tissues causing ductal structures to stand out, show ductal cutoff with a sensitivity of 84% and specificity of 97% for tumor.56
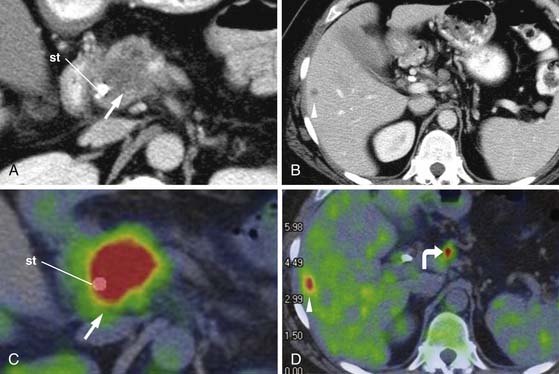
PET/CT is typically performed without intravenous contrast. PET/CT is commonly used to identify distant metastases because the lack of intravenous contrast prohibits staging local disease (Figure 11-16).
Endoscopic ultrasound (EUS) has a strong role in the detection and biopsy confirmation of pancreatic cancer. Using a combination of radial and curvilinear arrays with color Doppler, EUS with fine-needle aspiration (FNA) can be used to image and biopsy tumor in real time without the requirement of intravenous contrast. In a study at our institution, EUS-FNA was significantly more sensitive (99%) than MDCT (89-93%).49 A study from the Mayo Clinic reported a sensitivity of 86%, specificity of 94%, and positive predictive value of 100%, but negative predictive value of 86%.57 In our study, we found that the negative predictive value was only 70% and as low as 21% in the setting of biliary stents. At our institution, EUS examination is done before ERCP and both can be done at the same setting.
EUS can also be used to assess locoregional extension of tumor including vascular invasion. However, a prospective study of 62 patients showed helical CT to perform better than either MRI or EUS for assessing vascular invasion with an accuracy of 83% versus 75% for EUS and 74% for MRI. Another study showed a sensitivity of 63% and a specificity of 64% for vascular invasion. The sign of loss of the echoplane surrounding vessels had limited utility, with only 29% of cases with this sign having adherence of tumor to the vessel and none having actual invasion.58
Nodal Disease
All modalities show limitations regarding assessing nodal disease. Typically, lymph node size larger than 1 cm in short axis has been used on MRI and CT to differentiate normal from metastatic nodes but is nonspecific (see Figure 11-12). In a study by Valls and coworkers,58a only 3 of the 18 patients (16.7%) with nodal involvement identified at surgery were detected when a size criterion on CT of greater than 1.5 cm was utilized for adenopathy. Criteria for adenopathy such as hypodensity, ill-defined boundaries, or rounding of nodes improve specificity at the expense of sensitivity. On EUS, absence of an echogenic center is suggestive of metastatic disease. EUS with FNA improves assessment of metastatic nodes, but its limited field of view is such that para-aortic, mesenteric, and other nodes outside the surgical field are missed. The role of PET/CT is still being defined for nodal disease. Sensitivities of 46% to 71% and specificities of 63% to 100% have been reported.59–61 Overall, all current modalities are unable to detect micrometastases and have difficulty differentiating inflammatory nodes from malignancy.
Metastatic Disease
The most common sites of metastatic disease, as described previously, are the liver, peritoneum, and lungs, with bone metastases less common.40
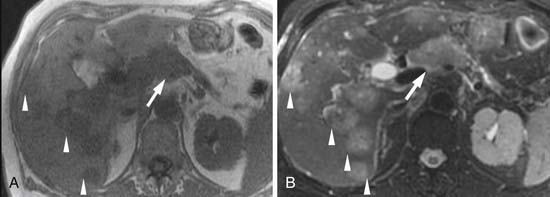
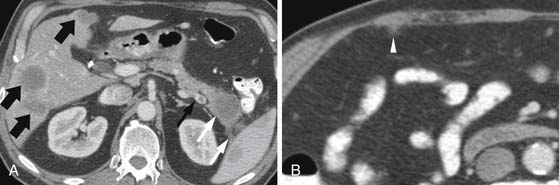
The assessment for liver metastases is most commonly made by CT and/or MRI (Figure 11-17; see also Figure 11-15). CT reportedly has a sensitivity of 75% to 87%.62–64 In a study comparing helical CT with MRI, the accuracy of CT was 87% versus 93.5% for MRI. Specific data are not available regarding liver-specific MRI contrast agents for detecting liver metastases from pancreatic cancer. Information on PET/CT is limited, but a study of 50 patients, 11 having liver metastases, showed that PET/CT had a sensitivity of only 46%, although this improved to 82% with the administration of intravenous contrast.65 Other studies have shown sensitivities of 68% to 70% for liver metastases (see Figure 11-16).66,67
Currently, all modalities are limited in detecting small peritoneal implants (Figure 11-18; see also Figure 11-17). Whereas laparoscopy has been utilized at some institutions to improve the detection of peritoneal implants, a meta-analysis suggested that laparoscopy may affect management in only 4% to 15% of patients after thin-section CT.68
Key Points Imaging
• Radiology report should include the level of certainty regarding metastatic disease and sites of potential metastatic disease.
• The report should describe in sufficient detail the nature of involvement of arteries and veins so that T status can be clearly understood.
• Report should describe suspicious nodal sites, especially if outside the operative field.
Clinician’s Perspective on Imaging
At our institution, EUS, CT, MRI, and PET/CT are complementary. EUS is the most sensitive for detecting, and EUS-FNA for confirming, small suspected pancreatic primary tumors, especially those isodense on CT scans. Baseline MDCT studies are important for localizing tumor, vascular staging, and following indeterminate small liver lesions. If liver findings are problematic, they are further worked up with MRI. We also use MRI when patients have allergies to iodinated contrast or renal insufficiency/renal failure. The use of gadolinium is typically precluded in patients with renal failure because of the risk of nephrogenic systemic fibrosis.69 MRI’s inherently superior soft tissue contrast provides useful information even without the administration of gadolinium. The role of PET/CT in the initial staging of pancreatic cancer is very limited at our institution.
Key Points Clinician’s perspective on imaging
• Surgeon needs to know the relationship of tumor to vessels and the length of venous involvement for planning venous graft placement.
• Oncologist needs to know size of tumor, extent of liver metastases, and sites of peritoneal disease
• Radiation oncologist needs to know the extent of disease craniocaudally as well as transversely, including degree of tumor spread, and all local sites of disease.
Treatment*
General Considerations
Only 20% of newly diagnosed patients with pancreatic cancer will have early-stage, potentially resectable disease.70–72 Of these, 75% to 80% will have CT-occult extrapancreatic disease that will eventually lead to recurrence. The 1-year survival rate of the patients presenting with advanced disease is a dismal 18%.
Resectable Disease: Surgical Considerations
Vascular Resection
We have found vascular resection (VR) for tumor involvement safe with survival similar to those who do not require it.73–75 In our experience, the vast majority of resections/reconstructions were of the PV or SMV-PV confluence, with a minority being short segments of the CHA or tangential resection of the inferior vena cava.74
Results of Surgery
At large centers, the ability to carry out complex resections and manage complications has greatly improved, increasing the fraction of patients who are resectable. At our institution, between 1990 and 2004, 724 pancreaticoduodenectomies were performed, 360 for pancreatic adenocarcinoma, with a median survival of 25.1 months.37 Ninety-three (25.8%) patients had major complications, including perioperative death (3 [0.83%]), need for reoperation, pancreaticojejunostomy leak, intra-abdominal hemorrhage, intra-abdominal fluid collection, cardiac arrhythmia, myocardial infarction, sudden cardiac death, pulmonary complications, gastrointestinal bleeding, and sepsis. A majority of our patients (318 [88.3%]) underwent adjuvant or neoadjuvant therapy, with 254 (70.5%) receiving preoperative therapy. One hundred and thirty (36.1%) required VR. Sixty patients (16.7%) had an R1 resection with a median survival of 21.5 months versus 27.8 months after R0 resection. Node-positive status was associated with a 21.6-month median survival versus 31.9 months for 174 (48.3%) node-negative patients. On analysis of predictors of survival, only the presence of major complications, N1 status, and blood loss were significant negative predictors.
Other specialized centers have had similar results. The largest experience, at Johns Hopkins, included 1175 patients.76 Their mortality fell from 30% in the 1970s to 1% in the 2000s. Perioperative morbidity was 38%; 15% had delayed gastric emptying; 42% had positive resection margins; and 78% had nodal disease. Median survival was 18 months with negative predictors of survival including tumor diameter 3 cm or greater and positive lymph nodes or resection margin. Adjuvant therapy improved survival. In a study of 696 patients at Memorial Sloan-Kettering, perioperative (90-day) mortality was 4%, and 28% had positive margins with median survival for node-positive patients of 16 months versus 27 months for node-negative.77 In 466 patients treated at the Mayo Clinic, median survival was 21.6 months, with adjuvant chemoradiation therapy increasing the median survival to 25.2 months.78
Chemotherapy/Radiation Therapy and Resectable Disease
In the adjuvant approach, resectable patients undergo surgery followed by chemotherapy and radiation therapy to treat the high percentage of patients who would otherwise develop local recurrence.79 The first prospective trial of adjuvant chemoradiation utilizing external beam radiation therapy (EBRT; 40 Gy) and concurrent 5-fluorouracil (5-FU) showed a median survival of 18 months. Experiences at single institutions have shown similar improvement, such as at Johns Hopkins which utilized 40 to 45 Gy EBRT and infusional 5-FU with a median patient survival of 20 months versus 14 months for those untreated.80 Unfortunately, postoperative morbidity and mortality limits the number of patients who can undergo therapy.
In the neoadjuvant approach, giving chemotherapy and radiation therapy ahead of surgery increases the percentage of patients who can undergo such therapy, treats early systemic disease, and decreases the microscopic resection margin positivity rate.81 At our institution, all potentially resectable patients undergo neoadjuvant therapy. However, indications for neoadjuvant therapy vary across institutions.81 At M. D. Anderson Cancer Center, a study utilizing gemcitabine and concurrent ERBT of 30 Gy had 86% of patients proceed to laparotomy and 73% undergo resection, with a median survival of 34 months for resected patients.81
Locally Advanced Pancreatic Cancer
In the absence of acute symptoms, chemotherapy (without radiation) is a good option for these patients. Two recent trials have compared chemotherapy alone with chemoradiation in locally advanced pancreatic cancer. The French FFCD/SFRO (Fédération Francophone de Carcinologie Digestive/Société Française de Radiothérapie Oncologie) trial compared EBRT (60 Gy in 30 fractions) plus concomitant 5-FU and cisplatin followed by gemcitabine until progression.71 The chemotherapy arm consisted of single-agent gemcitabine until progression. Median survival for chemotherapy was 13 months versus 8.6 months for the chemoradiation arm. This study was criticized, given the significantly increased toxicity noted in the chemoradiation arm (radiation therapy 60 Gy) and addition of cisplatin. An Eastern Cooperative Oncology Group (ECOG) study evaluating gemcitabine with and without radiation (ECOG 4201) accrued poorly, and early results in 74 patients showed an improvement in median overall survival with chemoradiation over chemotherapy (11 mo vs. 9.2 mo), but the chemoradiation arm had a significantly higher rate of grade 4 toxicity (41% vs. 6%).72
Metastatic Pancreatic Cancer
Gemcitabine, a ribonucleotide reductase inhibitor, is the approved standard therapy for advanced pancreatic adenocarcinoma. Burris and colleagues70 performed the landmark trial comparing single-agent gemcitabine with 5-FU that led to gemcitabine’s approval by the U.S. Food and Drug Administration (FDA) for improved clinical benefit response. Clinical benefit response was measured by pain medication need, weight gain, and improvement in performance status and was 23.8% in patients treated with gemcitabine compared with 4.8% for patients treated with 5-FU. The 1-year survival and median survival rates were 18% and 5.6 months for gemcitabine, and 2% and 4.4 months for 5-FU, respectively (P = .0025). Since then, several cytotoxic and targeted agents that have shown promise in preclinical, phase I, and single-arm phase II trials have been compared with gemcitabine and combined with gemcitabine but, unfortunately, have produced disappointing results in randomized phase III trials.
Erlotinib (a small-molecule epidermal growth factor receptor–tyrosine kinase inhibitor [EGFR-TKI]) was approved by the FDA in combination with gemcitabine for treatment of advanced pancreatic cancer (PA.3 phase III trial), albeit for a marginal improvement in overall survival.82 Meta-analyses suggest that doublet cytotoxic therapies (gemcitabine in combination with platinum agents) may be of value in very good performance status patients (ECOG 0-1), although currently, there are no published reports of any phase III randomized trials of combination cytotoxic doublets that has been clearly shown to be superior to gemcitabine alone. Two recent phase III trials of targeted agents, bevacizumab and cetuximab in combination with gemcitabine versus gemcitabine alone, did not show a survival benefit.
Key Points Therapy
• Two main schools of therapy for potential surgical candidates are preoperative and postoperative chemoradiation.
• Venous bypass grafts for venous (without arterial) tumor involvement have increased the pool of potential surgical candidates.
• In locally advanced disease, improving tumor control can be an important goal and can aid symptom palliation.
• For metastatic disease, prognosis remains very poor, with gemcitabine as standard therapy for advanced disease.
Surveillance
Monitoring Tumor Response
Imaging plays a vital role in identifying treatment response or failure. The earlier that treatment failure can be identified, the more rapidly patients can be changed to a potentially more effective treatment protocol. For preoperative treatment regimens, imaging can identify whether disease has progressed to being unresectable.
Recently, PET/CT has been suggested as a means to potentially detect earlier treatment response by identifying changes in glucose uptake by tumor.83,84 However, more work needs to be done because several published studies have been small and results have been conflicting. Although an earlier study by Nakata and associates84 of 14 patients suggested that a low SUV (standardized uptake value) was associated with improved survival, a subsequent study of 19 patients by Kuwatani and coworkers85 showed no significant difference. Kuwatani and coworkers85 suspected that, given how early pancreatic cancer metastasizes, measuring the uptake value of only the primary tumor was misleading and that follow-up studies should consider monitoring all tumor sites where it is possible to measure SUV. The Kuwatani study, in addition to CT and PET/CT, also utilized tumor markers. The tumor markers, such as CA19-9, and FDG-PET in their study appeared to provide similar information, although the study was too small to take into account problems such as patients with inherently low levels of CA19-9 production that would preclude its use in this patient subgroup. They also concluded that contrast-enhanced CT was more sensitive than either FDG-PET or tumor markers for predicting prognosis because of its superior ability to identify small metastatic sites (carcinomatosis, ascites, mesenteric metastases, and liver metastases) that are too small to be detectable by FDG uptake.84,85
Detection of Recurrence
CT is probably the modality used most to evaluate for recurrence. We have found it useful to obtain a follow-up contrast-enhanced thin-section MDCT study 3 to 4 months after surgery. Images are typically obtained in the portal venous phase at 2.5-mm thickness. Imaging earlier than 8 weeks is problematic because of postsurgical changes. A baseline CA19-9 level is also helpful. A study by Sperti and colleagues86 showed elevation of CA19-9 levels after surgery predictive of recurrence 1 to 10 months before evidence demonstrates it clinically or radiologically. However, care must be taken to identify those patients Lewis A-B-negative for whom CA19-9 is not an effective tumor marker.27
The most common sites of recurrence, in order, are liver, peritoneum, and locoregional.87,88 Close comparison with preoperative CT scans is helpful in identifying new small liver metastases, differentiating peritoneal disease from inflammatory changes, and differentiating pulmonary metastases from benign postinflammatory nodules.
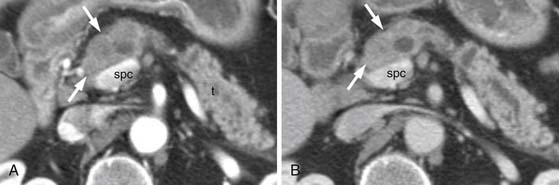
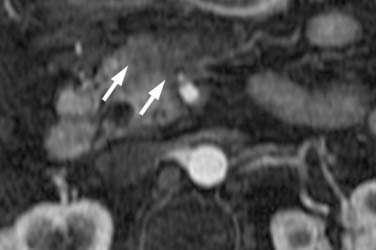
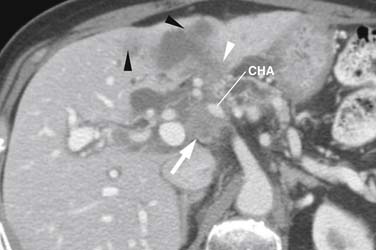
After surgery, nonspecific regions of soft tissue thickening are common near the CHA, SMA, and SMV. A study by Ishigami and associates89 showed such thickening to be present in all 44 post-pancreaticoduodenectomy patients; they speculated it was secondary to lymph node dissections. Close comparison with the baseline study is, therefore, necessary to identify early recurrence (Figure 11-19). Factors increasing suspicion include lymph node and/or margin positivity.89
An analysis of several studies has shown that PET/CT can identify sites suspicious for local recurrence when CT is equivocal.90,91 PET/CT is probably very helpful when no postoperative baseline study is available or postoperative changes are moderately extensive, providing regions where tumor can grow in an occult fashion. PET/CT also appeared to improve detection of peritoneal disease over CT, although the reason is unclear. One can speculate that PET/CT may be useful in differentiating common postoperative changes from underlying developing sites of peritoneal disease.91,92 However, PET/CT, without intravenous contrast, had a sensitivity for liver metastases of only 42% to 50% in two studies.91,92 Given the superior ability of CT and MRI to detect liver metastases, and given the practice of having a postoperative thin-section CT for comparison, we utilize PET/CT primarily when CT is negative and CA19-9 is rising.
Key Points Detection of recurrence
• Disease recurs more commonly distantly (i.e., liver, lung metastases) than locally.
• Local recurrence is more likely in the setting of positive surgical margin or positive nodal status.
• Baseline CT examination, no earlier than 8 weeks after surgery, is crucial for detecting local recurrence.
• Thin-section imaging (2-3 mm, potentially thinner) is important to detect local recurrence.
Complications of Therapy
Specific complications include the following. Extrapancreatic tumor extension can cause obstruction of the gastric outlet. Peritoneal disease, or obstruction of the SMV, can result in ascites. Jaundice can develop secondary to stent occlusion or recurrent/metastatic tumor; it can often be managed by ERCP or percutaneous biliary drainage. Thrombosis of the PV or SMV can cause ascites, chronic venous ischemic changes of bowel, PV thrombi to the liver, and altered liver perfusion.
Complications from pancreatic cancer surgery can be divided to acute (perioperative) and chronic. Acute changes include the following. Delayed gastric emptying, seen in 7% of patients, can be confirmed with nuclear medicine or fluoroscopic studies.93 Inflammatory complications include pancreatitis (incidence 9-10%), phlegmon, fluid collections, and abscess (incidence 4-10%).93–96 These typically occur in the setting of pancreatic fistula (in ≤ 17% of patients), or leak from an anastomosis or the jejunal stump. Close attention must, therefore, be paid to the gastrojejunostomy, pancreaticojejunostomy, and choledochojejunostomy as well as the jejunal stump. Leakage from the choledochojejunostomy can result in bilomas and abscesses, can be evaluated by Tc-HIDA (hepato-iminodiacetic acid) scans or ERCP, and can often be treated by stenting. Leakage from the jejunal stump, gastrojejunostomy, and pancreaticojejunostomy may show leakage of gastrointestinal contrast (although the amount of gastrointestinal contrast passing into the afferent loop draining the pancreas and the biliary tree is variable) (Figure 11-20). Close attention to the location of the anastomoses and fluid collections can aid in identifying the likely source.
There are also several chronic complications. Persistent pancreatic fistulae can also cause fat necrosis, phlegmon, pseudocysts, and pseudoaneurysms. Resulting mesenteric fibrosis can cause vascular strictures and consequent venous ischemic changes and varices (and potential gastrointestinal hemorrhage) and afferent loop obstruction. Stenosis of the choledochojejunostomy has an incidence of 3% after 1 year and 8% at 7 years.97 It can cause jaundice and pruritus and usually is treated by biliary stent placement and/or balloon dilation.
New Therapies
The future probably lays in returning to the drawing board for more vigorous basic research as well as the need to design better clinical trials (e.g., the use of randomized phase II trials, enriched trials for biomarkers to identify predictors of response). We are just now gaining insight into the underlying biologic characteristics of pancreatic cancer, and it clearly is a very complex process. A recent study using global genomic analyses has yielded insights into tumor pathogenesis and demonstrates that at least 12 signaling pathways and processes are altered in pancreas cancers—and our best hope for therapeutic advance for this lethal disease may be to target not individual genes but rather downstream mediators and key nodal points that are the drivers for tumorigenesis. Other potential pathways are to amplify the effects of tumor-suppressive pathways or to stimulate the immune response.98 There are emerging data from transgenic mouse models that drug delivery into a desmoplastic cancer may be a large challenge. Preclinical studies evaluating the role of tumor stroma are currently ongoing. There is considerable interest in studying the hedge hog pathway, expression of Hent-1 transport proteins (responsible for transport of gemcitabine into the cell), and tumor-stromal interactions. We are just beginning to better understand the very complex biology of this cancer, and biomarker-enriched drug discovery is warranted in preclinical and clinical settings.
Conclusion
Pancreatic cancer remains a difficult, challenging disease. Whereas surgical advances and preoperative therapy have increased the pool of surgical candidates, the prognosis for survival remains dismal. Imaging provides crucial information for accurately staging patients, assessing treatment response, detecting tumor recurrence, and identifying and managing complications. Ongoing trials are evaluating the merits of preoperative versus postoperative therapies. Growing knowledge of the biology of pancreatic cancer is being utilized to develop novel approaches to potentially intervene in this disease.
1. American Cancer Society. American Cancer Society Cancer Statistics Presentation. 2009. Available at www.cancer.org/Healthy/InformationforHealthCareProfessionals/cancer_statistic_2009_slides_rev.ppt (accessed December 29, 2009)
2. Royal R.E., Wolff R.A., Crane C.H. Pancreatic cancer. In: Devita V.T., Lawrence T.S., Rosenberg S.A., et al. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2008:3200.
3. Chang K.J., Parasher G., Christie C., et al. Risk of pancreatic adenocarcinoma: disparity between African Americans and other race/ethnic groups. Cancer. 2005;103:349-357.
4. Lowenfels A.B. Pancreas cancer: epidemiology and risk factors. In: Kelsen D.P., Daly J.M., Kern S.E., et al. Principles and Practice of Gastrointestinal Oncology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008:1008.
5. Lowenfels A.B., Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197-209.
6. Wynder E.L., Mabuchi K., Maruchi N., Fortner J.G. Epidemiology of cancer of the pancreas. J Natl Cancer Inst. 1973;50:645-667.
7. Everhart J., Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605-1609.
8. Michaud D.S., Giovannucci E., Willett W.C., et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921-929.
9. Stolzenberg-Solomon R.Z., Pietinen P., Taylor P.R., et al. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;155:783-792.
10. Michaud D.S., Skinner H.G., Wu K., et al. Dietary patterns and pancreatic cancer risk in men and women. J Natl Cancer Inst. 2005;97:518-524.
11. Hruban R.H., Petersen G.M., Ha P.K., Kern S.E. Genetics of pancreatic cancer. From genes to families. Surg Oncol Clin North Am. 1998;7:1-23.
12. Bartsch D.K., Kress R., Sina-Frey M., et al. Prevalence of familial pancreatic cancer in Germany. Int J Cancer. 2004;110:902-906.
13. Tersmette A.C., Petersen G.M., Offerhaus G.J., et al. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738-744.
14. Pancreas. In: Gray H., Strandring S., Ellis H., Berkovitz B.K.B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed, Edinburgh: Elsevier Churchill Livingstone; 2005:1231-1238.
15. Okahara M., Mori H., Kiyosue H., et al. Arterial supply to the pancreas; variations and cross-sectional anatomy. Abdom Imaging. 2010;35:134-142.
16. Washington M.K. Gross and microscopic anatomy of the pancreas. In: Von Hoff D.D., Evans D.B., Hruban R.H. Pancreatic Cancer. Boston: Jones and Bartlett; 2005:2-12.
17. Conlon K.C., Aremu M.A. Pancreas cancer: anatomy, staging systems, and techniques. In: Kelsen D.P., Daly J.M., Kern S.E., et al. Principles and Practice of Gastrointestinal Oncology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008:1008.
18. Solcia E., Capella C., Kloppel G. Tumors of the exocrine pancreas. In: Rosai J., Sobin L.H. Atlas of Tumor Pathology: Tumors of the Pancreas. Washington, DC: Armed Forces Institute of Pathology; 1997:31-130.
19. Iacobuzio-Donahue C.A., Ryu B., Hruban R.H., Kern S.E. Exploring the host desmoplastic response to pancreatic carcinoma: gene expression of stromal and neoplastic cells at the site of primary invasion. Am J Pathol. 2002;160:91-99.
20. Hruban R.H., Syed Z.A. Pathology of the exocrine pancreas. In: Von Hoff D.D., Evans D.B., Hruban R.H. Pancreatic Cancer. Boston: Jones and Bartlett; 2005:15-30.
21. Modolell I., Guarner L., Malagelada J.R. Vagaries of clinical presentation of pancreatic and biliary tract cancer. Ann Oncol. 1999;10(Suppl 4):82-84.
22. Kelsen D.P., Portenoy R., Thaler H., et al. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery. 1997;122:53-59.
23. Mujica V.R., Barkin J.S., Go V.L. Acute pancreatitis secondary to pancreatic carcinoma. Study Group Participants. Pancreas. 2000;21:329-332.
24. Howard J.M., Jordan G.L.Jr. Cancer of the pancreas. Curr Probl Cancer. 1977;2:5-52.
25. Saruc M., Pour P.M. Diabetes and its relationship to pancreatic carcinoma. Pancreas. 2003;26:381-387.
26. Hwang R.F., Grau A.M., Spitz F.R., et al. Pancreatic adenocarcinoma. In: Feig B.W., Berger D.H., Fuhrman G.M. The MD Anderson Surical Oncology Handbook. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:368-390.
27. Tempero M.A., Uchida E., Takasaki H., et al. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501-5503.
28. Jemal A., Siegel R., Ward E., et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66.
29. Ries L., Melbert D., Krapcho M. SEER cancer statistics review, 1975-2004, Based on November 2006 SEER data submission, posted to the SEER Web site. National Cancer Institute; 2007. Available at http://seer.cancer.gov/csr/1975_2004/results_merged/sect_22_pancreas.pdf
30. Tsiotos G.G., Farnell M.B., Sarr M.G. Are the results of pancreatectomy for pancreatic cancer improving? World J Surg. 1999;23:913-919.
31. Varadhachary G.R., Tamm E.P., Abbruzzese J.L., et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035-1046.
32. Fuhrman G.M., Leach S.D., Staley C.A., et al. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 1996;223:154-162.
33. Yeo C.J., Cameron J.L., Lillemoe K.D., et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355-366. discussion 366–368
34. Farnell M.B., Pearson R.K., Sarr M.G., et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618-628. discussion 628–630
35. Riediger H., Keck T., Wellner U., et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337-1344.
36. Ozaki H., Hiraoka T., Mizumoto R., et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16-22.
37. Raut C.P., Tseng J.F., Sun C.C., et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52-60.
38. Tamm E.P., Silverman P.M., Charnsangavej C., Evans D.B. Diagnosis, staging, and surveillance of pancreatic cancer. AJR Am J Roentgenol. 2003;180:1311-1323.
39. Rozenblum E., Schutte M., Goggins M., et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731-1734.
40. Borad M.J., Saadati H., Lakshmipathy A., et al. Skeletal metastases in pancreatic cancer: a retrospective study and review of the literature. Yale J Biol Med. 2009;82:1-6.
41. Kim T., Murakami T., Takahashi S., et al. Pancreatic CT imaging: effects of different injection rates and doses of contrast material. Radiology. 1999;212:219-225.
42. Schueller G., Schima W., Schueller-Weidekamm C., et al. Multidetector CT of pancreas: effects of contrast material flow rate and individualized scan delay on enhancement of pancreas and tumor contrast. Radiology. 2006;241:441-448.
43. Tublin M.E., Tessler F.N., Cheng S.L., et al. Effect of injection rate of contrast medium on pancreatic and hepatic helical CT. Radiology. 1999;210:97-101.
44. Fletcher J.G., Wiersema M.J., Farrell M.A., et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology. 2003;229:81-90.
45. Clark L.R., Jaffe M.H., Choyke P.L., et al. Pancreatic imaging. Radiol Clin North Am. 1985;23:489-501.
46. Agarwal B., Abu-Hamda E., Molke K.L., et al. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844-850.
47. DeWitt J., Jowell P., Leblanc J., et al. EUS-guided FNA of pancreatic metastases: a multicenter experience.[see comment]. Gastrointest Endosc. 2005;61:689-696.
48. Bronstein Y.L., Loyer E.M., Kaur H., et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol. 2004;182:619-623.
49. Tamm E.P., Loyer E.M., Faria S.C., et al. Retrospective analysis of dual-phase MDCT and follow-up EUS/EUS-FNA in the diagnosis of pancreatic cancer. Abdom Imaging. 2007;32:660-667.
50. Lu D.S., Reber H.A., Krasny R.M., et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439-1443.
51. Fayad L.M., Mitchell D.G. Magnetic resonance imaging of pancreatic adenocarcinoma. Int J Gastrointest Cancer. 2001;30:19-25.
52. Pamuklar E., Semelka R.C. MR imaging of the pancreas. Magn Reson Imaging Clin North Am. 2005;13:313-330.
53. Schima W., Ba-Ssalamah A., Kolblinger C., et al. Pancreatic adenocarcinoma. Eur Radiol. 2006;17:638-649.
54. Lall C.G., Howard T.J., Skandarajah A., et al. New concepts in staging and treatment of locally advanced pancreatic head cancer. AJR Am J Roentgenol. 2007;189:1044-1050.
55. Ly J.N., Miller F.H. MR imaging of the pancreas: a practical approach. Radiol Clin North Am. 2002;40:1289-1306.
56. Adamek H.E., Albert J., Breer H., et al. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356:190-193.
57. Wiersema M.J., Vilmann P., Giovannini M., et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095.
58. Aslanian H., Salem R., Lee J., et al. EUS diagnosis of vascular invasion in pancreatic cancer: surgical and histologic correlates. Am J Gastroenterol. 2005;100:1381-1385.
58a. Valls C., Andia E., Sanchez A., et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol. 2002;178:821-826.
59. Pakzad F., Groves A.M., Ell P.J. The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med. 2006;36:248-256.
60. Bares R., Klever P., Hauptmann S., et al. F-18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology. 1994;192:79-86.
61. Bares R., Dohmen B.M., Cremerius U., et al. Results of positron emission tomography with fluorine-18 labeled fluorodeoxyglucose in differential diagnosis and staging of pancreatic carcinoma [in German]. Radiologe. 1996;36:435-440.
62. Richter G.M., Simon C., Hoffmann V., et al. Hydrospiral CT of the pancreas in thin section technique [in German]. Radiologe. 1996;36:397-405.
63. Trede M., Rumstadt B., Wendl K., et al. Ultrafast magnetic resonance imaging improves the staging of pancreatic tumors. Ann Surg. 1997;226:397-405. discussion 405
64. Calculli L., Casadei R., Diacono D., et al. Role of spiral computerized tomography in the staging of pancreatic carcinoma [in German]. Radiol Med. 1998;95:344-348.
65. Strobel K., Heinrich S., Bhure U., et al. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med. 2008;49:1408-1413.
66. Frohlich A., Diederichs C.G., Staib L., et al. Detection of liver metastases from pancreatic cancer using FDG PET. J Nucl Med. 1999;40:250-255.
67. Diederichs C.G., Staib L., Vogel J., et al. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109-116.
68. Pisters P.W., Lee J.E., Vauthey J.N., et al. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88:325-337.
69. Perez-Rodriguez J., Lai S., Ehst B.D., et al. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment—report of 33 cases. Radiology. 2009;250:371-377.
70. Burris H.A.3rd, Moore M.J., Andersen J., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413.
71. Chauffert B., Mornex F., Bonnetain F., et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592-1599.
72. Loehrer P.J.S., Powell M.E., Cardenes H.R., et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201 [abstract]. J Clin Oncol. 2008;26:214s.
73. Evans D.B., Farnell M.B., Lillemoe K.D., et al. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1736-1744.
74. Tseng J.F., Raut C.P., Lee J.E., et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935-949. discussion 949–950
75. Al-Haddad M., Martin J.K., Nguyen J., et al. Vascular resection and reconstruction for pancreatic malignancy: a single center survival study. J Gastrointest Surg. 2007;11:1168-1174.
76. Winter J.M., Cameron J.L., Campbell K.A., et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199-1210. discussion 1210
77. House M.G., Gonen M., Jarnagin W.R., et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549-1555.
78. Corsini M.M., Miller R.C., Haddock M.G., et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol. 2008;26:3511-3516.
79. Westerdahl J., Andren-Sandberg A., Ihse I. Recurrence of exocrine pancreatic cancer—local or hepatic? Hepatogastroenterology. 1993;40:384-387.
80. Sohn T.A., Yeo C.J., Cameron J.L., et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579.
81. Hoffman J.P., Willett C.G., Cohen S.J. Pancreas cancer: clinical management. In: Kelsen D.P., Daly J.M., Kern S.E., et al. Principles and Practice of Gastrointestinal Oncology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008:377-392.
82. Moore M.J., Goldstein D., Hamm J., et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966.
83. Maisey N.R., Webb A., Flux G.D., et al. FDG-PET in the prediction of survival of patients with cancer of the pancreas: a pilot study. Br J Cancer. 2000;83:287-293.
84. Nakata B., Chung Y.S., Nishimura S., et al. 18F-fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer. 1997;79:695-699.
85. Kuwatani M., Kawakami H., Eto K., et al. Modalities for evaluating chemotherapeutic efficacy and survival time in patients with advanced pancreatic cancer: comparison between FDG-PET, CT, and serum tumor markers. Intern Med. 2009;48:867-875.
86. Sperti C., Pasquali C., Catalini S., et al. CA 19-9 as a prognostic index after resection for pancreatic cancer. J Surg Oncol. 1993;52:137-141.
87. Evans D.B., Varadhachary G.R., Crane C.H., et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496-3502.
88. Van den Broeck A., Sergeant G., Ectors N., et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600-604.
89. Ishigami K., Yoshimitsu K., Irie H., et al. Significance of perivascular soft tissue around the common hepatic and proximal superior mesenteric arteries arising after pancreaticoduodenectomy: evaluation with serial MDCT studies. Abdom Imaging. 2008;33:654-661.
90. Sperti C., Pasquali C., Bissoli S., et al. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J Gastrointest Surg. 2010;14:131-140.
91. Ruf J., Lopez Hanninen E., Oettle H., et al. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5:266-272.
92. Kitajima K., Murakami K., Yamasaki E., et al. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent pancreatic cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Mol Imaging Biol. 2010;12:452-459.
93. Grobmyer S.R., Pieracci F.M., Allen P.J., et al. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204:356-364.
94. Scialpi M., Scaglione M., Volterrani L., et al. Imaging evaluation of post pancreatic surgery. Eur J Radiol. 2005;53:417-424.
95. Schulick R.D. Complications after pancreaticoduodenectomy: intraabdominal abscess. J Hepatobiliary Pancreat Surg. 2008;15:252-256.
96. DeOliveira M.L., Winter J.M., Schafer M., et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931-937. discussion 937–939
97. Reid-Lombardo K.M., Ramos-De la Medina A., Thomsen K., et al. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg. 2007;11:1704-1711.
98. S.E. Kern, E. Gallmeier, M. Goggins, R.H. Hruban. Pancreatic cancer: molecular biology and genetics. D.P. Kelsen. J.M. Daly. S.E. Kern. et al. Principles and Practice of Gastrointestinal Oncology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008:329-342.