Pancreatic adenocarcinoma
Introduction
Adenocarcinoma of the pancreas accounts for 3% of new cancer cases per annum,1 yet it is the fourth leading cause of cancer-related death in Western countries. The insidious nature of the disease and its vagueness of presentation contribute to late diagnosis. Eighty per cent of patients have unresectable tumours at initial diagnosis. The overall survival at 5 years still remains at 6%, unchanged over the last four decades.1 However, in recent years improvements in preoperative imaging and staging modalities, coupled with advancements in adjuvant therapies with the use of immunomodulators and monoclonal antibodies, have resulted in some degrees of optimism. Progress has been made as the molecular basis of the disease is better understood. With such poor survival rates and recent high-profile media attention, a renewed interest in tackling this elusive cancer has arisen.
Epidemiology
An estimated 44 000 new cases of adenocarcinoma of the pancreas occurred in the USA in 2011, and almost 38 000 died in the same year.2 In the UK, 8085 people were diagnosed with pancreatic cancer and 8020 people died during 2009.3 The incidence of pancreatic cancer varies with age, sex and ethnicity. In 2008, the standardised incidence rate of pancreatic cancer was 3.9 per 100 000 population, while the standardised mortality rate was slightly lower at 3.7 per 100 000 population. Pancreatic cancer is the eleventh commonest cancer in males and eighth commonest cancer in females.4 The peak incidence for the disease occurs between the seventh and eighth decades of life, and is rare under the age of 30. In the American population, African and Hawaiian ethnicities confer a higher incidence than Caucasian, whereas Asian and Hispanic ethnic groups have a lower risk of developing the disease. The incidence of pancreatic cancer is rising, particularly in Europe, although this observation is subject to reporting bias related to improved diagnostics. However, pancreatic neoplasm must be detected at an early stage to enable the potential for curative treatment.
Risk factors (see Box 15.1)
Tobacco smoking is by far the leading preventable cause of pancreatic cancer, with an estimated 2.5-fold increase in risk when compared to non-smokers.5 In 1986, the International Agency for Research on Cancer (IARC) classified smoking as a proven carcinogen with respect to cancer of the pancreas. Observational studies suggest that a dose-dependent relationship exists, necessitating long-term exposure.5 However, smokers who have quit for more than 10 years no longer experience an increased risk.5 While the chemical cause is unclear, it is hypothesised that N-nitroso compounds in tobacco are carried to the pancreas in the blood. The time in which cigarette smoking exerts its negative influence is also subject to debate; however, observational studies seem to point towards the latter stages of carcinogenesis, particularly in the 15 years preceding development.
Diet and alcohol
Excessive body weight appears to increase the risk of pancreatic cancer. It has been shown that obesity has a positive association, with a relative risk of 1.72.6 Diets high in saturated fat have a suggested contributing role in carcinogenesis,6 although data are limited. Caffeine and meat preservatives have been suggested to have a negative association, but in recent years this has become more debated due to the studies having methodological flaws, and more recent studies demonstrating the opposite. Vitamin C, vitamin D and high-fibre diets have suggested protective associations with pancreatic neoplasm.7–9
Occupation
Workers exposed to ionising radiation, insecticides, aluminium, nickel, acrylamide and halogenated hydrocarbons are reported to have an increased risk of developing pancreatic adenocarcinoma. There is evidence of increased risk in people exposed to chlorinated hydrocarbon solvents (metal degreasing workers and dry cleaners), and those working in the paint and varnish industry and the textiles industry.10
Past medical history
Chronic pancreatitis is a progressive inflammatory process with associated irreversible histological changes. It is highly linked to excessive alcohol consumption, with up to an 18-fold increase in risk of pancreatic cancer compared to the general population. Quantifying the risk is still difficult due to confounding factors such as smoking, alcohol and diet.11
Diabetes has a positive association for pancreatic cancer. Meta-analysis has shown that type 2 diabetes increases the risk of pancreatic cancer by 82%.11 The high prevalence of diabetes in society excludes hyperglycaemia as a screening tool for pancreatic cancer.
Hereditary pancreatic cancer
Pancreatic carcinogenesis has an established genetic predisposition. Familial conditions such as Peutz–Jeghers syndrome, germ-line mutation in the STK11/LKB11 gene,13 BRCA2 expression14 and familial atypical multiple mole melanoma (p16/CDKN2A germ-line mutation) may predispose to pancreatic cancer.14 Links with hereditary non-polyposis colorectal cancer (Lynch syndrome), BRCA1 and von Hippel–Lindau have been suggested but not confirmed, as conferring increased risk.15 With the speed of developing technology, matched with reduced genetic diagnostic expense, one can foresee the potential for the discovery of further genes relating to familial pancreatic cancer.
Precursor lesions
Histologically distinct precursor lesions have been attributed to pancreatic carcinogenesis. Preneoplastic lesions are usually asymptomatic and can be incidentally discovered at the time of resection. They appear to follow a multi-step progression to invasive carcinoma.15 These precursor lesions include pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN).16 Because of their small size (usually < 5 mm), they are difficult to detect, making them elusive to computerised tomography (CT) or magnetic resonance imaging (MRI).15
Pan-INs are the most frequent preneoplastic lesions, observed in approximately 82% of pancreas with neoplasm.17 They are subclassified into PanIN-1, PanIN-2 and PanIN-3 depending upon the degree of cytological and architectural atypia.17 Each of these precursor lesions harbours a unique repertoire of clinicopathological and genetic characteristics that has an impact on the natural history and prognosis of these lesions.
Workers in Johns Hopkins University proposed pancreatic intraepithelial neoplasms (PanIN; 1A → 1B → 2 → 3) as the precursor lesions to invasive carcinoma.18 The model is analogous to that of ductal carcinoma in situ (DCIS) of the breast or adenomatous polyps in colorectal cancer. The lesions display atypical mucinous epithelium replacing the physiological cuboidal epithelium. The evidence for PanIN being a true premalignant state is largely circumstantial. These lesions were first described adjacent to resected adenocarcinoma. The more atypical PanIN-2 and -3 were seen exclusively in neoplastic pancreas. These lesions also display similar genetic aberrations to the frankly invasive samples. In particular, the percentage of p16 and K-ras mutations increases with the more atypical PanIN. These data have heralded development of a tumour genesis model involving sequential progression from PanIN-1a to invasive adenocarcinoma.19
Classically, evolution from precursor lesions to pancreatic neoplasm (ductal adenocarcinoma) involves diverse molecular changes (Fig. 15.1). Recent studies indentified at least 119 independent loci that may potentially play a role in tumour progression, including K-ras, TP53, p16/CDKN2A, MYC and AKT2.20 The K-ras gene product mediates signal transduction in a number of growth factor receptors. K-ras single point mutation is observed in 90–95% of pancreatic ductal adenocarcinoma, representing the most common mutation in this disease.21 K-ras is currently the focus of multiple ongoing studies to see if it can be utilised as a diagnostic tool.13 Altered epidermal growth factor receptor expression (EGFR) causing overexpression is thought to be an early event in pancreatic carcinogenesis.22
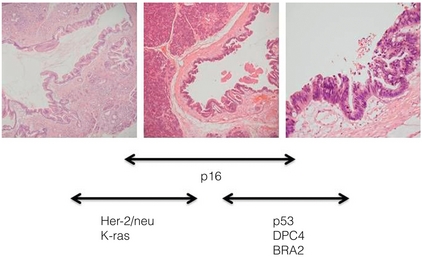
Figure 15.1 Diagrammatic representation of the multi-step progression to invasive carcinoma from low-grade neoplasm on the left to high-grade on the right. Images courtesy of Dr Paul Crotty.
Inactivation of numerous tumour suppressor genes, including p16/CDKN2A and TP53, plays a pivotal role in the development of pancreatic cancer. Loss of tumour suppression is noted in 70–95% of pancreatic neoplasm.17 Other targets including transforming growth factor-β (TGF-β) receptor genes, BRCA2, HER-2/NEU, DPC4, MKK4 and EBER-1 are currently under investigation. The discussion about these genes is outside the scope of this chapter. However, the best chance for cure in the treatment of pancreatic cancer lies with detecting these non-invasive lesions before progression to invasive carcinoma.
Presentation
The majority of patients present with vague and non-specific symptoms (Box 15.2). As a result, the disease is commonly widespread at diagnosis, and approximately 80% of patients present with unresectable disease.
Tumours in the body and tail of the pancreas usually present late. Pain is the most consistent symptom. Painless jaundice is seen in 13% of patients while 34% present with only pain and 46% present with both pain and jaundice. Weight loss and anorexia are observed in 7% of patients. Rarely, tumour invasion into stomach or duodenum can present as haematemesis and malaena. Patients may also present with late-onset diabetes mellitus and acute pancreatitis.23 Limited series have examined the screening of asymptomatic cohorts, with little evidence to support the introduction of population screening for pancreatic cancer.14 However, there may be a place for targeted screening of high-risk groups in the near future.
Investigation
Markers
There still remains no ideal tumour marker for pancreatic carcinoma. Carbohydrate antigen 19-9 (CA 19-9; 0–37 U/mL) exists in tissue as an epitope of sialylated Lewis a-type blood group antigen, and is the most widely utilised tumour marker. It was based on a monoclonal antibody to colorectal cancer cell lines. CA 19-9 is elevated in only approximately 50% of cases.24 Among symptomatic patients, CA 19-9 has a sensitivity of 81–85% and specificity of 81–90%.24 However, the positive predictive value remains low among the asymptomatic population, making it a very poor screening test. Falsely elevated CA 19-9 is documented in other neoplasms including gastric, colorectal, cholangiocarcinoma and urothelial malignancies, as well as benign conditions such as pancreatitis, hepatitis, thyroiditis and biliary obstruction. In addition patients expressing Lewis blood group antigens (a and b) may have elevated levels.25 CA 19-9 may be used to assess recurrence of disease, with a level higher than 500 U/mL signifying advanced disease.25 A level exceeding 243 U/mL for patients undergoing primary chemoradiotherapy for locoregionally advanced disease also indicates poorer median survival (7.1 vs. 12.3 months).26
Several other tumour markers are currently being investigated including carcinoembryonic antigen (CEA), K-ras, p53, CA242, CA50, SPAN-1, DU-PAN2, CAM-17.1 and a number of mucins (MUC1, MUC3, MUC4 and MUC5AC). They are proposed as having application in pancreatic neoplasms, although none of these markers are sensitive enough to be recommended for clinical use. CA242 shows promise as an independent prognostic factor.27
Diagnosis
Transabdominal (TA) ultrasound (US) is the initial investigation in the jaundiced patient. It is noted for its superior sensitivity for determining cholelithiasis over CT. Common bile duct dilatation (> 7 mm; > 10 mm in post-cholecystectomy patients) is an indirect sign, together with pancreatic duct dilatation (> 2 mm). The primary pancreatic lesion is often visible together with liver metastases and ascites if present. For lesions > 3 cm TAUS has approximately 95% sensitivity; however, this is considerably lower for smaller lesions.28 The main criticism of TAUS is machine quality difference and operator experience; thus it is user dependent.29 The role of colour Doppler US has been suggested to examine portal vein or superior mesenteric involvement. Ultrasound remains a useful imaging modality for the initial screening of the jaundiced patient, but further radiological modalities are necessary to examine the pancreas and assess resectability.
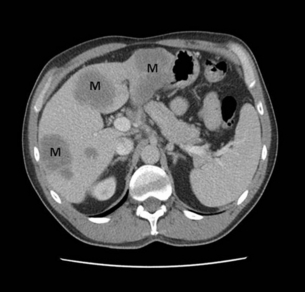
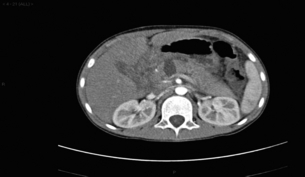
CT remains the most common staging modality. Conventional CT has been replaced by more sensitive dynamic CT with thinner slice/cuts (1–3 mm) with multidetector and 3D reconstruction. The sensitivity is approximately 90% for lesions greater than 2 cm, decreasing to approximately 60% for smaller lesions.30,31 CT allows for assessment of the primary lesion, its relationship to the remainder of the pancreas and peripancreatic vasculature, and determination of resectability (Figs 15.2 and 15.3). Direct evidence of a tumour is often seen as a hypodense mass, with other subtle signs such as pancreatic atrophy, deformity of the glandular contour or dilatation of the common bile and pancreatic ducts (Fig. 15.4). Metastatic lesions can be detected, and portal vein or superior mesenteric artery involvement can be determined.
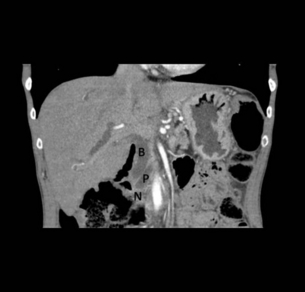
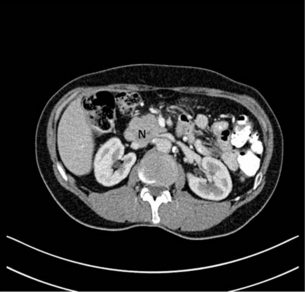
Figure 15.4 CT scan of coronal view demonstrating biliary duct (B), pancreatic duct (P), obstruction by pancreatic neoplasm (N) denoting the double duct sign.
MRI is mainly used as an adjuvant to other imaging modalities for planning treatment options. The combination of T1/T2-weighted imaging with magnetic resonance cholangiopancreatography (MRCP) is useful to visualise the primary tumour and its relationship to the biliary and pancreatic ducts, as well as peripancreatic vasculature. MRI/MRCP is considered to be equivalent to CT for assessing primary disease.33,34
Positron emission tomography (PET) shows accumulation of [18F]2-fluoro-2-deoxy-D-glucose (FDG) by tumour cells, and has the advantage of combining metabolic activity and imaging characteristics. PET-CT scanners are able to detect small pancreatic neoplasms up to 7 mm in diameter, and to diagnose metastatic disease in about 40%.35 PET is becoming a more common method of measuring tumour response to treatment and may help predict prognosis. However, FDG-PET is not accurate in pancreatic disease due to its reliance on normal glucose haemostasis. The combination of PET-CT has a sensitivity of 92%, and is superior to either modality alone.36,37
Endoscopic ultrasound (EUS) is becoming more commonly used in staging pancreatic cancer. It provides high-quality images and is noted to be more sensitive than CT for detecting small pancreatic lesions. EUS is also accurate in determining cancer involvement of the portal or splenic vein.38 It has similar efficacy to ERCP in defining small periampullary lesions. EUS may also help clarifying benign conditions mimicking cancer such as sclerosing pancreatitis or atypical choledocholithiasis. Fine-needle aspiration (FNA) of a lesion can be achieved with similar sensitivity and specificity to CT-guided FNA. However, the main drawbacks of EUS include cost, invasiveness and operator dependency.
Advanced staging techniques
Despite advances in non-invasive imaging, laparoscopic staging and ultrasound have a role in selected cases.38,39 Laparoscopy can be performed immediately before conversion to laparotomy40,41 or as an interval staging measure.42
The role of staging laparoscopy is dependent on institution protocol, and there still exists some considerable controversy regarding its use. Its aim is to identify radiographically occult metastatic disease via a minimally invasive approach to prevent non-therapeutic laparotomies. Laparoscopic examination allows for direct visualisation of intra-abdominal organs. It has been shown to be very sensitive in detecting small metastatic deposits < 3 mm on peritoneal and hepatic structures43 (Figs 15.5 and 15.6). The added value of laparoscopy over state-of-the-art dynamic multislice CT remains up to 20%.42
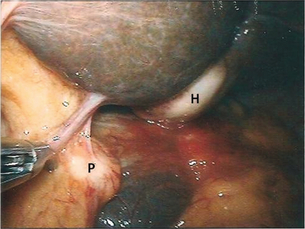
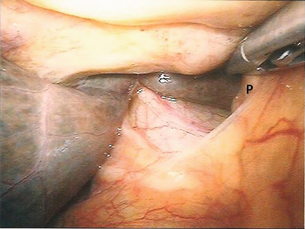
Figure 15.5 Staging laparoscopy demonstrating hepatic metastatic deposit (H) and peritoneal metastatic deposit (P).
Those who oppose minimal access staging suggest that a significant proportion of patients require surgical bypass and therefore laparoscopic staging should only be used if this would not be contemplated at laparotomy.43 Single-centre studies suggest that the need for subsequent operative palliation is less than 5%.44
Peritoneal cytology taken at the time of laparoscopic staging may also improve the accuracy of laparoscopic staging. In a prospective study of 150 consecutive patients with pancreatic carcinoma, unexpected metastases were found in 5–10%.48 Positive cytology is associated with advanced disease; it predicts unresectability of pancreatic adenocarcinoma and a decreased survival. As both radiological and endoscopic imaging will continue to advance, staging laparoscopy and LUS will have a selective role, especially in cases of equivocal findings.
Pathology
Ductal adenocarcinomas account for > 85% of all pancreatic neoplasms.
Treatment
Treatment options should be discussed at a multidisciplinary level, with emphasis on established guidelines. The American Joint Committee on Cancer TMN staging is outlined in Table 15.1. Figure 15.8 outlines our current treatment algorithm for patients with pancreatic cancer.
Table 15.1
American Joint Committee on Cancer TNM staging, 2010
| Tumour (T) | Node (N) | Metastasis (M) |
| Tx: Primary tumour cannot be assessed | Nx: Regional lymph nodes cannot be assesed | |
| T0: No evidence of primary tumour | N0: no regional nodes | M0: no metastases |
| Tis: Carcinoma in situ | ||
| T1: < 2 cm within pancreas | N1: Regional lymph node metastasis | M1: spread to distant organs or non-regional nodes (e.g. aortocaval) |
| T2: > 2 cm within pancreas | ||
| T3: Tumour extends beyond the pancreas but without involvement of the coeliac axis or the SMA | ||
| T4: Tumour involves the coeliac axis or the SMA (unresectable primary tumour) | ||
| Stage 0: Tis, N0, M0 | ||
| Stage IA: T1, N0, M0 | ||
| Stage IB: T2, N0, M0 | ||
| Stage IIA: T3, N0, M0 | ||
| Stage IIB: T1, N1, M0/T2, N1, M0/T3, N1, M0 | ||
| Stage III: T4, Any N, M0 | ||
| Stage IV: Any T, Any N, M1 |
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC, www.springer.com
Resection
If jaundice is present, then the controversy is whether preoperative biliary decompression should be undertaken. Evidence suggests an increased risk of perioperative sepsis, pancreatic fistula and wound infection.49,50 However, a recent meta-analysis questioned these previous findings.51 The authors’ practice is not to decompress the bile duct preoperatively, unless symptoms and signs of cholangitis or secondary signs of hyperbilirubinaemia are present. If a neoadjuvant approach is being considered, biliary stenting is required prior to commencing chemo/radiotherapy. Coagulopathy, if present, is treated with vitamin K prior to resection.
Patient selection is paramount, including cardiovascular and respiratory evaluation. Surgery with curative intent is associated with a median survival of 11–23 months, with approximately 10–18% alive at 5 years.52 Previously, pancreatic resections were associated with significant mortality; however, with advances in perioperative supportive care, mortality rates have now been reduced to less than 5% in high-volume centres.53
Pancreatico-duodenectomy
The remaining porta hepatis is dissected, and nodes are cleared. Cholecystectomy facilitates higher ligation of the bile duct, which is transected just proximal to the insertion of the cystic duct. It is our practice to send a biliary aspirate for routine culture and sensitivity as postoperative infective complications tend to involve enteric organisms.54
Reconstruction is undertaken with the biliary anastomosis followed by the pancreatic and finally the gastric. The most significant cause of morbidity is the development of pancreatic fistula, observed in up to 10–20% of cases.56
Pancreatico-jejunostomy and pancreatico-gastrostomy are the most commonly employed techniques for pancreatico-enteric reconstruction. These suggest a marginal decrease in fistula rates with the former, although differences are not clinically significant and have not induced a change in operative strategy, with jejunal reconstruction still favoured.57
Pylorus-preserving pancreatico-duodenectomy (PPPDR)
Many centres recommend a PPPDR approach, first described by Watson in 1942. It is believed to retain a functioning pylorus with an intact neurovascular supply, thus ensuring good gastrointestinal function and diminishing nutritive, dumping and bile reflux sequelae.59 Both pancreatico-duodenectomy and PPPDR have similar perioperative adverse events; however, in overall analysis PPPDR has decreased operating times, fewer blood transfusions, lower mortality and improved long-term patient survival.59 Detractors of PPPDR point to delayed gastric emptying as a potential cause for concern with this procedure.60
Extended lymph node and vascular dissection
It is the authors’ practice to perform extended dissection including aortocaval nodal clearance in the majority of cases. At presentation, most tumours have involvement of lymph nodes beyond the gland. Ishikawa et al. demonstrated increased median (but not long-term) survival following extended lymph node dissection.61 However, outside of Japan, findings of increased survival have not been validated. Studies have failed to demonstrate increased morbidity that one would expect with a radical operation, although in our experience there is invariably increased ascites in those who undergo extended lymphadenectomy. We believe that clearance of the left gastric and aortocaval nodes increases the specificity of staging and therefore predicted prognosis, and increases the likelihood of a negative surgical margin, although this remains controversial.
The role of extensive vascular resection has been proposed in certain cases. Pancreatico-duodenectomy with major vascular resection had been reported in recent years with acceptable outcomes, despite the increased challenging nature. Survival benefits are slight, and require further investigation.62
Laparoscopic pancreatectomy
Laparoscopic pancreatectomy remains one of the most challenging laparoscopic abdominal operations, and hence case series have low numbers. Two centres have shown total laparoscopic pancreatico-duodenectomy to be safe and feasible, with comparable results to the open approach.63,64 However, laparoscopic distal pancreatic resection is currently the most frequently performed laparoscopic pancreatic operation. It is associated with a higher likelihood of splenic preservation, increased operative time, decreased blood loss and decreased length of stay.63 With the further development of robot-assisted surgery, minimally invasive pancreatico-duodenectomy is likely to become more common.
Central pancreatectomy
The role of central pancreatectomy (CP) is rare and limited due to a low spectrum of indications. The surgery is historically reserved for chronic pancreatitis and traumatic injuries. More recently, it has been advocated for use in lesions of the pancreatic neck. Critics of its role cite higher rates of pancreatic anastomotic leakage. However, CP offers preserved functional elements (endocrine and exocrine) of the pancreas.65
Adjuvant therapies
Gemcitabine is becoming more favoured than 5- fluorouracil (5-FU) because its safety profile is better with similar efficacy.68
The role of adjuvant chemoradiation is less well defined, with conflicting outcomes from the trials. The Gastrointestinal Study Group (GITSG) showed a survival with 5-FU and radiotherapy, but the study size (n = 43) was criticised.69 The European Organization of Research and Treatment of Cancer (EORTC) trial showed no statistical survival benefit for those treated with adjuvant chemoradiation compared with an observation group.70 More recently, the Johns Hopkins–Mayo Clinic Collaborative study demonstrated that chemoradiation post pancreatico-duodenectomy was associated with improved survival.71
Neoadjuvant therapy
In recent years, many centres support the role of neoadjuvant therapy in the treatment of pancreatic cancer. Theoretical advantages include the delivery of chemotherapy or radiotherapy to well-oxygenated tissue, and hence early treatment of micrometastatic disease. Neoadjuvant therapy may help to identify patients who have more aggressive disease, and therefore would not be ideal surgical candidates. There is speculation that neoadjuvant chemoradiation decreases the risk of pancreatic leaks and makes pancreatic reconstruction easier.72 Detractors of neoadjuvant therapy claim that the delay in surgery may allow local disease to progress; however, this is difficult to prove. It has been suggested recently that neoadjuvant treatment should be targeted at patients with borderline pancreatic cancer with the aim to downstage the disease, allowing for resection at later date, with evidence of improved survival rates.72,73
References
1. Jemal, A., Siegel, R., Xu, J., et al, Cancer statistics 2010. CA Cancer J Clin 2010; 60:277–300. 20610543
2. American Cancer Society. Cancer facts and figures 2011. Available online: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2011
3. Cancer Research UKCancer statistics. London: CRUK, 2008.
4. Holly, E.A., Chaliha, I., Bracci, P.M., et al, Signs and symptoms of pancreatic cancer: a population based control study in the San Francisco Bay area. Clin Gastroenterol Hepatol 2004; 2:510–517. 15181621
5. Iodice, S., Gandini, S., Maisonneuve, P., et al, Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393(4):535–545. 18193270
6. Michaud, D.S., Giovannucci, E., Willett, W.C., et al, Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001; 286:921–929. 11509056
7. Dong, J., Zou, J., Yu, X.F., Coffee drinking and pancreatic cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2011;17(9):1204–1210. 21448427
8. Bulathsinghala, P., Syrigos, K.N., Saif, M.W., Role of vitamin D in the prevention of pancreatic cancer. J Nutr Metab 2010; 721365. 21274445
9. Olsen, G.W., Mandel, J.S., Gibson, R.W., et al, A case control study of pancreatic cancer and cigarettes, alcohol, coffee and diet. Am J Public Health 1989; 79:1016–1019. 2751016
10. Ojajarvi, I.A., Partanen, T.J., Ahlbom, A., et al, Occupational exposures and pancreatic cancer: a meta-analysis. Occup Environ Med 2000; 57:316–324. 10769297
11. Hurley, R., Ansary-Moghaddam, A., Berrington de Gonazalez, A., et al, Type II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92:2076–2083. 15886696
12. Hruban, R.H., Canto, M., Goggins, M., et al, Update on familial pancreatic cancer. Adv Surg 2010; 44:293–311. 20919528
13. Hemminski, A., Markie, D., Tomlinson, I., et al, A serine/threonine kinase gene defective in Peutz–Jegers syndrome. Nature 1998; 391:184–187. 9428765
14. Ozcelik, H., Schmocker, B., Di Nicola, N., et al, Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nat Genet 1997; 16:17–18. 9140390
15. Koorstra, J.B., Hustinx, S.R., Offerhaus, G.J., et al, Pancreatic carcinogenesis. Pancreatology 2008; 8:110–125. 18382097
16. Maitra, A., Fukushima, N., Takarori, K., et al, Precursors to invasive pancreatic cancer. Adv Anat Pathol 2005; 12:81–91. 15731576
17. Hruban, R.H., Maitra, A., Goggins, M., Update on pancreatic intra-epithelial neoplasia. Int J Clin Exp Pathol 2008; 1:306–316. 18787611
18. Singh, M., Maitra, A., Precursor lesions of pancreatic cancer: molecular pathology and clinical implications. Pancreatology 2007; 7:9–19. 17449961
19. Cubilla, A.L., Fitzgerald, P.J., Morphological lesions associated with human primary invasive non-endocrine pancreas cancer. Cancer Res 1976; 36:2690–2698. 1277176
20. Aguirre, A.J., Brennan, C., Bailey, G., et al, High resolution characterization of pancreatic adenocarcinoma genome. Proc Natl Acad Sci U S A 2004; 101:9067–9072. 15199222
21. Delpu, Y., Hanoun, N., Lulka, H., et al, Genetic and epigenetic alterations in pancreatic carcinogenesis. Curr Genomics. 2011;12(1):15–24. 21886451
22. Ozaki, N., Ohmuraya, M., Hirota, M., et al, Serine protease inhibitor Kazal type 1 promotes proliferation of pancreatic cancer cells through epidermal growth factor receptor. Mol Cancer Res 2009; 7:1572–1581. 19737965
23. Gullo, L., Tomassetti, P., Migliori, M., et al, Do early symptoms of pancreatic cancer exist that can allow an earlier diagnosis? Pancreas 2001; 22:210–213. 11249079
24. Goonetilleke, K.S., Siriwardena, A.K., Systematic review of carbohydrate antigen (CA19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 2007; 33:266–270. 17097848
25. Safi, F., Schlosser, W., Kolb, G., et al, Diagnostic value of CA 19-9 in patients with pancreatic cancer and nonspecific gastrointestinal symptoms. J Gastrointest Surg 1997; 1:106–112. 9834336
26. Micke, O., Bruns, F., Kurowski, R., et al, Predictive value of carbohydrate antigen 19-9 in pancreatic cancer treated with radiochemotherapy. Int J Radiat Oncol Biol Phys 2003; 57:90–97. 12909220
27. Moossa, A.R., Gamagami, R.A., Diagnosis and staging of pancreatic neoplasms. Surg Clin North Am 1995; 75:871–890. 7660251
28. Gandolfi, L., Torresan, F., Solmi, L., et al, The role of ultrasound in biliary and pancreatic disease. Eur J Ultrasound 2003; 16:141–159. 12573783
29. Karlson, B.M., Ekbom, A., Lindgren, P.G., et al, Abdominal US for diagnosis of pancreatic tumor: prospective cohort analysis. Radiology 1999; 213:107–111. 10540649
30. Barreiro, C.J., Lillemoe, K.D., Koniaris, L.G., Diagnostic laparoscopy for peri-ampullary and pancreatic cancer. What is the true benefit? J Gastrointest Surg 2002; 6:75–81. 11986021
31. Valls, C., Andia, E., Sanchez, A., Dual phase helical CT of pancreatic adenocarcinoma assessment of resectability before surgery. AJR Am J Roentgenol 2002; 178:821–826. 11906855
32. Katz, M.H., Savides, T.J., Moossa, A.R., et al, An evidence-based approach to the diagnosis and staging of pancreatic cancer. Pancreatology. 2005;5(6):576–590. 16110256
33. Schima, W., Függer, R., Schober, E., et al, Diagnosis and staging of pancreatic cancer: comparison of mangafodipir trisodium-enhanced MR imaging and contrast-enhanced helical hydro-CT. AJR Am J Roentgenol 2002; 179:717–724. 12185052
34. Park, H.S., Lee, J.M., Choi, H.K., et al, Pre-operative evaluation of pancreatic cancer; comparison of gadolinium enhanced dynamic MRI with MRCP versus MDCT. J Magn Reson Imaging 2009; 30:586–595. 19711405
35. Maemura, K., Takao, S., Shinchi, H., et al, Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg 2006; 13:435–441. 17013719
36. Wakabayashi, H., Nishiyama, Y., Otani, T., et al, Role of 18F-fluorodeoxyglucose positron emission tomography imaging in surgery for pancreatic cancer. World J Gastroenterol 2008; 14:64–69. 18176963
37. DeWitt, J., Devereaux, B., Chriswell, M., et al, Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med 2004; 141:753–763. 15545675
38. van Dijkum, E.J., de Wit, L.T., van Delden, O.M., et al, Staging laparoscopy and laparoscopic ultrasonography in more than 400 patients with upper gastrointestinal carcinoma. J Am Coll Surg 1999; 189:459–465. 10549734
39. Pisters, P.W., Lee, J.E., Vauthey, J.N., et al, Laparoscopy in the staging of pancreatic cancer. Br Surg 2001; 88:325–337. 11260096
40. White, R., Winston, C., Gonen, M., et al, Current utility of staging laparoscopy for pancreatic and peripancreatic neoplasm. J Am Coll Surg 2008; 206:445–450. 18308214
41. Holzman, M.D., Reintgen, K.L., Tyler, D.S., et al, The role of laparoscopy in the management of suspected pancreatic and periampullary malignancies. J Gastrointest Surg 1997; 1:236–244. 9834353
42. Jimenez, R.E., Warshaw, A.L., Rattner, D.W., et al, Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg 2000; 135:409–415. 10768705
43. Conlon, K.C., Dougherty, E., Klimstra, D.S., et al, The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223(2):134–140. 8597506
44. Espat, N.J., Brennan, M.F., Conlon, K.C., Patients with laparoscopically staged unresectable pancreatic adenocarcinoma do not require subsequent surgical biliary or gastric bypass. J Am Coll Surg 1999; 188:649–657. 10359358
45. Jeurnink, S.M., Eijck, C., Steyerberg, E.W., et al, Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol 2007; 7:18. 17559659
46. Lillemoe, K.D., Cameron, J.L., Hardacre, J.M., et al, Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg 1999; 230:322–330. 10493479 Two key papers arguing the role for and against prophylactic gastroenterostomy in palliation of pancreatic cancer.
47. Barabino, M., Santambrogio, R., Ceretti, A.P., et al, Is there still a role for laparoscopy combined with ultrasonography in the staging of pancreatic cancer. Surg Endosc 2011; 25:160–165. 20567851
48. Schmidt, J., Fraunhofer, S., Fleisch, M., et al, Is peritoneal cytology a predictor of unresectability in pancreatic carcinoma? Hepatogastroenterology. 2004;51(60):1827–1831. 15532836
49. Pisters, P.W., Hudec, W.A., Hess, K.R., et al, Effect of preoperative biliary decompression on pancreatico-duodenectomy-associated morbidity in 300 consecutive patients. Ann Surg 2001; 234:47–55. 11420482
50. van der Gaag, N.A., Kloek, J.J., de Castro, S.M., et al, Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg 2009; 13:814–820. 18726134
51. Wang, Q., Gurusamy, K.S., Lin, H., et al, Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev 2008; CD005444.. 18677779
52. Katz, M.H., Wang, H., Fleming, J.B., et al, Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 2009; 16:836–847. 19194760
53. McPhee, J.T., Hill, J.S., Whalen, G.F., et al, Perioperative mortality for pancreatectomy: a national perspective. Ann Surg 2007; 246:246–253. 17667503
54. Povoski, S.P., Karpeh, M.S., Conlon, K.C., et al, Pre-operative biliary drainage: impact on intraoperative bile cultures and infectious morbidity after pancreaticoduodenectomy. J Gastrointest Surg. 1999;3(5):496–505. 10482706
55. Verbeke, C.S., Menon, K.V., Redefining resection margin status in pancreatic cancer. HPB (Oxford). 2009;11(4):282–289. 19718354
56. Stojadinovic, A., Brooks, A., Hoos, A., et al, An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. Am Coll Surg. 2003;196(6):954–964. 12788434
57. Yeo, C.J., Cameron, J.L., Maher, M.M., et al, A prospective randomized trial of pancreatico-gastrostomy versus pancreatico-jejunostomy after pancreatico-duodenectomy. Ann Surg 1995; 222:580–592. 7574936
58. Conlon, K.C., Labow, D., Leung, D., et al, Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg 2001; 234:487–494. 11573042
59. Wenger, F.A., Jacobi, C.A., Haubold, K., et al, Gastrointestinal quality of life after duodenopancre- atectomy in pancreatic carcinoma. Preliminary results of a prospective randomized study: pancreatoduodenectomy or pylorus-preserving pancreatoduodenectomy. Chirurg 1999; 7:1454–1459. 10637702
60. Cooperman, A.M., Kini, S., Snady, H., et al, Current surgical therapy for carcinoma of the pancreas. J Clin Gastroenterol 2000; 31:107–113. 10993424
61. Ishikawa, O., Ohigashi, H., Sasaki, Y., et al, Adjuvant therapies in extended pancreatectomy for ductal adenocarcinoma of the pancreas. Hepatogastroenterology 1998; 45:644–650. 9684110
62. Al-Haddad, M., Martin, J.K., Nguyen, J., et al, Vascular resection and reconstruction for pancreatic malignancy: a single center survival study. J Gastrointest Surg 2007; 11:1168–1174. 17632763
63. Kendrick, M.L., Cusati, D., Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010; 145:19–23. 20083750
64. Palanivelu, C., Jani, K., Senthilnathan, P., et al, Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg 2007; 205:222–230. 17660068
65. Christein, J.D., Smoot, R.L., Farnell, M.B., Central pancreatectomy: a technique for the resection of pancreatic neck lesions. Arch Surg 2006; 141:293–299. 16549696
66. Oettle, H., Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer. JAMA 2007; 297:267–277. 17227978
67. Neoptolemos, J., Büchler, M., Stocken, D.D., et al. ESPAC-3(v2): a multicenter, international, open-label, randomized, controlled phase III trial of adjuvant 5-fluorouracil/folinic acid (5-FU/FA) versus gemcitabine (GEM) in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 27(Suppl. 18), 2009. [Abstract].
68. Regine, W.F., Winter, K.A., Abrams, R.A., et al, Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008; 299:1019–1026. 18319412
69. Neoptolemos, J.P., Stocken, D.D., Friess, H., et al, A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. 15028824
70. Klinkenbijl, J.H., et al, Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer co-operative group. Ann Surg. 1999;230(6):776–782. 10615932
71. Hsu, C.C., Herman, J.M., Corsini, M.M., et al, Adjuvant chemoradiation and chemotherapy for pancreatic adenocarcinoma: the Johns Hopkins–Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17(4):981–990. 20087786
72. Katz, M.H., Fleming, J.B., Lee, J.E., et al, Current status of adjuvant therapy for pancreatic cancer. Oncologist 2010; 15:1205–1213. 21045189
73. Callery, M.P., Chang, K.J., Fishman, E.K., et al, Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009; 16:1727–1733. 19396496