Chapter 110 Panax ginseng (Korean Ginseng)
Panax ginseng C.A. Meyer (family: Araliaceae)
Common names: Korean ginseng, Chinese ginseng, Asiatic ginseng, Oriental ginseng
 General Description
General Description
• Panax quinquefolius (American ginseng)
• Panax japonicus C.A. Meyer (Japanese ginseng)
P. ginseng C.A. Meyer is the most widely used and most extensively studied species.1,2
Its pharmacology is the major focus of this chapter.
Ginseng is often processed in two forms, white and red. White ginseng is the dried root whose peripheral skin is frequently peeled off. Red ginseng is the steamed root, which has a caramel-like color.2
There are many types and grades of ginseng and ginseng extracts, which vary according to the source, age, and parts of the root used, where it was grown, the time of harvesting, and the methods of preparation.1–3 Old, wild, well-formed roots are the most valued, whereas rootlets of cultivated plants are considered the lowest grade. High-quality preparations are usually in the form of extracts of the main root of plants between 4 and 6 years old that have been standardized for ginsenoside content (see later) and ratio to ensure optimum pharmacologic effect.
 Chemical Composition
Chemical Composition
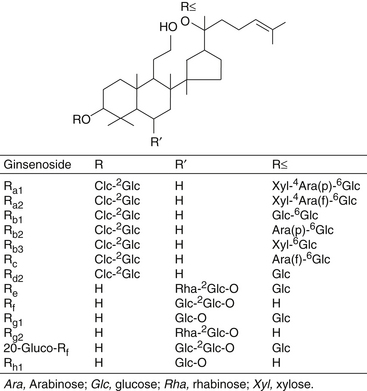
As can be seen from Figure 110-1, the ginsenosides differ primarily in their sugar groups.
Ginsenosides Rb1, Rb2, Rc, Re, and Rg1 are present in significant concentrations in Korean ginseng. In contrast, American ginseng (P. quinquefolius) contains primarily ginsenosides Rb1 and Re and does not contain ginsenosides Rf, Rb2, or, in some instances, Rg1.4 These features allow for easy detection of species with high-pressure liquid chromatography.
Other components of ginseng are as follows1,2:
• Free and glucoside-bound sterols (e.g., β-sitosterol and its β-glucoside)
• Polyacetylene derivatives β-elemene and panaxynol
• Low-molecular-weight polysaccharides
• Vitamins (e.g., thiamine, riboflavin, B12, nicotinic acid, pantothenic acid, biotin)
• Minerals (including germanium)5
• Simple sugars (glucose, fructose, sucrose, maltose, trisaccharides, etc.)
• Unique proteins (e.g., panaxagin, a protein that possesses antifungal, antiviral, translation-inhibiting, and ribonuclease activities)6
Although it was reported that ginseng contains large amounts of germanium (i.e., 300 ppm), a follow-up study using highly sensitive (detection limit of 1 ppb), flameless atomic absorption spectrometry combined with solvent extraction demonstrated that the highest concentration of germanium measured in samples of ginseng purchased in the Osaka market was only 6 ppb.5 More research is needed to accurately determine the germanium content of botanical medicines, because the reported concentrations vary widely. Such low levels suggest that a connection between the pharmacology of ginseng and its germanium content is unlikely.
 History and Folk Use
History and Folk Use
Perhaps the most famous medicinal plant of China, ginseng has been generally used alone or in combination with other herbs to restore the “Yang” quality. It has also been used as a tonic for its revitalizing properties, especially after a long illness. Conditions for which ginseng is used in folk medicine are shown in Box 110-1. It has been used as an alterative, anodyne, aperitif, aphrodisiac, cardiotonic, carminative, emetic, estrogenic, expectorant, gonadotrophic, nervine, sedative, sialogogue, stimulant, stomachic, and tranquilizer.1,2 As can be seen from this list, ginseng has been used for most conditions, reflecting a broad range of nutritional and medicinal properties.
 Pharmacology and Clinical Indications
Pharmacology and Clinical Indications
Over the years, ginseng has been reported to have numerous pharmacologic effects in humans and laboratory animals, including the following1,2,7:
• General stimulatory effects during stress
• Decrease in sensitivity to stress
• Increase in mental and physical capacity for work
• Improved endocrine system function
• Amelioration of radiation sickness, experimental neurosis, and cancer
• Enhanced protein synthesis and cell reproduction
• Improved glucose control in diabetes
• Modulation of various immune system parameters
Some of these actions are discussed in greater detail in the following sections.
Adaptogenic and Antistress Activity
Ginseng was originally investigated for its adaptogenic qualities. An adaptogen was defined in 1957 by the Russian pharmacologist I.I. Brekhman2 as a substance with the following properties:
• It must be innocuous and cause minimal disorders in the physiologic functions of an organism.
• It must have a nonspecific action (i.e., it should increase resistance to adverse influences through a wide range of physical, chemical, and biochemical factors).
• It usually has a normalizing action irrespective of the direction of the pathologic state.
According to tradition and scientific evidence, ginseng possesses this kind of equilibrating, tonic, antistress action, and so the term adaptogen is quite appropriate to describe its general effects.2,7,8
Ginseng delays the alarm phase response in Selye’s classic model of stress. Animal studies found that adrenal cholesterol levels were many times higher in animals given ginseng than in their matched controls, indicating greater tolerance of stress and delayed alarm phase response.9–11
Italian researchers studied the effect of a standardized ginseng extract, the ginsenoside composition of which was accurately determined, on the adrenal functions of rats exposed to cold.10 The ginseng extract significantly counteracted body temperature decline without affecting blood glucose or cortisone levels. In a group of adrenalectomized rats, the ginseng extract had no significant effects. Administration of hydrocortisone to the adrenalectomized rats did, however, cause body temperature to be maintained when the rats were exposed to cold.
Histologic findings in this study were as follows:
• Evidence of hyperfunctioning in the supraoptic and paraventricular nuclei of the hypothalamus in rats fed the ginseng extract
• Remarkable increase in corticotropic basophilic cells (adrenocorticotropic hormone [ACTH] producing) in the pars distalis of the pituitary gland
• Hyperplasia of the adrenal zona fasciculata, indicating that hyperfunctioning of the adrenal was promoted by the administration of the ginseng extract
Other researchers demonstrated that ginseng saponins significantly increased plasma ACTH and corticosteroids (in a parallel kinetic pattern).12,13 Because this effect could be blocked by dexamethasone (which acts on the hypothalamus and pituitary to prevent ACTH release), it was concluded that ginsenosides act predominantly on the hypothalamus or pituitary to promote secretion of ACTH. This conclusion was confirmed further by indirect studies. ACTH first stimulates an increase in cyclic adenosine monophosphate (cAMP) in the adrenal and then promotes corticosteroid synthesis. Ginseng administration was shown to increase adrenal cAMP in normal rats, but not in hypophysectomized rats.
• The antistress action of ginseng is greatly reduced by adrenalectomy.
• Ginseng continues to exert its antistress action after hypophysectomy only if ACTH is administered.
• Histologic and chemical evidence demonstrates a strong link between ginseng and the hypothalamic-pituitary-adrenal axis.
Ginseng may prove especially effective in restoration of normal adrenal function and prevention of adrenal atrophy associated with corticosteroid administration. In rats, ginseng was found to inhibit cortisone-induced adrenal and thymic atrophy.14
Antifatigue (Mental and Physical) Activity
Some of the first studies of ginseng’s adaptogenic activities were performed during the late 1950s and early 1960s by Brekhman and Dardymov7,8 in the Soviet Union and by Petkov15–17 in Bulgaria.
In one of Brekhman’s experiments, Soviet soldiers given an extract of ginseng ran faster in a 3-km race than those given a placebo. In another, radio operators tested after administration of ginseng extract transmitted text significantly faster and with fewer mistakes than those given placebo. These and similar results reported by European researchers, who demonstrated improvement in human physical and mental performance after administration of ginseng extracts, prompted researchers to confirm the results in experimental models using mice.2,7,8,15
In perhaps the best known of these experiments, mice were subjected to swimming in cold water or running up an apparently endless rope to determine whether ginseng could lengthen the time to exhaustion. The results indicated that ginseng possessed significant antifatigue activity, because a clearly dose-dependent increase in time to exhaustion was noted in mice receiving ginseng.2,8,18–20 In one study, the time to exhaustion was lengthened by up to 183% in the mice given ginseng 30 minutes before exercising compared with controls.8
Experimental animal studies indicated that much of the antifatigue action of ginseng was due to the stimulant effect of ginseng on the central nervous system (CNS). Stress coupled with ginseng ingestion induced alterations in energy metabolism during prolonged exercise.9,16–20
Ginseng has been shown to improve locomotor activity,21 modify electroencephalographic (EEG) tracings,16 improve metabolic activity in the CNS,22 and affect the hypothalamic-pituitary-adrenal axis (discussed later), all of which could be largely responsible for ginseng’s antifatigue activity in mental and physical performance. The CNS activity of ginseng is essentially different from that of usual stimulants. Although stimulants are active under most situations, ginseng reveals its stimulatory action only with the challenge of stress.22
On the physical level, ginseng’s antifatigue properties appear to be closely related to its ability to spare glycogen utilization in exercising muscle.9 Exercise physiologists clearly established that during prolonged exercise, the development of fatigue is closely related to the depletion of glycogen stores and the buildup of lactic acid, both in skeletal muscle and in the liver. If an adequate supply of oxygen is available to the working muscle, nonesterified fatty acids are the preferential energy substrate, thus sparing utilization of muscle glycogen, blood glucose, and, consequently, liver glycogen. The greater the ability to conserve body carbohydrate stores through mobilization and oxidizing of fatty acids, the greater the amount of time to exhaustion. Ginseng enhances fatty acid oxidation during prolonged exercise, thereby sparing muscle glycogen stores.9
Mental and physical antifatigue activity effects were demonstrated in both animal studies and double-blind clinical trials in humans. In one double-blind clinical study, nurses who switched from day to night duty rated themselves for competence, mood, and general well-being, and were evaluated with an objective test of psychophysical performance, blood cell counts, and blood chemistry analysis. The group administered ginseng demonstrated higher scores in competence, mood parameters, and objective psychophysical performance than those who received a placebo.23
From a clinical standpoint, ginseng’s antifatigue properties may be useful whenever fatigue or lack of vigilance is apparent. In particular, cancer patients who underwent chemotherapy, patients with chronic obstructive pulmonary disease (COPD), and athletes were all shown to benefit from ginseng use. In regards to COPD, in one double-blind study, 92 adults with COPD were randomly assigned to receive either ginseng or placebo.24 Pulmonary function tests, maximum voluntary ventilation, maximum inspiratory pressure, and maximal oxygen consumption were studied before treatment and every 2 weeks for the 3-month study. In the ginseng group, but not in the control group, all parameters were significantly higher than baseline and higher than the parameters in the placebo group. Maximum increases, in comparison with baseline, were forced vital capacity 32.5%, forced expiratory volume1.0 27.0%, maximum voluntary ventilation 40.4%, maximum inspiratory pressure 47.0%, and maximal oxygen consumption 37.5%. No side effects were observed.
In a large study in 290 cancer patients, subjects were randomized in a double-blind manner to receive American ginseng in doses of 750, 1000, or 2000 mg/day or placebo given in twice daily dosing over 8 weeks. Over twice as many patients on ginseng perceived a benefit and were satisfied with treatment over those on placebo.25 In another double-blind trial, 53 cancer patients were randomly assigned to receive ginseng (3000 mg/day) or placebo for 12 weeks. Quality of life was assessed using the World Health Organization Quality of Life Assessment-BREF (WHOQOL-BREF) and the General Health Questionnaire-12.26 After 12 weeks of therapy, the “psychological domain ” score of the WHOQOL-BREF was significantly improved in patients randomized to ginseng, compared with those randomized to placebo. There was a tendency for ginseng to improve the “physical health” and “environment” domain scores of the WHOQOL-BREF, compared with placebo. The General Health Questionnaire-12 total score was significantly improved in patients treated with ginseng than in those with placebo. These results from clinical trials indicate ginseng is of benefit in improving some aspects of mental and physical functioning in cancer patients.
Cognitive Performance
Standardized extracts of P. ginseng alone or in combination with Ginkgo biloba (a formula called Gincosan) were shown to improve cognitive performance in animal and clinical studies.27–36 In one of the earlier studies (a double-blind, crossover design) in university students in Italy, ginseng extract alone was compared with placebo in various tests of psychomotor performance. A favorable effect of ginseng relative to baseline performance was observed in attention (cancellation test), mental arithmetic, logical deduction, integrated sensorimotor function (choice reaction time), and auditory reaction time. However, statistically significant superiority of the ginseng group over the placebo group was noted only for mental arithmetic. It was interesting to note that during the course of the trial, the students taking ginseng reported a greater sensation of well-being.29 Later studies in college-aged students showed similar results with a clear dose dependency.30–32 However, unlike studies in older subjects, no positive effect on mood or quality of life has yet been demonstrated with ginseng administered to healthy young adults.
The benefits of ginseng on cognitive performance, mood, and quality of life assessment are considerably greater in middle-aged adults than in younger adults. In several studies of the administration of ginseng alone or in combination with G. biloba extract, significant improvements were noted, not only in tests of cognition and memory, but also in social functioning and mental health.33–35 In one study, however, the results attenuated after 4 weeks of use, indicating that cotherapy with G. biloba may be more efficacious or that cycling of the ginseng dosage may be necessary to produce a prolonged effect.35
Both G. biloba and P. ginseng have been shown to modulate aspects of cognitive performance, including effects on EEG recordings. One double-blind, placebo-controlled, balanced crossover experiment assessed, in 15 healthy volunteers, the effects of single doses of G. biloba extract (360 mg), a proprietary P. ginseng extract (200 mg G115), and an identical placebo on auditory-evoked potentials, contingent negative variation, and resting power within the δ, θ, α, and β wavebands.36 The results showed that ginseng use led to a significant shortening of the latency of the P300 component of the evoked potential. Both ginseng and ginkgo also led to significant reductions in frontal “eyes closed” θ and β activity, with additional reduction for ginseng in the α waveband. These findings demonstrated for the first time that P. ginseng can directly modulate EEG activity, and that these effects are more pronounced than those that follow ingestion of G. biloba. Additional studies with G115 also showed an ability to enhance cognitive function in normal subjects.37–39
In regards to improving cognitive performance in Alzheimer’s disease (AD), there appears to be some benefit based upon the results from an open label study.40 In the study, consecutive AD patients were randomly assigned to the ginseng (n = 58) or the control group (n = 39), and the ginseng group was treated with P. ginseng powder (4.5 g/day) for 12 weeks. Cognitive performances were monitored using the Mini-Mental State Examination (MMSE) and Alzheimer’s disease assessment scale (ADAS) during 12 weeks of the ginseng treatment and for 12 weeks after the ginseng discontinuation. MMSE and ADAS scales showed no baseline difference between the groups. After ginseng treatment, the cognitive subscale of ADAS and the MMSE scores began to show improvements and continued up to 12 weeks. After discontinuing ginseng, the improved ADAS and MMSE scores declined to the levels of the control group. These results suggest that P. ginseng is clinically effective in the cognitive performance of AD patients.
As an Ergogenic Aid
The benefits of ginseng on athletic performance are not clear. Results of later double-blind studies were negative. For example, one study, in which 36 healthy young men received a standardized ginseng extract (Ginsana) at a dosage of 400 mg/day, showed that ginseng had no effect on any exercise-related parameter (e.g., blood lactic acid concentration, heart rate, and perceived exertion).41 Similar results were seen in a study in women, and an even more comprehensive laboratory evaluation in 38 healthy adults also showed no improvement in any parameter.42,43 The totality of results at this time do not offer support for claims that ginseng improves athletic performance. However, other benefits were noted, such as an ability to protect against muscle injury and inflammation in athletes, reduced exercise-induced oxidative stress, reduced post-exercise plasma creatine kinase levels, as well as improved psychomotor performance at rest during a graded exercise test.44–47
Diabetes
Ginseng, used either alone or in combination with other botanicals, has a long folk use in the treatment of diabetes. This hypoglycemic activity was confirmed in both experimental and clinical trials. In one double-blind study in type 2 diabetes, ginseng therapy reduced fasting blood glucose levels, elevated mood, improved psychophysical performance, and lowered body weight and glycosylated hemoglobin.48
The chief constituents responsible for this effect are as follows49–53:
The ratio of ginsenosides also appears to be an important factor. In one human study, the ratio of protopanaxadiol/protopanaxatriol was the sole predictor of whether or not a ginseng preparation would exert hypoglycemic effects.54 It appears that it is important to utilize crude, standardized extracts having a higher protopanaxadiol/protopanaxatriol ratio, such as those from American ginseng extract, to produce the desired benefit.
Powdered American ginseng root (P. quinquefolius) at a dosage of about 3 g before each meal was shown to reduce postprandial blood sugar significantly in patients with type 2 diabetes. It was determined that American ginseng worked through the stimulation of pancreatic β-cells, with a subsequent increase in insulin secretion. This substance also has significant antioxidant properties and improves cognitive function. In addition, American ginseng was shown to possess nerve protection and regeneration properties. This could prove to be valuable in diabetes, in which peripheral and autonomic nerve damage so often occurs.55–57
It is interesting to note that ginseng raises serum cortisol levels in nondiabetic individuals but reduces serum cortisol levels in patients with diabetes.58 Because cortisol antagonizes insulin, this is presumably a beneficial effect. This action again demonstrates ginseng’s nonspecific balancing effect, which is baffling to researchers accustomed to investigating compounds with consistent pharmacologic effects.
In double-blind, randomized, crossover design study in 19 patients with well-controlled type 2 diabetes, each participant received the selected ginseng preparation (rootlets) and placebo at the selected dose (2 g/meal = 6 g/day) and mode of administration (preprandial oral agent [−40 minutes]) for 12 weeks as an adjunct to their usual antidiabetic therapy (diet and/or medications). Although there was no change in the primary end point, glycosylated hemogloblin, ginseng treatment decreased 75 g oral glucose tolerance test plasma glucose (OGTT-PG) indexes by 8% to 11% and fasting plasma insulin (PI) and 75 g OGTT-PG indexes by 33% to 38%, and increased the fasting insulin sensitivity index and 75 g OGTT-insulin sensitivity index by 33%, compared with placebo.59
In another double-blind study, improvements were seen with American ginseng supplementation on fasting blood sugar levels and insulin resistance in type 2 diabetic patients. Proposed mechanisms included effects on insulin release from pancreatic β-cells, insulin-stimulated glucose disposal, and increasing insulin sensitivity related to the peroxisome proliferator-activated receptor.60
Based upon current information, ginseng appears useful as an adjunctive therapy in the treatment of diabetes, both for its antihyperglycemic effect and for its ability to decrease the atherogenic index (see later). Although the root has received the most attention, animal studies indicate that extracts from the ginseng berry may even be more beneficial due to a higher concentration of ginsenoside Re.58,61
Reproductive Effects
Although it is claimed to be a “sexual rejuvenator” and aphrodisiac, human studies supporting this belief are scanty. In animal studies, however, ginseng was shown to have the following effects62,63:
• Promotes the growth of the testes and increases spermatogenesis in rabbits
• Accelerates the growth of the ovary and enhances ovulation in frogs
• Stimulates egg-laying in hens
• Facilitates lordotic response in female rats
• Increases gonadal weight in both male and female rats
• Raises testicular nucleic acid content in rats
• Increases sexual activity and mating behavior in male rats
These animal study results seem to support ginseng’s use as a fertility and virility aid.
In other experimental animal studies, ginseng was shown to increase testosterone levels while reducing prostate weight.64 This finding suggests that ginseng should have favorable effects in the treatment of benign prostatic hyperplasia; however, no clinical trials have yet been reported.
Ginsenosides were also shown to bind to human myometrial receptor proteins, and they apparently exert estrogen-like action on the vaginal epithelium. These activities are significant enough to prevent the atrophic vaginal changes associated with postmenopause and other menopausal symptoms.65
Other clinical indications involving the reproductive system (based on historical use and experimental evidence) are decreased sperm counts, testicular atrophy or hypofunction, other organic causes of male infertility, ovarian atrophy or hypofunction, amenorrhea, and other organic causes of female infertility. It should be noted that several reports of mastalgia were reported in women taking ginseng.66,67
Most of the later clinical studies involving reproductive effects focused on ginseng’s effect on erectile dysfunction (ED). A plausible mechanism involves evidence that ginsenosides can facilitate penile erection by directly inducing the vasodilatation and relaxation of penile corpus cavernosum, which is mediated by the release and/or modification of the release of nitric oxide from endothelial cells and perivascular nerves.68–70
In one double-blind, crossover study, 45 patients with clinically diagnosed ED showed significantly higher mean International Index of Erectile Function (IIEF) scores with Korean red ginseng than those who received placebo. In response to the global efficacy question, 60% of the patients answered that Korean red ginseng improved erection.70
In a double-blind, placebo-controlled trial, a tissue-cultured mountain ginseng extract (TMGE) or placebo was given to 86 male patients with ED. Over the course of 8 weeks, one group took 1000 mg of TMGE twice a day, and the other group took 1000 mg of placebo twice a day. The effects of the TMGE and the placebo were analyzed using the IIEF questionnaire. All 86 patients completed 8 weeks of treatment. The scores on the five domains of the IIEF after medication were significantly higher than the baseline scores in the group treated with TMGE, whereas no significant improvement was observed in the placebo group. Erectile function and overall satisfaction scores after medication were significantly higher in the TMGE group than in the placebo group.71
In another double-blind study, 60 patients presenting with mild or mild to moderate ED were given either P. ginseng at a dosage of 1000 mg three times daily or a placebo. The IIEF score after treatment was significantly higher in the ginseng group compared with that before the treatment. In contrast, there was no difference before and after the treatment in the placebo group.72
Menopause
Ginseng has also been a popular treatment for menopausal symptoms; however, clinical research is unconvincing. One double-blind study in 384 menopausal women was performed to compare the effects of a standardized ginseng extract with those of a placebo on quality of life, menopausal symptoms, and physiologic markers of menopause (follicle-stimulating hormone and estradiol levels, endometrial thickness, maturity index, and vaginal pH). Although the ginseng extract showed no statistically significant effects on the physiologic parameters, including vasomotor symptoms (hot flashes), it was associated with statistically significant improvements in mood and well-being.73
Cell-Proliferating, Antioxidant, and Antiaging Effects
Ginseng has a dual effect on cell growth: it stimulates cell division in an adequate nutritional environment but acts cytostatically under adverse conditions (as described in “Diabetes” section).73 Furthermore, ginseng yielded impressive results in lengthening the lifespan of cells in culture.74
This enhancement of cellular proliferation and function was shown in a variety of cell types (epithelial, hepatic, lymphocyte, fibroblast, thymic, etc.), but especially in nerve cells, which appears to be a result of potentiation of nerve growth factor by ginsenosides.3,75–77
Clinically, these results indicate a potential use of ginseng in healing damage to virtually all tissue types, but especially the brain. In animal studies, ginseng was shown to reduce oxidative stress, scavenge free radicals directly, protect endothelial cells from damage, and increase cellular antioxidant enzymes like superoxide dismutase.78–83 Again, these effects demonstrate ginseng’s nonspecific action. Its effect on improving arterial endothelial function is especially novel.84
Immunomodulating Effects
That ginseng possesses immunomodulating activity is evidenced by its ability to enhance the following immune functions85–92:
• Antibody plaque-forming cell response
• Circulatory antibody titer against sheep erythrocytes
• Natural killer (NK) cell activity
• The production of interferon
• Reticuloendothelial system proliferative and phagocytic functions
In addition to these immunostimulatory aspects, ginseng components were shown to exert antiallergy and anti-inflammatory effects in experimental models.93,94
From a clinical perspective, the long-term ingestion of ginseng by individuals with mild immunodeficiency may reduce the risk of viral infection. Its use in this manner is consistent with the historic use of ginseng by debilitated individuals. There is clinical evidence of benefit in these applications. Extracts of P. ginseng were found to stimulate NK function in normal individuals and patients with either chronic fatigue syndrome or AIDS.95 Ginseng was shown to prevent respiratory viral infections in a nursing home environment (89% relative risk reduction) and potentiate influenza vaccinations.96,97
It should be noted that large dosages of ginseng may be contraindicated in acute infections. The reason may be its in vitro inhibition of lymphocyte transformation (similar to cortisone) at high (i.e., more than 1 mg/mL), but not low concentrations.98,99 In vitro, ginseng at 1.6 mcg/mL was shown to inhibit phytohemagglutinin-induced transformation of peripheral blood lymphocytes to a greater degree than cortisone at 500 mcg/mL.98 The greatest level of inhibition, however, was observed when ginseng was used in combination with cortisone. These results suggest that ginseng at high doses may be effective against T-cell–mediated inflammatory diseases without producing glucocorticoid-like side effects. It also suggests that a lower dose of cortisone could be used if ginseng were given simultaneously.
When using ginseng, the clinician must remember: (1) that ginseng’s in vitro effect on lymphocyte proliferation is biphasic, that is, it exerts a strong inhibition at high concentrations and a moderate stimulation at low concentrations; and (2) that although ginseng has demonstrated significant inhibition of lymphocyte proliferation in vitro, this has not been the observed effect in vivo, in which lymphocyte proliferation has been enhanced.87 These effects may be related to a dose-dependent ginseng-induced elevation of interferon (which inhibits lymphocyte proliferation).
Anticancer Properties
Regular ginseng consumption may protect against cancer. In two large population studies, the risk for development of cancer was significantly lower among people who consumed ginseng on a regular basis. Ginseng extract and powder were shown to be more effective than fresh sliced ginseng, ginseng juice, or ginseng tea in reducing cancer risk. A statistically, highly significant dose–response relationship between ginseng intake and cancer risk was observed. These results support the preventive and anticancer effects of ginseng demonstrated in animal and in in vitro studies.100–102
Long-term oral administration of ginseng to newborn mice was shown to reduce the incidence and also to inhibit the proliferation of tumors induced by various chemical carcinogens, including 7,12-dimethylbenz[a]anthracene, urethane, and aflatoxin B1.45
The anticancer effects of ginseng in other experimental models can be summarized as follows102–106:
• The effect is observed only in slow-growing tumors, such as Ehrlich and sarcoma 180 ascites tumor.
• The effect is not observed in rapidly growing tumors, such as l1210, p388, and Walker carcinoma.
• There is no dose–response relationship or cumulative effect.
In addition to a possible role in cancer prevention, some researchers are investigating P. ginseng extracts as a possible adjunct in cancer treatment.107 Exerting some direct and indirect anticancer actions, ginseng may also offer protection against chemotherapy-induced damage to normal cells. In one animal study, P. ginseng extract was shown to protect against cisplatin nephrotoxicity.108
Cardiovascular Effects
From a clinical perspective, ginseng may offer some protection against atherosclerotic disease, further supporting its use as a general tonic. It may also possess a blood pressure–regulating effect via improvement in the endothelial dysfunction underlying many cases of hypertension.109
Ginseng administered to human subjects with hyperlipidemia was shown to reduce total serum cholesterol, triglycerides, and nonesterified fatty acid levels while raising serum high-density lipoprotein cholesterol levels. Platelet adhesiveness was also decreased.110
These results in humans confirmed earlier studies on rats fed high-cholesterol diets.111,112 The mechanism of action appears to be through accelerated degradation, conversion, and excretion of cholesterol and triglycerides despite increased lipogenesis and cholesterogenesis. Ginseng was also shown to be effective in inhibiting platelet aggregation and the conversion of fibrinogen to fibrin.113
Hepatic Effects
Obviously, any adaptogenic substance must affect the liver because of this organ’s central role in metabolic and detoxification reactions. Ginseng affects the liver in several ways. Perhaps the most important is its ability to produce a marked hyperplasia of the Kupffer cells of the liver and of the folliculi in the spleen and lymph nodes.10 The hyperplastic folliculi show an increase in the number and volume of light centers, thus demonstrating morphologic evidence of increased host defense capacity against a wide variety of external assaults. Because these cells are responsible for filtering out much of the toxins and debris from the portal circulation, increasing their number and activity could have profound effects.
Ginseng was also shown to increase nuclear RNA biosynthesis, indicating greater protein synthesis.3,114 Ginseng was shown to increase not only nuclear RNA synthesis, but also ribosomal and messenger RNA, the amount of rough endoplasmic reticulum, and the activity of RNA polymerase.3,114–116 These results indicate that ginseng activates virtually every step in protein biosynthesis. Since protein synthesis is often reduced in the elderly, the significance of the described effects on enhancement of hepatic protein synthesis could be extremely high. However, these results have yet to be confirmed by clinical studies.
Ginseng was also shown to reverse diet-induced fatty liver in animals and to possess significant antihepatotoxic action.117 The clinical indications of these hepatic actions of ginseng are quite broad and support its general tonic/adaptogen properties.
Radiation-Protecting Effects
Ginseng was shown to offer some protection against harmful radiation, both in vivo and in vitro, and to hasten recovery from radiation sickness.118,119 In the presence of ever-increasing environmental radiation contamination, ginseng may be an appropriate prophylactic against radiation exposure.
 Toxicology
Toxicology
The problem of quality control makes toxicology difficult to address. This is exemplified by a 1979 article in the Journal of the American Medical Association titled “Ginseng Abuse Syndrome.”120 In this article, a number of side effects of commercial preparations of ginseng were reported, including the following:
Studies performed on standardized extracts of ginseng demonstrated the absence of side effects and mutagenic or teratogenic effects.121–123 These studies differed markedly from the trial reported in JAMA, in that high-quality extracts were used. An extensive review of the safety of P. ginseng concluded, “Data from clinical trials suggest that the incidence of adverse events with ginseng monopreparations is similar to that with placebo.”120
 Dosage
Dosage
Currently, there is almost a total lack of quality control in ginseng products marketed in the United States. Independent research and published studies clearly documented a tremendous variation in the ginsenoside content of commercial preparations. Many products on the market contain only trace amounts of ginsenosides, and some formulations contain no ginseng at all. This situation has led to several problems, ranging from toxicity reactions (discussed later) to lack of medicinal effect. The widespread disregard for quality control in the health food industry has done much to tarnish the reputation of ginseng as well as other important botanicals.
We recommend the use of standardized ginseng preparations to ensure sufficient ginsenoside content, consistent therapeutic results, and reduced risk of toxicity. Products should be standardized in their ginsenoside content. The typical dose (taken one to three times daily) for general tonic effects should contain a saponin content of at least 5 mg of ginsenosides with an Rb1/Rg1 ratio of 2:1. For example, for a high-quality ginseng root powder containing 5% ginsenosides, the dose would be 100 mg.123
 Drug Interactions
Drug Interactions
Taking ginseng preparations may increase the effectiveness of insulin and drugs that lower blood sugar levels such as glyburide (Diabeta, Micronase). It is important to discuss proper monitoring of blood sugar levels with patients who have diabetes before prescribing ginseng. P. ginseng may potentiate the monoamine oxidase inhibitor phenelzine (Nardil) to produce manic-like symptoms.123
Ginseng may reduce the effectiveness of Coumadin (warfarin). In one double-blind study designed to evaluate the interactions between American ginseng and Coumadin (warfarin), twenty healthy volunteers received warfarin for 3 days during weeks 1 and 4.124 Beginning in week 2, patients were assigned to receive either American ginseng or placebo. The peak international normalized ratio statistically significantly decreased after 2 weeks of ginseng administration compared with placebo, with the difference between ginseng and placebo being zero. The international normalized ratio area under the curve, peak plasma warfarin level, and warfarin area under the curve were also statistically significantly reduced in the ginseng group compared with the placebo group. These results indicate a potential for ginseng reducing the effectiveness of warfarin; however, in a study in stroke patients, no interaction was noted.125 Until this interaction is further clarified, ginseng should only be used with careful monitoring, if at all, in patients on Coumadin.
1. Leung A.Y., Foster S. Encyclopedia of common natural ingredients used in food, drugs and cosmetics. New York: John Wiley; 1996. 277-281
2. Shibata S., Tanaka O., Shoji J., et al. Wagner H., Hikino H., Farnsworth N.R., eds., Economic and Medicinal Plant Research, Vol. 1. London: Academic Press. 1985:217–284.
3. Dong T.T., Cui X.M., Song Z.H., et al. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J Agric Food Chem. 2003;51:4617–4623.
4. Assinewe V.A., Baum B.R., Gagnon D., et al. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng). J Agric Food Chem. 2003;51:4549–4553.
5. Mino Y., Oto N., Sakao S., et al. Determination of germanium in medicinal plants by atomic absorption spectrometry with electrothermal atomization. Chem Pharm Bull (Tokyo). 1980;28:2687–2691.
6. Ng T.B., Wang H. Panaxagin, a new protein from Chinese ginseng, possesses anti-fungal, anti-viral, translation-inhibiting and ribonuclease activities. Life Sci. 2001;68:739–749.
7. Brekhman I.I., Dardymov I.V. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9:419–430.
8. Brekhman I.I., Dardymov I.V. Pharmacological investigation of glycosides from ginseng and Eleutherococcus. Lloydia. 1969;32:46–51.
9. Avakian E.V., Jr., Evonuk E. Effect of Panax ginseng extract on tissue glycogen and adrenal cholesterol depletion during prolonged exercise. Planta Med. 1979;36:43–48.
10. Bombardelli E., Cristoni A., Lietti A. The effect of acute and chronic (Panax) ginseng saponins treatment on adrenal function; biochemical and pharmacological. In: Proceedings of the 3rd International Ginseng Symposium. Seoul: Korean Ginseng Research Institute; 1980. 9-16
11. Fulder S.J. Ginseng and the hypothalamic-pituitary control of stress. Am J Chin Med. 1981;9:112–118.
12. Hiai S., Yokoyama H., Oura H. Features of ginseng saponin-induced corticosterone secretion. Endocrinol Jpn. 1979;26:737–740.
13. Hiai S., Yokoyama H., Oura H., et al. Evaluation of corticosterone secretion-inducing activities of ginsenosides and their prosapogenins and sapogenins. Chem Pharm Bull (Tokyo). 1983;31:168–174.
14. Tanizawa H., Numano H., Odani T., et al. Study of the saponin of Panax ginseng C.A. Meyer. I. Inhibitory effect on adrenal atrophy, thymus atrophy and the decrease of serum K+ concentration induced by cortisone acetate in unilateral adrenalectomized rats. Yakugaku Zasshi. 1981;101:169–173. [Japanese]
15. Petkov W. Pharmacology of the drug Panax ginseng. Arzneimittelforschung. 1959;9:305–311. [German]
16. Petkov W. On the mechanism of action of Panax ginseng C.A. Mey: on the problem of a pharmacology of reactivity.2. Arzneimittelforschung. 1961;11:288–295. 418-422: [German]
17. Petkov V. Effect of ginseng on the brain biogenic monoamines and 3’-5’-AMP system. Experiments in rats. Arzneimittelforschung. 1978;28:388–393. [German]
18. Saito H., Yoshida Y., Takagi K. Effect of Panax ginseng root on exhaustive exercise in mice. Jpn J Pharmacol. 1974;24:119–127.
19. Kaku T., Miyata T., Uruno T., et al. Chemico-pharmacological studies on saponins of Panax ginseng C.A. Meyer. II. Pharmacological part. Arzneimittelforschung. 1975;25:539–547.
20. Sterner W., Kirchdorfer A.M. Comparative work load tests on mice with standardized ginseng extract and a ginseng containing pharmaceutical preparation. Z Gerontol. 1970;3:307–312.
21. Cicero A.F., Bandieri E., Arletti R. Orally administered Panax notoginseng influence on rat spontaneous behaviour. J Ethnopharmacol. 2000;73:387–391.
22. Samira M.M., Attia M.A., Allam M., et al. Effect of the standardized ginseng extract G115 on the metabolism and electrical activity of the rabbit’s brain. J Int Med Res. 1985;13:342–348.
23. Hallstrom C., Fulder S., Carruthers M. Effect of ginseng on the performance of nurses on night duty. Comp Med East West. 1982;6:277–282.
24. Gross D., Shenkman Z., Bleiberg B., et al. Ginseng improves pulmonary functions and exercise capacity in patients with COPD. Monaldi Arch Chest Dis. 2002;57:242–246.
25. Barton D.L., Soori G.S., Bauer B.A., et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer. 2010;18:179–187.
26. Kim J.H., Park C.Y., Lee S.J. Effects of sun ginseng on subjective quality of life in cancer patients: a double-blind, placebo-controlled pilot trial. J Clin Pharm Ther. 2006;31:331–334.
27. Petkov V.D., Belcheva S., Petkov V.V. Behavioral effects of Ginkgo biloba L., Panax ginseng C.A. Mey. and Gincosan. Am J Chin Med. 2003;31:841–855.
28. Petkov V.D., Kehayov R., Belcheva S., et al. Memory effects of standardized extracts of Panax ginseng (G115), Ginkgo biloba (GK 501) and their combination Gincosan (PHL-00701). Planta Med. 1993;59:106–114.
29. D’Angelo L., Grimaldi R., Caravaggi M., et al. A double-blind, placebo-controlled clinical study on the effect of a standardized ginseng extract on psychomotor performance in healthy volunteers. J Ethnopharmacol. 1986;16:15–22.
30. Scholey A.B., Kennedy D.O. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum Psychopharmacol. 2002;17:35–44.
31. Kennedy D.O., Scholeya A.B., Wesnes K.A. Dose dependent changes in cognitive performance and mood following acute administration of ginseng to healthy young volunteers. Nutr Neurosci. 2001;4:295–310.
32. Cardinal B.J., Engels H.J. Ginseng does not enhance psychological well-being in healthy, young adults: results of a double-blind, placebo-controlled, randomized clinical trial. J Am Diet Assoc. 2001;101:655–660.
33. Coleman C.I., Hebert J.H., Reddy P. The effects of Panax ginseng on quality of life. J Clin Pharm Ther. 2003;28:5–15.
34. Ellis J.M., Reddy P. Effects of Panax ginseng on quality of life. Ann Pharmacother. 2002;36:375–379.
35. Wesnes K.A., Ward T., McGinty A., et al. The memory enhancing effects of a Ginkgo biloba/Panax ginseng combination in healthy middle-aged volunteers. Psychopharmacology (Berl). 2000;152:353–361.
36. Kennedy D.O., Scholey A.B. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700.
37. Reay J.L., Scholey A.B., Kennedy D.O. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum Psychopharmacol. 2010;25:462–471.
38. Reay J.L., Kennedy D.O., Scholey A.B. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained ‘mentally demanding’ tasks. J Psychopharmacol. 2006;20:771–781.
39. Reay J.L., Kennedy D.O., Scholey A.B. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19:357–365.
40. Lee S.T., Chu K., Sim J.Y., et al. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222–226.
41. Engels H.J., Wirth J.C. No ergogenic effects of ginseng (Panax ginseng C.A. Meyer) during graded maximal aerobic exercise. J Am Diet Assoc. 1997;97:1110–1115.
42. Engels H.J., Fahlman M.M., Wirth J.C. Effects of ginseng on secretory IgA, performance, and recovery from interval exercise. Med Sci Sports Exerc. 2003;35:690–696.
43. Engels H.J., Kolokouri I., Cieslak T.J., 2nd., et al. Effects of ginseng supplementation on supramaximal exercise performance and short-term recovery. J Strength Cond Res. 2001;15:290–295.
44. Cabral de Oliveira A.C., Perez A.C., Merino G., et al. Protective effects of Panax ginseng on muscle injury and inflammation after eccentric exercise. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:369–377.
45. Ziemba A.W., Chmura J., Kaciuba-Uscilko H., et al. Ginseng treatment improves psychomotor performance at rest and during graded exercise in young athletes. Int J Sport Nutr. 1999;9:371–377.
46. Kim S.H., Park K.S., Chang M.J., et al. Effects of Panax ginseng extract on exercise-induced oxidative stress. J Sports Med Phys Fitness. 2005;45:178–182.
47. Hsu C.C., Ho M.C., Lin L.C., et al. American ginseng supplementation attenuates creatine kinase level induced by submaximal exercise in human beings. World J Gastroenterol. 2005;11:5327–5331.
48. Sotaniemi E.A., Haapakoski E., Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care. 1995;18:1373–1375.
49. Ng T.B., Yeung H.W. Hypoglycemic constituents of Panax ginseng. Gen Pharmacol. 1985;16:549–552.
50. Waki I., Kyo H., Yasuda M., et al. Effects of a hypoglycemic component of ginseng radix on insulin biosynthesis in normal and diabetic animals. J Pharmacobiodyn. 1982;5:547–554.
51. Konno C., Sugiyama K., Kano M., et al. Isolation and hypoglycaemic activity of panaxans A, B, C, D and E, glycans of Panax ginseng roots. Planta Med. 1984;50:434–436.
52. Kimura M., Waki I., Tanaka O., et al. Pharmacological sequential trials for the fractionation of components with hypoglycemic activity in alloxan diabetic mice from ginseng radix. J Pharmacobiodyn. 1981;4:402–409.
53. Kimura M., Waki I., Chujo T., et al. Effects of hypoglycemic components in ginseng radix on blood insulin level in alloxan diabetic mice and on insulin release from perfused rat pancreas. J Pharmacobiodyn. 1981;4:410–417.
54. Sievenpiper J.L., Arnason J.T., Leiter L.A., et al. Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: the role of ginsenosides. J Am Coll Nutr. 2004;23:248–258.
55. Vuksan V., Sievenpiper J., Koo V.Y., et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–1013.
56. Vuksan V., Stavro M.P., Sievenpiper J.L., et al. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes. Diabetes Care. 2000;23:1221–1226.
57. Franz M.J., Bantle J.P., Beebe C.A., et al. Nutrition principles and recommendations in diabetes. Diabetes Care. 2004;27(Suppl 1):S36–S46.
58. Dey L., Xie J.T., Wang A., et al. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10:600–605.
59. Vuksan V., Sung M.K., Sievenpiper J.L., et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56.
60. Ma S.W., Benzie I.F., Chu T.T., et al. Effect of Panax ginseng supplementation on biomarkers of glucose tolerance, antioxidant status and oxidative stress in type 2 diabetic subjects: results of a placebo-controlled human intervention trial. Diabetes Obes Metab. 2008;10:1125–1127.
61. Attele A.S., Zhou Y.P., Xie J.T., et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858.
62. Nocerino E., Amato M., Izzo A.A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71(Suppl 1):S1–S5.
63. Kim C., Choi H., Kim C.C., et al. Influence of ginseng on mating behavior of male rats. Am J Chin Med. 1976;4:163–168.
64. Fahim M.S., Fahim Z., Harman J.M., et al. Effect of Panax ginseng on testosterone level and prostate in male rats. Arch Androl. 1982;8:261–263.
65. Punnonen R., Lukola A. Oestrogen-like effect of ginseng. BMJ. 1980;281:1110.
66. Yonezawa M. Restoration of radiation injury by intraperitoneal injection of ginseng extract in mice. J Radiat Res (Tokyo). 1976;17:111–113.
67. Palmer B.V., Montgomery A.C., Monteiro J.C. Gin Seng and mastalgia. BMJ. 1978;1:1284.
68. Murphy L.L., Lee T.J. Ginseng, sex behavior, and nitric oxide. Ann N Y Acad Sci. 2002;962:372–377.
69. Price A., Gazewood J. Korean red ginseng effective for treatment of erectile dysfunction. J Fam Pract. 2003;52:20–21.
70. Hong B., Ji Y.H., Hong J.H., et al. A double-blind crossover study evaluating the efficacy of Korean red ginseng in patients with erectile dysfunction: a preliminary report. J Urol. 2002;168:2070–2073.
71. Kim T.H., Jeon S.H., Hahn E.J., et al. Effects of tissue-cultured mountain ginseng (Panax ginseng CA Meyer) extract on male patients with erectile dysfunction. Asian J Androl. 2009;11:356–361.
72. De Andrade E., de Mesquita A.A., Claro Jde A., et al. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007;9:241–244.
73. Wiklund I.K., Mattsson L.A., Lindgren R., et al. Effects of a standardized ginseng extract on quality of life and physiological parameters in symptomatic postmenopausal women: a double-blind, placebo-controlled trial. Swedish Alternative Medicine Group. Int J Clin Pharmacol Res. 1999;19:89–99.
74. Fulder S.J. The growth of cultured human fibroblasts treated with hydrocortisone and extracts of the medicinal plant Panax ginseng. Exp Gerontol. 1977;12:125–131.
75. Yamamoto M., Masaka M., Yamada K., et al. Stimulatory effect of ginsenosides on DNA, protein and lipid synthesis in bone marrow and participation of cyclic nucleotides. Arzneimittelforschung. 1978;28:2238–2241.
76. Radad K., Gille G., Moldzio R., et al. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J Neural Transm. 2004;111:37–45.
77. Mizumaki Y., Kurimoto M., Hirashima Y., et al. Lipophilic fraction of Panax ginseng induces neuronal differentiation of PC12 cells and promotes neuronal survival of rat cortical neurons by protein kinase C dependent manner. Brain Res. 2002;950:254–260.
78. Fu Y., Ji L.L. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J Nutr. 2003;133:3603–3609.
79. Liu Z.Q., Luo X.Y., Sun Y.X., et al. Can ginsenosides protect human erythrocytes against free-radical-induced hemolysis? Biochim Biophys Acta. 2002;1572:58–66.
80. Siddique M.S., Eddeb F., Mantle D., et al. Extracts of Ginkgo biloba and Panax ginseng protect brain proteins from free radical induced oxidative damage in vitro. Acta Neurochir Suppl. 2000;76:87–90.
81. Kim Y.K., Guo Q., Packer L. Free radical scavenging activity of red ginseng aqueous extracts. Toxicology. 2002;172:149–156.
82. Kwan C.Y., Kwan T.K. Effects of Panax notoginseng saponins on vascular endothelial cells in vitro. Acta Pharmacol Sin. 2000;21:1101–1105.
83. Kim S.H., Park K.S. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48:511–513.
84. Jovanovski E., Jenkins A., Dias A.G., et al. Effects of Korean red ginseng (Panax ginseng C.A. Mayer) and its isolated ginsenosides and polysaccharides on arterial stiffness in healthy individuals. Am J Hypertens. 2010;23:469–472.
85. Jie Y.H., Cammisuli S., Baggiolini M. Immunomodulatory effects of Panax ginseng C.A. Meyer in the mouse. Agents Actions. 1984;15:386–391.
86. Gupta S., Agarwal S.S., Epstein L.B., et al. Panax. A new mitogen and interferon producer. Clin Res. 1980;28:504A.
87. Singh V.K., Agarwal S.S., Gupta B.M. Immunomodulatory activity of Panax ginseng extract. Planta Med. 1984;50:462–465.
88. Shin J.Y., Song J.Y., Yun Y.S., et al. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002;24:469–482.
89. Assinewe V.A., Amason J.T., Aubry A., et al. Extractable polysaccharides of Panax quinquefolius L. (North American ginseng) root stimulate TNFalpha production by alveolar macrophages. Phytomedicine. 2002;9:398–404.
90. Lim D.S., Bae K.G., Jung I.S., et al. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J Infect. 2002;45:32–38.
91. Cho J.Y., Kim A.R., Yoo E.S., et al. Ginsenosides from Panax ginseng differentially regulate lymphocyte proliferation. Planta Med. 2002;68:497–500.
92. Lim T.S., Na K., Choi E.M., et al. Immunomodulating activities of polysaccharides isolated from Panax ginseng. J Med Food. 2004;7:1–6.
93. Park E.K., Choo M.K., Han M.J., et al. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120.
94. Choo M.K., Park E.K., Han M.J., et al. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–522.
95. See D.M., Broumand N., Sahl L., et al. In vitro effects of Echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacol. 1997;35:229–235.
96. McElhaney J.E., Gravenstein S., Cole S.K., et al. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc. 2004;52:13–19.
97. Scaglione F., Cattaneo G., Alessandria M., et al. Efficacy and safety of the standardised ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold. Drugs Exp Clin Res. 1996;22:65–72.
98. Chong S.K., Brown H.A., Rimmer E., et al. In vitro effect of Panax ginseng on phytohaemagglutinin-induced lymphocyte transformation. Int Arch Allergy Appl Immunol. 1984;73:216–220.
99. Yeung H.W., Cheung K., Leung K.N. Immunopharmacology of Chinese medicine. 1. Ginseng induced immunosuppression in virus-infected mice. Am J Chin Med. 1982;10:44–54.
100. Yun T.K., Choi S.Y. Non-organ specific cancer prevention of ginseng: a prospective study in Korea. Int J Epidemiol. 1998;27:359–364.
101. Yun T.K. Panax ginseng—a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55.
102. Shin H.R., Kim J.Y., Yun T.K., et al. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control. 2000;11:565–576.
103. Kim H.S., Lee E.H., Ko S.R., et al. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004;27:429–435.
104. Liu W.K., Xu S.X., Che C.T. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000;67:1297–1306.
105. Yun T.K., Yun Y.S., Han I.W. Anticarcinogenic effect of long-term oral administration of red ginseng on newborn mice exposed to various chemical carcinogens. Cancer Detect Prev. 1983;6:515–525.
106. Lee K.D., Huemer R.P. Antitumoral activity of Panax ginseng extracts. Jpn J Pharmacol. 1971;21:299–302.
107. Chang Y.S., Seo E.K., Gyllenhaal C., et al. Panax ginseng: a role in cancer therapy? Integr Cancer Ther. 2003;2:13–33.
108. Liu S.J., Zhou S.W. Panax notoginseng saponins attenuated cisplatin-induced nephrotoxicity. Acta Pharmacol Sin. 2000;21:257–260.
109. Sung J., Han K.H., Zo J.H., et al. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000;28:205–216.
110. Yamamoto M., Uemura T., Nakama S., et al. Serum HDL-cholesterol-increasing and fatty liver-improving actions of Panax ginseng in high cholesterol diet-fed rats with clinical effect on hyperlipidemia in man. Am J Chin Med. 1983;11:96–101.
111. Yamamoto M., Kumagai A., Yamamura Y. Plasma lipid-lowering actions of ginseng saponins and mechanisms of the action. Am J Chin Med. 1983;11:84–87.
112. Joo C.N. The preventative effect of Korean (P. ginseng) saponins on aortic atheroma formation in prolonged cholesterol-fed rabbits. In: Proceedings of the 3rd International Ginseng Symposium. Seoul: Korean Ginseng Research Institute; 1980. 27-36
113. Matsuda H., Namba K., Fukuda S., et al. Pharmacological study on Panax ginseng C.A. Meyer. III. Effects of red ginseng on experimental disseminated intravascular coagulation. (2). Effects of ginsenosides on blood coagulative and fibrinolytic systems. Chem Pharm Bull (Toyko). 1986;34:1153–1157.
114. Oura H., Hiai S., Seno H. Synthesis and characterization of nuclear RNA induced by radix ginseng extract in rat liver. Chem Pharm Bull (Tokyo). 1971;19:1598–1605.
115. Oura H., Hiai S., Nabetani S., et al. Effect on ginseng extract on endoplasmic reticulum and ribosome. Planta Med. 1975;28:76–88.
116. Oura H., Nakashima S., Tsukada K., et al. Effect of radix ginseng extract on serum protein synthesis. Chem Pharm Bull (Tokyo). 1972;20:980–986.
117. Hikino H., Kiso Y., Kinouchi J., et al. Antihepatotoxic actions of ginsenosides from Panax ginseng roots. Planta Med. 1985;1:62–64.
118. Kim T.H., Lee Y.S., Cho C.K., et al. Protective effect of ginseng on radiation-induced DNA double strand breaks and repair in murine lymphocytes. Cancer Biother Radiopharm. 1996;11:267–272.
119. Ben-Hur E., Fulder S. Effect of Panax ginseng saponins and Eleutherococcus senticosus on survival of cultured mammalian cells after ionizing radiation. Am J Chin Med. 1981;9:48–56.
120. Siegel R.K. Ginseng abuse syndrome: problems with the panacea. JAMA. 1979;241:1614–1615.
121. Hess F.G., Jr., Parent R.A., Stevens K.R., et al. Effects of subchronic feeding of ginseng extract G115 in beagle dogs. Food Chem Toxicol. 1983;21:95–97.
122. Hess F.G., Jr., Parent R.A., Cox G.E., et al. Reproduction study in rats of ginseng extract G115. Food Chem Toxicol. 1982;20:189–192.
123. Coon J.T., Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Safety. 2002;25:323–344.
124. Yuan C.S., Wei G., Dey L., et al. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: a randomized, controlled trial. Ann Intern Med. 2004;141:23–27.
125. Lee S.H., Ahn Y.M., Ahn S.Y., et al. Interaction between warfarin and Panax ginseng in ischemic stroke patients. J Altern Complement Med. 2008;14:715–721.