24 Palmo-plantar hyperhidrosis
Summary and Key Features
• Palmar and plantar hyperhidrosis cause a significant impact on the quality of life for people who suffer from the disorder
• Botulinum toxin type A can produce hypohidrosis on the palms and soles for up to 6 months
• Ice or cooling devices are well-tolerated modes of anesthesia for the hands; however, regional nerve blocks are recommended prior to plantar injections
• Injections should be spaced 1–1.5 cm apart with 2–3 U of botulinum toxin A per injection site on average
• Success rates reach 90% for palmar hyperhidrosis with variable success for plantar hyperhidrosis despite similar injection techniques
• Postoperatively, patients may experience some weakness of thumb–index finger-pinch strength, which returns after 2–3 weeks; if injections are placed superficially and spaced properly, however, most will not
Patient evaluation
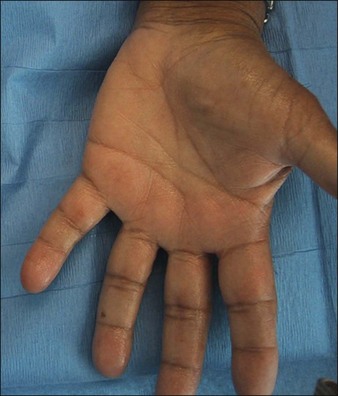
Most patients who present with palmar HH have had the condition since childhood or early adolescence with no known cause and report ‘sweaty palms’ that cause them social embarrassment. Solish & Haider reported evidence suggesting that an autosomal dominant inheritance pattern with variable expressivity such that a child born to a parent with palmar HH has a 25% chance of developing the disorder as well. Patients will go to great lengths to avoid shaking people’s hands and often keep their hands in their pockets. The hyperhidrosis can be so severe as to cause actual physical dripping and maceration and peeling of the skin itself (Fig. 24.1). Similarly, patients with plantar hyperhidrosis will report the need to change socks frequently or that they ‘slip’ in their shoes while walking. Interestingly, a study by Lear and colleagues suggested that spontaneous regression might occur over time as there is a low prevalence of the disorder in the elderly population. It is important for the physician to keep in mind the severe psychological impact HH has on patients, many of whom do not seek treatment due to embarrassment and yet decrease their leisure activities and suffer from depression because of it.
Prior to treatment, it is important to take a careful clinical history to ensure that the patient suffers from primary, not secondary, hyperhidrosis. In secondary hyperhidrosis, medications or systemic health problems can be responsible for sweating (Box 24.1) and the condition is usually generalized. If there is a suspicion of secondary hyperhidrosis, a complete blood count, fasting glucose level and thyroid function test are preliminary laboratory tests to be ordered.
Box 24.1
Select causes of secondary hyperhidrosis
 Febrile illness (e.g. chronic infections)
Febrile illness (e.g. chronic infections)
 Endocrine or metabolic condition (e.g. thyroid dysfunction, diabetes mellitus, menopause, pregnancy)
Endocrine or metabolic condition (e.g. thyroid dysfunction, diabetes mellitus, menopause, pregnancy)
 Neurological disorder (e.g. spinal cord injury, Parkinson disease, stroke)
Neurological disorder (e.g. spinal cord injury, Parkinson disease, stroke)
 Cardiovascular disorder (e.g. heart failure)
Cardiovascular disorder (e.g. heart failure)
 Medication use (e.g. antidepressants, antiemetics)
Medication use (e.g. antidepressants, antiemetics)
 Substance abuse (e.g. drug withdrawal, alcohol abuse)
Substance abuse (e.g. drug withdrawal, alcohol abuse)
 Neoplastic disease (e.g. pheochromocytoma, carcinoid tumors, Hodgkin lymphoma)
Neoplastic disease (e.g. pheochromocytoma, carcinoid tumors, Hodgkin lymphoma)
Scoring the impact of hyperhidrosis on the patient’s quality of life is important as well, not only to gauge treatment success but also to aide in obtaining insurance approval for treatment. The Hyperhidrosis Disease Severity Scale (HDSS) is a quick diagnostic tool that the practitioner can administer during the examination and was found by Solish and colleagues to be a reliable means of assessment (Table 24.1). HDSS scores should be followed to guide the choice of treatment and to determine whether treatment has made an impact (success should be considered a reduction in HDSS of 1 or more).
| Patient response | Score | Clinical interpretation |
|---|---|---|
| My sweating is never noticeable and never interferes with my daily activities | 1 | Mild |
| My sweating is tolerable but sometimes interferes with my daily activities | 2 | Moderate |
| My sweating is barely tolerable and frequently interferes with my daily activities | 3 | Severe |
| My sweating is intolerable and always interferes with my daily activities | 4 | Severe |
Marking the treatment areas
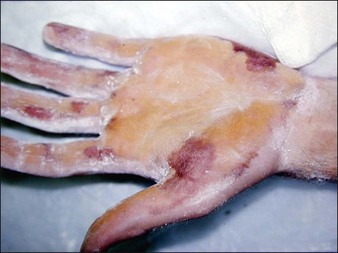
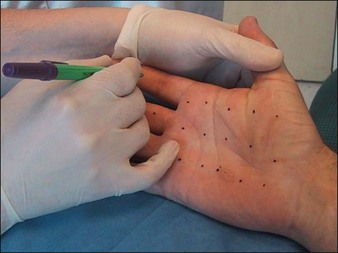
The Minor’s iodine-starch test can be used to estimate the areas of involvement that require treatment but should not be relied upon for precise quantitative measure of sweating. In brief, the palms or soles are first wiped with iodine (pre-packaged swabs of povidone-iodine are particularly useful for this) and allowed to dry. Next, the painted area is sprinkled with cornstarch powder. A colorimetric reaction ensues, resulting in a bluish-purple discoloration in areas of excess sweating that can then be marked for injection (Fig. 24.2). To ensure accuracy, patients should discontinue any topical therapies 5 days prior to iodine-starch testing. While this test is advocated for the axillae, we feel it is less helpful for the hands as the test usually demonstrates a positive reaction over the entire palm. For most patients, we advocated marking in a grid-like pattern with the iodine-starch test first (Fig. 24.3).
Dilution and injection
Though many dilution techniques exist, we find that for palmar and plantar injections, dilution of one vial of Botox® with 3 mL of bacteriostatic preserved saline results in a manageable concentration for injection (Table 24.2). With the 3 mL dilution, each 0.1 mL results in 3.3 U of Botox®. To prevent unnecessary waste of the toxin, we recommend removal of the lid and rubber stopper on the bottle vial so that no product is left behind, and use of hubless syringes. Since palmar and plantar skin is thick, needles dull more quickly after serial injection. Therefore, it is best to draw up the 100 U into six 0.5 mL syringes (B&D Ultra-Fine II 30-gauge hubless insulin needle syringes, Becton-Dickinson). Volumes of 0.05–0.1 mL (1.7–3.3 U) of Botox® should be deposited at each injection point spaced 1–1.5 cm apart because less diffusion occurs on the palms and soles compared with other body sites.
Table 24.2 Acceptable dilutions for botulinum toxin type A in the treatment of palmo-plantar hyperhidrosis
| Product | Dilution (mL) | Final concentration |
|---|---|---|
| Botox® | 2.5 | 4 U / 0.1 mL |
| Botox® | 3 | 3.3 U / 0.1 mL |
| Botox® | 5 | 2 U / 0.1 mL |
| Dysport® | 3 | 10 U / 0.1 mL |
Injection technique
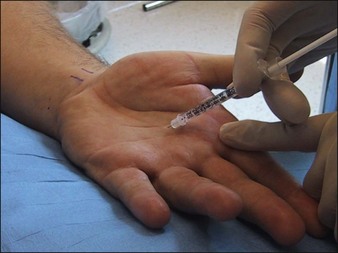
Proper injection depth for the palms and soles is at the junction of the dermis and subcutaneous tissue (the location of the eccrine glands). The needle should be inserted at an oblique angle and product placed into the deep dermis. Superficial injection decreases the probability that the neurotoxin will diffuse into the intrinsic muscles of the hands and cause weakness (Fig. 24.4). It is common to see a small zone of blanching around each injection point on the palms and soles; the wheal produced during neurotoxin injection in other parts of the body such as the axillae and forehead is not seen when performing palmar and plantar injections. Approximately 50–100 U of Botox is standard dosing for each palm depending on the size of the area; 100 U of Botox is reasonable to use per sole owing to the larger surface area. Though some authors recommend a range from 100 to 240 U of Botox per hand, one randomized, single-blind trial by Saadia and colleagues showed similar efficacy between 50 and 100 U of botulinum toxin type A for palmar HH. Of note, the higher dose was associated with an increased incidence of subjective hand weakness. For this reason, we recommend starting with 50 U per hand then reassessing the first-time patient at 1 month. Injections typically need to be repeated every 6 months.
Pearl 3
It should be noted that, in the palms and soles, neurotoxin has the tendency to backflow from the injection tract after injection. To minimize this, we recommend several tips such as keeping the needle bevel up, angling the needle more parallel to the skin surface and waiting for 1–2 seconds following injection to allow time for normalization of pressure, and making sure there are no air bubbles in the syringe, as reported by Fujita et al and Hayton et al (Box 24.2).
Methods to prevent backflow of neurotoxin during palmo-plantar injections
 Ensure there are no air bubbles in the syringe; this decreases inadvertent leakage of toxin when injecting.
Ensure there are no air bubbles in the syringe; this decreases inadvertent leakage of toxin when injecting.
 Keep the bevel of the needle up.
Keep the bevel of the needle up.
 Try to keep the angle of the needle as parallel as possible to the skin surface.
Try to keep the angle of the needle as parallel as possible to the skin surface.
 Advance the needle 2 mm prior to injection.
Advance the needle 2 mm prior to injection.
 Do not insert the needle into the skin with pressure on the plunger.
Do not insert the needle into the skin with pressure on the plunger.
 Wait 1–2 seconds following injection to allow time for normalization of pressure.
Wait 1–2 seconds following injection to allow time for normalization of pressure.
Fujita M, Mann T, Mann O, et al. Surgical pearl: use of nerve blocks for botulinum toxin treatment of palmar-plantar hyperhidrosis. Journal of the American Academy of Dermatology. 2001;45:587–589.
Glogau RG. Hyperhidrosis and botulinum toxin A: patient selection and techniques. Clinical Dermatology. 2004;22:45–52.
Grunfeld A, Murray CA, Solish N. Botulinum toxin for hyperhidrosis. American Journal of Clinical Dermatology. 2009;10:87–102.
Hamm H, Naumann M, Kowalski JW, et al. Primary focal hyperhidrosis: disease characteristics and functional impairment. Dermatology. 2006;212:343–353.
Hayton MJ, Stanley JK, Lowe NJ. A review of peripheral nerve blockade as local anaesthesia in the treatment of palmar hyperhidrosis. British Journal of Dermatology. 2003;149:447–451.
Hornberger J, Grimes K, Naumann M, et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. Journal of the American Academy of Dermatology. 2004;51:274–2786.
Kaufmann H, Saadia D, Polin C, et al. Primary hyperhidrosis: evidence for autosomal dominant inheritance. Clinics in Autonomic Research. 2003;13:96–98.
Lear W, Kessler E, Solish N, et al. An epidemiological study of hyperhidrosis. Dermatologic Surgery. 2007;33:S69–75.
Murray CA, Cohen JL, Solish N. Treatment of focal hyperhidrosis. Journal of Cutaneous Medicine and Surgery. 2007;11:67–77.
Naumann MK, Hamm J, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. British Journal of Dermatology. 2002;147:1218–1226.
Simonetta MM, Cauhepe C, Magues JP, et al. A double-blind, randomized, comparative study of Dysport vs. Botox in primary palmar hyperhidrosis. British Journal of Dermatology. 2003;149:1041–1045.
Solish N, Benohanian A, Kowalski JW. Canadian dermatology study group on health-related quality of life in primary axillary hyperhidrosis. Prospective open-label study of botulinum toxin type A in patients with axillary hyperhidrosis: effects on functional impairment and quality of life. Dermatologic Surgery. 2005;31:405–413.
Solish N, Haider A. Focal hyperhidrosis: diagnosis and management. Cutaneous Medicine Association Journal. 2005;172:169–175.
Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. Journal of the American Academy of Dermatology. 2004;51:241–248.
Walling HW. Primary hyperhidrosis increases the risk of cutaneous infection: a case-control study of 387 patients. Journal of the American Academy of Dermatology. 2009;61:242–246.
Walling HW, Swick BL. Treatment options for hyperhidrosis. American Journal of Clinical Dermatology. 2011;12:285–295.