23 Palliative Sedation
Terminology
Terminal sedation was described in the literature in 1991 as “as the intention of deliberately inducing and maintaining deep sleep, but not deliberately causing death in very specific circumstances.”1 However, a lack of consensus over the definition of terminal sedation exists amongst palliative care experts. In addition, it does not convey or emphasize the aim of sedation, to palliate symptoms, or the possibility that the patient may awaken or recover. In fact, terminal could be interpreted to mean the intentional ending of life. In one survey of 61 palliative care experts (59 physicians and two nurses, response rate of 87 percent) only 21 (40 percent) agreed that terminal sedation was “the intention of deliberately inducing and maintaining deep sleep, but not deliberately causing death in very specific circumstances.”2 In response to this continued lack of consensus, other terms such as sedation for intractable distress in the dying or sedation in the imminently dying arose as alternatives in the literature,3 and have evolved into the most commonly used term, palliative sedation.4 While there is not complete agreement on the optimal terminology or definition, most would agree that PST is “the use of sedative medications to relieve intolerable suffering from refractory symptoms by a reduction in patient consciousness.”5 PST is usually administered for intractable symptoms at the end of life. Because the addition of “therapy” to the term emphasizes that it is an accepted palliative treatment for symptoms rather than an end in and of itself, palliative sedation therapy is used in this chapter.
PST may be further described in terms of the level of sedation achieved, that is from mild to deep, as well as the intended duration, such as continuous versus intermittent or respite sedation. In general, the goal of PST is for the child to be in a state of conscious sedation, with relief from symptoms but still able to communicate. In rare situations requiring urgent relief of suffering, deep sedation may instead be the goal. If there is no intention of waking the patient, then continuous sedation is used. Both deep and continuous sedation can be recommended only for patients with an expected prognosis of hours to days without a treatable condition.6 Respite sedation is a subtype of palliative sedation therapy intended to be time-limited and temporary in nature. Such temporary respite from suffering may allow continued or future therapy to provide relief,7,8 or may provide relief and rest for a patient when their perception of intolerability is impacted by factors that create severe physical and emotional fatigue.6,7
A clear definition for refractory symptom is central to the discourse regarding palliative sedation. Cherny and Portenoy’s definition of refractory symptoms is widely accepted: “symptom[s] for which all possible treatment has failed, or it is estimated that no methods are available for palliation within the time frame and the risk-benefit ratio that the patient can tolerate.”7 Refractory symptoms can be categorized by the clinician using the diagnostic criteria as those symptoms in which further interventions are:
Despite controversy surrounding nomenclature as well as some of the specific indications for PST, this treatment modality is by and large accepted worldwide as an intervention for patients with refractory suffering at the end of life. In 2007, international guidelines and recommendations for standards were developed with representation from the United Kingdom, the Netherlands, Belgium, France, Germany, Switzerland, Finland, Canada, the United States, Argentina, South Africa, Israel, Japan, Australia and New Zealand.5 The European Association for Palliative Care developed a recommended 10-point framework for the use of palliative sedation based on existing guidelines, literature, and extensive peer review. This framework supports the use of palliative sedation as important and necessary, when used appropriately and ethically, in patients with otherwise refractory distress.6 However, literature regarding the provision of PST in the pediatric population is scant, and outside of one review,9 largely consists of case reports. Except where specified in this chapter, evidence presented reflects PST in adults. Issues regarding PST in children that require special consideration are discussed later in this chapter.
Setting the Stage
A variety of factors may influence the decision to initiate PST (Box 23-1). While PST is an effective palliative intervention for relief of suffering, the risks and potential controversy surrounding it require careful consideration of the situation from a variety of perspectives. This section describes some key issues that must be taken into account before this therapeutic option is employed. Communication with the family and entire care team around these issues is essential.
BOX 23-1 Factors Influencing the Decision to lnitiate Palliative Sedation Therapy
* Curlin FA, Lawrence RE, Chin MH, Lantos JD. Religion, conscience, and controversial clinical practices. N Engl J Med 356:593–600, 2007.
† Morita T, Akechi T, Sugawara Y, Chihara S, Uchitomi Y. Practices and attitudes of japanese oncologists and palliative care physicians concerning terminal sedation: a nationwide survey. J Clin Oncol 20:758–764, 2002.
Intolerable suffering
If refractory symptoms escalate to the point of intolerability, the predominant goal of care may shift from extension of life or preservation of function to that of alleviating of suffering. Suffering and distress are subjective phenomena (Fig. 23-1). As such, only the individual experiencing such suffering can truly deem his or her condition intolerable.10 However, many patients at the very end of life are unable to express whether their degree of suffering warrants sedation. In studies of adult palliative care patients, 22 percent to 49 percent of adult patients were not involved with the decision to undertake sedation for intolerable suffering.2,11,12 In these instances, proxies, in conjunction with the medical team, decided whether PST should be implemented. In pediatrics, parents most commonly serve as the surrogate decision makers for children. Skillful discussions with families regarding the distinction between the child’s suffering and their own are needed when PST is under consideration.
Developmental limitations or neurocognitive impairment are frequent conditions in children with life-threatening illnesses. In situations involving a pediatric patient, it is even more likely that a parent or guardian will make the decision about PST on behalf of the patient (Fig. 23-2). That is not to say that children should be routinely excluded from such discussions. Many children are able to consider significant decisions such as resuscitation status.13 The extent to which a particular child participates in a decision about PST depends greatly on the child as well as his or her family. Whether and how a child is involved in the determination of whether his or her suffering justifies PST should be decided by the family and care team on an individual basis, and include consideration of the extent to which the child can express his or her own will, understand the relevant information, and understand and acknowledge the implications of the choice.6
Distinguishing refractory symptoms from difficult symptoms
Before embarking on a consideration of PST, it is essential to distinguish symptoms that are challenging to manage from those that are not relieved by available measures within an acceptable timeframe and with acceptable toxicity. Further consideration of a symptom’s refractoriness should also include whether the patient’s medical team has enough expertise and experience to truly judge the symptom as refractory.5 Palliative care consultation, if available, should be a requisite condition whenever PST is considered. A palliative care team can help establish that all acceptable interventions to relieve suffering have been considered. In addition, a palliative care team can also ensure that the circumstances are considered from an interdisciplinary perspective, and that the physical, emotional, existential, and social dimensions of suffering have been thoroughly explored and addressed. If palliative care consultation is not an option, input from clinicians from different disciplines and with expertise in symptom control, ethics or the care of seriously ill children should be sought.
Symptoms Prompting Consideration of PST
Refractory physical symptoms such as pain, delirium and/or agitation, dyspnea, and nausea and/or vomiting may prompt consideration of PST. The published relative frequencies of physical symptoms invoking PST vary across settings and populations, and with the type of sedation studied.14,15 Large studies to determine the prevalence of indications for PST in children have not been conducted.
While the relative frequencies of refractory physical symptoms prompting PST may vary, refractory physical symptoms are generally accepted as potential indications for PST. This is largely due to the presence of well-established therapeutic strategies to be considered before the symptom is deemed refractory. Such physical symptoms also tend to occur late in the illness trajectory, when the proximity of death is incontrovertible.16
In contrast, distress from non-physical symptoms is less clear. In adults, such distress may involve feelings of meaninglessness and worthlessness; being a burden on others; dependency and/or the inability to take care of oneself; death anxiety, fear, and/or panic; a wish to control the time of death by oneself; and isolation or lack of social support.17 Therapeutic strategies are often less established and symptoms may fluctuate, complicating evaluation. These issues may all influence whether a nonphysical symptom is considered refractory, and whether the use of PST is proportionate to the suffering inflicted by the symptom. Finally, the beliefs of the members of the care team are likely to shape the determination of whether PST is acceptable treatment for refractory psychological and existential distress.18,19
Because nonphysical symptoms may not correlate with proximity to death, adults who receive PST for this indication may be sedated for a longer time before death. Some opponents of PST for psychological and existential distress argue that establishment of such suffering as refractory is difficult, and that it may be implemented earlier in the illness trajectory, with a concomitant increase in risk from PST and blurring of the distinction between PST and euthanasia.16,20,21 On the other hand, proponents of PST for psychological or existential suffering maintain that such suffering may cause more suffering than physical symptoms, with fewer therapeutic options. To facilitate decision making regarding PST and psychological and/or existential distress, there is a set of proposed clinical rules for consideration.16 This issue clearly requires further investigation prior to recommendation of it as a standard indication for PST in pediatrics.
Location of Care
The location of the child’s care should also be determined before initiating discussion of PST, because this is likely to affect PST option. In a multicenter study in Italy, wide variations in whether and how PST was implemented among centers was more reflective of differences in institutional policies and provider behavior rather than the patients’ preferences or needs.22 The availability of continuous palliative sedation or sedation to unconsciousness may be particularly uneven or unpredictable. Offering to relieve a child’s suffering through sedation when this is not a realistic option can leave a family feeling abandoned and the care team demoralized and guilty.23 Therefore, determining the range of options before presenting them to the family is recommended.
PST in adults is more commonly provided in inpatient settings, such as palliative care units. Institutional policies may restrict the availability of particular sedatives, such as propofol or dexmedetomidine, to the operating room, intensive care unit, or other specific clinical settings. Policies that permit the use of particular agents, such as midazolam or propofol, for the express purpose of PST in other settings do exist.24 Even with such policies, barriers such as logistical or clinical constraints and the healthcare team’s ethical or legal concerns may also determine whether PST is a viable option in certain settings.25
PST may also be implemented in the home setting; however, a high degree of cooperation and commitment from both staff and family is needed. Additionally, home-care staff are likely to be increasingly needed as the intensity of treatment increases.26 PST at home may present greater demands on home-care staff, with these individuals providing more emotional support for the family and experiencing more stress from the decision making.26
Artificial Hydration and Nutrition
Although provision of artificial hydration and nutrition (AHN) is not contraindicated during PST, it is unlikely to contribute to the patient’s comfort. It may actually detract from the goal of maximizing comfort by contributing to fluid overload or gastrointestinal discomfort.27 Discontinuation of AHN when PST is implemented has not been associated with shorter survival times,12 perhaps because patients with severely distressing and uncontrolled symptoms are unlikely to sustain themselves with oral intake for extended periods of time. In some cases where respite sedation is intended, ongoing provision of AHN may be consistent with the family’s primary goals. The interdisciplinary team approach is key in exploring the family’s personal or religious beliefs with regard to AHN. Some strongly believe that AHN be continued; in this case, AHN may be continued.
Communication with the Family Before and During PST
Frequent, open and honest communication with the family both before and during the provision of PST is crucial. Knowledge of end-of-life treatment options is highly variable in adult patients,28 and care teams should be aware of such variability when broaching the topic of PST with families. Decisions of great import, such as forgoing life-sustaining therapy, frequently require multiple conversations to reach consensus with families.29 Once initiated, PST is also associated with families expressing more concerns, so the importance of ongoing communication to readdress goals, plans, and the family’s needs persists, and may increase as duration of sedation increases.30
Bereaved family members of patients who received PST report having concerns about the patient’s distress, whether maximum efforts to control symptoms had been made before initiating PST, having opportunities to say important things to the patients before sedation, to understand their suffering, and for professionals to treat patients with dignity.31 Families also report feeling guilt, helplessness, and exhaustion during the process.
Opportunities, both initially and during follow-up conversations, are needed to address these concerns and feelings, which may compound the suffering that families endure during their loved one’s last days. Families report that information reduced their anxiety and confusion and gave them confidence that the staff was in control and would be able to help the patient.32 If care teams do not proactively create opportunities for ongoing communication to clarify the care plan, provide relevant information, and address questions during the provision of PST, then families may experience frustration and disappointment32 (Box 23-2).
Morita and colleagues have demonstrated that feeling the responsibility for the decision is an independent predictor of high distress in bereaved family members.33 It is therefore particularly important for the healthcare team to share the responsibility for the decision to administer PST, alleviating some of the burden that the family may feel. Frequent communication not only allows the transmission or clarification of information, but also allows for the development of rapport and the sense of a joint endeavor between the care team and family.
Concern that sedation may shorten life is common among family members,33 although several empirical studies have shown no differences in survival when patients who received PST are compared with those who did not.14,15,34–36 It is therefore important to discuss with families that PST, as with other forms of sedation, is generally very safe although rarely patients experience complications that may shorten life. The degree of focus on risks should also be influenced by the clinical context. For example, presentation of the risks and plans to reduce them, such as monitoring vital signs, may be different depending on whether there is a plan to reverse sedation and awaken the patient after a certain period of time. In any event, discussion of the risks should never leave families feeling as though they must choose between their child’s life and comfort.
Families should have the opportunity to meet with the care team every 24 to 48 hours, to revisit the plan of care and discuss concerns that may have arisen. All discussions should be documented in the medical record as they occur to maximize clear and consistent communication between staff members and the family (Box 23-3). Such documentation should be readily accessible by all members of the child’s care team, particularly if those administering PST are not directly part of ongoing discussions with the family regarding it. Special attention to regular interdisciplinary communication with families may be needed if PST is administered in the home. Such communication is important not only for the family, but also for staff providing direct care in the home. PST places higher demands on home-care staff.26 Routine interdisciplinary team meetings may lessen the burden that individual staff members in the home may feel.
BOX 23-3 Communication Documentation
Communication with the Child
Although conscious sedation may sometimes be the targeted level, the ability to communicate is diminished in the majority of patients who receive PST.37,38 The ability to communicate without hindrance with a loved one who is seriously ill is precisely why PST is not routinely used in patients facing the end of life.7 Most families deeply miss the ability to communicate with their loved one, and once the patient is comfortable and sedated, they often wish for opportunities to reawaken and communicate with their loved one.8,32 Such longing may lead to feelings of anger, frustration, and disappointment. Families should be encouraged to continue to talk to their child and to participate in their care if desired. Some family members may be hesitant to touch their child. To the extent that they are comfortable doing so, family members should be encouraged to do certain things for their child, such as washing and dressing, or holding or massaging the child (Fig. 23-3). The use of respite sedation may be another strategy for permitting meaningful interactions with their loved one, if interrupted sedation does not evoke a crisis of uncontrolled suffering.
During PST, families are also acutely aware of the manner in which the care team interacts with their loved one. Even small gestures, such as talking to the child and attending to the environment, conveys compassion for the child as well as the message that the child will continue to receive the same attentive care received before sedation. A respectful manner that preserves the individual’s dignity and individuality is needed from all members of the care team. When respectful interactions that preserve the child’s dignity and individuality are not provided by all members of the care team, it may deeply impact families and their subsequent bereavement.31,32
Team Communication
Caring for a patient with intractable suffering can strain every member of a care team physically, emotionally, and morally. Although PST is a widely accepted therapy for otherwise intractable suffering at the end of life in many countries,2,5,19,39 including Japan, Europe, Canada, and the United States, support for PST may not necessarily be unanimous, particularly so when PST is administered for non-physical suffering. Serious disagreement among team members can jeopardize a team’s ability to function as a cohesive unit in effectively caring for a patient who is suffering at the end of life. It is imperative that all members have the freedom to share their views with others on the team in a non-judgmental and respectful environment. Those who object or who are significantly distressed by providing palliative sedation should be given the opportunity to change patient assignments without concern for repercussions. For those who continue to provide care for the child, frequent opportunities to debrief are essential.
Even when support for PST is unanimous, evidence exists that caring for patients who are receiving it may place a significant strain on care team members. For example, nurses report a significant burden providing PST for patients.40,41 Among a study of 3187 nurses in Japan, 30 percent reported wanting to leave their current work situation at least occasionally due to sedation-related burden.40 In qualitative work with nurses in the Netherlands, others found that a significant number of nurses believed that PST shortened life or was equivalent to euthanasia.41 Similarly, concern that sedation may shorten life is not infrequent among physicians.2,39 The Japan study also demonstrated that physicians who report higher levels of emotional exhaustion and depersonalization, indicators of professional burnout, were more likely to choose deep, continuous sedation for patients with refractory physical and psychological distress.39 Strategies to prevent occupational overload, including provision of PST by an interdisciplinary team and opportunities for staff to debrief in a supportive, nonjudgmental environment, are clearly needed and should be considered routine whenever PST is used.
Documentation
The importance of documentation whenever PST is considered cannot be underestimated. Outcomes of interdisciplinary assessments in determination of a symptom’s refractoriness, discussions with families regarding the PST option, and ongoing assessments of clinicians caring for a child receiving PST must all be thoroughly and routinely documented (see Box 23-3). Such documentation should be readily accessible by all members of the child’s care team. Documentation is particularly important for caregivers who may not have had participated in discussions, but are involved in providing sedation.
Physician Considerations
Choice of sedative
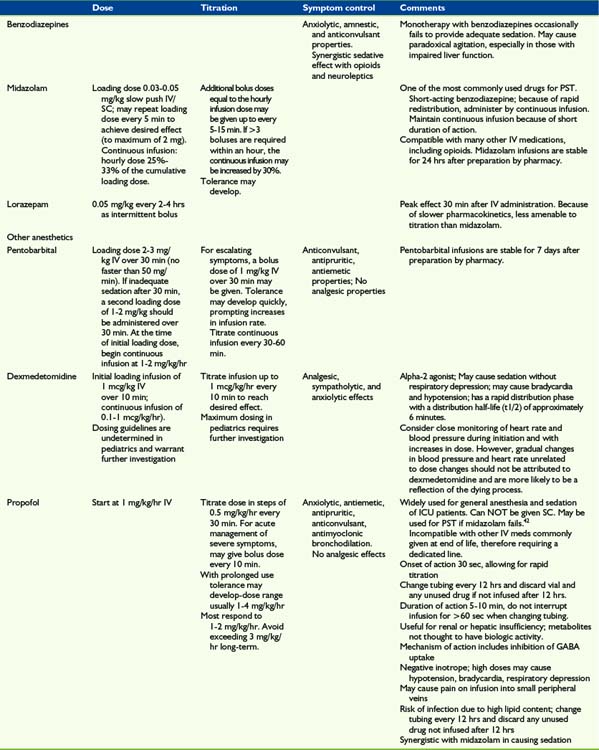
In the absence of controlled trials evaluating sedatives for PST, the drug choice is largely empirical. The choice of sedative is often driven by the particular clinical scenario, including the nature of the refractory symptoms, location of care, and whether continuous or respite sedation is desired. A rapid onset and short duration of action are properties that are frequently desirable for medications used to deliver PST (Table 23-1 and Box 23-4). The sedatives used in PST are usually benzodiazepines, neuroleptics, or barbiturates, although in recent years successful use of other anesthetic agents such as propofol has been reported.42,43
BOX 23-4 Guidelines for Administration of Palliative Sedation Therapy
While opioids are sometimes used expressly for their sedating properties, they are not effective in producing reliable, prolonged sedation. Furthermore, escalating opioids may have neuroexcitatory side effects such as delirium, hyperalgesia, or myoclonus. Therefore, opioids should not be delivered expressly for the purpose of achieving palliative sedation. However, for children already receiving opioids, they should be continued to provide ongoing analgesia and to prevent symptoms of withdrawal. The use of dexmedetomidine may permit discontinuation of opioids and benzodiazepines, as discussed later. Although ketamine has sedative and analgesic properties, it may cause unpleasant dysphoric or dissociative reactions, particularly in older children, and is not recommended as monotherapy for PST. Neuroleptics such as chlorpromazine, haloperidol, and methotrimeprazine are often cited in the PST literature,14,15 but do not by themselves reliably induce unconsciousness. However, for agitated delirium, a neuroleptic in conjunction with another agent, such as a benzodiazepine, may be a reasonable choice.
Midazolam and barbituates such as pentobarbital were some of the first agents reported to be used for PST.44,45 In the absence of delerium, midazolam is the agent of choice and is the most commonly used agent for PST.46 It has a rapid onset and short duration of action, facilitating titration. Pentobarbital also has a short onset of action and may alternatively be used, but it has a long half-life and is not readily reversed. Because of these properties, pentobarbital is not ideal for respite sedation.
Propofol and dexmedetomidine may be used for PST but are often not available outside the operating room or intensive care unit. If available, propofol is frequently an excellent choice because its rapid onset and duration of action allow for close control of sedation in response to symptoms and for variable depths of sedation.43 In fact, its onset and duration of action is 5 to 10 minutes, shorter than that of midazolam. A recent case series reported that propofol was able to provide relief of symptoms and conscious sedation by careful titration of the dose in nine of 22 patients.42 The remaining patients had symptoms that necessitated doses that kept them asleep. Propofol also has many other beneficial properties, including anxiolytic, antiemetic, antipruritic, anticonvulsant, and antimyoclonic effects.
Dexmedetomidine acts in the descending pain modulating system on post-synaptic alpha-2 receptors.47 It is associated with less delirium than midazolam in intubated ICU patients,48 has analgesic effects, and frequently allows patients to be sedated but communicative and cooperative.47 Dexmedetomidine is used for sedation during withdrawal of ventilatory support49 and may hold promise as a new agent for PST. If dexmedetomidine is used for sedation, opioids and benzodiazepines may be discontinued. Because this approach is novel, use of dexmedetomidine and discontinuation of opioids and benzodiazepines should be considered only in consultation with an anesthesiologist or a clinician with expertise in pain medicine.
Other physician considerations
The physician should document the outcome of the discussion with the family in the medical record (see Box 23-3), including the indication for PST, desired outcomes, and the family’s agreement with the plan. The physician should also ensure that a DNR order is in the chart. Once sedation has been titrated to relieve symptoms, the physician should ensure that clear parameters for sedative titration and notifying the physician are documented and discussed with the care team. The parameters are the initial infusion rate, rate of continuous infusion, time interval for infusion increases, dose and time interval for bolus doses, indications for infusion rate increases or bolus dose administration. The physician should also discontinue all orders not contributing to the patient’s comfort or goals of care. During ongoing PST, the physician should evaluate the child, and document efficacy of PST and occurrence and outcomes of ongoing discussions with the family and staff. Finally, even after PST has been initiated, the physician should continue to ensure ongoing interdisciplinary assessment of the patient for new and ongoing sources of distress.
Monitoring
Patient monitoring must consider goals of treatment, effectiveness of the intervention in relieving symptoms, and the degree of sedation achieved. For continuous sedation when there is no intent to awaken the patient, and a DNR order is in place, cardiopulmonary monitoring is usually not necessary, and the key monitored parameters are those that reflect the patient’s comfort.6 For example, respiratory rate may be monitored as a measure of respiratory distress. However, for respite sedation when death may not be imminent, monitoring of routine physiologic parameters such as heart rate, blood pressure, respiratory rate, and oxygen saturation may be in order. Observational assessments and physical exam can be used to make adjustments to therapy and the desired effects.
Monitoring sedation
To minimize the risk of treatment failure during PST, the degree of sedation should be regularly assessed.50 Awareness in patients undergoing surgery or conscious sedation is a rare event, but when it occurs it may be intensely distressing for patients. A large, multicenter study of adults undergoing PST in Japan found that 49 percent of patients awoke after falling into a deeply sedated state. Although awakening in this instance was defined as eye-opening, any movement of the extremities, or speech, it usually did not indicate distress.38
During the initiation and titration of therapy, the level of sedation is monitored frequently. Once sedation has been achieved, the level of sedation should be evaluated and documented at least every 4 hours. The level of sedation may be documented as mild, intermediate or deep or using a tool such as the Ramsay Scale.51 Consistent use of a sedation scale may be useful in monitoring the degree of sedation because comfort as well as degree may guide subsequent dosing of the sedative (Box 23-5).
BOX 23-5 Monitoring Recommendations
Ethical Considerations
PST has been criticized as a slow form of euthanasia. Euthanasia is the deliberate termination of a patient’s life by active intervention, at the request of the patient in otherwise uncontrollable suffering. The aim of PST is the relief of suffering, and not the shortening of life.5 The major distinguishing factor between slow euthanasia and palliative sedation is the intent of the clinician and care providers. The intent of the clinician, patient, and family receiving PST is to relieve suffering, not to cause death. Another distinguishing factor is the use of the lowest dose of medication in PST to relieve suffering, versus the use of lethal doses in euthanasia. The amount of medication, dose, and route for PST needs to be slowly titrated and the patient frequently monitored to establish the minimal level of sedation required to relieve the amount of intolerable suffering. The doctrine of double effect is often used to justify PST, but invocation of the doctrine is often unnecessary and overemphasizes an association between PST and hastening death.
Controversy with PST will continue to exist, which makes it imperative to have guidelines and frameworks to assist with the implementation of PST. As stated by Cherny for the European Association of Palliative Care, “inattention to potential risks and problematic practices can lead to harmful and unethical practice.”6 Each patient situation will vary and questions related to the ethics of PST will need to be evaluated. Ethics consultation is highly advisable in instances where disagreement exists, or consultation would support the patient, family, and healthcare team members in the decision-making process.
Summary
During the past decade, inroads in understanding the end-of-life experiences of children have been made. However, the presumed rarity of sedation in pediatric palliative care may pose a particular challenge for investigators aiming to further elucidate this treatment for children. While numerous retrospective and a few prospective studies in adults have been published,52 the pediatric palliative sedation literature is remarkably sparse. Practice is largely based on extrapolation from adult studies and anecdotal knowledge. Further study of this area is needed in order to understand the prevalence, practices, and needs of children with refractory suffering for whom PST might bring relief.
1 Enck R.E. Drug-induced terminal sedation for symptom control. Am J Hosp Palliat Care. 1991;8:3-5.
2 Chater S., Viola R., Paterson J., Jarvis V. Sedation for intractable distress in the dying: a survey of experts. Palliat Med. 1998;12:255-269.
3 Krakauer E.L., Penson R.T., Truog R.D., King L.A., Chabner B.A., Lynch T.J.Jr. Sedation for intractable distress of a dying patient: acute palliative care and the principle of double effect. Oncologist. 2000;5:53-62.
4 Jackson W.C. Palliative sedation vs. terminal sedation: what’s in a name? Am J Hosp Palliat Care. 2002;19:81-82.
5 de Graeff A., Dean M. Palliative sedation therapy in the last weeks of life: a literature review and recommendations for standards. J Palliat Med. 2007;10:67-85.
6 Cherny N.I., Radbruch L. European Association for Palliative Care (EAPC) recommended framework for the use of sedation in palliative care. Palliat Med. 2009;23:581-593.
7 Cherny N.I., Portenoy R.K. Sedation in the management of refractory symptoms: guidelines for evaluation and treatment. J Palliat Care. 1994;10:31-38.
8 Morita T., Inoue S., Chihara S. Sedation for symptom control in Japan: the importance of intermittent use and communication with family members. J Pain Symptom Manage. 1996;12:32-38.
9 Kenny N.P., Frager G. Refractory symptoms and terminal sedation of children: ethical issues and practical management. J Palliat Care. 1996;12:40-45.
10 Cassell E.J. Diagnosing suffering: a perspective. Ann Intern Med. 1999;131:531-534.
11 Rietjens J.A., van Delden J.J., van der Heide A., et al. Terminal sedation and euthanasia: a comparison of clinical practices. Arch Intern Med. 2006;166:749-753.
12 Hasselaar J.G., Reuzel R.P., van den Muijsenbergh M.E., et al. Dealing with delicate issues in continuous deep sedation: varying practices among Dutch medical specialists, general practitioners, and nursing home physicians. Arch Intern Med. 2008;168:537-543.
13 Hinds P.S., Drew D., Oakes L.L., et al. End-of-life care preferences of pediatric patients with cancer. J Clin Oncol. 2005;23:9146-9154.
14 Chiu T.Y., Hu W.Y., Lue B.H., Cheng S.Y., Chen C.Y. Sedation for refractory symptoms of terminal cancer patients in Taiwan. J Pain Symptom Manage. 2001;21:467-472.
15 Ventafridda V., Ripamonti C., De Conno F., Tamburini M., Cassileth B.R. Symptom prevalence and control during cancer patients’ last days of life. J Palliat Care. 1990;6:7-11.
16 Cherny N.I. Commentary: sedation in response to refractory existential distress: walking the fine line. J Pain Symptom Manage. 1998;16:404-406.
17 Morita T. Palliative sedation to relieve psycho-existential suffering of terminally ill cancer patients. J Pain Symptom Manage. 2004;28:445-450.
18 Rietjens J.A., Buiting H.M., Pasman H.R., van der Maas P.J., van Delden J.J., van der Heide A. Deciding about continuous deep sedation: physicians’ perspectives: a focus group study. Palliat Med. 2009;23:410-417.
19 Curlin F.A., Lawrence R.E., Chin M.H., Lantos J.D. Religion, conscience, and controversial clinical practices. N Engl J Med. 2007;356:593-600.
20 Sanft T., Hauser J., Rosielle D., et al. Physical pain and emotional suffering: the case for palliative sedation. J Pain. 2009;10:238-242.
21 Rousseau P.C. Palliative sedation and the fear of legal ramifications. J Palliat Med. 2006;9:246-247.
22 Peruselli C., Di Giulio P., Toscani F., et al. Home palliative care for terminal cancer patients: a survey on the final week of life. Palliat Med. 1999;13:233-241.
23 Quill T.E., Lo B., Brock D.W., Meisel A. Last-resort options for palliative sedation. Ann Intern Med. 2009;151:421-424.
24 Elsayem A., Curry Iii E., Boohene J., et al. Use of palliative sedation for intractable symptoms in the palliative care unit of a comprehensive cancer center. Support Care Cancer. 2009;17:53-59.
25 Braun T.C., Hagen N.A., Clark T. Development of a clinical practice guideline for palliative sedation. J Palliat Med. 2003;6:345-350.
26 Rosengarten O.S., Lamed Y., Zisling T., Feigin A., Jacobs J.M. Palliative sedation at home. J Palliat Care. 2009;25:5-11.
27 Siden H., Tucker T., Derman S., Cox K., Soon G.S., Hartnett S., Straatman L. Pediatric enteral feeding intolerance: a new prognosticator for children with life-limiting illness? J Palliat Care. 2009;25(3):213-217.
28 Silveira M.J., DiPiero A., Gerrity M.S., Feudtner C. Patients’ knowledge of options at the end of life: ignorance in the face of death. JAMA. 2000;284:2483-2488.
29 Garros D., Rosychuk R.J., Cox P.N. Circumstances surrounding end of life in a pediatric intensive care unit. Pediatrics. 2003;112:e371.
30 van Dooren S., van Veluw H.T., van Zuylen L., Rietjens J.A., Passchier J., van der Rijt C.C. Exploration of concerns of relatives during continuous palliative sedation of their family members with cancer. J Pain Symptom Manage. 2009;38:452-459.
31 Morita T., Ikenaga M., Adachi I., et al. Concerns of family members of patients receiving palliative sedation therapy. Support Care Cancer. 2004;12:885-889.
32 Brajtman S. The impact on the family of terminal restlessness and its management. Palliat Med. 2003;17:454-460.
33 Morita T., Ikenaga M., Adachi I., et al. Family experience with palliative sedation therapy for terminally ill cancer patients. J Pain Symptom Manage. 2004;28:557-565.
34 Stone P., Phillips C., Spruyt O., Waight C. A comparison of the use of sedatives in a hospital support team and in a hospice. Palliat Med. 1997;11:140-144.
35 Morita T., Tsunoda J., Inoue S., Chihara S. Effects of high dose opioids and sedatives on survival in terminally ill cancer patients. J Pain Symptom Manage. 2001;21:282-289.
36 Maltoni M., Pittureri C., Scarpi E., et al. Palliative sedation therapy does not hasten death: results from a prospective multicenter study. Ann Oncol. 2009;20:1163-1169.
37 Mercadante S., Intravaia G., Villari P., Ferrera P., David F., Casuccio A. Controlled sedation for refractory symptoms in dying patients. J Pain Symptom Manage. 2009;37:771-779.
38 Morita T., Chinone Y., Ikenaga M., et al. Efficacy and safety of palliative sedation therapy: a multicenter, prospective, observational study conducted on specialized palliative care units in Japan. J Pain Symptom Manage. 2005;30:320-328.
39 Morita T., Akechi T., Sugawara Y., Chihara S., Uchitomi Y. Practices and attitudes of Japanese oncologists and palliative care physicians concerning terminal sedation: a nationwide survey. J Clin Oncol. 2002;20:758-764.
40 Morita T., Miyashita M., Kimura R., Adachi I., Shima Y. Emotional burden of nurses in palliative sedation therapy. Palliat Med. 2004;18:550-557.
41 Rietjens J.A., Hauser J., van der Heide A., Emanuel L. Having a difficult time leaving: experiences and attitudes of nurses with palliative sedation. Palliat Med. 2007;21:643-649.
42 Lundstrom S., Zachrisson U., Furst C.J. When nothing helps: propofol as sedative and antiemetic in palliative cancer care. J Pain Symptom Manage. 2005;30:570-577.
43 McWilliams K., Keeley P.W., Waterhouse E.T. Propofol for terminal sedation in palliative care: a systematic review. J Palliat Med. 2010;13:73-76.
44 Truog R.D., Berde C.B., Mitchell C., Grier H.E. Barbiturates in the care of the terminally ill. N Engl J Med. 1992;327:1678-1682.
45 Burke A.L., Diamond P.L., Hulbert J., Yeatman J., Farr E.A. Terminal restlessness: its management and the role of midazolam. Med J Aust. 1991;155:485-487.
46 Cowan J.D., Walsh D. Terminal sedation in palliative medicine: definition and review of the literature. Support Care Cancer. 2001;9:403-407.
47 Soares L.G., Naylor C., Martins M.A., Peixoto G. Dexmedetomidine: a new option for intractable distress in the dying. J Pain Symptom Manage. 2002;24:6-8.
48 Riker R.R., Shehabi Y., Bokesch P.M., et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499.
49 Kent C.D., Kaufman B.S., Lowy J. Dexmedetomidine facilitates the withdrawal of ventilatory support in palliative care. Anesthesiology. 2005;103:439-441.
50 Davis M.P. Does palliative sedation always relieve symptoms? J Palliat Med. 2009;12:875-877.
51 Ramsay M.A., Savege T.M., Simpson B.R., Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656-659.
52 Hasselaar J.G., Verhagen S.C., Vissers K.C. When cancer symptoms cannot be controlled: the role of palliative sedation. Curr Opin Support Palliat Care. 2009;3:14-23.