Chapter 51 Palliation of Malignant Pancreaticobiliary Obstruction
![]() Video related to this chapter’s topics: Palliation of Malignant Biliary Obstruction with Self-Expanding Metallic Stent
Video related to this chapter’s topics: Palliation of Malignant Biliary Obstruction with Self-Expanding Metallic Stent
Epidemiology
Of all pancreaticobiliary malignancies, pancreatic adenocarcinoma has the highest incidence, with around 30,000 new cases annually in the United States. It ranks fifth among the leading causes of cancer-related deaths.1,2 Only 10% of patients are suitable candidates for resection, and the overall 5-year survival rate is less than 4%.3,4 The incidence of gallbladder carcinomas is 1 per 100,000 person-years. The survival rate is only slightly higher than that of pancreatic carcinoma.2 Patients most likely to survive are patients in whom early cancer was detected in a postcholecystectomy specimen. Klatskin’s tumors also have a poor prognosis, with less than 10% of patients surviving 5 years after being diagnosed and most patients dying in the first year.5 The number of potentially resectable tumors is low, ranging from 10% to 20%. In ampullary carcinoma, biliary obstruction usually develops early in the course of the disease. Tumors are usually small, and radical resection is possible in most cases, with an overall 5-year survival rate of 50%.6
Pathogenesis
A detailed discussion of the pathogenesis of pancreaticobiliary malignancies is beyond the scope of this chapter; however, several epidemiologic studies have identified risk factors for the development of pancreaticobiliary malignancies. Tobacco smoking doubles the risk of pancreatic cancer.7,8 Patients with chronic pancreatitis have an increased risk for developing pancreatic cancer that is estimated at 4% per 20 years.9 The risk of developing pancreatic cancer in patients with hereditary pancreatitis is 50%, with smoking as an important risk modifier.10,11 Etiologic factors for cholangiocarcinoma include primary sclerosing cholangitis and hepatolithiasis.12,13 Gallstone disease is the most important risk factor for gallbladder cancer.14
Pathology
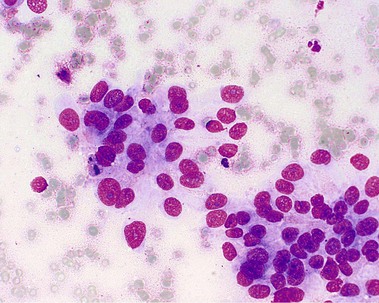
About 90% of pancreaticobiliary malignancies are ductal adenocarcinomas (Fig. 51.1). Most of these tumors arise from the pancreatic head. Other exocrine malignancies are mucinous cyst adenocarcinoma and acinar cell carcinomas. Endocrine tumors include gastrinoma and insulinoma. Metastases of a primary tumor (mammary, lung, and melanoma) and lymphoma should be considered because of important treatment implications (e.g., chemotherapy). Mesenchymal tumors are extremely rare.
Cytologic brushings are easy to obtain and widely used. Specificity approaches 100%, but sensitivity is 30% to 60%.15,16 The sensitivity in cholangiocarcinoma is greater than in pancreatic carcinoma. Forceps biopsy requires endoscopic sphincterotomy and is associated with a slightly increased risk of complications.17 Biopsy specimens of ampullary tumors can be obtained directly. Sampling of ductal fluid is a simple method, but the sensitivity is very low, and it is not used very often. Several studies have shown that sensitivity can be increased by combining different techniques of tissue sampling.16,18 Endoscopic ultrasound (EUS)–guided fine needle aspiration biopsy has an excellent sensitivity of 85% to 90% and specificity of virtually 100%.19 Although these tests may be useful in making the diagnosis of carcinoma, a negative test cannot rule out malignant disease. Percutaneous fine needle aspiration biopsy is another accurate method for confirmation of malignancy, with a sensitivity of 60% to 90%.20 However, needle tract seeding has been described, and this technique should be used only for tissue confirmation in the case of unresectable disease.
Differential Diagnosis
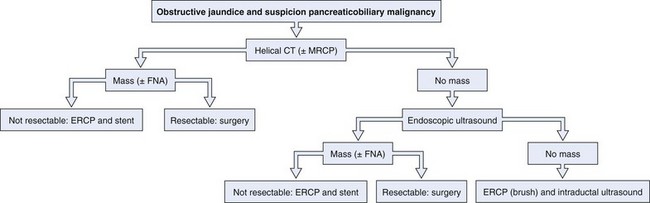
An enlarged pancreatic head may be caused either by pancreatitis or by carcinoma. The patient’s history and clinical presentation contribute to making a diagnosis. Autoimmune pancreatitis is an increasingly recognized condition and may mimic a malignant tumor. Differential diagnosis is based on a distinct clinical picture with a diffusely enlarged pancreas with a nondilated pancreatic duct, elevated IgG4 levels, and a prompt response to corticosteroid therapy with improvement of clinical symptoms including jaundice and resolution of morphologic abnormalities on cross-sectional imaging.21 Cystic lesions of the pancreas may be benign (pancreatic pseudocyst or serous cystadenoma), premalignant (mucinous cystadenoma), or malignant (cystadenocarcinoma). Radiologic imaging is used to characterize these lesions. EUS in combination with fine needle aspiration and fluid analysis may increase accuracy of the diagnosis further. In the case of a suspicious stricture in the mid–bile duct or proximal bile duct, a gallbladder carcinoma should be included in the differential diagnosis. It is important to exclude benign causes of strictures, such as Mirizzi’s syndrome, primary and secondary sclerosing cholangitis, and postoperative conditions. An algorithm for the diagnosis of pancreaticobiliary cancer is presented in Fig. 51.2.
Treatment
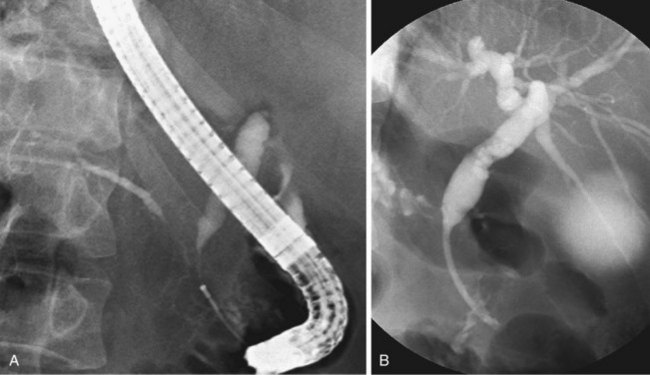
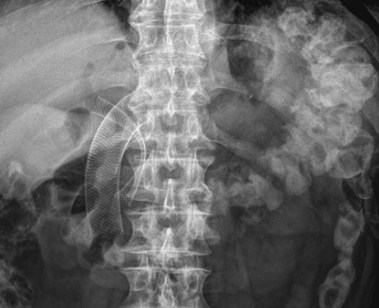
Since the introduction of endoscopic biliary stent therapy in 1980, the palliative treatment of pancreaticobiliary malignancies has changed considerably. At the present time, endoscopic stent placement to relieve jaundice is well established and is considered the preferred treatment (Fig. 51.3). Compared with percutaneous and surgical drainage, endoscopic biliary stent therapy is associated with lower morbidity and mortality rates.22–24 The main problem of endoscopic biliary drainage is late stent occlusion, which necessitates stent exchange. The technical success rate of endoscopic biliary drainage is 70% to 90% and is higher for distal tumors compared with more proximal malignancies involving the bifurcation. The complication rate of therapeutic ERCP is 5% to 10%.25,26
Indications and Contraindications
The indications for ERCP with a drainage procedure by stent placement include jaundice, fever, and pruritus. Biliary stent placement has also been shown to improve symptoms of anorexia and quality of life.27,28 It has been suggested that preoperative biliary drainage may improve surgical outcome after pancreaticoduodenectomy, but this has not been substantiated in clinical trials.29,31 In a randomized study comprising 202 patients with pancreatic head carcinoma comparing direct surgery with delayed surgery after biliary drainage, surgical outcome and complication rates were not affected by preoperative stent placement. The overall complication rate in the delayed surgery group with preoperative stent placement was significantly increased, mainly owing to stent-related complications. The outcome of this study strongly argues against standard preoperative drainage in patients with pancreatic head cancer in whom immediate surgery is planned. Preoperative drainage is indicated, however, when operative resection is not imminent. One common example is preoperative drainage performed with neoadjuvant chemotherapy. Some authors argue for preferential insertion of an expandable metal stent owing to their comparable durable patency.32,33 There are no absolute contraindications. Coagulation disorders are a relative contraindication and should be corrected before ERCP.
Overview of Stents for Biliary Drainage
Plastic Stents
The median patency of a conventional 10-Fr plastic stent is 3 to 6 months. The incidence of stent occlusion is 20% to 50%.34–36 The initial event in stent blockage is adherence of proteins and bacteria to the inner wall of the stent and subsequent formation of a biofilm. Bacteria are introduced into the biliary system during transpapillary placement of the stent. Sludge forms from the accumulation of bacteria, which produce β-glucuronidase and form calcium bilirubinate and calcium palmitate.37–39 Many efforts have been made to prolong stent patency, some of which are discussed in the following paragraphs.
Stent Diameter
The first biliary stents that were placed were only 7-Fr or 8-Fr in diameter because of limitations of the diameter of the working channel of the endoscope (2.8 mm). When side-viewing endoscopes with large-diameter working channels (4.2 mm) were introduced in 1980, it became possible to insert large-bore plastic stents.40 Larger stents (10-Fr) perform better than smaller stents (7-Fr)41 apparently because of the higher flow rate, as predicted by Poiseuille’s law, and less stasis with larger diameter stents. Theoretically, bile flow rate is proportional to the internal diameter raised to the fourth power; even a small increase in diameter results in a substantial increase in flow capacity.42 In contrast to this hypothesis, the use of larger diameter plastic stents of 11.5 Fr or 12 Fr did not result in further improvements in stent patency.43–45
Stent Design
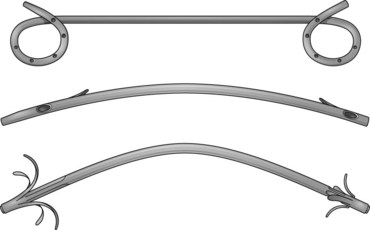
The first biliary stents had a pigtail configuration at the proximal end to provide better anchorage. Straight stents were developed because of their improved bile flow characteristics compared with pigtail stents (Fig. 51.4).42,46,47 Huibregtse and Tytgat48 developed the Amsterdam-type stent—a straight design with two side holes to facilitate biliary drainage and two side flaps to prevent dislocation—which has been the standard type of stent since 1980.
Sludge in plastic stents mainly accumulates around side holes.37,49 This accumulation seems to be the result of higher intraluminal flow turbulence and decreased flow rates.42 Soehendra and others50,51 postulated that elimination of side holes might improve patency rates and designed the so-called Teflon Tannenbaum stent (a straight stent without side holes and with multiple proximal and distal side flaps to prevent dislocation). At first, uncontrolled results were encouraging, with patency rates comparable to metal stents, but randomized trials could not confirm these initial results.52–54 Omitting side holes in a standard-design polyethylene stent also did not improve stent patency.55
Stent Material
Different materials have been used for stent construction, including polyethylene, polyurethane, and polytef (Teflon). In vitro studies have shown a direct relationship between the coefficient of friction and the amount of encrusted material. Teflon has the lowest friction coefficient and the best potential for preventing stent clogging.37 Initially, Teflon Tannenbaum stents showed a favorable patency rate.50,51 A randomized study comparing Amsterdam-type stents made from polyethylene and Teflon did not show a difference in stent patency.56 Other controlled clinical trials also could not confirm the superiority of Teflon material in a Tannenbaum-design stent.52–54
Scanning electron microscopy of out-of-package biliary stents has shown that the inner surface smoothness of plastic stents is highly variable. This variability is possibly a result of the manufacturing process of plastic stents by extrusion. Only the polyurethane stent was found to have an extremely smooth surface.57 Two new polymers were introduced with an ultrasmooth surface, Vivathane and Hydromer. Both materials have been shown to reduce bacterial adherence in vitro.58,59 In addition, the Hydromer stent has not only a smooth texture but also a coating that absorbs water and provides a hydrophilic sheath. Because bacteria initially attach by hydrophobic interactions, this coating could potentially lower bacterial adhesion and increase stent patency. However, the encouraging results of in vitro studies could not be confirmed in prospective clinical trials.60,61
Stent Coating
Priming the inner surface of a stent with a coating comprising some form of antiadhesion property may reduce biofilm formation and stent clogging. Antibiotics, antithrombotics, silver, and hydrophilic coating all were effective in reducing bacterial colonization in vitro.59,62,63 However, clinical studies using antibiotic-coated or hydrophilic-coated stents did not show any benefit.61
Stent Position
Placing the stent entirely within the common bile duct has the theoretical advantage of preserving the barrier function of the sphincter of Oddi; this prevents duodenal reflux of food and bacteria into the stent and biliary tree. This so-called inside stent approach can be performed only when a free margin of 1 to 2 cm is maintained between the distal end of the stricture and the papilla. With this parameter in mind, about one-third of patients with malignant obstructive jaundice are potential candidates for such treatment.64 However, no difference was found in stent performance in a randomized trial. In the inside stent group, stent migration occurred significantly more often.65
Antibiotics
In vitro studies showed that antibiotic treatment reduced bacterial adherence to plastic stents.66 In a prospective randomized study with ciprofloxacin, no difference in stent patency was found.67 In another study, rotating antibiotics (cycles of 2 weeks of ampicillin, metronidazole, and ciprofloxacin) were combined with ursodeoxycholic acid, and no difference in stent patency was shown.68 Only one small pilot study showed a reduced rate of stent blockage with norfloxacin plus ursodeoxycholic acid.69 Other studies combining antibiotics and bile salts (ofloxacin and ursodeoxycholic acid, ciprofloxacin, and Rowachol) did not show a longer duration of stent patency.70,71 There is no compelling evidence that stent patency benefits from antibiotic prophylaxis.
Aspirin
Animal studies in prairie dogs showed that aspirin inhibits mucous glycoprotein secretion by blocking prostaglandin synthesis.72 In a clinical study, the use of aspirin reduced the content of all sludge components, although no effect was shown on stent patency.73 No further studies using aspirin have been performed.
Bile Salts
Bile salts have a potent antibacterial effect and may stimulate bile flow. Because bacteria attach by hydrophobic interactions, hydrophobic bile salts (deoxycholate, taurodeoxycholate) inhibit initial bacterial attachment, as was shown in experimental studies.74 However, hydrophobic bile salts are not well tolerated. Hydrophilic bile salts such as ursodeoxycholate, which are better tolerated, have a minimal effect on bacterial adhesion. Except for one small pilot study, different prospective clinical studies using ursodeoxycholic acid alone or combining ursodeoxycholic acid with antibiotics could not show a difference in stent patency.68–71
Stent Exchange
Some endoscopists prefer to schedule patients for elective stent exchange every 3 to 4 months. The optimal time interval is unknown.75,76 Prophylactic stent exchange requires a repeat (clinically not indicated) endoscopy and has to be compared with the risks of watchful waiting and the risk of (severe) cholangitis. Because most patients do not develop stent occlusion before dying of the underlying disease, most endoscopists favor an expectant management strategy.
Stent Cleaning
Some endoscopists have proposed leaving an occluded stent in situ and cleaning the obstructed lumen with a cytology brush or flushing with saline instead of performing stent replacement.77 However, stent cleaning carries the risk of inducing biliary sepsis by actively introducing the biofilm of the stent and bacteria from the duodenum into the biliary tract. Stent cleaning is not recommended.
Self-Expanding Metal Stent
The diameter of biliary stents was restricted by the size of the instrumentation channel of the endoscope until the development of self-expanding metal stents. All currently available expandable stents are made of metal. They differ in the way they are braided, the size of the mesh, the metal used, and their rigidity. At the present time, different types of self-expanding metal stents are available from various manufacturers (Fig. 51.5). To date, the most experience has been gained with the self-expanding Wallstent (Boston Scientific, Natick, MA). This stent is delivered in a collapsed configuration on an 8-Fr delivery system. When deployed, it expands to a final diameter of 30 Fr (approximately 10 mm) and shortens about 30% in length. The final diameter is achieved after 1 week, when equilibrium is achieved between the dilating force of the stent and the resistance of the bile duct wall and tumor. These large-caliber self-expanding metal stents of 30 Fr remain patent for longer than plastic stents but do not prevent blockage indefinitely. Metal stents with a 6-mm diameter occlude significantly more frequently than 10-mm (30-Fr) metal stents, showing that size is the most important determining factor for stent patency.78
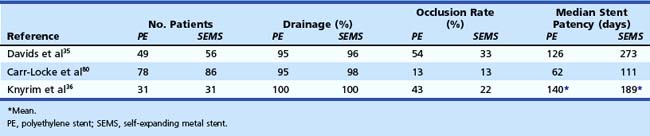
Because of their design, self-expanding metal stents have much less surface to which bacteria can adhere. The mechanism of stent blockage differs from that seen in plastic stents and includes tumor ingrowth through the interstices of the stent or overgrowth of the end of the stent and intima hyperplasia. Several studies have shown a median stent patency of about 6 to 9 months (Table 51.1).35,36,76,79,80 Self-expanding metal stents are more difficult to insert, uncovered metal stents cannot be removed after deployment, and initial costs are high (about $1000). Various types of self-expanding metal stents are available to date.
Wallstent
The initial endoscopic placement experience was reported in 1989.81 The Wallstent is made from stainless steel alloy filaments braided in a tubular mesh configuration. In the early phase of development, technical problems mainly involved the restraining membrane failing to retract completely, but this is now rarely seen.82 The first randomized trial comparing plastic stents and the Wallstent was performed by Davids and coworkers.35 Wallstent patency was superior to patency of plastic stents, with a median duration of 9 months. These results were confirmed in several other studies.36,76,83 The Wallstent is offered in two diameters (8 mm and 10 mm) and various lengths (40 mm, 60 mm, 80 mm, and 100 mm). The Wallstent is also available with a covering designed to resist tumor ingrowth.
Covered Self-Expanding Metal Stent
Tissue ingrowth through the meshes of the stent is responsible for stent occlusion in about 22% to 33% of patients.35,36 To overcome this problem, self-expanding metal stents have been covered with a polyurethane or silicone membrane. Results of various stents in studies are contradictory.84–86 At first, many prospective cohort studies could not confirm a lower rate of tumor ingrowth while using covered metal stents.87,88 However, in a prospective comparative study, stent obstruction owing to tumor ingrowth occurred significantly less frequently with covered stents compared with uncovered stents.89 Data with regard to the risk of complications are scarce, but stent migration, cholecystitis, and pancreatitis seem to occur at a slightly higher rate.88–90 There seems to be no benefit from endoscopic papillotomy before deployment of covered metal stents with regard to the prevention of pancreatitis, whereas migration rates may increase.91 Covered stents should not be used intrahepatically because of occlusion of hepatic side branches by the covering membrane.
Plastic versus Metal Stent
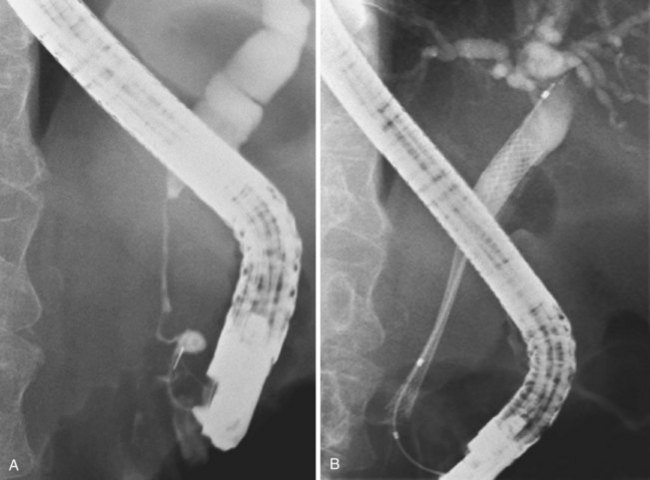
Self-expanding metal stents have a longer duration of patency than plastic stents and ideally should be placed in all patients. The high initial costs have limited their use in different health care settings worldwide. In a cost-effective approach, the choice between a plastic and metal stent depends mainly on an estimate of patient survival. Tumor size seems to be a reliable predictor of survival. Prat and coworkers92 claimed that in the case of a tumor greater than 30 mm, a polyethylene stent should be placed because of shorter expected survival. The presence and number of liver metastases have also been shown to be independently related to prognosis.93,94 Comparative studies did not show any benefit of self-expanding metal stents compared with polyethylene stents in the first 3 months after insertion.35,76 It seems reasonable to insert a polyethylene stent in patients with a life expectancy of less than 3 months (Fig. 51.6). If expected survival is 3 to 6 months, a self-expanding metal stent should be considered (Fig. 51.7). Different authors have shown this strategy to be cost-effective.35,95–98 Patients who present with early clogging of a polyethylene stent (within 1 month) should also receive a self-expanding stent, regardless of their life expectancy, although this has not been proved in prospective studies.99
Antibiotics before Stent Placement Procedure
Drainage of the biliary tree is the mainstay of therapy for patients with cholangitis. There is controversy about the routine use of preprocedure antibiotic prophylaxis.100–102 Preoperative administration of antibiotics should definitely be started in a patient with fever. Because failure to drain the entire biliary tree is the most important risk factor associated with cholangitis after ERCP, antibiotic prophylaxis should also be administered in a highly selective group of patients in whom incomplete drainage is anticipated, such as patients with a hilar malignancy or primary sclerosing cholangitis.103,104 Prophylaxis can be given as a single, adequate dose shortly before the procedure. If contrast agent is injected in the biliary tract but obstruction cannot be relieved, antibiotic therapy should be continued (or started) until drainage is established.
Technique of Stent Placement
A large-channel (4.2-mm) side-viewing therapeutic endoscope is introduced into the second portion of the duodenum. Standard cannulation of the papilla of Vater is performed by a ball-tip or cone-tip catheter; eventually cannulation can be attempted with a guidewire inserted in the ball-tip catheter. If this approach fails, a double-lumen sphincterotome with a guidewire (cannulatome) should be used. Use of this device may aid in achieving an optimal angle for bile duct cannulation. If a sphincterotome is unsuccessful, a precut sphincterotomy is performed to obtain biliary access.105 With the use of all these different techniques, deep cannulation is achieved in up to 95% of patients.
Plastic Stents
Management of Plastic Stent Occlusion
A clogged plastic stent can be removed with the use of a snare or dormia basket. It is important to keep the position of the endoscope in line with the common bile duct. When a snare is used, the stent is caught in the snare and removed through the instrumentation channel of the endoscope. When a dormia basket is used, the stent is pulled close to the endoscope, and both the endoscope and the stent are withdrawn. When massive tumor invasion is present in the duodenum, and difficult stent exchange is anticipated because of a nonoptimal scope position, it can be helpful to leave the occluded stent in place and use it as a guide for common bile duct cannulation and introduction of a second stent. Soehendra and coworkers106 described a technique that enables the removal of a clogged stent while maintaining the original pathway to the bile duct. A ball-tip catheter is positioned at the distal end of the stent, and the stent is cannulated with the guidewire. A Soehendra retriever is introduced over the guidewire, and the tip is screwed into the distal end of the stent. The retriever is pulled out along with the stent, leaving the guidewire in place.
Self-Expanding Metal Stents
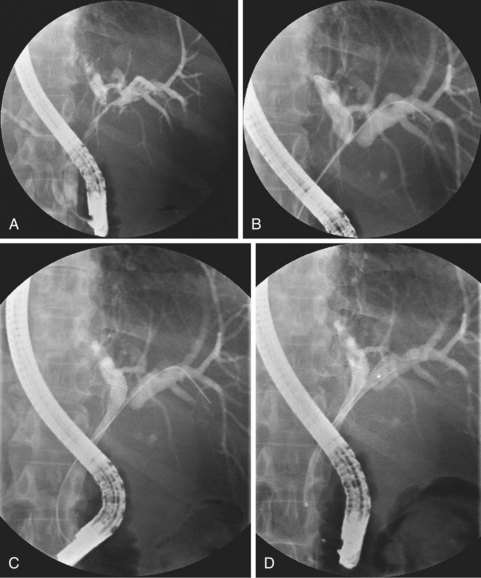
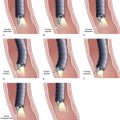
Deployment reduces the length of certain self-expanding metal stents up to about 30%. It is important to correct the position of the expanding stent constantly under fluoroscopic control, which usually means that one has to pull the insertion device outward while deploying the stent. When the expanding metal stent bridges the papilla, in the case of a distal stenosis, the endoscopic image is used to keep a fixed distance of about 1 cm between the papillary orifice and the distal margin of the stent. Stent diameter expands to 8 to 10 mm, and the available deployed lengths are 40 mm, 60 mm, 80 mm, and 100 mm. In case of a complex hilar stricture in which both liver lobes are drained by two or more self-expanding endoprostheses, the procedure is as follows.107 First, two stiff guidewires are introduced, one in each liver lobe. If appropriate, dilation of a stricture is performed over one of the guidewires. An expanding metal stent is inserted over the guidewire into the left system and deployed. Finally, an expanding metal stent is inserted into the right system alongside the first stent and deployed under fluoroscopic control (Fig. 51.8). Although technically difficult, it is also possible to insert a second self-expanding metal stent through the meshes of a formerly placed self-expanding stent.108 In such a case, a guidewire is introduced, and the mesh is dilated using a balloon catheter before passing the second constrained stent and deploying it.
Management of Occlusion of a Self-Expanding Metal Stent
A noncovered self-expanding metal stent can be removed without problems within the first days after deployment by grasping it with a forceps or a snare. After this time, the stent becomes embedded in the tumor tissue, and extraction can become extremely difficult, although successful removal has been reported after months.109 Stent obstruction is mainly due to tumor ingrowth through the interstices of the stent or overgrowth of the ends of the stent. Management of stent occlusion consists of placement of a polyethylene stent or a second self-expanding metal stent through the occluded self-expanding metal stent. Another strategy is mechanical cleaning by using a balloon and flushing, but this is effective only in cases of sludge formation. Covered stents can be removed more easily for a much longer period compared with uncovered stents.109,110 Covered stents can be grasped at their distal end with a snare or with a forceps in case a retrieval loop is present.
Intrahepatic Biliary Obstruction
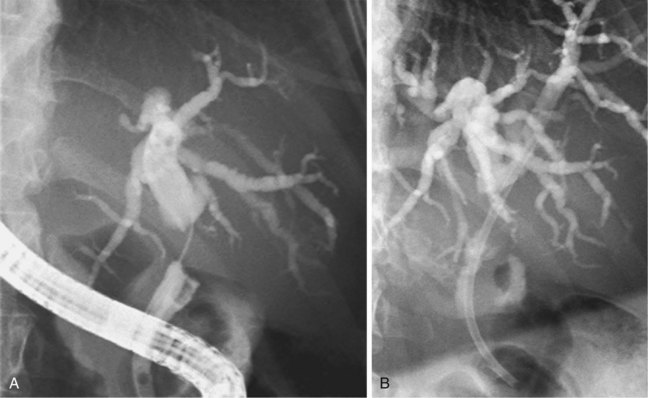
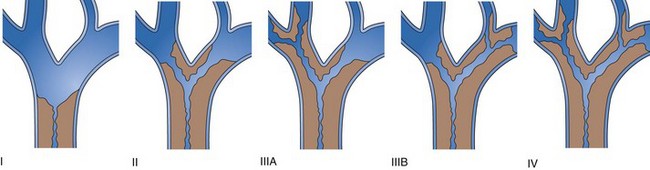
Strictures at the level of the hepatic confluence account for about 20% of malignant bile duct obstruction and mainly consist of primary cholangiocarcinoma, gallbladder neoplasms, and metastatic spread to hilar nodes. Cholangiocarcinoma arising at the hilar level is also referred to as Klatskin’s tumor and is classified according to the degree of involvement of the intrahepatic bile ducts (Fig. 51.9).111 Stent placement in the proximal biliary tree is more challenging and is associated with lower success rates than stent placement for distal common bile duct stenosis. Drainage can be achieved either endoscopically (retrograde) or percutaneously (antegrade). Procedure-induced cholangitis caused by contrast agent injection in undrained biliary branches is the main complication and occurs in 30% of cases.112–114 The current management strategy (depending on local services available) is first to attempt endoscopic drainage; when this strategy is unsuccessful, percutaneous drainage offers additional opportunities.115–117 When internal drainage fails, an external drain can be left in situ, minimizing the risk of cholangitis.
Unilateral versus Bilateral Drainage
There is controversy whether to drain one or both liver lobes in Bismuth type II, III, and IV strictures. In Bismuth type I, one stent always suffices because the left and right ducts communicate, and drainage is complete. Theoretically, at least 25% of the liver volume must be drained to achieve biochemical improvement and relief of symptoms.118 Concerns about unilateral drainage include the inability to relieve jaundice and the potential for bacterial contamination in the undrained lobe. The worst treatment results seem to be obtained in patients with cholangiographic opacification of both lobes but drainage of only one.119
In a prospective randomized trial comparing unilateral with bilateral hepatic duct drainage, the latter procedure was associated with a significantly higher rate of complications because of the higher rate of early cholangitis.120 In per-protocol analysis, the rates of successful drainage, complications, and mortality did not differ between the two groups. Magnetic resonance cholangiopancreatography (MRCP)–guided endoscopic stent placement in Bismuth III and IV malignancies was associated with a low morbidity and mortality in an uncontrolled study.121 The intention was to place a unilateral stent in one of both lobes, guided by the MRCP picture, and to avoid entry and contrast agent injection in the contralateral lobe. In patients in whom, by accident, guidewire entry (50%) or contrast agent injection (20%) occurred in the contralateral liver lobe, stents were placed bilaterally. This treatment strategy resulted in a very low cholangitis rate of only 6%. A more recent study evaluated selective unilateral MRCP-targeted or CT-targeted drainage, and no episodes of cholangitis were observed.122 The message seems to emerge that unilateral drainage is appropriate when unilateral cannulation and opacification has been achieved. If the contralateral lobe is (unintentionally) opacified or probed, it should also be drained to avoid cholangitis.
Plastic versus Self-Expanding Metal Stent
By design, expandable stents may be more suitable than plastic stents for draining hilar tumors. The stent lumen is much wider, and, more importantly, intrahepatic side branches can drain through the metal meshes. Self-expanding metal stents that were inserted via the percutaneous route showed a higher rate of treatment efficacy than plastic stents.115,123 No randomized studies comparing endoscopic and percutaneous insertion of self-expanding metal stents in hilar strictures are available. Additional proof of the superiority of self-expanding metal stents over plastic stents is suggested by a retrospective series of patients with nonresectable hilar cholangiocarcinoma in whom plastic stents were replaced by metal expandable stents during stent treatment.124 Successful palliation without the need for further biliary reintervention was achieved in most patients (69%). In a prospective multicenter observational cohort study in patients with a malignant hilar biliary obstruction, it was observed that metallic stent performance was superior to plastic stent performance for hilar tumor palliation with respect to short-term outcomes (30 days), independent of disease severity, Bismuth class, or drainage quality.125
For primary bilobar drainage, a specialty “Y” stent has been introduced consisting of two uncovered Niti-S Y-type biliary stents (TaeWoong Medical Co. Ltd.). The first self-expanding metal stent has a radiologically marked segment with wider mesh holes in its middle part, through which the second stent is advanced into the contralateral liver lobe.126 A potential drawback of the placement of a metal stent is that introduction of additional stents in the case of treatment failure may become difficult. However, a technique for introducing a second stent through the wire mesh of the first stent has been described.108
Duodenal Stenosis
Duodenal stenosis resulting from pancreaticobiliary malignancies occurs in 10% to 20% of patients.127 Presenting symptoms include nausea and vomiting resulting from gastric outlet obstruction. Duodenal stenosis is usually a late event and occurs in patients in poor general condition who have already received a biliary endoprosthesis.128 Surgical bypass has a significant procedure-related mortality of 10% and related morbidity and prolonged hospital stay.24,129,130 Endoscopic stent treatment for duodenal obstruction with bile duct stent placement may be an effective alternative. Placement of duodenal stents has a high technical success rate without major procedure-related complications.131–134 Stent placement is performed under simultaneous endoscopic and fluoroscopic control. Patients are usually able to tolerate a liquid diet immediately after stent placement. Full stent deployment may take a few days, during which time soft foods are allowed.
The results of a retrospective study in 95 patients suggest that duodenal stent placement is associated with better short-term outcomes, and gastrojejunostomy is associated with better long-term outcomes.135 The choice of treatment modality may depend on the life expectancy of the patient. One study reported simultaneous endoscopic decompression of biliary and duodenal obstruction with similar success rates compared with duodenal stent placement alone.136 Because of the difficulty of accessing the biliary tree endoscopically through the mesh wall of a duodenal stent, an expanding metal biliary stent should preferably be placed before the duodenal stent is introduced (Fig. 51.10). In expert hands, however, it seems also feasible to drain the biliary tree endoscopically through the meshes of a metal duodenal stent.137 If endoscopic biliary stent treatment fails, the remaining treatment options are percutaneous stent placement, combined percutaneous and endoscopic management, and surgical bypass.
Complications
Early Complications
Early complications are defined as complications that occur less than 1 week after the conclusion of the procedure. The rate of complications is 5% to 10% for therapeutic ERCP with a mortality rate of up to 1%.25,26,138 Cotton and coworkers25 introduced a grading system in which complications are graded as mild, moderate, and severe, and these guidelines are still widely used. The most frequent early complication is cholangitis, probably resulting from introduction of bacteria into the biliary tract during the procedure. Cholangitis is reported in approximately 10% to 15% of patients in most series. It occurs more often after endoscopic procedures for complex hilar strictures when incomplete drainage is achieved. The same holds true for patients with primary sclerosing cholangitis. In these high-risk procedures, antibiotics should be administered prophylactically and continued for a few days after the procedure.
Pancreatitis develops after ERCP in about 5% to 7% of patients. It is defined as new-onset or increased abdominal pain lasting at least 24 hours after ERCP, with associated elevation in serum amylase or lipase to at least three to five times normal.25,26,139 Most cases are mild, are self-limiting, and require only intravenous fluids and gut rest. Serious cases may evolve into (infected) necrotizing pancreatitis with multiorgan failure.
The rate of postsphincterotomy bleeding is about 0.2% to 5% with an associated mortality rate less than 1%.140 Bleeding is usually obvious immediately after sphincterotomy but can be delayed for hours or several days. Most episodes of delayed bleeding are managed successfully by conservative measures and blood transfusions if the hemoglobin level decreases significantly. Postsphincterotomy bleeding usually occurs at the apex of the sphincterotomy site and can be managed endoscopically with injection of epinephrine.
Retroperitoneal perforation occurs in less than 1% of cases in most series. It may be caused by standard sphincterotomy, precut sphincterotomy, or guidewire manipulation. Most cases are diagnosed or suspected during ERCP. These perforations mostly heal with conservative measures and do not usually result in clinical symptoms.141 Conservative treatment measures consist of giving nothing by mouth, antibiotic treatment, and nasogastric suction. About 20% to 30% of patients require surgery. In cases of peritoneal perforation caused by the duodenoscope, prompt exploratory laparotomy, with repair or oversewing of the defect in the duodenal wall, is mandatory.142
Late Complications
The primary late complication of stent placement is occlusion of the endoprosthesis, which can occur in 50% of cases.35,36 These patients present clinically with a flulike syndrome with cholestasis, frank cholangitis, or jaundice. Treatment consists of exchange of the occluded stent or, in the case of an occluded self-expanding metal stent, insertion of a polyethylene stent or second self-expanding metal stent (see sections on management of plastic stent occlusion and management of self-expanding metal stent occlusion) through the obstructed expanding stent. Plastic stent migration, either proximally or distally, may occur in 10% of cases.143
Future Trends
Photodynamic Therapy
Photodynamic therapy (PDT) involves the administration of a photosensitizer, which is activated with a laser light and causes necrosis of the exposed tissue. Preliminary results suggest prolonged survival and stent patency for PDT in cholangiocarcinoma at the hilum.144–146 Controlled trials are under way. Previously, it was thought that PDT was incompatible with uncovered expanding stents because stents may occlude by necrotic tumor tissue.147 However, this does not seem to be a major issue, although the light dose should be adjusted to counteract the reduction of light transmittance caused by the metal.148 PDT has also been used successfully to recanalize metal stents that were blocked by tumor ingrowth.149
Drug-Coated Biliary Stents
Covering biliary stents with chemotherapeutic agents, delivering chemotherapy directly to the tumor tissue, at least in theory should give protection against tumor ingrowth, overgrowth, or both. For optimal therapeutic effects, these drugs should be released over a longer time with good penetration in tissue and without systemic toxicity. Carboplatin and paclitaxel have been shown to inhibit cell proliferation in vitro.150,151 Carboplatin-coated plastic stents have been used with promising preliminary results in a few patients.151 Placement of a metallic stent covered with a paclitaxel-incorporated membrane in patients with malignant biliary obstruction proved to be feasible, safe, and effective.152 Median patency was 270 days (range 68 to 810 days), and cumulative patency rates at 3 months, 6 months, and 12 months were 100%, 71%, and 36%. Whether drug-eluting stents represent an advancement in the treatment of patients with malignant biliary strictures remains to be proven in prospective comparative trials.
Transgastric Endoscopic Ultrasound–Guided Biliary Drainage
EUS-guided hepaticogastrostomy has been reported by several groups as an alternative treatment to percutaneous biliary drainage or surgical bypass in the case of failed ERCP.153,154 A dilated biliary duct in the left lobe is punctured by a 19-gauge needle under EUS guidance. Next, a guidewire is advanced, and the needle is removed. A cytotome is introduced over the wire to create a fistulous tract by the use of electrocautery. Successful long-term drainage has been reported with plastic stents and covered metal stents.
Endoscopic Ultrasound–Guided Plexus Neurolysis
Celiac plexus neurolysis is used to control pain in patients with pancreatic cancer. The injected agent usually includes a local anesthetic (bupivacaine or lidocaine) and a neurolytic (phenol or alcohol). Comparative studies with conventional pain management (uptitration of opioids) or percutaneous celiac plexus neurolysis are lacking. Side effects occur in about 3% of cases and are usually mild, the most frequent being asymptomatic hypotension.155 Bilateral injection of the neurolytic agent (i.e., right and left sides of the celiac trunk) has shown to be more effective than central injection (i.e., in the midline and anterior to the celiac artery).156 Reliable data with regard to the efficacy of EUS-guided celiac plexus neurolysis in cancer are unavailable, but a meta-analysis of percutaneous celiac plexus neurolysis showed long-lasting benefit for 70% to 90% of patients.157 For the best results, it is recommended to perform celiac plexus neurolysis not too late in the course of the disease when pain has become unbearable. The central effects of chronic pain lead to hypersensitization and unresponsiveness to pain treatments. EUS may reduce neurologic complications (because of the anterior approach) compared with the percutaneous technique, although no comparative studies have been performed.158
1 Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88:2398-2424.
2 Michaud DS. The epidemiology of pancreatic, gallbladder, and other biliary tract cancers. Gastrointest Endosc. 2002;56:S195-S200.
3 Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465.
4 Rosewicz S, Wiedenmann B. Pancreatic carcinoma. Lancet. 1997;349:485-489.
5 Bismuth H, Castaing D, Traynor O. Resection or palliation: Priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39-47.
6 Monson JR, Donohue JH, McEntee GP, et al. Radical resection for carcinoma of the ampulla of Vater. Arch Surg. 1991;126:353-357.
7 Bueno de Mesquita HB, Maisonneuve P, Moerman CJ, et al. Life-time history of smoking and exocrine carcinoma of the pancreas: A population-based case-control study in The Netherlands. Int J Cancer. 1991;49:816-822.
8 Gold EB, Goldin SB. Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am. 1998;7:67-91.
9 Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437.
10 Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169-170.
11 Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: An overview. Cancer Detect Prev. 2003;27:87-93.
12 Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321-327.
13 Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: Recent progress. Part 1. Epidemiology and etiology. J Gastroenterol Hepatol. 2002;17:1049-1055.
14 Lowenfels AB, Maisonneuve P, Boyle P, et al. Epidemiology of gallbladder cancer. Hepatogastroenterology. 1999;46:1529-1532.
15 Mansfield JC, Griffin SM, Wadehra V, et al. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671-677.
16 Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51:383-390.
17 Pugliese V, Conio M, Nicolo G, et al. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: A prospective study. Gastrointest Endosc. 1995;42:520-526.
18 Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: Results of a prospective study. Gastrointest Endosc. 1995;42:565-572.
19 Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut. 1999;44:720-726.
20 Linder S, Blasjo M, Sundelin P, et al. Aspects of percutaneous fine-needle aspiration biopsy in the diagnosis of pancreatic carcinoma. Am J Surg. 1997;174:303-306.
21 Finkelberg DL, Sahani D, Deshpande V, et al. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670-2676.
22 Speer AG, Cotton PB, Russell RC, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62.
23 Andersen JR, Sorensen SM, Kruse A, et al. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132-1135.
24 Smith AC, Dowsett JF, Russell RC, et al. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet. 1994;344:1655-1660.
25 Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest Endosc. 1991;37:383-393.
26 Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918.
27 Ballinger AB, McHugh M, Catnach SM, et al. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut. 1994;35:467-470.
28 Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: A prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56:835-841.
29 Lai EC, Mok FP, Fan ST, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195-1198.
30 Sewnath ME, Birjmohun RS, Rauws EA, et al. The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am Coll Surg. 2001;192:726-734.
31 Martignoni ME, Wagner M, Krahenbuhl L, et al. Effect of preoperative biliary drainage on surgical outcome after pancreatoduodenectomy. Am J Surg. 2001;181:52-59.
32 Mullen JT, Lee JH, Gomez HF, et al. Pancreaticoduodenectomy after placement of endobiliary metal stents. J Gastrointest Surg. 2005;9:1094-1104.
33 Chen VK, Arguedas MR, Baron TH. Expandable metal biliary stents before pancreaticoduodenectomy for pancreatic cancer: A Monte-Carlo decision analysis. Clin Gastroenterol Hepatol. 2005;3:1229-1237.
34 Shepherd HA, Royle G, Ross AP, et al. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: A randomized trial. Br J Surg. 1988;75:1166-1168.
35 Davids PH, Groen AK, Rauws EA, et al. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492.
36 Knyrim K, Wagner HJ, Pausch J, et al. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207-212.
37 Coene PP, Groen AK, Cheng J, et al. Clogging of biliary endoprostheses: A new perspective. Gut. 1990;31:913-917.
38 Leung JW, Ling TK, Kung JL, et al. The role of bacteria in the blockage of biliary stents. Gastrointest Endosc. 1988;34:19-22.
39 Speer AG, Cotton PB, Rode J, et al. Biliary stent blockage with bacterial biofilm: A light and electron microscopy study. Ann Intern Med. 1988;108:546-553.
40 Huibregtse K. Endoscopic biliary and pancreatic drainage. Stuttgart: Georg Thieme; 1988.
41 Speer AG, Cotton PB, MacRae KD. Endoscopic management of malignant biliary obstruction: Stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest Endosc. 1988;34:412-417.
42 Rey JF, Maupetit P, Greff M. Experimental study of biliary endoprosthesis efficiency. Endoscopy. 1985;17:145-148.
43 Pereira-Lima JC, Jakobs R, et al. Endoscopic biliary stenting for the palliation of pancreatic cancer: Results, survival predictive factors, and comparison of 10-French with 11.5-French gauge stents. Am J Gastroenterol. 1996;91:2179-2184.
44 Kadakia SC, Starnes E. Comparison of 10 French gauge stent with 11.5 French gauge stent in patients with biliary tract diseases. Gastrointest Endosc. 1992;38:454-459.
45 Siegel JH, Pullano W, Kodsi B, et al. Optimal palliation of malignant bile duct obstruction: Experience with endoscopic 12 French prostheses. Endoscopy. 1988;20:137-141.
46 Scheeres D, O’Brien W, Ponsky L, et al. Endoscopic stent configuration and bile flow rates in a variable diameter bile duct model. Surg Endosc. 1990;4:91-93.
47 Leung JW, Del Favero G, Cotton PB. Endoscopic biliary prostheses: A comparison of materials. Gastrointest Endosc. 1985;31:93-95.
48 Huibregtse K, Tytgat GN. Palliative treatment of obstructive jaundice by transpapillary introduction of large bore bile duct endoprosthesis. Gut. 1982;23:371-375.
49 Dowidar N, Kolmos HJ, Matzen P. Experimental clogging of biliary endoprostheses: Role of bacteria, endoprosthesis material, and design. Scand J Gastroenterol. 1992;27:77-80.
50 Seitz U, Vadeyar H, Soehendra N. Prolonged patency with a new-design Teflon biliary prosthesis. Endoscopy. 1994;26:478-482.
51 Binmoeller KF, Seitz U, Seifert H, et al. The Tannenbaum stent: A new plastic biliary stent without side holes. Am J Gastroenterol. 1995;90:1764-1768.
52 Catalano MF, Geenen JE, Lehman GA, et al. “Tannenbaum” Teflon stents versus traditional polyethylene stents for treatment of malignant biliary stricture. Gastrointest Endosc. 2002;55:354-358.
53 England RE, Martin DF, Morris J, et al. A prospective randomised multicentre trial comparing 10 Fr Teflon Tannenbaum stents with 10 Fr polyethylene Cotton-Leung stents in patients with malignant common duct strictures. Gut. 2000;46:395-400.
54 Terruzzi V, Comin U, De Grazia F, et al. Prospective randomized trial comparing Tannenbaum Teflon and standard polyethylene stents in distal malignant biliary stenosis. Gastrointest Endosc. 2000;51:23-27.
55 Sung JJ, Chung SC, Tsui CP, et al. Omitting side-holes in biliary stents does not improve drainage of the obstructed biliary system: A prospective randomized trial. Gastrointest Endosc. 1994;40:321-325.
56 van Berkel AM, Boland C, Redekop WK, et al. A prospective randomized trial of Teflon versus polyethylene stents for distal malignant biliary obstruction. Endoscopy. 1998;30:681-686.
57 van Berkel AM, van Marle J, van Veen H, et al. A scanning electron microscopic study of biliary stent materials. Gastrointest Endosc. 2000;51:19-22.
58 McAllister EW, Carey LC, Brady PG, et al. The role of polymeric surface smoothness of biliary stents in bacterial adherence, biofilm deposition, and stent occlusion. Gastrointest Endosc. 1993;39:422-425.
59 Jansen B, Goodman LP, Ruiten D. Bacterial adherence to hydrophilic polymer-coated polyurethane stents. Gastrointest Endosc. 1993;39:670-673.
60 Costamagna G, Mutignani M, Rotondano G, et al. Hydrophilic hydromer-coated polyurethane stents versus uncoated stents in malignant biliary obstruction: A randomized trial. Gastrointest Endosc. 2000;51:8-11.
61 van Berkel AM, Bruno MJ, Bergman JJ, et al. A prospective randomized study of hydrophilic polymer-coated polyurethane versus polyethylene stents in distal malignant biliary obstruction. Endoscopy. 2003;35:478-482.
62 Leung JW, Liu Y, Cheung S, et al. Effect of antibiotic-loaded hydrophilic stent in the prevention of bacterial adherence: A study of the charge, discharge, and recharge concept using ciprofloxacin. Gastrointest Endosc. 2001;53:431-437.
63 Leung JW, Lau GT, Sung JJ, et al. Decreased bacterial adherence to silver-coated stent material: An in vitro study. Gastrointest Endosc. 1992;38:338-340.
64 Liu Q, Khay G, Cotton PB. Feasibility of stent placement above the sphincter of Oddi (“inside-stent”) for patients with malignant biliary obstruction. Endoscopy. 1998;30:687-690.
65 Pedersen FM, Lassen AT, Schaffalitzky de Muckadell OB. Randomized trial of stent placed above and across the sphincter of Oddi in malignant bile duct obstruction. Gastrointest Endosc. 1998;48:574-579.
66 Leung JW, Liu YL, Desta TD, et al. In vitro evaluation of antibiotic prophylaxis in the prevention of biliary stent blockage. Gastrointest Endosc. 2000;51:296-303.
67 Sung JJ, Sollano JD, Lai CW, et al. Long-term ciprofloxacin treatment for the prevention of biliary stent blockage: A prospective randomized study. Am J Gastroenterol. 1999;94:3197-3201.
68 Ghosh S, Palmer KR. Prevention of biliary stent occlusion using cyclical antibiotics and ursodeoxycholic acid. Gut. 1994;35:1757-1759.
69 Barrioz T, Ingrand P, Besson I, et al. Randomised trial of prevention of biliary stent occlusion by ursodeoxycholic acid plus norfloxacin. Lancet. 1994;344:581-582.
70 Halm U, Schiefke, Fleig WE, et al. Ofloxacin and ursodeoxycholic acid versus ursodeoxycholic acid alone to prevent occlusion of biliary stents: A prospective, randomized trial. Endoscopy. 2001;33:491-494.
71 Luman W, Ghosh S, Palmer KR. A combination of ciprofloxacin and Rowachol does not prevent biliary stent occlusion. Gastrointest Endosc. 1999;49:316-321.
72 Lee SP, Carey MC, LaMont JT. Aspirin prevention of cholesterol gallstone formation in prairie dogs. Science. 1981;211:1429-1431.
73 Smit JM, Out MM, Groen AK, et al. A placebo-controlled study on the efficacy of aspirin and doxycycline in preventing clogging of biliary endoprostheses. Gastrointest Endosc. 1989;35:485-489.
74 Sung JY, Shaffer EA, Lam K, et al. Hydrophobic bile salt inhibits bacterial adhesion on biliary stent material. Dig Dis Sci. 1994;39:999-1006.
75 Frakes JT, Johanson JF, Stake JJ. Optimal timing for stent replacement in malignant biliary tract obstruction. Gastrointest Endosc. 1993;39:164-167.
76 Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1-7.
77 Matsuda Y, Shimakura K, Akamatsu T. Factors affecting the patency of stents in malignant biliary obstructive disease: Univariate and multivariate analysis. Am J Gastroenterol. 1991;86:843-849.
78 Loew BJ, Howell DA, Sanders MK, et al. Comparative performance of uncoated, self-expanding metal biliary stents of different designs in 2 diameters: Final results of an international multicenter, randomized, controlled trial. Gastrointest Endosc. 2009;70:445-453.
79 Lammer J. Biliary endoprostheses: Plastic versus metal stents. Radiol Clin North Am. 1990;28:1211-1222.
80 Carr-Locke D, Ball TJ, Conners PJ. Multicenter randomized trial of Wallstent biliary prosthesis versus plastic stents. Gastrointest Endosc. 1993;39:310-316.
81 Huibregtse K, Cheng J, Coene PP, et al. Endoscopic placement of expandable metal stents for biliary strictures—a preliminary report on experience with 33 patients. Endoscopy. 1989;21:280-282.
82 Bethge N, Wagner HJ, Knyrim K, et al. Technical failure of biliary metal stent deployment in a series of 116 applications. Endoscopy. 1992;24:395-400.
83 Lammer J, Hausegger KA, Fluckiger F, et al. Common bile duct obstruction due to malignancy: Treatment with plastic versus metal stents. Radiology. 1996;201:167-172.
84 Shim CS, Lee YH, Cho YD, et al. Preliminary results of a new covered biliary metal stent for malignant biliary obstruction. Endoscopy. 1998;30:345-350.
85 Isayama H, Komatsu Y, Tsujino T, et al. Polyurethane-covered metal stent for management of distal malignant biliary obstruction. Gastrointest Endosc. 2002;55:366-370.
86 Smits ME. New Developments in endoscopic biliary and pancreatic drainage [thesis]. Amsterdam: Thesis Publishers; 1995.
87 Yoon WJ, Lee JK, Lee KH, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996-1000.
88 Fumex F, Coumaros D, Napoleon B, et al. Similar performance but higher cholecystitis rate with covered biliary stents: Results from a prospective multicenter evaluation. Endoscopy. 2006;38:787-792.
89 Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-734.
90 Nakai Y, Isayama H, Komatsu Y, et al. Efficacy and safety of the covered Wallstent in patients with distal malignant biliary obstruction. Gastrointest Endosc. 2005;62:742-748.
91 Artifon EL, Sakai P, Ishioka S, et al. Endoscopic sphincterotomy before deployment of covered metal stent is associated with greater complication rate: A prospective randomized control trial. J Clin Gastroenterol. 2008;42:815-819.
92 Prat F, Chapat O, Ducot B, et al. Predictive factors for survival of patients with inoperable malignant distal biliary strictures: A practical management guideline. Gut. 1998;42:76-80.
93 Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182.
94 Pereira-Lima JC, Jakobs R, Maier M, et al. Endoscopic stenting in obstructive jaundice due to liver metastases: Does it have a benefit for the patient? Hepatogastroenterology. 1996;43:944-948.
95 Yeoh KG, Zimmerman MJ, Cunningham JT, et al. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999;49:466-471.
96 Arguedas MR, Heudebert GH, Stinnett AA, et al. Biliary stents in malignant obstructive jaundice due to pancreatic carcinoma: A cost-effectiveness analysis. Am J Gastroenterol. 2002;97:898-904.
97 Moss AC, Morris E, Leyden J, et al. Do the benefits of metal stents justify the costs? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2007;19:1119-1124.
98 Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: A prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-995.
99 van Berkel AM, Bergman JJ, Waxman I, et al. Wallstents for metastatic biliary obstruction. Endoscopy. 1996;28:418-421.
100 van den Hazel SJ, Speelman P, Dankert J, et al. Piperacillin to prevent cholangitis after endoscopic retrograde cholangiopancreatography: A randomized, controlled trial. Ann Intern Med. 1996;125:442-447.
101 Sauter G, Grabein B, Huber G, et al. Antibiotic prophylaxis of infectious complications with endoscopic retrograde cholangiopancreatography: A randomized controlled study. Endoscopy. 1990;22:164-167.
102 Harris A, Chan AC, Torres-Viera C, et al. Meta-analysis of antibiotic prophylaxis in endoscopic retrograde cholangiopancreatography (ERCP). Endoscopy. 1999;31:718-724.
103 Motte S, Deviere J, Dumonceau JM, et al. Risk factors for septicemia following endoscopic biliary stenting. Gastroenterology. 1991;101:1374-1381.
104 Deviere J, Motte S, Dumonceau JM, et al. Septicemia after endoscopic retrograde cholangiopancreatography. Endoscopy. 1990;22:72-75.
105 Bruins Slot W, Schoeman MN, Disario JA, et al. Needle-knife sphincterotomy as a precut procedure: A retrospective evaluation of efficacy and complications. Endoscopy. 1996;28:334-339.
106 Soehendra N, Maydeo A, Eckmann B, et al. A new technique for replacing an obstructed biliary endoprosthesis. Endoscopy. 1990;22:271-272.
107 Dumas R, Demuth N, Buckley M, et al. Endoscopic bilateral metal stent placement for malignant hilar stenoses: Identification of optimal technique. Gastrointest Endosc. 2000;51:334-338.
108 Neuhaus H, Gottlieb K, Classen M. The “stent through wire mesh technique” for complicated biliary strictures. Gastrointest Endosc. 1993;39:553-556.
109 Familiari P, Bulajic M, Mutignani M, et al. Endoscopic removal of malfunctioning biliary self-expandable metallic stents. Gastrointest Endosc. 2005;62:903-910.
110 Shin HP, Kim MH, Jung SW, et al. Endoscopic removal of biliary self-expandable metallic stents: A prospective study. Endoscopy. 2006;38:1250-1255.
111 Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170-178.
112 Deviere J, Baize M, de Toeuf J, et al. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34:95-101.
113 Ducreux M, Liguory C, Lefebvre JF, et al. Management of malignant hilar biliary obstruction by endoscopy: Results and prognostic factors. Dig Dis Sci. 1992;37:778-783.
114 Polydorou AA, Cairns SR, Dowsett JF, et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32:685-689.
115 Stoker J, Lameris JS, van Blankenstein M. Percutaneous metallic self-expandable endoprostheses in malignant hilar biliary obstruction. Gastrointest Endosc. 1993;39:43-49.
116 Gordon RL, Ring EJ, LaBerge JM, et al. Malignant biliary obstruction: Treatment with expandable metallic stents—follow-up of 50 consecutive patients. Radiology. 1992;182:697-701.
117 Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: A percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55-62.
118 Dowsett JF, Vaira D, Hatfield AR, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96:1180-1186.
119 Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354-362.
120 De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: Results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553.
121 Hintze RE, Abou-Rebyeh H, Adler A, et al. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40-46.
122 Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41-49.
123 Wagner HJ, Knyrim K, Vakil N, et al. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction: A prospective and randomized trial. Endoscopy. 1993;25:213-218.
124 Cheng JL, Bruno MJ, Bergman JJ, et al. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: Efficacy of self-expandable metallic Wallstents. Gastrointest Endosc. 2002;56:33-39.
125 Perdue DG, Freeman ML, DiSario JA, et al. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: A prospective multicenter observational cohort study. J Clin Gastroenterol. 2008;42:1040-1046.
126 Lee JH, Kang DH, Kim JY, et al. Endoscopic bilateral metal stent placement for advanced hilar cholangiocarcinoma: A pilot study of a newly designed Y stent. Gastrointest Endosc. 2007;66:364-369.
127 Watanapa P, Williamson RC. Surgical palliation for pancreatic cancer: Developments during the past two decades. Br J Surg. 1992;79:8-20.
128 Yates MR3rd, Morgan DE, Baron TH. Palliation of malignant gastric and small intestinal strictures with self-expandable metal stents. Endoscopy. 1998;30:266-272.
129 van der Schelling GP, van den Bosch RP, Klinkenbij JH, et al. Is there a place for gastroenterostomy in patients with advanced cancer of the head of the pancreas? World J Surg. 1993;17:128-132.
130 Wong YT, Brams DM, Munson L, et al. Gastric outlet obstruction secondary to pancreatic cancer: Surgical vs endoscopic palliation. Surg Endosc. 2002;16:310-312.
131 Nevitt AW, Vida F, Kozarek RA, et al. Expandable metallic prostheses for malignant obstructions of gastric outlet and proximal small bowel. Gastrointest Endosc. 1998;47:271-276.
132 Soetikno RM, Lichtenstein DR, Vandervoort J, et al. Palliation of malignant gastric outlet obstruction using an endoscopically placed Wallstent. Gastrointest Endosc. 1998;47:267-270.
133 Venu RP, Pastika BJ, Kini M, et al. Self-expandable metal stents for malignant gastric outlet obstruction: A modified technique. Endoscopy. 1998;30:553-558.
134 van Hooft JE, Uitdehaag MJ, Bruno MJ, et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): A prospective multicenter study. Gastrointest Endosc. 2009;69:1059-1066.
135 Jeurnink SM, Steyerberg EW, Hof G, et al. Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: A comparison in 95 patients. J Surg Oncol. 2007;96:389-396.
136 Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc. 2003;17:457-461.
137 Mutignani M, Tringali A, Shah SG, et al. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007;39:440-447.
138 Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: A prospective multicenter study. Am J Gastroenterol. 2001;96:417-423.
139 Testoni PA, Bagnolo F. Pain at 24 hours associated with amylase levels greater than 5 times the upper normal limit as the most reliable indicator of post-ERCP pancreatitis. Gastrointest Endosc. 2001;53:33-39.
140 Foutch PG. A prospective assessment of results for needle-knife papillotomy and standard endoscopic sphincterotomy. Gastrointest Endosc. 1995;41:25-32.
141 Enns R, Eloubeidi MA, Mergener K, et al. ERCP-related perforations: Risk factors and management. Endoscopy. 2002;34:293-298.
142 Stapfer M, Selby RR, Stain SC, et al. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 2000;232:191-198.
143 Johanson JF, Schmalz MJ, Geenen JE. Incidence and risk factors for biliary and pancreatic stent migration. Gastrointest Endosc. 1992;38:341-346.
144 Dumoulin FL, Gerhardt T, Fuchs S, et al. Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2003;57:860-867.
145 Zoepf T, Jakobs R, Arnold JC, et al. Photodynamic therapy for palliation of nonresectable bile duct cancer—preliminary results with a new diode laser system. Am J Gastroenterol. 2001;96:2093-2097.
146 Ortner M. Photodynamic therapy for cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8:137-139.
147 Shah SK, Mutignani M, Costamagna G. Therapeutic biliary endoscopy. Endoscopy. 2002;34:43-53.
148 Wang LW, Li LB, Li ZS, et al. Self-expandable metal stents and trans-stent light delivery: Are metal stents and photodynamic therapy compatible? Lasers Surg Med. 2008;40:651-659.
149 Pereira SP, Ayaru L, Rogowska A, et al. Photodynamic therapy of malignant biliary strictures using meso-tetrahydroxyphenylchlorin. Eur J Gastroenterol Hepatol. 2007;19:479-485.
150 Kalinowski M, Alfke H, Kleb B, et al. Paclitaxel inhibits proliferation of cell lines responsible for metal stent obstruction: Possible topical application in malignant bile duct obstructions. Invest Radiol. 2002;37:399-404.
151 Mezawa S, Homma H, Sato T, et al. A study of carboplatin-coated tube for the unresectable cholangiocarcinoma. Hepatology. 2000;32:916-923.
152 Suk KT, Kim JW, Kim HS, et al. Human application of a metallic stent covered with a paclitaxel-incorporated membrane for malignant biliary obstruction: Multicenter pilot study. Gastrointest Endosc. 2007;66:798-803.
153 Bories E, Pesenti C, Caillol F, et al. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy. 2007;39:287-291.
154 Will U, Thieme A, Fueldner F, et al. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39:292-295.
155 O’Toole TM, Schmulewitz N. Complication rates of EUS-guided celiac plexus blockade and neurolysis: Results of a large case series. Endoscopy. 2009;41:593-597.
156 Sahai AV, Lemelin V, Lam E, et al. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: A comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326-329.
157 Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: A meta-analysis. Anesth Analg. 1995;80:290-295.
158 Gunaratnam NT, Sarma AV, Norton ID, et al. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316-324.