CHAPTER 246 Pain, Complications, and Iatrogenic Injury in Nerve Surgery
Pain and Peripheral Nerve Injury
Neuropathic pain and neuralgia are two common terms that many clinicians use interchangeably. Their definitions remain broad and include pain related to an injury or abnormality of a peripheral nerve (i.e., neuropathic pain) and pain in the cutaneous distribution of a peripheral nerve (i.e., neuralgia).1 There are additional categories of peripheral nerve-related pain, summarized in Table 246-1. Although some categories have distinct etiologies, symptoms, and treatments, significant overlap remains, as does continued disagreement with the classification system currently being used.1
The etiology of peripheral neuropathic pain is likely multifactorial.2 In general, the following processes likely contribute:
The exact relationship between sympathetic outflow and pain remains uncertain, however, transient improvement in some patients after sympathectomy strongly supports this connection.4 Pathophysiologic changes in the spinal cord and periaqueductal gray matter can centralize pain so that pain remains despite nerve blocks and other peripheral treatments being applied.
Diagnosis and classification of pain is predominantly based on the history and physical examination. Response to trial medications, autonomic changes, and temporary improvement with nerve blocks may help refine the cause. When taking a history, the location, quality (e.g., burning, paresthetic, crushing), and any exacerbating and relieving maneuvers, should be discussed with the patient. With peripheral nerve entrapment, pain is often referred adjacent to, and along, the distribution of the compressed nerve. For example, the description of aching discomfort in the wrist and forearm, along with nocturnal symptoms, including paresthesias in the median nerve distribution, are characteristic of carpal tunnel syndrome. Pain and tenderness may also be present at the entrapment site (e.g., near the fibular head with peroneal nerve entrapment at the knee). When peripheral nerves without cutaneous sensory afferents are injured, numbness and paresthesias do not occur; however, a deep aching pain may be present not only at the point of entrapment but also within any joints the entrapped nerve carries proprioceptive sensation from (e.g., dorsal wrist pain when the posterior interosseous nerve is compressed in the proximal forearm; shoulder pain with the early stages of suprascapular entrapment neuropathy). When significant damage to a motor-sensory nerve occurs, concomitant sensory abnormalities, including paresthesias, hypoesthesias, and hyperesthesias, are characteristically present in the nerve’s sensory territory.5 The clinician must keep in mind that sensory loss may be unreported because of more severe, overlying pain.
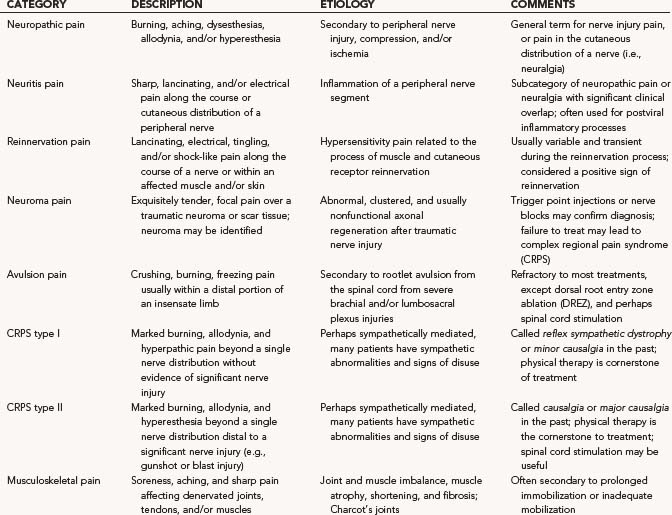
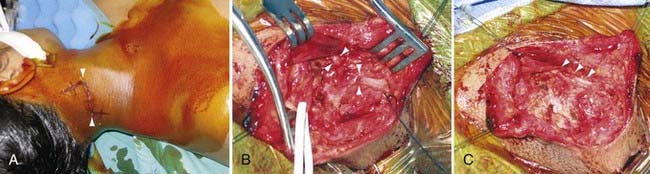
Autonomic disturbance, in part, may also cause significant pain in patients with peripheral nerve injuries and is a hallmark of both type I (formerly known as reflex sympathetic dystrophy) and type II (formerly known as causalgia) complex regional pain syndrome (CRPS) (Fig. 246-1).6 CRPS type I usually occurs after a minor injury to the extremity (e.g., sprained ankle), whereas CRPS type II occurs after significant damage to a major mixed nerve (e.g., gunshot wound). Severe burning pain, careful attempts to protect the involved extremity from movement or manipulation, and evidence of autonomic overactivity (or underactivity) are features of both CRPS types I and II. Trophic changes such as dry skin, nail loss or nail changes, and vascular changes such as skin flushing and discoloration and temperature fluctuations are hallmarks of this disarrayed autonomic activity. Furthermore, denervation hypersensitivity to circulating or local catecholamines may also exacerbate nerve pain and likely contributes to worsen pain during periods of environmental and emotional stress.
Management of neuropathic pain remains challenging, with many patients having only partial or minimal relief with treatment. Those with complicated, chronic, or refractory nerve pain should be referred to a pain center where a comprehensive evaluation and treatment plan can be instituted based on a multidisciplinary consensus. In general, treatment options may be divided into the following categories: physical therapy, pharmacotherapy, local and proximal nerve blocks (e.g., sympathectomy), psychological counseling, local surgical intervention (e.g., neuroma removal), and peripheral and central nervous system stimulation. A stepwise trial of progressively more invasive treatments should be recommended based on the clinical situation. Stimulation has increased in popularity in recent years. It has been proved effective for postherpetic neuralgia and CRPS and less effective, but commonly used, for peripheral neuropathy, avulsion pain, and failed back syndrome.7 Peripheral nerve stimulation has for the most part been replaced by spinal cord stimulation for pain in the extremities. This is because of the ease of spinal stimulator trials and permanent implantation; however, occipital and frontalis peripheral nerve stimulation remain excellent options for neuralgia affecting these nerves.8 An important concept for peripheral nerve stimulation is that the pain should be solely localized to the distribution of the affected nerve for the stimulation to be effective.8 Central nervous system ablative surgery also maintains a role in contemporary pain management.7 Indications include dorsal root entry zone (DREZ) ablation for avulsion pain and radiofrequency ablation of the descending sensory trigeminal tract for treatment of anesthesia dolorosa affecting the trigeminal nerve and for certain forms of cancer pain.
Complications and Their Avoidance in Peripheral Nerve Surgery
Avoiding complications during peripheral nerve surgery often requires an understanding of principles and techniques that are distinct from those used for the brain and spine.9 Furthermore, because most neurosurgery training programs do not perform a high volume of peripheral nerve surgery, many residency graduates do not possess the experience and understanding needed to minimize complications during peripheral nerve surgery, even though many will subsequently perform these procedures in their practice.10 In this section, we review how fundamental errors, such as misdiagnosis, misidentification, poor operative timing, and other operative errors, may predispose to complications. The complications for several types of peripheral nerve surgery (e.g., brachial plexus repair, carpal tunnel release) are summarized in Table 246-2. A more in-depth review can be found elsewhere.11,12
| NERVE | COMPLICATIONS |
|---|---|
| Brachial plexus | Misdiagnosis of rootlet avulsion, incomplete dissection causing misidentification, iatrogenic nerve injury (e.g., phrenic nerve), vascular injury, clavicular nonunion (if osteotomized), thoracic duct injury, chylothorax, hemothorax, or pneumothorax |
| Median nerve at carpal tunnel | Iatrogenic nerve injury (e.g., palmar cutaneous branch, thenar motor branch, ulnar nerve), injury to palmar arterial arch, misdiagnosis (e.g., C6 radiculopathy), wound pain, complex regional pain syndromes (CRPS) type 1, incomplete decompression |
| Ulnar nerve at cubital tunnel | Iatrogenic injury to the posterior division of the medial antebrachial cutaneous nerve causing elbow numbness, neuroma formation, recurrent symptoms secondary to iatrogenic compression against the medial intermuscular septum (or arcade of Struthers) with transposition |
| Nerve sheath tumors | Numbness, paralysis (e.g., foot drop), neuropathic pain, wound hematoma, iatrogenic injury to nearby nerves during exposure and retraction, inappropriate resection of salvageable fascicles |
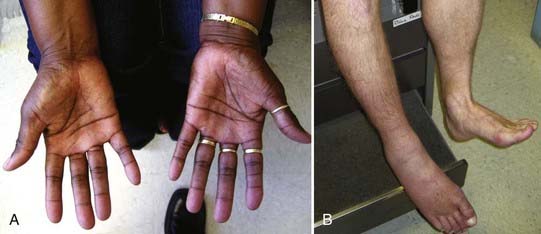
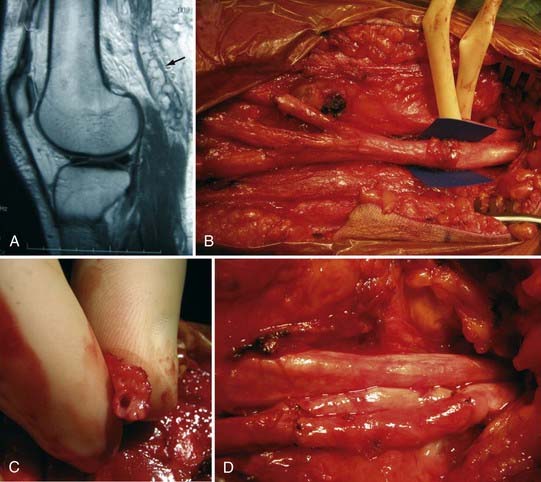
More than for most neurosurgical diseases, lesion localization in the peripheral nervous system relies on an accurate history and physical examination. In fact, many peripheral nerve operations are performed without radiographic confirmation of the diagnosis. When investigating most peripheral nerve entrapments or injuries, most imaging modalities, including plain films, computed tomography (CT) scans, magnetic resonance imaging (MRI), and ultrasound, either have poor sensitivity and specificity, are not yet fully evaluated as diagnostic tools in the peripheral nervous system, do not provide information that changes management, or have a poor predictive value for clinical outcomes. Nevertheless, imaging plays an important role in peripheral nerve surgery. CT myelography or MRI are necessary when investigating root avulsion, and CT or MRI is necessary for the exclusion of mass lesions, including nerve sheath tumors, ganglion cysts, and lipomas. The clinician should have a low threshold for ordering an imaging study when a structural lesion is suspected. Without the correct preoperative diagnosis, opportunities to repair nerve injuries may be missed, inappropriate and potentially dangerous surgeries may be performed, and a patient may be offered more invasive therapy when a simpler option is available (Fig. 246-2).
For more severe nerve injuries in continuity (axonotmesis), which by definition cause wallerian degeneration and possible axonal regeneration, acceptable outcomes may also occur without surgical intervention. In these instances, the clinician should closely monitor, both with serial neuromuscular examinations and electromyography, the patient’s progress. If no recovery occurs by about 3 months, prompt operative exposure and intraoperative nerve recordings across the injury are indicated to rule out the need for graft repair of a nonconducting neuroma-in-continuity.13 Not doing so may lead to a missed opportunity for repair and poor outcome.
When a neuroma-in-continuity is encountered during surgery, it is not possible by palpation and visual inspection to know whether the injured segment contains viable axons, which have not, as of yet, reached their target muscles. In general, neuromas-in-continuity conducting evoked potentials should not be resected because their recovery following external neurolysis alone is better than that seen with grafting or primary repair.14 Use of nerve stimulation alone with selective electromyographic recordings from muscle is not recommended because sampling from a few areas of muscle can miss axons innervating other areas not being recorded. Also, because some axons that have regenerated through the damaged segment may not have reached a muscle yet, these would be missed. Although a positive finding, it only takes a hundred or so regenerating nerve fibers reaching muscle to have an evoked muscle action potential, and yet thousands are necessary to have good recovery of function. When resecting nonconducting neuromas-in-continuity, one should make certain to trim back the nerve ends until healthy, pouting fascicles are apparent and good bleeding points are encountered. One should not accept leaving behind residual neuroma in hopes of achieving end-to-end repair or allowing shorter graft length; doing so will result in poor regeneration.
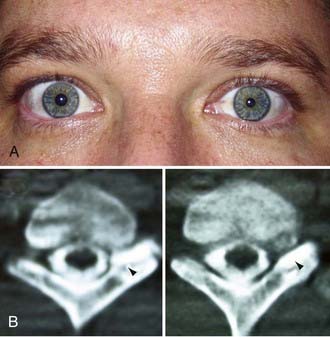
It is understood that clean, sharp nerve transections should be repaired urgently (i.e., in less than 72 hours). However, one should avoid performing immediate repairs of blunt transections.15 Blunt transections include chainsaw, gunshot (although most gunshots do not cause transections), degloving wounds with substantial tissue loss, and other high-speed, penetrating wounds, both clean and contaminated. In addition to the transection, they also have a significant blunt or stretch component. These injuries should be repaired about 2 to 3 weeks after injury so that any contusive or stretch damage to the nerve ends, which commonly occurs with blunt transections, has time to demarcate and visually manifest. Using this delayed approach, one avoids inadvertently coapting damaged, and eventually neuromatous, nerve ends (Fig. 246-3). This approach also avoids repairing nerves in a contaminated wound. Bluntly transected nerves that are found during an emergent exploration for concurrent vascular or orthopedic injuries should be tacked down to adjacent fascial planes, which helps minimize nerve end retraction. The wound is then reexplored about 2 to 3 weeks later, and the nerves may then be repaired without fear of having coapted nonviable nerve.
Iatrogenic Peripheral Nerve Injuries
Nerve injury may occur during almost any invasive procedure.16 Nerves may be directly damaged in the operative field or compressed secondary to poor patient positioning on the operating room table, especially for longer procedures under general anesthesia.
Iatrogenic Injury during Nonperipheral Nerve Surgery
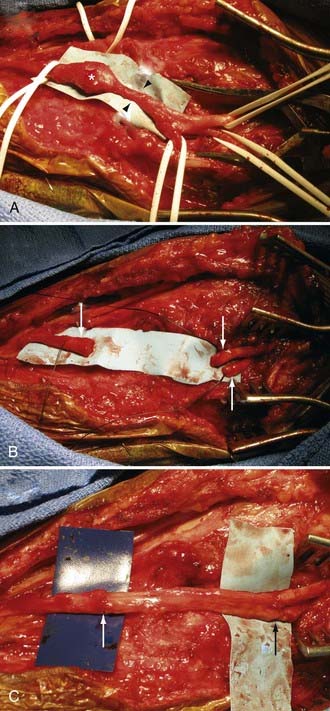
Considering peripheral nerves course throughout the body, they may be injured during almost any open or percutaneous procedure, including bedside venous and arterial punctures. Some of the more common types of iatrogenic nerve injuries are summarized in Table 246-3. During surgery, peripheral nerves may be damaged from excessive retraction (usually by a sharp, self-retaining retractor), coagulation, transection, postoperative swelling, or accidental stapling or ligature of a nerve (Figs. 246-4 and 246-5).
| NERVE | MECHANISM | COMMENTS |
|---|---|---|
| Spinal accessory nerve | Injured during cervical lymph node biopsy | May be misdiagnosed considering the levator scapulae also shrugs the shoulder; minimized with use of intraoperative stimulation before biopsy |
| Ilioinguinal nerve | Injured during inguinal hernia repair | May occur with open or laparoscopic hernia repairs; genitofemoral and iliohypogastric nerves may also be affected |
| Sciatic nerve | Injured during hip replacement | Secondary to retraction, transection, suture misplacement, or postoperative swelling |
| Peroneal nerve | Injured during knee replacement and gynecological procedures | Secondary to retraction, transection, suture misplacement, or postoperative swelling or improper padding while in stirrups |
| Ulnar nerve | Compression or stretch injury related to operative positioning | May be from direct nerve compression in retrocondylar groove or from prolonged flexion of the elbow |
| Brachial plexus | Stretch injury related to operative positioning | May occur after prone spine surgery with shoulders pulled down for fluoroscopy exposure, or from excessive abduction and external rotation in the supine position |
| Lateral femoral cutaneous nerve | Compressive injury from prolonged spine procedures in the prone position | Common after long spine surgery procedure in the prone position, but resolves spontaneously in most patients. May be injured while taking of iliac crest graft |
Iatrogenic Injury during Peripheral Nerve Surgery
Table 246-2 summarizes the more common iatrogenic nerve injuries during peripheral nerve surgery; an in-depth review can be found elsewhere.11,12 Two general concepts of nerve surgery that help minimize iatrogenic nerve injury are the proper manipulation of scar tissue and adequate exposure.
Iatrogenic Injury Secondary to Patient Positioning
Since the advent of modern surgery under anesthesia, iatrogenic positional nerve injuries have occurred. These injuries are secondary to stretch or compression and continue to occur despite commonplace preventive measures.17 With time, most positional neuropathies improve; however, many do not achieve a full recovery despite an optimal regimen of physical therapy and the judicious use of operative nerve evaluation, decompression, and repair.
Positional injuries involving peripheral nerves are usually secondary to direct or indirect compression.18 This compression can either be from pressure on a nerve from a hard surface (e.g., ulnar nerve compression on an operating table or armboard) or secondary to traction on a nerve causing pressure at a remote anatomic location (e.g., an arm abducted and extended at the shoulder causing the brachial plexus to be compressed under the pectoralis against the coracoid process or humeral head). Traction, or stretch per se, may also cause nerve injury.19 Practically speaking, positional nerve injuries are caused by placing the affected extremity in a suboptimal position (causing nerve compression) or from inadequate padding of the operating table. Risk factors include thin or cachectic patients, diabetes mellitus, hereditary predisposition to pressure palsies, long surgical procedures, prone or other complicated patient positions, and the presence of subclinical nerve compression before surgery.20
As soon as a positional palsy is suspected, an immediate history and neuromuscular examination should be performed. Regardless of postoperative pain and sedation issues, the most thorough evaluation should be obtained. The timing of the events should be carefully recorded. Any evidence of direct nerve injury within the operative field or inflammatory neuritis should be considered because the prognosis and treatment of these lesions are different from those of positional palsy. The examination should document any neurological deficits as well as any early pressure sores, bruising, or erythema that may be secondary to positional compression. An electrodiagnostic test should be performed about 1 month after the injury. In the case of postoperative ulnar palsies, an immediate electrodiagnostic evaluation may be indicated because there may be antecedent evidence of denervation. If the patient has had a rapid improvement during this first month, electrodiagnostics can be cancelled. Further detail on the diagnosis and management of positional injuries may be found elsewhere.21
Burchiel KJ, Ochoa JL. Surgical management of post-traumatic neuropathic pain. Neurosurg Clin N Am. 1991;2:117-126.
Cooper DE, Jenkins RS, Bready L, Rockwood CAJr. The prevention of injuries of the brachial plexus secondary to malposition of the patient during surgery. Clin Orthop Relat Res. 1988;228:33-41.
Denny-Brown D, Brenner C. Paralysis of nerve by direct pressure and by tourniquet. Arch Neurol Psych. 1944;51:1-26.
Denny-Brown D, Doherty M. Effects of transient stretching of peripheral nerve. Arch Neurol Psych. 1945;54:116-129.
Devor M. The pathophysiology and anatomy of damaged nerve. In: Wall PD, Melzack R, Bonica JJ, editors. Textbook of Pain. New York: Churchill Livingstone; 1984:49-64.
Furlan AD, Lui PW, Mallis A. Chemical sympathectomy for neuropathic pain: does it work? Case report and systemic literature review. Clin J Pain. 2001;17:327-336.
Horwitz N, Rizzoli H. Postoperative Complications of Extracranial Neurological Surgery. Baltimore: Williams & Wilkins; 1987. 1-403
Kim DH, Cho YJ, Tiel RL, Kline DG. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98:1005-1016.
Kline DG. Timing for exploration of nerve lesions and evaluation of the neuroma-in-continuity. Clin Orthop Relat Res. 1982;163:42-49.
Kretschmer T, Antoniadis G, Braun V, et al. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg. 2001;94:905-912.
Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124:2347-2360.
Maniker A, Passannante M. Peripheral nerve surgery and neurosurgeons: results of a national survey of practice patterns and attitudes. J Neurosurg. 2003;98:1159-1164.
Merskey H, Bogduk N. Pain terms. In: Mersky H, Bogduk N, editors. Classification of Chronic Pain. Seattle: IASP Press; 1998:207-213.
O’Neill OR, Burchiel KJ. Role of the sympathetic nervous system in painful nerve injury. Neurosurg Clin N Am. 1991;2:127-136.
Raslan AM, McCartney S, Burchiel KJ. Management of chronic severe pain: spinal neuromodulatory and neuroablative approaches. Acta Neurochir. 2007;97:33-41.
Russell SM, Kline DG. Complication avoidance in peripheral nerve surgery: preoperative evaluations of nerve injuries and brachial plexus exploration—part 1. Neurosurgery. 2006;59:441-447.
Russell SM, Kline DG. Complication avoidance in peripheral nerve surgery: injuries, entrapments, and tumors of the extremities—part 2. Neurosurgery. 2006;59:449-456.
Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008;5:100-106.
Tiel RL, Happel LT, Kline DG. Nerve action potential recording method and equipment. Neurosurgery. 1996;39:312-319.
Warner MA, Warner ME, Martin JT. Ulnar neuropathy: incidence, outcome, and risk factors in sedated or anesthetized patients. Anesthesiology. 1994;81:1332-1340.
Winfree CJ, Kline DG. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5-18.
1 Merskey H, Bogduk N. Pain terms. In: Mersky H, Bogduk N, editors. Classification of Chronic Pain. Seattle: IASP Press; 1998:207-213.
2 Burchiel KJ, Ochoa JL. Surgical management of post-traumatic neuropathic pain. Neurosurg Clin N Am. 1991;2:117-126.
3 Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124:2347-2360.
4 Furlan AD, Lui PW, Mallis A. Chemical sympathectomy for neuropathic pain: does it work? Case report and systemic literature review. Clin J Pain. 2001;17:327-336.
5 Devor M. The pathophysiology and anatomy of damaged nerve. In: Wall PD, Melzack R, Bonica JJ, editors. Textbook of Pain. New York: Churchill Livingstone; 1984:49-64.
6 O’Neill OR, Burchiel KJ. Role of the sympathetic nervous system in painful nerve injury. Neurosurg Clin N Am. 1991;2:127-136.
7 Raslan AM, McCartney S, Burchiel KJ. Management of chronic severe pain: spinal neuromodulatory and neuroablative approaches. Acta Neurochir. 2007;97:33-41.
8 Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008;5:100-106.
9 Horwitz N, Rizzoli H. Postoperative Complications of Extracranial Neurological Surgery. Baltimore: Williams & Wilkins; 1987. 1-403
10 Maniker A, Passannante M. Peripheral nerve surgery and neurosurgeons: results of a national survey of practice patterns and attitudes. J Neurosurg. 2003;98:1159-1164.
11 Russell SM, Kline DG. Complication avoidance in peripheral nerve surgery: preoperative evaluations of nerve injuries and brachial plexus exploration—part 1. Neurosurgery. 2006;59:441-447.
12 Russell SM, Kline DG. Complication avoidance in peripheral nerve surgery: injuries, entrapments, and tumors of the extremities—part 2. Neurosurgery. 2006;59:449-456.
13 Tiel RL, Happel LT, Kline DG. Nerve action potential recording method and equipment. Neurosurgery. 1996;39:312-319.
14 Kim DH, Cho YJ, Tiel RL, Kline DG. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98:1005-1016.
15 Kline DG. Timing for exploration of nerve lesions and evaluation of the neuroma-in-continuity. Clin Orthop Relat Res. 1982;163:42-49.
16 Kretschmer T, Antoniadis G, Braun V, et al. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg. 2001;94:905-912.
17 Cooper DE, Jenkins RS, Bready L, Rockwood CAJr. The prevention of injuries of the brachial plexus secondary to malposition of the patient during surgery. Clin Orthop Relat Res. 1988;228:33-41.
18 Denny-Brown D, Brenner C. Paralysis of nerve by direct pressure and by tourniquet. Arch Neurol Psych. 1944;51:1-26.
19 Denny-Brown D, Doherty M. Effects of transient stretching of peripheral nerve. Arch Neurol Psych. 1945;54:116-129.
20 Warner MA, Warner ME, Martin JT. Ulnar neuropathy. Incidence, outcome, and risk factors in sedated or anesthetized patients. Anesthesiology. 1994;81:1332-1340.
21 Winfree CJ, Kline DG. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5-18.