31 Pain Assessment and Management
The goals of this chapter are to describe the role of the interdisciplinary team (IDT), the importance of quality improvement, the epidemiology of pain in children with life-limiting illnesses, pain assessment and measurement, the myths surrounding pain management, and pain management guidelines. The reader is referred to other references for more detailed discussion of pediatric analgesic pharmacology.2,3
Pain Management, The Interdisciplinary Team, and The Child with a Life-Threatening Illness
The World Health Organization (WHO) mandates that a certain standard of pain management be available to every child receiving palliative care irrespective of location.4 As an extension of the WHO document, individual countries and groups of countries are declaring standards of pain management related to pediatric palliative care. Quality of life is often related to a child’s experience of pain and is therefore vital that the child receive excellent pain assessment and management. Successful pain management often needs the combined efforts of an IDT within the palliative care team of the medical, nursing, social work, play therapy, physiotherapy, occupational therapy disciplines, among others.
The Interdisciplinary Team and the Alleviation of Suffering
Support for parents includes education about anticipated potential symptoms. Knowledge will increase a sense of control. This in turn lessens anxiety and this may positively affect the child’s pain experience. Competent and compassionate care can alleviate a child’s pain and suffering. This can be achieved through a trusting, consistent, and honest relationship among all involved in providing care, and the family and the child. Because children are especially vulnerable and depend on adults to act as their advocates we must support the humane and competent treatment of pain and suffering at all times (Table 31-1).
TABLE 31-1 Clinical Background and Responsibilities in Pediatric Pain Management for Children Receiving Palliative Care
| Professional background | Contribution toward pain management |
|---|---|
| Pediatric palliative care physician | Primarily responsible for the assessment, diagnosis, and management of physical pain, including the prescription of pharmacologic, and non-pharmacologic management. May have a role in the alleviation of the psychological and existential components of pain. |
| Pediatric palliative care nurse | Primarily responsible for implementing and monitoring pain management through ongoing assessment and measurement. Also has a role in advocating for the child’s pain management and a role in the alleviation of the psychological and existential components of pain. |
| Pediatric palliative care social worker | Primarily responsible for the social domain of care of the child and family, especially when affecting pain management. Has a major role advocating for the child and family, including pain management. |
| Pediatric palliative care child-life therapist | Primarily responsible for the use of play as a therapeutic means of self-expression. This may include the expression of pain severity and suffering through therapeutic play. |
| Pediatric palliative care spiritual care provider | Primarily responsible for assessing and managing the spiritual components of patient receiving palliative care and the existential suffering related to dying. Suffering related to the spirit may be related to the experience of physical pain. |
Epidemiology of Pain in Children with Life-Threatening Illnesses
Pain and other symptoms at the end of a child’s life
Pain and other physical and psychological symptoms are highly prevalent in children at the end of life. The proxy report of nurses documented the symptoms of dying children, using a modified Memorial Symptom Assessment Scale.5 A mean of 11.1 ± 5.6 symptoms was documented per child. At least half of the children had six symptoms; the most frequent ones being lack of energy, pain, drowsiness, skin changes, irritability, and extremity swelling. Lack of energy was the most distressing symptom for nearly one-third of the children. Nervousness, worry, and dysesthetic extremities were notably distressing, although not frequent. Most children were described in the health professionals’ notes as being “always comfortable” to “usually comfortable” in the last week (64%), day (76.6%), and hour (93.4%) of life. A retrospective chart review documented the signs and symptoms occurring at the end of life in 28 children dying from cancer in Japan. All children experienced anorexia, 82.1% had dyspnea, and 75% had pain. Other symptoms included fatigue (71.4%), nausea and/or vomiting (57.1%), constipation (46.4%), and diarrhea (21.4%).6 This symptom profile parallels that of the North American reviews of the symptoms of dying children.7–9
Pain syndromes related to tumors in children with cancer
Despite the predominance of treatment-related pain, a number of children have pain related to a tumor, despite the initial response of their pain to treatment. One-third of the pain experienced by patients in the hospital setting was tumor-related pain, but less than 20% of the pain experienced by outpatients was caused by tumor.10 Direct tumor involvement of bone, hollow viscera, or nerves are more common causes of pain in adult patients with cancer than in children. Such tumor involvement commonly results in somatic, visceral, and neuropathic pain, respectively. Somatic pain is typically well-localized and is frequently described as aching or gnawing. Examples of somatic pain include pain associated with primary or metastatic bone disease or postsurgical incision pain. Visceral pain results from the infiltration, compression, distension, or stretching of thoracic and abdominal viscera by primary or metastatic tumor. This pain is poorly localized, often described as deep squeezing and pressure and may be associated with nausea, vomiting, and diaphoresis, particularly when acute. An example of visceral pain includes pain associated with tumor of the liver, either primary such as hepatoblastoma, or metastatic, such as neuroblastoma. Neuropathic pain most commonly results from tumor compression or infiltration of peripheral nerves or the spinal cord. Chemical- or radiation-induced injury also may result in this sort of pain. The clinical features of pain resulting from neural injury include:
The pattern of symptoms, based on the self-reports of children aged 10 to 18 treated for cancer, was studied.11 Children were surveyed across the spectrum of illness and included newly diagnosed patients, those receiving a bone marrow transplant, and those receiving palliative care. It showed that children with cancer are very symptomatic and are often highly distressed by their symptoms. A prevalence rate greater than 35% was noted for the symptoms of pain, drowsiness, nausea, cough, anorexia, lack of energy, and psychological symptoms. In-patients reported being more symptomatic than their out-patient cohorts. Children with solid tumors were more symptomatic than children with other malignancies. Pain, nausea, and anorexia were clustered as being highly distressing symptoms.11 Children 7 to 12 years of age, also treated for cancer, similarly self-reported their symptoms. The most prevalent symptoms were pain, difficulty sleeping, itch, nausea, fatigue, and anorexia.12
Pain in children with cystic fibrosis at the end of life
A retrospective chart review at a tertiary-care hospital summarized the end-of-life care of patients more than 5 years of age and dying from cystic fibrosis in the United States.13 Increasing pain for this patient population may signal advanced, progressive disease.14 Twenty-five percent of these patients had been receiving opioids for the treatment of chronic headache and/or chest pain for more than their last 3 months of life. When opioids were used for the treatment of breathlessness and/or chest pain, the proportion increased to 86%. Chest, head, extremity, abdomen, and back pain were the most common pain locations during end-of-life care.14
Pain and other symptoms in children with neurodegenerative illnesses
Pain, breathlessness, and oral symptoms such as secretions were highlighted as the most common symptoms by caregivers proxy reports for children in the last month of life at an in-patient hospice.15 Half of the children were non-communicative. Neurodegenerative illness was the major diagnostic category in this in-patient hospice population. Common sources of pain in children with cognitive and physical impairment include muscle spasticity and problems of the musculoskeletal system, such as hip dislocation or kyphoscoliosis.
Pain and other symptoms in children with HIV/AIDS
HIV/AIDS is known to cause pain and other symptoms for multiple reasons, including primary treatments, associated infections, and other complications.16 Possible causes of pain include bowel dysfunction, cachexia, pancreatitis and sequelae of infection. In a U.S,-based study, 59% of HIV-infected children reported that their pain impacted negatively on their lives.17
Pathophysiology of Tumor-Related Pain in Childhood Cancer
Mechanisms for persistent neuropathic pain after damage to peripheral tissues include:
Pain Assessment in Children with Life-Threatening Illnesses
The pain assessment of the child receiving palliative care may be a complex process. Regular pain assessment of the child receiving post-operative pain management is standard practice at most children’s healthcare facilities. It is a less-established practice that the child with progressive illness receives a regular pain assessment. Pain may still not be thought of or asked about when the child’s condition is rare, poorly understood, and/or impairs cognition; and clinicians may erroneously believe that if patients do not volunteer information about pain, then it is not a relevant clinical issue.18 In addition, children and their caregivers may not volunteer information about pain due to a fear that it may indicate progressive disease. Continually assessing a child’s pain is an essential component of competent pain management in pediatric palliative care.
Pain measurement as part of pain assessment
Unidimensional Self-Report Measures
Self-report measures of pain in children have largely focused on the assessment of acute pain severity. Generally the data support the use of visual analogue scales (VAS) or faces scales for children over the age of 5.19 VAS have been used in the assessment of pediatric cancer pain; frequently they have anchors of no pain and the worst pain possible. To use such scales, children must understand proportionality, to be able to conceptualize their pain experience along a continuum and be able to translate that understanding to the visual representations on the line and the anchors (Fig. 31-1).
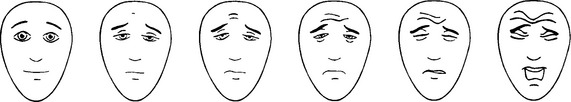
Fig. 31-1 Visual Scale for Assessment of Pediatric Pain.
(Hicks CL, von Baeyer CL, Spafford P, van Korlaar I, Goodenough B. Faces Pain Scale-Revised: Toward a Common Metric in Pediatric Pain Measurement. Pain 2001; 93:173-183. With the instructions and translations as found on the website: www.painsourcebook.ca. This figure has been reproduced with permission of the International Association for the Study of Pain® [IASP®].)
Similar strategies, such as Likert scales with anchor points of 1, no pain, and 5, extreme pain, have been used to assess pain in children with cancer.20 However, research on the use of verbal rating scales with children 9 years and older has not clearly established the utility of this approach over visual analog scales.21 Other investigators have used visual cues, such as different pictures of a child’s face that are graded from neutral or happy expressions for no pain to sad or distressed expressions for extreme pain.22,23
Behavioral Observation Measures
The subjective distress of acute pain, particularly after traumatic medical procedures, often manifests itself in certain facial expressions, verbal, and motor responses. Behavioral methods for assessing pain in children require independent raters recording the physical behaviors of children in pain, as well as the frequency of the occurrence.24 Behavioral measures of pain in children consist of observation checklists in which a trained observer records the occurrence of certain behaviors. The frequency and duration of the behaviors that occur during the medical procedures are scored to produce a numerical value that represents the child’s overall distress. This value is an integrated index of a child’s anxiety, fear, distress, and pain, but children’s behavioral scores have been interpreted as their global pain scores.25
The Gauvain-Piquard rating scale26 is an observation scale designed to assess chronic pain in pediatric oncology patients aged 2 to 6 years. The lack of operational definitions and the low kappa coefficients question the utility of this scale. The scale consists of 17 items:
Pain Measurement in Children with Neurocognitive Impairment
A substantial number of children who die have an illness that results in cognitive impairment. Many neurodegenerative diseases impact profoundly on the child’s ability to verbally communicate. The physical aspects of certain illnesses, such as grimacing or hypertonia, can mimic features or behaviors commonly attributed to pain. In one post-operative pain study, 24 children aged 3 to 19 years with cognitive impairment were rated by their caregivers and researchers as to their perceived intensity of the child’s pain pre- and post-surgery.27 Familiarity with an individual child was not necessary for observers to have congruent pain measurements. Pain cues reported by 29 caregivers of non-communicative children aged 2 to 12 years with life-threatening conditions were compared against a checklist of 203 items. This study yielded a common core set of six pain cues. These are screaming and/or yelling, crying, distressed facial expression, tense body, difficult to comfort, and flinching when touched.28
MultiDimensional Symptom Assessment Scales
The Memorial Symptom Assessment Scale 10-18, modified from an adult version, was developed for children aged 10 to 18 years with cancer. In a mean of 11 minutes, the majority of children were able to answer questions about how severe, frequent, and distressing they found their symptoms.11 For the younger child with cancer, the scale was modified and trialed in 7 to 12 year olds.12 On this scale, pain is one of many symptoms assessed in 3 dimensions: severity, frequency, and distress.
Adequate Pain Management at the End of Life is Achievable
Goals for pain management
The goals for pain management for the child receiving palliative care are:
Pain relief with what would be considered conventional analgesic doses and routes is achievable to fulfill these goals for the vast majority of children facing pain as a consequence of advanced illness. This was well documented in a 1995 study of a pediatric oncology population, where the records of 199 children and young adults dying of malignancy were reviewed. Only 6% of these patients required what would be considered massive doses of an opioid infusion, defined as 100-fold the usual post-operative opioid requirement.29 Of that small proportion of patients, there were a few instances where extraordinary doses of analgesia, the use of unusual routes such as opioid infusions given via the subarachnoid route, or the provision of sedation was required to ensure comfort at end of life.29 Similarly, regional anesthetic techniques are infrequent in treating pain at end of life for children with cancer diagnoses.30 A review conducted over a 5-year period assessed the opioid doses used in children (n = 42) dying at a pediatric hospice. The parental morphine equivalents ranged from 0.001-73.9 mg/kg/hr, with a median of 0.085 mg/kg/hr.31
Myths and misperceptions about pain in children
The myth that children either do not experience pain, or do not experience pain as much as adults, has until recently inhibited progress in pain management for children. Since the 1980s there has been a growing movement toward improved pain control for infants, children, and adolescents. This movement was partly a response to the weight of evidence indicating that poor pain control negatively influenced outcome in post-operative neonates.32 It was also partly due to improved measures of pain severity in infants and children and a critical mass of clinicians with developing expertise in this area. This latter has seen the development of interdisciplinary pain services in many pediatric centers around the world. In most cases centers have a close professional affiliation with pediatric palliative care services.
Addiction is a psychological and behavioral syndrome characterized by drug craving and aberrant drug use. Some parents fear that exposure to an opioid will result in their child subsequently becoming a drug addict. The incidence of opioid addiction was examined prospectively in 12,000 hospitalized adult patients who received at least one dose of a strong opioid.33 There were only four documented cases of subsequent addiction in patients who did not have a history of drug abuse. These data suggest that iatrogenic opioid addiction is an uncommon problem in adults. This observation is also consistent with a large worldwide experience with opioid treatment of cancer pain in childhood.
Analgesic studies at the end of a child’s life
The need to improve pain management in dying children is demonstrated by data indicating that pain is often not adequately assessed and treated effectively.7 Improvement in pain management will be dependent not only on advances in pediatric analgesic therapeutics but also on strategies to correct the barriers to the adequate treatment of pain in these children. Few analgesic studies have been performed in children receiving palliative care. One reason pertains to the heterogeneous nature of pain in this population, and that children with cancer tend to receive therapies directed at control of their tumors until very late in the course of their illnesses. They are frequently very ill and highly symptomatic. These variables make it less likely that a subpopulation of children receiving palliative care exists who have a stable, chronic pattern of pain amenable to evaluation in a trial. The lack of an appropriate analgesic study design to account for small numbers of subjects is a further impediment to progress in pain management for these children.
The Evidence from Analgesic Studies
Most of the analgesic studies of opioids performed in children with cancer34–42 have been previously performed in adults. Most of these studies had small numbers of subjects, few were controlled studies, and did not use validated pain severity assessment scales. Most did not demonstrate differences between pediatric and adult data. Significantly, however, one study40 demonstrated age differences in morphine pharmacokinetics compared with the adult population and recommended a starting total daily dose of morphine of 1.5 to 2.0 mg/kg/day to provide plasma concentrations >12ng/ml in children with cancer pain unrelieved by analgesics used for mild to moderate pain.
Historically, the status of pediatric analgesic studies has improved, as have psychometric data for measures of pain se-verity. This has resulted in a greater sophistication of pediatric analgesic studies in this patient population. For example, PCA morphine was compared with continuous infusion morphine for the relief of mucositis pain in patients aged 12 to 18 years. This study used randomized controlled trial methodology.43 Less morphine intake and fewer opioid side effects were demonstrated in the morphine PCA group.
With small patient numbers, a novel pediatric analgesic study used randomized, double-blind, three period cross-over methodology.44 The safety and efficacy of a clinical protocol for the administration of opioids by PCA for mucositis pain after bone marrow transplantation was demonstrated. In this small study, hydromorphone was not superior to morphine in terms of analgesia or the side-effect profile. The clearances of hydromorphone and morphine in the children studied were generally greater than those previously recorded, but this finding may be related to disease or treatment variables. Apart from clearance, the morphine pharmacokinetics in the study population were similar to those previously recorded. In addition, hydromorphone may be less potent in this population of children than indicated by adult equipotency tables.45
Pain Management Guidelines
Guidelines for the management of pain in children with life-threatening conditions need to be created at a local level. The extent to which clinical guidelines promote improvement is unknown. However, a recent Swedish questionnaire surveyed all the pediatric departments about their pain management practices for cancer.46 It showed that 63% of physicians follow the analgesic ladder approach recommended in the World Health Organization (WHO) guidelines.46
Analgesics
Analgesics can be divided into three groups of drugs: non-opioid analgesics, opioid analgesics, and adjuvant analgesics. The prescription of these drugs for children with cancer pain is based on the WHO analgesic ladder, which emphasizes pain intensity as the guide to choice of analgesic, rather than etiologic factors. In other words, the prescription of analgesics should be according to pain severity, ranging from acetaminophen and non-steroidal anti-inflammatory drugs for mild pain to opioids for moderate to severe pain. The choice of analgesics is individualized to achieve an optimum balance between analgesia and side effects (Table 31-2).
Acetaminophen
Acetaminophen is one of the most commonly used nonopioid analgesics in children with cancer. It has a potential for hepatic and renal injury,47 but this is uncommon in therapeutic doses. Unlike aspirin, acetaminophen does not have an association with Reye Syndrome. The antipyretic action of acetaminophen may be contraindicated in neutropenic patients in whom it is important to monitor fever. Pediatric dosing of acetaminophen has been based on the antipyretic dose-response. Oral dosing of 15 mg/kg every 4 to 6 hours is recommended, with a maximum daily dose of 60 mg/kg/day for patients of normal or average build.
There are no data on the safety of chronic acetaminophen administration in children. In Australia, New South Wales Health Policy mandates that paracetamol, or acetaminophen, should not be administered to children for more than 48 hours without a medical review.48 Intravenous paracetamol is available as a therapeutic analgesic option in some countries. Its use has been documented in the context of pediatric post-operative pain management49 and practice guidelines are evolving.50
Aspirin and Nonsteroidal Anti-inflammatory Drugs (NSAID)
Choline magnesium trisalicylate (Trilisate) has been widely recommended because of reports in adults of minimal effects on platelet function in vitro and experimental studies showing minimal gastric irritation in rats, in contrast to aspirin.51 The studies do not include medically frail patients with thrombocytopenia or other morbidities.
The cyclooxygenase-2 (COX-2) inhibitors target a specific isoenzyme involved in the generation of prostanoids, which contribute to pain and inflammation. Although celecoxib and meloxicam have undergone some limited trials in children with rheumatoid arthritis and post-operative pain,52,53 their role in pediatric pain management is unclear. Rofecoxib was removed from the international market because of increased risk of cardiovascular events in adults.54
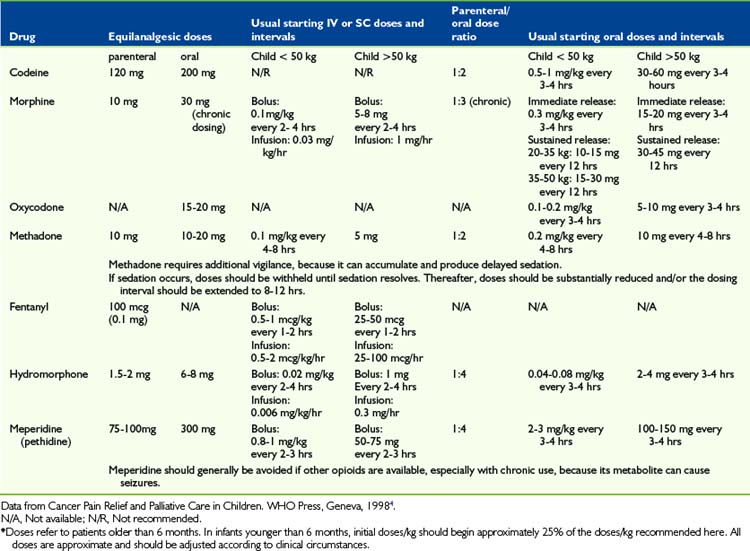
Codeine
In pediatrics, codeine is commonly administered via the oral route and in combination with acetaminophen. It is prescribed for mild to moderate pain. Codeine is typically administered in pediatrics in oral doses of 0.5-1 mg/kg every 4 hours for children older than 6 months. Pharmacogenetic studies have demonstrated that 4% to 14% of the population lacks the hepatic enzyme responsible for the conversion of codeine to morphine. A pediatric study has shown that 35% of children showed inadequate conversion of codeine to morphine.55 The prescription of codeine as an analgesic in pediatrics is declining.
Oxycodone
Oxycodone is used for moderate to severe pain in children with cancer. Oxycodone may be available only as an oral preparation in combination with acetaminophen in some countries. The total daily acetaminophen dose may be the limiting factor in dose escalation of these products. Oxycodone has a higher clearance value and a shorter elimination half life, t1/2, in children aged 2 to 20 years than adults.56,57 Oxycodone is available as a long-acting preparation in some countries.
Morphine
Morphine is one of the most widely used opioids for moderate to severe cancer pain in children. Evolving data indicate that a variable human analgesic response of morphine may be explained in part by genetic variation and different µ-opioid receptor neurotransmitter responses.58
The binding of morphine to plasma protein is age dependent. In premature infants, less than 20% is bound to plasma proteins.59,60 Within the neonatal period for term infants, the volume of distribution is linearly related to age and body surface area,59–61 but after the neonatal period the values are approximately the same as adults.62,63
Morphine clearance is delayed in the first 1 to 3 months of life. The half-life of morphine, t1/2, changes from values of 10 to 20 hours in preterm infants to values of 1 to 2 hours in preschool aged children.62,63 Therefore starting doses in very young infants should be reduced to approximately 25% to 30% on a per kg basis relative to dosing recommended for older children.
Following oral dosing, morphine has a significant first-pass metabolism in the liver. An oral-to-parenteral potency ratio of approximately 3:1 is commonly encountered during chronic administration.64 A typical starting dose for immediate release oral morphine in opioid-naive subjects is 0.3 mg/kg every 4 hours. Typical starting intravenous infusion rates are 0.02-0.03mg/kg/hr beyond the first 3 months of life, and 0.015mg/kg/hr in younger infants. Sustained release preparations of morphine are available for children and permit oral dosing either twice or three times daily. Crushing sustained-release tablets produces an immediate release of morphine. This limits its use in children who must chew tablets.
Hydromorphone
Hydromorphone is an alternative opioid when the dose escalation of morphine is limited by side effects or volume restriction is needed for parenteral administration. It is available for oral, intravenous, subcutaneous, epidural, and intrathecal administration. Adult studies indicate that intravenous hydromorphone is 5 to 8 times as potent as morphine. A double-blind, randomized crossover comparison of morphine to hydromorphone using PCA in children and adolescents with mucositis following bone marrow transplantation showed that hydromorphone was well tolerated and had a potency ratio of approximately 6:1 relative to morphine in this setting.44 Because of its high potency and aqueous solubility, hydromorphone is convenient for subcutaneous infusion. Little is known about the pharmacokinetics of hydromorphone in infants.
Fentanyl
Fentanyl is a synthetic opioid that is approximately 50 to 100 times more potent than morphine during acute intravenous administration. The half-life of this opioid is prolonged in preterm infants undergoing cardiac surgery,65 but comparable values with those of adults are reached within the first months of life.66–69 The clearance of fentanyl is higher in toddlers and children than in adults. (For younger infants, including those undergoing abdominal surgery, the clearance is not higher than in adults.68,69) Fentanyl may also be used for continuous infusion for selected patients with dose-limiting side effects from morphine. Rapid administration of high doses of IV fentanyl may result in chest wall rigidity and severe ventilatory difficulty.
Oral transmucosal fentanyl produces a rapid onset of effect and escapes first-pass hepatic clearance. Oral transmucosal fentanyl for sedation and/or analgesia during bone marrow biopsy and/or aspiration and lumbar puncture was found safe and effective, although the frequency of vomiting may be a limiting factor in its tolerability.70 Its use for breakthrough cancer pain in adults has been described.71
In a small study using a clinical protocol, the utility, feasibility, and tolerability of transdermal fentanyl was demonstrated in children with cancer pain.39 The mean clearance and volume of distribution of transdermal fentanyl are the same for both adults and children, but the variability is higher in adults.39 A subsequent larger study confirmed the effectiveness of this analgesic for children.72
Meperidine
Meperidine has been used for procedural and postoperative pain in children. It is a short half-life synthetic opioid. Neonates have a slower elimination of meperidine than children and young infants.73–77 Normeperidine is the major metabolite of meperidine. This can cause CNS excitatory effects, including tremors and convulsions,78 particularly in patients with impaired renal clearance. Meperidine is therefore not generally recommended for children with chronic pain but may be an acceptable alternative to fentanyl for short painful procedures.
Methadone
Methadone is a synthetic opioid that has a long and variable half-life. Following single parenteral doses, its potency is similar to that of morphine. In children receiving post-operative analgesia, methadone produced a more prolonged analgesia than morphine.79,80 Because of its prolonged half-life, methadone has a risk of delayed sedation and overdose occurring several days after initiating treatment.
The oral:parenteral potency ratio of methadone is approximately 1.5 to 2:1. Frequent patient assessment is the key to safe and effective use of methadone. If a patient becomes comfortable after initial doses, the dose should be reduced or the interval extended to reduce the likelihood of subsequent somnolence. If a patient becomes oversedated early in dose escalation, it is recommended to stop dosing, not just reduce the dose, and to observe the patient until there is increased alertness. Although as needed dosing is discouraged for most patients with cancer pain, some clinicians find this approach a useful way to establish a dosing schedule for methadone.79,80 Methadone remains a long-acting agent when administered either as an elixir or as crushed tablets. Unique issues with conversion between other opioids and methadone are detailed below in the section on “Opioid Switching.”
Routes and methods of analgesic administration
Topical
The eutectic mixture of local anesthetics, EMLA, is a topical preparation that provides local anesthesia to the skin, dermis, and subcutaneous tissues if applied under an occlusive dressing for at least one hour. It has been shown to be useful for procedural pain, including lumbar puncture81 and central venous port access82 in children with cancer. Preliminary studies of topical amethocaine for percutaneous analgesia before venous cannulation in children have demonstrated promising safety and efficacy data.83 The newer generation of topical local anesthetics promise a quicker onset of action and are being reviewed.84
Subcutaneous
The subcutaneous route is an alternative route of administration for children with either no or poor intravenous access. Solutions are generally concentrated so that infusion rates do not exceed 1-3 ml/hr.85 An application of a topical local anesthetic agent is recommended prior to the placement of a subcutaneous needle. A small catheter or butterfly needle (27 gauge) may be placed under the skin of the thorax, abdomen, or thigh, with sites changed approximately every 3 days.
Rectal
Rectal administration is discouraged in the pediatric cancer population because of concern regarding infection and because of the great variability of rectal absorption of morphine.86 It may be useful in the home care of the dying child when no other route is available. Slow-release morphine tablets can be administered via the rectum.
Patient-Controlled Analgesia
PCA has been used successfully for the management of prolonged oropharyngeal mucositis pain following bone marrow transplantation in children and adolescents.43,44,87 A controlled comparison of staff-controlled continuous infusion (CI) of morphine and PCA in adolescents with severe oropharyngeal mucositis found that the PCA group had equivalent analgesia but less sedation and less difficulty concentrating.43
The Pediatric Pain Crisis
A pain crisis in a child is an emergency and may require treatments beyond conventional means. A specific diagnosis must be made, as therapies directed at the primary cause may be more effective in the long term. Management requires the clinician to be at the patient’s bedside to titrate incremental intravenous opioid doses every 10 to 15 minutes until effective analgesia has been attained. The analgesic effect of opioids increase in a log-linear function, with incremental opioid dosing required until either analgesia is achieved or somnolence occurs.64 A continuous infusion of opioid may need to be commenced to maintain this level of analgesia. The initial infusion rate is often based on the opioid administered as a loading dose rather than the starting doses typically referred to in practical reference manuals.64 An alternative to a continuous infusion of opioid is intermittent parenteral opioid, especially in the setting of an unpredictable pain syndrome.
Breakthrough pain in children
Breakthrough or rescue doses are additional doses of opioid incorporated into the analgesic regime to allow for additional analgesia if required by the patient. Breakthrough doses of opioid may be calculated as approximately 5% to 10% of the total daily opioid requirement and may be administered orally every hour.64 Given the frequency with which additional analgesia may be required for severe pain, it may be convenient for some children to self-administer breakthrough opioid doses using a PCA device. Data suggest that 7-year-old children of normal intelligence can use PCA effectively to provide analgesia post-operatively.88
A prospective study determined the prevalence, characteristics, and impact of breakthrough pain in children with cancer.44 Twenty-seven pediatric inpatients with cancer aged 7 to 18 years who had severe pain requiring treatment with opioids participated in this study. The children responded to a structured interview, Breakthrough Pain Questionnaire for Children, designed to characterize breakthrough pain in children. Measures of pain, anxiety, and depressed mood were completed. Fifty-seven percent of the children experienced one or more episodes of breakthrough pain during the preceding 24 hours, each episode lasting seconds to minutes, occurring three of four times a day and most commonly characterized as sharp and shooting by the children. Younger children, those 7 to 12 years, had a significantly higher risk of experiencing breakthrough pain compared to teenagers. No statistical difference could be shown between children with and without breakthrough pain in regard to anxiety and depression. The most effective treatment of an episode of breakthrough pain was a PCA opioid bolus dose. It is clear that further studies of breakthrough pain in children and more effective treatment strategies in this age group are necessary.89
Opioid dose escalation
Opioid switching
The usual indication for switching to an alternative is dose-limiting opioid side effects that prevent dose escalation. An observation is that a switch from one opioid to another is often accompanied by change in the balance between analgesia and side effects.90 A favorable change in opioid analgesia to side-effect profile will be experienced if there is less cross-tolerance at the opioid receptors mediating analgesia than at those mediating adverse effects.91 An opioid switch may permit better analgesia with less opioid side effects.92 There are emerging pediatric data on the practice of opioid rotation in children with cancer. Following a review of opioid prescription at a pediatric hospital for the above indications, opioid rotation was employed in 9% of all opioid prescriptions, with a positive impact on side-effect control and without a significant change in pain scores.93
Following a prolonged period of regular dosing with one opioid, equivalent analgesia may be attained with a smaller dose of a second opioid than that calculated from an equianalgesic table. An opioid switch is usually accompanied by a reduction in the equianalgesic dose, approximately 50% for short half-life opioids. In contrast to short half-life opioids, the doses of methadone required for equivalent analgesia after switching may be of the order of 10% to 20% of the equianalgesic dose of the previously used short half-life opioid. Protocols for methadone dose conversion and titration have been documented in adults.94,95 These protocols have been incorporated into a very convenient opioid dose conversion program available on the web at www.globalrph.com/narcoticonv.htm. Methadone is both extremely useful and challenging to titrate, both because of its variable metabolism and because of its combined action as a mu-opioid and as an NMDA receptor antagonist. In our view, if there is a pain emergency and pain remains difficult to manage despite a rapid and aggressive opioid dose escalation, a trial of methadone should be strongly considered in many cases.
Side effects of opioids
Children with many life-threatening conditions commonly experience the overlapping symptoms of fatigue, mental clouding, sleep disturbance, depressed mood, and daytime somnolence. Evaluation and treatment of these symptoms should be broad-based and not limited to a narrow medical model. A recent study suggested that as pain in children with advanced illness is more aggressively treated with opioids, the complex of fatigue-associated symptoms becomes more common.96
In some cases, fatigue and somnolence may be improved by simplifying regimens to reduce the cumulative burden of sedating medications. Antihistamines have minimal evidence for efficacy for the treatment of opioid-induced pruritus, and they do contribute to sedation. We have been unable to identify controlled studies of the less-sedating antihistamines for pruritus or for many of the common uses of antihistamines, such as for premedication prior to administration of blood products. Data on the effectiveness of stimulants for the treatment of fatigue and somnolence in children with advanced cancer is limited to one case series.97
Although constipation is eminently treatable, studies from adult hospices with formal and fairly aggressive bowel regimens still report refractory constipation as a common problem. Oral naloxone has been studied in adults with refractory constipation, with some variability in recommended dose and in response rates. Two novel opioid antagonist drugs have been designed to provide a more mechanism-based approach to treatment of opioid-induced bowel dysfunction.98 Methylnaltrexone is a quaternized derivative of the opioid antagonist naltrexone that is excluded from the central side of the of blood-brain barrier. It can be given by intravenous or subcutaneous routes. Alvimopan is an orally-administered enterally constrained opioid antagonist. Methylnaltrexone is now available in the United States, albeit at a retail cost of approximately $40 per dose. Pediatric experience to date is limited, though anecdotally the safety and efficacy has appeared quite good. Methylnaltrexone may be especially useful for the child receiving high dose opioids with refractory constipation who cannot tolerate an enteral laxative regimen. Based on favorable anecdotal experience, methylnaltrexone may also be considered for two other situations:
NMDA receptor antagonists
NMDA-receptor antagonists depress central sensitization to painful stimuli in animal experiments and in humans.99–102 Dextromethorphan, dextrorphan, ketamine, memantine, and amantadine, among others, have been shown to have NMDA-receptor antagonist activities. The clinical usefulness of some of these medications is compromised by a high adverse side effect to analgesic ratio. There are limited data of their utility in pediatrics, other than procedural pain management. Clinical usage is increasing, particularly for severe neuropathic pain and rapid opioid dose escalation and perceived tolerance. A small case series described low-dose ketamine infusions for children with poorly controlled pain due to cancer.103
Other approaches to intractable pediatric cancer pain
The experience of using regional anesthesia for children with intractable pain is limited. A retrospective study of children with terminal cancer30 showed that regional anesthesia may be appropriate in a highly select subset of children. The indications for regional anesthesia in this group were mostly related to either dose-limiting side effects of opioids or opioid unresponsiveness in patients where pain was confined to one region of the body. Rapid intravenous opioid dose reduction was required in some cases.30 In the years since publication of that case series, some technical aspects of regional anesthesia in this setting have evolved.104
Other modalities of pain management
Although prominent in clinical practice, there is little in the published literature about such modalities as radiotherapy, radiopharmaceuticals, and transcutaneous electrical nerve stimulation (TENS), generally used concurrently with other pain management techniques. A case series reported some benefit for 29 children with symptomatic metastatic neuroblastoma sites treated with palliative radiotherapy.105 Similarly, the use of strontium-89 was reported for pain relief in children treated for metastatic cancer but the numbers were too small to make suggestions for clinical care.106
Adjuvant Analgesics
Adjuvant analgesics are a heterogeneous group of drugs that have a primary indication other than pain but are analgesic in some painful conditions.107 Adjuvant analgesics are commonly, but not always, prescribed with primary analgesic drugs. Common classes of these agents include antidepressants, anticonvulsants, neuroleptics, psychostimulants, antihistamines, corticosteroids, and centrally acting skeletal muscle relaxants.
Antidepressants
Data from adult studies have guided the use of antidepressants as adjuvant analgesics in pediatrics. Tricyclic antidepressants have been used for a variety of pain conditions in adults, including postherpetic neuralgia,108 diabetic neuropathy,109 tension headache,110 migraine headache,111 rheumatoid arthritis,112 chronic low back pain,113 and cancer pain.114 Antidepressants are potentially effective in relieving neuropathic pain. With very similar results for anticonvulsants, it is still unclear which drug class should be the first choice.115
Baseline hematology and biochemistry tests, including liver function tests, and an electrocardiogram (ECG) to exclude Wolff-Parkinson-white syndrome or other cardiac conduction defects have been recommended prior to starting treatment with tricyclic antidepressants.116 The measurement of antidepressant plasma concentration allows confirmation of compliance and ensures that optimization of dosage has occurred before discontinuing. An ECG is recommended periodically during long-term use or if standard mg/kg dosages are exceeded.117
Psychostimulants
Dextroamphetamine potentiates opioid analgesia in postoperative adult patients118 and methylphenidate counteracts opioid-induced sedation119 and cognitive dysfunction120 in advanced cancer patients. Psychostimulants may allow dose escalation of opioids in patients who have somnolence as a dose-limiting side effect.107 The potential side effects of methylphenidate include anorexia, insomnia, and dysphoria. The use of dextroamphetamine and methylphenidate was reported in a retrospective survey of 11 children receiving opioids for a variety of indications, including cancer pain.97 Somnolence was reduced in these patients without significant adverse side effects.
Corticosteroids
Corticosteroids may produce analgesia by a variety of mechanisms, including anti-inflammatory effects and reduction of tumor edema. It may potentially produce analgesia by a reduction of spontaneous discharge in injured nerves.121 Dexamethasone tends to be used most frequently because of its high potency, longer duration of action, and minimal mineralocorticoid effect. Corticosteroids may have a role in bone pain due to metastatic bone disease,122 cerebral edema due to either primary or metastatic tumor,123 or epidural spinal cord compression.124
Anticonvulsants
The mechanism of action of anticonvulsants in controlling lancinating pain is not known but is probably related to reducing paroxysmal discharges of central and peripheral neurons. Anticonvulsants are effective in relieving neuropathic pain. With very similar results for antidepressants, it is still unclear which drug class should be the first choice.115 The use of phenytoin, carbamazepine, and valproate may be problematic in the pediatric cancer population due to their potential adverse effects on the hematological profile. Gabapentin and pregabalin have a generally good safety profile, and may benefit some children with neuropathic pain. Some children experience adverse effects on mood with gabapentin, pregabalin, or any other of the anticonvulsants.
Neuroleptics
Methotrimeprazine, a phenothiazine, has been reported as being analgesic for adult cancer pain.126 Methotrimeprazine is not considered to be a substitute for opioid analgesia. The mechanism by which methotrimeprazine produces analgesia, and its role as an adjuvant agent in pediatric cancer pain, is unclear. It may be useful as an adjuvant analgesic in a patient disseminated cancer who also experiences pain associated with anxiety, restlessness, or nausea107 (Table 31-3).
TABLE 31-3 Dosage Guidelines for Commonly Used Adjuvant Analgesics
| Adjuvant analgesic | Starting dose | Dose guideline |
|---|---|---|
| Amitriptyline | 5 mg | 0.5 – 1.0 mg/kg/day |
| Nortriptyline | 5 mg | 0.5 -1.0 mg/kg/day |
| Gabapentin | 10 mg/kg/day | Increasing dose every day until maximum 30 mg/kg/day reached. Then reassess. |
| Gabapentin | 100 mg at night for patients > 50 kg | Increase dose every 2 to 7 days depending on the clinical setting. In 2 days begin twice daily dosing. Escalate to 3 times daily dosing, with half the daily dose at night, as tolerated or until reaching a full TID dose of 600 mg / 600 mg/ 1200 mg |
| Pregabalin | 25 mg BID for patients > 50 kg | Increase dose every 2 to 7 days, depending on the clinical setting, as tolerated or until reaching 150 mg BID |
Sedation in Pediatric Palliative Care
The European Association for Palliative Care (EAPC) has reviewed the use of sedation in palliative care including the indications, risks, benefits and potential for abuse, injudicious use and substandard clinical practice in 2009.127 (See Chapter 23 for more information.) The guidelines given may inform clinical services in the development of policies and procedures to promote ethical decision making and promote and protect the interests of patients, their families and healthcare providers. Many of the principles in this review may be applicable to pediatric practice (Box 31-1).
BOX 31-1 Framework for the Development of Sedation Guidelines
Adapted from Ozen, S et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010 May; 69(5):798–806.
The use of sedation for refractory pain generally assumes that therapies beyond the conventional have been used or are impractical and that there is no acceptable means of providing analgesia without compromising consciousness. Given the principles of pain management outlined above, this is an extremely rare practice in pediatrics. Continuous deep sedation should be considered only if the patient is in the very terminal stages, with an expected prognosis of hours or days at most.127 Transient respite sedation may be considered earlier in the illness trajectory to provide temporary relief while waiting for more effective analgesic therapies to take effect.
The trade-off between sedation and inadequate pain relief requires the consideration of the wishes of the child, as appropriate, and his or her family. The ethical issues surrounding prolonged sedation in pediatrics, including the principle of double effect, have been previously discussed.128–130 The continuation of high-dose opioid infusions in these circumstances is recommended to avoid situations in which a patient may have unrelieved pain but inadequate clarity to express pain perception. A variety of drugs have been used in this setting, including barbiturates, benzodiazepines, and phenothiazines.129,131
1 Wall P.D., Melzack R. Textbook of pain, ed 5. London: Elsevier Churchill Livingstone, 2006.
2 Collins J.J., Stevens M.M., Berde C.B. Pediatric cancer pain. In: Sykes N., Bennett M.I., Yung K.K., editors. Cancer pain. ed 2. London: Hodder and Stroughton; 2008:345-358.
3 Greco C., Berde C.B. Acute pain management in children. In Ballantyne J., Rathmell J., Fishman S., editors: Bonica’s pain management in children, ed 4, Philadelphia: Lippincott Williams and Wilkins, 2009.
4 Cancer pain relief and palliative care in children. Geneva: WHO, 1998. Ref Type: Pamphlet
5 Drake R., Frost J., Collins J.J. The symptoms of dying children. J Pain Symptom Manage. 2003;27(7):6-10.
6 Hongo T., Watanabe C., Okada S. Analysis of the circumstances at the end of life in children with cancer: symptoms, suffering and acceptance. Pediatr Int. 2003;45:60-64.
7 Wolfe J., Grier H.E., Klar N., et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342(5):326-333.
8 McCallum D.E., Byrne P., Bruera E. How children die in hospital. J Pain Symptom Manage. 2000;20(6):417-423.
9 Belasco J., Danz P., Drill A., Schmid W., Burkey E. Supportive care: palliative care in children, adolescents, and young adults. J Palliat Care. 2000;16:39-46.
10 Miser A.W., Dothage P., Wesley R.A., et al. The prevalence of pain in a pediatric and young adult population. Pain. 1987;29:265-266.
11 Collins J.J., Byrnes M.E., Dunkel I., Foley K.M., Lapin J., Rapkin B., et al. The Memorial Symptom Assessment Scale (MSAS): validation study in children aged 10-18. J Pain Symptom Manage. 2000;19(5):363-367.
12 Collins J.J., Devine T.B., Dick G., Johnson E.A., Kilham H.K. The measurement of symptoms in young children with cancer: the validation of the Memorial Symptom Assessment Scale in children aged 7-12. J Pain Symptom Manage. 2002;23(1):10-16.
13 Robinson W.M., Ravilly S., Berde C.B., Wohl M.E. End-of-life care in cystic fibrosis. Pediatrics. 1997;100:205-209.
14 Ravilly S., Robinson W., Suresh S., Wohl M.E., Berde C.B. Chronic pain in cystic fibrosis. Pediatrics. 1996;98:741-747.
15 Hunt A.M. A survey of signs, symptoms and symptom control in 30 terminally ill children. Dev Med Child Neurol. 1990;32:347-355.
16 Oleske J.M., Czarniecki L. Continuum of palliative care: lessons from caring for children infected with HIV-1. Lancet. 1999;354:1287-1290.
17 Hirschfeld S., Moss H., Dragisic K., Pizzo P.A. Pain in pediatric immunodeficiency virus infection: incidence and characteristics in a single-institution pilot study. Pediatrics. 1996;98:449-452.
18 McGrath P.J., Frager G. Psychological barriers to optimal pain management in infants and children. Clin J Pain. 1996;12:135-141.
19 Bieri D., Reeve R.A., Champion G.D., Addicoat L., Ziegler J.B. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990;41(2):139-150.
20 LeBaron S., Zeltzer L. Assessment of acute pain and anxiety in children and adolescents by self-reports, observer reports and a behavior checklist. J Consult Clin Psychol. 1984;52:729-738.
21 Savedra M., Gibbons P., Tesler M., et al. How do children describe pain? A tentative assessment. Pain. 1982;14:95-104.
22 Kuttner L., Bowman M., Teasdale M. Psychological treatment of distress, pain and anxiety for children with cancer. Dev Behav Pediatr. 1988;9:374-381.
23 Manne S.L., Bakeman R., Jacobsen P., et al. Adult and child interaction during invasive medical procedures: sequential analysis. Health Psychol. 1992;11:241-249.
24 Jay S.M., Ozolins M., Elliott C., Caldwell S. Assessment of children’s distress during painful medical procedures. J Health Psych. 1983;2:133-147.
25 Shacham S., Daut R. Anxiety or pain: what does the scale measure? J Consult Clin Psychol. 1981;49(468):469.
26 Gauvain-Piquard A., Rodary C., Rezvani A., Lemerle J. Pain in children aged 2-6 years: a new observational rating scale elaborated in a pediatric oncology unit: a preliminary report. Pain. 1987;31:177-188.
27 Breau L.M., Finley G.A., McGrath P.J., Camfield C.S. Validation of the Non-communicating Children’s Pain Checklist-Postoperative Version. Anesthesiology. 2002;96(3):523-526.
28 Stallard P., Williams A., Velleman R., Lenton S., McGrath P.J. Brief report: behaviors identified by caregivers to detect pain in noncommunicating children. Pediatr Psychology. 2002;27:209-214.
29 Collins J.J., Grier H.E., Kinney H.C., Berde C.B. Control of severe pain in terminal pediatric malignancy. J Pediatr. 1995;126(4):653-657.
30 Collins J.J., Grier H.E., Sethna N.F., Berde C.B. Regional anesthesia for pain associated with terminal malignancy. Pain. 1996;65:63-69.
31 Siden H., Nalewajek P. High dose opioids in pediatric palliative care. J Pain Symptom Manage. 2003;25(5):397-399.
32 Anand K.J., Hansen D.D., Hickey P.R. Hormonal metabolic stress response in neonates undergoing surgery. Anesthesiology. 1990;73:661-670.
33 Porter J., Lick J. Addiction is rare in patients treated with narcotics [letter]. N Engl J Med. 1980;302:123.
34 Miser A.W., Moore L., Greene R. Prospective study of continuous intravenous and subcutaneous morphine infusions for therapy-related or cancer-related pain in children and young adults with cancer. Clin J Pain. 1986;2:101-106.
35 Miser A.W., Dothage J.A., Miser J.S. Continuous intravenous fentanyl for pain control in children and young adults with cancer. Clin J Pain. 1987;2:101-106.
36 Miser A.W., Miser J.S. The use of oral methadone to control moderate and severe pain in children and young adults with malignancy. Clin J Pain. 1985;1:243-248.
37 Miser A.W., Miser J.S., Clark B.S. Continuous intravenous infusion of morphine sulfate for control of severe pain in children with terminal malignancy. J Pediatr. 1980;96(5):930-933.
38 Miser A.W., Davis D.M., Hughes C.S., Mulne A.F., Miser J.S. Continuous subcutaneous infusion of morphine in children with cancer. Am J Dis Child. 1983;137(4):383-385.
39 Collins J.J., Dunkel I., Gupta S.K., et al. Transdermal fentanyl in children with cancer: feasibility, tolerability, and pharmacokinetic correlates. J Pediatr. 1999;134:319-323.
40 Hunt A.M., Joel S., Dick G., Goldman A. Population pharmacokinetics of oral morphine and its glucuronides in children receiving morphine as immediate-release liquid or sustained-release tablets. J Pediar. 1999;135(1):47-55.
41 Noyes M., Irving H. The use of transdermal fentanyl in pediatric oncology palliative care. Am J Hosp Palliat Care. 2004;18(6):411-416.
42 Hunt A.M., Goldman A., Devine T.B., Phillips M. Transdermal fentanyl for pain relief in a paediatric palliative care population. Palliat Med. 2001;15(5):405-412.
43 Mackie A.M., Coda B.C., Hill H.F. Adolescents use patient controlled analgesia effectively for relief from prolonged oropharyngeal mucositis pain. Pain. 1991;46:265-269.
44 Collins J.J., Geake J., Grier H.E., et al. Patient-controlled analgesia for mucositis pain in children: a three-period crossover study comparing morphine and hydromorphone. J Pediatr. 1996;129(5):722-728.
45 Cherny N.I., Chang V., Frager G., Ingham J.M., Tiseo P.J., Popp B., et al. Opioid pharmacotherapy in the management of cancer pain. Cancer. 1995;76(7):1283-1292.
46 Ljungman G., Kreugar A., et al. Treatment of pain in pediatric oncology: a Swedish nationwide survey. Pain. 1996;68:385-394.
47 Sandler D.P., Smit J.C., Weinberg C.R., et al. Analgesic use and chronic renal disease. N Engl J Med. 1989;320:1238-1243.
48 Paracetamol use. NSW Health, 2009. Available from: URL: www.health.nsw.gov.au/policies/pd/2006/PD2006_004.html Accessed June 25, 2010
49 Wurthwein G., Koling S., Reich A., et al. Pharmacokinetics of intravenous paracetamolin children and adolescents under major surgery. Eur J Clin Pharmacol. 2005;60:883-888.
50 NSW Therapeutic Advisory Group I. IV paracetamol—where does it sit in hospital practice? Australia: NSW, 2005. Sep
51 Stuart J.J., Pisko E.J. Choline magnesium trisalicylate does not impair platelet aggregation. Pharnatheraoeutica. 1981;2:547.
52 Foeldvari I., Burgos-Varos R., Thon A., Tuerck D. High response rate in the phase 1/11study of meloxicam in juvenile rheumatoid arthritis. J Rheumatol. 2002;29:1079-1083.
53 Stempak D., Gammon J., Klein J., et al. Single-dose and steady-state pharmacokinetics of celecoxib in children. Clin Pharmacol Ther. 2006;72:490-497.
54 Mukherjee D., Nissen S.E., Topol E.J. Risk of cardiovascular events associated with selectiev COX -2 inhibitors. JAMA. 2001;286:954-959.
55 Williams D., Patel A., Howard R.F. Pharmacogenetics of codeine metabolism in an urban population of children and its implication for analgesic reliability. Br J Anaesth. 2002;89:839-845.
56 Poyhia R., Seppala T. Lipid solubility and protein binding of oxycodone in vitro. Pharmacol Toxicol. 1994;74:23-27.
57 Pelkonen O., Kaltiala E.H., Larmi T.KL. Comparison of activities of drug metabolizing enzymes in human fetal and adult liver. Clin Pharmacol Ther. 1973;14:840-846.
58 Ross J., Riley J., Welsh K. Genetic variation in the catechol-o-methyl-transferase gene is associated with response to morphine in cancer patients. In: Flor H., Kalso E., Dostrovsky J.O., editors. Proceedings of the 11th World Congress on Pain. Seattle: IASP Press; 2006:461-467.
59 McRorie T.I., Lynn A., Nespeca M.K. The maturation of morphine clearance and metabolism. Am J Dis Child. 1992;146:972-976.
60 Bhat R., Chari G., Gulati A., et al. Pharmacokinetics of a single dose of morphine in pre-term infants during the first week of life. J Pediatr. 1990;117:477-481.
61 Pokela M.L., Olkkala K.T., Seppala T. Age-related morphine kinetics in infants. Dev Pharmacol Ther. 1993;20:26-34.
62 Stanski D.R., Greenblatt D.J., Lowenstein E. Kinetics of intravenous and intramuscular morphine. Clin Pharmacol Ther. 1978;24:52-59.
63 Olkkola K.T., Maunuksela E.L., Korpela R., Rosenberg P.H. Kinetics and dynamics of postoperative intravenous morphine in children. Clin Pharmacol Ther. 1988;44(2):128-136.
64 Cherny N.I., Foley K.M. Nonopioid and opioid analgesic pharmacotherapy of cancer pain. Cherny N.I., Foley K.M., editors. Hematol Oncol Clin North Am. 1996: 79-102.
65 Collins C., Koren G., Crean P., et al. Fentanyl pharmacokinetics and hemodynamic effects in preterm infants during ligation of patent ductus arteriosus. Anesth Analg. 1985;64:1078-1080.
66 Koren G., Goresky G., Crean P., et al. Unexpected alterations in fentanyl pharmacokinetics in children undergoing cardiac surgery: age related or disease related? Dev Pharmacol Ther. 1986;9:183-191.
67 Koren G., Goresky G., Crean P., et al. Pediatric fentanyl dosing based on pharmacokinetics during cardiac surgery. Anesth Analg. 1984;63:577-582.
68 Johnson K., Erickson J., Holley F., Scott J. Fentanyl pharmacokinetics in the pediatric population. Anesthesiology. 1984;61(3A):A441.
69 Gauntlett I.S., Fisher D.M., Hertzka R.E., et al. Pharmacokinetics of fentanyl in neonatal humans and lambs: effects of age. Anesthesiology. 1988;69:683-687.
70 Schechter N.L., Weisman S.J., Rosenblum M., et al. The use of oral transmucosal fentanyl citrate for painful procedures in children. Pediatrics. 1995;95:335-339.
71 Payne R., Coluzzi P., Hart L., Simmonds M., Lyss A., Rauck R., et al. Long-term safety of oral transmucosal fentanyl citrate for breakthrough cancer pain. J Pain Symptom Manage. 2001;22(1):575-583.
72 Hunt A., Goldman A., Devine T., Phillips M. Transdermal fentanyl for pain relief in a paediatric palliative care population. Palliat Med. 2001;15(5):405-412.
73 Tamsen A., Hartvig P., Fagerlund C., et al. Patient-controlled analgesic therapy, part 1: pharmacokinetics of pethidine in the pre- and postoperative periods. Clin Pharmacokinet. 1982;7:149-163.
74 Hamunen K., Maunuksela E.L., Seppala T., et al. Pharmacokinetics of IV and rectal pethidine in children undergoing ophthalmic surgery. Br J Anaesth. 1993;71:823-826.
75 Koska A.J., Kramer W.G., Romagnoli A., et al. Pharmacokinetics of high dose meperidine in surgical patients. Anesth Analg. 1981;60:8-11.
76 Pokela M.L., Olkkala K.T., Kovisto M., et al. Pharmacokinetics and pharmacodynamics of intravenous meperidine in neonates and infants. Clin Pharmacol Ther. 1992;52:342-349.
77 Mather L.E., Tucker G.T., Pflug A.E., et al. Meperidine kinetics in man: intravenous injection in surgical patients and volunteers. Clin Pharmacol Ther. 1975;17:21-30.
78 Kaiko R.F., Foley K.M., Grabinsky P.Y., et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180-185.
79 Berde C.B., Sethna N.F., Holzman R.S., Reidy P., Gondek E.J. Pharmacokinetics of methadone in children and adolescents in the perioperative period. Anesthesiology. 1987;67:A519.
80 Berde C.B., Beyer J.E., Bournaki M.C., Levin C.R., Sethna N.F. Comparison of morphine and methadone for prevention of postoperative pain in 3- to 7-year-old children. J Pediatr. 1991:136-141.
81 Kapelushnik J., Koren G., Solh H., et al. Evaluating the efficacy of EMLA in alleviating pain associated with lumbar puncture: comparison of open and double-blinded protocols in children. Pain. 1990;42:31-34.
82 Miser A.W., Goh T.S., Dose A.M., et al. Trial of a topically administered local anesthetic (EMLA cream) for pain relief during central venous port accesses in children with cancer. J Pain Symptom Manage. 1994;9(4):259-264.
83 Van Kan H.JM., Egberts A.CG., Rijnvos W.PM., Ter Pelkwijk N.J., Lenderink A.W. Tetracaine versus lidocaine-prilocaine for preventing venipuncture-induced pain in children. Am J Obstet Gynecol. 1997;54:388-392.
84 Houck CS, Sethna NF: Transdermal analgesia with local anesthetics in children: review, update and future directions, Expert Rev Neurother
85 Bruera E., Brenneis C., Michaud M., et al. Use of the subcutaneous route for the administration of narcotics in patients with cancer pain. Cancer. 1988;62:407-411.
86 Gourlay G. Fatal outcome with use of rectal morphine for postoperative pain control in an infant. Br Med J. 1992;304:766-767.
87 Dunbar P.J., Buckley P., Gavrin J.R., Sanders J.E., Chapman C.R. Use of patient-controlled analgesia for pain control for children receiving bone marrow transplants. J Pain Symptom Manage. 1995;10:604-611.
88 Berde C.B., Lehn B.M., Yee J.D., et al. Patient controlled analgesia in children and adolescents: a randomized, prospective comparison with intramuscular morphine for postoperative analgesia. J Pediatr. 1991;118:460-466.
89 Friedrichsdorf S., Finney D., Bergin M., Stevens M., Collins J.J. Breakthrough pain in children with cancer. J Pain Symptom Manage. 2007;34(2):209-216.
90 Galer B.S., Coyle N., Pasternak G.W., et al. Individual variability in the response to different opioids: report of five cases. Pain. 1992;49:87-91.
91 Portenoy R.K. Opioid tolerance and responsiveness: research findings and clinical observations. In: Gebhart G.F., Hammond D.I., Jensen T.S., editors. Progress in pain research and management. Seattle: IASP Press; 1994:615-619.
92 Indelicato R.A., Portenoy R.K. Opioid rotation in the managment of refractory cancer pain. J Clin Oncol. 2003;21:87-91.
93 Drake R., Longworth J., Collins J.J. Opioid rotation in children with cancer. J Palliat Med. 2004;7(3):419-422.
94 Inturrisi C.E., Portenoy R.K., Max M., Colburn W.A., Foley K.M. Pharmacokinetic-pharmacodynamic relationships of methadone infusions in patients with cancer pain. Clin Pharmacol Ther. 1990;47:565-577.
95 Ripamonti C., Groff L., Brunelli C., et al. Switching from morphine to oral metahadone in treating cancer pain: what is the equianalgesic dose ratio? J Clin Oncol. 1998;16:3216-3221.
96 Ullrich C., Dusel V., Hilden J., et al. Recognition and treatment of fatigue in children with advanced cancer. Pediatr Blood Cancer. 52(6), 2009.
97 Yee J.D., Berde C.B. Dextroamphetamine or methylphenidate as adjuvants to opioid analgesia for adolescents with cancer. J Pain Symptom Manage. 1994;9:122-125.
98 Berde C.B., Nurko S. Opioid side effects: mechanism-based therapy. N Engl J Med. 2008;358(22):2332-2343.
99 Eide P.K., Jorum E., Stubhaug A., et al. Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind cross-over comparison with morphine and placebo. Pain. 1994;58:347-354.
100 Persson J., Axelsson G., Hallin R.G., et al. Beneficial effects of ketamine in a chronic pain state with allodynia. Pain. 1995;60:217-222.
101 Nelson K.A., Park K.M., Robinovitz E., et al. High dose dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48:1212-1218.
102 Eisenberg E., Pud D. Can patients with chronic neuropathic pain be cured by acute administration of the NMDA-receptor antagonist amantadine? Pain. 1994;74:37-39.
103 Finkel J.C., Pestieau S.R., Quezado Z.M. Ketamine as an adjuvant for treatment of cancer pain in children and adolescents. J Pain. 2007;8(6):515-521.
104 Carullo V., Carpino E., Weldon C., Berde C.B. Intraspinal analgesia via implanted ports for refractory pain in paediatric advanced cancer. ISPP Publications, 2010. In press
105 Paulino A.C. Palliative radiotherapy in children with neuroblastoma. Pediatr Hematol Oncol. 2003;20(2):111-117.
106 Charron M., Brown M., Rowland P., Mirro J. Pain palliation with strontium-89 in children with metastatic disease. Med Pediatr Oncol. 1996;26(6):393-396.
107 Lussier D., Portenoy R.K. Adjuvant analgesics in pain management. In: Hanks G.WC., Cherny N.I., Christiakis N.A., Fallon M., Kaasa S., Portenoy R.K., editors. Oxford textbook of palliative medicine. ed 4. Oxford University Press; 2009:706-733.
108 Watson C., Evans R., Reed K., et al. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32:671-673.
109 Max M.B. Antidepressants as analgesics. In: Fields H.L., Liebeskind J.C., editors. Progress in pain research and pain management. Seattle: IASP Press; 1994:229-246.
110 Diamond S., Baltes B. Chronic tension headache treatment with amitriptyline: a double blind study. Headache. 1971;11:110-116.
111 Couch J., Ziegler D., Hassanein R. Amitriptyline in the prophylaxis of migraine: effectiveness and relationship of antimigraine and antidepressant effects. Neurology. 1976;26:121-127.
112 Frank R., Kashani J., Parker J., et al. Antidepressant analgesia in rheumatoid arthritis. J Rheumatology. 1988;15:1632-1638.
113 Ward N. Tricyclic antidepressants for chronic low back pain: mechanisms of action and predictors of response. Spine. 1986;11:661-665.
114 Magni G. The use of antidepressants in the treatment of chronic pain. Drugs. 1991;42(5):730-748.
115 McQuay H.J., Tramer M., Nye B.A., et al. A systematic review of antidepressants in neuropathic pain. Pain. 2003;68:217-227.
116 Heiligenstein E., Gerrity S. Psychotropics as adjuvant analgesics. In: Schechter N.L., Berde C.B., Yaster M., editors. Pain in infants, children, and adolescents. Baltimore: Williams and Wilkins; 1993:173-177.
117 Biederman J., Baldessarini R.J., Wright V., et al. A double-blind placebo controlled study of desipramine in the treatment of ADD:II. Serum drug levels and cardiovascular findings. J Am Acad Child Adolesc Psychiatry. 1989;28:903-911.
118 Forrest W.H., Brown B.W., Brown C.R., et al. Dextroamphetamine with morphine for the treatment of postoperative pain. N Engl J Med. 1977;296(13):712-715.
119 Bruera E., Miller M.J., Macmillan K., Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain. 1992;48:163-166.
120 Bruera E., Faisinger R., MacEachern T., Hanson J. The use of methylphenidate in patients with incident pain receiving regular opiates: a preliminary report. Pain. 1992;50:75-77.
121 Watanabe S., Bruera E. Corticosteroids as adjuvant analgesics. J Pain Symptom Manage. 1994;9:442-445.
122 Tannock I., Gospodarowicz M., Meakin W., et al. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol. 1989;7(5):590-597.
123 Weinstein J.D., Toy F.J., Jaffe M.E., Goldberg H.I. The effect of dexamethasone on brain edema in patients with metastatic brain tumors. Neurology. 1973;23:121-129.
124 Greenberg H.S., Kim J., Posner J.B. Epidural spinal cord compression from metastatic tumor: results with a new treatment protocol. Ann Neurol. 1980;8:361-366.
125 Khurana D.S., Riviello J., Helmers S., et al. Efficacy of gabapentin therapy in children with refractory partial seizures. J Pediatr. 1996;128:829-833.
126 Beaver W.T., Wallenstein S., Houde R.W. A comparison of the analgesic effects of methotrimeprazine and morphine in patients with cancer. Clin Pharmacol Ther. 1966;7:436-446.
127 Cherny N.I., Radbruch L. European Association for Palliative Care (EAPC) recommended framework for the use of sedation in palliative care. Palliat Med. 2009;23(7):581-593.
128 Truog R.D., Berde C.B., Mitchell C., Grier H.E. Barbiturates in the care of the terminally ill. N Engl J Med. 1992;327:1678-1682.
129 Kenny N.P., Frager G. Refractory symptoms and terminal sedation in children: ethical issues and practical management. J Palliat Care. 1996;12:40-45.
130 Truog R.D., Burns J.P., Shurin S.B., Emanuel E.J. Ethical considerations in pediatric oncology. In: Pizzo P.A., Poplack D.G., editors. Principles and practice of pediatric oncology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2002:1411-1430.
131 Siever B.A. Pain management and potentially life-shortening analgesia in the terminally ill child: the ethical implications for pediatric nurses. J Pediatr Nurs. 1994;5:307-312.