3.2 Paediatric neurotrauma
Introduction
Paediatric neurotrauma is a common presenting problem in emergency medicine practice. This chapter will deal principally with paediatric head injury or, more correctly, traumatic brain injury (TBI), and spinal cord injury. Spinal injury is also covered in Chapter 24.3.
Epidemiology
TBI covers a spectrum of injury from trivial to lethal. It is the leading cause of morbidity and mortality in paediatric trauma.1 The number of children admitted to hospital in a recent study indicated an annual rate in Australia of 232 per 100 000 for those aged 0–4 years, 158 per 100 000 for those aged 5–9 years and 203 per 100 000 for those aged 10–14 years.2 These figures are higher than the overall, age standardised, annual rate of between 140 and 150 per 100 000 population.2,3 It is important to remember that these figures only reflect hospital admissions and the true incidence of TBI in the community is much higher.4
In Australia the most common cause of TBI is falls, followed by motor vehicle related accidents.2 A much smaller percentage result from being struck by objects, crushed, assaulted (non-accidental injury) and other miscellaneous causes. Males consistently outnumber females, with most studies reporting approximately a 2:1 ratio for all age groups. The overall incidence of spinal cord injury in Australia for children aged 0 to 14 years is unknown.4,5 The age standardised incidence in the 15+-year-old population is 14 per million of population per year, with a male to female ratio averaging 4:1, but peaking at 9:1 in the 15–24-year age group.5
Pathophysiology
Children have unique anatomical, physiological and developmental differences when compared to adults. They have a large head to body ratio, leading to a high centre of gravity (falls) and to the head being the primary ‘target’ for trauma. The skull is thinner and more plastic and thus transmits rather than attenuates impact.6,7 Skull fractures are therefore more common in children and importantly, serious brain injury can occur without an associated skull fracture.8–12
In children, the dura is more closely adherent to the skull compared to adults, making extradural haematomas less common in children, particularly in infants.7,13 Unfused sutures and an open fontanelle can expand to accommodate intracranial haemorrhage or cerebral oedema.7 Some authors have thought children to be more prone to ‘malignant cerebral swelling’ that can cause rapid and sometimes fatal deterioration even after minor TBI.9,14,15 However, this view has recently been questioned and swelling may be no more common in children than in adults.16
Physiologically, children have a lower systolic and mean arterial blood pressure, which implies a lower cerebral perfusion pressure (CPP). This, in turn, may cause problems with maintaining adequate cerebral perfusion if they have raised intracranial pressure. Infants and small children may become hypovolaemic with large intracranial bleeds. This is not seen in larger children or adults.6
Cerebral blood flow is often very low and may approach ischaemic levels following more severe TBI.17 This may be related to a low brain metabolic rate in comatose patients, increased intracerebral pressure and vasospasm. Autoregulation of cerebral blood flow (CBF) may be lost following TBI, and in this setting CPP largely determines CBF. This underscores the importance of maintaining an adequate CPP in the head-injured patient, especially those with more severe injuries.
Head injury, as opposed to TBI, may be described as extra-axial or intra-axial. Extra-axial injury refers to pathology outside the brain parenchyma.7,18 Extra-axial structures include the skull, structures between the skull and brain and the ventricular spaces within the brain. Common extra-axial lesions include skull fractures and extradural, subdural, subarachnoid and intraventricular haemorrhages. Extra-dural haemorrhage occurs, as its name implies, outside the dura. Medical literature often refers to this as ‘epidural haemorrhage’ but in Australasia the term ‘epidural’ is generally used only to describe lesions outside the dura of the spinal cord. Intra-axial injuries are true TBIs and include contusion, laceration, haemorrhage and diffuse axonal injury (DAI). DAI may result in considerable disability with little to see on radiological investigation.7
Classification
The generally accepted method of classifying severity of TBI is by using the Glasgow Coma Scale (GCS), although other measures such as duration of unconsciousness or amnesia are sometimes used. The GCS was first described for adults in 1974 and scores three variables: eye opening, verbal response and motor response (see ‘examination’ and Table 3.2.1).19 It has proved to be a very useful tool in rating severity of TBI and prognosis.7
The problem with using the GCS on young children, particularly those aged less than 2 years, is that the best verbal response is limited by their language development. In an attempt to overcome this difficulty, modified GCSs have been proposed, including the so-called Child Coma Scale (CCS).9,20 It is important to note that, unlike the GCS, the CCS has never been properly validated and many studies of head injury in children deliberately exclude those aged less than 2 years.
TBI is usually divided into three categories: mild, moderate and severe.
Mild TBI (GCS 14 to 15)
Mild TBI, sometimes termed ‘minor’ TBI, was originally defined as head trauma patients with a GCS from 13 to 15 (and/or varying periods of loss of consciousness (LOC) and amnesia).21 The problem with this definition is that patients with GCS 13 have a significantly higher risk of intracranial injury, with subsequent risks for deterioration and neurosurgery, than patients with a GCS of 14 or 15. They more properly belong in the moderate head injury group.15,22
The original definition of GCS 13 to 15 continues to be used in international and Australasian literature but the recognised definition of mild TBI in Australasia is GCS of 14 or 15.14 Some authors believe that even this is too liberal and the definition of mild TBI should be restricted to patients with a GCS of 15.22,23
Approximately 80% of children with TBI will fall into this category.24 The reported incidence of intracranial haemorrhage (ICH) varies between 4 and 7% in children with GCS 15, and increases to approximately 10% in children with GCS 14. 10,15,25,26 The overall mortality in this group is reported to be as high as 2%.15 These figures may be subject to significant selection bias.
The terms ‘minimal’ or ‘trivial’ TBI are sometimes used to describe a subgroup of mild TBI who meet the following criteria: GCS 15, normal neurological examination and no signs of a skull fracture.23,25,27 Transient LOC or amnesia does not exclude patients from this subgroup.
Assessment
History
The following historical information is relevant and should be ascertained if possible:
The possibility of non-accidental injury (NAI) must always be considered in children with skull fractures or intracranial injuries. Be particularly vigilant with children aged <2 years, when a parent or carer has delayed seeking medical care or the stated mechanism is not in keeping with degree of injury observed.12
Past history that is particularly relevant in the context of neurotrauma is:
Glasgow Coma Scale (GCS)
If a painful stimulus is needed to test motor function, apply pressure to the supra-orbital margin to test for localisation of pain. This is superior to a ‘sternal rub’. To test for withdrawal or abnormal flexion/extension, use a pen or pencil to apply pressure to finger-nail or toe-nail beds. Abnormal flexion is usually termed ‘decorticate posturing’ and abnormal extension termed ‘decerebrate posturing’ although this was discouraged in the original description of the GCS as it implied a specific physio-anatomic correlation.19 The best score should be recorded after testing all four limbs and any discrepancy between limbs should be recorded separately.5
Table 3.2.1 allows comparison of the GCS and the CCS used by Hahn et al and others.9,29 The CCS has never been properly validated and assessment of verbal response is somewhat subjective, particularly in children aged less than 6 months.20
A rapid assessment of a child’s neurological disturbance can also be made using the AVPU scale, which denotes the child’s response to stimuli (Table 3.2.2). The child who demonstrates a non-purposeful response to pain (withdrawal, flexor or extensor responses) has a level of consciousness consistent with a GCS of <9 and the unresponsive child, a GCS of 3.
Assess the child carefully for signs of trauma to the head, neck and thoracolumbar spine. A skull vault fracture may be suggested by scalp haematoma, crepitus or palpable depression. A basilar skull fracture should be suspected in the presence of ‘racoon eyes’, Battle’s sign (bruising around the mastoid process), haemotympanum or CSF rhinorrhoea/otorrhoea. Any sign of trauma above the clavicles increases the likelihood of intracranial pathology being detected on computerised tomography (CT) scanning.30
Investigations
Laboratory
In patients with moderate or severe TBI the following should be checked:
Radiological
Skull X-rays
Before the widespread availability of CT scanning, skull X-rays were often used to screen or help risk stratify which children should be observed in hospital or have a brain CT scan.8,29 While it is acknowledged that children with a skull X-ray demonstrating a fracture have a higher incidence of ICH compared to those without a fracture, many children without fractures also have an ICH.9–11,15,29 To compound this problem, the reported sensitivity and specificity of skull X-rays in detecting a fracture is reported to be as low as 21% and 53% respectively.33 Emergency department staff need to be familiar with the interpretation of paediatric skull X-rays in order to minimise the misinterpretation of sutures or vascular markings as fractures and vice versa.
The role of plain films is very limited in seriously injured patients but may still have a role in helping to risk stratify very young children (age <2) with head trauma, who can be difficult to keep still for CT scanning without sedation or general anaesthetic and in those in whom possible NAI is suspected.34,35 Skull X-rays may also be useful in screening for depressed skull fractures or penetrating skull injury, particularly in environments where CT scanning is not readily available.
Cervical and thoracolumbar spine X-rays
These should be obtained for any child with suspected cervical or thoracolumbar trauma, or evidence of spinal cord injury, and those in whom the spine cannot be cleared clinically. Young children (age <10 years) are more prone to high cervical spine injuries whereas older children, like adults, are more prone to lower cervical spine injuries.36 Spinal cord injury without radiological abnormality (SCIWORA) was thought to be principally a paediatric phenomenon but is in fact seen much more often in adults.37
CT scan
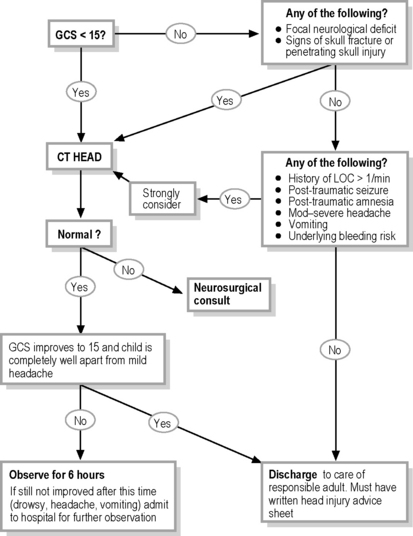
In cases of TBI, CT scan is the modality of choice for imaging the skull for fractures and brain for acute haemorrhage, oedema, mass effect, pneumocephalus and hydrocephalus.7,38 It is rapid, inexpensive and can accommodate a wide range of life support and monitoring equipment. Any child with a persistent GCS ≤14, clinical evidence of a skull fracture or penetrating skull injury should have an emergent cerebral CT scan (Table 3.2.3; see also ‘mild TBI’). The child needs to be accompanied by appropriate staff and monitoring equipment to the CT scan environment to continue optimal monitoring and rapidly manage any potential complications.
∗ The duration LOC does not correlate well with the risk of intracranial pathology and anything greater than transient LOC (i.e. >1 min) should be considered significant.12,23
The CT is also used as the initial investigation of choice to further evaluate suspected spinal injury although it cannot exclude ligamentous injury and it provides limited information on injury to the spinal cord itself.39,40
Magnetic resonance imaging (MRI)
MRI is superior to CT scanning for detecting cerebral oedema, contusion and diffuse axonal injury in cases of TBI.7 It is also superior to CT scanning for visualising the posterior fossa and brainstem regions. It is the investigation of choice for spinal cord injury and the actual patterns of haemorrhage and oedema within the cord carry prognostic significance.39,41 MRI imaging of ligamentous tissues can be used to investigate possible spine instability. However, clinical correlation with MRI findings in ‘mild’ cases is still lacking.40
Management
Mild TBI (GCS 14, 15)
The management of mild TBI is controversial. The debate primarily focuses on the question of whether or not children in this group can be risk stratified and managed clinically without further investigation, or whether they need to have a cerebral CT scan regardless of clinical findings. Concern stems from the observation that even children with a GCS of 15 and a normal neurological examination can harbour clinically significant intracranial pathology with attendant risks for subsequent deterioration and death. One study of 429 children with mild TBI found that 16% of the children with GCS 15 and no LOC had significant IC injury.42 Of these, 1.4% required neurosurgical intervention and 2% died.
On the other hand, CT scanning involves a significant radiation dose and concern has been expressed about the subsequent lifetime risk of radiation-induced malignancies which may be as high as 1 per 1500 scans in very young children.43 Also, if very young, the child may require sedation or a general anaesthetic, with their attendant risks of apnoea, hypoxia, aspiration and prolonged sedation.12 In addition to the radiation risks, the cost of scanning all children with head trauma would be considerable.
The observation has been made that the greatest benefit in treating patients with TBI is not with aggressive management of the severely injured, but in preventing deterioration and complications in those with mild or moderate injuries who appear to be at low risk.12
Risk factors for IC injury or deterioration, such as LOC, amnesia, headache, vomiting, seizures and focal neurological deficit have been studied in an attempt to identify those children with mild TBI who do not require a CT scan.11,15,18,26,30,35,42,44–47 The presence of any of these risk factors increases the likelihood of intracranial pathology and it is important to note that a child can have all of them and still have a GCS of 15. Prospective studies evaluating such clinical decision rules for imaging children with TBI are still to be completed.
The risk of developing clinically significant IC pathology following the initial trauma decreases over time. Although current Australasian guidelines for children suffering mild TBI suggest discharge after 4 hours of observation if the child has a GCS of 15 and is asymptomatic,14 a large study of over 28 000 children admitted to hospital following head injury demonstrated that 6 hours of post-injury observation was required to identify all children who were likely to deteriorate.48
It has been estimated that a child with trivial head injury, who has no LOC and who is completely well (i.e. none of the above mentioned symptoms or signs) has a less than 1:5000 chance of significant IC pathology and can be discharged to the care of a responsible adult without further investigation.23
Based upon current knowledge, the management and disposition algorithm shown in Fig. 3.2.1 is suggested.
Moderate and Severe TBI
Airway control, oxygenation and ventilation
It is important to rapidly identify and correct hypoxia as this is a significant contributor to secondary brain injury.49–52 Supplemental oxygen should be applied in order to maximise oxygen delivery to any ischaemic tissue and the child’s SaO2 should be monitored.
SaO2 and PaO2 are not good indicators of adequate ventilation and continuous ETCO2 monitoring should occur if the child is intubated. The child should be ventilated to maintain an ETCO2 in the low–normal range (i.e. aiming for an ETCO2 of 35 mmHg). Hypercarbia secondary to inadequate ventilation may result in cerebral vasodilation and secondary increase in ICP. Hypocarbia causes cerebral vasoconstriction and therefore decreases ICP, but increases the risk of causing or exacerbating cerebral hypoperfusion with secondary ischaemia.53 Routine ‘prophylactic hyperventilation’ in adults has been shown to worsen outcome and presumably does so in children. Therefore hyperventilation to a PaCO2 of 25 mmHg should be reserved for the child who is rapidly deteriorating with signs of increased intracranial pressure or cerebral herniation, such as new onset pupil asymmetry or rapidly decreasing GCS.
Circulation
Hypotension is the single most significant factor contributing to secondary brain insult.49,51 Hypotension is defined as an SBP <90 mmHg in adults and a SBP <5th percentile for age in children.49–52,54 One or more episodes of hypotension from time of injury through resuscitation at least doubles mortality and significantly increases morbidity.49,51,54–56
Despite studies focusing upon SBP, the true objective in the patient with TBI is to maintain an adequate CPP. This is the difference between mean arterial pressure (MAP) and intracranial pressure (ICP) [i.e. CPP = MAP – ICP]. The normal ICP is 0–10 mmHg and an ICP of 20 mmHg is generally regarded as the threshold for initiating specific therapy to reduce it. The optimal CPP is uncertain but consensus opinion recommends not less than 50 mmHg and not greater than 70 mmHg, as aggressive attempts to maintain CPP > 70 mmHg may also worsen outcomes.57 In the pre-hospital and emergency department settings, the ICP is unknown so treatment is purely empirical. It is reasonable to aim for a MAP of between 70 and 90 mmHg that would maintain a CPP between 50 and 70 mmHg (i.e. allowing for ICPs up to 20 mmHg).
Hypertonic saline
Hypertonic saline solutions have been used for initial resuscitation, as maintenance fluid and as specific treatment for raised ICP. In addition to rapidly restoring circulating volume, increasing blood pressure and reducing ICP, these solutions appear to have important and beneficial immuno-modulatory and neuro-chemical effects that may reduce secondary brain injury.57,59
A suggested regimen for the use of 3% saline for either initial resuscitation or to rapidly decrease ICP is to give a 5 mL kg–1 bolus, repeated if necessary according to patient response. It would appear that rapid changes in serum sodium concentration do not cause complications such as central pontine myelinolysis in humans, and most studies do not place an upper limit on serum sodium concentration.59,60
Mannitol
Mannitol has been used as both a resuscitation fluid (plasma expander) and as therapy for acute deterioration secondary to increasing ICP. Boluses of 0.25 g kg–1 to 1 g kg–1 body weight have been used successfully for short-term reduction of ICP.61 Mannitol acts as an osmotic diuretic and can lead to subsequent problems with hypovolaemia and acute renal failure via acute tubular necrosis. A loop diuretic such as furosemide is sometimes used in addition to mannitol for treatment of acute rises in ICP. A urinary catheter is essential in any patient receiving mannitol or diuretics.
Prophylactic anti-seizure therapy
Seizures that occur within seven days of a TBI are termed early post-traumatic seizures (EPTS) and those thereafter are late post-traumatic seizures (LPTS).62 The overall incidence of seizures in children with TBI is probably between 5 and 15% but rises with increasing severity of TBI, occurring up to 40% of the time in those with a GCS ≤8.51,62–64 Greater than 95% of PTS are early and approximately 80% of these occur within the first 24 hours.
Seizures may cause or exacerbate secondary brain injury by increasing cerebral metabolic demands, increasing intracerebral pressure and by causing or exacerbating cerebral hypoxia.63,64
The incidence of EPTS can be reduced by the use of prophylactic anticonvulsants such as phenytoin. Their use should be considered65 in those with:
It should be noted, however, that reduced EPTS does not translate into reduced mortality65,66 and it remains to be seen whether or not an overall improved level of functioning occurs in those survivors given prophylactic anticonvulsant therapy. It should also be noted that prophylactic anticonvulsants do not alter the incidence of LPTS and their routine use after 7 days is not recommended.65
The management of active seizures should be with benzodiazepines and should follow the guidelines discussed in Chapter 8.3.
Steroids
Various steroids have been used in an attempt to improve outcome from TBI. To date no overall improvements have been identified and they may actually worsen outcome. Routine use is no longer recommended.67–69
Thermoregulation: prophylactic hypothermia and prevention of hyperthermia
Mild hypothermia (32–35°C) is known to decrease ICP and, in animal models, has been shown to be neuroprotective.59,70,71 Outcomes in humans have not been consistently better and mild hypothermia may increase complications such as sepsis, pneumonia, bleeding and mortality in the TBI child.59,72 Research is ongoing in this area and prophylactic hypothermia should be considered in consultation with local PICU intensivists regarding individual cases.
Hyperthermia is associated with a worse outcome in children with severe TBI.72 It is not known if actively cooling the patient alters outcome and further research needs to be conducted in this area. In the meantime it would seem reasonable to attempt to cool a febrile brain-injured child.
Spinal cord injury
There is a great paucity of research into the optimal management of acute spinal cord injury in children. Therefore there are insufficient data to support diagnostic or treatment standards.40 However, the principles of management of acute spinal cord injury are considered to be no different from TBI. The focus of therapy is to prevent secondary injury. Attention should be paid to the maintenance of strict spinal immobilisation, adequate oxygenation, ventilation, blood pressure and good supportive care as per moderate-severe TBI.
The use of high-dose steroids in acute spinal injury is controversial.73,74 The issue is further complicated in the paediatric population by the fact that children <13 years were excluded from the major trials of steroids for spinal cord injury.40 Routine use of high-dose steroids for spinal cord injury is no longer recommended by the Neurosurgical Society of Australasia.14 If steroids are used, in consultation with local paediatric neurosurgical practice, they should be administered within 8 hours of injury. Practice currently varies between institutions. The recommended dosing schedule is: methylprednisolone 30 mg kg–1 bolus over 15 minutes followed by a 45-minute break, then 5.4 mg kg–1 hr–1 continuous infusion for 23 hours.14
Family considerations
There are considerable immediate stresses during the initial stabilisation phases on the parents and family of the injured child. The family requires appropriate support and explanation whilst in the emergency department. If possible, it is useful to provide a dedicated staff member to be with them. The family should be kept well informed of the child’s status and its proposed management by a designated senior medical member of the resuscitation team and consideration should be given to allowing parents into the resuscitation room to be with their child (see Chapter 2.1). Premature or vague conclusions of prognosis should be avoided until all relevant assessments and investigations have been made.
1 Martin C., Falcone R. Pediatric traumatic brain injury: an update of research to understand and improve outcomes. Curr Opin Pediatr. 2008;20:294-299.
2 O’Connor P. Hospitalisation due to traumatic brain injury, Australia 1997–98. Australian Institute of Health and Welfare; 2002.
3 Khan F., Baguley I., Cameron D. Rehabilitation after traumatic brain injury. Med J Aust. 2003;178(6):290-295.
4 Research Centre for Injury Studies. Spinal cord injury, Australia 1995/6. Australian Injury Prevention Bulletin. 18, 1998.
5 O’Connor. December Spinal cord injury, Australia, 1999-00. Australian Institute of Health and Welfare; 2001.
6 Anderson V., Catroppa C., Morse S., et al. Outcome from mild head injury in young children: A prospective study. J Clin Exp Neuropsychol. 2001;23(6):705-717.
7 Poussaint T., Moeller K. Imaging of paediatric head trauma. Neuroimaging Clin N Am. 2002;12:271-294.
8 Lazar L., Erez I., Gutermacher M., et al. Brain concussion produces transient hypokalemia in children. J Pediatr Surg. 1997;32(1):88-90.
9 Hahn Y., McLone D. Risk factors in the outcome of children with minor head injury. Pediatr Neurosurg. 1993;19:135-142.
10 Lloyd D., Carty H., Patterson M., et al. Predictive value of skull radiography for intracranial injury in children with blunt head injury. Lancet. 1997;349:821-824.
11 Quayle K., Jaffe D., Kuppermann N., et al. Diagnostic testing for acute head injury in children: When are head computed tomography and skull X-rays indicated? Pediatrics. 1997;99:e11.
12 Schutzman S., Greenes D. Paediatric minor head trauma. Ann Emerg Med. 2001;37(1):65-74.
13 Moura dos Santos A., Plese J., Ciquini O., et al. Extradural hematomas in children. Pediatr Neurosurg. 1994;21:50-54.
14 Neurological Society of Australasia and Royal Australasian College of Surgeons. The Management of Acute Neurotrauma in Rural and Remote Locations, 2nd ed.. 2009.
15 Keskil I., Baykaner M., Ceviker N., et al. Assessment of mortality associated with mild head injury in the paediatric age group. Childs Nerv Syst. 1995;11:467-473.
16 Lang D., Teasdale G., MacPherson P., et al. Diffuse brain swelling after head injury: More often malignant in adults than children? J Neurosurg. 1994;80:675-680.
17 Bratton S., Chestnut R., Ghajar J., et al. Cerebral perfusion thresholds. J Neurotrauma. 2007;24:S59-64.
18 Ratan S., Pandey R., Ratan J. Association among duration of unconsciousness, Glasgow Coma Scale, and cranial computed tomography abnormalities in head injured children. Clin Pediatr. 2001:375-378. July
19 Teasdale G., Jennett B. Assessment of coma and impaired consciousness. Lancet. July 1974:81-83.
20 Simpson D., Reilly P. Paediatric Coma Scale. Lancet. August 1982:450.
21 Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87.
22 Culotta V., Sementilli M., Gerold K., et al. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38(2):245-250.
23 Committee on quality improvement, American Academy of Paediatrics. The management of minor closed head injury in children. Paediatrics. 1999;104(6):1407-1415.
24 Murgio A., Patrick P., Andrade F., et al. International study of emergency department care for paediatric traumatic brain injury and the role of CT scanning. Childs Nerv Syst. 2001;17:257-262.
25 Davis R., Mullen N., Makela M., et al. Cranial computed tomography scans in children after minimal head injury with loss of consciousness. Ann Emerg Med. 1994;24(4):640-645.
26 Schunk J., Rodgerson J., Woodward G. The utility of head computed tomographic scanning in paediatric patients with normal neurologic examination in the emergency department. Pediatr Emerg Care. 1996;12(3):160-165.
27 Roddy S., Cohn S., Moller B., et al. Minimal head trauma in children revisited: Is routine hospitalisation required? Pediatrics. 1998;101(4):575-577.
28 Johnson D., Krishnamurthy S. Severe paediatric head injury: Myth, magic, and actual fact. Pediatr Neurosurg. 1998;28:167-172.
29 Hahn Y., Chyung C., Bartherl M., et al. Head injuries in children under 36 months of age. Demography and outcome. Childs Nerv Syst. 1988;4:34-40.
30 Haydel M., Preston C., Mills T., et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343(2):100-105.
31 Vavilala M., Dunbar P., Rivara F., et al. Coagulopathy predicts poor outcome following head injury in children less than 16 years of age. Journal of Neurosurgical Anaesthetics. 2001;13(1):13-18.
32 Keller M., Fendya D., Weber T. Glasgow Coma Scale predicts coagulopathy in paediatric trauma patients. Semin Pediatr Surg. 2001;10(1):12-16.
33 Homer C., Kleinman L. Technical report: Minor head injury in children. Pediatrics. 1999;104(6):1380.
34 Gruskin K., Schutzman S. Head trauma in children younger than 2 years: Are there predictors for complications? Arch Pediatr Adolesc Med. 1999;153(1):15-20.
35 Schutzman S., Barnes P., Duhaime A.-C., et al. Evaluation and management of children younger than two years old with apparently minor head trauma: proposed guidelines. Pediatrics. 2001;107:983-993.
36 Eleraky M., Theordore N., Adams M., et al. Paediatric cervical spine injuries; Report of 102 cases and review of the literature. J Neurosurg (Spine 1). 2000;92:12-17.
37 Hendey G., Wolfson A., Mower W., et al. Spinal cord injury without radiographic abnormality; Results of the national emergency X-ray utilization study in blunt cervical trauma. J Trauma. 2002;53(1):1-4.
38 Schwartz D., Reisdorff E. Emergency Radiology. USA: McGraw-Hill; 2000.
39 Sledge J., Dain A., Hyman J. Use of magnetic resonance imaging in evaluating injuries to the paediatric thoracolumbar spine. J Pediatr Orthop. 2001;21(3):288-293.
40 Hadley M. Management of paediatric cervical spine and spinal cord injuries. Neurosurgery. 2002;50(3):S85-99.
41 Frank J., Lim C., Flynn J., et al. The efficacy of magnetic resonance imaging in paediatric cervical spine clearance. Spine. 2002;27(11):1176-1179.
42 Simon B., Letourmeau P., Vitorino E., et al. Paediatric minor head trauma: Indications for computed tomographic scanning revisited. J Trauma. 2001;51(2):231-238.
43 Brenner D., Hall E. Computed Tomography – An increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
44 Mandera M., Wencel T., Bazowski P., et al. How should we manage children after mild head injury? Childs Nerv Syst. 2000;16:156-160.
45 Ng S., Toh E., Sherrington C. Clinical predictors of abnormal computed tomography scans in paediatric head injury. J Paediatr Child Health. 2002;38:388-392.
46 Batchelor J., McGuiness A. A meta-analysis of GCS 15 head injured patients with loss of consciousness or post traumatic amnesia. Emerg Med J. 2002;19:515-519.
47 Simon B., Letourneau P., Vitorino E., et al. Pediatric minor head trauma: Indications for computed tomographic scanning revisited. J Trauma. 2001;51(2):231-238.
48 Sainsbury C., Sibert J. How long do we need to observe head injuries in hospital? Arch Dis Child. 1984;59:856-959.
49 Philip S., Udomphorn Y., Kirham F., et al. Cerebrovascular Pathophysiology in Pediatric Trauma Brain Injury. J Trauma. 2009;67(2):S128-34.
50 Chiaretti A., De Benedictis R., Della Corte F., et al. The impact of initial management on the outcome of children with severe head injury. Childs Nerv Syst. 2002;18:54-60.
51 Chiaretti A., Piastra M., Pulitano S., et al. Prognostic factors and outcome of children with severe head injury: An 8 year experience. Childs Nerv Syst. 2002;18:129-136.
52 Ong L., Selladurai B., Dhillon M., et al. The prognostic value of the Glasgow Coma Scale, hypoxia and computerised tomography in outcome prediction of paediatric head injury. Pediatr Neurosurg. 1996;24:285-291.
53 Skippen P., Seear M., Poskitt K., et al. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25(8):1402-1409.
54 Traumatic Brain Injury Guidelines Taskforce. Hypotension. J Neurotrauma. 2000;17(6/7):591-595.
55 Winchell R., Simons R., Hoyt D. Transient systolic hypotension. A serious problem in the management of head injury. Arch Surg. 1996;131:533-539.
56 Kokoska E., Smith G., Pittman T., et al. Early hypotension worsens neurological outcome in paediatric patients with moderately severe head trauma. J Pediatr Surg. 1998;33(2):333-338.
57 Banks C., Furyk J. Review article: Hypertonic saline use in the emergency department. Emerg Med Australas. 2008;20:294-305.
59 Walker P., Harting M., Baumgartner J., et al. Modern approaches to pediatric brain injury therapy. J Trauma. 2009;67(2):S120-7.
60 Peterson B., Khanna S., Fisher B., et al. Prolonged hypernatremia controls elevated intracranial pressure in head-injured paediatric patients. Crit Care Med. 2000;28(4):1136-1143.
61 Bratton S., Chestnut R., Ghajar J., et al. Hyperosmolar therapy. J Neurotrauma. 2007;24:S14-20.
62 Ong L., Dhillon M., Selladurai B., et al. Early post-traumatic seizures in children: Clinical and radiological aspects of injury. J Paediatr Child Health. 1996;32:173-176.
63 Ratan S., Kulshreshtha R., Pandey R. Predictors of posttraumatic convulsions in head injured children. Pediatr Neurosurg. 1999;30:127-131.
64 Chiaretti A., Benedictis R., Polidori G., et al. Early post-traumatic seizures in children with head injury. Childs Nerv Syst. 2000;16:862-866.
65 Bratton S., Chestnut R., Ghajar J., et al. Antiseizure prophylaxis. J Neurotrauma. 2007;24:S83-6.
66 Haltiner A., Newell D., Temkin N., et al. Side effects and mortality associated with use of phenytoin for early posttraumatic seizure prophylaxis. J Neurosurg. 1999;91:588-592.
67 Bratton S., Chestnut R., Ghajar J., et al. Steroids. J Neurotrauma. 2007;24:S91-5.
68 Alderson P., Roberts I. Corticosteroids for acute traumatic brain injury (review). Cochrane Database Syst Rev. (4):2009.
69 Roberts I. Aminosteroids for acute traumatic brain injury. Cochrane Database Syst Rev. (4):2009.
70 Sydenham E., Roberts I., et al. Hypothermia for traumatic brain injury. Cochrane Database of Systematic Reviews. (4):2009.
71 Hutchison J., Ward R., Lacroix J. Hypothermia: Therapy after traumatic brain injury in children. N Engl J Med. 2009;358:2447-2456.
72 Natale J., Joseph J., Helfaer M., et al. Early hyperthermia after traumatic brain injury in children; Risk factors, influence on length of stay, and effect on short-term neurologic status. Crit Care Med. 2000;28(7):2608-2615.
73 Hurlbert R. The role of steroids in acute spinal cord injury; An evidence-based analysis. Spine. 2001;26(24 Suppl.):S39-46.
74 Canadian Association of Emergency Physician Position Statement. Steroids in acute spinal cord injury. 2003. www.caep.ca. [Revised 22 January 2003]
Beattie T. Minor head injury. Arch Dis Child. 1997;77:82-85.
Bratton S., Chestnut R., Ghajar J., et al. Hyperventilation. J Neurotrauma. 2007;24:S87-90.
Bratton S., Chestnut R., Ghajar J., et al. Blood pressure and oxygenation. J Neurotrauma. 2007;24:S7-13.
Davis R., Hughes M., Gubler D., et al. The use of cranial CT scans in the triage of paediatric patients with mild head injury. Paediatrics. 1995;95(3):345-349.
Mitchell K., Fallat M., Raque G., et al. Evaluation of minor head injury in children. J Pediatr Surg. 1994;29(7):851-854.
Smally A. Management of minor closed head injury in children. Paediatrics. 2001;107(5):1231.
Temkin N., Dikmen S., Anderson G., et al. Valproate therapy for prevention of posttraumatic seizures: A randomised trial. J Neurosurg. 1999;91:593-600.
Traumatic Brain Injury Guidelines Taskforce. Glasgow Coma Scale score. J Neurotrauma. 2000;17(6/7):563-570.
Traumatic Brain Injury Guidelines Taskforce. Pupillary diameter and light reflex. J Neurotrauma. 2000;17(6/7):583-590.