25 Paediatric Considerations in Critical Care
After reading this chapter, you should be able to:
• consider and anticipate the specific needs of critically ill infants and children
• describe common conditions that lead to critical illness in infants and children
• discuss and apply the age-appropriate assessment, monitoring and management of critically ill infants and children
• identify age-appropriate parameters and care required by critically ill infants and children who require ventilation
• discuss psychological and emotional care required by critically ill infants and children, and their family
Introduction
In mid 2007 the Australian paediatric population was estimated to be 4.1 million children aged 0–14 years,1 with the New Zealand paediatric population 0–15 years reported at 894,400.2 Approximately 559,000 children were admitted to Australian public hospitals in 2007–20083 with over 8300 children in Australia and New Zealand requiring admission to intensive care units (ICUs) in 2008.4 During the same period, there were over 145,000 total admissions to Australian and New Zealand ICUs.5 Children represent 5.7% of all ICU admissions in Australia and New Zealand. Over half of these children required mechanical ventilation,4 compared with around 41% of adults in intensive care.5 While just over half (52.5%) of critically ill children were admitted to specialist paediatric ICUs, a significant number were managed in or retrieved from adult ICUs.4 Geographical distances and the centralised nature of paediatric services can influence whether a child is nursed in a general or paediatric ICU. In many circumstances, children will respond effectively to initial resuscitation, particularly support of breathing and fluid resuscitation, and may not require transfer to a specialist paediatric unit. Paediatric clinical advice, support and information are available from children’s hospitals and specialist paediatric retrieval services and should be sought as early as possible in the absence of paediatric trained staff, or when the need for transfer to a higher level of care becomes apparent.
The age distribution of children in ICUs has remained the same for a number of years, with the figures from 2008 showing that children under the age of five years represent just over 66% of admissions, with almost 59% of this age group under 12 months of age and 26% under four weeks of age. Boys make up 58% of children admitted to ICUs.4 The overall ICU mortality rate is 11% for Australia and 13% for New Zealand,4 however the paediatric mortality rate is 3%.5 Over the past 30 years, although length of stay and severity of illness to the PICUs have not essentially changed, mortality has halved while disability has increased.6
Anatomical and Physiological Considerations in Children
• Children have increased surface area to volume ratio compared with adults, which leads to increased heat loss and insensible fluid losses, placing infants and children at increased risk of developing hypothermia and dehydration. Providing an environment that maintains the infant and small child’s body temperature is essential. Avoid exposing infants and children more than necessary; use warming blankets, open care systems for all newborns and infants under 4 kg and overhead heaters when exposure is unavoidable. Temperature monitoring is required when using any heating devices to avoid iatrogenic thermal injury.
• Lower glycogen stores and increased metabolic rate predispose infants to hypoglycaemia. There are few standard doses in paediatric ICU; rather, medication doses and fluid requirements are calculated on age and kilograms of body weight. Weight of infants and children should therefore be estimated as accurately as possible. The Broselow tape measure is a colour-coded method to estimate weight and is particularly accurate in children ≤ 25 kg.7,8 Some differences may occur in estimated weight of children of different origin.7
• Fluid requirements are based on body weight, and aim to ensure adequate hydration while preventing fluid overload. Maintenance intravenous (IV) fluids for infants and young children typically require the addition of glucose. Common IV maintenance fluids used are 0.45% sodium chloride with either 2.5% glucose or 5% glucose and 0.9% sodium chloride with 5% glucose.9 Isotonic sodium chloride is recommended as the first choice fluid bolus in paediatric resuscitation.10 Table 25.1 provides a guide for fluid maintenance requirements of children based on body weight.
• Excluding the newborn period, normal values for all blood gas and serum electrolyte levels are the same as adult levels. Creatinine and urea levels will vary with age.
• Methods of oxygen delivery (humidified if possible) for infants include via nasal prongs (maximum rate 2 L/min) or a head box. A head box can reliably deliver a required percentage of oxygen, but visualisation of the infant is often compromised and there is a sense of separation between the parents and the infant. Comforting, touching and regular nursing assessment are more easily achieved when nasal prongs are used. Hudson masks and partial non-rebreather masks are available in paediatric sizes.
Central Nervous System
Many central nervous system functions, such as locomotion and hand–eye coordination, will take from months to years, to fully develop. Functions of the cerebral cortex are particularly underdeveloped, with myelination of all major nerve tracts continuing throughout infancy.11 Consequently, assessment and management priorities will be dictated by the level of neurological maturity of the infant or child. As with adults, neurological dysfunction in infants and children may be primary or secondary. The plasticity inherent in the brain of the infant may compensate for injury more readily than older children and adults in some circumstances, with other areas of the infant’s brain taking over function. Because the eight cranial bones are not yet fused, infants’ skulls cope with both birth and ongoing growth, which is greatest in the first two years of life. In the first year, the cartilaginous sutures fuse at two points to form the posterolateral fontanelle. The larger anterior fontanelle closes during the second year as bone is laid down. By around five years of age, the sutures of the child’s skull are completely fused.12 However the thinner skull will provide less protection to underlying tissues than the adult skull.
A common misconception is that the Monro-Kellie doctrine (see Chapter 16) does not apply to young children and infants with a more compliant skull. While slow rises in intracranial volume may be accommodated over time in children under three years of age, they will usually be accompanied by growing head circumference, making routine measurement of head circumference in children under three years of age an important assessment. However, the less rigid skull of the older child will not compensate for acute rises in intracranial volume, and the child will display symptoms of neurological compromise.12 Normal ranges of intracranial pressure (ICP) and cerebral perfusion pressure (CPP) have not been formally studied in infants and children, but are presumed to be lower than in adults, reaching adult range by adolescence. Values that are commonly used to guide treatment are age-related and are displayed in Table 25.2.
| Age | Desirable minimum CPP |
|---|---|
| Infants under 1 year | 45–55 mmHg |
| Children 1–10 years | >55 mmHg |
| Children over 10 years | >65 mmHg |
Adapted from (9).
Cardiovascular System
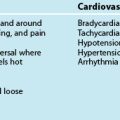
In infants, approximately 70% of the haemoglobin is fetal haemoglobin (HbF), allowing greater amounts of oxygen to be carried for any given PaO2. Circulating blood volume per kilogram decreases with age; in the infant, circulating volume is approximately 85 mL/kg, with total body water accounting for 70% of body mass, adjusting to the adult values of 65 mL/kg and total body water of 60%.13 The apex beat is heard at the fourth intercostal space, mid-clavicle, and by around seven years of age the left ventricle has grown and the apex beat can be heard at the fifth intercostal space, as in adults. An infant’s cardiac output is approximately 500 mL/min, which, relative to body weight, is about twice that of an adult.14 Heart rate is a major determinant of cardiac output in infants and young children, as there is limited ability to increase stroke volume. Tachycardia is an early sign of distress, but bradycardia is an ominous sign in infants and young children, as they are more dependent on a high heart rate to maintain cardiac output. In infants, bradycardia requires resuscitation.13
Arterial blood pressure should be appropriate for age, weight and clinical condition. Mean arterial pressure is generally used. Monitoring blood pressure using correct cuff sizes is important because incorrect cuff size is a common cause of inaccurate blood pressure readings in children. Diastolic blood pressure is recorded at Korotkoff sound 5 (K5); age-related parameters for mean blood pressure are displayed in Table 25.3. Tachycardia in the absence of fever is a more reliable sign than hypotension, as up to 25% of the child’s circulating volume may be lost before hypotension occurs. Hypotension is thus a late sign in children and may indicate late decompensated shock, particularly following fluid delivery.14
| Age | Mean BP (mmHg) |
|---|---|
| Term | 40–60 |
| 3 months | 45–75 |
| 6 months | 50–90 |
| 1 year | 50–90 |
| 3 years | 50–90 |
| 7 years | 60–90 |
| 10 years | 60–90 |
| 12 years | 65–95 |
| 14 years | 65–95 |
Adapted from (9).
Paediatric Considerations for Cardiovascular Assessment
Cardiovascular assessment in children includes clinical parameters that are similar to those observed in adults. The normal values are, however, age and weight dependent. Urine volume in infants should average 2 mL/kg/hr, with 1 mL/kg/hr in children and 0.5–1 mL/kg/hr in adolescents. Other indirect evidence of poor systemic perfusion in infants may include:15
Indirect evidence of poor systemic perfusion in children is irritability, then disorientation or lethargy. Clinical signs of reduced cardiac output, typically seen in shock, are similar to adults.16
Respiratory System
The newborn’s larynx is just one-third of the diameter of the adult larynx.17 Narrow nasal passages, in combination with being obligatory nose-breathers up to 5–6 months of age, means that infants may experience respiratory distress if nasal passages become oedematous or contain secretions such as mucus or blood. With the airway of an infant measuring around 6 mm in diameter at the level of the cricoid, obstruction is more likely. The paediatric airway is characterised and differentiated from an adult airway by the following features:13,17
• more acute angle of airway, particularly notable when attempting to visualise with a laryngoscope
• a more cephalad larynx that moves distally as the neck grows
• the cricoid ring is the narrowest portion of the airway
• smaller lower airways, less developed with fewer alveoli
• true alveoli not present until 2 months, with full complement developed by around eight years of age
• little smooth muscle present in airways
• little collateral ventilation in airways, as the pores of Kohn are not fully developed until about eight years of age (see Figures 25.1 and 25.2).
Paediatric Respiratory Assessment
Newborn infants have a respiratory rate of approximately 40 breaths/min, generating an average tidal volume of 16 mL/kg and minute volume of 0.64 L/min.18 The thoracic cavity of infants and children is characterised by a thin chest wall that is highly compliant, with poorly developed intercostal and accessory muscles. The diaphragm is the most important muscle for infants and children in respiration, with abdominal muscles also used. The compliant chest wall prevents generation of high intrathoracic pressures, meaning that infants and young children are unable to significantly increase tidal volume; rather, they increase minute volume by breathing faster. This means that tachypnoea is a normal response to illness in infants and children, and a slowing respiratory rate in children may indicate impending collapse rather than clinical improvement.18,19
Assessing airway patency is important. Talking and crying indicates that the infant or child is maintaining their own airway. Adventitious airway noises in children include wheeze, stridor and grunting. In infants, grunting may be heard and is an attempt by the baby to produce positive end-expiratory pressure (PEEP). Infants and children who are grunting, gasping or unconscious need urgent assessment for possible endotracheal intubation.19
Other observed signs of respiratory distress in infants and children up to about eight years old include head bobbing in infants, nasal flaring, and paradoxical chest movement observed in several locations on the chest and known as recessions. Recessions can be observed at the costal margin, or subcostal; between the ribs, or intercostal; at the sternum, or sternal; and at the trachea, called tracheal tug. Oral feeding is difficult for infants in moderate to severe respiratory distress due to limitations associated with sucking and breathing at the same time. In addition, tachypnoea greater than 60–80 breaths/min may lead to vomiting and aspiration.20 For these reasons, initial enteral feeding might not be possible or desirable, so nutrients should be given as parenteral nutrition (PN) until enteral feeding is tolerated.21
Gastrointestinal Tract
There are few differences between the child’s and adult’s gastrointestinal tract outside the neonatal period; although a palpable liver below the costal margin is a normal finding. It will be up to 3 cm below the costal margin in normal infants, decreasing to 1 cm by 4–5 years of age, and should no longer be palpable in adolescents. In the neonate, a relative pancreatic amylase deficiency means utilisation of starches is less effective. Fats are also absorbed less well; the reason why higher-fat milks such as cow’s milk are not ideal for infants. Protein synthesis and storage is however enhanced in the neonate.13
As the infant liver is not completely mature at birth, gluconeogenesis is deficient, causing low and unstable blood sugar level in the first weeks of life. The infant is therefore reliant on fat stores until normal feeding is established.13 Formation of plasma proteins and clotting factors are likely to be inadequate in the first weeks of life, thus all newborns in Australasia are given vitamin K shortly after birth to prevent bleeding. Blood glucose monitoring and provision of early nutrition are essential aspects of care, especially for infants. Children normally have increased metabolic demands to achieve growth but have fewer energy stores than adults.
Other Systems and Considerations
Genitourinary System
The small developing pelvic bones of infants and young children cause adult pelvic organs, such as the bladder, to be located in the lower abdominal cavity. Urine output in children is calculated in mL/kg bodyweight/hour. In infants with immature kidney function and limited ability to conserve water, urine output should be 1–2 mL/kg/h. In the first month of life, infants have the capacity to concentrate urine to only 1.5 times their plasma osmolality, while adults can concentrate their urine to 3–4 times their plasma osmolality. The higher metabolic rate of infants means that they produce twice the acid that an adult will, leading to a tendency to acidosis in critical illness.22 By six months of age, normal urine output should be 1 mL/kg/h, and by adolescence 0.5–1 mL/kg/h is considered normal. Catheterisation is generally required in critically ill infants and children for accurate hourly measurement of urine output. Where this is not possible, particularly where small sizes of indwelling catheters are not readily available, weighing nappies will provide an interim estimate of urine output. Inserting feeding tubes in place of a urinary catheter is not recommended.
Musculoskeletal System
Children have less developed musculature than adults, with less protection from external forces that collide with the child. Conversely, a child’s skeleton is more cartilaginous than adults and therefore more pliable. As a result, rib fractures rarely accompany chest trauma in children while lung contusions are common.23 The skeleton in children changes from less cartilaginous in nature at infancy to complete ossification and adult features during adolescence, so daily calcium requirements increase over childhood and adolescence.24
Integumentary System
Infants have a thinner epidermis, dermis and subcutaneous tissue that will continue to mature. This results in a greater susceptibility to absorption of chemicals, injury from adhesive tapes and any shearing force, and loss of water and heat, particularly in the newborn period.22 Critically ill children are more likely to develop pressure areas on the occiput, ear, sacrum, heel, or thigh; 50% of pressure ulcers in children are associated with equipment pressing or rubbing on the skin.25 A commonly used tool for assessing risk of development of pressure areas in children is the modified Braden Q scale. This shorter version includes three subscales (mobility, sensory perception, tissue perfusion/oxygenation) with a cutoff score of 7 and has comparable psychometric properties to the adult Braden scale26 (see Chapter 6). However, recent evidence suggests that the Glamorgan paediatric pressure ulcer risk assessment scale may perform better than the Braden Q scale.27,28 The Glamorgan scale includes ten subscales: anaemia, equipment pressing, mobility, poor peripheral perfusion, pyrexia, serum albumin, surgery in last 4 weeks, weight < 10th centile, continence, and nutrition.27
Developmental Considerations
Admission to ICU is very stressful for paediatric patients29 as well as for their family.30–33 The stressors, combined with the effects of critical illness, can lead to disturbances in normal child development and attachment. The psychological needs of children and families are not always met.34 Factors that affect the psychosocial wellbeing of a critically ill child include loss of usual routines and self-control; family presence and role; family and friends’ visits, comfort and the ICU environment.29,35–37
Knowledge and understanding of developmental psychology can help nurses assess and plan care for the critically ill child.38,39 Identification of internal strengths, external supports and environmental modification can facilitate coping and reduce stress in these children.40 Parental support is an important coping mechanism of infants and children during periods of stress.36 Strategies to facilitate coping in children of all ages include:
• facilitating parental presence at all times, including during invasive procedures and resuscitation41,42
• maintaining normal routines and rituals as much as possible, including story reading, bedtime routines and presence of favourite toys
• providing appropriate analgesia and sedation as well as non-pharmacological interventions
• providing opportunities for play and activities unrelated to treatment.
Erikson’s psychosocial theory is helpful for understanding childhood development.43 Erikson’s theory asserts that people experience eight ‘psychosocial crisis stages’ which significantly affect their development and personality. The first five stages are presented below.
Infants (Stage 1)
The first year of life is concerned with developing a sense of trust, which lays the foundation for all future relationships.38,43 More specifically, the affective exchanges between the infant and the primary caregiver provide a foundation for neurological development and lead to the creation of neural networks (particularly in the right hemisphere) that will influence the infant’s personality and relationships with others throughout life.44,45 Generally, up to the age of six months, infants are able to cope with limited separation from their mothers; however, changes to usual routine create anxiety and stress.43 From around 6–18 months of age separation is the major fear, with changes to usual routine and environment resulting in anxiety.43 Therefore, critically ill infants require parental presence and maintenance of normal routines, including breastfeeding, as much as is practicable.
Toddlers (Stage 2)
The toddler period, between 12 months and three years of age, is a time for establishing autonomy and independence. Control over bodily functions, increasing ability to communicate, ability to view the self as separate from others, and being able to tolerate brief separation from the mother are all developmental characteristics during this period.46 Toddlers tend to be egocentric in how they view the world, so illness, procedures and separation from parents may be perceived as punishment.43 Their thinking processes include transduction, animism and ritual.39 Transductive thinking allows a child to link unrelated objects or events, such as separation and endotracheal suction if suction occurs after the parent leaves the room. Animism attributes lifelike traits to inanimate objects, so the ventilator becomes a hissing monster, or monitoring leads may be trying to trap them. Many toddlers have varying levels of ritual or sameness, including always eating off the same plate, different foods that should not be touched, or a security toy or blanket. Regression, or loss of recently-acquired skills such as toileting, may also occur during critical illness, creating further distress. When caring for a critically ill toddler, encourage parental presence and maintain as many of the usual rituals and routines as possible to facilitate coping.47
Preschool Children (Stage 3)
Children from 3–5 years of age fall into the preschool period of development. This period is characterised by discovery, inventiveness, curiosity, and the development of culturally- and socially-acceptable behaviour.38,39,43 Preschoolers can generally verbalise their needs reasonably well.48 While thought processes become less ritualistic and negative, they are still egocentric and magical thinking emerges, thus ideas about causality and linking events may be faulty. Fears, both real and imagined, are prevalent during this period.39 For example, fears of monsters or being hurt may occur. They may also feel guilty as a result of illness.43 There is, however, greater understanding of the passage of time, so parents can leave the preschooler for defined short periods. Hospitalisation remains difficult, but preschoolers respond to anticipatory preparation and concrete explanations.38
School-Age Children (Stage 4)
Children between 6 and 11 years are usually referred to as being of school age. This period represents a widening of the sphere of influence from parents/family to include the school environment and peers.39 A transition from egocentric thinking to concrete operations occurs,38,39 with children becoming more independent and achievement-oriented for their sense of self-worth. In the ICU, school-age children may have a distorted or fantasy-laden view, and will need concrete explanations. Sicker children are less able to cope with the ICU environment and are more likely to regress, which can have a significant impact on their sense of self-worth. Modesty and privacy is imperative at this age.49 Preadolescence occurs between 10 and 11 years, and represents a time of turmoil and emotional upheaval.39
Adolescents (Stage 5)
Adolescence is considered a time of transition from childhood to adulthood. It is a developmental stage rather than an age group, but is typically represented by children aged 12–18 years, or teenagers. Internal changes relate to emotional upheaval, search for autonomy, and transition of thought process from concrete to abstract.43 External changes relate to physical changes, such as the emergence of secondary sex characteristics with a related preoccupation on bodily functions and image.38
A goal in adolescence is to develop an integrated sense of self, achieved through managing the conflicting demands of family and peers. Peer identity is essential to psychological growth and development, as is the gradual shift from family to peer orientation. The peer group provides a way for the adolescent to self-evaluate and to bolster self-esteem. Adolescents also target authority figures with retaliation and defiance. Conversely, adolescents will seek out non-parental adults, such as a teacher or relative, to obtain approval and acceptance.50 Slote has described a process associated with adolescent illness.50 The first is hopelessness and helplessness provoked by the equipment and environment. Adolescents often think they will not get better, and need to be given clear information about the expected course of the illness. They also need to be included as much as possible in decision making and encouraged to participate in their own care. Feeling helpless and defenceless is contrary to their normal feelings of invincibility and may result in antisocial behaviours. The adolescent must learn to accept that the quest for autonomy has been temporarily interrupted. Acknowledging his/her feelings and setting clear behavioural limits can help an adolescent cope.50 Adolescents will also experience fear and anxiety. This can be offset by clear explanations and acknowledgement of feeling through articulation and reflection. Concerns for body image is also paramount, particularly fear of mutilation and scarring. Physical appearance is important for acceptance into the peer group and for self-esteem.50 In summary, in addition to considering age-related physical characteristics, critical care nurses need to also consider the developmental level of the child when providing care.
Comfort Measures
Critically ill infants and children are particularly vulnerable to pain. If pain remains unrelieved it may cause short- and long-term physiological and psychological complications, such as increased risk of mortality and morbidity.51–53 The assessment of pain in children is particularly challenging,51 but the use of valid pain and sedation assessment tools may be useful for the management of pain in critically ill children.54,55 Prevention of procedural pain is important not only to avoid pain-related complications and emotional trauma, but also to facilitate the procedure.56 Target sedation level according to the child’s clinical status may help maintain comfort without compromising haemodynamic and respiratory status,57 as well as minimising other undesirable effects of analgesics and sedatives.
Pain and Sedation Assessment
Recent advances in pain and sedation assessment show that they remain problematic in paediatric critical care and highlight the need for routine assessment, documentation and effective communication of the pain and sedation scores. Numerous pain assessment instruments have been developed, but few have been validated for the paediatric critical care population. These latter include the PICU-MAPS and the COMFORT behaviour scale. The PICU-MAPS is a multidimensional scale developed for critically ill children, including five categories of physiological and behavioural items, providing a possible pain score between 0 (no pain) and 10 (maximum pain).58,59 The COMFORT scale has been validated in several studies in PICU60,61 and comprises seven behavioural items, where only six are rated (alertness, calmness/agitation, respiratory response or crying, physical movement, muscle tone, and facial tension), generating a possible score between 6 and 30. In combination with pain, assessment of sedation is paramount and the State Behavioral Scale (SBS) is particularly relevant to evaluate the level of sedation in infants and children in ICU.62 It consists of a six-level responsiveness continuum, ranging from −3 (unresponsive) to +2 (agitated), with a neutral state ‘awake and able to calm’ of 0.
Pain and Sedation Management
Painful procedures should be minimised when possible. Some nonpharmacological therapies have been shown to be beneficial alone in managing mild pain or in combination with drug therapy in infants and young children. These therapies may include non-nutritive sucking (e.g. finger or pacifier) with or without sucrose (for infants up to 4 months), swaddling, music therapy,63 and distraction with or without parental presence.64
Pharmacological treatment of pain and sedation in infants and children should be tailored to the child’s need and condition. Continuous opioid (morphine) infusions are used at the lowest effective dose and minimum duration based on regular pain assessment. Fentanyl boluses are not recommended in neonates as they may cause glottic and chest wall rigidity.65 Sedation management in children is similar to that in adults, except for the use of propofol, which should be used with caution in children. Although there is no strong evidence, propofol infusion in children has been associated with sudden myocardial failure and death.66 More recent data shows that propofol has an acceptable safety profile and could be used in children for short term deep sedation under close monitoring of the airways.67 Use of dexmedetomidine in paediatrics is promising, but additional safety and efficacy studies need to be carried out before routine use as a sedative agent can be recommended in children.68 Indications for the use of neuromuscular blocking agents in children, monitoring of the effects and management are similar to adult practice.69
Family Issues and Consent
When children are admitted to an ICU, the whole family is affected by the hospitalisation. ‘Family-centred care’ (FCC) provides a framework for the care of children and their family in hospital. FCC means that during a hospital stay, nursing care is ‘planned around the whole family, not just the individual child, and in which all the family members are recognised as care recipients’.70, p. 1318 Parents should receive unbiased information at regular times, be involved in the decision-making process and the care of their child; this parent–professional collaboration should be facilitated at all levels of healthcare.70,71 As the developmental issues highlight, parents are essential to a child’s coping with critical illness. Critically ill children are particularly vulnerable to short- and long-term emotional and psychological sequelae, but parental presence and participation in care can make a difference.72
Parents need to feel involved in their child’s care, which includes the need for information, communication, understanding the child’s illness and being part of the decision-making process.31,34,73,74 A partnership between staff and parents is the ideal situation, but parents often need to be reminded on how to maintain the parental role and how they can effectively care for both their child’s and their own psychological health.75 Parents should be allowed to be present during potentially stressful situations such as endotracheal suction, cannulation and resuscitation if they choose to, providing adequate support from a nurse or another designated health care worker is given.41 Being present at the end of their child’s life may help them accept the death.42 Not allowing parents to be present during procedures is a form of paternalism that goes against the right of the patient.64 Parents should however be informed that it is their right to leave if they wish.
Consent and Assent
Except for emergency treatment, parents or legal guardians need to consent to all aspect of medical care, including preventive, diagnostic or therapeutic measures for children. The legal age of consent differs between legislations but is 18 years in major European countries and all Australian states, except New South Wales and South Australia, where the legal age for consent is 14 and 16 years, respectively.76 However, a young person with the emotional maturity and intellectual capacity to agree to medical procedures, in circumstances where he or she is not legally authorised or lacks sufficient understanding for giving consent competently can provide informed assent.77,78 To be considered competent, young people must be able to understand the nature of the decision as well as the consequences of making or not making the decision.78 Whenever possible, it is recommended to obtain the child’s assent for treatment or procedures. Children, even when deemed not competent, have the right to be informed and, when appropriate, to be asked for their permission. However refusal of treatment by a child has no legal bearing when a parent has consented. Importantly, parents may also refuse consent, and in that case national laws and legal mechanisms for resolving disputes may be used.77,79
The Child Experiencing Upper Airway Obstruction
General Description and Clinical Manifestations
• a longer inspiratory phase with unchanged expiratory phase
• recessions of the chest wall
Observing and listening to the child’s symptoms without disturbing them will provide important clues about the level and degree of obstruction the child is experiencing. The aim is to assess the child without causing further distress, as a crying, agitated child can further increase the degree of obstruction and work of breathing, leading to respiratory collapse.80 The Paediatric Assessment Triangle (PAT) is a useful tool to facilitate rapid assessment of the child’s appearance, work of breathing, and skin circulation.81 Stridor indicates obstruction in the upper airway, while wheeze is suggestive of lower airway disease. When stridor is also associated with a barking cough, it is likely to be croup. A softer stridor in a child who looks systemically unwell may indicate epiglottitis. When a previously well child presents with a sudden onset of stridor, it is likely to indicate foreign body aspiration, and eliciting the history of a sudden choking episode can clarify the diagnosis.19,80,82
Congenital Airway Abnormalities
Laryngomalacia is the most common cause of stridor in the newborn period. Stridor is produced by flaccid, soft laryngeal cartilage and aryepiglottic folds that collapse into the glottis on inspiration.83 An inspiratory stridor, usually high-pitched, will be present. It may be intermittent, may decrease when the patient is placed prone with the neck extended, may increase with agitation, and is usually present from birth or the first weeks of life. The infant’s cry is usually normal. Feeding problems may be associated with increased respiratory distress. Laryngoscopy confirms the laryngomalacia diagnosis. Treatment is supportive, with only a small proportion of infants requiring airway reconstructive surgery unless respiratory distress interferes with feeding and growth, in which case a tracheostomy may be indicated.84
A laryngeal web is made of membrane that typically spreads between the vocal cords, with an inspiratory stridor present soon after birth. Diagnosis is confirmed by laryngoscopy. Treatment involves lysis in the case of thin membranous webs while a tracheostomy may be required for a thicker fibrotic web. Laryngeal webs can also develop after contracting illnesses such as diphtheria, and are occasionally reported in otherwise normal adults, typically at intubation for an operative procedure.85
Tracheomalacia and tracheobronchomalacia involve malformed cartilage rings, with lack of rigidity and an oval shape to the lumen. Secondary tracheomalacia is associated with prolonged intubation and prematurity and presents within the first year of life.17 Malacias are characterised by wheezing and stridor on expiration, with collapse of the tracheal or bronchial lumen. Diagnosis is confirmed by fluoroscopy and bronchoscopy, which demonstrate tracheal collapse on expiration. As the infant grows, cartilaginous development improves the airway by about two years of age, but a number of children will require airway stenting or reconstructive surgery.83
Vascular rings result from congenital malformations of the intrathoracic great vessels, resulting in compression of the airways.83 Infants present with stridor at birth or within the first few weeks of life. Other symptoms include wheezing, cough, cyanosis, recurrent bronchopulmonary infections, and dysphagia. Diagnosis may be confirmed by CT scan, MRI scan or endoscopy, which reveals indentations secondary to the extrinsic pressure of the vasculature.84 The anatomy of the vascular malformations is determined by angiography. Treatment is surgical correction of the vascular malformation.84
Monitoring and Diagnostics
Lateral airway X-rays are unlikely to be helpful in the setting of croup and epiglottitis and, when they are likely to involve separating the child from a parent, are potentially harmful and not recommended.19 When there is a less dramatic presentation of the infant or child, or when the diagnosis is not clear, as in the case of an inhaled foreign body, then a chest X-ray may be diagnostic.
Managing the Paediatric Airway
A child’s airway may be managed in a number of ways. Simple positioning may be all that is required to manage an infant’s airway. Children will often assume an upright sitting position and may become more distressed if placed into the supine position, thus when possible the best position for an infant or child with upper airway obstruction may be sitting on their parent’s lap. Because of the anatomy and physiology of the respiratory tract, avoid extending the head of infants. Chin-lift and jaw-thrust are useful airway adjuncts in children to maintain an airway and to facilitate use of a bag–valve–mask. It may be necessary to use an oropharyngeal airway or nasopharyngeal airway, laryngeal masks and endotracheal intubation in an unconscious or sedated infant.19,80
Intubation
Intubation may be required to manage airway obstruction.86 Uncuffed endotracheal tubes (ETT) have been favoured in paediatric practice over cuffed tubes. Inflating the cuff of a regular ETT can cause damage in prepubescent children, as the subglottic area is the narrowest portion of their airways. The recent availability of a paediatric-specific ETT with microcuff and markings to ensure correct placement below the glottis has facilitated ventilation when a leak is undesirable, including in the child with facial and airway burns,87 and when using inhaled nitric oxide and for high frequency ventilatory strategies such as oscillation ventilation. Equipment necessary for paediatric intubation are shown in Figure 25.3. Figure 25.4 shows a range of sizes of uncuffed ETTS: 2.5 mm to 5.5 mm, that should be available in 0.5 mm increments, while cuffed ETTs are now available in sizes from 3 mm through to 9 mm. Selecting the correct ETT size includes having the recommended tube size plus tubes that are 0.5 mm larger and smaller than that. For children over 1 year of age, several formulae exist to calculate appropriate tube sizes, but the age-based and the fifth fingernail width-based predictions of ETT size are the most accurate.88 Table 25.4 provides a guide for ETT sizes, suction catheter size and nasogastric tube size for different-aged infants and children.
Practice tip
To calculate ETT tube size and length, use the following formula from the 2010 Australian and New Zealand Resuscitation Guidelines:89
• For term newborns ≥3 kg: size 3.0 mm or 3.5 mm (uncuffed tubes) or 3.0 mm (cuffed tubes)
• For infant up to 6 months: size 3.5 mm or 4.0 mm (uncuffed tubes) or 3.5 mm (cuffed tubes)
• For infant 7 to 12 months: size 4.0 mm (uncuffed tubes) or 3.5 mm (cuffed tubes)
• For children over 1 year: Uncuffed tubes: size (mm) = age (years)/4 + 4 or Cuffed tubes: size (mm) = age (years)/4 + 3.5
The most common method used to intubate children is the rapid-sequence method. Rapid-sequence intubation is performed where the child may have a full stomach and is at risk of aspiration during intubation.90 It involves the practically simultaneous administration of hypnotic medication and a muscle relaxant immediately before intubation.92,93 The main advantages of this method include good airway visualisation with a relaxed jaw, open immobile vocal cords, and the elimination of all movement, including gagging and coughing.90
Specific Conditions Affecting the Upper Airway
Bacterial and viral infections of the upper airway are common in children. Croup is the most common infection causing upper airway obstruction in children. Epiglottitis is now rarely seen since immunisation against Haemophilus influenzae type b (Hib) was introduced into the immunisation schedule for all Australian and New Zealand children. However, it is important to distinguish epiglottitis from croup in order to initiate appropriate management. Other less common infectious causes of upper airway obstruction seen in young children include bacterial tracheitis and retropharyngeal abscess, while diseases thought to have disappeared, such as Lemierre’s syndrome and diphtheria have not been completely eradicated.94
Infection of the lymphoid tissue around the nodes draining the nasopharynx, sinuses and eustachian tubes may cause pus to accumulate in the retropharyngeal space, leading to a retropharyngeal abscess. Presenting symptoms include history of upper respiratory tract infection (URTI), sore throat, fever, toxic appearance, meningismus, stridor, dysphagia, and difficulty handling secretions.94 Diagnosis is usually made on bronchoscopy. Treatment involves surgical drainage and antibiotic administration. Short-term intubation may be required until swelling has resolved following surgery.
Croup
Croup (laryngotracheobronchitis) is used to describe a set of symptoms caused by acute swelling causing obstruction in the upper airway (larynx, trachea and bronchi) from inflammation and oedema, caused mostly by the parainfluenza or influenza viruses.84,95 Croup occurs in approximately 2% of Australian children, generally aged 1–4 years, and in winter months.96 Recent advances in croup management have been responsible for a fall in the number of children requiring hospitalisation and intubation. Possible complications of croup include respiratory failure, respiratory arrest, hypoxic damage, secondary bacterial infection, acute pulmonary oedema, persistence or recurrence.84
Clinical manifestations
Croup is characterised by a barking or seal-like cough, inspiratory stridor and hoarse voice.97 The severity of croup is assessed based on increased respiratory rate, increased heart rate, altered mental state, work of breathing and stridor. Stridor at rest is noted in moderate to severe croup and is often quite loud. If a child’s stridor becomes softer but the work of breathing remains increased, it should be treated as an emergency as the obstruction may become more severe.98 The symptoms of croup are listed and compared with those of epiglottitis in Table 25.5. Diagnosis is made on physical assessment and the history of the illness.
| Croup | Epiglottitis | |
|---|---|---|
| Aetiology | Viral | Bacterial |
| Age | 6 months–3 years | Infancy through adulthood |
| Onset | Subacute (over days) | Acute (over hours) |
| Fever | Mild (±38°C) | Severe (>38.5°C) |
| Cough | Present (often barking or seal-like) | Absent |
| Drooling | Absent | Present |
| Activity | Distressed | Lethargic |
| Colour | Pale/sick | Toxic |
| Obstruction | +++ | + |
| Stridor | Inspiratory, high-pitched | Expiratory snore |
| Sore throat | Uncommon | Common |
| Position | Any | Tripod; sitting up |
| Course | Gradual worsening or improvement | Unpredictable; fatal if not treated |
| Season | Autumn–winter | Throughout the year |
Adapted from (95).
Management
Management of croup depends on the severity of the upper airway obstruction and close cardiorespiratory observation and monitoring is essential. Children with moderate to severe croup should be given face-mask oxygen and allowed to adopt the position which they find most comfortable. Strategies such as positioning the child in a parent’s lap and holding the face-mask close to their face may limit their distress and can have beneficial effects on oxygenation.97
The use of steroids in combination with nebulised adrenaline is responsible for significant improvement of symptoms in children within 12 hours of administration, abating the need for intubation in the vast majority of cases.97,98 Nebulised adrenaline is efficacious to reduce airway inflammation, with effects seen within five minutes and lasting up to two hours. Although inhalations can be repeated, the benefits lessen with subsequent treatments. Adrenaline does not alter the course of croup.
Epiglottitis
Epiglottitis is inflammation of the epiglottis, frequently involving surrounding structures, with the classic description of a swollen, cherry-red, softened and floppy epiglottis, which tends to fall backwards, obstructing the airway.99 Obstruction also occurs circumferentially, from the oedematous, inflamed aryepiglottic folds surrounding the larynx. It is typically caused by Hib and since the introduction of childhood immunisation programmes to protect against Hib infection, the incidence has dropped from 23.8 cases per 100,000 children in 1991 to 2.81 per 100,000 in 2002 in the UK.100 A similar pattern was observed in Australia with a drop in prevalence rate from 22.7 to 3.3 per 100,000 children in 1998 and 2008, respectively.101,102 Hib infection can cause meningitis, septicaemia, septic arthritis and cellulitis as well as epiglottitis. The disease process and development of major symptoms progress rapidly over a few hours and an untreated child may become acutely obstructed. A child will make a full recovery without sequelae if diagnosis and treatment are appropriate and timely. Supraglottitis has emerged in recent times as a more accurate description of a similar range of symptoms as epiglottitis, and has been linked with the herpes virus and other organisms, requiring treatment with aciclovir and vancomycin.99
Clinical manifestations
The child with epiglottitis presents looking unwell with a fever, is unable to swallow secretions, drooling saliva and refusing to talk or swallow. The child may maintain an upright position, usually leaning with the head extended, supporting a sitting position with the arms stretched out behind in what is known as the tripod position. Hypoxaemia is usually present. Sudden respiratory arrest followed by cardiac arrest, can occur unpredictably. Cardiac arrest is likely to be asystolic in rhythm due to either vagal stimulation or hypoxia secondary to airway obstruction.94
Management
The most important aspect in the management of epiglottitis is rapid diagnosis and minimal handling of the child until an airway is in place. Children with epiglottitis require urgent intubation because acute airway obstruction followed by cardiac arrest is a potential hazard. Thus, the aim of management at this time is to keep the child as calm as possible until the airway is secured.19,99 The child should be nursed propped up with pillows or on a parent’s lap while arrangements are made for the insertion of an ETT. Procedures such as cannulation and examination of the throat should be avoided until the child’s airway is secure.95
Prophylaxis with antibiotics is required for families and household contacts if there is an infant under 12 months of age and/or a child in the household under the age of five years who is not fully immunised. Where the infected child has attended childcare for more than 18 hours each week, it is recommended that staff and other children at the centre also receive antibiotic prophylaxis.82
Foreign Body Aspiration
Aspiration of a foreign body into the upper airway is another relatively common cause of obstruction in children. Infants tend to swallow food items such as nuts and seeds, while toddler-aged children tend to swallow coins, teeth, and small toys or toy parts.103 An inhaled foreign body is likely to have a rapid onset with no previous symptoms. Sometimes the diagnosis is missed for days, weeks or even months, and the child’s symptoms may be non-specific, such as a cough with or without blood-stained sputum.83,103
Clinical Manifestations
Sudden onset of coughing, gagging and an audible stridor in a previously-well infant or child is suggestive of an inhaled foreign body.83 However, an accurate history – such as a recent coughing or choking episode – is the most sensitive factor in making a diagnosis of inhaled foreign body.
Management
Management will depend on the location and level of the aspirated foreign body, as it may have lodged in the pharynx, oesophagus, larynx, trachea or bronchial tree. Coughing is encouraged for mild airway obstruction.104 Up to five back blows may be successful in dislodging the foreign body, which may be followed by up to five chest thrusts and back blows. Direct laryngoscopy and removal of a foreign body using Magill forceps may be required for an acute episode when back blows and chest thrusts have been unsuccessful. When the foreign body has lodged below the carina, for the majority of children diagnosis and definitive treatment will consist of removal of the foreign body via a bronchoscopy under general anaesthesia.83
The Child Experiencing Lower Airway Disease
Lower airway disease in children is a common reason for admission to ICU. Infants under 12 months usually present with bronchiolitis or pneumonia. Asthma is more common in older children, but infants nearing 12 months of age may develop asthma105 and there is often confusion between bronchiolitis and asthma at this age.106
Specific Conditions
Bronchiolitis and asthma are commonly seen in children, and the management of each condition is discussed below. National and worldwide clinical guidelines for these conditions have been developed and are continually updated.107,108
Bronchiolitis
Viral bronchiolitis in infancy is characterised by obstruction of the small airways, resulting in air trapping and respiratory distress in infants less than 12 months of age. It is the most common severe respiratory infection in infancy, although the course is usually mild to moderate and is self-limiting, usually requiring no treatment. Severe infection represents less than 5% of all cases and is usually associated with prematurity or congenital heart disease.95 Respiratory syncytial virus (RSV) causes 90% of bronchiolitis cases.109 Other causative agents are parainfluenza virus types 1, 2 and 3, influenza B, adenovirus types 1, 2 and 5 and Mycoplasma. RSV invades the epithelial cells of the bronchioles, spreading via cell fusion and the creation of syncytia. This results in destruction of the epithelium and patches of necrosis. The debris associated with epithelial shedding and mucus production lead to small airway blockage and the clinical features of bronchiolitis.109
In temperate regions of Australia and New Zealand, most cases occur between late autumn and early spring, with sporadic cases throughout the year. There is a paradoxical relationship between the incidence of RSV and other viral pathogens causing bronchiolitis. RSV epidemics occur when other respiratory pathogen epidemics are diminishing, and vice versa. Although there are limited data on the actual incidence of bronchiolitis, laboratory isolation data in New South Wales estimate that about 1000 infants are hospitalised with bronchiolitis each year. The majority of these are under six months old.110 There is also a higher incidence of bronchiolitis in the Indigenous population of Australia111,112 and more severe illness when compared to non-Indigenous.113 Younger children with one or more comorbidities were at higher risk of complications.111 RSV infection occurs throughout the year, with an annual peak during the winter months.111,114
When bronchiolitis occurs, the highest risk for hospitalisation is infants under six months of age, those with exposure to tobacco smoke and underlying conditions such as congenital heart disease, prematurity and low socioeconomic group.20,102,115 Severe disease, requiring admission to a paediatric ICU, is associated with prematurity, particularly in infants with chronic lung disease or a history of ventilation in the newborn period and congenital heart disease.
Clinical manifestations
Bronchiolitis is a clinical diagnosis; non-isolation of a causative viral agent does not exclude the diagnosis. The clinical features of bronchiolitis are variable, and may include URTI symptoms such as rhinorrhoea (runny nose) and an irritating cough. Within three days the infant develops tachypnoea and respiratory distress, which may be mild, moderate or severe. An expiratory wheeze is often present and auscultation usually reveals fine to coarse crackles. Fever is present in approximately 50% of infants. In very young, premature or low-birthweight infants, apnoea is often the presenting symptom, which then develops into severe respiratory distress.116 The clinical course of bronchiolitis is usually 7–10 days; however, the effects of severe illness may last much longer. Respiratory distress is present. Indications for intensive care admission include frequent and/or prolonged apnoeas; hypoxaemia despite administration of oxygen; haemodynamic instability; an obviously tiring infant or decreased level of consciousness.20,117
Asthma
Asthma is a disease of the lower respiratory tract characterised by mucosal and immune system dysfunction. There is a complex interaction between bronchial wall cells, inflammatory mediators and the nervous system. The chronic inflammatory process causes narrowing of bronchial airways, thus obstructing airflow. This leads to episodes of wheezing, breathlessness and chest tightening that are usually reversible.106
Development of childhood asthma results from a combination of genetic, environmental and socioeconomic risk factors.118–122 Increasing prevalence of asthma over the past 20–30 years may be linked to a higher incidence of genetic predisposition, independent of environmental factors. Some studies have identified links between asthma and various regions of the human genome, but the linkages are not consistent. The CD14 gene shows the best promise of linkage, with increased expression or promotion of this gene associated with atopy and asthma in early to late childhood.123 Certain racial groups, such as African-Americans, when compared to Americans of European origin, are also more likely to develop asthma and have complications, particularly those traditionally from tropical regions.124 Once asthma has developed, there are triggers that may precipitate an attack. These include viral illnesses, particularly respiratory viruses, tobacco smoke exposure, house dustmites, exercise, pet hair, food and environmental allergens.
Asthma is one of the commonest paediatric presentations to emergency departments and its worldwide prevalence is growing with differences between populations.125 It is reported that as many as 20–30% of children in Westernised countries, including Australia and New Zealand, will develop wheeze or asthma symptoms;126 the current disease rate is between 9.6% in the US,127 29.7% in the UK,128 and 31% in Australia.1 Asthma prevalence is increasing,126 is higher in boys129,130 and in urban areas, but its mortality has declined over the past two decades, from 1–2/100,000 down to 0.8/100,000.126
Clinical manifestations
ICU admission is required when children present with respiratory failure due to an asthma exacerbation. Obesity and genetic predisposition may be important in reacting to β2-agonist therapy.131 These children exhibit clinical features associated with respiratory distress. Pulsus paradoxus, a phenomenon of palpable changes in blood pressure that occur with respirations, may also be present and can also be noted on plethysmography. Arterial blood gas analysis usually reveals initially a mild respiratory alkalosis and hypoxaemia; however, more severe asthma may show combined respiratory and metabolic acidosis and hypercapnia as the child tires and is unable to eliminate carbon dioxide.133
Assessment and management
Assessment of asthma severity is based on criteria such as the degree of respiratory failure as evidenced by cyanosis, length of sentences between breaths, retractions and hypoxia, as well as level of consciousness and degree of pulsus paradoxus. There are many scores available to assist in determining the severity of asthma, including the National Asthma Campaign guidelines, the Pulmonary Index Score, the Respiratory Failure Score and the Modified Dyspnoea Scale. Whatever method is used, assessments should be frequent and response to treatment recorded (see Table 25.6). Severe asthma that worsens and/or does not respond to treatment warrants admission to a paediatric ICU.130
β2-agonists, anticholinergics and steroids form the foundation of acute severe asthma management, but for children over 40 kg and those who have reached puberty it may be more appropriate to administer IV adrenaline. β2-agonists act by relaxing bronchial smooth muscle, improving mucociliary transport and inhibiting mediator release. In severe to life-threatening asthma, nebulised salbutamol is preferred.134 Inhaled salbutamol combined with magnesium sulfate improves pulmonary function.135 Adverse effects of β2-agonists’ administration include hypokalaemia, tachycardia, tremors, agitation and hyperglycaemia. Mild lactic acidosis may also occur. Intravenous salbutamol infusion should be considered when there is severe, life-threatening asthma refractory to inhaled treatment. Inhaled salbutamol may be discontinued once IV infusion has commenced, but should be reestablished before ceasing the infusion. In acute severe episodes, salbutamol is usually given every 20 minutes; if there is little response, continuous nebuliser therapy may be required. In this instance, a feeding tube is inserted into the nebuliser and the chamber replenished as it empties. Anticholinergics, in combination with β2-agonists, improve lung function by augmenting the action of β2-agonists, blocking irritant receptors and bronchodilation of larger airways.136
Corticosteroids decrease airway inflammation, enhancing the β2-agonists’ effects, and reduce mucus production. Oral and intravenous methods of administration are similarly efficacious. The effects of systemic steroids are apparent within 3–4 hours of administration, with maximal benefit achieved within 6–12 hours. There is little evidence to support giving inhaled steroids during an acute episode.137
Magnesium sulphate promotes smo3oth muscle relaxation by inhibiting uptake of calcium. Intravenous magnesium sulfate has demonstrated efficacy in acute severe asthma and inhaled magnesium sulphate combined with a β2-agonist results in improved pulmonary function.135
Aminophylline has shown some benefit in regards to improved lung function in severe asthma that is unresponsive to inhaled bronchodilators and steroids. It is a bronchodilator, improving diaphragmatic contractility and a central respiratory stimulant. However, the narrow therapeutic window and side effects of induced nausea and/or vomiting represent a non-negligible risk of complication, thus its use should be limited to managing asthma not responsive to other agents.138
Ventilation may be required when there is profound hypoxaemia, severe muscle fatigue or decreased level of consciousness.130,139 However, as asthmatic children are at higher risk of complications such as barotrauma and air trapping, there is a higher risk of death associated with ventilation in this group of patients. Non-invasive positive pressure ventilation (NPPV) is the first choice, with some evidence that it rapidly corrects gas exchange abnormalities and assists with respiratory muscle fatigue.140–142 The contraindications for NPPV include cardiac/respiratory arrest, severe encephalopathy, haemodynamic instability, facial surgery/deformity, high risk for aspiration, nonrespiratory organ failure, severe upper gastrointestinal bleeding, unstable arrhythmia and upper airway obstruction.142
Intubation may be necessary when signs of deterioration are present, such as elevated carbon dioxide levels, exhaustion, alteration of mental status, haemodynamic instability and refractory hypoxaemia.142 Because of high airway pressures, a cuffed endotracheal tube should be used.
Children with acute asthma may have a raised metabolic rate and increased insensible losses, together with reduced oral intake. With increased intrathoracic pressure due to air trapping, even mild dehydration may compromise cardiac output. Therefore, adequate fluid replacement is necessary. In addition, pulmonary secretions will thicken and plug the airways if fluid intake is inadequate. Maintenance fluids should be provided until the child’s condition and oral intake improve.143
Nursing the Ventilated Child
Principles of mechanical ventilation were covered in Chapter 15. Issues such as gastric decompression, adequate analgesia and sedation and undertaking steps to prevent accidental extubation are similar to those for adults. Specific considerations for ventilating infants and children include:
• Most children are oxygenated before, during and after suctioning with 100% O2.144 The child’s clinical status is monitored throughout the procedure.
• Heated humidification is preferred in children as they have limited respiratory reserve and are prone to airway blockage.145,146
• Endotracheal suctioning does not require normal saline instillations.147–149
• To prevent iatrogenic atelectasis, the suction catheter size should be less than or equal to two-thirds the internal diameter of the ETT. Suction pressure should be limited to −60 mmHg (−8 kPa) for infants, and up to −200 mmHg (−27 kPa) for adolescents. A suction regulator is useful to monitor the amount of applied negative pressure, as too much can result in atelectasis.
• Restraints may be required to limit the movement of the child, with the aim of preventing accidental extubation rather than maintaining the child in an immobile state. Restraints may be physical, such as arm boards or hand ties; or chemical, such as sedation. Accidental extubation is a medical emergency.
Modes of Ventilation
There are many modes of ventilation (see Chapter 15 for more details). This section includes information specifically related to paediatric ventilation. As with adults, arterial blood gases should be taken about 15–20 minutes after initiating mechanical ventilation.
Volume Ventilation of Children
Typically, volume ventilation is not used in infants under 5 kg due to the small tidal volumes, which risk being lost in the distensible tubing and leaking around the ETT. In addition, most volume ventilators do not have a constant fresh gas flow, so the infant has to work harder to trigger a breath. Some of the newer models of ventilator have attempted to overcome these problems. Steps in beginning volume ventilation for a child are as follows:150
1. Set the tidal volume at <8 mL/kg. This is a protective lung strategy approach151 and can be increased if needed.
2. Set the rate at 20 breaths/min. This is lower than physiological norm for infants, but the slightly larger tidal volumes will compensate.
3. Set the FiO2 at <0.6 and titrate according to oxygen saturation and blood gases.
4. Set the PEEP at 5 cm. This is slightly higher than physiological norm.
5. Set the trigger sensitive enough to allow the infant or child to trigger a breath without working too hard. If a continuous fresh gas flow is available, then this is preferable. If autocycling occurs, gradually decrease the trigger-setting sensitivity until the autocycling stops.
Pressure Ventilation of Children
The pressure ventilation mode is most commonly used in infants weighing under 5 kg or with children who have a large leak around the ETT. Steps in beginning pressure ventilation for a child are as follows and should be based on arterial blood gases:150
1. Set the peak inspiratory pressure (PIP) at 18–20 cmH2O.
2. Set the positive end expiratory pressure (PEEP) at 5 cmH2O.
3. Set the rate at 20 breaths/min.
4. Set the FiO2 at <0.6 and titrate according to oxygen saturation and blood gases.
5. Set the trigger sensitive enough to trigger a breath. Most pressure ventilators have a constant fresh gas flow, which allows the child to breathe spontaneously without increased effort.
Non-invasive Ventilation
Non-invasive ventilation (NIV) refers to ventilatory support without an artificial airway in the trachea (see Chapter 15). In critically ill children with respiratory failure, NIV may be used to reduce the need for intubation. However, the evidence for its use in children is weak152 and often extrapolated from adults.153 Some studies showed that NIV decreases the rate of ventilator-associated pneumonia and reduces oxygen requirement in children with lower airway diseases when compared to conventional ventilation140,154 and may be recommended as the first line ventilation strategy.142
High-frequency Oscillatory Ventilation
HFOV is delivered primarily by the Sensor Medics 3100A (Mayo Healthcare Australia). This ventilator uses a diaphragm piston unit to actively move gas into and out of the lung, and requires a non-compliant breathing circuit. A major difference between HFOV and other forms of ventilation is that there is active expiration with oscillation versus passive expiration for conventional ventilation.150,155 Unlike conventional ventilation, which uses bulk flow to deliver gas to the lungs, using smaller-than-dead-space tidal volumes utilises the mechanisms of pendelluft, Taylor dispersion, asymmetrical velocity profiles, cardiogenic mixing and, to a very limited extent, bulk flow.155 These are all terms used to describe the distribution of gas when rapid rates and tiny volumes are used.
Ventilation is dependent on amplitude (a determinant of tidal volume) much more than rate. With the Sensor Medics oscillator, paradoxically lowering frequency (Hz) improves CO2 removal. This is thought to occur because the oscillating diaphragm is able to move through a greater distance, thus increasing tidal volume by providing more inspiratory time and a longer expiratory time.155
The principal determinants of oxygenation are the same as those for conventional ventilation. Therefore, as with conventional ventilation, the alveoli must be opened and prevented from collapsing if hypoxaemia is to be corrected. HFOV achieves this through delivering a high mean airway pressure without imposing a large tidal volume.150 It thus avoids overdistension and the risk of barotrauma.
Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO) is an alternative method of providing ventilatory and/or cardiac support. When used to support ventilation, ECMO allows the lungs to rest and heal. Ventilation settings are reduced to minimal to minimise the iatrogenic effects of positive pressure.156,157 There are two main methods of ECMO: veno-venous and venoarterial. In veno-venous ECMO, large-bore cannulas are placed in large veins, such as the internal jugular or femoral.158 The more common form of ECMO in paediatrics, venoarterial, utilises the right internal jugular to drain blood and the right common carotid artery for blood return.158 Alternative placement of cannulas for venoarterial ECMO after heart surgery is the right atrium and aorta. Venoarterial ECMO allows support of both circulation and ventilation. Essentially, blood is drained from the ‘venous’ line, pumped through a membrane to oxygenate the blood and remove CO2, then returned through a filter via the ‘arterial’ cannula.158
Children are considered for ECMO if they have potentially reversible lung or cardiac injury, or shock that has not responded to conventional therapies.159–161 Contraindications include irreversible brain or CNS injury, immunodeficiency or severe coagulopathy. Outcomes are generally positive, but ECMO centres need to maintain their competence by performing the procedure often.
The Child Experiencing Shock
Mortality rate for septic shock in children is reported at around 9%.162 A detailed description of shock is given in Chapter 21, with specific paediatric considerations addressed here. Hypovolaemic, cardiogenic and septic shock (also termed distributive shock) are the most common types of shock in children. Cardiogenic shock is rare and is seen mainly after open-heart surgery and severe myocarditis or untreated shock. The infant with an undiagnosed congenital heart defect, in particular lesions that rely on the ductus arteriosis – known as duct-dependent lesions – can present in shock.162 As infants and children presenting in hypovolaemic shock are likely to respond to fluid resuscitation alone, they may not require transfer to a specialist paediatric centre. However, children presenting with septic shock or cardiogenic shock will require transfer to a specialist paediatric centre for ongoing management, and contact should be made to initiate goal-directed therapy as soon as possible. Those children who do not respond to fluid volume alone will require invasive haemodynamic monitoring and possible pharmacological intervention. The development of shock in a hypovolaemic patient is considered to indicate losses of at least 30 mL/kg.162
Septic shock was responsible for about 8% of all deaths of children in Australian and New Zealand ICUs in 2008.4 Causes of septic shock in infants and children are often different from those in adolescents and adults. The commonest infecting organisms are often age-related in children, and are listed in Table 25.7. Infants and children with either congenital or acquired immunocompromise are at greater risk of developing septic shock.16 Meningococcal sepsis remains the leading cause of septic shock in developed countries such as Australia and New Zealand.
TABLE 25.7 Organisms causing sepsis in newborns, infants and children
| Age group | Common organisms causing sepsis |
|---|---|
| Newborns |
Clinical Manifestations
There are many similarities between children and adults in the clinical manifestations of shock (see Chapter 21). However, there are three major differences:163
1. Children with systemic inflammatory response syndrome have either abnormal temperature or elevated white cell count (or both) plus either abnormal heart rate or elevated respiratory rate (or both).
2. In addition to the symptoms of cardiovascular dysfunction seen in adults, children may also present with a normal blood pressure with no inotrope requirements, but have two of the following: unexplained metabolic acidosis, increased lactate, oliguria, prolonged capillary refill time, or core to peripheral temperature gap >3°C.
3. Systemic hypotension is not necessary to make the diagnosis of septic shock.
Other specific factors for children that are not relevant in the adult population include a higher risk of sepsis in preterm infants and in infants with cardiac defects or chronic lung disease.162
Patient Assessment and Diagnostic
Assessment of the child with shock is based on clinical assessment, not on chemical test as recommended in adult shock.162 Ideally, shock should be diagnosed before hypotension occurs. Hypothermia or hyperthermia and altered neurological status, which provides information about perfusion pressure and peripheral vasodilation (warm shock) or vasoconstriction with capillary refill >2 sec (cold shock) are clinical signs of shock in children.162
Careful respiratory and cardiovascular assessment is required, as described in this chapter and Chapters 9 and 13. Monitoring of children experiencing shock is the same as for adults (see Chapter 21). It consists of continuous monitoring of heart rate, SvO2 saturation, quality of peripheral pulses, capillary refill, level of consciousness, peripheral skin temperature, urine output as indirect measures of cardiac output (CO) as well as serial blood gas and electrolyte analysis.162
Diagnosis of septic shock can be difficult in children. When present, non-blanching rash is a specific sign of meningococcal septicaemia.166
However, a certain proportion of children will present with non-specific symptoms or signs of infection, such as fever, vomiting, lethargy, irritability, or headache and the conditions may be difficult to distinguish from other infections.14,15,166 Laboratory testing of samples of blood, urine, stool, sputum, cerebrospinal fluid and any obvious wounds or lesions is standard practice in adults and children.
Management of Shock
Early recognition of shock, institution of appropriate goal-directed therapy and targeting the causative agent remain the mainstay of managing septic shock in children as in adults. Goal-directed therapies such as oxygen therapy, fluid resuscitation, maintenance of acceptable blood pressure, and institution of pharmacological treatment and other supportive treatments to achieve therapeutic goals are practised in managing shock in children, and are linked to better outcomes.162,163
Large amounts of fluid may be required by children despite peripheral oedema or absence of overt fluid loss.16 Early aggressive fluid resuscitation will improve survival in children with hypovolaemic and septic shock, particularly if received within the first hour, when hypotension has not yet developed.19 Intravascular access in children can be difficult and umbilical venous access in newborns and intraosseous access in children can be used before the placement of central lines.162,167 The use of the EZ-IO® (Vidacare Corporations, Texas) paediatric intraosseous needle set and driver system has become common in practice.168,169 Other kinds of manually inserted intraosseous needles are available, and regardless of type, intraosseous needles all allow rapid access to the intramedullary capillary network, facilitating delivery of fluids, drugs and blood products. The site of choice in infants and children is the proximal tibia, 2–3 cm below the tibial tuberosity.170 Once sited, a syringe must be attached to aspirate and ascertain correct placement. Fluid boluses can then be given via syringe into the intramedullary space with the aim of restoring circulating volume which will in turn facilitate venous access with improvement of peripheral perfusion.171
Similarly to adults, after appropriate volume resuscitation has been given and symptoms of shock are not resolving or hypotension is developing, then inotropes and vasopressors are recommended.162 Inotropic drugs that are recommended in children include dopamine, adrenaline and noradrenaline. Vasodilators, including sodium nitroprusside or nitroglycerin, are used to recruit microcirculation; type III phosphodiesterase inhibitors are used to improve cardiac contractility. If shock persists and there is a risk for adrenal insufficiency, hydrocortisone therapy is recommended.162 ECMO may also be considered for a child who appears to be developing irreversible shock.162
Monitoring of blood glucose is essential in all critically ill infants and children. In septic shock hyperglycaemia may be present, which has been linked to higher mortality rates in paediatric septic shock.162 Blood glucose should be monitored and maintained within normal ranges (80–150 mg/dL) with appropriate use of insulin and glucose administration.104,162
The Child Experiencing Acute Neurological Dysfunction
There are many reasons why an infant or child can present with an acute episode of neurological dysfunction. Common presentations to an ICU include meningitis,173,174 encephalitis,174 seizures and encephalopathy175–177 (see also Chapter 17). Assessment and recognition of the clinical features and management of the various causes of neurological dysfunction in children are the keys to achieving good outcomes.
Neurological Assessment
To assess a child’s level of consciousness, several different scales can be used. The Glasgow Coma Scale (GCS) is commonly used,178 but the Glasgow Coma Motor subscore is more appropriate for children.179 Another reliable scale is the Full Outline of Unresponsiveness (FOUR) score; it includes four parameters (eye response, motor response, pupil reflexes, and breathing) rated on a 0 to 4 scale, giving a possible score situated between 0 (completely unresponsive) and 16.180 The FOUR score and the GCS are both able to predict in-hospital morbidity and poor outcome at the end of hospitalisation.
Other neurological assessment parameters include:
• Pupils: assess size, reaction and symmetry.
• Posture: abnormal flexion posturing, often referred to as decorticate posturing, is a flexion response of the arms with either flexion or extension of the legs, while abnormal extension posturing, often referred to as decerebrate posturing, is an extension response of all limbs, where arms rotate externally. Both abnormal flexion and extension posturing in a previously normal child may indicate raised intracranial pressure.
• Meningism: this is indicated by neck stiffness in a child and full/bulging fontanelle in an infant.
Seizures
Seizures are covered in Chapter 17. The various aetiologies of seizures in children include febrile convulsions, CNS infection such as meningitis or encephalitis, metabolic imbalances, drugs, trauma or epilepsy. Seizures in children are common, with about 4–10% of children having an unprovoked seizure without recurrence.181 Children between the ages of six months and six years are more likely to develop seizures.182 Children, particularly those under five years, are at higher risk, as the developing brain has a lower threshold for seizures.181 Febrile convulsions occur in 2–5% of children, commonly between the ages of 6 and 60 months.183 Non-febrile seizures are typically more common in the neonatal period, with the incidence falling with age.182
Management
Management of the paediatric patient with seizures is similar to management of the adult (see Chapter 17), but there are some specific paediatric considerations. The paediatric patient who is suffering seizures is more susceptible than an adult to hypoglycaemia. Hypoglycaemia may lead to secondary brain injury during and after seizures. Blood sugar levels should always be checked in children suffering from seizures and intravenous fluids containing glucose administered.182,184
The care of the seizing or post-ictal child is generally supportive and includes monitoring for signs of ongoing seizures, administration of appropriate anticonvulsants, and regular assessment of neurological function. In young infants, seizures may be difficult to determine and may include stiffening, staring and lip smacking rather than obvious clonic activity.185,186
Meningitis
Meningitis is an acute inflammation of the meninges that usually develops over 1–2 days. A fulminant form of meningitis caused by Neisseria meningitidis or meningococcal disease may develop over several hours. Organisms causing bacterial meningitis vary by age group. In infants under three months of age, group b Streptococcus, E. coli, Streptococcus pneumoniae and Listeria are the most likely agents. In children over three months of age meningococcus, Haemophilus influenzae type b and Streptococcus pneumoniae are more common.172 The most common causes of viral meningitis in infants and children include herpes simplex virus and the enteroviruses.187 Tuberculous meningitis, while still rare, is becoming more common, particularly in immigrant families or those with recent travel to affected areas. Bacterial meningitis continues to have a poorer outcome than other forms of meningitis, despite advances in therapy.188
Incidence
Data on the incidence of meningitis in Australia is limited to the major bacterial types, particularly for infants and children over two months of age. Hib, meningococcal and pneumococcal infections are all notifiable. Since the introduction of the Hib vaccine in1993, Hib infection has fallen to 1.2/100,000 in 2005.189 Of all reports of infection only 28% were meningitis, and the majority of infections were in children under two years of age.189
Meningococcus is the main cause of meningitis in children. It occurs seasonally, with the main peak in Australia between June and October. Serogroups A, B and C account for 90% of cases in Australia, with serogroup B causing 66% of disease.189 There are two main peaks of disease. The 0–4-years age group represents 31% of all cases and with 17% occurring in the 15–19-years age group.189 The incidence of meningococcal disease in children aged 0–4 years is 10/100,000. Of children with invasive meningococcal disease, 47% have meningitis, with or without sepsis.190,191 The mortality rate of meningococcal meningitis in children under five years of age is below 1%; with sepsis present, the rate increases up to 10–15%.
The incidence of invasive pneumococcal disease (IPD) has significantly dropped since the introduction of routine vaccination, with a reported rate of 23.4 cases per 100,000 children aged less than five years in 2005.192 The highest peak of IPD is seen in children aged one year with a rate fluctuating between 26.5, 37 and 51/100,000 in Australia, North America and Europe, respectively.193–196 The highest Australian incidence occurs in the Northern Territory, with Indigenous children at highest risk.197 Other risk factors include extreme prematurity, chronic lung disease, trisomy 21 (Down syndrome), diabetes and cystic fibrosis. Clinical manifestations or symptoms vary with the age of the child, duration of the illness and history of antibiotic use for the current illness. These are outlined in Table 25.8.
| Infants under 3 months | Infants over 3 months and children |
|---|---|
Management
Initial management of the infant or child with meningitis includes assessment and management of the airway, breathing, circulation and disability. Once the initial resuscitation has been completed, consideration should be given to correcting any biochemical abnormalities. In particular, blood sugar level should be checked and corrected in the early management phase. Once meningitis is suspected, a lumbar puncture (LP) is generally performed to confirm diagnosis, but if the child is haemodynamically unstable or has ongoing seizures, problems with ventilation or signs of raised intracranial pressure, the LP should be delayed and blood cultures obtained.166,199
Steroid use in meningitis has some benefit in reducing morbidity in adults,195 but not in children.200 However, it was shown to reduce the risk of severe hearing loss in children with bacterial meningitis.201
Encephalitis
The most common type of encephalitis in children is acute viral encephalitis, and the causative agent is usually herpes symplex virus (HSV).202 Left untreated, HSV is almost uniformly fatal, with over half of survivors experiencing significant long-term morbidity.174,203–205 Other causes of encephalitis in children include:
• enteroviruses (e.g. enterovirus 71, coxsackievirus, polio and echovirus)
The incidence of acute encephalitis is over 10 cases per 100,000 children.206 Children under one year of age are at higher risk of developing encephalitis. Other risk factors include immune dysfunction and exposure to risk animals, or specific geographic location. For example, Murray Valley encephalitis and Kunjin viruses are endemic in the Kimberley region of the Northern Territory, and Japanese B virus has been reported on Cape York Peninsula; it is endemic in southeast Asia.207
Encephalitis symptoms are similar to meningitis, but often with a much slower onset. Progressively worsening headache, fever and decreased level of consciousness or behavioural changes characterise encephalitis. Focal neurological signs and seizures may indicate involvement of the meninges or spinal cord.208
Management
Prompt administration of aciclovir, if HSV is the suspected cause, is warranted due to high mortality and morbidity rates. Other viruses are also treated with aciclovir. Ganciclovir is useful for resistant organisms, but is more toxic.208,209 Intensive care management involves supporting ventilation and managing neurological complications such as seizures and cerebral oedema. If the child is unconscious on presentation, the disease course will be more severe.203
Gastrointestinal and Renal Considerations in Children
Many critically ill infants and children are also at risk of developing complications involving the gastrointestinal tract (GIT). Although primary acute renal failure (ARF) is relatively rare in critically ill children, the incidence of renal impairment secondary to the underlying illness is recognised increasingly in children, although a paucity of prospective studies remains problematic. The total incidence of ARF in PICU is currently between 4.5% and 10%.210,211 Diagnoses associated with primary ARF in children are haemolytic uraemic syndrome, oncological diagnoses and following congenital cardiac surgery.210,212 The RIFLE criteria used to describe kidney injury in adults (see Chapter 18) also applies to children.211 Both primary and acquired kidney injury in children are associated with increased length of stay and increased mortality. Consequently, continuous renal replacement therapy (CRRT) should be considered earlier in management than has previously been the case. Critically ill children experiencing, or at risk of developing, acute renal failure will benefit from prompt transfer to specialty PICU.
The child’s GIT will need protection from developing GIT ulceration and bleeding in critical illness. A potentially fatal complication, stress ulceration and bleeding, has a current incidence of around 10% in critically ill children.213 Clinically significant bleeding that causes haemodynamic instability or the need for transfusion is reported in about 1.6% of children in PICU.213 The same treatments can be used in both children and adults, with no one agent, dose or regimen standing out as better for minimising bleeding and ulceration or leading to fewer complications such as pneumonia.213
Nutritional Considerations
The aims of nutrition in critically ill children are two-fold. First, children are at particular risk of malnutrition because they are growing, have greater energy requirements for their weight and less storage capacity than adults. Second, children are at particular risk of developing protein–calorie malnutrition, which can lead to immunodysfunction, increased risk of infections, morbidity and death in those children with organ dysfunction.214
In addition, nutrition is important to maintain gut mucosa integrity, prevent the development of hypo- and hyperglycaemia, assist with maintenance of immune function and in modulating the immune response as well as providing energy.214 One barrier to achieving adequate nutrition for critically ill children is the fluid restrictions that are routine practice in the ICU, so liberalising fluids where possible for enteral nutrition to be maximised should be considered.
Other recent studies have found a reduction in nosocomial infections in children when enteral feeds have been used; transpyloric tube feeding versus gastric feeding and nutritional support teams result in improved nutritional delivery and enhanced enteral feeding rates in critically ill children.215–218
Supplements and Feeding
Whilst promising work has been undertaken in adult critical care with the supplementation of feeds and total parenteral nutrition (TPN) with supplements including amino acids such as L-arginine, glutamine, taurine, nucleotides, omega-3 and omega-6 fatty acids, carnitine, antioxidants, prebiotics and probiotics, the same outcomes have not been reproduced in children to date.214 The evidence for additives in enteral feeding is not clearcut in children and therefore routine supplementation for critically ill infants and children is not common practice.
Intravenous Therapy for Children
Until enteral feeding is established, critically ill infants and children will require maintenance IV fluids. Traditionally, hypotonic fluids – fluids containing a concentration of sodium lower than normal serum sodium – have been administered as maintenance fluids. These included the hypotonic formulation of 0.225% sodium chloride with 3.75% glucose. Over the past decade this formulation has largely been replaced with 0.45% sodium chloride and 2.5% glucose as iatragenic hyponatraemia has been observed in otherwise-well children having surgery.219,220 It has been common paediatric practice to use only 500 mL IV bags in children for safety reasons. In the modern era across westernised countries, use of volumetric IV pumps and burettes have also been standard paediatric practice, although changes to larger volumes of IV formulations for children will need to be closely monitored.
The use of hypotonic fluids is under review in many countries, and changes underway in Australia will see 500 mL bags of IV fluids change to 1000 mL bags in all children’s hospitals, with increased level of monitoring of weight and serum electrolytes recommended. Hypotonic fluids are implicated in hospital-acquired hyponatraemia219,220 and for critically ill children, the capacity to excrete additional free water is often impaired. In addition, a number of common conditions seen in the ICU increase secretion of antidiuretic hormone (ADH), including pain, nausea and infections of the CNS, the GIT, the lung and post surgery, thus promoting the retention of water.220 The risk of developing cerebral oedema is increased in children, who also have an increased body tissue water content and studies indicate that there is an increased risk of developing acute hyponatraemia leading to seizures.
Glucose Control in Children
Hyperglycaemia is associated with a worse outcome in infants and children requiring ICU admission,221 however, the predisposition to hypoglycaemia in children has meant that aggressive treatment of hyperglycaemia is not yet commonplace in critically ill children as it has been in adults in ICU. Hypoglycaemia is documented to occur more frequently in two groups of nondiabetic children: those requiring mechanical ventilation and those requiring inotropic support.222 In the study cited here, hypoglycaemia was an independent predictor of increased mortality. While studies are yet to recommend tight glucose control in the paediatric population, monitoring for hypoglycaemia continues to be an important assessment parameter, particularly in sicker children who require ventilatory support, inotropic support and where enteral feeding may be contraindicated. Hypoglycaemia may be an indicator of worsening organ function, therefore further research needs to focus on the safety of insulin therapy in the nondiabetic critically ill child before aggressive management of hyperglycaemia can be recommended.222
Liver Disease in Children
Liver failure is relatively rare in children. It often arises as a primary problem in children from countries where viral hepatitis is endemic, is associated with paracetamol overdose, and chronic liver disorders, toxins, autoimmune disease, malignancies, vascular and biliary tree malformations as well as unidentified causes.223 Chapter 19 contains more detail on liver function and dysfunction. There are varying severities and forms of liver failure. Infants and children experiencing fulminant hepatic failure and hepatic encephalopathy, regardless of underlying cause, are critically ill, and require transfer to a specialist PICU for ongoing management and possible liver transplantation. Mortality rate is strongly linked with the development of cerebral oedema and intracranial hypertension, and is reported to be as high as 50% where cerebral oedema occurs.224 Many critically ill infants and children are at risk of developing some degree of liver dysfunction; therefore, liver function of all critically ill children requires careful monitoring and management. Clinical manifestations and management of infants and children with liver failure are similar to those of adults.
In summary, the mainstay of management in children with fulminant liver failure is liver transplantation. All children with fulminant disease should be transferred to a paediatric centre as soon as the diagnosis of liver failure is made. Children are at particular risk of developing protein–calorie malnutrition, which can lead to immunodysfunction, increased risk of infections, morbidity and death in children with organ dysfunction.214
Paediatric Trauma
Trauma is the leading cause of death in children and young adults in all developed countries; in the developing world it is second only to deaths from infections.4,225 The approach to management of trauma in children is the same as in adults. For further details on trauma systems and trauma management, see Chapter 23. While there has been some evidence from North America that specialist paediatric trauma centres produce better outcomes for children suffering traumatic injuries,23 the largely spread-out and relatively small population distribution in Australia and New Zealand means that children will often need to be treated initially in adult settings.4
Incidence and Patterns of Injury in Children
Across Australia in 2007–2008 almost 68,000 children were hospitalised as the result of injury, accounting for 12% of all paediatric admissions, with 15% of all children’s deaths attributable to injury.3,225 In 2008, injury accounted for around 7.1% of paediatric admissions to Australian and New Zealand ICUs, with a 4.8% mortality rate, accounting for 29% of all deaths in ICUs in the 1–15-years age group.4
Injury patterns in children differ from adults, with traumatic brain injury, blunt trauma and more diffuse injuries more common in children. There is a bimodal injury pattern associated with age, with peak incidence occurring in children aged 1–4 years and a second peak during adolescence and young adulthood, reflecting the different activities associated with each group.1,226 Infants and young children have a decreased sense of danger and reduced ability to protect themselves, while adolescents have increased exposure to higher risk activities in conjunction with exposure to alcohol, drugs and motor vehicles.227,228 Children who live in regional and rural areas have increased rates of traumatic injuries and deaths from trauma, as do children from lower socioeconomic backgrounds. The same pattern is reflected in Australian and New Zealand statistics.225,229 Time of day and seasonal factors play a role in childhood injury, with children more likely to be injured between 3pm and 5pm, coinciding with the end of the school day, and during summer months, where the incidence of submersion injuries increases.5,226,230
Injury-related deaths in children are highest in the transport deaths category, followed by immersion and assault.1 Motor vehicle accidents involving children as passengers, pedestrians or cyclists are the commonest cause of injury in Australian children, with driveway injuries involving four-wheel drive or light commercial vehicles more likely to be fatal.225 Trauma associated with the use of all terrain vehicles such as quad bikes are becoming increasingly common, particularly in rural areas.228,229 For children under 14, falling from one level to another, such as falling from a window, was the most common form of falls-related injury.231
Immersions are another leading cause of death in children 0–4 years of age, with around 29% of near-drownings in Australia in this age group, peaking in the summer months. Boys outnumber girls, with two-thirds being boys. Infants are more likely to drown in the bath, 1–3-year-olds are most likely to drown in a backyard swimming pool and older children drown in open waterways such as dams and rivers. In Australia, in 2008–2009 there were a total of 302 deaths from drowning, including 50 children. This figure has increased over the previous five years.230
Homicide and assault of children remains low in Australia when compared with other developed economies,231 however it is listed as the third-leading cause of death from injury in children.1,231,232 Spinal injury rates for children are reportedly low at around 1% but are associated with significant mortality and disability.233 Paediatric spinal injury statistics in Australia and New Zealand are not currently reported, as current reporting only captures patients cared for in dedicated adult spinal units,3 however, the incidence is considered to be low, as it does not feature in the annual report of the Australian and New Zealand Paediatric Intensive Care (ANZPIC) Registry.4
Risk Factors
The kinetic forces involved in injury are associated with a more diffuse injury pattern and a greater incidence of multiple trauma in children, as more of the child’s body is subjected to the traumatic forces.227 Children generally have less subcutaneous fat and musculature, providing less protection to the liver, kidneys, and spleen, leading to a higher incidence of lung contusions and abdominal trauma.234 In addition, the relatively large head size of the infant, particularly, and the child leads to a high incidence of head injury.227
Primary Survey and Resuscitation
Initial stabilisation of children who have experienced a traumatic injury is likely to have occurred in the field. Once at the hospital, the primary survey is conducted to assess for, detect and stabilise the child with life-threatening injuries. Undertaking a primary survey and resuscitation uses the same structured approach in children and adults. Chapters 22 and 23 cover emergency presentations and trauma management, however, specific paediatric considerations are highlighted below.
Children sustaining trauma to the head, just as adults, are managed with cervical spine precautions including a collar, until the spine has been radiologically and clinically cleared.9,233 A selection of paediatric hard collars should be available and the measuring guide used to ensure good fit. As the collar can cause neck flexion in infants and small children, the child’s torso may need to be elevated with a folded blanket to maintain a neutral neck position.19 The head and neck are usually immobilised, with head blocks (e.g. rolled towels) placed either side of the head to maintain in-line stabilisation and tapes applied to the forehead and chin to prevent movement. The combative, uncooperative child will not tolerate this, and the actions are likely to increase the child’s agitation and movement. The critical care nurse can position themselves to maintain in-line stabilisation while talking and soothing the child, or ideally where parents are present, seek their assistance to console their child. Specific paediatric trauma boards are available that are designed to maintain the child’s head in a neutral position.
Fluid resuscitation is a controversial area of practice in paediatric trauma, where therapies have been generally less-well-studied than in adults. Current recommendations from the Advanced Paediatric Life Support Group indicate that fluid boluses should be given initially in 10 mL/kg amounts until uncontrolled bleeding has been assessed for and ruled out.15 However, in a child with a traumatic brain injury at risk of secondary brain injury from hypotension, this more conservative approach may not be appropriate. Where more than 20 mL/kg is required, immediate surgical assessment for bleeding is indicated.15
Exposure of the child, with temperature control, is necessary to assess the child completely for injuries.234,235 As hypothermia can develop quickly in children, overhead heating sources and blankets are ideally used to keep the child warm. Hypothermia in trauma patients is associated with increased risk for coagulopathy and mortality, as in adults, so providing warmth is essential paediatric trauma nursing care.236 The child’s right to privacy and dignity should also be considered and exposure minimised.
Secondary Survey
Undertaking a secondary survey is similar in children and adults and is described in Chapter 22. Specific paediatric considerations are highlighted below.
In children, particularly those under one year of age, if injuries and the accompanying history do not seem to match, non-accidental injury should be considered and noted.226 History should be obtained from the child where possible, from any witnesses to the accident, and ambulance officers if they attended. Parents or caregivers will provide details of the child’s past medical history, any medications and any known allergies.
Specific Conditions
Specific injuries that are seen in children are discussed briefly under the headings of traumatic brain injury, chest trauma and abdominal trauma. Obtaining an accurate history of the accident or events leading up to an injury is useful in determining the type of injuries that children may have sustained. Regardless of aetiology, where a child has been involved in a motor vehicle accident (MVA) or sustained a fall, there are likely to be multiple injuries and the situation should be treated as such until other injuries have been considered and excluded.227
Traumatic Brain Injury
Traumatic brain injury (TBI) is a leading cause of deaths and injury worldwide in children. In Australia and other developed economies, children experience the greatest number of head injuries of any age group.23 TBI is often associated with MVA where the child is a vehicle occupant, a pedestrian or a cyclist, with falls and with near-drowning. TBI is described in detail in Chapter 17.
In 2008, 273 children were admitted to Australian and New Zealand ICUs with a diagnosis of head trauma. About 10% of all children aged 1–15 years of age who died in Australasian ICUs had sustained a traumatic brain injury.4 Age and gender are the most significant risk factors for TBI, with peak incidence in the 0–4-years group and in males.237,238 Other factors to consider in children are the increased tendency of the immature brain of children to experience disruption of the blood–brain barrier and, unlike adults, for an increased cerebral blood volume to lead to cerebral oedema due to higher brain water content.11
In 2003, the Society of Critical Care Medicine published guidelines on the management of paediatric brain injury, however little paediatric research evidence underpinned these as they were essentially based on extrapolation from adult research evidence and expert opinion. Since the clinical manifestations of TBI in children are very similar to those in adults, management is also very similar. One significant change recommended in the 2003 guidelines was tight control of CO2, in recognition of hypocarbia as a major secondary brain injury factor. The practice of hyperventilation should be avoided as it is associated with regional cerebral ischaemia.239
In terms of assessment, the GCS modified for children has previously been described in this book. Indications for intracranial pressure (ICP) monitoring in children include all infants and children with a severe head injury, which equates to a GCS score of 8 or below that persists following adequate cardiopulmonary resuscitation, and those children who present with abnormal motor posturing and hypotension.237 Combined with invasive haemodynamic monitoring, targeted therapy to manage both ICP and CPP remains an important part of treatment. While thresholds for treating intracranial hypertension in children have not been studied, it has been known since the 1980s that prolonged intracranial hypertension or high ICP levels will worsen outcome. An ICP of 20 mmHg is considered high in children, with 15 mmHg considered high in infants. ICPs of these values are the usual cut-off points and are likely to be treated with the aim of lowering ICP while maintaining an adequate CPP.9,239
Diagnostics
Diagnostic techniques240 and clinical management of children with TBI241 mirror those in adults (see Chapter 17). The smaller size of children means that diagnostics such as mixed cerebral venous saturation and direct brain oxygen saturation are not yet common practice in paediatrics. A high index of suspicion for spinal injuries in paediatric TBI should be maintained, as spinal cord injury without radiological abnormality (SCIWORA) on plain X-rays is a feature of paediatric spinal injury.233 CT scans are available in more centres than MRI, but involves radiation exposure to the young spine. Conjecture remains around CT imaging versus MRI in children.233
Treatment
Several of the therapies used in the treatment of the child with severe head injury are controversial, as they have not undergone rigorous scientific evaluation. Essentially treatment of TBI in children is identical to adult management: minimising intracranial hypertension, maintaining optimal CPP while preventing secondary injury from hypoxia, hypercarbia, and hypotension while reducing the risk of iatrogenesis from treatment. Decompressive craniectomy is considered for intractable intracranial hypertension to avoid herniation.235,242
Hypothermia has not yet been shown to make a difference in outcome in children, as it has in newborns and adults with hypoxic-ischaemic brain injury. Moderate hypothermia (temperature maintained from 32–34°C) has been studied with disappointing results,243 thought to be associated with inadequate length of cooling (24 hours). A multicentre study is planned to commence in the future to provide moderate hypothermia for 76 hours with slow controlled rewarming.
Outcomes from traumatic brain injury in children are associated with the severity of the initial injury and the presence and control of secondary brain injury, as in adults. Hypotension and hypoxia prehospital admission are strongly linked to mortality and poor functional outcome, with some emerging evidence that hypertension in the first 24 hours may also predict poor outcomes at one year post-injury.244
Chest Trauma
Thoracic injuries in children rarely occur in isolation with traumatic injuries and are often accompanied by head and neck injuries. There is some evidence that thoracic injuries are indicative of a more severe injury; they have been associated with higher mortality.227 Injury to the heart and great vessels in particular is associated with higher mortality. The combination of head injury and thoracic injury is also known to have higher mortality. Most chest injuries in children are sustained as a consequence of MVAs.225 The pattern of injury in children is predominantly one of blunt trauma. Lung contusions are the commonest thoracic injury seen in children. As the ribcage is much more compliant in children, ribs are rarely broken, but they can damage underlying structures such as the lungs, so pulmonary contusions, pneumothorax and haemothorax are often seen. The clinical manifestations, approach to assessment, monitoring and management of children sustaining thoracic trauma is similar to that in adults, and is discussed in Chapter 23. Children with thoracic injuries are generally managed in a specialist trauma centre equipped to manage children.245
Abdominal Trauma
Abdominal trauma in children is a leading cause of death when combined with head injury.246 Blunt trauma from MVAs is the most common mechanism of injury, but bicycle handlebars may also inflict a significant injury.247 The liver and spleen are the most commonly injured organs in abdominal trauma and can usually be managed non-surgically. The abdominal organs are relatively large in children, with less musculature and a more compliant ribcage, meaning that there can be injury to underlying organs with no apparent external injury.234 Blunt trauma is common, while penetrating injury is less common, resulting from gunshot and stab wounds. These injuries are associated with older children and adolescents, though a thinner body wall may result in greater underlying organ damage, particularly if the flank is penetrated.234
During the primary survey, the child’s abdomen should be exposed and may reveal signs such as bruising from bicycle handles, tyre marks, abrasions or contusions. Abdominal distension is a less reliable sign in children, as distension may be from air that is swallowed from pain and crying. However, as in adults, the primary survey may not include the abdomen if other immediately life-threatening injuries are present, such as thoracic and/or head injuries. These injuries will take precedence, so it may not be until the secondary survey can be undertaken that abdominal injuries are considered. The monitoring and management of children sustaining abdominal trauma is very similar to that of adults,248 and is discussed in Chapter 23.
A number of clinical indicators will determine the need for a CT scan of the abdomen. These include all children with multiple injuries, children experiencing pain and tenderness over the abdomen, gross haematuria with a minor injury, children with a haemoglobin below 100 g/L and children who require fluid resuscitation with no obvious source of blood loss. Diagnostic peritoneal lavage and FAST sonography are rarely used in children because of the poor sensitivity of the test to detect the presence of intraabdominal injuries in children.249 However, when FAST sonography is combined with elevated liver transaminases, the sensitivity and specificity of the screening increases to 88% and 98%, respectively.250 Monitoring of blood in urine is a simple, useful technique to detect bladder and kidney injuries. Management of abdominal trauma generally requires only haemodynamic and laboratory monitoring in conjunction with supportive therapies such as fluid replacement, monitoring of urine output, and pain management with the aim of detecting signs of haemorrhage.14,251
Summary
Case study
Research vignette
Abstract
Setting
The setting was an eight-bed paediatric intensive care unit in an inner city teaching hospital.
Learning activities
Learning activities are all based on the case study.
1. Consider the information provided in the first paragraph of the case study. What injuries can you predict in children travelling in a rear car seat?
2. On arrival in the ED, Lisa has a GCS score of 4 and requires immediate intubation. Calculate the ETT size for Lisa. Should the ETT be placed orally or nasally? Should a cuffed or uncuffed ETT be placed? Justify your answers.
3. Lisa’s mean blood pressure was initially low, and she required initial fluid resuscitation. What fluids could have been considered for Lisa to restore her circulating blood volume while considering her lung and brain injuries?
4. What is an appropriate ventilation strategy for Lisa, keeping in mind her lung and head injuries and associated treatment?
5. Will permissive hypercapnoea be considered for Lisa? Provide a rationale for your answer.
6. The specialist retrieval teams were unable to retrieve Lisa for some hours due to inclement weather. Consider where Lisa would be best managed within your own facility, should this situation arise. Imagine your facility is located a minimum of 6-hour drive from a major paediatric trauma centre. Consider the availability of resources (human and technological) that would be available in various clinical areas to manage Lisa for 12 hours until retrieval could be accomplished.
The Children’s Hospital, Westmead http://www.chw.edu.au/
John Hunter Children’s Hospital, Newcastle http://www.hnehealth.nsw.gov.au/servs_facil/john_hunter_childrens.htm
Mater Children’s Hospital, Brisbane http://www.mater.org.au/healthServices/MCH.asp
Princess Margaret Hospital for Children, Perth http://wchs.health.wa.gov.au/
Royal Children’s Hospital, Brisbane http://www.health.qld.gov.au/rch/default.asp
Royal Children’s Hospital, Melbourne http://www.rch.org.au/rch
Starship Children’s Hospital, Auckland http://www.starship.org.nz/
Sydney Children’s Hospital, Randwick http://www.sch.edu.au/
Women’s and Children’s Hospital, Adelaide http://www.wch.sa.gov.au/
1 Australian Institute of Health and Welfare (AIHW). A picture of Australia’s children 2009. Canberra: AIHW; 2009.
2 Statistics New Zealand. National Population Estimates: March 2010 quarter. Auckland: New Zealand Government; 2010. p. 1–10
3 Australian Institute of Health and Welfare (AIHW). Australian hospital statistics 2008–09, 17th edn. Canberra: AIHW; 2010.
4 Alexander J, Tregea S, Slater A. Australian and New Zealand Paediatric Intensive Care Registry 2008. Herston, QLD: Australian and New Zealand Intensive Care Society; 2010.
5 ANZICS Centre for Outcome and Resource Evaluation. 2008 Annual Report. Melbourne: Australian and New Zealand Intensive Care Society, Centre for Outcome and Resource Evaluation; 2010.
6 Namachivayam P, Shann F, Shekerdemian L, Taylor A, van Sloten I, et al. Three decades of intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010;11(5):549–555.
7 Ramarajan N, Krishnamoorthi R, Strehlow M, Quinn J, Mahadevan SV. Internationalizing the Broselow Tape: How Reliable Is Weight Estimation in Indian Children. Acad Emerg Med. 2008;15(5):431–436.
8 So TY, Farrington E, Absher RK. Evaluation of the accuracy of different methods used to estimate weights in the pediatric population. Pediatrics. 2009;123(6):e1045–e1051.
9 PICU Policy and Procedure Committee. Severe traumatic brain injury practice guideline. Sydney: The Children’s Hospital, Westmead; 2008.
10 Boluyt N, Bollen CW, Bos AP, Kok JH, Offringa M. Fluid resuscitation in neonatal and pediatric hypovolemic shock: a Dutch Pediatric Society evidence-based clinical practice guideline. Intensive Care Med. 2006;32(7):958–961.
11 Jenkins LW, Kochanek PM. Developmental Neurobiology, Neurophysiology and the PICU. In: Nichols DG, ed. Rogers’ textbook of pediatric intensive care. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008:810–825.
12 Vernon-Levett P. Intracranial dynamics. In: Curley M, Moloney-Harmon P. Critical care nursing of infants and children. 2nd edn. Philadelphia: Saunders; 2001:323–368.
13 Blackburn ST. Maternal, fetal & neonatal physiology: a clinical perspective, 3rd edn. Missouri: Saunders Elsevier; 2007.
14 Nadel S, Kisson T, Ranjit S. Recognition and initial management of shock. In: Nichols DG, ed. Rogers’ textbook of pediatric intensive care. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008:372–383.
15 Advanced Life Support Group. The child in shock. In: Mackway-Jones KME, Phillips B, Wieteska S. Advanced paediatric life support: the practical approach. 4th edn. Boston: BMJ Books; 2005:97–114.
16 Kelley D. Hypovolaemic shock: an overview. Crit Care Nurs Q. 2005;28(1):2–19.
17 Kubba H, Moores T. Developmental anatomy of the airway. Anaesth Intensive Care. 2006;7(5):158–160.
18 Curley MAQ, Thompson J. Oxygenation and ventilation. In: Curley M, Moloney-Harmon P. Critical care nursing of infants and children. 2nd edn. Philadelphia: Saunders; 2001:233–308.
19 Advanced Life Support Group. The child with breathing difficulties. In: White C, Wieteska S, Williams K, Wyllie J, Young S. Advanced paediatric life support: the practical approach. 4th edn. Oxford: Blackwell Publishing; 2005:73–96.
20 Parker MJ, Allen U, Stephens D, Lalani A, Schuh S. Predictors of major intervention in infants with bronchiolitis. Pediatr Pulmonol. 2009;44(4):358–363.
21 Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Saugstad OD, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med. 2007;35(3):175–186.
22 MacGregor J. Introduction to the anatomy and physiology of children. London: Routledge; 2000.
23 Baird JS, Cooper A. Multiple trauma. In: Nichols DG, ed. Rogers’ textbook of pediatric intensive care. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008:384–407.
24 De Carvalho WB, Leite HP. Nutritional support in the critically ill child. In: Nichols DG, ed. Rogers’ textbook of pediatric intensive care. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008:1500–1515.
25 Willock J, Harris C, Harrison J, Poole C. Identifying the characteristics of children with pressure ulcers. Nurs Times. 2005;101(11):40–43.
26 Curley MAQ, Razmus I, Roberts K, Wypij D. Predicting pressure ulcer risk in pediatric patients: the Braden Q scale. Nurs Res. 2003;52(1):22–23.
27 Anthony D, Willock J, Baharestani M. A comparison of Braden Q, Garvin and Glamorgan risk assessment scales in paediatrics. J Tissue Viability. 2010;19(3):98–105.
28 Willock J, Baharestani MM, Anthony D. The development of the Glamorgan paediatric pressure ulcer risk assessment scale. J Wound Care. 2009;18(1):17–21.
29 Rennick JE, Rashotte J. Psychological outcomes in children following pediatric intensive care unit hospitalization: a systematic review of the research. J Child Health Care. 2009;13(2):128–149.
30 Colville G, Darkins J, Hesketh J, Bennett V, Alcock J, Noyes J. The impact on parents of a child’s admission to intensive care: Integration of qualitative findings from a cross-sectional study. Intensive Crit Care Nurs. 2009;25(2):72–79.
31 Foster M, Whitehead L, Maybee P. Parents’ and health professionals’ perceptions of family centred care for children in hospital, in developed and developing countries: A review of the literature. Int J Nurs Stud. 2010;47(9):1184–1193.
32 Harrison TM. Family-centered pediatric nursing care: state of the science. J Pediatr Nurs. 2010;25(5):335–343.
33 Melnyk BM. Coping with unplanned childhood hospitalization: the mediating functions of parental beliefs. J Pediatr Psychol. 1995;20(3):299–312.
34 Sturdivant L, Warren NA. Perceived met and unmet needs of family members of patients in the pediatric intensive care unit. Crit Care Nurs Q. 2009;32(2):149–158.
35 Colville G. The psychologic impact on children of admission to intensive care. Pediatr Clin North Am. 2008;55(3):605–616.
36 Melnyk BM, Alpert-Gillis L, Feinstein NF, Crean HF, Johnson J, et al. Creating opportunities for parent empowerment: program effects on the mental health/coping outcomes of critically ill young children and their mothers. Pediatrics. 2004;113(6):597–607.
37 Melnyk BM, Crean HF, Feinstein NF, Fairbanks E, Alpert-Gillis LJ. Testing the theoretical framework of the COPE program for mothers of critically ill children: an integrative model of young children’s post-hospital adjustment behaviors. J Pediatr Psychol. 2007. [Cited Feb 2011]. Available from http://www.mrw.interscience.wiley.com/cochrane/clcentral/articles/007/CN-00586007/frame.html
38 Dunkel CS, Sefcek JA. Eriksonian lifespan theory and life history theory: An integration using the example of identity formation. Rev Gen Psychol. 2009;13(1):13–23.
39 Piaget J. The child’s conception of the world: A 20th-century classic of child psychology. Lanham (MD): Rowman & Littlefield Publishers; 2007.
40 Roberts CA. Unaccompanied hospitalized children: a review of the literature and incidence study. J Pediatr Nurs. 2010;25(6):470–476.
41 Dudley NC, Hansen KW, Furnival RA, Donaldson AE, Van Wagenen KL, Scaife ER. The effect of family presence on the efficiency of pediatric trauma resuscitations. Ann Emerg Med. 2009;53(6):777–784.
42 Tinsley C, Hill JB, Shah J, Zimmerman G, Wilson M, et al. Experience of families during cardiopulmonary resuscitation in a pediatric Intensive Care Unit. Pediatrics. 2008;122(4):e799–e804.
43 Erikson EH. Identity and the life cycle. New York: Norton & Company, Inc.; 1994.
44 Schore AN. Affect regulation and the origin of the self: The neurobiology of emotional development. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1994.
45 Schore JR, Schore AN. Modern attachment theory: The central role of affect regulation in development and treatment. Clin Soc Work J. 2008;36(1):9–20.
46 Beilin H, Pufall PB. Piaget’s theory prospects and possibilities. Lawrence Erlbaum Associates, Inc.: Hillsdale (NJ), 1992.
47 Wheeler HJ. The importance of parental support when caring for the acutely ill child. Nurs Crit Care. 2005;10(2):56–62.
48 Coyne I. Consultation with children in hospital: children, parents’ and nurses’ perspectives. J Clin Nurs. 2006;15(1):61–71.
49 Hockenberry M, Wilson D. Wong’s essentials of pediatric nursing, 8th edn. St. Louis: Mosby; 2009.
50 Slote RJ. Psychological Aspects of Caring for the Adolescent Undergoing Spinal Fusion for Scoliosis. Orthop Nurs. 2002;21(6):19–31.
51 Ramelet AS, Huijer Abu-Saad H, McDonald S, Rees N. The challenges of pain measurement in critically ill young children: A comprehensive review. Aust Crit Care. 2004;17(1):33–45.
52 Bouza H. The impact of pain in the immature brain. J Matern Fetal Med. 2009;22(9):722–732.
53 Walker SM. Pain in children: recent advances and ongoing challenges. Br J Anaesth. 2008;101(1):101–110.
54 O’Connor M, Bucknall T, Manias E. Sedation management in Australian and New Zealand intensive care units: doctors’ and nurses’ practices and opinions. Am J Crit Care. 2010;19(3):285–295.
55 Ista E, de Hoog M, Tibboel D, van Dijk M. Implementation of standard sedation management in paediatric intensive care: effective and feasible? J Clin Nurs. 2009;18(17):2511–2520.
56 Neuhauser C, Wagner B, Heckmann M, Weigand MA, Zimmer K-P. Analgesia and sedation for painful interventions in children and adolescents. Dtsch Arztebl Int. 2010;107(14):241–247.
57 Jin HS, Yum MS, Kim SL, Shin HY, Lee EH, et al. The efficacy of the COMFORT scale in assessing optimal sedation in critically ill children requiring mechanical ventilation. J Korean Med Sci. 2007;22(4):693–697.
58 Ramelet AS, Abu-Saad H, Bulsara M, McDonald S, Rees N. Clinical validation of the Multidimensional Assessment Pain Scale (MAPS) in preverbal critically ill children. Paediatr Anaesth. 2007;17(12):1156–1165.
59 Ramelet AS, Rees N, McDonald S, Bulsara M, Huijer Abu-Saad H. Development and preliminary psychometric testing of the Multidimensional Assessment of Pain Scale: MAPS. Paediatr Anaesth. 2007;17(4):333–344.
60 Johansson M, Kokinsky E. The COMFORT behavioural scale and the modified FLACC scale in paediatric intensive care. Nurs Crit Care. 2009;14(3):122–130.
61 van Dijk M, Peters WB, van Deventer P. The COMFORT Behavior Scale. A tool for assessing pain and sedation in infants. Adv J Nurs. 2005;105(1):33–36.
62 Curley MAQ, Harris SK, Fraser KA, Johnson RA, Arnold JH. State Behavioral Scale: A sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7(2):107–114.
63 Golianu B, Krane E, Seybold J, Almgren C, Anand KJS. Non-Pharmacological Techniques for Pain Management in Neonates. Semin Perinatol. 2007;31(5):318–322.
64 Kuzin JK, Yborra JG, Taylor MD, Chang AC, Altman CA, Whitney GM, et al. Family-member presence during interventions in the intensive care unit: Perceptions of pediatric cardiac intensive care providers. Pediatrics. 2007;120(4):e895–e901.
65 Spence K, Henderson-Smart D, New K, Evans C, Whitelaw J, Woolnough R, et al. Evidenced-based clinical practice guideline for management of newborn pain. J Paediatr Child Health. 2010;46(4):184–192.
66 Kang TM. Propofol infusion syndrome in critically ill patients. Ann Pharmacother. 2002;36(9):1453–1456.
67 Vespasiano M, Finkelstein M, Kurachek S. Propofol sedation: intensivists’ experience with 7304 cases in a children’s hospital. Pediatrics. 2007;120(6):e1411–e1417.
68 Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Paediatr Drugs. 2008;10(1):49–69.
69 Playfor S, Jenkins I, Boyles C, Choonara I, Davies G, et al. Consensus guidelines for sustained neuromuscular blockade in critically ill children. Paediatr Anaesth. 2007;17(9):881–887.
70 Shields L, Pratt J, Hunter J. Family centred care: a review of qualitative studies. J Clin Nurs. 2006;15(10):1317–1323.
71 Corlett J, Twycross A. Negotiation of parental roles within family-centred care: a review of the research. J Clin Nurs. 2006;15(10):1308–1316.
72 Davidson JE, Powers K, Hedayat KM, Tieszen M, Kon AA, et al. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004–2005. Crit Care Med. 2007;35(2):605–622.
73 Jackson C, Cheater FM, Reid I. A systematic review of decision support needs of parents making child health decisions. Health Expect. 2008;11(3):232–251.
74 Lam LW, Chang AM, Morrissey J. Parents’ experiences of participation in the care of hospitalised children: A qualitative study. Int J Nurs Stud. 2006;43(5):535–545.
75 Cleveland LM. Parenting in the neonatal intensive care unit. J Obstet Gynecol Neonatal Nurs. 2008;37(6):666–691.
76 NHRMC.gov.au. National Statement on Ethical Conduct in Human Research (2007). Canberra: National Health and Medical Research Council [updated Sep 2009; cited Feb 2011]. Available from http://www.nhmrc.gov.au/publications/synopses/e72syn.htm
77 De Lourdes Levy M, Larcher V, Kurz R, the members of the Ethics Working Group of the CESP. Informed consent/assent in children. Statement of the Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP). Eur J Pediatr. 2003;162(9):629–633.
78 Roberson AJ. Adolescent informed consent: ethics, law, and theory to guide policy and nursing research. J Nurs Law. 2007;11(4):191–196.
79 Munro ER, Ward H. Balancing parents’ and very young children’s rights in care proceedings: decision-making in the context of the Human Rights Act 1998. Child Fam Social Work. 2008;13(2):227–234.
80 RCH.org. Clinical practice guidelines: croup (laryngotracheobronchitis). Melbourne: Royal Children’s Hospital Melbourne [updated 2008 May 21; cited 2010 Nov 25]. Avalaible from http: //www.rch.org.au/clinicalguide/cpg.cfm?doc_id=5141
81 Dieckmann RA, Brownstein D, Gausche-Hill M. The pediatric assessment triangle: a novel approach for the rapid evaluation of children. Pediatr Emerg Care. 2010;26(4):312–315.
82 de Caen A, Duff J, Coovadia AH, Luten R, Thompson AE. Airway management. Nichols DG, ed. Rogers’ textbook of pediatric intensive care, 4th edn, Philadelphia: Lippincott Williams & Wilkins, 2008.
83 Bruce IA, Rothera MP. Upper airway obstruction in children. Paediatr Anaesth. 2009;19(Suppl1):88–99.
84 Doherty GM. Acute and chronic airway obstruction in children. Anaesth Intensive Care. 2009;10(4):191–195.
85 Schmidt MH, Riley RH, Hee GY. Difficult double-lumen tube placement due to laryngeal web. Anaesth Intensive Care. 2010;38(1):194–196.
86 Khemani RG, Markovitz BP, Curley MA. Characteristics of children intubated and mechanically ventilated in 16 PICUs. Chest. 2009;136(3):765–771.
87 Sheridan RL. Uncuffed endotracheal tubes should not be used in seriously burned children. Pediatr Crit Care Med. 2006;7(3):258–259.
88 Turkistani A, Abdullah KM, Delvi B, Al-Mazroua KA. The ‘best fit’ endotracheal tube in children – comparison of four formulae. Middle East J Anesthesiol. 2009;20(3):383–387.
89 Resus.org.au. ARC Guideline 12.6 Techniques in paediatric advanced life support. Melbourne: Australian Resuscitation Council [updated Dec 2010; cited Feb 2011]. Available from http://www.resus.org.au/
90 Zelicof-Paul A, Smith-Lockridge A, Schnadower D, Tyler S, Levin S, et al. Controversies in rapid sequence intubation in children. Curr Opin Pediatr. 2005;17(3):355–362.
91 Shann F. Drug doses, 15th edn. Parkville: Royal Children’s Hospital; 2010.
92 Ching KY, Baum CR. Newer agents for rapid sequence intubation: etomidate and rocuronium. Pediatr Emerg Care. 2009;25(3):200–207.
93 Perry JJ, Lee JS, Sillberg VA, Wells GA. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev 2008. CD002788.
94 Jenkins IA, Saunders M. Infections of the airway. Paediatr Anaesth. 2009;19(Suppl1):118–130.
95 Shah S, Sharieff GQ. Pediatric respiratory infections. Emerg Med Clin North Am. 2007;25(4):961–979.
96 Chang AB, Chang CC, O’Grady K, Torzillo PJ. Lower respiratory tract infections. Pediatr Clin North Am. 2009;56(6):1303–1321.
97 Bjornson CL, Johnson DW. Croup. Lancet. 2008;371(9609):329–339.
98 Rajapaksa S. Croup – assessment and management. Aust Fam Physician. 2010;39(5):280–282.
99 Glynn F, Fenton J. Diagnosis and management of supraglottitis (Epiglottitis). Curr Infect Dis Rep. 2008;10(3):200–204.
100 Garner D, Weston V. Effectiveness of vaccination for haemophilus influenzae type b. Lancet. 2003;361(1):395–396.
101 Hanna JN, Wild BE, Sly PD. The epidemiology of acute epiglottitis in children in Western Australia. J Paediatr Child Health. 1992;28(6):459–464.
102 Grimwood K, Cohet C, Rich FJ, Cheng S, Wood C, Redshaw N, et al. Risk factors for respiratory syncytial virus bronchiolitis hospital admission in New Zealand. Epidemiol Infect. 2008;136(10):1333–1341.
103 Wheeler DS, Dauplaise DJ, Guiliano JS. An infant with fever and stridor. Pediatr Emerg Care. 2008;24(1):46–49.
104 Resus.org.au. ARC Guideline 12.7. Management after resuscitation in paediatric advanced life support. Melbourne: Australian Resuscitation Council [updated 2010 Dec; cited Feb 2011]. Available from http: //www.resus.org.au/
105 Turner SW, Young S, Goldblatt J, Landau LI, Le Souëf PN. Childhood asthma and increased airway responsiveness: a relationship that begins in infancy. Am J Respir Crit Care Med. 2009;179(2):98–104.
106 Bradley E, Chipps BE. Asthma in Infants and Children. Clin Cornerstone. 2008;8(4):44–61.
107 Myers TR. Guidelines for asthma management: a review and comparison of 5 current guidelines. Respir Care. 2008;53(6):751–769.
108 GINAsthma.org. Global strategy for the diagnosis and management of asthma in children 5 years and younger. US: Global Initiative for Asthma [updated 2009 Dec; cited Feb 2011]. Available from http: //www.ginasthma.org/index.asp
109 Checchia P. Identification and management of severe respiratory syncytial virus. Am J Health Syst Pharm. 2008;65(23):S7–12.
110 Lister S, McIntyre P, Menzies R. The epidemiology of respiratory syncytial virus infections in NSW chilren, 1992–1997. NSW Public Health Bull. 2000;11(7):119–123.
111 Dede A, Isaacs D, Torzillo PJ, Wakerman J, Roseby R, Fahy R, et al. Respiratory syncytial virus infections in Central Australia. J Paediatr Child Health. 2010;46(1–2):35–39.
112 Roche P, Lambert S, Spencer J. Respiratory syncytial virus. Commun Dis Intell. 2003;27(1):117–122.
113 Bailey EJ, Maclennan C, Morris PS, Kruske SG, Brown N, Chang AB. Risks of severity and readmission of Indigenous and non-Indigenous children hospitalised for bronchiolitis. J Paediatr Child Health. 2009;45(10):593–597.
114 Whitehall J, Bolisetty S, Whitehall J, Francis F, Norton R, Patole S. High rate of indigenous bronchiolitis and palivuzumab. J Paediatr Child Health. 2001;37(4):416–417.
115 Eidelman AI, Megged O, Feldman R, Toker O. The burden of respiratory syncytial virus bronchiolitis on a pediatric inpatient service. Isr Med Assoc J. 2009;11(9):533–536.
116 Vicencio AG. Susceptibility to bronchiolitis in infants. Curr Opin Pediatr. 2010;22(3):302–306.
117 Turner T, Wilkinson F, Harris C, Mazza D. Evidence based guideline for the management of bronchiolitis. Aust Fam Physician. 2008;37(6):S6–13.
118 Patel MM, Miller RL. Air pollution and childhood asthma: recent advances and future directions. Curr Opin Pediatr. 2009;21(2):235–242.
119 Mitchell DK, Adams SK, Murdock KK. Associations among risk factors, individual resources, and indices of school-related asthma morbidity in urban, school-aged children: a pilot study. J Sch Health. 2005;75(10):375–383.
120 Martel MJ, Rey E, Malo JL, Perreault S, Beauchesne MF, et al. Determinants of the incidence of childhood asthma: a two-stage case-control study. Am J Epidemiol. 2009;169(2):195–205.
121 Meurer JR, Lustig JV, Jacob HJ. Genetic aspects of the etiology and treatment of asthma. Pediatr Clin North Am. 2006;53(4):715–725.
122 Claudio L, Stingone JA, Godbold J. Prevalence of childhood asthma in urban communities: the impact of ethnicity and income. Ann Epidemiol. 2006;16(5):332–340.
123 Munthe-Kaas MC, Torjussen TM, Gervin K, Lodrup Carlsen KC, Carlsen KH, Granum B, et al. CD14 polymorphisms and serum CD14 levels through childhood: A role for gene methylation? J Allergy Clin Immunol. 2010;125(6):1361–1368.
124 Cornell A, Shaker M, Woodmansee DP. Update on the pathogenesis and management of childhood asthma. Curr Opin Pediatr. 2008;20(5):597–604.
125 Weinmayr G, Weiland SK, Björkstén B, Brunekreef B, Büchele G, et al. Atopic sensitization and the international variation of asthma symptom prevalence in children. Am J Respir Crit Care Med. 2007;176(6):565–574.
126 Anandan C, Nurmatov U, Van Schayck OCP, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010;65(2):152–167.
127 CDC.org. FastStats: Asthma. Atlanta: Centers for Disease Control and Prevention [updated 2010 Oct 27; cited Dec 2010]. Available from http://www.cdc.gov/nchs/fastats/asthma.htm
128 Watson L, Turk F, James P, Holgate ST. Factors associated with mortality after an asthma admission: a national United Kingdom database analysis. Respir Med. 2007;101(8):1659–1664.
129 Gurka MJ, Blackman JA, Heymann PW. Risk of childhood asthma in relation to the timing of early child care exposures. J Pediatr. 2009;155(6):781–787.
130 Lyell PJ, Villanueva E, Burton D, Freezer NJ, Bardin PG. Risk factors for intensive care in children with acute asthma. Respirology. 2005;10(4):436–441.
131 Schramm CM, Carroll CL. Advances in treating acute asthma exacerbations in children. Curr Opin Pediatr. 2009;21(3):326–332.
132 National Asthma Council Australia. Asthma Management Handbook 2006. Melbourne. Australia Ltd: National Asthma Council; 2006.
133 Babl FE, Sheriff N, Borland M, Acworth J, Neutze J, et al. Paediatric acute asthma management in Australia and New Zealand: practice patterns in the context of clinical practice guidelines. Arch Dis Child. 2008;93(4):307–312.
134 Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev 2006. CD000052.
135 Blitz M, Blitz S, Beasely R, Diner B, Hughes R et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev 2005. CD003898.
136 Everard M, Bara A, Kurian M, N’Diaye T, Ducharme F, Mayowe V. Anticholinergic drugs for wheeze in children under the age of two years. Cochrane Database Syst Rev 2005. CD001279.
137 Smith M, Iqbal Shaikh MSI, Rowe BH, N’Diaye T. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev 2003 CD002886.
138 Mitra AAD, Bassler D, Watts K, Lasserson TJ, Ducharme FM. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database Syst Rev 2005. CD001276.
139 Carroll CL, Smith SR, Collins MS, Bhandari A, Schramm CM, Zucker AR. Endotracheal intubation and pediatric status asthmaticus: site of original care affects treatment. Pediatr Crit Care Med. 2007;8(2):91–95.
140 Mayordomo-Colunga J, Medina A, Rey C, Diaz JJ, Concha A, Los Arcos M, et al. Predictive factors of non invasive ventilation failure in critically ill children: A prospective epidemiological study. Intens Care Med. 2009;35(3):527–536.
141 Essouri S, Chevret L, Durand P, Haas V, Fauroux B, Devictor D. Non invasive positive pressure ventilation: Five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med. 2006;7(4):329–334.
142 Stather D, Stewart T. Clinical review: Mechanical ventilation in severe asthma. Crit Care. 2005;9(6):581–587.
143 Yang KD. Consensus, Limitations and Perspectives on Pediatric Asthma Treatment. Pediatr Neonatol. 2010;51(1):5–6.
144 Morrow BM, Argent AC. A comprehensive review of pediatric endotracheal suctioning: Effects, indications, and clinical practice. Pediatr Crit Care Med. 2008;9(5):465–477.
145 Kelly M, Gillies D, Todd DA, Lockwood C. Heated humidification versus heat and moisture exchangers for ventilated adults and children. Cochrane Database Syst Rev 2010. CD004711.
146 Nagaya K, Okamoto T, Nakamura E, Hayashi T, Fujieda K. Airway humidification with a heated wire humidifier during high-frequency ventilation using Babylog 8000 plus in neonates. Pediatr Pulmonol. 2009;44(3):260–266.
147 McKinley D, Kinney S. The challenges of completing a randomised controlled trial evaluating the use of saline during endotracheal suction in the paediatric intensive care unit … The Asia Pacific Critical Care 2008 Congress. Aust Crit Care. 2009;22(1):62–63.
148 Paratz JD, Stockton KA. Efficacy and safety of normal saline instillation: a systematic review. Physiotherapy. 2009;95(4):241–250.
149 Copnell B, Tingay DG, Mills JF, Dargaville PA. Endotracheal suction techniques that effectively remove secretions do not preserve lung volume. Aust Crit Care. 2009;22(1):61.
150 Tarczy-Hornoch P, Mayock DE, Jones D, Meo H, Zerom B, Woodrum D. Mechanical ventilators. Washington: University of Washington Academic Medical Center; Seattle: Seattle Children’s Hospital [updated 2008 Aug 7; cited Feb 2011]. Available from http://depts.washington.edu/nicuweb/NICU-WEB/vents.stm#Authors
151 Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev 2007. CD003844.
152 Shah PS, Ohlsson A, Shah JP. Continuous negative extrathoracic pressure or continuous positive airway pressure for acute hypoxemic respiratory failure in children. Cochrane Database Syst Rev 2008. CD003699.
153 Schönhofer B, Kuhlen R, Neumann P, Westhoff M, Berndt C, Sitter H. Clinical Practice Guideline: Non-Invasive Mechanical Ventilation as Treatment of Acute Respiratory Failure. Dtsch Arztebl Int. 2008;105(24):424–433.
154 Floret D. Non-invasive ventilation as primary ventilatory support for infants with severe bronchiolitis. Intensive Care Med. 2008;34(9):1608–1614.
155 Cools F, Henderson-Smart DJ, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev 2009. CD000104.
156 Frenckner B, Radell P. Respiratory failure and extracorporeal membrane oxygenation. Semin Pediatr Surg. 2008;17(1):34–41.
157 Greenough A. Role of ventilation in RSV disease: CPAP, ventilation, HFO, ECMO. Paediatr Respir Rev. 2009;10(Suppl 1)):26–28.
158 Keckler SJ, Laituri CA, Ostlie DJ, St Peter SD. A review of venovenous and venoarterial extracorporeal membrane oxygenation in neonates and children. Eur J Pediatr Surg. 2010;20(1):1–4.
159 Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev 2008. CD001340.
160 Maclaren G, Butt W, Best D, Donath S, Taylor A. Extracorporeal membrane oxygenation for refractory septic shock in children: one institution’s experience. Pediatr Crit Care Med. 2007;8(5):447–451.
161 Morini F, Goldman A, Pierro A. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: a systematic review of the evidence. Eur J Pediatr Surg. 2006;16(6):385–391.
162 Brierley J, Carcillo JA, Choong K, Cornell T, DeCaen A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666–688.
163 Skippen P, Kissoon N, Waller D, Northway T, Krahn G. Sepsis and Septic Shock: Progress and Future Considerations. Indian J Pediatr. 2008;75(6):599–607.
164 Beardsmore CS, Westaway J, Killer H, Firmin RK, Pandya H. How does the changing profile of infants who are referred for extracorporeal membrane oxygenation affect their overall respiratory outcome? Pediatrics. 2007;120(4):762–768.
165 Donovan C, Blewitt J. An overview of meningitis and meningococcal septicaemia. Emerg Nurse. 2009;17(7):30–36.
166 Nice.org.uk.. Bacterial meningitis and meningococcal septicaemia. London: National Collaborating Centre for Women’s and Children’s Health, Commissioned by the National Institute for Health and Clinical Excellence [updated 2010 Sep; cited Feb 2011]. Available from http: //www.nice.org.uk/nicemedia/live/13027/49437/49437.pdf
167 The International Liaison Committee on Resuscitation. The International Liaison Committee on Resuscitation (ILCOR) consensus on science with treatment recommendations for pediatric and neonatal patients: pediatric basic and advanced life support. Pediatrics. 2006;117(5):e955–e977.
168 Tobias J, Ross A. Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use. Anesth Analg. 2010;110(2):391–401.
169 Sunde G, Heradstveit B, Vikenes B, Heltne J. Emergency intraosseous access in a helicopter emergency medical service: a retrospective study. Scand J Trauma Resusc Emerg Med. 2010;18(1):52.
170 Eslami P. Intraosseous access. New York: WebMD [updated 2010 Jan 27; cited Dec 2010]. Available from http://emedicine.medscape.com/article/940993-overview
171 Luck R, Haines C, Mull C. Intraosseous Access. J Emerg Med. 2010;39(4):468–475.
172 Ackerman A. Meningococcal sepsis in children: persistent problem; new insights? Crit Care Med. 2010;38(1):316–317.
173 Dash N, Al Khusaiby S, Behlim T, Mohammadi A, Mohammadi E, Al Awaidy S. Epidemiology of meningitis in Oman, 2000–2005. East Mediterr Health J. 2009;15(6):1358–1364.
174 Cherry JD. Recognition and management of encephalitis in children. Adv Exp Med Biol. 2009;634:53–60.
175 Kawano G, Iwata O, Iwata S, Kawano K, Obu K, et al. Determinants of outcomes following acute child encephalopathy and encephalitis: pivotal effect of early and delayed cooling. Arch Dis Child. 2010. [Cited Feb 2011]. Available from http://adc.bmj.com/content/early/2010/06/16/adc.2009.180554.short?rss=1
176 Okanishi T, Maegaki Y, Ohno K, Togari H. Underlying neurologic disorders and recurrence rates of status epilepticus in childhood. Brain Dev. 2008;30(10):624–628.
177 Nishiyama I, Ohtsuka Y, Tsuda T, Inoue H, Kunitomi T, Shiraga H, et al. An epidemiological study of children with status epilepticus in Okayama, Japan. Epilepsia. 2007;48(6):1133–1137.
178 Newgard CD, Rudser K, Atkins DL, Berg R, Osmond MH, et al. The availability and use of out-of-hospital physiologic information to identify high-risk injured children in a multisite, population-based cohort. Prehosp Emerg Care. 2009;13(4):420–431.
179 Van de Voorde P, Sabbe M, Rizopoulos D, Tsonaka R, De Jaeger A, et al. Assessing the level of consciousness in children: A plea for the Glasgow Coma Motor subscore. Resuscitation. 2008;76(2):175–179.
180 Cohen J. Interrater reliability and predictive validity of the FOUR score coma scale in a pediatric population. J Neurosci Nurs. 2009;41(5):261–267.
181 Bonkowsky JL, Guenther E, Srivastava R, Filloux FM. Seizures in children following an apparent life-threatening event. J Child Neurol. 2009;24(6):709–713.
182 Chen CY, Chang YJ, Wu HP. New-onset seizures in pediatric emergency. Pediatr Neonatol. 2010;51(2):103–111.
183 American Academy of Pediatrics. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121(6):1281–1286.
184 Mirski MAA. Seizures and status epilepticus in the critically ill. Crit Care Clin. 2007;24(1):115–147.
185 Abou Khaled KJ, Hirsch LJ. Advances in the management of seizures and status epilepticus in critically ill patients. Crit Care Clin. 2007;22(4):637–659.
186 Abou Khaled KJ, Hirsch LJ. Updates in the management of seizures and status epilepticus in critically ill patients. Neurol Clin. 2008;26(2):385–408.
187 Chin RFM, Neville BGR, Scott RC. Meningitis is a common cause of convulsive status epilepticus with fever. Arch Dis Child. 2005;90(1):66–69.
188 Husain EH, Al-Shawaf F, Bahbahani E, El-Nabi MH, Al-Fotooh KA, et al. Epidemiology of childhood meningitis in Kuwait. Med Sci Monitor. 2007;13(5):CR220–CR223.
189 Department of Health and Ageing. Australia’s notifiable diseases status, 2008: Annual report of the National Notifiable Diseases Surveillance System 2008. Commun Dis Intell. 2010 Sep;34(3):157–225.
190 Baumer JH. Guideline review: management of invasive meningococcal disease, SIGN. Arch Dis Child Educ Pract Ed. 2009;94(2):46–49.
191 Branco RG, Amoretti CF, Tasker RC. Meningococcal disease and meningitis. J Pediatr (Rio J). 2007;83(2 Suppl):S46–S53.
192 Centers for Disease Control and Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease – United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005;54(36):893–897.
193 Roche PW, Krause V, Cook H, Barralet J, Coleman D, et al. Invasive pneumococcal disease in Australia, 2006. Commun Dis Intell. 2008;32(1):18–30.
194 Centers for Disease Control and Prevention. Invasive Pneumococcal Disease in Children 5 Years After Conjugate Vaccine Introduction – Eight States, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57(6):144–148.
195 Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14(3):e197–e209.
196 Clarke SC, Jefferies JM, Smith AJ, McMenamin J, Mitchell TJ, Edwards GFS. Potential impact of conjugate vaccine on the incidence of invasive pneumococcal disease among children in Scotland. J Clin Microbiol. 2006;44(4):1224–1228.
197 Mackenzie G, Leach A, Carapetis J, Fisher J, Morris P. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis. 2010;10:304.
198 De Cauwer HG, Eykens L, Hellinckx J, Mortelmans LJ. Differential diagnosis between viral and bacterial meningitis in children. Eur J Emerg Med. 2007;14(6):343–347.
199 Mercier JC. Clinical signs suggestive of bacterial meningitis in infants. Med Mal Infect. 2009;39(7–8):452–461.
200 Prasad K, Singh MB. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2008. CD:002244.
201 van de Beek D, de Gans J, McIntyre P, Prasad K. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2007. CD004405.
202 Seltz LB, Cohen E, Weinstein M. Risk of bacterial or herpes simplex virus meningitis/encephalitis in children with complex febrile seizures. Pediatr Emerg Care. 2009;25(8):494–497.
203 Vial C, Pozzetto B, Essid A, Stephan JL, Chabrier S. Acute encephalitis: report on 32 consecutive pediatric cases observed in one hospital. Med Mal Infect. 2007;37(4):208–214.
204 Clarke M, Newton RW, Klapper PE, Sutcliffe H, Laing I, Wallace G. Childhood encephalopathy: viruses, immune response, and outcome. Dev Med Child Neurol. 2006;48(4):294–300.
205 Sanefuji M, Ohga S, Kira R, Nomura A, Torisu H, et al. Epstein-Barr virus-associated meningoencephalomyelitis: intrathecal reactivation of the virus in an immunocompetent child. J Child Neurol. 2008;23(9):1072–1077.
206 Jmor F, Emsley H, Fischer M, Solomon T, Lewthwaite P. The incidence of acute encephalitis syndrome in Western industrialised and tropical countries. Virol J. 2008;5(1):134.
207 Fischer M, Lindsey N, Staples JE, Hills S. Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59(RR-1):1–27.
208 Fitch MT, Abrahamian FM, Moran GJ, Talan DA. Emergency department management of meningitis and encephalitis. Infect Dis Clin. 2008;22(1):33–52.
209 Kneen R, Jakka S, Mithyantha R, Riordan A, Solomon T. The management of infants and children treated with aciclovir for suspected viral encephalitis. Arch Dis Child. 2010;95(2):100–106.
210 Bailey D, Phan V, Litalien C, Ducruet T, Merouani A, Lacroix J, et al. Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8(1):29–35.
211 Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38(3):933–939.
212 Hackbarth RM, Maxvold NJ, Bunchman TE. Acute renal failure and end-stage renal disease. In: Nichols DG, ed. Rogers’ textbook of pediatric intensive care. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008:1661–1676.
213 Reveiz L, Guerrero-Lozano R, Camacho A, Yara L, Mosquera PA. Stress ulcer, gastritis and gastrointestinal bleeding prophyulaxis in critically ill pediatric patients: A systematic review. Pediatr Crit Care Med. 2010;11(1):124–132.
214 Gathinji M, Harris ZL. Principles of nutrition and metabolism. In: Nichols DG, ed. Rogers’ textbook of pediatric intensive care. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008:1488–1499.
215 Gurgueira G, Leite HP, Taddei J, De Carvalho W. Outcomes in a pediatric intensive care unit before and after the implementation of a nutrition support team. J Parenter Enteral Nutr. 2005;29(3):176–185.
216 van der Kuip M, Oosterveld M, van Bokhorst-de van der Schueren M, de Meer K, Lafeber H, Gemke R. Nutritional support in 111 pediatric intensive care units: a European survey. Intens Care Med. 2004;30(9):1807–1809.
217 Meert K, Daphtary K, Metheny N. Gastris versus small bowel feeding in critically ill children receiving mechanical ventilation. Chest. 2004;126(3):872–878.
218 Petrillo-Albarano T, Pettignano R, Asfaw M, Easley K. Use of a feeding protocol to improve nutritional support through early, aggressive, enteral nutrition in the pediatric intensive care unit. Pediatr Crit Care Med. 2006;7(4):340–344.
219 Eulmesekian PG, Perez A, Minces PG, Bohn D. Hospital-acquired hyponatraemia in postoperative pediatric patients: Prospective observational study. Pediatr Crit Care Med. 2010;11(4):479–483.
220 Montanana PA, Alapont MI, Ocon AP, Lopex PO, Prats JLL, Parreno JDT. The use of isotonic fluids as maintenance therapy prevents iatrogenic hyponatraemia in pediatrics: A randomized, controlled open study. Pediatr Crit Care Med. 2008;9(6):589–597.
221 Yung M, Wilkins B, Norton L, Slater A. Glucose control, organ failure and mortality in pediatric intensive care. Pediatr Crit Care Med. 2008;9(2):147–152.
222 Faustino EVS, Bogue CW. Relationship between hypoglycaemia and mortality in critically ill children. Pediatr Crit Care Med. 2010;11(6):690–698.
223 Tissieres P, Devictor DJ. Fulminant hepatic failure and transplantation. In: Nichols DG, ed. Rogers’ textbook of pediatric intensive care. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008:1535–1549.
224 Farmer DG, Venick RS, McDiarmid SV, Duffy JP, Kattan O, et al. Fulminant hepatic failure in children: superior and durable outcomes with liver transplantation over 25 years at a single center. Ann Surg. 2009;250(3):484–493.
225 Australian Institute of Health and Welfare (AIHW). Injury among young Australians. Canberra: AIHW; 2008.
226 Henley G, Harrison JE. Injury deaths, Australia 2004–05. Adelaide: Australian Institute of Health and Welfare; 2009.
227 Mikrogianakis A, Valani R, Cheng A. The hospital for sick children manual of pediatric trauma. Philadelphia: Lippincott Williams & Wilkins; 2008.
228 Curran J, O’Leary C. Paediatric trauma associated with all-terrain vehicles. Ir Med J. 2008;101(2):55–57.
229 Kreisfeld R. Hospitalised farm injury among children and young people, Australia 2000–01 to 2004–05. Adelaide: Australian Institute of Health and Welfare; 2008.
230 Royal Life Saving Society Australia. The national drowning report 2009. Sydney: Royal Life Saving; 2010.
231 Helps YLM, Pointer SC. Child injury due to falls from playground equipment, Australia 2002–04. Adelaide: Australian Institute of Health and Welfare; 2006.
232 Lamont A. Child deaths from abuse and neglect in Australia. Canberra: Australian Institute of Family Studies; 2010.
233 Hutchings L, Atijosan O, Burgess C, Willett K. Developing a spinal clearance protocol for unconscious pediatric trauma patients. J Trauma. 2009;67(4):681–686.
234 Sandler G, Leishman S, Branson H, Buchan C, Holland AJ. Body wall thickness in adults and children – relevance to pentrating trauma. Injury. 2010;41(5):506–509.
235 Froese NR. Special considerations in paediatric intensive care. Anaesth Intensive Care. 2009;10(10):514–521.
236 Waibel BH, Durham CA, Newell MA, Schlitzkus LL, Sagraves SG, Rotondo MF. Impact of hypothermia in the rural, pediatric trauma patient. Pediatr Crit Care Med. 2010;11(2):199–204.
237 Ducrocq SC, Meyer PG, Orliaguet GA, Blanot S, Laurent-Vannier A, et al. Epidemiology and early predictive factors of mortality and outcome in chlidren with severe traumatic brain injury: Experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7(5):461–467.
238 Australian Institute of Health and Welfare (AIHW). Injury among young Australians. Canberra: AIHW; 2008.
239 Carney NA, Chestnut R, Kochanek PM. Guidelines for the acute medical management of severe traumatic brain injury in infants, children and adolescents. Pediatr Crit Care Med. 2003;4(Suppl 3):S1.
240 Kapapa T, Konig K, Pfister U, Sasse M, Woischneck D, et al. Head trauma in children, part 1: Admission, diagnostics, and findings. J Child Neurol. 2010;25(2):146–156.
241 Orliaguet GA, Meyer PG, Baugnon T. Management of critically ill children with traumatic brain injury. Paediatr Anaesth. 2008;18(6):455–461.
242 Foster K, Stocker C, Schibler A. Controversies of prophylactic hypothermia and the emerging use of brain tissue oxygen tension monitoring and decompressive craniectomy in traumatic brain-injured children. Aust Crit Care. 2010;23(1):4–11.
243 Hutchinson JS, Ward RE, Lacroix J. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–2456.
244 Salorio CF, Slomine BS, Guerguerian AM, Christensen JR, White JRM, et al. Intensive care unit variables and outcome after pediatric traumatic brain injury: A retrospective study of survivors. Pediatr Crit Care Med. 2008;9(1):47–54.
245 National Trauma Registry Consortium (Australia and New Zealand). The National Trauma Registry (Australia & New Zealand) Report: 2005. Herston: National Trauma Registry Consortium (Australia & New Zealand); 2008.
246 Bayreuther J, Wagener S, Woodford M, Edwards A, Lecky F, et al. Paediatric trauma: injury pattern and mortality in the UK. Arch Dis Child Educ Pract Ed. 2009;94(2):37–41.
247 Aleman KB, Meyers MC. Mountain biking injuries in children and adolescents. Sports Med. 2010;40(1):77–90.
248 Zwingmann J, Schmal H, Sudkamp NP, Strohm PC. [Injury severity and localisations seen in polytraumatised children compared to adults and the relevance for emergency room management]. Zentralbl Chir. 2008;133(1):68–75.
249 Holmes JF, Gladman A, Chang CH. Performance of abdominal ultrasonography in pediatric blunt trauma patients: a meta-analysis. J Pediatr Surg. 2007;42(9):1588–1594.
250 Sola JE, Cheung MC, Yang R, Koslow S, Lanuti E, Seaver C, et al. Pediatric FAST and elevated liver transaminases: An effective screening tool in blunt abdominal trauma. J Surg Res. 2009;157(1):103–107.
251 Javouhey E, Iguerin AC, Martin JL, Floret D, Chiron M. Management of severely injured children in road accidents in France: Impact of acute care organization on the outcome. Pediatr Crit Care Med. 2009;10(4):472–478.

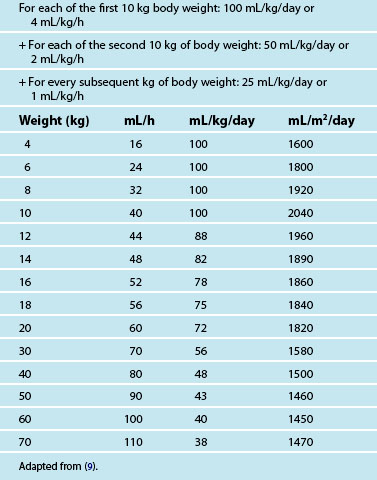
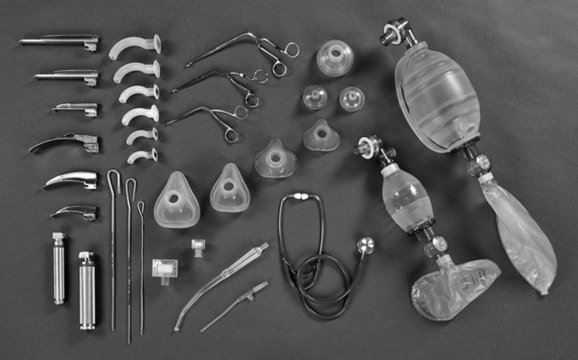
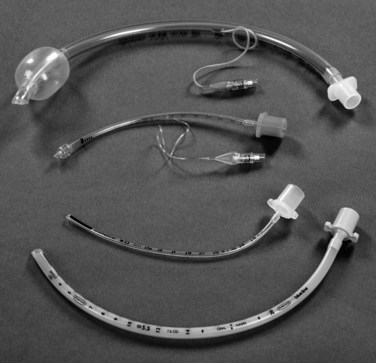
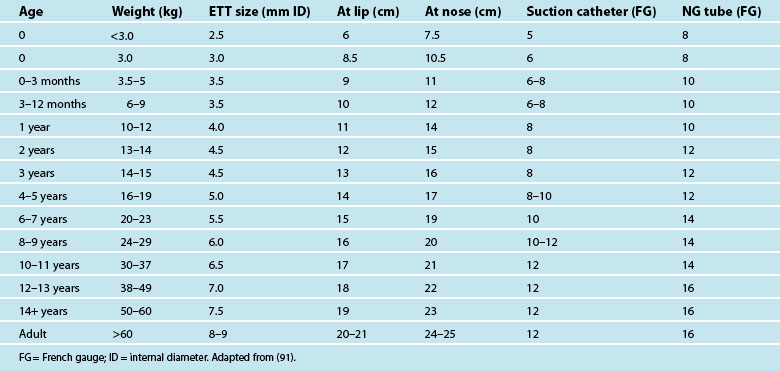
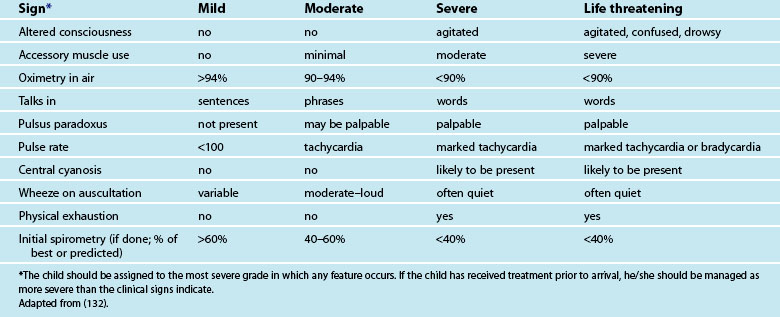
 months post-injury. At her first review with rehabilitation specialists three months post-injury she was moving independently, tremor no longer apparent, could recall three of seven elements after 10 minutes, was animated and social in her interactions. However, ongoing frontal lobe type behaviours of impulsiveness and poor attention span were also noted. Ongoing formal assessment was planned semiannually, including review of her first two terms at school. Returning home has allowed extended family members to assist the family’s recovery and rehabilitation. Lisa’s parents continue to share parenting and care for both children.
months post-injury. At her first review with rehabilitation specialists three months post-injury she was moving independently, tremor no longer apparent, could recall three of seven elements after 10 minutes, was animated and social in her interactions. However, ongoing frontal lobe type behaviours of impulsiveness and poor attention span were also noted. Ongoing formal assessment was planned semiannually, including review of her first two terms at school. Returning home has allowed extended family members to assist the family’s recovery and rehabilitation. Lisa’s parents continue to share parenting and care for both children.