2.3 Paediatric advanced life support (PALS, APLS)
Introduction
Definition of ALS
The recommendations for advanced CPR given here are based on publications of the Australian Resuscitation Council,1 the European Resuscitation Council,2 the American Heart Association3 and the International Liaison Committee on Resuscitation (ILCOR).4 They are intended for use by medical and nursing personnel in hospital and by ambulance personnel in the field.
Diagnosing cardiac arrest
Healthcare personnel (doctors and nurses) have difficulty diagnosing cardiac arrest in infants and children if they rely on pulse palpation alone. Their accuracy is approximately 80% with a sensitivity of 0.85 and specificity of 0.65,5 which means that in 15% of circumstances they would not give CPR when needed and would give it in 35% when not needed. While application of CPR is not harmful when there is a circulation, the withholding of CPR when there is none dooms the patient to die. The time taken to diagnose cardiac arrest is longer than hitherto realised6 – as a group, healthcare personnel take an average of 15 seconds to exclude cardiac arrest by finding a pulse but 30 seconds to diagnose real cardiac arrest by the absence of a pulse. However, the accuracy and expediency of diagnosis are related to experience and training. Only experienced personnel who palpate pulses on a daily basis are able to detect a real pulse within 10 seconds but they, like inexperienced personnel, are unable to quickly diagnose cardiac arrest by the lack of a pulse and need on average about 25 seconds to confirm it. Clinical guidelines advise to spend no more than 10 seconds on pulse palpation and to combine whatever information is gained with observable signs of circulation such as responsiveness, movement and presence or absence of normal respiration. In short, if the patient is unresponsive and not breathing normally there is no point wasting time on pulse palpation (it is inaccurate and time consuming). Instead give CPR immediately.
Oxygen, ventilation and advanced airway support
Ventilation
Self-inflating bags
These bags should not be used to provide supplemental oxygen to a spontaneously breathing patient with a mask placed near or loosely over the face. With Laerdal and Partner bags, negligible amounts of oxygen (0.1–0.3 L min–1) issue from the patient valve when 5–15 L min–1 of oxygen is introduced into bags unconnected to patients.7 The patient valve is unlikely to open unless the mask is sealed well on the face. Although not recommended, if they are used in this way, it is vital to ensure that the patient valve opens or the reservoir bags deflates in unison with the chest movement.
Rates and ratios of external cardiac compression and ventilation in ALS
The techniques of external cardiac compression and expired air resuscitation (mouth-to-mouth) or rescue breathing are described in Section 2.2. The recommended ratio of external cardiac compression to ventilation in basic life support by a single rescuer is 30:21–4 which if able to be repeated 5 times in 2 minutes, with pauses for ventilations, would yield approximately 75 compressions and 5 breaths per minute.
Advanced airway support
Tracheal intubation
The trachea should be intubated as soon as practicable but it can be deferred if successful bag–mask ventilation can be given and should not be undertaken by inexperienced personnel out-of-hospital8 because of complications and poorer outcomes compared with use of bag–mask ventilation. Nonetheless, intubation has numerous advantages, which include establishment and maintenance of the airway, facilitation of mechanical ventilation, titration of oxygen therapy, minimisation of the risk of pulmonary aspiration, enablement of tracheal suction, provision of a route for the administration of selected drugs and preferred for transport and long-term ventilation. Regurgitation of gastric contents is common during cardiac arrest.
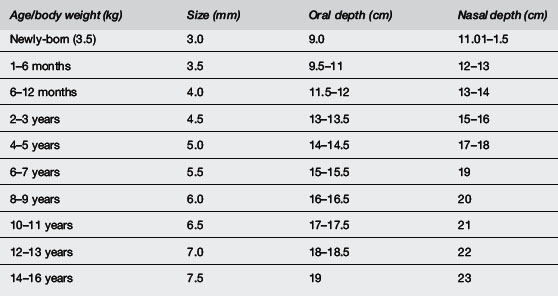
Endotracheal tube size (Table 2.3.1)
Uncuffed sizes are 2.5 mm for a premature newborn <1 kg, 3.0 mm for infants 1–3.5 kg, 3.5 mm for infants >3.5 kg and up to age of six months, size 4 mm for infants seven months to one year (Table 2.3.1). The approximate size may be chosen for children over one year by the formula: size (mm) = age (years)/4 + 4. Tubes one size larger and smaller should be readily available. The correct size should allow a small leak on application of moderate pressure but also enable adequate pulmonary inflation. If the lungs are non-compliant, however, it may be necessary to insert a tube without a leak or insert a cuffed tube. Appropriate- sized cuffed tubes may be estimated by the formula: size (mm) = age (years)/4 + 3.5.
Laryngeal mask airway (LMA)
These have been used for resuscitation by medical, nursing and ambulance personnel trained in their selection and insertion. They may be used to maintain an airway and are a suitable alternative to the use of airway opening manoeuvres and use of oropharyngeal and nasopharyngeal airways. They are useful to establish an airway in the setting of airway obstruction or failed intubation.9 An intubating LMA serves as a conduit for intubation.
However, the role of LMA in provision of mechanical ventilation remains uncertain. Like bag–mask ventilation, they do not protect the airway from aspiration, which occurs commonly during cardiopulmonary resuscitation. They are a suitable alternative to a face mask as a means to give ventilation before endotracheal intubation and when intubation is difficult. This is a better technique when the operator is unskilled in the use of LMA and intubation. Although insertion of an LMA is easier to learn than endotracheal intubation, training should not replace mastery of bag–mask ventilation. They should not be used in semi-conscious patients or when the gag reflex is present and are not suitable for long-term use or use during transport when endotracheal intubation is far preferable. They are subject to dislodgment during movement and transport. Appropriate sizes according to body weight are given in Table 2.3.2.
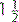
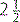
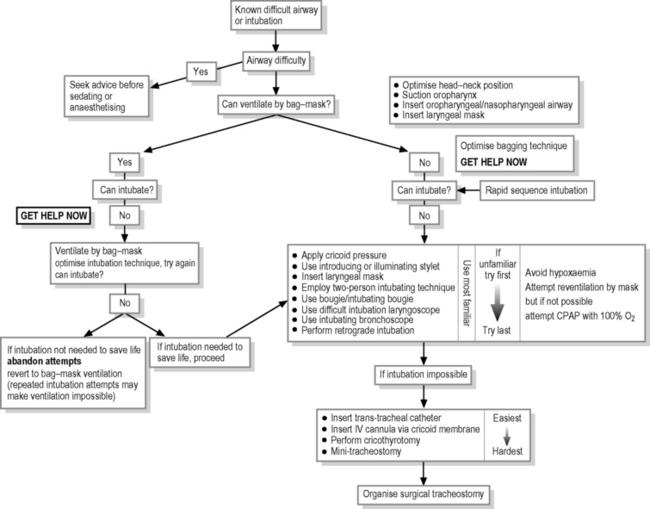
Management of the difficult airway
Resuscitators must be well-skilled in the management of the airway and provision of bag–mask ventilation. It is vital to possess good basic resuscitation techniques (see Chapter 2.2 on basic life support), to be familiar with equipment, to have skilled assistance and to have appropriate equipment ready-to-hand. They must also be familiar with manoeuvres to overcome difficulties with airway maintenance, bag–mask ventilation and intubation. Resuscitators must also have a pre-conceived plan to cope with difficult and failed (impossible) intubation, especially when bag–mask ventilation fails (Fig. 2.3.1).
Monitoring
Vascular access
Other techniques
Surgical cutdown onto a long saphenous, saphenofemoral junction or basilic is a valuable skill sometimes required in traumatic exsanguination. Very occasionally, injection into the superior sagittal sinus of an infant1 may be the only vascular access available. Any pre-existing functioning line can be used provided it does not contain any drug or electrolyte, which may have caused the CPA.
Fluid therapy
Resuscitation drugs
Calcium
Calcium should not be administered during acute resuscitation unless the cause of collapse is due to hypocalcaemia, hyperkalaemia, calcium channel blocker overdose or hypermagnesaemia. Although intimately involved in myocardial excitation-contraction coupling, it is not useful and possibly harmful, by causing cell death, in the regular treatment of asystole, electromechanical dissociation and ventricular fibrillation. Its use is associated with poor outcomes10 and it should not be used without a definite indication. If it is indicated, the dose is 0.2 mL kg–1 of 10% calcium chloride or 0.7 mL kg–1 of 10% calcium gluconate and it may be repeated after 10 minutes according to the serum level if possible.
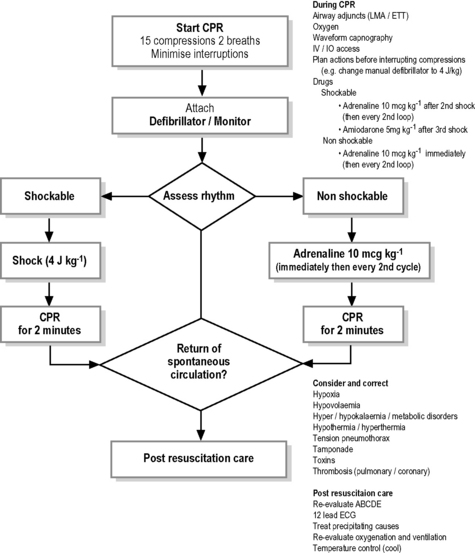
Management of pulseless arrhythmias (Fig. 2.3.2)
The following discussion assumes that mechanical ventilation with oxygen and external cardiac compression (ECC) have been commenced and continued if an adequate pulse rate is not detectable. The treatment of pulseless arrhythmias (ventricular fibrillation, ventricular tachycardia, asystole, electromechanical dissociation and pulseless electrical activity) are summarised in Fig. 2.3.2
Ventricular fibrillation and pulseless ventricular tachycardia
Either monophasic or biphasic waveforms may be used. The optimum dose of external DC shock in terms of achieving first shock success with minimal damage to the myocardium is unknown. Some guidelines recommend a dose of 2–4 J kg−1.3,4 The dose of 2 J kg–1 is considered too little by other guidelines1,2 which advise 4 J kg–1. This is supported by a recent study which showed that 2 J kg–1 converted only about 50% of patients to a perfusing rhythm.12 In contrast to previous recommendations, it is now recommended to give one shock followed immediately by uninterrupted CPR for 2 minutes without pausing to determine if another shock is required. Only in monitored witnessed onset of VF and immediate availability of defibrillation (first dose within 30 seconds) is a stack of up to three shocks (each 4 J kg–1)1,2 without intervening CPR recommended. If ROSC has not occurred within 10 seconds of any of the 3 shocks CPR should be given. DC shock may be more successful if front and back placement of pads is used. Whatever position, use of pads rather than paddles enables minimal disruption to continuous external cardiac compression.
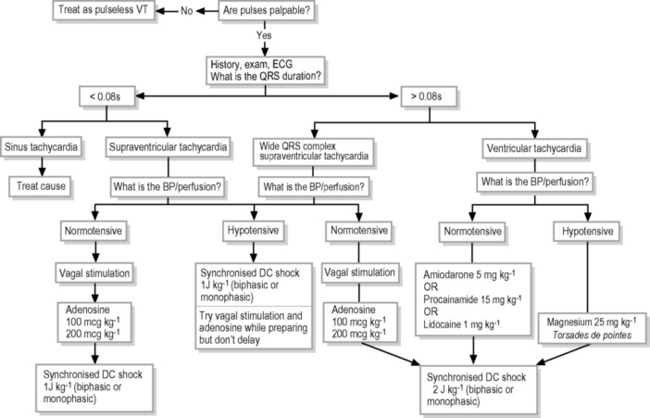
Management of pulsatile dysrhythmias
Supraventricular tachycardia
If haemodynamically stable (adequate perfusion and blood pressure), initial treatment of SVT should be vagal stimulation. For infants and young children, application to the face of a plastic bag filled with iced-water is often effective, or alternatively submersion of the face into a slurry of ice and water in a bowl. Older children may be treated with carotid sinus massage or asking them to perform a Valsalva manoeuvre – such as blowing through a narrow straw. If unsuccessful, give adenosine initially at 100 mcg kg–1 IV by rapid bolus (max dose 6 mg), increasing to 200 mcg kg–1 or 300 mcg kg–1. In older children, it is important to describe the transient feeling of ’chest heaviness or breathing difficulty or fearfulness‘ that may accompany adenosine administration, and/or precede the adenosine with a small amnestic dose of midazolam. If unsuccessful, give synchronised DC shock (cardioversion)13 initially at 0.5?1.0 J kg–1 but subsequently up to 2 J kg–1. If at the outset SVT is accompanied by haemodynamic instability, proceed to cardioversion (synchronised 1.0 J kg–1) immediately, although vagal stimulation or adenosine (IV or IO) may be used, provided they do not delay cardioversion. Verapamil should not be used to treat SVT in infants and should be avoided in children because it induces hypotension by vasodilation and negative inotropic effect.
Post-resuscitation management
Survival and neurological outcome is better when deliberate hypothermia is used after cardiac arrest. ILCOR has recommended therapeutic hypothermia (32–34°C) for 12–24 hours for adults and children1 who remain unconscious with spontaneous circulation after out-of-hospital cardiac arrest when the initial rhythm was ventricular fibrillation, and suggested that for any other rhythm, or cardiac arrest in hospital, such cooling may also be beneficial. The optimal duration of such requirement, however, is unknown but clinical studies among newborns suggest 72 hours.14 Inadvertently hypothermic patients, provided temperature is above 32°C or greater should not be actively warmed and hyperthermia should be aggressively treated. If deliberate hypothermia is employed, shivering should be prevented with sedation and/or neuromuscular blockade. Seizures should be actively sought and treated with anticonvulsant.
Cessation of CPR
Long-term outcome from paediatric CPR is poor, with approximately 5–10% of patients surviving out-of-hospital arrest15 and 25–50% in-hospital cardiac arrest.16,17 The decision to cease CPR should be based on a number of factors including the duration of resuscitation, its quality, response to treatment, pre-arrest status of the patient, remediable factors, likely outcome if ultimately successful, opinions of personnel familiar with the patient and, whenever appropriate, the wishes of informed parents. In general, unless hypothermia or drug toxicity exists, survival to normality is most unlikely if there has been a failure to respond to full CPR after 30 minutes and several doses of adrenaline, unless environmental hypothermia was an important aetiological or consequential factor. In the newly-born infant, discontinuation of treatment is appropriate if CPR does not establish a spontaneous circulation within 15 minutes.1 Family members should be kept informed, allowed to be present or asked if they want to be present during resuscitation (see Chapter 2.1).
Prevention of cardiorespiratory arrest
Of course, hospital personnel should be well-trained and organised to treat unexpected cardiorespiratory arrest, but the far preferable course of action is prevention. Some paediatric hospitals have instituted ‘Rapid Response Team’ systems or ‘Medical Emergency Team’ systems, which can respond rapidly to deterioration in a patient’s condition before cardiorespiratory arrest occurs, with consequent significant reductions in unexpected cardiac arrest (38%) mortality18,19 (21%) and unplanned admission to intensive care.
1 . Australian Resuscitation Council Guidelines. Melbourne. p. 12.1–12.7. http://www.resus.org.au
2 Biarent D., Bimgham R., Eich C., et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 6. Paediatric life support. Resuscitation. 2010;81:1364-1388.
3 Kleinman M.E., Chameides L., Schexnayder S.M., et al. Part 14: Pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S876-S908.
4 de Caen A.R., Kleinman M.E., Chameides L., et al. Part 10: Paediatric basic and advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2010;81:e213-e259.
5 Tibballs J., Russell P. Reliability of pulse palpation by healthcare personnel to diagnose paediatric cardiac arrest. Resuscitation. 2009;80:61-64.
6 Tibballs J., Weeranatna C. The influence of time on the accuracy of healthcare personnel to diagnose paediatric cardiac arrest by pulse palpation. Resuscitation. 2010;81(6):671-675.
7 Carter B.G., Fairbank B., Tibballs J., et al. Oxygen delivery using self-inflating resuscitation bags. Pediatr Crit Care Med. 2005;6:125-128.
8 Gausche M., Lewis R.J., Stratton S.J., et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA. 2000;283:783-790.
9 Benumot J.L. Laryngeal mask airway and the ASA difficult airway algorithm. Anesthesiology. 1996;84:686-699.
10 Srinivasan M., Morris M.C., Helfaer M.A., et al. Calcium use during in-hospital pediatric cardiopulmonary resuscitation: a report from the National Registry of Cardiopulmonary Resuscitation. Pediatrics. 2008;121:e114-e151.
11 Stiell I.G., Hebert P.C., Wells G.A., et al. Vasopressin versus epinephrine for inhospital cardiac arrest: A randomised controlled trial. Lancet. 2001;358:105-109.
12 Tibballs J., Carter B., Kiraly N.J., et al. External and internal biphasic DC shock doses for pediatric ventricular fibrillation and pulseless ventricular tachycardia. Pediatr Crit Care Med. 2011;12:14-20.
13 Tibballs J., Carter B., Kiraly N.J., et al. Biphasic DC shock cardioverting doses for paediatric atrial dysrhythmias. Resuscitation. 2010;81:1101-1104.
14 Kochanek P.M., Fink E., Bell M.J., et al. Therapeutic hypothermia: applications in pediatric cardiac arrest. J Neurotrauma. 2009;26:421-427.
15 Deasy C., Bernard S.A., Cameron P., et al. Epidemiology of paediatric out-of-hospital cardiac arrest in Melbourne, Australia. Resuscitation. 2010;81:1095-1100.
16 Tibballs J., Kinney S. A prospective study of outcome of in-patient paediatric cardiopulmonary arrest. Resuscitation. 2006;71:310-318.
17 Meert K.L., Donaldson A., Nadkarni V., et al. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:544-553.
18 Chan P.S., Jain R., Nallmothu B.K., et al. Rapid response teams. A systematic review and meta- analysis. Arch Intern Med. 2010;170:18-26.
19 Tibballs J., Brilli R.J. Pediatric RRSs. In: De Vita M.A., Hillman K., Bellomo R., editors. Textbook of Rapid Response Systems: Concepts and Implementation. New York: Springer; 2011:231-243.